Abstract
Background
The purpose of this study was to assess the effectiveness and safety of a model predictive control (MPC) algorithm for an artificial pancreas system in outpatients with type 1 diabetes.
Methods
We searched PubMed, EMBASE, Cochrane Central, and the Web of Science to December 2021. The eligibility criteria for study selection were randomized controlled trials comparing artificial pancreas systems (MPC, PID, and fuzzy algorithms) with conventional insulin therapy in type 1 diabetes patients. The heterogeneity of the overall results was identified by subgroup analysis of two factors including the intervention duration (overnight and 24 h) and the follow-up periods (< 1 week, 1 week to 1 month, and > 1 month).
Results
The meta-analysis included a total of 41 studies. Considering the effect on the percentage of time maintained in the target range between the MPC-based artificial pancreas and conventional insulin therapy, the results showed a statistically significantly higher percentage of time maintained in the target range in overnight use (10.03%, 95% CI [7.50, 12.56] p < 0.00001). When the follow-up period was considered, in overnight use, the MPC-based algorithm showed a statistically significantly lower percentage of time maintained in the hypoglycemic range (−1.34%, 95% CI [−1.87, −0.81] p < 0.00001) over a long period of use (> 1 month).
Conclusions
Overnight use of the MPC-based artificial pancreas system statistically significantly improved glucose control while increasing time maintained in the target range for outpatients with type 1 diabetes. Results of subgroup analysis revealed that MPC algorithm-based artificial pancreas system was safe while reducing the time maintained in the hypoglycemic range after an overnight intervention with a long follow-up period (more than 1 month).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-022-00962-2.
Keywords: Artificial pancreas, Algorithm, Model predictive control, Hypoglycemia, Type 1 diabetes
Background
Type 1 diabetes (T1D), which occurs mainly in children and adolescents, is caused by insulin deficiency due to the auto-immune destruction of beta cells in the pancreas [1]. T1D patients require intensive management early in the disease to achieve HbA1c levels close to normal [2]. The biggest problem with T1D management is the occurrence of severe hypoglycemia and ketoacidosis [3]. In particular, hypoglycemia is known as a symptom that occurs frequently in patients with T1D due to inadequate dosage and timing of sulfonylureas or insulin used for treatment. [4]. Continuous blood glucose monitoring and insulin dose adjustment are keys to reducing such complications [5]. Clinical research on insulin therapy is needed for effective treatment and the prevention of complications. In addition, studies according to the follow-up period are necessary to obtain clinical evidence for identifying the risk factors [6].
Conventional insulin therapy includes continuous subcutaneous insulin infusion (CSII) and sensor-augmented pumps (SAP). CSII is a treatment that delivers insulin at a preselected basal infusion rate by the sensor. It is not administered automatically according to blood glucose. Instead, it is injected by calculating and setting the dose according to manually measured blood sugar level. It does not provide blood glucose data. BG measurements are used to make treatment decisions [7]. SAP consists of a combination of CGM and CSII, a glucose sensor introduced into insulin pump therapy [8, 9]. This is capable of monitoring treatment and response of the patients [10]. CSII and SAP have the advantage of being able to control the infusion rate as much as needed and continuously deliver insulin. However, the disadvantage is that if the pump does not work properly, the risk of complications by administering too little or too much insulin can be high [11]. To compensate for these shortcomings, artificial pancreas systems have been developed. The real strength of the artificial pancreas system is in regulating basal insulin injections to change the glycemic status (both hyperglycemia and hypoglycemia). That is, improving metabolic control without increasing the risk of hypoglycemia in T1D patients by aiming to increase the proportion of time in the target range [12].
An artificial pancreas is an automated system that automatically measures blood glucose levels to achieve the target range and injects insulin into the blood accordingly. This system consists of three devices including: (1) a sensor, such as a continuous glucose monitor (CGM) that transmits data to an algorithm after measuring blood glucose; (2) an algorithm that analyzes the data and calculates the required insulin injection dose, and (3) an insulin infusion pump that delivers insulin according to the algorithm [13]. Among these, at the core of artificial pancreas technology is an algorithm that calculates the amount of insulin required to maintain a patient's glucose level within the target range [14]. There are three main types of control algorithms for glucose regulation, model predictive control (MPC), proportional integral derivative (PID), and fuzzy logic (FL). The MPC algorithm predicts future glucose levels to bring current blood glucose levels into the target range. The PID algorithm analyzes the deviation of the measured glucose from the target range to calculate the amount of insulin to deliver. The fuzzy algorithm quickly mimics the insulin dose calculates made by clinical experts based on monitoring data [15]. The main role of an algorithm is to keep blood glucose level in a safe range. Therefore, the type of algorithm is considered an important factor in safe blood glucose control.
Several meta-analyses have compared artificial pancreas systems and conventional insulin therapy according to the intervention period [16–18] and, hormone type [16, 17]. Weisman et al [16] and Karageorgiou et al [19] evaluated the effectiveness and safety of artificial pancreas algorithm types, but reported no details regarding which algorithms affected the outcomes. In a recent clinical study (Haidar et al. [20]), an MPC-based artificial pancreas system (69%) showed a greater effect on the percentage of time maintained in the target blood glucose range than a sensor-augmented pump (61%). Pinsker and colleagues directly compared the effectiveness of artificial pancreas systems according to algorithm types (MPC vs PID) with conventional insulin therapy [21]. The mean difference was greater for the MPC (74.4%) than for the PID algorithm (63.7%). To the best of our knowledge, no previous meta-analysis has analyzed the influence of algorithm types on outcomes and compared them according to the follow-up period. This systematic review and meta-analysis aimed to determine whether an MPC algorithm-based artificial pancreas system might be more effective and safe than conventional insulin therapy in terms of the risk of hypoglycemia and maintaining glucose levels within the target range in outpatients with T1D. Moreover, to identify the finding of the previous studies [16, 21] that the MPC algorithm performed well or better than PID in terms of safe and effective glucose management, additional meta-analysis has analyzed the influence of algorithm types on outcomes.
Methods
Search strategy
We searched PubMed, EMBASE, Web of Science (WoS), and the Cochrane Central Register of Controlled Trials to December 2021. For the search terms, mesh terms and natural languages were used, including: “((((artificial pancreas[MeSH Terms]) OR (diabetes mellitus[MeSH Terms])) AND (diabetes mellitus, type 1[MeSH Terms])) AND (controlled clinical trials, randomized[MeSH Terms])) AND (algorithm[MeSH Terms])”, “Artificial pancreas OR Closed-loop system AND Diabetes mellitus type 1 AND Clinical trial AND Randomized clinical trial”. This study was reported according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement (PROSPERO, number 2021:CRD42021268271) (PRISMA Checklist–Additional file 1: Table S1).
Inclusion and exclusion criteria
Studies that met the following criteria were included: (1) type 1 diabetes outpatients including non-pregnant adults, children, and infants; (2) interventions using an algorithm-based artificial pancreas; (3) comparisons using conventional insulin therapy (SAP or insulin pumps); (4) outcomes including the outcome variables of the percentage of time maintained in the target blood glucose range (3.9–10 mmol), the percentage of time maintained in the hypoglycemic range (< 3.9 mmol), and the daily insulin dose; (5) Randomized clinical trials (RCTs) including crossover and parallel studies. Studies meeting the following criteria were excluded: (1) non-RCT studies; (2) duplicated documents; (3) review papers or letters; (4) clinical trial protocol studies, and (5) studies that did not report at least one of the outcomes.
Selection of studies
Two reviewers (Kang and Hwang) selected the studies. The studies were screened by title and then selected by abstract and content. Disagreements regarding the original study were discussed, and jointly reviewed to reach a consensus. In the meta-analysis, only specified studies for each purpose were selected from included studies. In the meta-analysis to assess the effectiveness and safety of the MPC algorithm, only studies comparing the MPC-based artificial pancreas system with conventional insulin therapy were selected from included studies. In additional analysis of algorithm types, studies comparing algorithm-based artificial pancreas with conventional insulin therapy were selected from included studies.
Data extraction
Data including the study, year, patients, devices, intervention, comparator, duration, and follow-up period were extracted. A standardized format was used by two independent reviewers (Kang and Hwang). Disagreements regarding the original study were discussed, and jointly reviewed to reach a consensus.
Quality assessment
Risk of bias (RoB) is the likelihood that a characteristic of the study design or study conduct will give erroneous results. The RoB is evaluated according to random sequence generation, allocation concealment, blinding of the participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other biases. It was assessed as high, low, or unclear using the Cochrane risk of bias tool.
Outcome measures
The primary outcome was the percentage of time maintained in the target blood glucose range (3.9–10 mmol). The secondary outcomes were the percentage of time maintained in the hypoglycemic range (< 3.9 mmol) and the daily insulin dose. If the primary and second outcome data were reported separately, they were analyzed separately in this study. Studies done over a 24 h period reported 24 h results and studies done overnight reported overnight results. If the adult and pediatric data were reported separately, they were analyzed separately, and if the single and dual hormone data were reported separately, they were analyzed separately.
Statistical analysis
The forest plot is one of the most useful tools for providing a visual summary of the analysis results. It graphically presents estimates of the overall effect size and confidence intervals of the included studies. The mean difference (MD) was calculated for all results, and a random-effects model was used. Ninety-five percent confidence intervals (CI) were calculated for all analyses, and the significance level was 0.05 (p < 0.05). When presented as an interquartile range (IQR) value, the standard deviation (SD) was calculated as (q3-q1)/1.35 according to Cochrane’s recommendation [22, 23]. The heterogeneity was evaluated by I2. If it was 50% or more, heterogeneity was identified, and 75% or more, heterogeneity was identified as high. Subgroup analysis was conducted to identify heterogeneity according to the intervention duration (overnight and 24 h), follow-up period (< 1 week, 1 week to 1 month, and > 1 month), and algorithm types (MPC, PID, and Fuzzy). Sensitivity analysis was performed on the primary outcome, which was the percentage of time maintained in the target blood glucose range to explore the cause of high heterogeneity. Meta-analysis was analyzed using Revman 5.3 software, and statistical analysis was performed through SPSS Statistics 25.
Results
Characteristics of the included studies
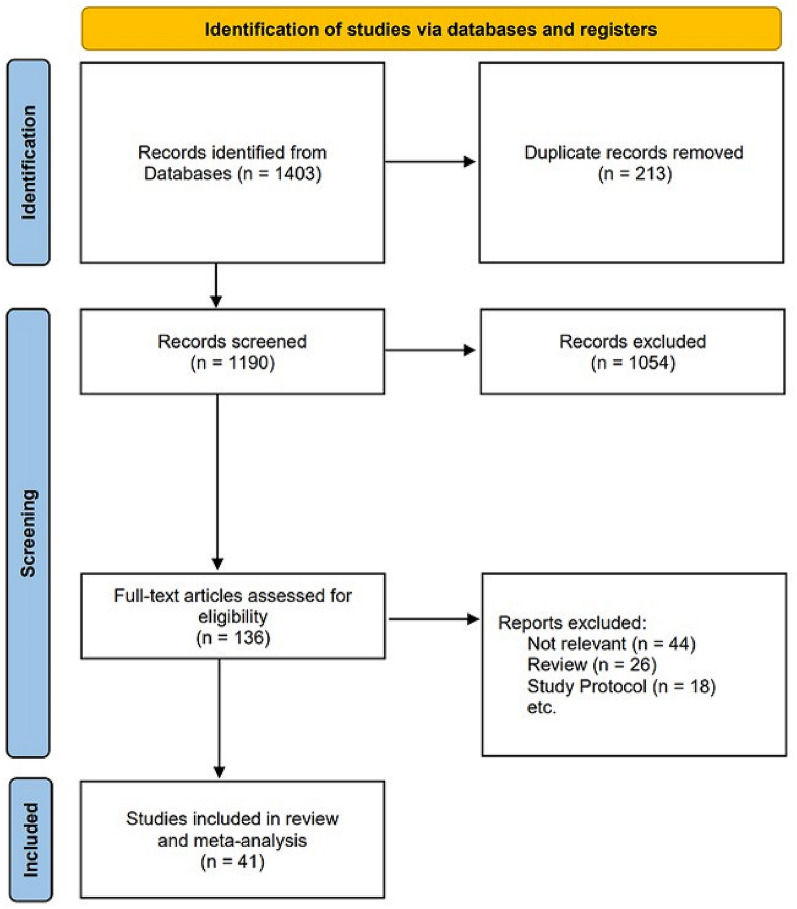
Figure 1 shows a flowchart of the study selection process. A total of 1,403 studies were searched, 213 duplicate studies were removed and 1,190 studies were screened. Of the studies, 1,054 were excluded by the title and abstract contents, and the full-text of 136 studies was evaluated. Unrelated documents such as reviews, letters, and clinical trial protocols were excluded. Most clinical trials performed artificial pancreas system (MPC, PID, and Fuzzy) vs. conventional insulin therapy. To analyze the effectiveness and safety of MPC algorithm-based artificial pancreas systems and the influence of algorithm type on outcomes, inclusion criteria were set as studies that compared artificial pancreas system with conventional insulin therapy. A total of 41 documents were finally included.
Fig. 1.
Flowchart of the study selection
Table 1 shows the characteristics of 41 studies [20, 24–62] included in the systematic review (1,398 total patients). Nine trials compared insulin pumps, and 32 trials compared sensor-augmented pumps. Thirty-three trials used the MPC algorithm, 6 trials used the PID algorithm, and 2 trials used the fuzzy algorithm. The artificial pancreas system was used overnight in 15 trials and 24 h in 26 trials. According to the follow-up period, 18 trials reported periods of less than 1 week, 14 trials reported between 1 week and 1 month, and 9 trials reported periods of more than 1 month. The detailed characteristics are given in Additional file 1: Table S2.
Table 1.
Characteristics of the included studies
| No | Study | Year | Patients | Intervention | Algorithm | Comparator | Duration | Follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | Anderson et al. [24] | 2019 | 42 | DiAs USS with Dexcom | MPC | SAP | 24 h | 4 weeks |
| 2 | Bally et al. [25] | 2017 | 29 | Florence with freestyle navigator | MPC | SAP | 24 h | 4 weeks |
| 3 | Benhamou et al. [26] | 2019 | 63 | Hybrid closed-loop system | MPC | SAP | 24 h | 12 weeks |
| 4 | Blauw et al. [27] | 2016 | 10 | Inreda diabetic | PID | Pump | 24 h | 4 days |
| 5 | Breton et al. [28] | 2020 | 101 | t:slim X2 insulin pump, Dexcom with Control-IQ Technology | MPC | SAP | 24 h | 16 weeks |
| 6 | Breton et al. 2 [29] | 2017 | 32 | t:AP pump or Roche Accu-Chek Spirit Combo pump, Dexcom with DiAs | MPC | SAP | 24 h | 120 h |
| 7 | Brown et al. [30] | 2019 | 168 | t:slim X2 insulin pump with Control-IQ Technology, Tandem Diabetes Care, Dexcom | MPC | SAP | 24 h | 6 months |
| 8 | Brown et al. 2 [31] | 2017 | 40 | DiAs USS with Dexcom | MPC | SAP | Overnight | 5 days |
| 9 | Brown et al. 3 [32] | 2015 | 10 | Accu-Chek Spirit Combo pump or personal pump, Dexcom with DiAs system | PID | SAP | Overnight | 5 days |
| 10 | Chernavvsky et al. [33] | 2016 | 16 | DiAs USS with Dexcom | MPC | Pump | 24 h | 1 day |
| 11 | De bock et al. [34] | 2018 | 12 | Medtronic MiniMed Hybrid Closed Loop System | MPC | Pump | 24 h | 7 days |
| 12 | De boer et al. [35] | 2017 | 12 | DiAs USS with Dexcom | MPC | SAP | 24 h | 3 days |
| 13 | Del Favero et al. [36] | 2016 | 30 | Accu-Chek Spirit Combo pump or personal pump, Dexcom with DiAs system | MPC | SAP | 24 h | 72 h |
| 14 | El-Khatib et al. [37] | 2017 | 39 | Two(one for insulin, one for glucagon) t:Slim infusion pumps, Dexcom | MPC | Pump | 24 h | 11 days |
| 15 | Elleri et al. [38] | 2013 | 12 | SEVEN PLUS; Dexcom | MPC | Pump | Overnight | 36 h |
| 16 | Forlenza et al. [39] | 2017 | 19 | DiAs | MPC | SAP | 24 h | 2 weeks |
| 17 | Forlenza et al. 2 [40] | 2017 | 28 | Medtronic PHHM | MPC | SAP | Overnight | 21 nights |
| 18 | Haidar et al. [20] | 2021 | 36 | Dexcom CGM system, t:slim TAP3 insulin pump | MPC | SAP | 24 h | 12 days |
| 19 | Hovorka et al. [41] | 2014 | 16 | Florence with FreeStyle Navigator | MPC | SAP | Overnight | 21 days |
| 20 | Huyett et al. [42] | 2017 | 10 | DiAs with Dexcom | MPC | SAP | 24 h | 72 h |
| 21 | Kovatchev et al. [43] | 2020 | 125 | Accu-Chek Spirit Combo insulin pump, Dexcom CGM system, and inControlAP | MPC | SAP | Overnight | 3 months |
| 22 | Kovatchev et al. 2 [44] | 2020 | 78 | Accu-Chek Spirit Combo insulin pump, Dexcom CGM system, and inControlAP | MPC | SAP | Overnight | 10 months |
| 23 | Kovatchev et al. 3 [45] | 2014 | 18 | Tandem t:slim pump, with DiAs system | PID | SAP | 24 h | 40 h |
| 24 | Kropff et al. [46] | 2015 | 32 | Accu-Chek Spirit Combo insulin pump, Dexcom CGM system | MPC | SAP | Overnight | 12 weeks |
| 25 | Leelarathna et al. [47] | 2014 | 17 | Florence with FreeStyle Navigator | MPC | SAP | 24 h | 8 days |
| 26 | Ly et al. [48] | 2016 | 21 | Medtronic MiniMed Hybrid Closed Loop System | PID | SAP | Overnight | 5–6 days |
| 27 | Ly et al. 2 [49] | 2015 | 21 | Medtronic MiniMed Hybrid Closed Loop System | PID | SAP | 24 h | 6 days |
| 28 | Ly et al. 3 [50] | 2014 | 20 | Medtronic MiniMed Hybrid Closed Loop System | PID | SAP | Overnight | 5–6 days |
| 29 | Nimri et al. [51] | 2014 | 24 | MD-Logic system with Medtronic Paradigm Veo pump | Fuzzy | SAP | Overnight | 6 weeks |
| 30 | Nimri et al. 2 [52] | 2014 | 15 | MD-Logic system with Medtronic Paradigm Veo pump | Fuzzy | SAP | Overnight | 4 days |
| 31 | Renard et al. [53] | 2018 | 23 | DiAs with Dexcom | MPC | SAP | 24 h | 2 days |
| 32 | Russell et al. [54] | 2016 | 19 | Two(one for insulin, one for glucagon) t:Slim infusion pumps, Dexcom | MPC | Pump | 24 h | 5 days |
| 33 | Russell et al. 2a [55] | 2014 | 20 | Two(one for insulin, one for glucagon) t:Slim infusion pumps, Dexcom | MPC | Pump | 24 h | 5 days |
| 34 | Russell et al. 2b [55] | 2014 | 32 | Two(one for insulin, one for glucagon) t:Slim infusion pumps, Dexcom | MPC | Pump | 24 h | 5 days |
| 35 | Sherr et al. [56] | 2020 | 11 | Omnipod hybrid closed loop system | MPC | Pump | Overnight | 7 days |
| 36 | Spaic et al. [57] | 2017 | 30 | Medtronic PHHM | MPC | SAP | Overnight | 21 nights |
| 37 | Tauschmann et al. [58] | 2018 | 86 | Medtronic hybrid closed loop system | MPC | SAP | 24 h | 12 weeks |
| 38 | Tauschmann et al. 2 [59] | 2016 | 12 | Florence with freestyle navigator | MPC | SAP | 24 h | 3 weeks |
| 39 | Tauschmann et al. 3 [60] | 2016 | 12 | Florence with freestyle navigator | MPC | SAP | 24 h | 7 days |
| 40 | Thabit et al. [61] | 2015 | 33 | Florence with freestyle navigator | MPC | SAP | 24 h | 12 weeks |
| 41 | Thabit et al. 2 [62] | 2014 | 24 | Florence with freestyle navigator | MPC | SAP | Overnight | 4 weeks |
MPC Model Predictive Control, PID Proportional Integral Derivative, SAP Sensor-Augmented Pump, CGM Continuous Glucose Monitoring, DiAs Diabetes Assistant
Assessment of risk of bias
The results of the bias evaluation are presented in Additional file 1: Figures S1, S2. Of the 41 studies, only 13 studies had a low risk, and 4 studies showed a high risk. Three of 4 studies were rated at high risk due to insufficient data. One was at high risk due to the small number of patients and lack of patient information.
Primary outcome
Thirty-three comparisons with 1,311 patients were pooled to analyze the effectiveness of glucose control of the MPC-based artificial pancreas system. The percentage of time maintained in the target blood glucose range was 12.57% ([MD], 95% CI [9.63, 15.50] p < 0.00001), higher than that of, conventional insulin therapy (Additional file 1: Table S3). However, the heterogeneity was high (I2 = 89%).
Secondary outcomes
Thirty comparisons with 1,237 patients were pooled to analyze the safety according to time maintained in hypoglycemia range in the MPC-based artificial pancreas system. The percentage of time maintained in the hypoglycemic range was −1.12% ([MD], 95% CI [-1.50, -0.75] p < 0.00001) in the MPC algorithm-based artificial pancreas system, lower than that with the conventional insulin therapy (Additional file 1: Table S3). However, the heterogeneity still existed (I2 = 64%).
Sixteen comparisons with 724 patients reported U/day or U/8 h were pooled for evaluating the daily insulin dose in the MPC-based artificial pancreas system. The daily insulin dose in the MPC algorithm-based artificial pancreas system showed a statistically significant decrease in 24 h interventions ([MD], −1.24U, 95% CI [−2.43, −0.06] p = 0.04), compared to conventional insulin therapy (Additional file 1: Table S4).
Subgroup analysis of intervention timing (24 h versus overnight) in studies using the MPC algorithm
Subgroup analysis was performed to identify the cause of the heterogeneity in the percentage of time maintained in the target blood glucose range and hypoglycemic range, when restricted to studies using the MPC algorithm with sufficient data. The protocol for the subgroup analysis is shown in Additional file 1: Figure S3. We subdivided the studies according to the timing of the intervention (24 h or overnight).
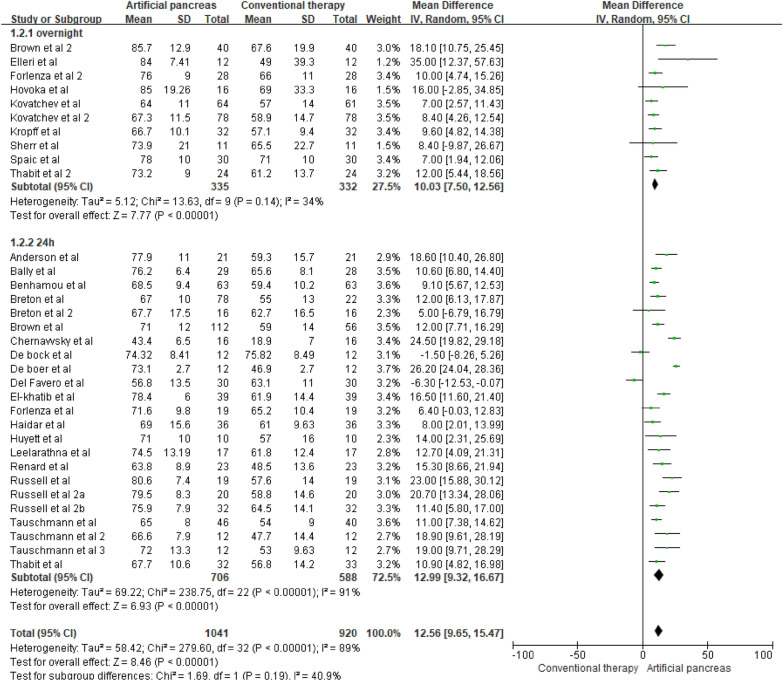
Figures 2 and 3 show the forest plots of MPC-based artificial pancreas systems versus conventional insulin therapy for the percentage of time maintained in the target blood glucose range (23 24 h studies and 10 overnight studies) and hypoglycemic range (20 24 h studies and 10 overnight studies), respectively. Differences in the percentage of time maintained in the target blood glucose range were higher in studies with 24 h of intervention ([MD], 12.99%, 95% CI [9.32, 16.67] p < 0.00001) compared to overnight interventions ([MD], 10.03%, 95% CI [7.50, 12.56] p < 0.00001, Fig. 2). However, there was high heterogeneity (I2 = 91%) in the subgroup of studies with 24 h interventions, which requires further exploration.
Fig. 2.
Mean difference in time maintained in the target blood glucose range according to the intervention duration (MPC algorithm-based artificial pancreas)
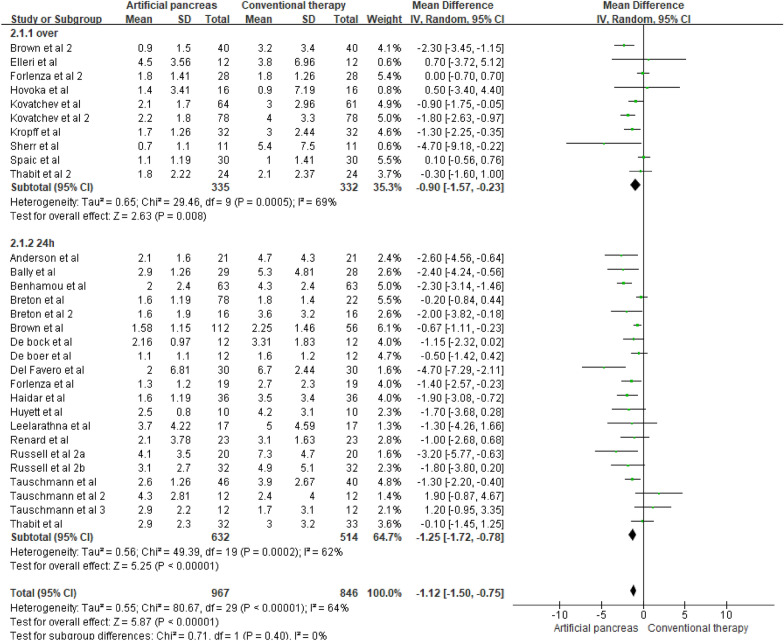
Fig. 3.
Mean difference in time maintained in the hypoglycemic range according to the intervention duration (MPC algorithm-based artificial pancreas)
Differences in the reductions in hypoglycemia were higher with 24 h of intervention ([MD], −1.25%, 95% CI [−1.72, −0.78] p < 0.00001) compared to overnight interventions ([MD], −0.90%, 95% CI [−1.57, −0.23] p = 0.008, Fig. 3). However, there was moderate heterogeneity between the trials within each of these subgroups (overnight: I2 = 69%; 24 h: I2 = 62%). Therefore, the validity of the treatment effect estimate for each subgroup is uncertain, as the individual trial results were inconsistent.
Subgroup analysis of follow-up periods in studies using the MPC algorithm
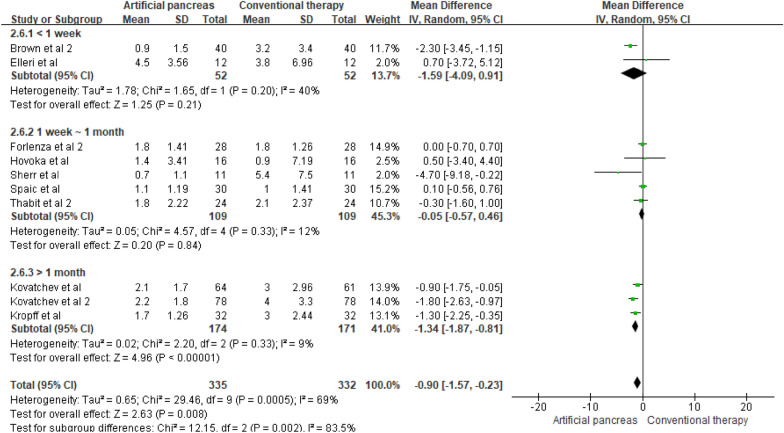
If there was heterogeneity in the subgroup analysis according to the intervention duration, we subdivided the studies by follow-up period. The percentage of time maintained in the hypoglycemic range in the long-term (G3) group with overnight interventions was −1.34% ([MD], 95% CI [−1.87, −0.81] p < 0.00001, Fig. 4) lower, showing a statistically difference. In contrast, there was no significant difference between these subgroups in the time maintained in the target blood glucose range and hypoglycemic range in studies with 24 h intervention (Additional file 1: Figures S4 and S5).
Fig. 4.
Mean difference in time maintained in the hypoglycemic range according to the follow-up period (artificial pancreas (MPC-overnight))
Additional analysis of algorithm types (MPC, PID, and Fuzzy) for primary and secondary outcomes
To verify findings of previous studies [16, 21], we performed additional meta-analysis for different algorithm types (MPC, PID, and Fuzzy) compared to conventional insulin therapy. The MPC algorithm-based artificial pancreas system was associated with a higher percentage of time maintained in the target blood glucose range ([MD], 12.57%, 95% CI [9.63, 15.50] p < 0.00001) than the PID algorithm-based artificial pancreas system ([MD], 9.59%, 95% CI [−3.67, 22.85] p < 0.00001). Reductions of hypoglycemia were associated with a higher in studies using the PID ([MD], -5.24%, 95% CI [−16.06, 5.58] p < 0.00001) and Fuzzy ([MD], -20.80%, 95% CI [−64.12, 22.52] p = 0.0007) algorithms than in studies using the MPC ([MD], -1.12%, 95% CI [−1.50, −0.75] p < 0.00001) algorithm. However, studies using the PID algorithm (3 studies, 52 patients) and the fuzzy algorithm (2 studies, 90 patients) had far smaller numbers of trials and participants than studies using the MPC algorithm (30 studies, 1,185 patients), meaning that the analysis was unlikely to produce useful findings (Additional file 1: Table S3).
Sensitivity analysis
Sensitivity analysis was performed to explore the cause of the high heterogeneity (93%) in the percentage of time maintained in the target blood glucose range (Additional file 1: Figure. S4). In the analysis, only 13 studies with low risk were included, and 28 studies with risk of bias due to insufficient data or unclear results were excluded. The sensitivity analysis, confirmed that heterogeneity was reduced to 32% (Additional file 1: Figure. S6).
Publication bias
Publication bias was evaluated by a funnel plot (Additional file 1: Figures S7–S9). Studies with a small sample size are grouped at the bottom of the graph, and studies with a large sample size are grouped at the top. If there was publication bias, the overall appearance was asymmetrical. The funnel plot in this study showed a symmetrical pattern, indicating no publication bias.
Discussion
This study aimed to determine whether an MPC algorithm-based artificial pancreas system might be more effective than conventional insulin therapy in terms of hypoglycemia risk and maintaining glucose levels within the target range in outpatients with T1D. Most clinical trials compared artificial pancreatic systems (MPC, PID, and Fuzzy) and conventional insulin therapy for the percentage of time maintained in the target and hypoglycemic range. Since artificial pancreas systems might be influenced by the algorithm type and follow-up period, a meta-analysis including these variables is required. In previous studies, meta-analyses were performed according to the intervention period [16–18] or hormone type [16, 17]. To the best of our knowledge, no previous meta-analysis has determined effects of algorithm type on outcomes or compared them according to the follow-up period. We hypothesized that the MPC algorithm would have an influence on reducing hypoglycemia. The main result highlights are as follows. The percentage of time maintained in the target range in MPC-based artificial pancreas systems was high when they were used overnight (10.03% [7.50, 12.56] p < 0.00001) and for 24 h (12.99% [9.32, 16.67] p < 0.00001). The percentage of time maintained in the hypoglycemia range in MPC-based artificial pancreas system was low when used overnight and for more than 1 month (−1.34% [−1.87, −0.81] p < 0.00001). Therefore, it is considered that an MPC-based artificial pancreas system could improve glucose control while reducing the risk of hypoglycemia, compared to conventional insulin therapy in long-term (> 1 month) use. Moreover, to verify findings of previous studies [16, 21] showing that the MPC algorithm performed well or better than PID in terms of safe and effective glucose management, we performed additional meta-analysis for different algorithm types compared to conventional insulin therapy. The MPC-algorithm group showed more improvement in the time maintained in the target blood glucose range than the PID algorithm group (p < 0.00001). This finding was consistent with a recent subgroup analysis in a meta-analysis study [16], in which PID algorithms had substantially less improvement in the percentage of time maintained in the target blood glucose range than MPC and fuzzy logic algorithms.
The percentage of time maintained in the target blood glucose range was analyzed to confirm the effect of blood glucose control. Compared to conventional insulin therapy, MPC-based artificial pancreas system showed high percentages of time maintained in the target blood glucose range when used overnight and 24 h. The basic principle of MPC is that the model is used to predict the impact of a control shift on future output, and optimization is performed to select the best set of current and control shifts to achieve the goal. The main advantages of MPC are that (a) restrictions on insulin delivery rates are explicitly controlled in the calculations, (b) a general framework that can include the effects of meals, exercise, and other events that are functions of the time of day, and (c) it is flexible enough to include targets ranging from set points to zones [63]. These characteristics of MPC are considered to have a great impact on improving blood glucose control in T1D patients.
Hypoglycemia is a typical complication of type 1 diabetes management systems. To compare the influence on hypoglycemia according to MPC algorithm, the effect size was analyzed for the percentage of time maintained in the hypoglycemic range. Subgroup analysis was additionally performed according to intervention period and follow-up period to identify the cause of the heterogeneity. The analyses showed that the MPC algorithm-based artificial pancreas systems statistically significantly reduced the time in hypoglycemia during overnight use for more than 1 month. The MPC algorithm has important characteristics of being customizable to the patient. In particular, it is considered to have a significant effect on hypoglycemia because it can learn the details of a patient's daily life (e.g., timing, and the duration and intensity of meals and exercise) to optimize insulin infusions. It is considered that the nature of the MPC algorithm would enable rapid recovery from hypoglycemia even overnight when the risk of hypoglycemia could be pronounced [16]. In addition, an automatic insulin delivery algorithm that can learn and adapt to patients’ growth, development, and lifestyle changes could enable the long-term use of artificial pancreas systems [64]. The MPC algorithm can be a suitable condition for long-term blood glucose control. Therefore, it is considered that the MPC-based artificial pancreas system showed a statistically significant decreased risk of hypoglycemia in patients who used it for a long-time (> 1 month).
Daily insulin dose was analyzed to determine the association with risk factors such as the occurrence of hypoglycemia. The results showed a statistically significant difference between the MPC-based artificial pancreas system and conventional insulin therapy in the 24 h group. MPC has safeguards that can be individualized using the insulin basal rate, insulin sensitivity factor, and the time of action for each patient's insulin [65]. To achieve a target blood glucose level, it uses a preprogrammed rate of injection as an initial estimate of the insulin required. As blood glucose levels increase, MPC steps up insulin infusion but works carefully at suboptimal levels [66]. According to these characteristics, the MPC algorithm seems to be able to achieve safely control blood glucose levels by reducing the daily insulin dose.
This study had some limitations. The number of large-scale (n > 100) clinical studies was small. Further meta-analysis evidence on MPC algorithm-based artificial pancreas systems using a large sample size and a long follow-up duration through individual trials is needed. Although it was confirmed that the MPC-based artificial pancreas system could statistically significantly improve glycemic control, heterogeneity existed due to age (especially adult or paediatric) [16, 55] or product type of intervention. To decrease heterogeneity, additional clinical trials on age or DIY systems are needed in the future.
Conclusion
The aim of this study was to analyze whether MPC algorithm-based artificial pancreas systems were effective and safe for people with type 1 diabetes. The percentage of time maintained in the target blood glucose range was high in studies with overnight interventions. The percentage of time maintained in the hypoglycemic range was low in studies with overnight interventions when long follow-up period (more than 1 month) was considered.
Supplementary Information
Additional file 1. “Risk of bias graph”. “Risk of bias summary”. “Subgroup analysis protocol”. “Mean difference in time maintained in the target blood glucose range according to the follow-up period (artificial pancreas (MPC-24h))”. “Mean difference in time maintained in the hypoglycemic range according to the follow-up period (artificial pancreas (MPC-24h))”. “Sensitivity analysis of only studies with a low risk of bias”. “Funnel plot of studies evaluating the percentage of time maintained in the target blood glucose range (3.9-10mmol)”. “Funnel plot of studies evaluating the percentage of time maintained in the hypoglycemic range (<3.9mmol)”. “Funnel plot of studies evaluating the daily insulin dose”. “PRISMA CheckList 2014”. “Detailed characteristic of the included studies”. “Mean difference in time maintained in the target blood glucose range and hypoglycemic range according to the timing of intervention and algorithm type”. “Mean difference in daily insulin dose(U) according to the intervention duration (overnight and 24h) and algorithm type (MPC, PID, and fuzzy) (artificial pancreas vs conventional insulin therapy)”. The supplementary figures show additional data analyzed by meta-analysis. The supplementary tables show the data reviewed systematically through the checklist, detailed study characteristics table, and additional meta-analysis data of primary and second outcomes.
Acknowledgements
Not applicable.
Abbreviations
- MPC
Model Predictive Control
- T1D
Type 1 diabetes
- EMBASE
Excerpta medica data-BASE
- PID
Proportional integral derivation
- CSII
Continuous subcutaneous insulin infusion
- SAP
Sensor augmented pump
- CGM
Continuous glucose monitoring
- FL
Fuzzy Logic
- MeSH
Medical subject headings
- PRISMA
Preferred reporting items for systematic review and meta-analyses
- PROSPERO
International prospective register of systematic reviews
- RCT
Randomized controlled trial
- RoB
Risk of bias
- MD
Mean difference
- CI
Confidence intervals
- IQR
Interquartile range
- SD
Standard deviation
Author contributions
Kang screened the publications, performed statistics, and drafted the manuscript. Hwang screened the publications, revised the first version of the manuscript. Kwon helped perform statistics and analyze the data. Kim supervised our study and approved the final manuscript for submission. All authors read and approved the final manuscript.
Funding
This work was supported by the training program for advanced medical device industry professional manpower of Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea, but the sponsor had no role in the design or conduct of this research.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicting interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Su Lim Kang, Email: slkang2047@gmail.com.
Yoo Na Hwang, Email: yoona7747@gmail.com.
Ji Yean Kwon, Email: jykwon@dongguk.edu.
Sung Min Kim, Email: sungmin2009@gmail.com.
References
- 1.Brawerman G, Thompson PJ. Beta cell therapies for preventing type 1 diabetes: from bench to bedside. Biomoleculs. 2020;10:1–20. doi: 10.3390/biom10121681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38:971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D exchange clinic registry. Endocr Res. 2013;98(8):3411–3419. doi: 10.1210/jc.2013-1589. [DOI] [PubMed] [Google Scholar]
- 4.Nakhleh A, Shehadeh N. Hypoglycemia in diabetes: an update on pathophysiology, treatment, and prevention. World J Diabetes. 2021;12(12):2036–2049. doi: 10.4239/wjd.v12.i12.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S61–70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 7.ABCD Diabetes Technology Network UK. BEST PRACTICE GUIDE: Continuous subcutaneous insulin infusion (CSII) A clinical guide for adult diabetes services. http://irep.ntu.ac.uk/id/eprint/34046/. Accessed 8 Nov 2022.
- 8.Boscari F, Avogaro A. Current treatment options and challenges in patients with Type 1 diabetes: pharmacological, technical advances and future perspectives. Rev Endocr Metab Disord. 2021;22:217–240. doi: 10.1007/s11154-021-09635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schönauer M, Thomas A. Sensor-augmented pump therapy—on the way to artificial pancreas. Avances en Diabetología. 2010;26:143–6. doi: 10.1016/S1134-3230(10)63002-5. [DOI] [Google Scholar]
- 10.Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 11.Selam JL. Evolution of diabetes insulin delivery devices. J Diabetes Sci Technol. 2010;4(3):505–513. doi: 10.1177/193229681000400302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito S, Santi E, Mancini G, et al. Efficacy and safety of the artificial pancreas in the paediatric population with type 1 diabetes. J Transl Med. 2018;16(176):1–7. doi: 10.1186/s12967-018-1558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesavadev J, Saboo B, Krishna MB, Krishnan G. Evolution of insulin delivery devices: From syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther. 2020;11:1251–1269. doi: 10.1007/s13300-020-00831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovatchev B. Automated closed-loop control of diabetes: the artificial pancreas. Bioelectron Med. 2018;4(14):1–12. doi: 10.1186/s42234-018-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trevitt S, Simpson S, Wood A. Artificial pancreas device systems for the closed-loop control of type 1 diabetes: What systems are in development? J Diabetes Sci Technol. 2016;10(3):714–723. doi: 10.1177/1932296815617968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501–512. doi: 10.1016/S2213-8587(17)30167-5. [DOI] [PubMed] [Google Scholar]
- 17.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:1–15. doi: 10.1136/bmj.k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai X, Zc Luo, Zhai L, Wp Zhao, Huang F. Artificial pancreas as an effective and safe alternative in patients with type 1 diabetes mellitus: a systematic review and Meta-Analysis. Diabetes Ther. 2018;9:1269–1277. doi: 10.1007/s13300-018-0436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karageorgiou V, Papaioannou TG, Bellos I, et al. Effectiveness of artificial pancreas in the non-adult population: a systematic review and network meta-analysis. Metabolism. 2019;90:20–30. doi: 10.1016/j.metabol.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Haidar A, Legault L, Raffray M, et al. Comparison between closed-loop insulin delivery system(the Artificial Pancreas) and sensor-augmented pump therapy: a randomized-controlled crossover trial. Diabetes Technol Ther. 2021;23(3):168–174. doi: 10.1089/dia.2020.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinsker JE, Lee JB, Dassau E, et al. Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care. 2016;39:1135–1142. doi: 10.2337/dc15-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weir CJ, Butcher I, Assi V, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol. 2018;18(25):1–14. doi: 10.1186/s12874-018-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration, updated March. 2011. https://handbook-5-1.cochrane.org/. Accessed 17 Oct 2021.
- 24.Anderson SM, Buckingham BA, Breton MD, et al. Hybrid closed-loop control is safe and effective for people with type 1 diabetes who are at moderate to high risk for hypoglycemia. Diabetes Technol Ther. 2019;21(6):356–363. doi: 10.1089/dia.2019.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bally L, Thabit H, Kojzar H, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol. 2017;5:261–270. doi: 10.1016/S2213-8587(17)30001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benhamou PY, Franc S, Reznik Y, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digital Health. 2019;1:e17–25. doi: 10.1016/S2589-7500(19)30003-2. [DOI] [PubMed] [Google Scholar]
- 27.Blauw H, van Bon AC, Koops R, DeVries JH. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016;18:671–677. doi: 10.1111/dom.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breton MD, Kanapka LG, Beck RW, et al. A randomized trial of closed-loop control in children with type 1 diabetes. New England J Med. 2020;383(9):836–45. doi: 10.1056/NEJMoa2004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breton MD, Chernavvsky DR, Forlenza GP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40:1644–1650. doi: 10.2337/dc17-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707–1717. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown SA, Breton MD, Anderson SM, et al. Overnight closed loop control improves glycemic control in a multicenter study of adults with type 1 diabetes. J Clin Endocrinol Metab. 2017;102(10):3674–3682. doi: 10.1210/jc.2017-00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown SA, Kovatchev BP, Breton MD, et al. Multinight “Bedside” closed-loop control for patients with type 1 diabetes. Diabetes Technol Ther. 2015;17(3):203–209. doi: 10.1089/dia.2014.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chernavvsky DR, De Boer MD, Keith-Hynes P, et al. Use of an artificial pancreas among adolescents for a missed snack bolus and an underestimated meal bolus. Pediatr Diabetes. 2016;17:28–35. doi: 10.1111/pedi.12230. [DOI] [PubMed] [Google Scholar]
- 34.de Bock M, Dart J, Hancock M, Smith G, Davis EA, Jones TW. Performance of medtronic hybrid closed-loop iterations, results from a randomized trial in adolescents with type 1 diabetes. Diabetes Technol Ther. 2018;19(5):293–298. doi: 10.1089/dia.2018.0161. [DOI] [PubMed] [Google Scholar]
- 35.DeBoer MD, Breton MD, Wakeman C, et al. Performance of an artificial pancreas system for young children with type 1 diabetes. Diabetes Technol Ther. 2017;19(6):1–6. doi: 10.1089/dia.2016.0424. [DOI] [PubMed] [Google Scholar]
- 36.Del Favero S, Boscari F, Messori M, et al. Randomized summer camp crossover trial in 5- to 9-year-old children: Outpatient wearable artificial pancreas is feasible and safe. Diabetes Care. 2016;39:1180–1185. doi: 10.2337/dc15-2815. [DOI] [PubMed] [Google Scholar]
- 37.El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389(10067):369–380. doi: 10.1016/S0140-6736(16)32567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elleri D, Allen JM, Kumareswaran K, et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes. Diabetes Care. 2013;36:838–844. doi: 10.2337/dc12-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forlenza GP, Deshpande S, Ly TT, et al. Application of zone model predictive control artificial pancreas during extended use of infusion set and sensor: a randomized crossover-controlled home-use trial. Diabetes Care. 2017;40:1096–1102. doi: 10.2337/dc17-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forlenza GP, Raghinaru D, Cameron F, et al. Predictive hyperglycemia and hypoglycemia minimization: In-home double-blind randomized controlled evaluation in children and young adolescents. Pediatr Diabetes. 2018;19(3):420–428. doi: 10.1111/pedi.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37:1204–1211. doi: 10.2337/dc13-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huyett LM, Ly TT, Forlenza GP, et al. Outpatient closed-loop control with unannounced moderate exercise in adolescents using zone model predictive control. Diabetes Technol Ther. 2017;19(6):1–9. doi: 10.1089/dia.2016.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovatchev B, Anderson SM, Raghinaru E, et al. Randomized controlled trial of mobile closed-loop control. Diabetes Care. 2020;43:607–615. doi: 10.2337/dc19-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovatchev BP, Kollar L, Anderson SM, et al. Evening and overnight closed-loop control versus 24/7 continuous closed-loop control for type 1 diabetes: a randomised crossover trial. Lancet Digital health. 2020;2(2):e64–73. doi: 10.1016/S2589-7500(19)30218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37:1789–1796. doi: 10.2337/dc13-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kropff J, Del Favero S, Place J, et al. 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol. 2015;3(12):939–947. doi: 10.1016/S2213-8587(15)00335-6. [DOI] [PubMed] [Google Scholar]
- 47.Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931–1937. doi: 10.2337/dc13-2911. [DOI] [PubMed] [Google Scholar]
- 48.Ly TT, Keenan B, Roy A, et al. Automated overnight closed-loop control using a proportional-integral-derivative algorithm with insulin feedback in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Technol Ther. 2016;18(6):377–384. doi: 10.1089/dia.2015.0431. [DOI] [PubMed] [Google Scholar]
- 49.Ly TT, Roy A, Grosman B, et al. Day and night closed-loop control using the integrated medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015;38:1205–1211. doi: 10.2337/dc14-3073. [DOI] [PubMed] [Google Scholar]
- 50.Ly TT, Breton MD, Keith-Hynes P, et al. Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care. 2014;37:2310–2316. doi: 10.2337/dc14-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: Randomized crossover trial. Diabetes Care. 2014;37:3025–3032. doi: 10.2337/dc14-0835. [DOI] [PubMed] [Google Scholar]
- 52.Nimri R, Muller I, Atlas E, et al. Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial – interim analysis. Pediatric Diabetes. 2014;15:91–99. doi: 10.1111/pedi.12071. [DOI] [PubMed] [Google Scholar]
- 53.Renard E, Tubiana-Rufi N, Bonnemaison-Gilbert E, et al. Closed-loop driven by control-to-range algorithm outperforms threshold-low-glucose-suspend insulin delivery on glucose control albeit not on nocturnal hypoglycaemia in prepubertal patients with type 1 diabetes in a supervised hotel setting. Diabetes Obes Metab. 2019;21:183–187. doi: 10.1111/dom.13482. [DOI] [PubMed] [Google Scholar]
- 54.Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4(3):233–243. doi: 10.1016/S2213-8587(15)00489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. New England J Med. 2014;371:313–25. doi: 10.1056/NEJMoa1314474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherr JL, Buckingham BA, Forlenza GP, et al. Safety and performance of the omnipod hybrid closed-loop system in adults, adolescents, and children with type 1 diabetes over 5 days under free-living conditions. Diabetes Technol Ther. 2020;22(3):174–184. doi: 10.1089/dia.2019.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spaic T, Driscoll M, Raghinaru D, et al. Predictive hyperglycemia and hypoglycemia minimization: In-home evaluation of safety, feasibility, and efficacy in overnight glucose control in type 1 diabetes. Diabetes Care. 2017;40:359–366. doi: 10.2337/dc16-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392:1321–1329. doi: 10.1016/S0140-6736(18)31947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tauschmann M, Allen JM, Wilinska ME, et al. Home use of day-and-night hybrid closed-loop insulin delivery in suboptimally controlled adolescents with type 1 diabetes: a 3-week, free-living, randomized crossover trial. Diabetes Care. 2016;39:2019–2025. doi: 10.2337/dc16-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tauschmann M, Allen JM, Wilinska ME, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2016;39:1168–1174. doi: 10.2337/dc15-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129–2140. doi: 10.1056/NEJMoa1509351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thabit H, Lubina-Solomon A, Stadler M, et al. Home use of closed loop insulin delivery improves overnight glucose control in adults with type 1 diabetes: a four-week multicentre randomised crossover study. Lancet Diabetes Endocrinol. 2014;2(9):701–709. doi: 10.1016/S2213-8587(14)70114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wayne BB. Algorithms for a closed-loop artificial pancreas: the case for model predictive control. J Diabetes Sci Technol. 2013;7(6):1632–1643. doi: 10.1177/193229681300700624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi D, Dassau E, Doyle FJ. Multivariate learning framework for long-term adaptation in the artificial pancreas. Bioeng Transl Med. 2019;4(1):61–74. doi: 10.1002/btm2.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boiroux D, Duun-Henriksen AK, Schmidt S, et al. Overnight control of blood glucose in people with type 1 diabetes. Int Fed Autom Control. 2012;39:503–512. doi: 10.3182/20120829-3-HU-2029.00106. [DOI] [Google Scholar]
- 66.Wilinska ME, Budiman ES, Taub MB, et al. Overnight closed-loop insulin delivery with model predictive control: assessment of hypoglycemia and hyperglycemia risk using simulation studies. J Diabetes Sci Technol. 2009;3(5):1109–1120. doi: 10.1177/193229680900300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. “Risk of bias graph”. “Risk of bias summary”. “Subgroup analysis protocol”. “Mean difference in time maintained in the target blood glucose range according to the follow-up period (artificial pancreas (MPC-24h))”. “Mean difference in time maintained in the hypoglycemic range according to the follow-up period (artificial pancreas (MPC-24h))”. “Sensitivity analysis of only studies with a low risk of bias”. “Funnel plot of studies evaluating the percentage of time maintained in the target blood glucose range (3.9-10mmol)”. “Funnel plot of studies evaluating the percentage of time maintained in the hypoglycemic range (<3.9mmol)”. “Funnel plot of studies evaluating the daily insulin dose”. “PRISMA CheckList 2014”. “Detailed characteristic of the included studies”. “Mean difference in time maintained in the target blood glucose range and hypoglycemic range according to the timing of intervention and algorithm type”. “Mean difference in daily insulin dose(U) according to the intervention duration (overnight and 24h) and algorithm type (MPC, PID, and fuzzy) (artificial pancreas vs conventional insulin therapy)”. The supplementary figures show additional data analyzed by meta-analysis. The supplementary tables show the data reviewed systematically through the checklist, detailed study characteristics table, and additional meta-analysis data of primary and second outcomes.
Data Availability Statement
Not applicable.