Abstract
An abdominal aortic aneurysm (AAA) is a localized dilation of the aorta located in the abdomen that poses a severe risk of death when ruptured. The cause of AAA is not fully understood, but degradation of medial elastin due to elastolytic matrix metalloproteinases is a key step leading to aortic dilation. Current therapeutic interventions are limited to surgical repair to prevent catastrophic rupture. Here we report the development of injectable supramolecular nanofibers using peptide amphiphile molecules designed to localize to AAA by targeting fragmented elastin, matrix metalloproteinase 2 (MMP-2), and membrane type 1 matrix metalloproteinase. We designed four targeting peptide sequences from X-ray crystallographic data and incorporated them into PA molecules via solid phase peptide synthesis. After co-assembling targeted and diluent PAs at different molar ratios, we assessed their ability to form nanofibers using transmission electron microscopy and to localize to AAA in male and female Sprague Dawley rats using light sheet fluorescence microscopy. We found that three formulations of the PA nanofibers were able to localize to AAA tissue, but the MMP-2 targeting PA substantially outperformed the other nanofibers. Additionally, we demonstrated that the MMP-2 targeting PA nanofibers had an optimal dose of 5 mg (~12 mg/kg). Our results show that there was not a significant difference in targeting between male and female Sprague Dawley rats. Given the ability of the MMP-2 targeting PA nanofiber to localize to AAA tissue, future studies will investigate potential diagnostic and targeted drug delivery applications for AAA.
Keywords: Peptide amphiphile, abdominal aortic aneurysm, targeted nanomaterials, matrix metalloproteinases, self-assembly
Graphical Abstract

Abdominal aortic aneurysm (AAA) is the most common aortic pathology, and accounts for ~10,000 deaths annually in the United States.1, 2 Key associations with AAA are advanced age, male sex, cigarette smoking, atherosclerosis, hypertension, and a genetic predisposition.3–6 When ruptured, AAAs have a mortality rate approaching 90%, and are estimated to account for 1 in 20 sudden deaths in the United States.2 Treatment is predominantly limited to surgical intervention, and elective repairs have a 30-day mortality risk of 1–5% depending on the type of surgery.2, 7 For ruptured AAA, up to 50% of patients never reach the hospital, and of the patients who reach the operating room, an additional 50% do not survive.2 Analyses of aneurysmal tissue from discarded surgical specimens reveal fragmentation of medial elastin8–11 and increased levels of matrix metalloproteases as pathological findings.12–17
A widely reported approach to generate AAA in animal models involves the use of calcium chloride (CaCl2) applied to the periadventitial surface of the aorta which results in a consistent dilation of the exposed aorta.18, 19 One advantage of this model over others is the ability to induce AAA in any animal model regardless of genetic background, including transgenics and knockouts.20–22 Importantly, aneurysm development occurs over a prolonged period, making it possible to examine the mechanistic underpinnings of the process. While it is recognized that no single model, whether in large (pigs) or small (mice, rats) animals, perfectly replicates human aneurysms on a temporal and molecular scale, similarities observed between rat and human AAAs provide validation that CaCl2-induced aneurysms recapitulate key hallmarks of clinical AAA pathophysiology including fragmentation of medial elastin and increased levels of matrix metalloproteinase 2 (MMP-2) and membrane type 1 matrix metalloproteinase (MT1-MMP) during aneurysm development.2, 23–27 Therefore, this model provides an ideal means to induce AAA and evaluate the efficacy of a nanoscale targeting platform.
Of the currently available pharmacological interventions and recent clinical trials, little evidence has shown that these treatments prevent or reduce AAA enlargement.2, 28 Doxycycline, which has shown benefits in animal models,29–31 was unfortunately shown to be ineffective in a phase III clinical trial in humans.32 Thus, better pharmacological intervention is needed. One promising avenue is the use of nanomedicine in vascular therapies.33, 34 Nanomedicine offers an alternative approach for drug delivery of compounds which might otherwise have cytotoxic or other deleterious effects when administered systemically. Using vascular targeting moieties, nanoparticles can significantly increase drug efficacy by concentrating drug delivery to the site of interest, thereby reducing the systemic dose while locally increasing the effective dose. Our laboratory has already developed targeted nanotherapeutics using peptide amphiphile (PA) technology to prevent restenosis, target atherosclerosis, and stop hemorrhage.33–38 PA molecules consist of a peptide sequence attached to an alkyl tail, where in an aqueous environment, hydrophobic collapse of these aliphatic tails results in molecular aggregation. Subsequent intermolecular hydrogen bonding among the peptide segments drives elongation into one-dimensional nanofibers.39 Depending on processing conditions and peptide sequence, these assemblies can produce dynamic one-dimensional nanofibers highly reminiscent of those observed in the extracellular matrix (ECM).40, 41 Based on our review of the literature and crystal structures, we selected three proteins with the potential to target aortic aneurysms: MMP-2, MT1-MMP, and fragmented elastin.
MMPs are a large family of zinc-dependent proteases that break down the components of the ECM,42 and a number of MMPs are involved in AAA development.18, 43–47 Specifically, MMP-2 and MT1-MMP both play key roles in the pathophysiology of AAA.12–17 MMP-2 is a particularly attractive target due to its increased levels very early in AAA pathophysiology, and because it is largely produced within the media of aneurysmal tissue.2, 15, 23–25 Additionally, the majority of clinical presentations of AAA are classified as small aneurysms.48 MT1-MMP is also an interesting target due to its role in MMP-2 activation. Furthermore, MMP-2 and MT1-MMP have been shown to be critical for aneurysm development in mouse knockout models.18 Using the surface contact sites with tissue inhibitor of metalloproteinase 2, we designed three peptide sequences, two to target MMP-2 (RGAPPKQEFLDIE) and (RSDGSCAWYR) and one to target MT1-MMP (LWMDWVEKNIN). Another key feature of AAA is fragmentation of medial elastin. Elastin fibers are composed of an insoluble polymer of tropoelastin that makes up the bulk dry weight of arteries.49 While elastin is incredibly resilient, calcification50–52 and enzymatic degradation23, 53–55 can fragment elastin and lead to a number of pathologies including AAA. Fragmentation of elastin disrupts its fibrillin coating, which exposes the amorphous core of elastin and allows the elastin to be targeted. Our aim is to design a suite of peptides that can be incorporated into PA molecules that subsequently form PA nanofibers in an aqueous environment and can also target key pathological features of AAA tissue. We hypothesize that intravenously administered PA nanofibers incorporating epitopes that recognize features of aneurysmal tissue will target AAA.
Results and Discussion
AAA Model Characterization.
Before designing targeted PA nanofibers, we confirmed the presence of key pathophysiological features of abdominal aortic aneurysms (AAA). Aneurysms were induced in adult (>13 week) Sprague Dawley rats (Figure 1A). We measured the presence of key features of human AAA including aortic dilation (Figure 1B & 1C and Figure S1), as well as levels of MMP-2 (Figure 1D & 1E), MT1-MMP (Figure 1F & 1G), and medial elastin (Figure 1H). Photomicrographs and ultrasound showed a steady dilation of ~7–8% per week resulting in a 43.7±1.5% increase in diameter for male rats and a 38.5±2.9% increase for female rats by day 35 (Figure 1C, n=72 males, 15 females, p=0.155). Additionally, levels of MMP-2 and MT1-MMP were significantly higher in the region of the aorta exposed to CaCl2 (p<0.05, n=6, Figure 1E & 1G), along with increased fragmented elastin (Figure 1H). Our findings are consistent with previous studies of increased levels of MMP-2, MT1-MMP and fragmented elastin.18, 47, 50 Presence of these proteins also recapitulated features found in human AAA.18, 47, 50 Importantly these results indicate that all three proteins are present and are potentially viable strategies for targeting AAA.
Figure 1.
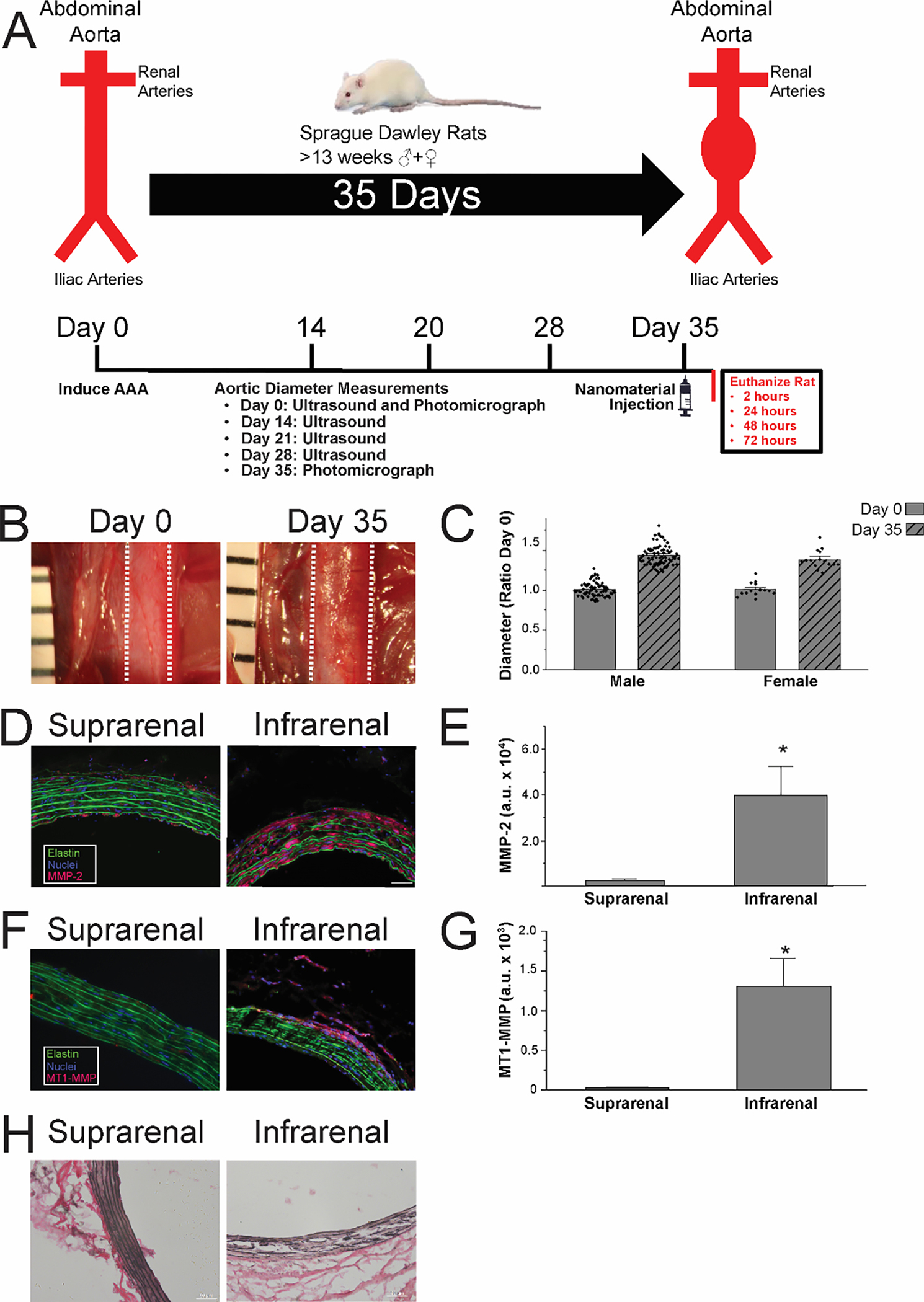
Study design and AAA model characterization. (A) AAA study outline of key experimental time points. (B) Photomicrographs of aorta in situ before and 35 days after CaCl2 exposure. Dashed lines indicate aortic margins. (C) Measurements of aortic diameter before and after CaCl2 exposure. (D) Immunofluorescence imaging of suprarenal and infrarenal aorta stained for MMP-2. (E) Quantification of MMP-2 staining, *p<0.05. (F) Immunofluorescence imaging of suprarenal and infrarenal aorta stained for MT1-MMP. (G) Quantification of MT1-MMP staining, *p<0.05. (H) Photomicrographs of suprarenal and infrarenal aorta stained for collagen via Ver Hoeff Van Gieson (VVG) stain.
Targeting Peptide/Peptide Amphiphile (PA) Design, and Synthesis.
After we demonstrated that pathological features were present in the AAA tissue, we designed targeting peptide sequences using X-ray crystal structures from the Protein Data Bank (Figure S2A & S2B). Two peptides were designed from contact sites between TIMP-2 and MMP-2 located on the C-terminal domain of MMP-2. An additional peptide was derived from a surface contact site between MMP-2 and MT1-MMP which flanks the catalytic domain of MT1-MMP. For the fragmented elastin, we used a peptide sequence from the elastin-binding domain of the 67-kDa elastin-binding protein (EBP).56 EBP binds chaperones and aids in coacervation and elastic fiber assembly of tropoelastin monomers at the cell surface and ECM.56–59 PA molecules were synthesized containing four separate targeting peptides (Figure 2A and Table S1), which were designed to be co-assembled with diluent PA and fluorescently labeled diluent PA (Figure 2B & 2C and Table S1). Selection of diluent PA backbone was determined by which terminus (N- or C-terminus) of the targeted peptide contained more amino acid residues involved in hydrogen bonding with their respective target protein. PA molecules were determined to be >95% pure by liquid chromatography and calculated masses were confirmed by electrospray ionization-mass spectrometry (Figure S3).
Figure 2.
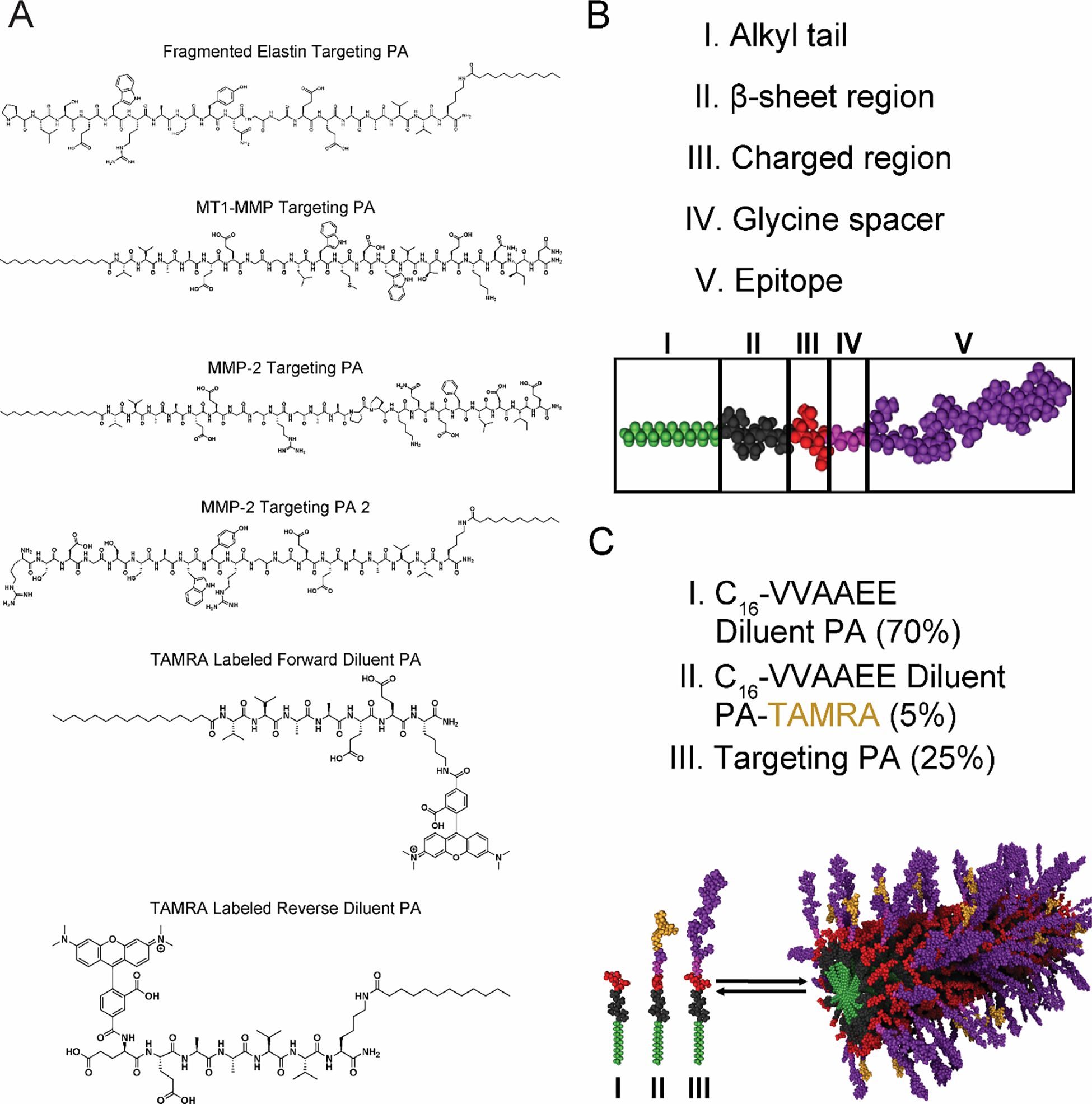
Peptide amphiphile design and synthesis. (A) The chemical structure of peptide amphiphile (PA) molecules synthesized containing the derived targeting peptides. (B) Molecular graphics simulation of the structure of the PA molecule and (C) a PA nanofiber co-assembly. TAMRA = 5-carboxytetramethylrhodamine.
Transmission Electron Microscopy (TEM) Analysis of PA Nanostructures.
After PA molecules were synthesized, nanofiber formation was analyzed using transmission electron microscopy (TEM). TEM analyses were conducted at a range of co-assemblies with diluent PA and fluorescently labeled diluent PA molecules (Figure 3A). TEM data demonstrated that the MT1-MMP targeting PA and the fragmented elastin targeting PA formed fibers at all ratios examined, while both MMP-2 targeting PA molecules formed nanofibers at the lower co-assembly ratios and aggregates at higher ratios (Figure 3A). Additionally, the scrambled and altered hydrogen bonding MMP-2 PA formed fibers at the 25 mole% co-assemblies. Direct measurements of TEM images revealed average fiber lengths of ~174 nm for the 25 mole% fragmented elastin targeting PA, ~160 nm for the 95 mole% MT1-MMP targeting PA, and ~290 nm for 25 mole% MMP-2 targeting PA (Figure 3C).
Figure 3.
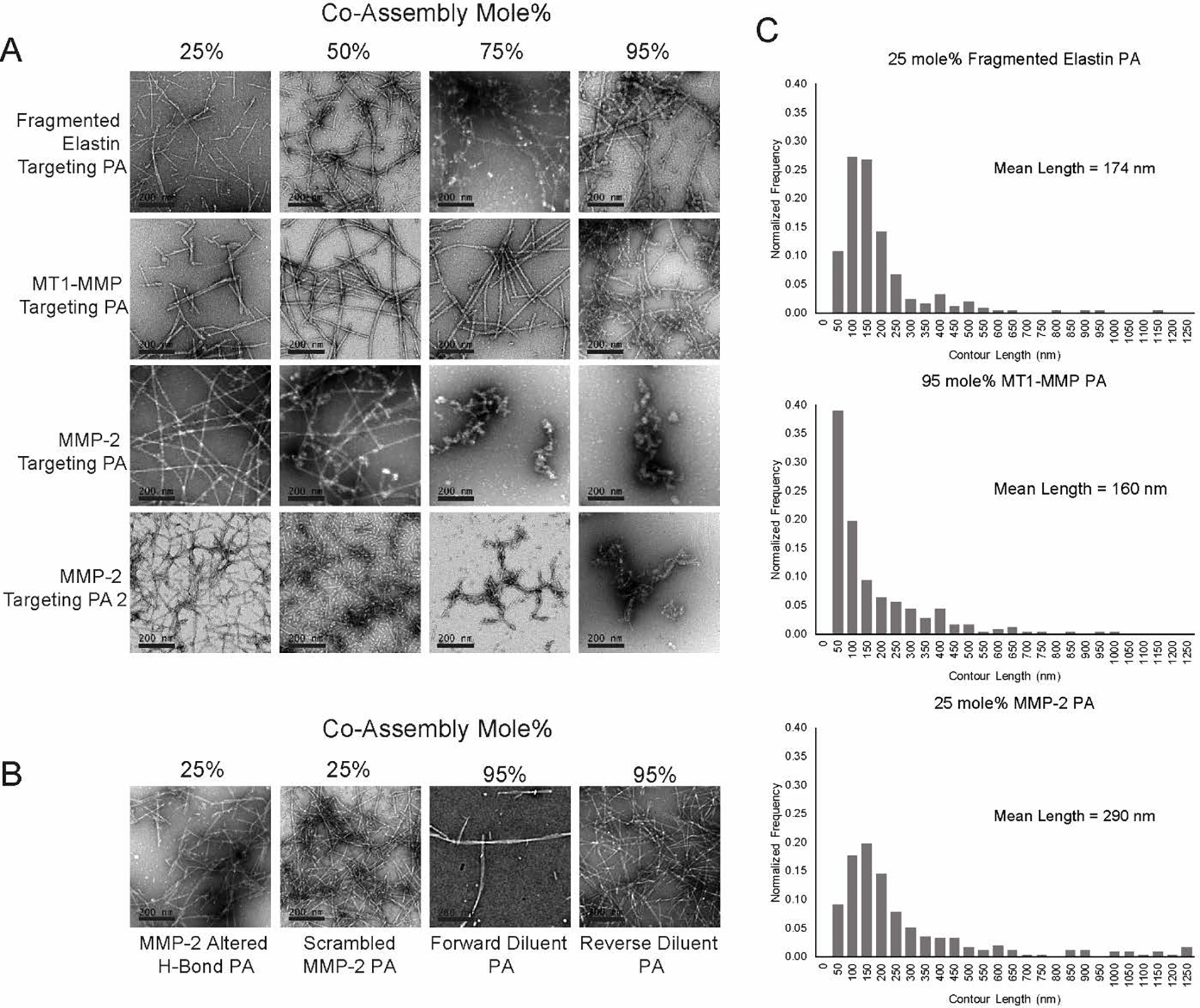
Nanofiber formation screening. (A) Conventional transmission electron microscopy (TEM) images of PA co-assemblies consisting of 25 mole% to 95 mole% targeting PA. (B) TEM images of control and diluent PA co-assemblies. (C) Histogram of fiber length and mean fiber length for 25 mole% fragmented elastin PA, 95 mole% MT1-MMP PA, and 25 mole% MMP-2 PA co-assemblies.
X-ray Scattering and Circular Dichroism Analysis of PA Nanostructures.
After observing nanostructures using TEM, PA molecules were examined with X-ray scattering and CD spectroscopy (Figure 4 and S4). Small-angle X-ray scattering (SAXS) analysis of the MMP-2 targeting PA co-assembled at 25 mole% showed the data fit well to a polydisperse core-shell cylinder model, revealing a nanofiber diameter of ~12 nm was the dominant structure present (Figure 4A). SAXS data for the 95 mole% MT1-MMP targeting PA co-assembly fit well to a sticky core-shell micelle with a diameter of ~6.2 nm (Figure S4B). For the fragmented elastin targeting PA molecules, SAXS data demonstrated ratios of the 25 mole% fragmented elastin targeting PA co-assemblies fit well to a polydisperse core-shell cylinder model with a diameter of ~5.4 nm (Figure S4C). Critical aggregation concentration of the MMP-2 targeting PA co-assembly calculated via Nile Red assay was ~35 μM. Wide-angle X-ray scattering (WAXS) measurements confirmed the internal order of the 25 mole% MMP-2 targeting PA co-assembly with a characteristic peak at approximately 1.35 Å−1, corresponding to a d-spacing of 4.65 Å characteristic of β-sheet hydrogen bonding (Figure 4B).36, 60 The presence of β-sheet hydrogen bonding within the 25 mole% MMP-2 PA nanofibers was further confirmed by broad negative ellipticity at 218 nm and positive ellipticity at 200 nm using circular dichroism (CD) spectroscopy (Figure 4C).61 Interestingly as the amount of MMP-2 targeting PA increased, the spectrum took on the characteristics of a random coil with strong negative ellipticity at 200 nm for the 95 mole% MMP-2 targeting PA nanofiber co-assembly (Figure S4A).61 WAXS and CD spectroscopy data confirmed β-sheet hydrogen bonding for the MMP-2 targeting PA at the 25 mole% co-assembly (Figure 4B & 4C). These findings from CD spectroscopy help explain the diminishing fiber formation of higher molar concentrations of the MMP-2 targeting PA. For the MT1-MMP targeting PA, WAXS data confirmed β-sheet hydrogen bonding at 25 and 50 mole% co-assemblies, but not 95% (Figure S4B). WAXS data also confirmed β-sheet hydrogen bonding for all three fragmented elastin targeting PA co-assemblies (Figure S4C). CD spectra for MT1-MMP and fragmented elastin targeting PA confirmed presence of β-sheets at 25 mole% and mixed α-helical profile at 50 and 95 mole% (Figure S4B & S4C). For the fragmented elastin targeting PA fiber, WAXS had at a peak at 1.35 Å−1 across the concentrations tested, but the CD spectroscopy was more indicative of a mixed β-sheet and α-helical structure (Figure S4C).
Figure 4.
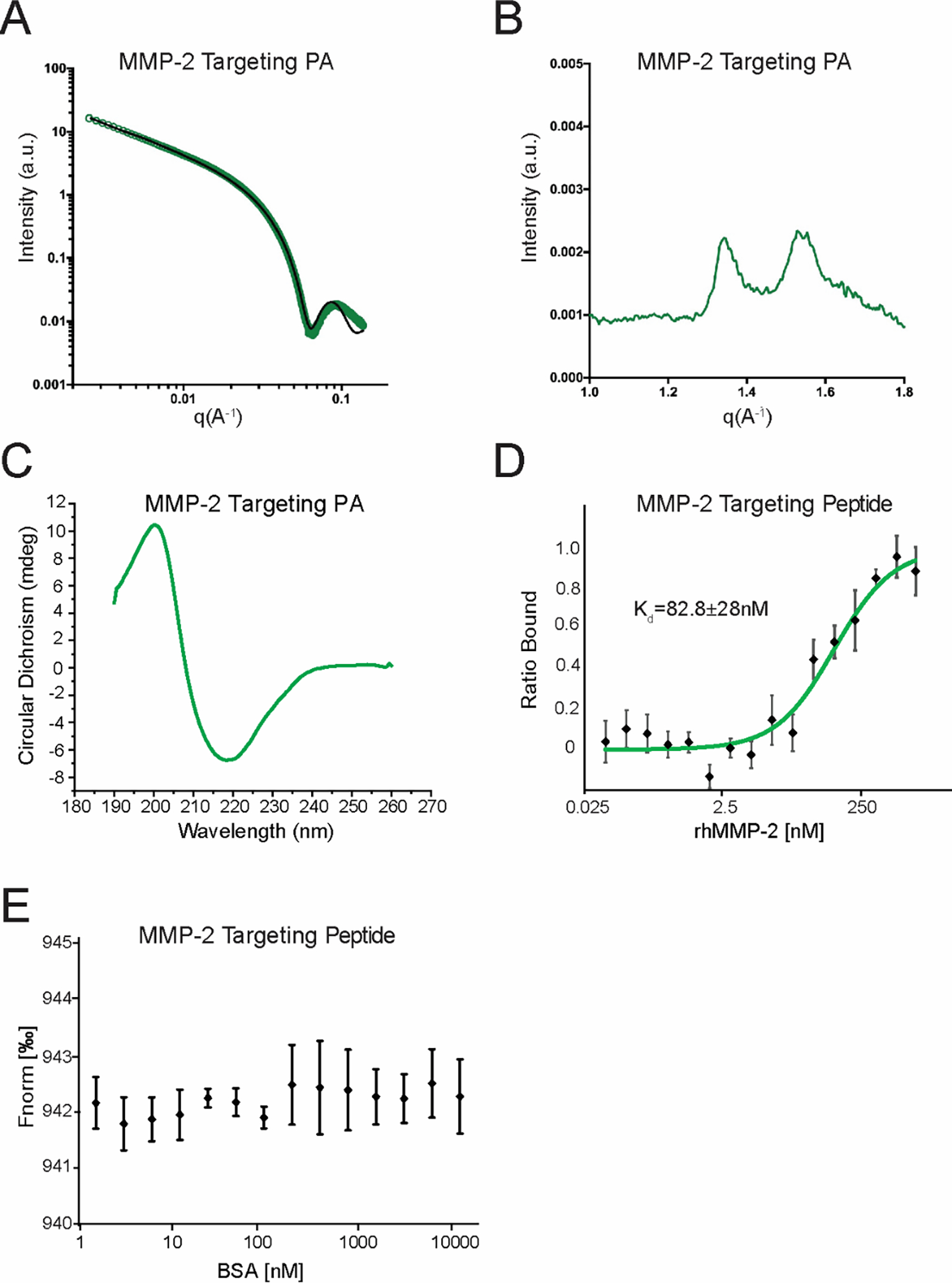
MMP-2 targeting PA nanofiber material characterization. (A) Small-angle X-ray scattering (SAXS) analysis of the 25 mole% MMP-2 targeting PA with polydisperse core-shell cylinder modeling overlaid. (B) Wide-angle X-ray scattering (WAXS) analysis of the 25 mole% MMP-2 targeting PA shows peaks typical of β-sheets. (C) Circular dichroism (CD) spectroscopy of the 25 mole% MMP-2 targeting PA shows maximum and minimum characteristic of β-sheets. MicroScale Thermophoresis analysis of MMP-2 targeting peptide shows binding kinetics to (D) rhMMP-2 and (E) BSA.
After elucidating structural features of the MMP-2 targeting PA, we measured the binding kinetics of the MMP-2 targeting peptide using MicroScale Thermophoresis (MST). These data revealed that our MMP-2 targeting peptide binds to rhMMP-2 with a Kd of 82.8±28.8 nM (Figure 4D) and does not bind nonspecifically to bovine serum albumin (Figure 4E).
AAA Targeting by PA Nanofibers.
Next, all PA molecules that could be efficiently synthesized, and formed nanofibers, were tested for AAA targeting ability. We chose not to pursue the second MMP-2 targeting PA since the first MMP-2 targeting PA was an order of magnitude more efficient to synthesize via Fmoc chemistry. These PA specimens included the fragmented elastin targeting PA at 25, 50, and 95 mole%, the MT1-MMP targeting PA at 25, 50, and 95 mole%, and MMP-2 targeting PA at 25 mole% (Figure S5 and S9). Prior to injection, all tubes containing PA nanofiber solutions were inverted to ensure that the solution flowed and had not formed a gel. We then investigated targeting of fluorescently labeled nanoparticles using light sheet fluorescence microscopy (LSFM). The results for the most efficient targeting nanofibers are summarized including MMP-2 PA 25 mole%, MT1-MMP PA 25 mole%, and fragmented elastin targeting PA 95 mole% in Figure 5. When administered to aneurysmal rats, PAs targeted to all three markers of AAA (MMP-2, MT1-MMP, and fragmented elastin) localized to the site of aneurysm. Of these nanofibers, MMP-2 targeting PA showed the strongest targeting (3.5×108±3.5×107 μm3/mm3 PA/tissue, n=6), significantly outperforming both MT1-MMP (1×107±2.4×106, n=4) and the fragmented elastin targeting PA nanofibers (5×106±1.1×106, n=4; p<0.001) and representing ~40× the localization within the aorta. This could be due to a number of factors, but we hypothesize that it is most likely due to the number of residues involved in surface contact sites between our targeting peptide and MMP-2 compared to the MT1-MMP. 62 While there is no published crystal structure of elastin, it may also be the case that the fragmented elastin targeting PA does not interact with elastin as closely as our MMP-2 targeting PA interacts with MMP-2. Alternatively, because the MMP-2 targeting PA has a more uniform βbeta-sheet characteristic, it is possible that alterations in the secondary peptide structure play a role in the diminished efficacy of the MT1-MMP and fragmented elastin targeting PA nanostructures. For instance, our laboratory has previously demonstrated enhanced in vivo targeting of type IV collagen with PA nanofibers compared to spheres.33 In the current study, targeting of MMP-2 was lost when the sequence of the MMP-2 targeting peptide was scrambled, or the residues involved in hydrogen bonding with the target were altered (Figure 5 and Figure S5).
Figure 5.

AAA targeting by PAs. (A) Light sheet fluorescence microscopy (LSFM) images of rat aorta 2 hours post PA injection. White color represents tissue autofluorescence, and red to yellow represents the fluorescence of TAMRA-labeled PA. Fragmented elastin targeting PA is 25 mole%, MT1-MMP is 95 mole%, and MMP-2 Targeting PA is 25 mole%. (B) Plotted mean data of the ratio of PA volume to tissue volume for each AAA-targeting PA. *p= 5.61×10−8 vs. MMP-2 Scrambled PA.
The AAA targeting methods employed here used approaches not previously reported. Our design covalently conjugates a targeting peptide sequence to the PA backbone. Using PA molecules offers several benefits over more classical active targeting techniques, namely, the PA material is very stable, comparatively inexpensive to produce, and does not require tagging of an antibody to a nanocarrier. Prior efforts to target fragmented elastin have relied on antibody-based approaches;63 however, antibodies are larger, more complex, and more susceptible to denaturation than our peptide-based materials. The structure of a supramolecular PA nanofiber offers a high number of potential MMP-2 targeting PA molecules per nanoparticle compared to other nanomaterial geometries. As mentioned previously, we have shown that this enhanced in vivo targeting of type IV collagen with PA nanofibers compared to spheres.33
MMP-2 Targeting PA Dosage Optimization.
To determine the minimum dose of PA that would still form nanofibers after intravenous injection, we performed a Nile Red Assay to determine the critical aggregation concentration of the 25 mole% MMP-2 targeting PA (35±4 μM, n=3, Figure 6A). Assuming a blood volume of ~7% of the weight of our rats,64 we selected a minimum dose of 2.5 mg. Any dose below this could potentially be too dilute to form nanofibers. To avoid potential gelation prior to injection, we selected a maximum dose of 10 mg of nanofiber. Both the 5 mg (1.24×108±9.8×107 μm3/mm3, n=6) and 10 mg dose (1.32×108±7.05×107 μm3/mm3, n=5) show significantly higher levels of targeting than the 2.5 mg dose (4.63×106±6×105 μm3/mm3, n=5; p<0.05). There was no significant difference between the 5 mg and 10 mg dose. Given that our data show targeting saturated at a dose of 5 mg, which corresponds to ~12 mg/kg (Figure 6B & 6C), we selected this dose for subsequent studies. Additionally, because female rodents express different levels of MMP-2 following AAA induction,65, 66 we also examined the efficacy of targeting in female rats. We found that targeting efficacy was similar in both male and female rats (Figure S6).
Figure 6.
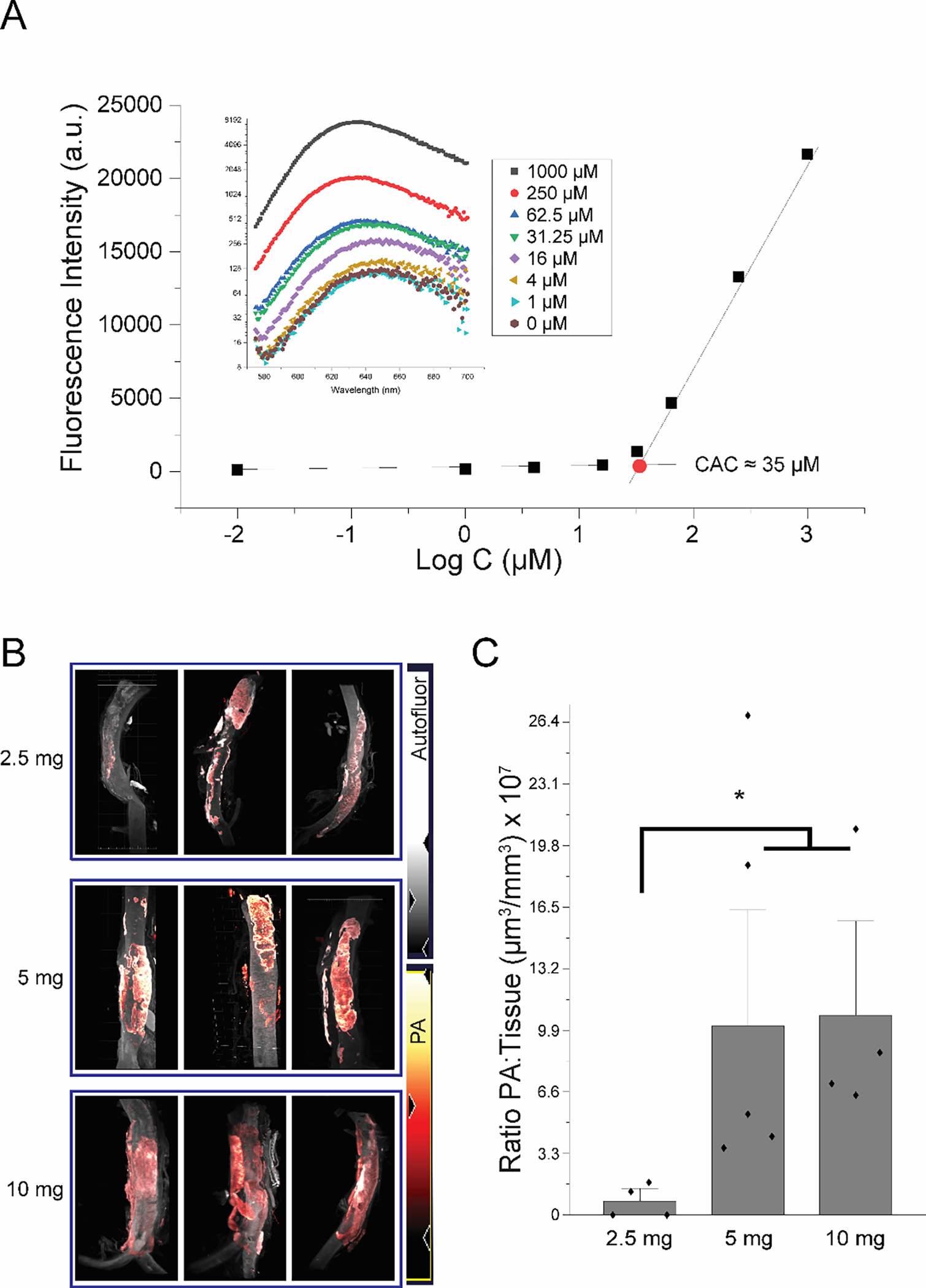
Targeting PA dosage optimization. (A) Nile Red Assay to determine critical aggregation concentration of 25 mole% MMP-2 targeting PA co-assemblies. (B) LSFM images of rat aorta 2 hours post PA injection of either 2.5 mg, 5 mg, or 10 mg of 25 mole% MMP-2 targeting PA. (C) Plotted mean data of the ratio of PA volume to tissue volume for each dose of PA.
MMP-2 Targeted PA Binding Duration.
When injected at 35 days after AAA induction, the MMP-2 targeting PA nanofiber remained localized at the aneurysm for at least 24 hours post injection (~10% of 2 hour post injection, n=4, Figure 7). Minimal PA fluorescence was observed at 72 hours post injection (0.1% of 2-hour post injection, n=4, Figure 7B). While previous investigators have directly targeted the catalytic site of MMP-2,67 we describe a different approach for binding MMP-2 which does not target the catalytic site of the enzyme. Exosite targeting is desirable because MMP-2 has been reported to have a dual role of degradation of medial elastin alongside a less well understood role in preserving aneurysmal tissue in a state of hypertension.68
Figure 7.

MMP-2 targeted PA localization duration. (A) LSFM images of rat aorta 2 hours, 24 hours, 48 hours, and 72 hours post injection of 5 mg 25 mole% MMP-2 targeting PA. (B) Plotted mean data of the ratio of PA volume to tissue volume for each time point.
MMP-2 Targeting PA Predominantly Localizes to the Aorta.
The next study was designed to examine biodistribution of the MMP-2 targeting PA nanofibers. Of the organs examined (Figure S7), the MMP-2 targeting PA was distributed primarily in the kidney, liver, spleen, lung, and aneurysmal aorta, but was not found in the non-aneurysmal aorta (Figure 8). PA was not detected in significant quantities within organs or tissues other than the infrarenal aorta, kidney, liver, spleen, and lungs. Of the organs and tissues examined, we found that the PA nanofiber initially was found abundantly in the kidney (7.7×107±2.2×107 μm3/mm3, n=4, Figure 8B) and liver (4.6×107±6.2×106μm3/mm3, n=4, Figure 8B) compared to other organs. We also observed fluorescence in the urine indicating elimination (Figure S7). These data indicate that the PA molecules are being eliminated via renal clearance.69 Additionally, hematoxylin and eosin (H&E) staining revealed no significant fibrosis in any of the tissues examined (Figure S8). Given the high signal to noise ratio (~4.5 fold higher in aneurysmal tissue compared to the next highest tissue) and their persistence within the AAA tissue for at least 48 hours, the MMP-2 targeting PA nanofibers demonstrate potential as a diagnostic tool. In principle, a similar approach of incorporating radiotracers or other contrast agents could be used to detect MMP-2 in the early phase of AAA development, opening multiple therapeutic options to treat aneurysms in early-stage AAA.
Figure 8.
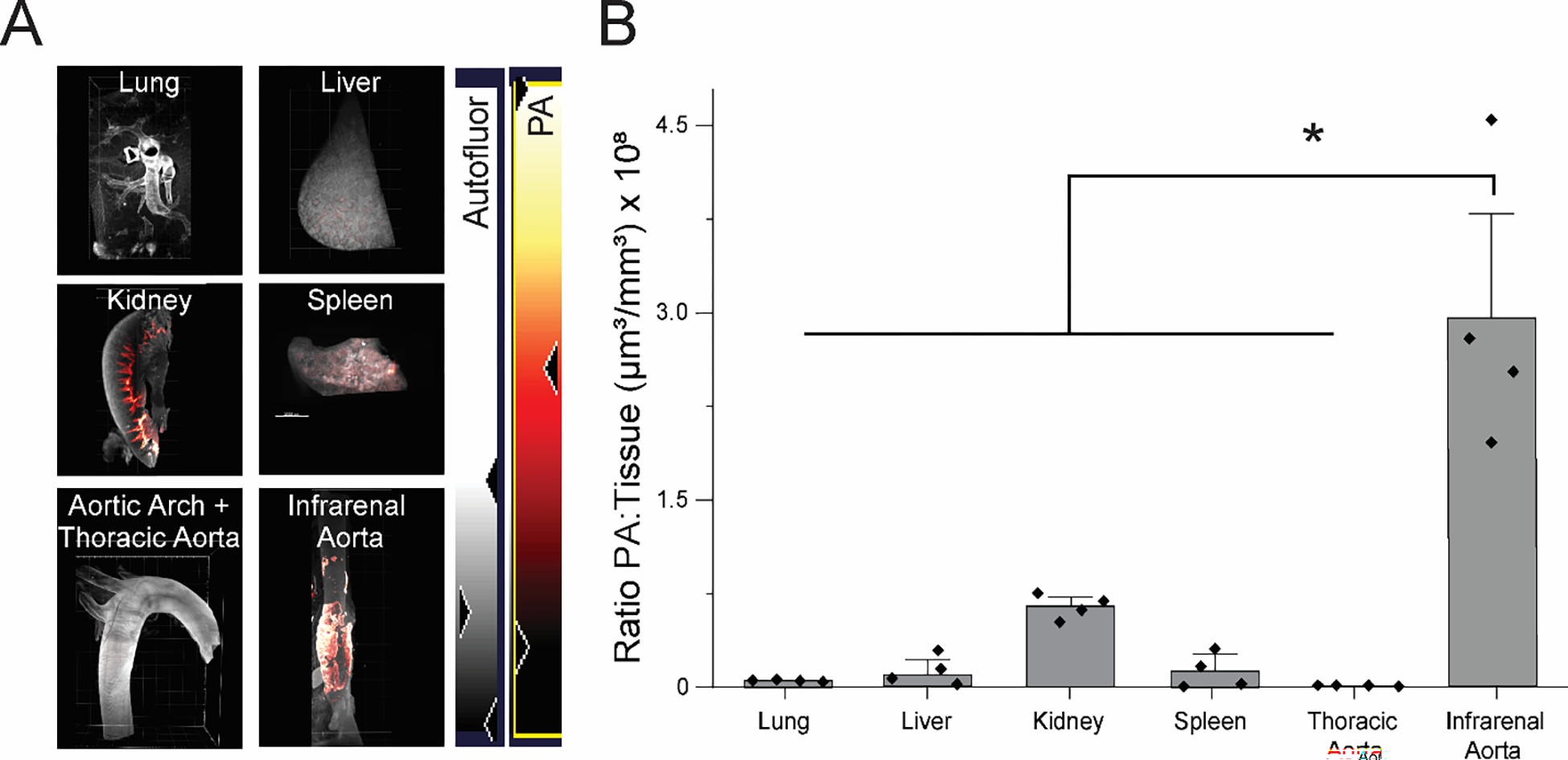
MMP-2 targeting PA predominantly localizes to the aorta. (A) Organ distribution of 25 mole% MMP-2 targeting PA in the kidney, liver, lung, spleen, and aorta of Sprague Dawley rats 2 hours post injection. White color represents autofluorescence and red to yellow represents TAMRA-labeled PA. (B) Plotted mean data of the ratio of PA to tissue in each organ.
Limitations.
There are limitations in our current study. First, as each PA nanofiber was co-assembled as a mixture of at least two PA molecules and is held together by noncovalent bonds, it is possible that fluorescently labeled PA molecules are not evenly distributed throughout the nanofibers. In this case, we could see a lack of targeting that does not correspond to localization of the targeting PA, but instead is an effect of a subpopulation of nanofibers containing the fluorescent PA molecules but not the targeting PA molecules. Despite this possibility, we chose to label a PA not involved with targeting to avoid any potential decrease in targeting capacity arising from directly labeling the targeting PA. Additionally, techniques such as SAXS are an average across all of the structures within the sample and scattering is strongest from larger assemblies versus smaller assemblies or PA monomers. This makes deconvolution of SAXS scattering patterns very difficult to distinguish between a larger assembled PA nanofiber relative to any smaller molecular structures that are present. Further, we see very nice fitting with a core-shell cylinder model to our data (Figure 4A), and fiber formation using TEM (Figure 3). WAXS and CD spectroscopy (Figure 4B & 4C and Figure S4) demonstrate β-sheet formation, which also suggests that this is most likely the dominant structure in our sample, and that contributions from other assemblies, micelles, aggregates, ‘unassembled peptides’ is small/negligible. Further, the Nile Red assay (Figure 6A) indicates that the PA molecules are well above their CAC, which indicates that a significant portion of molecules are self-assembling to form structures. CACs in the micromolar range also suggest these molecules have a high propensity for nanofiber assembly. Finally, we did not examine the hemocompatibility, cytokine profile, direct measurements of PA stability, or cellular uptake data for our targeting nanofibers, but we will be exploring this in future studies after the incorporation of therapeutic and diagnostic compounds into our MMP-2 targeting platform.
Conclusions
In conclusion, we have demonstrated exosite targeting of MMP-2 as an effective method to target AAA. Further, the measured high affinity of the MMP-2 targeting peptide to recombinant MMP-2 in vitro provides strong evidence that LSFM is a viable method to investigate targeting and biodistribution of fluorescently labeled nanoparticles. Additionally, we have shown that these targeting peptide amphiphile nanofibers are not affected by sex in Sprague Dawley rats. Our results provide evidence that our MMP-2 targeting PA nanofiber could be used as a diagnostic tool and targeted drug delivery system for AAA.
Methods
Study Approval.
All animal procedures follow the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication 85–23, 1996). The University of North Carolina at Chapel Hill (UNC-CH) institutional animal care and use committee reviewed and approved all animal experiments.
Rat Model of Abdominal Aortic Aneurysm (AAA).
Figure 1A outlines the key time points for animal experiments: AAA induction timeline, aortic diameter measurements, nanofiber injection, and sacrifice time points. Adapting a model from Ikonomidis, et al., AAA was induced aseptically in male and female Sprague Dawley rats (Envigo, Indianapolis, IN, USA) greater than 13 weeks old by anesthetizing with inhaled isoflurane, performing a midline laparotomy, locating the infrarenal aorta, and exposing a 10 mm segment with a 0.5M CaCl2-soaked surgical sponge (18105-03, Fine Science Tools; Foster City, CA, USA) for a total of 30 minutes (exchanging the sponge once after 15 minutes).70 Following CaCl2 exposure, the abdomen and skin were closed with 4–0 Vicryl sutures. Aortic diameter was measured with ultrasound (Vevo 2100, Visual Sonics; Toronto, Ontario, Canada) to monitor AAA before injecting targeted PA nanofibers. Additionally, we measured aortic diameter with photomicrographs (Nikon SMZ645, Nikon Instruments Inc.; Melville, NY, USA) prior to AAA induction and during a terminal surgical procedure. Fluorescently labeled AAA targeting PA nanofibers including the fragmented elastin targeting PA at 25, 50, and 95 mole%, the MT1-MMP targeting PA at 25, 50, and 95 mole%, and MMP-2 targeting PA at 25 mole%, were administered via tail vein injection.
Tissue Processing.
Following PA nanofiber injection, rats were euthanized at predetermined time points (Figure 1A) by overdose with isoflurane followed by bilateral thoracotomies. The rats were systemically perfused through the ascending aortic arch with 400–600 mL of phosphate buffered saline (PBS) followed by 300 mL of 2% paraformaldehyde solution (w/v in 1× PBS) at a pressure of ~100 mmHg using an IV bag hung 139 cm above the rat’s body. After perfusion, aorta, lung, liver, kidney, spleen, heart, gastrointestinal tract, thymus, tail, brain, and spinal cord were removed and post fixed for 2 hours in a 2% paraformaldehyde solution (w/v in 1× PBS). Following post fixation, tissues were embedded in 1% agarose solution (16500–500, Thermo Scientific; Carlsbad, CA, USA) prepared in triacetate ethylenediaminetetraacetic acid (EDTA) buffer or transferred to a 30% sucrose solution (w/v in DI H2O) overnight.
After embedding in agarose, tissues were optically cleared using the iDISCO+ protocol.64 Briefly, samples were serially dehydrated in methanol solutions increasing at 20% increments and exchanged every hour until 100% was reached, followed by incubation overnight in 100% methanol. The next day, tissues were delipidized for 3 hours on a plate shaker in a 2:1 (v/v) mixture of dichloromethane (DCM) to methanol and then switched to 100% DCM for two 15-minute washes. Finally, tissues were placed in 100% dibenzyl ether (DBE) to render the specimens optically clear. Samples remained in DBE until ready for imaging.
Alternatively, tissues that were incubated in sucrose overnight were placed in cryomolds containing optimal cutting temperature (OCT, Thermo Scientific; Waltham, MA, USA) compound and flash frozen in the vapor phase of liquid nitrogen. Samples were stored at −80°C or placed in a CryoStar NX70 Cryostat (Thermo Scientific; Waltham, MA, USA) at −20°C and sectioned at a thickness of 5 μm.
Tissue Staining.
Specimens of aortic tissue were stained for elastin (Modified Verhoeff Van Gieson Elastic Stain HT25A, Sigma Aldrich; St. Louis, MO, USA), MT1-MMP (ab51704, Abcam; Cambridge, UK), and MMP-2 (ab37150, Abcam). Elastic stain was performed according to the kit manufacturer’s protocol. For both immunofluorescence stains, aortic tissue was blocked with 1.5% bovine serum albumin in PBST (1× PBS + 0.2% Tween-20), stained with primary MMP-2 (1:200) or MT1-MMP (1:400) for 2 hours at room temperature, and then washed 3 times with 1× PBS. Tissues were then stained with goat anti-rabbit secondary antibody carrying AlexaFluor 647 (1:2000 A32733, Thermo Scientific; Waltham, MA, USA) for 1 hour, washed with 1× PBS, and counterstained with 0.5μg/mL 4′,6-diamidino-2-phenylindole (DAPI). Specimens were then mounted with Vectashield (H-120-10 Vector Labs, San Francisco, CA, USA) and sealed with clear fingernail polish.
Imaging.
Imaging for light sheet fluorescence microscopy (LSFM) was performed using a LaVision BioTec Ultramicroscope II (LaVision BioTec GmbH; Bielefeld, Germany) as previously described 71, with the following modifications. Cleared agarose-tissue blocks mounted in a custom sample holder were submerged in 100% DBE. Artery images were acquired at 1.26× mag (0.63× zoom) with a 10 ms exposure, using the two-light-sheet configuration with both left and right light sheets, with the horizontal focus centered in the middle of the field of view, a numerical aperture of 0.039 (sheet thickness at horizontal focus = 19 μm), and a light sheet width of 100%. Pixel size was 4.96 μm and Z-slice spacing was 9.5 μm. Two channels were imaged: tissue autofluorescence with 488 nm laser excitation and a Chroma ET525/50m emission filter at 70% laser power and TAMRA channel with 561 nm laser excitation and a Chroma ET600/50m emission filter at 40% laser power. Focus in both channels was ensured by use of the chromatic correction module on the instrument. To minimize sample bleaching during initial imaging setup we lowered laser power and increased exposure times. Tissues studied for targeting and biodistribution include aorta, thymus, heart, lung, liver, kidney, spleen, skeletal muscle, gastrointestinal tract (stomach/small intestine/colon), brain, spinal cord, and tail.
Imaging Analysis.
Targeting and bio-distribution were analyzed using the surface analyzer tool in IMARIS 9.5 (Oxford Instruments 2021, Oxford, UK). For tissues other than the abdominal aorta the whole sample was analyzed. For all tissue other than the spleen, tissue volume was measured with the 488 nm laser with a surface detail of 20 μm. For the spleen, the 647 nm laser was used due to high auto-fluorescence with the 488 nm laser. To measure TAMRA signal, we used the 561 nm laser with a surface detail of 2.5 μm and measured voxels above an absolute threshold intensity of 250. For the AAA localization, aortic tissue volume was measured by creating a region of interest around the dilated portion of the aorta. Values for the biodistribution and targeting studies are reported as a ratio of fluorescent PA positive tissue (μm3) vs. tissue volume (mm3). For the lung tissue, specimens were taken from the left lung, the right inferior lobe, and right superior lobe. For other large organs, tissue specimens were taken from 3 equally spaced regions of the tissue. Additionally, to account for baseline differences in the aorta between males and females, data were normalized to baseline aorta diameters obtained using ultrasound for each sex.
PA Synthesis and Co-assembly.
PAs and peptides were synthesized using standard fluorenylmethyloxycarbonyl (Fmoc) solid-phase synthesis conditions as described previously.11 Briefly, PAs were synthesized using a CEM model Liberty Blue Microwave Assisted Peptide Synthesizer on rink amide MBHA resin. Automated coupling reactions were performed using 4 equiv. Fmoc-protected amino acid, 4 equiv. of N,N’-diisopropylcarbodiimide (DIC), and 8 equiv. ethyl(hydroxyimino)cyanoacetate (Oxyma pure). Removal of the Fmoc groups was achieved with 20% 4-methylpiperidine in DMF. Peptides were cleaved from the resin using standard solutions of 95% TFA, 2.5% water, 2.5% triisopropylsilane (TIPS) and precipitated with cold ether. PAs were then then purified by reverse-phase HPLC on a Waters Prep150 or Shimadzu Prominence HPLC using a water/acetonitrile (each containing 0.1% NH4OH) gradient. Eluting fractions containing the desired peptide were confirmed by mass spectrometry using an Agilent 6520 QTOF LCMS (Agilent Technologies; Santa Clara, CA, USA). Confirmed fractions were pooled and the acetonitrile was removed by rotary evaporation before freezing and lyophilization. Purity of lyophilized products was tested by LCMS on an Agilent 6520 QTOF LCMS. Each targeting PA molecule was co-assembled with matched diluent PA (either C16-VVAAEE or EEAAVV-K-C12) and a TAMRA labeled diluent. PA molecules were weighed and then mixed in a solution of 1,1,1,3,3,3-hexafluoroisopropanol (HFIP, Sigma 105228) at a concentration of 1–10 mg/mL to produce a clear solution. The HFIP was then removed under vacuum using a Schlenk line. The co-assembled PA molecules were then dissolved in deionized water, flash frozen, and lyophilized. As needed, aliquots of co-assembled nanofibers were dissolved in 1× Hanks Balanced Salt Solution (HBSS) prior to injection.
Nile Red Assay.
Critical aggregation concentration (CAC) for PA nanofibers was measured with a Nile Red Assay as previously reported.33 Nile Red dye undergoes a blue shift in fluorescence in increasingly hydrophobic environments found in the core of the amphiphilic nanofibers. MMP-2 targeting PA was prepared at 25 mole% with a C16V2A2E2 diluent PA and diluted from 1 mM to 100 nM in 1× PBS containing 2.5 μM Nile Red (ab228553, Abcam; Cambridge, UK). PAs were aliquotted in triplicate onto a 96-well plate, excited at 550 nm, and the fluorescence read from 580 to 750 nm. The CAC was determined by plotting the log of the concentration with the corresponding maximum fluorescence intensity and calculating the concentration at the intersection of the baseline and tangent line to the rising curve.
MicroScale Thermophoresis.
A binding kinetics assay was performed using a Monolith NT.115 microscale thermophoresis (MST) device (Nanotemper; Cambridge, MA, USA). Our targeting MMP-2 targeting peptide and a scrambled peptide were synthesized with a fluorescein isothiocyanate (FITC) (FITC-AA-RGAAPKQEFLDIE) using Fmoc chemistry. Subsequently, 60 nM stock solutions of peptides and a 3.33 μM solution of recombinant human MMP-2 (Sigma Aldrich) were prepared in a 0.05% solution of Tween-20. The rhMMP-2 was then prepared in serial dilution from 3.3 μM to 100 pM and then combined with stock peptide solutions in standard Monolith capillaries (MO-K022).
X-Ray Analysis.
Experiments were performed at beamline 5-ID-D of the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) Synchrotron Research Center at the Advanced Photon Source, Argonne National Laboratory. We dissolved PA samples in 1× HBSS at 10 mg/mL immediately prior to measuring. Data was collected by irradiating each sample for 5 frames of 5 second exposure with and X-ray energy of 17keV (I=0.83 Å). We used the following sample to detector distances: 201.25 mm small-angle X-ray scattering and 8508.4 mm wide-angle X-ray scattering. Scattering intensity measurements were recorded in the interval 0.002390 < q < 4.4578 Å-1. The wave vector q is defined as = (4π/λ) sin(θ/2), where θ is the scattering angle. Azimuthal integration (Fit2D) was used to average 2D scattering images to produce 1D profiles of intensity versus q. A syringe pump was used to oscillate solutions to avoid PA sample damage during beam exposure. HBSS scattering intensity values were subtracted from the PA samples using the Irena SAS macro.72 The resulting plots were fitted using NCNR analysis macro to a polydisperse core–shell cylinder model.
Circular Dichroism Spectroscopy.
PA samples were analyzed at 0.5 mM in 0.02 M tris buffer at pH 7.5 with a 0.1 mm pathlength Suprasil quartz cuvette (Sigma-Aldrich) using a Chirascan-plus circular dichroism spectrometer (Applied Photophysics; Beverly, MA, USA). Samples were analyzed at 20°C from 190 to 260 nm with 0.5 nm step size and analysis time of 1.25 s per data point. Spectrum data were averaged from two scans with the tris buffer values subtracted.
Transmission Electron Microscopy (TEM).
Conventional TEM images were taken on a FEI Tecnai T-12 TEM (ThermoFisher Scientific; Hillsboro, OR, USA) at 80 kV with an Orius® 2k×2k CCD camera (Gatan, Inc.; Pleasanton, CA, USA). PAs at 0.5 mg/mL in 1× HBSS were prepared for TEM by negative staining. Briefly, 8 μL samples were incubated onto 400-mesh copper grids covered with a thin carbon film and previously treated with glow discharge. After 3 min, samples were stained with 2% uranyl acetate (w/v in DI H2O) for 2–3 min and air-dried before imaging. Nanofiber lengths were determined as previously described.73
Figures.
All figures were generated using Adobe Illustrator CC 2021 (25.2.3).
Supplementary Material
Acknowledgments
This study was supported, in part, by funding from the University of North Carolina School of Medicine. TDC acknowledges funding support from an American Australian Association Fellowship and the Center for Regenerative Nanomedicine at the Simpson Querrey Institute for BioNanotechnology at Northwestern University. We would like to acknowledge technical support provided by several core facilities at UNC Chapel Hill including: the UNC Microscopy Services Lab, the Small Animal Imaging Facility at the Biomedical Research Imaging Center, the R. L. Juliano Bioinformatics Core facility, and the Neuroscience Microscopy Core. The TEM studies were supported by a grant to JDG from the National Institutes of Health (5R0)-ES031635. The Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. Research reported in this publication was supported in part by the North Carolina Biotech Center Institutional Support Grant 2016-IDG-1016. MST and CD spectroscopy experiments were conducted at the UNC Macromolecular Interactions Facility which is supported by the National Cancer Institute of the National Institutes of Health under award number P30CA016086. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. X-ray experiments were carried out at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the Advanced Photon Source (APS). DND-CAT is supported by Northwestern University, E.I. DuPont de Nemours & Co., and The Dow Chemical Company. Use of APS, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. We would like to personally thank M. Seniw at the Simpson Querrey Institute for BioNanotechnology for peptide amphiphile nanofiber illustration design. Peptide amphiphile synthesis was performed in the Peptide Synthesis Core Facility of the Simpson Querrey Institute at Northwestern University. The U.S. Army Research Office, the U.S. Army Medical Research and Materiel Command, and Northwestern University provided funding to develop this facility and ongoing support is being received from the Soft and Hybrid Nanotechnology Experimental (SHyNE) (NSF ECCS-2025633).
Footnotes
Supporting Information
Additional experimental data, including a range of conditions tested to induce abdominal aortic aneurysms, a diagram of the crystal structures used to derive targeting peptides, electrospray ionization mass spectroscopy of all targeting and control PA molecules, X-ray scattering and circular dichroism for all targeting PA co-assemblies tested in animals, complete LSFM AAA targeting data, LSFM data comparing male and female rats, LSFM images of all tissue examined for presence of 25 mole% MMP-2 targeting PA nanofiber, H&E stains of tissue after injection of 25 mole% MMP-2 targeting PA nanofiber, conventional fluorescence microscopy of AAA targeting of 50 mole% fragmented elastin targeting PA nanofiber, and a table of all peptide amphiphiles and peptides used in the study.
References
- (1).CDC. Aortic Aneurysm Fact Sheet. 2016. https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_aortic_aneurysm.htm (accessed 2018 10/30).
- (2).Keisler B; Carter C Abdominal Aortic Aneurysm. Am Fam Physician 2015, 91 (8), 538–543. [PubMed] [Google Scholar]
- (3).Elkalioubie A; Haulon S; Duhamel A; Rosa M; Rauch A; Staels B; Susen S; Van Belle E; Dupont A Meta-Analysis of Abdominal Aortic Aneurysm in Patients with Coronary Artery Disease. Am J Cardiol 2015, 116 (9), 1451–1456. DOI: 10.1016/j.amjcard.2015.07.074. [DOI] [PubMed] [Google Scholar]
- (4).Argyriou C; Georgiadis GS; Kontopodis N; Pherwani AD; Van Herwaarden JA; Hazenberg C; Antoniou GA Screening for Abdominal Aortic Aneurysm During Transthoracic Echocardiography: A Systematic Review and Meta-Analysis. Eur J Vasc Endovasc Surg 2018, 55 (4), 475–491. DOI: 10.1016/j.ejvs.2018.01.003. [DOI] [PubMed] [Google Scholar]
- (5).Upchurch GR Jr.; Schaub TA Abdominal Aortic Aneurysm. Am Fam Physician 2006, 73 (7), 1198–1204. [PubMed] [Google Scholar]
- (6).Shteinberg D; Halak M; Shapiro S; Kinarty A; Sobol E; Lahat N; Karmeli R Abdominal Aortic Aneurysm and Aortic Occlusive Disease: A Comparison of Risk Factors and Inflammatory Response. Eur J Vasc Endovasc Surg 2000, 20 (5), 462–465. DOI: 10.1053/ejvs.2000.1210. [DOI] [PubMed] [Google Scholar]
- (7).Annambhotla S; Bourgeois S; Wang X; Lin PH; Yao Q; Chen C Recent Advances in Molecular Mechanisms of Abdominal Aortic Aneurysm Formation. World J Surg 2008, 32 (6), 976–986. DOI: 10.1007/s00268-007-9456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kimura T; Yoshimura K; Aoki H; Imanaka-Yoshida K; Yoshida T; Ikeda Y; Morikage N; Endo H; Hamano K; Imaizumi T; Hiroe M; Aonuma K; Matsuzaki M Tenascin-C Is Expressed in Abdominal Aortic Aneurysm Tissue with an Active Degradation Process. Pathol Int 2011, 61 (10), 559–564. DOI: 10.1111/j.1440-1827.2011.02699.x. [DOI] [PubMed] [Google Scholar]
- (9).Henderson EL; Geng YJ; Sukhova GK; Whittemore AD; Knox J; Libby P Death of Smooth Muscle Cells and Expression of Mediators of Apoptosis by T Lymphocytes in Human Abdominal Aortic Aneurysms. Circulation 1999, 99 (1), 96–104. [DOI] [PubMed] [Google Scholar]
- (10).Lopez-Candales A; Holmes DR; Liao S; Scott MJ; Wickline SA; Thompson RW Decreased Vascular Smooth Muscle Cell Density in Medial Degeneration of Human Abdominal Aortic Aneurysms. Am J Pathol 1997, 150 (3), 993–1007. [PMC free article] [PubMed] [Google Scholar]
- (11).Didangelos A; Yin X; Mandal K; Saje A; Smith A; Xu Q; Jahangiri M; Mayr M Extracellular Matrix Composition and Remodeling in Human Abdominal Aortic Aneurysms: A Proteomics Approach. Mol Cell Proteomics 2011, 10 (8), M111 008128. DOI: 10.1074/mcp.M111.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Stather PW; Sidloff DA; Dattani N; Gokani VJ; Choke E; Sayers RD; Bown MJ Meta-Analysis and Meta-Regression Analysis of Biomarkers for Abdominal Aortic Aneurysm. Br J Surg 2014, 101 (11), 1358–1372. DOI: 10.1002/bjs.9593. [DOI] [PubMed] [Google Scholar]
- (13).Vasic N; Glumac S; Pejic S; Amidzic LJ; Tadic Latinovic LJ; Dozic B; Hinic S; Maksimovic Z Expression of Matrix Metalloproteinases and Endogenous Inhibitors in Abdominal Aortic Aneurysm and Aortoiliac Occlusive Disease (Syndrome Leriche). Folia Biol (Praha) 2017, 63 (5–6), 209–216. [DOI] [PubMed] [Google Scholar]
- (14).Moran CS; McCann M; Karan M; Norman P; Ketheesan N; Golledge J Association of Osteoprotegerin with Human Abdominal Aortic Aneurysm Progression. Circulation 2005, 111 (23), 3119–3125. DOI: 10.1161/CIRCULATIONAHA.104.464727. [DOI] [PubMed] [Google Scholar]
- (15).Okada Y; Katsuda S; Okada Y; Nakanishi I An Elastinolytic Enzyme Detected in the Culture Medium of Human Arterial Smooth Muscle Cells. Cell Biol Int 1993, 17 (9), 863–869. DOI: 10.1006/cbir.1993.1149. [DOI] [PubMed] [Google Scholar]
- (16).Ciavarella C; Alviano F; Gallitto E; Ricci F; Buzzi M; Velati C; Stella A; Freyrie A; Pasquinelli G Human Vascular Wall Mesenchymal Stromal Cells Contribute to Abdominal Aortic Aneurysm Pathogenesis through an Impaired Immunomodulatory Activity and Increased Levels of Matrix Metalloproteinase-9. Circ J 2015, 79 (7), 1460–1469. DOI: 10.1253/circj.CJ-14-0857. [DOI] [PubMed] [Google Scholar]
- (17).Chan CYT; Cheuk BLY; Cheng SWK Abdominal Aortic Aneurysm-Associated MicroRNA-516a-5p Regulates Expressions of Methylenetetrahydrofolate Reductase, Matrix Metalloproteinase-2, and Tissue Inhibitor of Matrix Metalloproteinase-1 in Human Abdominal Aortic Vascular Smooth Muscle Cells. Ann Vasc Surg 2017, 42, 263–273. DOI: 10.1016/j.avsg.2016.10.062. [DOI] [PubMed] [Google Scholar]
- (18).Longo GM; Xiong W; Greiner TC; Zhao Y; Fiotti N; Baxter BT Matrix Metalloproteinases 2 and 9 Work in Concert to Produce Aortic Aneurysms. J Clin Invest 2002, 110 (5), 625–632. DOI: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Gertz SD; Kurgan A; Eisenberg D Aneurysm of the Rabbit Common Carotid Artery Induced by Periarterial Application of Calcium Chloride in Vivo. J Clin Invest 1988, 81 (3), 649–656. DOI: 10.1172/JCI113368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Patelis N; Moris D; Schizas D; Damaskos C; Perrea D; Bakoyiannis C; Liakakos T; Georgopoulos S Animal Models in the Research of Abdominal Aortic Aneurysms Development. Physiol Res 2017, 66 (6), 899–915. [DOI] [PubMed] [Google Scholar]
- (21).Trollope A; Moxon JV; Moran CS; Golledge J Animal Models of Abdominal Aortic Aneurysm and Their Role in Furthering Management of Human Disease. Cardiovasc Pathol 2011, 20 (2), 114–123. DOI: 10.1016/j.carpath.2010.01.001. [DOI] [PubMed] [Google Scholar]
- (22).Lysgaard Poulsen J, J. S, Lindholt JS. Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. European Journal of Vascular and Endovascular Surgery 2016, 52 (4), 487–499, Systematic Review. [DOI] [PubMed] [Google Scholar]
- (23).Klaus V; Tanios-Schmies F; Reeps C; Trenner M; Matevossian E; Eckstein HH; Pelisek J Association of Matrix Metalloproteinase Levels with Collagen Degradation in the Context of Abdominal Aortic Aneurysm. Eur J Vasc Endovasc Surg 2017, 53 (4), 549–558. DOI: 10.1016/j.ejvs.2016.12.030. [DOI] [PubMed] [Google Scholar]
- (24).Siennicka A; Zuchowski M; Kaczmarczyk M; Cnotliwy M; Clark JS; Jastrzebska M Spatial Differences of Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 within Abdominal Aortic Aneurysm Wall and Intraluminal Thrombus. J Physiol Pharmacol 2016, 67 (6), 903–910. [PubMed] [Google Scholar]
- (25).Davis V; Persidskaia R; Baca-Regen L; Itoh Y; Nagase H; Persidsky Y; Ghorpade A; Baxter BT Matrix Metalloproteinase-2 Production and Its Binding to the Matrix Are Increased in Abdominal Aortic Aneurysms. Arterioscler Thromb Vasc Biol 1998, 18 (10), 1625–1633. [DOI] [PubMed] [Google Scholar]
- (26).Tekin G; Isbir S; Sener G; Cevik O; Cetinel S; Dericioglu O; Arsan S; Cobanoglu A The Preventive and Curative Effects of Melatonin against Abdominal Aortic Aneurysm in Rats. J Vasc Surg 2018, 67 (5), 1546–1555. DOI: 10.1016/j.jvs.2017.04.028. [DOI] [PubMed] [Google Scholar]
- (27).Jones JA; Ruddy JM; Bouges S; Zavadzkas JA; Brinsa TA; Stroud RE; Mukherjee R; Spinale FG; Ikonomidis JS Alterations in Membrane Type-1 Matrix Metalloproteinase Abundance after the Induction of Thoracic Aortic Aneurysm in a Murine Model. Am J Physiol Heart Circ Physiol 2010, 299 (1), H114–124. DOI: 10.1152/ajpheart.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Pharmaceuticals NPN ACZ885 for the Treatment of Abdominal Aortic Aneurysm (AAA). 2016. https://clinicaltrials.gov/ct2/show/study/NCT02007252?rslt=With&type=Intr&cond=Aortic+Aneurysm&rank=1§=X370156 (accessed 2018 October 31).
- (29).Abdul-Hussien H; Hanemaaijer R; Verheijen JH; van Bockel JH; Geelkerken RH; Lindeman JH Doxycycline Therapy for Abdominal Aneurysm: Improved Proteolytic Balance through Reduced Neutrophil Content. J Vasc Surg 2009, 49 (3), 741–749. DOI: 10.1016/j.jvs.2008.09.055. [DOI] [PubMed] [Google Scholar]
- (30).Kaito K; Urayama H; Watanabe G Doxycycline Treatment in a Model of Early Abdominal Aortic Aneurysm. Surg Today 2003, 33 (6), 426–433. DOI: 10.1007/s10595-002-2513-0. [DOI] [PubMed] [Google Scholar]
- (31).Yu M; Chen C; Cao Y; Qi R Inhibitory Effects of Doxycycline on the Onset and Progression of Abdominal Aortic Aneurysm and Its Related Mechanisms. Eur J Pharmacol 2017, 811, 101–109. DOI: 10.1016/j.ejphar.2017.05.041. [DOI] [PubMed] [Google Scholar]
- (32).Baxter BT; Matsumura J; Curci J; McBride R; Blackwelder WC; Liu X; Larson L; Terrin ML; Investigators NT Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA(3)CT): Design of a Phase IIb, Placebo-Controlled, Double-Blind, Randomized Clinical Trial of Doxycycline for the Reduction of Growth of Small Abdominal Aortic Aneurysm. Contemp Clin Trials 2016, 48, 91–98. DOI: 10.1016/j.cct.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Moyer TJ; Kassam HA; Bahnson ES; Morgan CE; Tantakitti F; Chew TL; Kibbe MR; Stupp SI Shape-Dependent Targeting of Injured Blood Vessels by Peptide Amphiphile Supramolecular Nanostructures. Small 2015, 11 (23), 2750–2755. DOI: 10.1002/smll.201403429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bahnson ES; Kassam HA; Moyer TJ; Jiang W; Morgan CE; Vercammen JM; Jiang Q; Flynn ME; Stupp SI; Kibbe MR Targeted Nitric Oxide Delivery by Supramolecular Nanofibers for the Prevention of Restenosis after Arterial Injury. Antioxid Redox Signal 2016, 24 (8), 401–418. DOI: 10.1089/ars.2015.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Morgan CE; Dombrowski AW; Rubert Pérez CM; Bahnson ES; Tsihlis ND; Jiang W; Jiang Q; Vercammen JM; Prakash VS; Pritts TA; Stupp SI; Kibbe MR Tissue-Factor Targeted Peptide Amphiphile Nanofibers as an Injectable Therapy to Control Hemorrhage. ACS Nano 2016, 10 (1), 899–909. DOI: 10.1021/acsnano.5b06025. [DOI] [PubMed] [Google Scholar]
- (36).Klein MK; Kassam HA; Lee RH; Bergmeier W; Peters EB; Gillis DC; Dandurand BR; Rouan JR; Karver MR; Struble MD; Clemons TD; Palmer LC; Gavitt B; Pritts TA; Tsihlis ND; Stupp SI; Kibbe MR Development of Optimized Tissue-Factor-Targeted Peptide Amphiphile Nanofibers to Slow Noncompressible Torso Hemorrhage. ACS Nano 2020, 14 (6), 6649–6662. DOI: 10.1021/acsnano.9b09243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Mansukhani NA; Peters EB; So MM; Albaghdadi MS; Wang Z; Karver MR; Clemons TD; Laux JP; Tsihlis ND; Stupp SI; Kibbe MR Peptide Amphiphile Supramolecular Nanostructures as a Targeted Therapy for Atherosclerosis. Macromol Biosci 2019, 19 (6), e1900066. DOI: 10.1002/mabi.201900066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Peters EB; Tsihlis ND; Karver MR; Chin SM; Musetti B; Ledford BT; Bahnson EM; Stupp SI; Kibbe MR Atheroma Niche-Responsive Nanocarriers for Immunotherapeutic Delivery. Adv Healthc Mater 2019, 8 (3), e1801545. DOI: 10.1002/adhm.201801545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Clemons TD; Stupp SI Design of Materials with Supramolecular Polymers. Prog Polym Sci 2020, 111, 101310. DOI: 10.1016/j.progpolymsci.2020.101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Yuan CQ; Ji W; Xing RR; Li JB; Gazit E; Yan XH Hierarchically Oriented Organization in Supramolecular Peptide Crystals. Nat Rev Chem 2019, 3 (10), 567–588. DOI: 10.1038/s41570-019-0129-8. [DOI] [Google Scholar]
- (41).Yuan CQ; Levin A; Chen W; Xing RR; Zou QL; Herling TW; Challa PK; Knowles TPJ; Yan XH Nucleation and Growth of Amino Acid and Peptide Supramolecular Polymers through Liquid-Liquid Phase Separation. Angew Chem Int Edit 2019, 58 (50), 18116–18123. DOI: 10.1002/anie.201911782. [DOI] [PubMed] [Google Scholar]
- (42).Zitka O; Kukacka J; Krizkova S; Huska D; Adam V; Masarik M; Prusa R; Kizek R Matrix Metalloproteinases. Curr Med Chem 2010, 17 (31), 3751–3768. [DOI] [PubMed] [Google Scholar]
- (43).Curci JA; Liao S; Huffman MD; Shapiro SD; Thompson RW Expression and Localization of Macrophage Elastase (Matrix Metalloproteinase-12) in Abdominal Aortic Aneurysms. J Clin Invest 1998, 102 (11), 1900–1910. DOI: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Newman KM; Ogata Y; Malon AM; Irizarry E; Gandhi RH; Nagase H; Tilson MD Identification of Matrix Metalloproteinases 3 (Stromelysin-1) and 9 (Gelatinase B) in Abdominal Aortic Aneurysm. Arterioscler Thromb 1994, 14 (8), 1315–1320. [DOI] [PubMed] [Google Scholar]
- (45).Wilson WR; Anderton M; Schwalbe EC; Jones JL; Furness PN; Bell PR; Thompson MM Matrix Metalloproteinase-8 and −9 Are Increased at the Site of Abdominal Aortic Aneurysm Rupture. Circulation 2006, 113 (3), 438–445. DOI: 10.1161/CIRCULATIONAHA.105.551572. [DOI] [PubMed] [Google Scholar]
- (46).Duellman T; Warren CL; Peissig P; Wynn M; Yang J Matrix Metalloproteinase-9 Genotype as a Potential Genetic Marker for Abdominal Aortic Aneurysm. Circ Cardiovasc Genet 2012, 5 (5), 529–537. DOI: 10.1161/CIRCGENETICS.112.963082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Xiong W; Knispel R; MacTaggart J; Greiner TC; Weiss SJ; Baxter BT Membrane-Type 1 Matrix Metalloproteinase Regulates Macrophage-Dependent Elastolytic Activity and Aneurysm Formation in Vivo. J Biol Chem 2009, 284 (3), 1765–1771. DOI: 10.1074/jbc.M806239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Aggarwal S; Qamar A; Sharma V; Sharma A Abdominal Aortic Aneurysm: A Comprehensive Review. Exp Clin Cardiol 2011, 16 (1), 11–15. [PMC free article] [PubMed] [Google Scholar]
- (49).Halper J; Kjaer M Basic Components of Connective Tissues and Extracellular Matrix: Elastin, Fibrillin, Fibulins, Fibrinogen, Fibronectin, Laminin, Tenascins and Thrombospondins. Adv Exp Med Biol 2014, 802, 31–47. DOI: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- (50).Basalyga DM; Simionescu DT; Xiong W; Baxter BT; Starcher BC; Vyavahare NR Elastin Degradation and Calcification in an Abdominal Aorta Injury Model: Role of Matrix Metalloproteinases. Circulation 2004, 110 (22), 3480–3487. DOI: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Dao HH; Essalihi R; Bouvet C; Moreau P Evolution and Modulation of Age-Related Medial Elastocalcinosis: Impact on Large Artery Stiffness and Isolated Systolic Hypertension. Cardiovasc Res 2005, 66 (2), 307–317. DOI: 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- (52).Bailey M; Pillarisetti S; Jones P; Xiao H; Simionescu D; Vyavahare N Involvement of Matrix Metalloproteinases and Tenascin-C in Elastin Calcification. Cardiovasc Pathol 2004, 13 (3), 146–155. DOI: 10.1016/S1054-8807(04)00009-2. [DOI] [PubMed] [Google Scholar]
- (53).Jones GT; Tromp G; Kuivaniemi H; Gretarsdottir S; Baas AF; Giusti B; Strauss E; Van’t Hof FN; Webb TR; Erdman R; Ritchie MD; Elmore JR; Verma A; Pendergrass S; Kullo IJ; Ye Z; Peissig PL; Gottesman O; Verma SS; Malinowski J; Rasmussen-Torvik LJ; Borthwick KM; Smelser DT; Crosslin DR; de Andrade M; Ryer EJ; McCarty CA; Bottinger EP; Pacheco JA; Crawford DC; Carrell DS; Gerhard GS; Franklin DP; Carey DJ; Phillips VL; Williams MJ; Wei W; Blair R; Hill AA; Vasudevan TM; Lewis DR; Thomson IA; Krysa J; Hill GB; Roake J; Merriman TR; Oszkinis G; Galora S; Saracini C; Abbate R; Pulli R; Pratesi C; Saratzis A; Verissimo AR; Bumpstead S; Badger SA; Clough RE; Cockerill G; Hafez H; Scott DJ; Futers TS; Romaine SP; Bridge K; Griffin KJ; Bailey MA; Smith A; Thompson MM; van Bockxmeer FM; Matthiasson SE; Thorleifsson G; Thorsteinsdottir U; Blankensteijn JD; Teijink JA; Wijmenga C; de Graaf J; Kiemeney LA; Lindholt JS; Hughes A; Bradley DT; Stirrups K; Golledge J; Norman PE; Powell JT; Humphries SE; Hamby SE; Goodall AH; Nelson CP; Sakalihasan N; Courtois A; Ferrell RE; Eriksson P; Folkersen L; Franco-Cereceda A; Eicher JD; Johnson AD; Betsholtz C; Ruusalepp A; Franzen O; Schadt EE; Bjorkegren JL; Lipovich L; Drolet AM; Verhoeven EL; Zeebregts CJ; Geelkerken RH; van Sambeek MR; van Sterkenburg SM; de Vries JP; Stefansson K; Thompson JR; de Bakker PI; Deloukas P; Sayers RD; Harrison SC; van Rij AM; Samani NJ; Bown MJ Meta-Analysis of Genome-Wide Association Studies for Abdominal Aortic Aneurysm Identifies Four New Disease-Specific Risk Loci. Circ Res 2017, 120 (2), 341–353. DOI: 10.1161/CIRCRESAHA.116.308765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Takagi H; Manabe H; Kawai N; Goto SN; Umemoto T Circulating Matrix Metalloproteinase-9 Concentrations and Abdominal Aortic Aneurysm Presence: A Meta-Analysis. Interact Cardiovasc Thorac Surg 2009, 9 (3), 437–440. DOI: 10.1510/icvts.2009.208835. [DOI] [PubMed] [Google Scholar]
- (55).Eliason JL; Hannawa KK; Ailawadi G; Sinha I; Ford JW; Deogracias MP; Roelofs KJ; Woodrum DT; Ennis TL; Henke PK; Stanley JC; Thompson RW; Upchurch GR Jr. Neutrophil Depletion Inhibits Experimental Abdominal Aortic Aneurysm Formation. Circulation 2005, 112 (2), 232–240. DOI: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- (56).Blanchevoye C; Floquet N; Scandolera A; Baud S; Maurice P; Bocquet O; Blaise S; Ghoneim C; Cantarelli B; Delacoux F; Dauchez M; Efremov RG; Martiny L; Duca L; Debelle L Interaction between the Elastin Peptide Vgvapg and Human Elastin Binding Protein. J Biol Chem 2013, 288 (2), 1317–1328. DOI: 10.1074/jbc.M112.419929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Hinek A; Rabinovitch M 67-kD Elastin-Binding Protein Is a Protective “Companion” of Extracellular Insoluble Elastin and Intracellular Tropoelastin. J Cell Biol 1994, 126 (2), 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Hinek A; Mecham RP; Keeley F; Rabinovitch M Impaired Elastin Fiber Assembly Related to Reduced 67-kD Elastin-Binding Protein in Fetal Lamb Ductus Arteriosus and in Cultured Aortic Smooth Muscle Cells Treated with Chondroitin Sulfate. J Clin Invest 1991, 88 (6), 2083–2094. DOI: 10.1172/JCI115538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Hinek A; Pshezhetsky AV; von Itzstein M; Starcher B Lysosomal Sialidase (Neuraminidase-1) Is Targeted to the Cell Surface in a Multiprotein Complex That Facilitates Elastic Fiber Assembly. J Biol Chem 2006, 281 (6), 3698–3710. DOI: 10.1074/jbc.M508736200. [DOI] [PubMed] [Google Scholar]
- (60).Edelbrock AN; Clemons TD; Chin SM; Roan JJW; Bruckner EP; Alvarez Z; Edelbrock JF; Wek KS; Stupp SI Superstructured Biomaterials Formed by Exchange Dynamics and Host-Guest Interactions in Supramolecular Polymers. Adv Sci (Weinh) 2021, 8 (8), 2004042. DOI: 10.1002/advs.202004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Dodero VI; Quirolo ZB; Sequeira MA Biomolecular Studies by Circular Dichroism. Front Biosci (Landmark Ed) 2011, 16, 61–73. DOI: 10.2741/3676. [DOI] [PubMed] [Google Scholar]
- (62).Morgunova E; Tuuttila A; Bergmann U; Tryggvason K Structural Insight into the Complex Formation of Latent Matrix Metalloproteinase 2 with Tissue Inhibitor of Metalloproteinase 2. Proc Natl Acad Sci U S A 2002, 99 (11), 7414–7419. DOI: 10.1073/pnas.102185399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Sinha A; Shaporev A; Nosoudi N; Lei Y; Vertegel A; Lessner S; Vyavahare N Nanoparticle Targeting to Diseased Vasculature for Imaging and Therapy. Nanomedicine 2014, 10 (5), 1003–1012. DOI: 10.1016/j.nano.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Renier N; Wu Z; Simon DJ; Yang J; Ariel P; Tessier-Lavigne M Idisco: A Simple, Rapid Method to Immunolabel Large Tissue Samples for Volume Imaging. Cell 2014, 159 (4), 896–910. DOI: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- (65).DiMusto PD; Lu G; Ghosh A; Roelofs KJ; Su G; Zhao Y; Lau CL; Sadiq O; McEvoy B; Laser A; Diaz JA; Wakefield TW; Henke PK; Eliason JL; Upchurch GR Jr. Increased Pai-1 in Females Compared with Males Is Protective for Abdominal Aortic Aneurysm Formation in a Rodent Model. Am J Physiol Heart Circ Physiol 2012, 302 (7), H1378–1386. DOI: 10.1152/ajpheart.00620.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).DiMusto PD; Lu G; Ghosh A; Roelofs KJ; Sadiq O; McEvoy B; Su G; Laser A; Bhamidipati CM; Ailawadi G; Henke PK; Eliason JL; Upchurch GR Jr. Increased Jnk in Males Compared with Females in a Rodent Model of Abdominal Aortic Aneurysm. J Surg Res 2012, 176 (2), 687–695. DOI: 10.1016/j.jss.2011.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Sela-Passwell N; Kikkeri R; Dym O; Rozenberg H; Margalit R; Arad-Yellin R; Eisenstein M; Brenner O; Shoham T; Danon T; Shanzer A; Sagi I Antibodies Targeting the Catalytic Zinc Complex of Activated Matrix Metalloproteinases Show Therapeutic Potential. Nat Med 2011, 18 (1), 143–147. DOI: 10.1038/nm.2582. [DOI] [PubMed] [Google Scholar]
- (68).Shen M; Lee J; Basu R; Sakamuri SS; Wang X; Fan D; Kassiri Z Divergent Roles of Matrix Metalloproteinase 2 in Pathogenesis of Thoracic Aortic Aneurysm. Arterioscler Thromb Vasc Biol 2015, 35 (4), 888–898. DOI: 10.1161/ATVBAHA.114.305115. [DOI] [PubMed] [Google Scholar]
- (69).Choi HS; Liu W; Misra P; Tanaka E; Zimmer JP; Itty Ipe B; Bawendi MG; Frangioni JV Renal Clearance of Quantum Dots. Nat Biotechnol 2007, 25 (10), 1165–1170. DOI: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Ikonomidis JS; Gibson WC; Gardner J; Sweterlitsch S; Thompson RP; Mukherjee R; Spinale FG A Murine Model of Thoracic Aortic Aneurysms. J Surg Res 2003, 115 (1), 157–163. DOI: 10.1016/s0022-4804(03)00193-8. [DOI] [PubMed] [Google Scholar]
- (71).Buglak NE; Lucitti J; Ariel P; Maiocchi S; Miller FJ; Bahnson ESM Light Sheet Fluorescence Microscopy as a New Method for Unbiased Three-Dimensional Analysis of Vascular Injury. Cardiovasc Res 2021, 117 (2), 520–532. DOI: 10.1093/cvr/cvaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Ilavsky J; Jemian PR Irena: Tool Suite for Modeling and Analysis of Small-Angle Scattering. Journal of Applied Crystallography 2009, 42, 347–353. DOI: 10.1107/S0021889809002222. [DOI] [Google Scholar]
- (73).Sato K; Ji W; Palmer LC; Weber B; Barz M; Stupp SI Programmable Assembly of Peptide Amphiphile Via Noncovalent-to-Covalent Bond Conversion. J Am Chem Soc 2017, 139 (26), 8995–9000. DOI: 10.1021/jacs.7b03878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


