Abstract
Background
This meta-analysis was conducted to evaluate the effect of fractional flow reserve (FFR) on clinical outcomes after coronary artery bypass grafting (CABG).
Methods
Five online databases were searched for studies that (1) enrolled patients who underwent isolated CABG or CABG with aortic valve replacement and (2) demonstrated the effect of an FFR-guided strategy on major adverse cardiac events (MACE) after surgery based on a randomized controlled trial or adjusted analysis. MACE included cardiac death, acute myocardial infarction (MI), and repeated revascularization. The primary outcomes were all MACE outcomes and a composite of all-cause death and MI, and the secondary outcomes were the individual MACE outcomes. Publication bias was assessed using a funnel plot and the Egger test.
Results
Six articles (3 randomized and 3 non-randomized studies n=1,027) were selected. MACE data were extracted from 4 studies. The pooled analyses showed that the risk of MACE was not significantly different between patients who underwent FFR-guided CABG and those who underwent angiography-guided CABG (hazard ratio [HR], 0.80; 95% CI, 0.57–1.12). However, the risk of the composite of death or MI was significantly lower in patients undergoing FFR-guided CABG (HR, 0.62; 95% CI, 0.41–0.94). The individual MACE outcomes were not significantly different between FFR-guided and angiography-guided CABG.
Conclusion
FFR-guided CABG might be beneficial in terms of the composite outcome of death or MI compared with angiography-guided CABG although data are limited.
Keywords: Fractional flow reserve, Coronary artery bypass grafting, Statistics, Meta-analysis
Introduction
The functional significance of coronary artery stenosis (CAS) in treatment outcomes after coronary revascularization has been recently emphasized because of differences in the anatomic severity of CAS and functional ischemia of the subtending myocardium [1,2].
The fractional flow reserve (FFR) has been widely used to evaluate the functional significance of CAS; in particular, FFR is widely used in the decision-making process for percutaneous coronary intervention (PCI) [3,4]. In the presence of discordance between the anatomic severity and hemodynamic significance of CAS, a strategy based on the hemodynamic significance of coronary stenosis assessed by FFR demonstrated better clinical outcomes than a strategy based on angiographic severity [3,5,6].
However, data regarding the effect of an FFR-guided strategy on outcomes after coronary artery bypass grafting (CABG) are limited. Therefore, this meta-analysis was conducted to evaluate the effect of FFR-guided decision-making on clinical outcomes after CABG.
Methods
Data source and literature search
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [7]. Full-text articles evaluating the effect of FFR on clinical outcomes after CABG were searched for in the MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Web of Science, and Scopus databases on January 28, 2022 without any restrictions on the language or publication year. The following keywords and medical subject heading terms were searched in MEDLINE: (“Fractional Flow Reserve, Myocardial” [MeSH Terms] OR “Fractional Flow Reserve” [Title/Abstract]) AND (“Coronary Artery Bypass” [MeSH Terms]) OR “Coronary artery bypass” [Title/Abstract] OR “Coronary revascularization” [Title/Abstract] OR “Coronary artery revascularization” [Title/Abstract] OR “Myocardial revascularization” [Title/Abstract]). The search strategies for the other databases were adapted from this strategy.
Institutional Review Board approval was not necessary due to the nature of the meta-analysis.
Study selection
Studies were selected independently by 2 reviewers (Y.K. and H.Y.H.) based on the selection criteria. Any disagreements were resolved via a discussion with the third author. The studies were selected by screening first the titles and abstracts and then the full texts.
Studies that compared clinical outcomes after FFR-guided CABG with those after angiography-guided CABG were included. When duplicate publications with overlapping study populations were found, the most appropriate article was selected.
Data extraction
The study characteristics and the patients’ baseline data were extracted independently by 2 reviewers (Y.K. and H.Y.H.). Data regarding study outcomes were extracted independently by 2 reviewers (M.J.J. and H.Y.H.). Any disagreements were resolved by discussion with the third author (S.H.S.).
Assessment of quality
The overall study quality was assessed independently by 2 reviewers (M.J.J. and H.Y.H.) using the Revised Cochrane Risk-of-Bias tool (RoB2) for randomized controlled trials (RCTs) and the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) for non-randomized studies (NRSs) [8,9]. In the RoB2, each of the 5 domains was assessed with judgments (low, some concerns, or high), and the overall risk of bias (ROB) was determined as the worst ROB in the 5 domains. In the ROBINS-I, each of the 7 domains was rated with a judgment (low, moderate, serious, or critical) and the overall ROB was defined as the highest ROB level in the 7 domains. Any disagreements between the reviewers were resolved by discussion with the third author (S.H.S.).
Statistical analysis
Major adverse cardiac events (MACE) after surgery were defined as all-cause death, myocardial infarction (MI), and repeated revascularization. The primary outcomes were all MACE outcomes and a composite of all-cause death and MI. The secondary outcomes were the individual outcomes of MACE.
For studies reporting results from both multivariable and propensity-score matching (PSM) analyses, PSM estimates were selected for the present analyses, and the number of patients in the study was counted as the number of patients included in the PSM analysis. For studies that did not report the composite of death or MI, the number of composite outcomes was drawn from individual outcome data. Statistical heterogeneity among the studies was assessed using the chi-square test and I2 statistic. I2 values of 25%, 50%, and 75% are indicators of low, moderate, and high heterogeneity, respectively [10]. A random-effects model with the DerSimonian and Laird method was used when substantial heterogeneity was found (I2>50%); otherwise, a fixed-effects model was planned using the inverse variance method.
Outcomes were compared and presented as hazard ratios (HRs) with 95% confidence intervals (CIs). For studies reporting the number of events without HRs, the HRs and 95% CIs were calculated from the number of events according to the formula [11]. Pooled estimates from RCTs and NRSs were presented. Subgroup differences were assessed using the Cochran Q test for heterogeneity. A funnel plot and the Egger test for asymmetry were applied to assess the possibility of publication bias for the primary outcomes, but not for the secondary outcomes because of the small number of studies included [12].
All analyses were performed using R ver. 3.6.2 (meta package; The R Foundation for Statistical Computing, Vienna, Austria). Two-sided p-values <0.05 were considered to indicate statistical significance.
Results
Identification of the studies
The database search detected 9,225 articles. After reviewing the titles and abstracts, publications not related to the study objectives or those without clinical outcomes were excluded (n=9,214) and 11 full manuscripts were reviewed. Five studies were excluded because the inclusion criteria were not met (n=4) or due to duplicate data (n=1). Therefore, 6 studies were ultimately included in this review (Fig. 1) [13-18].
Fig. 1.
Flow diagram based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Study characteristics and patient populations
Among the 6 studies involving 1,027 patients, 5 studies [13-17] enrolled isolated CABG patients, while the other study included patients who underwent CABG and aortic valve replacement [18]. Three studies [15-17] presented the results of RCTs (n=378) and the other 3 reported the outcomes of NRSs (n=649). The cut-off value of FFR was 0.8 in all studies. The clinical follow-up duration ranged from 6 to 86 months (Table 1). On average, the patients were in their 60s or 70s, and more than 70% of the patients were male. Dyslipidemia (54%–86%) and hypertension (56%–78%) were the most common comorbidities (Table 2).
Table 1.
Characteristics of the included studies
| Study | Operative period | Country | Study type | Study population | Cut-off of FFR | Follow-up (mo) | Type of surgery | Statistical methods | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Total | FFR | CAG | ||||||||
| Fournier et al. [13] (2018) | 2006–2010 | Belgium | NRS | 396 | 198 | 198 | 0.8 | 86 | Isolated CABG | PSM |
| Moscona et al. [14] (2018) | 2014–2016 | USA | NRS | 109 | 14 | 95 | 0.8 | 18 | Isolated CABG | UV |
| Thuesen et al. [15] (2018) | 2014–2016 | Denmark | RCT | 97 | 49 | 48 | 0.8 | 6 | Isolated CABG | RCT |
| Toth et al. [16] (2019) | 2012–2016 | Europe | RCT | 172 | 88 | 84 | 0.8 | 12 | Isolated CABG | RCT |
| Rioufol et al. [17] (2021) | - | France | RCTa) | 109 | 54 | 55 | 0.8 | 12 | Isolated CABG | RCTa) |
| Di Gioia et al. [18] (2016) | 2002–2010 | Belgium | NRS | 144 | 41 | 103 | 0.8 | 60 | CABG+AVR | PSMb) |
FFR, fractional flow reserve; CAG, coronary angiography; NRS, non-randomized study; CABG, coronary artery bypass graft surgery; PSM, propensity score matching; UV, univariate analysis; RCT, randomized controlled trial; AVR, aortic valve replacement.
a)The study design was an RCT, but the enrolled patients underwent either a percutaneous intervention or CABG. b)The study design was PSM, but the enrolled patients underwent various treatments, and data from patients who underwent CABG+AVR were analyzed.
Table 2.
Patients’ characteristics
| Study | Age (yr) | Male (%) | BMI (kg/m2) | Smoking (%) | Hypertension (%) | Diabetes (%) | Dyslipidemia (%) | LVEF (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| FFR | CAG | FFR | CAG | FFR | CAG | FFR | CAG | FFR | CAG | FFR | CAG | FFR | CAG | FFR | CAG | ||||||||
| Fournier et al. [13] (2018) | 65±12 | 66±10 | 82 | 79 | 28±4 | 27±3 | 42 | 47 | 78 | 74 | 21 | 22 | 65 | 65 | 70±12 | 70±14 | |||||||
| Moscona et al. [14] (2018) | 59 | 64 | 64 | 76 | 32 | 29 | 72 | 62 | 72 | 78 | 57 | 44 | 57 | 59 | 51±13 | 52±5 | |||||||
| Thuesen et al. [15] (2018) | 66±6 | 65±9 | 88 | 89 | 28±4 | 27±4 | 27 | 17 | 67 | 67 | 22 | 23 | 86 | 75 | - | - | |||||||
| Toth et al. [16] (2019) | 67±8 | 67±7 | 83 | 79 | - | - | 53 | 42 | 77 | 70 | 35 | 40 | 80 | 79 | - | - | |||||||
| Rioufol et al. [17] (2021)a) | 65±10 | 66±11 | 85 | 82 | 28±5 | 27±5 | 24 | 26 | 58 | 61 | 31 | 32 | 60 | 61 | 55±12 | 56±11 | |||||||
| Di Gioia et al. [18] (2016)b) | 73±10 | 73±9 | 72 | 69 | 27±4 | 27±4 | 38 | 32 | 59 | 56 | 24 | 24 | 56 | 54 | 69±17 | 69±17 | |||||||
Values are presented as mean±standard deviation or %.
BMI, body mass index; LVEF, left ventricular ejection fraction; FFR, fractional flow reserve; CAG, coronary angiography.
a)Data from the entire population of study patients who underwent either percutaneous intervention or coronary artery bypass grafting. b)Data from the entire population of study patients who underwent medical, interventional, or surgical treatment for combined aortic stenosis and coronary artery disease.
Quality of the included studies
The ROB of all 3 RCTs was considered low for all 5 domains [15-17]; thus, the overall ROB was judged to be low. Two NRSs were judged to have an overall moderate ROB [13,18], whereas the other NRS had a high ROB based on the “bias due to confounding” domain [14]. All other ROB items were determined to be as low in all 3 NRSs (Tables 3, 4).
Table 3.
Quality assessment of the included studies by revised Cochrane Risk-of-Bias tool for randomized trials
| Study | Quality assessment by revised Cochrane Risk-of-Bias tool for randomized trials | Overall | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Bias arising from the randomization process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported result | ||
| Thuesen et al. [15] (2018) | Low | Low | Low | Low | Low | Low |
| Toth et al. [16] (2019) | Low | Low | Low | Low | Low | Low |
| Rioufol et al. [17] (2021) | Low | Low | Low | Low | Low | Low |
Table 4.
Quality assessment of the included studies by Risk of Bias in Non-randomized Studies of Interventions for Non-randomized Studies
| Study | Quality assessment by Risk of Bias in Non-randomized Studies of Interventions for Non-randomized Studies | Overall | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Bias due to confounding | Bias in selection of participants into the study | Bias in measurement of interventions | Bias due to departures from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | ||
| Fournier et al. [13] (2018) | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Moscona et al. [14] (2018) | High | Low | Low | Low | Low | Low | Low | High |
| Di Gioia et al. [18] (2016) | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
Primary outcomes
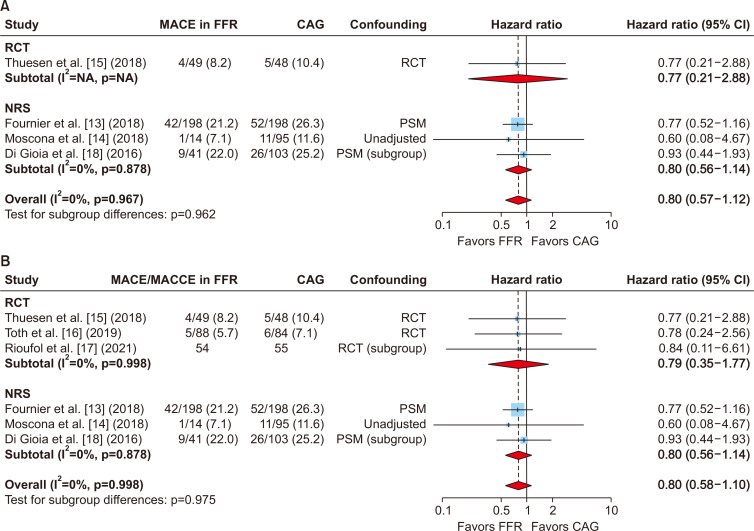
MACE data were extracted from 4 studies [13-15,18], while the other 2 studies presented data regarding major adverse cardiac and cerebrovascular events (MACCE) that included MACE and stroke. Although the pooled analyses of MACE in 746 patients from 4 studies favored FFR-guided CABG, the risk reduction was not statistically significant (HR, 0.80; 95% CI, 0.57–1.12) (Fig. 2). The results were similar when the pooled analysis included the MACE data from 4 studies and the MACCE data from the other 2 studies (HR, 0.80; 95% CI, 0.58–1.10) (Fig. 2).
Fig. 2.
(A) Pooled analysis of the risk of the primary endpoint, major adverse cardiac events (MACE), after fractional flow reserve (FFR)-guided coronary artery bypass grafting (CABG) compared with angiography-guided CABG in randomized controlled trials (RCTs) and non-randomized studies (NRSs). The pooled estimates from the RCTs and NRSs showed that the decrease in MACE risk was not statistically significant in the FFR-guided CABG group. (B) A similar finding was obtained when the pooled analysis was performed for MACE or major adverse cardiac and cerebrovascular events (MACCE) in 6 studies. HR, hazard ratio; CI, confidence interval.
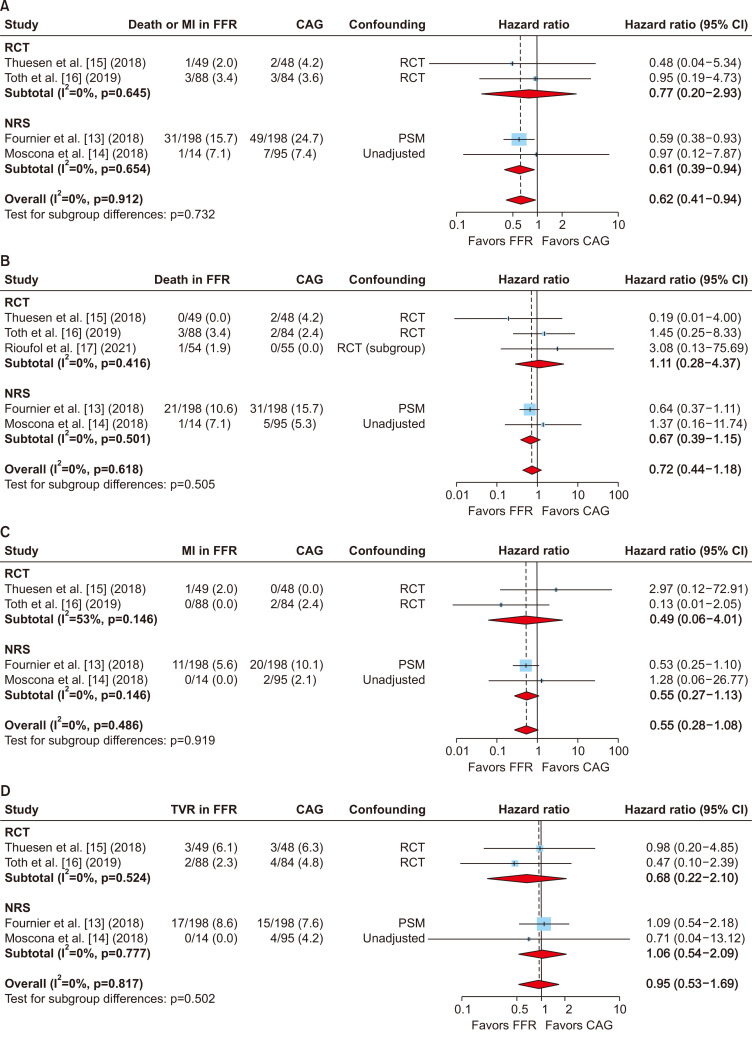
The composite of death or MI was extracted from 4 studies involving 774 patients [13-15,18]. In a study reporting only individual outcomes, it was unclear whether all the events occurred in different patients or whether a patient experienced both MI and death in angiography-guided CABG [16]. Therefore, the number of patients who experienced these composite events in angiography-guided CABG could have been either 3 or 4. To avoid bias of double-counting and to provide more conservative results, it was counted as 3 rather than 4. Despite this conservative approach, the pooled analysis demonstrated that FFR-guided CABG was significantly associated with a 38% reduction in the occurrence of the composite of death or MI (HR, 0.62; 95% CI, 0.41–0.94) (Fig. 3).
Fig. 3.
Pooled analysis of the risk of outcomes. (A) Composite of death or myocardial infarction (MI), (B) death, (C) MI, and (D) repeated revascularization after fractional flow reserve (FFR)-guided coronary artery bypass grafting (CABG) compared with angiography-guided CABG in randomized controlled trials (RCTs) and non-randomized studies (NRSs). The pooled estimates from the RCTs and NRSs showed that the risk of the composite of death or MI was significantly lower in the FFR-guided CABG group compared with the angiography-guided CABG group. HR, hazard ratio; CI, confidence interval. (Continued on next page).
Secondary outcomes
Data regarding death, MI, and repeated revascularization were extracted from 5 [13-17], 4 [13-16], and 4 studies [13-16], respectively. The pooled analyses demonstrated that FFR-guided CABG tended to be favored for the secondary outcomes although the results were not statistically significant (Fig. 3).
Publication bias
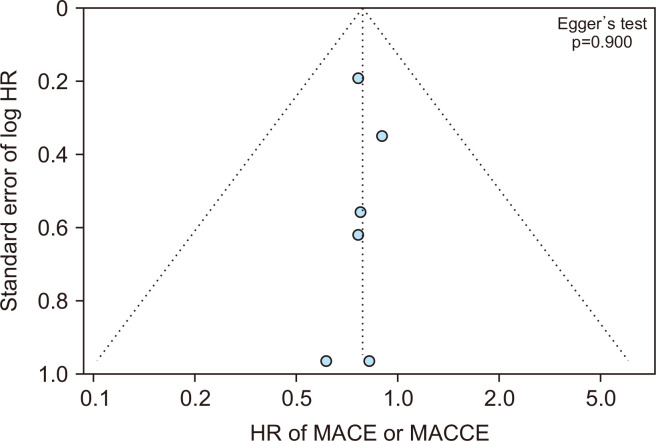
A funnel plot and the Egger test for asymmetry suggested no publication bias for the primary outcomes (Fig. 4).
Fig. 4.
A funnel plot and the Egger test for asymmetry suggested no publication bias for major adverse cardiac events (MACE) or major adverse cardiac and cerebrovascular events (MACCE). HR, hazard ratio.
Discussion
The present meta-analysis demonstrated that FFR-guided CABG might be associated with a 38% reduction in the occurrence of the composite of death or MI compared with angiography-guided CABG despite fewer revascularizations being performed in the FFR-guided group.
The FFR is the ratio of maximal blood flow in a stenotic portion of the coronary artery to that in a proximal part of the artery with a normal flow pattern, and 0.75–0.80 is recommended as the cut-off value to distinguish the functional significance of CAS [4,19]. The FFR-guided approach has been suggested in the decision-making process for PCI, as a discordance exists between the anatomic severity and hemodynamic significance of CAS [1,2]. Previous studies have demonstrated better clinical outcomes after PCI following the FFR-guided approach than with angiography-guided PCI [3,4,6]. Based on this evidence, FFR is now the gold standard for assessing the physiological lesion severity of CAS [20].
Contrary to the role of the FFR in PCI, scarce evidence shows the benefits of the FFR-guided approach during CABG compared with classical angiography-guided CABG [13,17,21]. The theoretical advantages of FFR-guided CABG include (1) the need for fewer anastomoses and the ease of the CABG grafting strategy, and (2) a high possibility of off-pump CABG by deferring a functionally insignificant moderate lesion that is difficult to expose without the aid of cardiopulmonary bypass.
Previous studies have shown that FFR-guided CABG resulted in a higher graft patency rate with significant reductions in overall death, angina, or MI [13,22]. However, other studies have demonstrated that FFR use did not improve clinical outcomes after CABG [17,21]. In addition, the risk for future adverse events when the CAS in a deferred lesion progresses after surgery was suggested as a concern regarding the FFR-guided revascularization strategy [15].
Due to great interest in this field, several meta-analyses have compared the results of FFR-guided CABG with those of the angiography-guided approach, despite the limited data regarding the role of FFR in the surgical setting [23-27]. However, previous meta-analyses failed to search all relevant references to identify recent studies [16,17]. The present meta-analysis conducted an extensive search of all relevant studies, including very recent studies. Although this study did not demonstrate a significant benefit of FFR-guided CABG in terms of MACE or MACCE, the pooled analysis demonstrated that FFR-guided CABG resulted in a 38% reduction in the occurrence of a composite of death or MI after surgery.
Previous studies have suggested that an occluded graft that has been anastomosed to vessels with noncritical CAS during angiography-guided CABG may be clinically silent because of sufficient coronary blood flow from the native coronary artery [28,29]. However, bypass grafts linked to functionally nonsignificant coronary vessels have a greater chance of flow competition and low wall shear stress, which accelerate atherosclerotic plaque formation in the grafted vessels [29,30]. This could explain the increased risk of the composite endpoint of death or MI after angiography-guided CABG, although the individual outcomes did not reach statistical significance, possibly due to the relatively small numbers of events and enrolled patients in these analyses.
Study limitations
The present study has several limitations that should be noted. First, the number of included studies was small and not all studies were RCTs. Second, although a funnel plot and the Egger test showed statistical insignificance, publication bias could not be ruled out. Third, the follow-up durations in the included studies may not have been long enough. Fourth, 1 study included patients who underwent PCI as well as those with CABG, and another study included patients who underwent concomitant aortic valve replacement. The heterogeneity of these 2 studies could affect the results of the analyses. Fifth, 1 study included a large number of patients, and this might have affected the study results [13]. Therefore, the results of the present study should be interpreted with caution.
Conclusion
The FFR-guided grafting strategy during CABG might be associated with a reduced risk of the composite of death or MI compared with angiography-guided CABG, although the data are limited.
Acknowledgments
The authors thank Prof. Chang Wook Nam of Keimyung University Dongsan Hospital for advice in writing the Comments section.
Article information
Author contributions
Conceptualization: HYH, MJJ. Data curation: HYH, MJJ. Formal analysis: MJJ. Methodology: HYH, MJJ. Project administration: HYH, SHS, MJJ. Visualization: HYH, MJJ. Writing–original draft: YK, HH. Writing–review & editing: SHS, HYH, MJJ. Final approval of the manuscript: YK, HH, SHS, HYH, MJJ.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Toth GG, Toth B, Johnson NP, et al. Revascularization decisions in patients with stable angina and intermediate lesions: results of the international survey on interventional strategy. Circ Cardiovasc Interv. 2014;7:751–9. doi: 10.1161/CIRCINTERVENTIONS.114.001608. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001608. [DOI] [PubMed] [Google Scholar]
- 2.Toth G, Hamilos M, Pyxaras S, et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014;35:2831–8. doi: 10.1093/eurheartj/ehu094. https://doi.org/10.1093/eurheartj/ehu094. [DOI] [PubMed] [Google Scholar]
- 3.Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–11. doi: 10.1016/j.jacc.2007.01.087. https://doi.org/10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 4.Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24. doi: 10.1056/NEJMoa0807611. https://doi.org/10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 5.Ciccarelli G, Barbato E, Toth GG, et al. Angiography versus hemodynamics to predict the natural history of coronary stenoses: fractional flow reserve versus angiography in multivessel evaluation 2 substudy. Circulation. 2018;137:1475–85. doi: 10.1161/CIRCULATIONAHA.117.028782. https://doi.org/10.1161/CIRCULATIONAHA.117.028782. [DOI] [PubMed] [Google Scholar]
- 6.van Nunen LX, Zimmermann FM, Tonino PA, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet. 2015;386:1853–60. doi: 10.1016/S0140-6736(15)00057-4. https://doi.org/10.1016/S0140-6736(15)00057-4. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group, author. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. https://doi.org/10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Sterne JA, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. https://doi.org/10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 9.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. https://doi.org/10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. https://doi.org/10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly C, Anwer S, Welton NJ, Dias S, Ades AE. Meta-analysis of event outcomes: guideline methodology document 3. NICE Guidelines Technical Support Unit; Bristol: 2021. [Google Scholar]
- 12.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x. https://doi.org/10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 13.Fournier S, Toth GG, De Bruyne B, et al. Six-year follow-up of fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circ Cardiovasc Interv. 2018;11:e006368. doi: 10.1161/CIRCINTERVENTIONS.117.006368. https://doi.org/10.1161/CIRCINTERVENTIONS.117.006368. [DOI] [PubMed] [Google Scholar]
- 14.Moscona JC, Stencel JD, Milligan G, et al. Physiologic assessment of moderate coronary lesions: a step towards complete revascularization in coronary artery bypass grafting. Ann Transl Med. 2018;6:300. doi: 10.21037/atm.2018.06.31. https://doi.org/10.21037/atm.2018.06.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thuesen AL, Riber LP, Veien KT, et al. Fractional flow reserve versus angiographically-guided coronary artery bypass grafting. J Am Coll Cardiol. 2018;72:2732–43. doi: 10.1016/j.jacc.2018.09.043. https://doi.org/10.1016/j.jacc.2018.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Toth GG, De Bruyne B, Kala P, et al. Graft patency after FFR-guided versus angiography-guided coronary artery bypass grafting: the GRAFFITI trial. EuroIntervention. 2019;15:e999–1005. doi: 10.4244/EIJ-D-19-00463. https://doi.org/10.4244/EIJ-D-19-00463. [DOI] [PubMed] [Google Scholar]
- 17.Rioufol G, Derimay F, Roubille F, et al. Fractional flow reserve to guide treatment of patients with multivessel coronary artery disease. J Am Coll Cardiol. 2021;78:1875–85. doi: 10.1016/j.jacc.2021.08.061. https://doi.org/10.1016/j.jacc.2021.08.061. [DOI] [PubMed] [Google Scholar]
- 18.Di Gioia G, Pellicano M, Toth GG, et al. Fractional flow reserve-guided revascularization in patients with aortic stenosis. Am J Cardiol. 2016;117:1511–5. doi: 10.1016/j.amjcard.2016.02.023. https://doi.org/10.1016/j.amjcard.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson TB, Jr, Chen C, Buch AN. Fractional flow reserve-guided coronary bypass surgery: should surgeons use it? Curr Opin Cardiol. 2013;28:654–60. doi: 10.1097/HCO.0b013e32836581a3. https://doi.org/10.1097/HCO.0b013e32836581a3. [DOI] [PubMed] [Google Scholar]
- 20.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. https://doi.org/10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 21.Bruno F, D'Ascenzo F, Marengo G, et al. Fractional flow reserve guided versus angiographic guided surgical revascularization: a meta-analysis. Catheter Cardiovasc Interv. 2021;98:E18–23. doi: 10.1002/ccd.29427. https://doi.org/10.1002/ccd.29427. [DOI] [PubMed] [Google Scholar]
- 22.Toth G, De Bruyne B, Casselman F, et al. Fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circulation. 2013;128:1405–11. doi: 10.1161/CIRCULATIONAHA.113.002740. https://doi.org/10.1161/CIRCULATIONAHA.113.002740. [DOI] [PubMed] [Google Scholar]
- 23.Casselman F, Van der Merwe J, Ferrara A, Barbato E. The present day potential role of fractional flow reserve-guided coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2016;151:926–32. doi: 10.1016/j.jtcvs.2015.12.021. https://doi.org/10.1016/j.jtcvs.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Timbadia D, Ler A, Sazzad F, Alexiou C, Kofidis T. FFR-guided versus coronary angiogram-guided CABG: a review and meta-analysis of prospective randomized controlled trials. J Card Surg. 2020;35:2785–93. doi: 10.1111/jocs.14880. https://doi.org/10.1111/jocs.14880. [DOI] [PubMed] [Google Scholar]
- 25.Jayakumar S, Bilkhu R, Ayis S, Nowell J, Bogle R, Jahangiri M. The role of fractional flow reserve in coronary artery bypass graft surgery: a meta-analysis. Interact Cardiovasc Thorac Surg. 2020;30:671–8. doi: 10.1093/icvts/ivaa006. https://doi.org/10.1093/icvts/ivaa006. [DOI] [PubMed] [Google Scholar]
- 26.Changal K, Patel M, Salman F, Nazir S, Gupta R. Meta-analysis comparing angiography-guided versus FFR-guided coronary artery bypass grafting. Am J Cardiol. 2020;135:184–5. doi: 10.1016/j.amjcard.2020.09.002. https://doi.org/10.1016/j.amjcard.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Toth GG, Collet C, Thuesen AL, et al. Influence of fractional flow reserve on grafts patency: systematic review and patient-level meta-analysis. Catheter Cardiovasc Interv. 2022;99:730–5. doi: 10.1002/ccd.29864. https://doi.org/10.1002/ccd.29864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyd JH. FFR 4 CABG: more than a vanity plate. J Thorac Cardiovasc Surg. 2016;151:933–4. doi: 10.1016/j.jtcvs.2015.12.031. https://doi.org/10.1016/j.jtcvs.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Pellicano M, De Bruyne B, Toth GG, Casselman F, Wijns W, Barbato E. Fractional flow reserve to guide and to assess coronary artery bypass grafting. Eur Heart J. 2017;38:1959–68. doi: 10.1093/eurheartj/ehw505. https://doi.org/10.1093/eurheartj/ehw505. [DOI] [PubMed] [Google Scholar]
- 30.Brown AJ, Teng Z, Evans PC, Gillard JH, Samady H, Bennett MR. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol. 2016;13:210–20. doi: 10.1038/nrcardio.2015.203. https://doi.org/10.1038/nrcardio.2015.203. [DOI] [PubMed] [Google Scholar]