Abstract
Introduction
The increasing number of people with diabetes and the unclear long-term safety and effectiveness of newer and older blood-glucose-lowering treatments emphasize the need for more pharmaco-epidemiological studies in this field. A prospective, regularly updated cohort of people with diabetes would provide quick and up-to-date information regarding prevalence, treatment, safety and effectiveness. The current aim was to describe the design of the DIAbetes MANagement and Treatment (DIAMANT) cohort.
Methods
The DIAMANT cohort is a population-based, dynamic, prospective cohort of persons with diabetes. It contains real-world data (RWD) from general practitioners (GP), including diagnoses, symptoms, examinations, communication to/from specialists and medication. Diabetes is defined as a recorded diabetes diagnosis or a prescription of drugs used in diabetes. The cohort is part of the national infrastructure of “Stichting Informatievoorziening voor Zorg en Onderzoek” (STIZON) and is linked to other data sources.
Results
Currently, the cohort enables access to information of 89,883 patients in 2004 to 344,914 in 2020 (6% T1D, 84% T2D and 10% unclassified type of diabetes), with 193,931 participants still registered as being present in the GP practice (active) in 2020. The frequency of follow-up of persons with diabetes is practice dependent. The Dutch guidelines advise 2–4 contacts per year with a more extensive yearly check-up. The DIAMANT cohort is updated several times a year. Anonymised data from the DIAMANT cohort are available to researchers.
Discussion
The DIAMANT cohort provides the opportunity to gain RWD insights into the treatment and outcomes among people with diabetes in daily general practice. The data can be enriched by established linkages to other data sources (eg, hospital data, the Perinatal Registry, the Cancer Registry). The DIAMANT cohort serves as a start of a national infrastructure to study, manage and provide personalised care in order to ultimately improve care and outcomes for people with diabetes.
Keywords: diabetes, type 2 diabetes, epidemiology, follow-up, prospective cohort, real-world data
Introduction
Despite better awareness, new developments in diabetes treatment and preventive programs, the number of people with diabetes is still rising from 463 million in 2019 to an expected increase to 578 million worldwide by 2030.1,2 In the Netherlands, more than 1.1 million persons were living with diabetes in 2017 and this prevalence is estimated to rise to 1.3 million persons in 2030.3
Type 1 diabetes (T1D) accounts for 5–10% of all diabetes cases, and type 2 diabetes (T2D) for 90–95%. T2D emerges alongside cultural and social changes in most countries. Increased overweight and obesity, sedentary lifestyle and unhealthy eating plans4 are associated with high morbidity and mortality.5–7 With a prevalence of approximately 6%, T2D is one of the most prevalent chronic diseases in the Netherlands.
The increasing number of people with diabetes and the unclear long-term safety and effectiveness of newer and older blood-glucose-lowering treatments emphasize the need for more pharmaco-epidemiological studies in this field. This resulted in the initiation of the DIAbetes MANagement and Treatment (DIAMANT) cohort in 2019,8 containing real-world data (RWD) from electronic general practitioner (GP) information systems. The cohort is embedded in a defined population of Dutch GP practices, thereby enabling comparing patients with diabetes with regional matched controls without diabetes.
The DIAMANT cohort aims to gain real-life insights into the treatment and outcomes among people with diabetes in daily general practice in the Netherlands, with the ambition to prevent diabetes-related complications. In addition, the cohort is part of the national infrastructure of “Stichting Informatievoorziening voor Zorg en Onderzoek” (STIZON), which enables linkages to other data sources to study, manage and support personalised care to improve care and outcomes for people with diabetes ultimately.
Materials and Methods
The DIAMANT cohort represents one of the first cohorts enabling access to information about patients with diabetes and controls in over 1000 out of approximately 4500 GP offices in the Netherlands. In the Dutch healthcare system, it is mandatory for each person to be registered with a GP. GPs act as gatekeepers to secondary and tertiary care. Therefore, the cohort includes nearly all persons with diabetes in the general population of GP practices participating in STIZON. Here, diabetes is defined as a recorded diagnosis of diabetes (International Classification of Primary Care [ICPC] code T90) or a prescription of drugs used in diabetes (Anatomical Therapeutic Chemical [ATC] code A10). The age and sex distribution of the population covered by the GP practices that participate in the national infrastructure STIZON are representative of the Dutch population (Table S1).
STIZON collects and maintains identifiable patient data retrieved from various healthcare providers.9,10 Because the DIAMANT cohort is embedded in the GP practices of STIZON, there is no fixed time window for in- and exclusion. Effectively, the cohort included patients from 2004 onwards. People are followed from the start of their registration in the electronic file of the participating GP practice (cohort entry date) until the end of registration, death or end of the study period, whichever occurred first.
Study Population
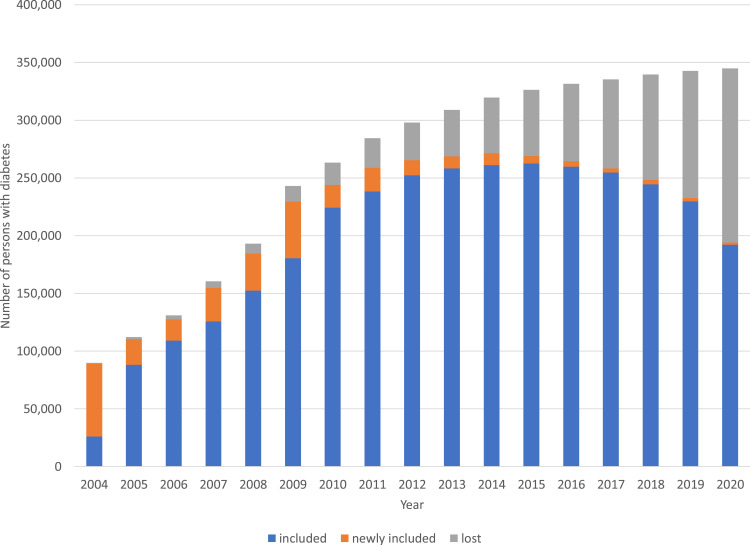
The number of persons included in the cohort increased with the number of participating GPs from 89,883 in 2004 to 344,914 in 2020 (Figure 1). Cohort and patient characteristics of the persons in the DIAMANT cohort, stratified by type of diabetes, are shown in Table 1. The last recorded ICPC code for T1D or T2D defined the type of diabetes (T90.01 for T1D and T90.02 for T2D). In addition, people with ≥2 consecutive prescriptions of non-insulin drugs used in diabetes (ATC code A10B) within six months were also defined as T2D. In the cohort, 289,284 (84%) persons had T2D, 20,035 (6%) had T1D, and the type of diabetes of the remaining 35,595 (10%) persons was not recorded. Overall, the participants were aged 57.5 (standard deviation [SD]: 16.7) years, and 50% were female. The median (interquartile range [IQR]) follow-up duration was 9.5 (4.7–14.7) years.
Figure 1.
Number of persons with diabetes registered on January 1st of each year (2004–2020). The number of persons already included, persons newly included, and persons who were lost to follow-up are presented for each year.
Table 1.
Cohort and Patient Characteristics of the Participants in the DIAMANT Cohort
| All | T1D | T2D | Unclassified Type of Diabetes | |
|---|---|---|---|---|
| N = 344,914 | N = 20,035 | N = 289,284 | N = 35,595 | |
| n (%) | n (%) | n (%) | n (%) | |
| Sex, females | 172,571 (50) | 9298 (46) | 138,046 (48) | 25,227 (71) |
| Age at index date (years)* | ||||
| 0–4 | 2288 (1) | 829 (4) | 671 (<0.5) | 788 (2) |
| 5–17 | 6270 (2) | 4377 (22) | 1038 (<0.5) | 855 (2) |
| 18–44 | 58,235 (17) | 8121 (41) | 32,381 (11) | 17,733 (50) |
| 45–64 | 153,385 (44) | 4304 (21) | 141,236 (49) | 7845 (22) |
| 65–74 | 75,442 (22) | 1364 (7) | 69,863 (24) | 4215 (12) |
| 75–84 | 39,700 (12) | 797 (4) | 35,945 (12) | 2958 (8) |
| ≥85 | 9594 (3) | 243 (1) | 8150 (3) | 1201 (3) |
| Mean (SD) | 57.5 (16.7) | 35.5 (21.8) | 60.4 (13.8) | 46.3 (20.7) |
| Year of index date* | ||||
| <2004 | 77,995 (23) | 8244 (41) | 65,795 (23) | 3956 (11) |
| 2004–2006 | 50,212 (15) | 2348 (12) | 44,928 (16) | 2936 (8) |
| 2007–2010 | 73,073 (21) | 3683 (18) | 63,185 (22) | 6205 (17) |
| 2011–2015 | 82,097 (24) | 3324 (17) | 68,834 (24) | 9939 (28) |
| 2016–2020 | 61,537 (18) | 2436 (12) | 46,542 (16) | 12,559 (35) |
| SES | ||||
| Low | 131,666 (38) | 6789 (34) | 112,367 (39) | 12,510 (35) |
| Middle | 109,632 (32) | 6683 (33) | 91,193 (32) | 11,756 (33) |
| High | 102,623 (30) | 6498 (32) | 84,914 (29) | 11,211 (31) |
| Unknown | 993 (<0.5) | 65 (<0.5) | 810 (<0.5) | 118 (<0.5) |
| History duration (years), median (IQR)† | 0.9 (0.0–5.9) | 0.0 (0.0–3.1) | 0.8 (0.0–5.7) | 3.5 (0.0–8.4) |
| Follow-up duration (years), median (IQR)‡ | 9.5 (4.7–14.7) | 12.6 (6.4–20.2) | 9.8 (5.2–14.9) | 5.2 (2.0–10.2) |
Notes: *Among persons with T2D, index date was defined as the date of the first recorded diagnosis for T2D or the second prescription, whichever occurred first. Among persons with T1D, index date was defined as the date of the first recorded diagnosis for T1D. Among persons with an unknown type of diabetes, index date was defined as the date of the first recorded for diabetes diagnosis or the first prescription, whichever occurred first. †Time between the cohort entry and index date; ‡Time between index date and the end of registration, death or end of the study period.
Abbreviations: T1D, type 1 diabetes; T2D, type 2 diabetes; SES, socioeconomic status; IQR, interquartile range.
T2D
In the Netherlands, GPs are primarily responsible for the care of T2D. Most people with T2D receive integrated diabetes care within a primary care setting organised by GP care groups. Health insurers fund the integrated diabetes care programmes based on a bundled payment per patient per year.11 Complex T2D patients, ie, if glycaemic control is insufficient despite intensive insulin treatment with optimal titration, are referred to secondary care. Table 2 presents the baseline characteristics of all T2D patients in the DIAMANT cohort. Overall, the mean age was 60.4 (SD: 13.8) years, and 48% were female. The mean body mass index (BMI) was 30.5 (SD: 5.5) kg/m2, and the mean HbA1c was 55.9 (SD: 19.3) mmol/mol.
Table 2.
Baseline Characteristics of the T2D Participants in the DIAMANT Cohort
| Overall | |
|---|---|
| N = 289,284 | |
| n (%) | |
| Sex, females | 138,046 (48) |
| Age at index date (years)*, mean (SD) | 60.4 (13.8) |
| Year of index date* | |
| <2004 | 65,795 (23) |
| 2004–2006 | 44,928 (16) |
| 2007–2010 | 63,185 (22) |
| 2011–2015 | 68,834 (24) |
| 2016–2020 | 46,542 (16) |
| SES | |
| Low | 112,367 (39) |
| Middle | 91,193 (32) |
| High | 84,914 (29) |
| Unknown | 810 (0.5) |
| Physical examination, mean ± SD† | |
| BMI (kg/m2) | 30.5 ± 5.5 |
| Systolic blood pressure (mmHg) | 140.9 ± 19.9 |
| Diastolic blood pressure (mmHg) | 82.3 ± 11.0 |
| Laboratory, mean ± SD† | |
| HbA1c (mmol/mol) | 55.9 ± 19.3 |
| HbA1c >53 mmol/mol‡ | 62,792 (37) |
| Total cholesterol | 5.1 ± 1.3 |
| HDL-cholesterol | 1.2 ± 0.4 |
| LDL-cholesterol | 3.0 ± 1.0 |
| Triglycerides | 2.1 ± 1.7 |
| Creatinine | 80.6 ± 24.6 |
| Renal function | |
| MDRD (eGFR) | 79.4 ± 21.8 |
| Cockcroft-Gault | 102.3 ± 40.7 |
| Creatinine clearance | 84.9 ± 35.8 |
| Foot examination, measured† | |
| Inspection | 54,709 (19) |
| Circulation | 51,582 (18) |
| Monofilament examination | 51,992 (18) |
| Risk foot ulcers (Sims classification) | 55,114 (19) |
| Eye examination, measured† | |
| Fundoscopy | 52,457 (18) |
| Diabetic retinopathy | 45,507 (16) |
| Lifestyle and risk factors† | |
| Smoking status | |
| Current | 27,684 (10) |
| Never | 33,954 (12) |
| Former | 50,559 (17) |
| Alcohol consumption** | 73,522 (25) |
| Nutritional pattern*** | 45,057 (16) |
| Physical activity | |
| Complies with norm | 28,133 (10) |
| Less than norm | 21,476 (7) |
| Inactive | 2387 (1) |
| Physical activity advice given | 50,351 (17) |
| Relevant conditions† | |
| Diabetic retinopathy | 2044 (1) |
| Angina pectoris | 2390 (1) |
| Acute myocardial infarction | 2269 (1) |
| Other / chronic ischeamic heart disease | 1404 (<0.5) |
| Hypertension uncomplicated | 17,050 (6) |
| Hypertension complicated | 2955 (1) |
| Transient ischaemic attack | 1166 (<0.5) |
| Stroke/cerebrovascular accident | 100 (<0.5) |
| Cerebral infarction | 402 (<0.5) |
| Intermittent claudication | 1269 (<0.5) |
| Aortic aneurysm | 558 (<0.5) |
| Diabetic neuropathy | 1572 (1) |
| Down / depressed | 1157 (<0.5) |
| Depression | 2615 (1) |
| Renal impairment / renal insufficiency | 3841 (1) |
| Albuminuria / proteinuria | 1896 (1) |
| Medication† | |
| Drugs used in diabetes | 148,621 (51) |
| Antihypertensives | 2726 (1) |
| Diuretics | 58,127 (20) |
| Beta blocking agents | 73,629 (25) |
| Calcium channel blockers | 37,672 (13) |
| Agents acting on the renin–angiotensin system | 95,593 (33) |
| Lipid modifying agents | 116,773 (40) |
| Influenza vaccines | 33,796 (12) |
Notes: *Index date was defined as the date of the first recorded diagnosis for T2D or the second prescription, whichever occurred first; †Determined in the year after index date; **Recorded via alcohol consumption or Five-Shot questionnaire; ***Recorded via four nutrition-related questions. ‡Among patients with a known HbA1c value.
Abbreviations: T2D, type 2 diabetes; SD, standard deviation; SES, socioeconomic status; BMI, body mass index; HbA1c, glycosylated haemoglobin; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
T1D
The medical supervision of people with T1D in the Netherlands is usually performed in secondary care. However, since the GP is the gatekeeper of the Dutch healthcare system, some summarised information of these people is stored in the GP registry, but usually not entirely in the standardised format of a structured diagnostic coding system.
Unclassified Type of Diabetes
People with only one prescription of drugs used in diabetes or with ≥2 prescriptions in more than six months and without a recorded diagnosis of diabetes during the entire observation period were defined as people with an unclassified type of diabetes. Also, people with a recorded diagnosis of diabetes without the specification of type (ICPC code T90) were defined as people with an unclassified type of diabetes. Although the type of diabetes is essential to know, these people were kept in the cohort for several reasons. Firstly, it is possible that the GP retrospectively registers the type of diabetes. Once the DIAMANT cohort is updated, this new information becomes available, and the person may then retrospectively be classified as T1D or T2D. Secondly, when diabetes-free controls are needed for a study, people with an unknown/missing type of diabetes can easily be excluded from the pool of potential controls. Thirdly, other types of diabetes, such as latent autoimmune diabetes in adults (LADA) and maturity-onset diabetes of the young (MODY), are currently included under the unclassified type of diabetes, but future studies might focus on these types as well.
Available Data
The DIAMANT cohort contains the structured information of the electronic GP information systems, ie, examinations, medication, diagnoses (including symptoms), referrals to specialists and journals. A selection of this information is cleaned, standardised, harmonised, and readily available for studies. This selection is defined by the e-Diabetes Mellitus core set (Table S2),12 part of the integrated diabetes care programme. It includes patient information, examinations, diagnoses and medication. However, structured information outside the e-Diabetes Mellitus core set, for instance, information about other co-morbidities, treatment, referrals and journal text, is also available in the electronic GP information system and, therefore, in the DIAMANT cohort.
Examinations
Examinations are based on “Nederlands Huisartsen Genootschap” (NHG) codes (issued by the Dutch College of GPs) and include physical and laboratory examinations, questionnaire information and lifestyle and risk factors.13 The diabetes core set includes the following variables on physical examination: BMI, systolic (SBP) and diastolic blood pressure (DBP), foot inspection, circulation in left and right foot, monofilament examination, risk assessment for foot ulcers (Sims classification14), fundoscopy and diabetic retinopathy. Variables on laboratory results are HbA1c, total cholesterol, high density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, triglycerides, creatinine level, urinary albumin or urine albumin-to-creatinine ratio and renal function expressed as modification of diet in renal disease (MDRD), Cockcroft-Gault or creatinine clearance. Lifestyle and risk factors include smoking status, smoking cessation, alcohol consumption, nutritional pattern, healthy nutrition advice, physical activity and therapy adherence (prevention cardiovascular disease [CVD]) (ie, caregiver’s impression of how the patient complies with the medication agreements to prevent CVD). The exact codes for identifying these examinations are supplied in Table S2.
Medication
All GP prescribed medication records include information on product type, prescription date, strength, dosage regimen, quantity and route of administration. Drug prescriptions are coded according to the G-Standaard, which is provided by the Medicines Information Centre of the Royal Dutch Pharmacists Association (KNMP).15 The G-Standaard includes brand and generic names, dosing regimen information, timing and estimated duration of use, and references to the World Health Organisation (WHO) ATC Classification System.16 The diabetes core set describes glucose-lowering drugs, antihypertensives, diuretics, beta-blocking agents, calcium channel blockers, agents acting on the renin–angiotensin system (RAAS), lipid-modifying agents, and influenza vaccines. The exact codes for identifying these medications are available in Table S2.
Diagnoses
Recorded diagnoses and symptoms are coded according to the ICPC,17 which can be mapped to the International Classification of Diseases (ICD)-10 codes, and entered as free text. The diagnoses described by the diabetes core set include mainly micro- and macrovascular complications: diabetic retinopathy, angina pectoris, acute myocardial infarction, different/chronic ischemic heart disease, hypertension with/without organ damage, transient ischemic attack, cerebral vascular accident, cerebral infarction, intermittent claudication, aorta aneurysm, diabetic neuropathy, renal dysfunction/insufficiency and albuminuria/proteinuria. Other diagnoses in the diabetes core set are a down/depressed feeling, depression and diagnosis of T1D and T2D. The exact codes for identifying these diagnoses are described in Table S2.
Referrals to Specialists
The GP registers referrals and the associated correspondence. In addition to referring individual patients and maintaining an overview, the data regarding referrals are used for reports in the context of the indicators, quality policy (practice accreditation) and quality management.
Journal Text
In addition to these structured data, the GP recorded unstructured data are called “free text”. Dutch GPs usually register the patient contacts according to the guideline of the Dutch college of GPs.18 Consultations are summarised in free text structured by the so-called SOEP registration (S: subjective/reason of encounter, O: observation and examination results, E: evaluation, P: treatment plan). The reason for the encounter and the evaluation are also coded according to ICPC codes.17 The contacts are grouped in so-called ‘episodes’ with ICPC coding combined with explanatory free text.
The free text information is not included in the DIAMANT cohort by default due to privacy reasons. However, if the needed permission is granted, this information can be made available.
Linkage to Other Sources
Linkages with other databases have been performed to enrich the DIAMANT cohort (Table 3 for additional sources). The type of additional data depends on the database to which it is linked. The extent of the geographical and temporal overlap determines the size of the sub-selection for which the additional information is available.
Table 3.
Overview of the Implemented Linkages Between DIAMANT and Other Databases
| Additional Information | |
|---|---|
| Out-patient Pharmacy Database | Data on all GP or specialist prescribed healthcare products dispensed by the community pharmacies.10 |
| In-patient Pharmacy Database | Data on all drug dispensing from the hospital pharmacy, given during hospitalisation.10 |
| Clinical Laboratory Database | Results of tests performed on clinical specimens, requested by GPs or specialists.10 |
| Hospital Database | Data on all hospitalizations for more than 24 hours or for which a bed is required, outpatient visits and high budget impact medication. Data are obtained from the Dutch Hospital Data Foundation.10 |
| Cancer Registry | Data on all newly diagnosed cancer cases. Data are obtained from the Dutch Comprehensive Cancer Organization.10 |
| Pathology Registry | Excerpts of histological, cytological and autopsy examinations. Data are obtained from PALGA.10 |
| Perinatal Registry | Data on pregnancies, birth and neonatal outcomes. Data are obtained from Perined.10 |
| Diabetes Care System (DCS) | Detailed data on T2D, eg microvascular complications and biobank (including genome-wide SNP genotyping [GWAS]) samples from the DCS cohort.25 |
| Central Bureau of Genealogy (CBG) | Data regarding vital status. |
Follow-Up
Diabetes care at the GP is based on the T2D guideline for GPs, issued by the Dutch College of GPs (NHG guideline T2D).19 The guideline advises two to four contacts per year with several monitoring examinations that should be performed minimally once a year (see ‘Examinations’ below). Although the healthcare insurance companies promote this monitoring regimen and even give (financial) incentives to stimulate implementation, GPs are free to individualise. Secondary care follows roughly the same regimen for T1D and is advised to send a care summary to the GP at least once a year.
The DIAMANT cohort is updated several times a year with the data from the electronic GP information systems. Information can be updated both prospectively and retrospectively. This means that previously generated results from the DIAMANT cohort can be slightly different when updates have been made.
As of December 31st, 2020, 193,931 out of 344,914 participants (56%) were still registered as being present in the GP practice (active) (Figure 1 and Table S3). Participants can become inactive when they die or change to a GP practice that does not participate in STIZON. Table S4 presents the characteristics of participants still active in the cohort and those lost to follow-up. Due to its dynamic nature and an inclusion period of more than 15 years, it is evident that those lost to follow-up differ from those still active in the cohort.
Findings to Date
Data from the DIAMANT cohort and its source population have already been used in several studies. We briefly discuss some of these studies here.
Prevalence of T2D
A study into the prevalence of diabetes was performed for the period 1999–2014. The study showed an increase in the prevalence of diabetes in the Netherlands from 1.8% in 1999 to 4.9% in 2014. Changes in age demographics per sex could partly explain this increase.20
Treatment Patterns Across Europe
The population of the DIAMANT cohort was part of a study into similarities and differences of T2D treatment in daily practice across Europe. This study obtained prescriptions for drugs used in diabetes treatment from electronic databases in the Netherlands, Spain, Italy, France and the United Kingdom (UK) during a 5-year study period. Results showed that metformin monotherapy was the most common initial therapy during the study period. Differences in treatment patterns were reflected by differences between national guidelines in these countries.21
T2D and Cancer Risk
The DIAMANT cohort has been linked to the Netherlands Cancer Registry (NCR) to study relations between T2D and cancer. The relation between T2D and colorectal cancer risk has been examined. T2D patients were compared with matched patients without T2D, showing that T2D patients had a 1.3 times higher risk of developing colorectal cancer. Men with T2D showed a 1.4 times higher risk of developing distal colon cancer, and women with T2D had a 1.6 times higher risk of developing proximal colon cancer.22
Another study investigated the risk of women with T2D being diagnosed with more advanced stages of breast cancer by comparing breast cancer patients with T2D to matched breast cancer patients without diabetes. Results showed that women with T2D were at an increased risk of being diagnosed with more advanced tumour stages (odds ratio 1.28 [95% confidence interval 1.13–1.44]) and higher-grade tumours (1.22 [1.08–1.39]) as compared to women without diabetes. However, women with T2D were less often diagnosed with a progesterone receptor-negative tumour than women without diabetes (0.77 [0.67–0.89]).23
Data Access
Access to the data from the DIAMANT cohort is possible for researchers. After approval of a data request by the steering committee of DIAMANT, a collaboration agreement will be signed, and access to the data can be obtained via a virtual machine (VM). For more information, please contact: www.diabetes-diamant.nl.
Ethics Approval
The institutional review board of STIZON, Utrecht, Netherlands and the Medical Ethical Committee of the Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, Netherlands, approved the study.
Strengths and Weaknesses
The DIAMANT cohort is one of the first population-based cohorts where data are accessible from individual GP practices enabling the extraction of anonymised data into a single cohort of specified data in line with General Data Protection Regulation (GDPR) requirements. The main strengths of the DIAMANT cohort are its size, completeness and long follow-up, its dynamic prospective design, the possibility to link to other databases, and compare outcomes to persons not suffering from diabetes as controls.
Due to the Dutch healthcare system, nearly every citizen is registered with one GP practice,24 resulting in a complete, population-based patient file in the electronic GP information systems. In addition, GPs are incentivised financially to record the information regarding T2D correctly.11 Because of these two reasons, the DIAMANT cohort serves as a reliable source to study not only diabetes itself but also to calculate epidemiological measures, such as incidence and prevalence.
Included patients can be followed for an extended period. In addition, the data are updated regularly, allowing the opportunity to study real-life insights.
A limitation of the DIAMANT cohort is that the data are a true reflection of daily practice and therefore are not collected primarily for research purposes but are recorded in the routine of primary care. Although the primary care guideline advises monitoring at least once a year, missing values do exist. The standard data include information items considered necessary or obligatory (eg, weight) but may lack items not (yet) considered important in current daily practice (eg, level of education). However, the national infrastructure of which the DIAMANT cohort is part makes it possible to link additional (diabetes) data not yet captured in the cohort (Table 3). Although the financial incentive to record diabetes data has ensured that the data on patients with diabetes are very rich, this incentive may also have led to the diagnosis of diabetes being recorded too quickly. This might have resulted in some people in the cohort with pre-diabetes rather than diabetes. The advantage is that these people can always be excluded when executing a study with some additional inclusion criteria.
Overall, the infrastructure and the cohort are highly suitable to study, manage, and provide information to improve care and outcomes for people with diabetes.
Conclusion
The DIAbetes MANagement and Treatment (DIAMANT) cohort has promising potential to aid researchers to gain real-life insights into the treatment and outcomes among people with diabetes in daily general practice in the Netherlands, with the ambition to prevent diabetes-related complications. In addition, the cohort is part of the national infrastructure of STIZON, which enables linkages to other data sources to study, manage and support personalised care to improve care and outcomes for people with diabetes ultimately.
Acknowledgments
We would like to thank all the healthcare providers contributing information to the PHARMO Data Network.
Disclosure
J.O., K.S. and R.H. are employees of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for government and related health care authorities and several pharmaceutical companies. The authors report no other conflicts of interest in this work.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. Brussels: International Diabetes Federation; 2019. [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 3.Nielen M, Poos R, Korevaar J. Diabetes Mellitus in Nederland Prevalentie En Incidentie: Heden, Verleden En Toekomst. Utrecht: Nivel; 2020. [Google Scholar]
- 4.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- 5.Mooy JM, Grootenhuis PA, de Vries H, et al. Prevalence and determinants of glucose intolerance in a Dutch caucasian population. The Hoorn study. Diabetes Care. 1995;18(9):1270–1273. doi: 10.2337/diacare.18.9.1270 [DOI] [PubMed] [Google Scholar]
- 6.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105–113. doi: 10.1016/S2213-8587(14)70219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overbeek JA. Type 2 diabetes, its pharmacological treatment and associations with cancer: (Pharmaco) epidemiological studies based on data from the PHARMO DIAMANT cohort: Amsterdam UMC – locatie VUmc, Vrije Universiteit. Amsterdam Public Health Research Institute; 2019. [Google Scholar]
- 9.Stichting Informatievoorziening voor Zorg en Onderzoek (STIZON); 2022. Available from: https://stizon.nl/. Accessed April 7, 2022.
- 10.Kuiper JG, Bakker M, Penning-van Beest FJA, Herings RMC. Existing data sources for clinical epidemiology: the PHARMO database network. Clin Epidemiol. 2020;12:415–422. doi: 10.2147/CLEP.S247575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Struijs JN, de Jong-van Til JT, Lemmens LC, Drewes HW, de Bruin SR, Baan CA. Three years of bundled payment for diabetes care in the Netherlands. Bilthoven: national Institute for public health and the environment (RIVM) ministry of health, welfare and Sport; 2012.
- 12.e-Diabetes Mellitus kernset (samenvatting) [e-Diabetes Mellitus core set]. Diabetesvereniging Nederland, ndf & Nederlands Huisartsen Genootschap; 2013. Available from: https://www.zorgstandaarddiabetes.nl/wp-content/uploads/2014/01/e-Diabetes-Mellitus-kernset-samenvatting-DEF.pdf. Accessed November 21, 2022.
- 13.Bepalingenviewer: tabel Diagnostische Bepalingen versie [Examination viewer: table diagnostic examinations] 37 (14 juli 2021). Bepalingenclusters 16 (25 februari 2021); 2021. Available from: https://bepalingen.nhg.org/labcodes/determinations. Accessed April 7, 2022.
- 14.Nijenhuis-Rosien L, Kleefstra N, van Dijk PR, et al. Laser therapy for onychomycosis in patients with diabetes at risk for foot ulcers: a randomized, quadruple-blind, sham-controlled trial (LASER-1). J Eur Acad Dermatol Venereol. 2019;33(11):2143–2150. doi: 10.1111/jdv.15601 [DOI] [PubMed] [Google Scholar]
- 15.Z-Index. Available from: https://www.z-index.nl/. Accessed April 7, 2022. Dutch.
- 16.World Health Organization. Anatomical therapeutic chemical classification system; 2021. Available from: http://www.whocc.no/atc_ddd_index. Accessed April 7, 2022.
- 17.International Classification of Primary Care; 2022. Available from: https://www.nhg.org/themas/artikelen/icpc. Accessed April 7, 2022. Dutch.
- 18.Nederlands Huisartsen Genootschap (NHG). Adequate dossiervorming met het elektronisch patiëntdossier (ADEPD); 2019.
- 19.Barents ESE, Bilo HJG, Bouma M, et al. NHG-Standaard Diabetes mellitus type 2; 2021.
- 20.Overbeek JA, van der Heijden AW, Herings RM, Nijpels G. Meer dan verdubbeling van de prevalentie van diabetes - Diabetes mellitus in Nederland in de periode 1999-2014 [Prevalence of diabetes mellitus in the Netherlands more than doubled in the period 1999–2014]. Ned Tijdschr Geneeskd. 2017;160:D673. Dutch. [PubMed] [Google Scholar]
- 21.Overbeek JA, Heintjes EM, Prieto-Alhambra D, et al. Type 2 diabetes mellitus treatment patterns across Europe: a population-based multi-database study. Clin Ther. 2017;39(4):759–770. doi: 10.1016/j.clinthera.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 22.Overbeek JA, Kuiper JG, van der Heijden A, et al. Sex- and site-specific differences in colorectal cancer risk among people with type 2 diabetes. Int J Colorectal Dis. 2019;34(2):269–276. doi: 10.1007/s00384-018-3191-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overbeek JA, van Herk-Sukel MPP, Vissers PAJ, et al. Type 2 diabetes, but not insulin (Analog) treatment, is associated with more advanced stages of breast cancer: a national linkage of cancer and pharmacy registries. Diabetes Care. 2019;42(3):434–442. doi: 10.2337/dc18-2146 [DOI] [PubMed] [Google Scholar]
- 24.Faber MJ, Burgers JS, Westert GP. A sustainable primary care system: lessons from the Netherlands. J Ambul Care Manage. 2012;35(3):174–181. doi: 10.1097/JAC.0b013e31823e83a4 [DOI] [PubMed] [Google Scholar]
- 25.van der Heijden AA, Rauh SP, Dekker JM, et al. The Hoorn Diabetes Care System (DCS) cohort. A prospective cohort of persons with type 2 diabetes treated in primary care in the Netherlands. BMJ Open. 2017;7(5):e015599. [DOI] [PMC free article] [PubMed] [Google Scholar]