Abstract
To determine the effects of castration on growth performance, serum hormone levels, cecal microbiota composition, and metabolites in cattle. A total of 18 Holstein bulls and steers were divided into bull and steer groups and randomly assigned to 3 pens (3 cattle per pen, and each cattle were separated by a fence) to determine the average daily gain (ADG), daily dry matter intake (DMI), and feed efficiency (G/F). After the finishing trial, six cattle per group were randomly slaughtered. Serum was collected to measure the hormone concentration, and the cecal content was collected to measure the pH, short-chain fatty acids, and digestive enzyme activities. Metagenome sequencing and untargeted metabolomics were used to investigate the microbiota composition, functional profiles, and differential metabolites of the cecal contents. We found that castration significantly decreased ADG, DMI, and G/F in cattle (P < 0.05). The serum testosterone, thyroxine, growth hormone (P < 0.05), and triiodothyronine (P < 0.01) concentrations significantly decreased in the steer group when compared to those of the bull group. The activities of cellulase, xylanase, pectinase, and β-glucosidase (P < 0.05) significantly decreased in the steer group, whereas the activities of lipase and α-amylase significantly increased. Moreover, castration significantly decreased the relative abundance of Ruminococcaceae_bacterium, Treponema_porcinum, Oscillibacter_sp. (P < 0.05), and Alistipes_senegalensis (P < 0.01), whereas the relative abundance of Phocaeicola_plebeius (P < 0.05) was significantly increased. Also, the relative abundance of Phocaeicola_plebeius was negatively correlated with testosterone levels, and the function of the cecal microbiota was enriched in the GH29 and GH97 families in the steer group. Metabolomic analysis indicated that castration increased the levels of L-valine, L-phenylalanine, L-aspartic acid, L-isoleucine, L-lysine, methionine, L-glutamic acid, and L-leucine, while decreasing the levels of α-ketoglutaric acid through the 2-oxocarboxylic acid metabolism pathway. In addition, α-ketoglutaric acid was negatively correlated with Oscillibacter_sp. (P < 0.01). Overall, castration can inhibit cattle growth by altering the composition of the cecal microbiota. Therefore, this study provides a theoretical and practical basis for improving the growth performance of steers.
Keywords: castration, cecal, Holstein bull, microbiome, metabolome
1. Castration can inhibit growth in Holstein cattle.
2. This study provides a theoretical and practical basis for improving the growth performance of steers.
Introduction
The castration of male cattle is widely conducted in the beef cattle industry. This is because it can increase the meat marbling score and improve meat quality. Castration can also reduce aggressive behavior in cattle during the rearing phase, which is beneficial for production management (Álvarez-Rodríguez et al., 2017). However, studies have shown that castration led to changes in the concentration of hormones in the body, mainly the sex hormones, which greatly influence growth (Lee et al., 1990; Álvarez-Rodríguez et al., 2017). Whon et al. (2021) reported that castration of male cattle decreased the serum testosterone levels and growth performance.
The cecum is an important fermentation and absorption organ in the hindgut of ruminants (Myer et al., 2015) and is involved in various biological functions, such as water and electrolyte absorption, chyme retention, and microbial fermentation (Xu et al., 2020). Therefore, the hindgut has an important effect on animal production and health (Gressley et al., 2011). In particular, when anterior gastric digestion is limited, the potentially digestible fiber that is not degraded by the rumen can undergo secondary fermentation in the cecum and be absorbed (Hoover, 1978). Short-chain fatty acids (SCFAs) can be transported into the blood through the cecal epithelium, providing energy to the host (Kirat and Kato, 2010), and up to 8.6% of metabolizable energy in steers is provided by the cecum as an additional energy source for host metabolism (Siciliano-Jones and Murphy, 1989). Thus, changes in the cecal microbiota can regulate the growth performance of monogastric animals, such as pigs (Zhang et al., 2020b), chickens (Li et al., 2020b), and geese (Yin and Huang, 2016), but there have been limited reports on the effect on growth performance in cattle.
Previous studies have focused on carcass and meat quality after castration (Bong et al., 2012; Krause et al., 2020). Therefore, there are few studies on the effects of cecal microbiota on growth performance after castration in cattle. In this study, we hypothesized that castration alters the cecal microbiota composition of Holstein bulls and that these changes modulate the cecal metabolites and affect growth performance. We aimed to elucidate the mechanism by which castration inhibits the growth performance of steers using metagenome sequencing and metabolomics, and thus, provide a theoretical and practical basis for improving the growth performance of steers.
Materials and Methods
This study was conducted at Gansu Huarui Agriculture Co. Ltd., Zhangye, Gansu, China. The animal sampling was approved by the Institutional Animal Care and Use Committee of Gansu Agricultural University under permit no. GSAU-Eth-AST-2022-035.
Animals, diets, and experimental design
Nine Holstein bulls, with an initial average body weight of 341.41 ± 4.04 kg (aged 10.5 ± 0.3 months), and nine Holstein steers, with an initial average body weight of 345.13 ± 6.89 kg (aged 10.2 ± 0.1 months) and which were castrated at 2 months of age, were used in a single factor experimental design. The experimental cattle were divided into bull and steer groups and were randomly assigned to three pens (three cattle per pen, and each cattle were separated by a fence; n = 9) per group, and there were no statistically significant differences in the initial body weight between the two groups (P > 0.05). The experiment consisted of a 30-d acclimation period and 270-d experimental period. The body weight was established on an empty stomach after 12 h at 0, 30, 60, 90, 120, 150, 180, 210, 240, and 270 d to determine the average daily gain (ADG). Then, the daily feed offered and orts were weighed to calculate the daily dry matter intake (DMI). The feed efficiency (G/F) was also determined. The cattle were fed a total mixed ration consisting of corn silage and a grain mixture to meet the Nutrient Requirement of Beef Cattle, 8th Revised Edition, by the Committee on Nutrient Requirements of Beef Cattle and the National Research Council (2016). The nutritional composition of the feed was changed monthly (Supplementary Table S1), and cattle were fed twice daily at 08:00 and 16:00. All the cattle had access to food and water ad libitum during the experimental period. The finishing trial lasted 270 d, after which six cattle (two cattle per pen) per group were randomly slaughtered.
Sample collection
At the end of the experimental period, the cattle were slaughtered after fasting for 12 h. Initially, 5 mL of blood was collected from the jugular vein before slaughter, and the serum was collected and stored at −20 °C. After slaughter, the skin, head, and feet were removed, and the abdominal cavity was immediately opened. A small hole was cut in the cecal pouch using a sterile sharp surgical knife, 5 mL of cecal content was collected from each animal using a sterile spoon, transferred into a sterile tube, immediately frozen using liquid nitrogen, and stored at −80 °C until DNA extraction. Another 15 mL of cecal content was sampled and stored in a sterilized container at −20 °C for the measurement of the digestive enzyme activities.
Serum hormones measurement
The serum samples were thawed at 4 °C for 3 h and mixed thoroughly before analysis. Following the manufacturer’s instructions, ELISA kits were used to measure the levels of testosterone, triiodothyronine, thyroxine, growth hormone, and somatostatin in the serum (Beijing Sino-uk Institute of Biological Technology, Beijing, China), which inter-assay coefficient of variation (CV) was less than 9% and the intra-assay CV was less than 4.5%.
pH and short-chain fatty acids measurement
The pH value of the cecal contents was immediately measured using an Ark Technology PHS-10 portable acidity meter (Chengdu, China). Then, high-performance gas chromatography (GC-2010; Agilent, Kyoto, Japan) was used to analyze the SCFA concentrations of the cecal contents following the procedure described by Zhang et al. (2021). Briefly, the sample supernatant was injected into an AT-FFAP capillary column (50 m × 0.32 mm × 0.25 µm). The column temperature was maintained at 60 °C for 1 min, increased to 115 °C at 5 °C/min, and then increased to 180 °C at 15 °C/min. The injector and detector temperatures were set to 260 °C and 250 °C, respectively.
Digestive enzyme activities measurement
The cecal contents and homogenate medium mixture were placed in an ultrasonic pulp refiner to obtain a 10% homogenization buffer. It was then centrifuged at 8,000 × g at 4 °C for 10 min, and the supernatant was subjected to β-glucosidase, cellulase, xylanase, pectinase, lipase, and α-amylase enzyme assays to determine their concentrations. In addition, the enzyme activities were measured using the colorimetry method according to the manufacturer’s instructions for the reagent kits (Biosino Biotechnology Co. Ltd., Beijing, China; Mindray BS-240 automatic biochemical analyzer, Mindray, Shenzhen, China). The inter-assay CV was less than 9% and the intra-assay CV was less than 4.5%.
DNA extraction and metagenomic sequencing
Metagenomic DNA was extracted from the cecal contents according to the manufacturer’s instructions (Omega Bio-tek, Norcross, GA, US). The purity and concentration of the DNA were determined using a NanoDrop2000 and TBS-380, respectively, and the quality was checked on a 1% agarose gel. Then, the DNA was fragmented to an average size of approximately 480 bp using a Covaris M220 (Gene Company Limited, China) for paired-end library construction. Next, paired-end sequencing was performed on an Illumina HiSeq 3000 platform (Illumina Inc., San Diego, CA, USA) at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The clean reads were generated by trimming the paired-end Illumina reads of splice sequences and removing low-quality reads (< 50 bp in length, with a quality value < 20, or with N bases) using the fastp software (version 0.20.0) (Chen et al., 2018). The reads were mapped to the Bos taurus reference genome assembly using the BWA software (version 0.7.17) (Li and Durbin, 2009), and reads with high alignment similarity were removed. Also, contigs with lengths ≥ 300 bp were assembled using Multiple Megahit (version 1.1.2) (Li et al., 2015) to produce the final assembly results. The open reading frames (ORFs) were predicted using MetaGene (Noguchi et al., 2006), and the predicted genes (length ≥ 100 bp) were retrieved and translated into amino acid sequences using the NCBI translation table. Furthermore, a non-redundant gene catalog cluster analysis was constructed using CD-HIT (version 4.6.1) (Fu et al., 2012) with 90% sequence identity and 90% coverage. After quality control, all the high-quality paired-end reads were mapped to the non-redundant gene catalog with 95% identity using SOAPaligner (version 2.21) (Li et al., 2008), and the abundance of each gene in each metagenomic sample was evaluated. Subsequently, the non-redundant gene catalog was aligned to the NCBI non-redundant protein sequence database using BLASTP (version 2.2.28+) (Altschul et al., 1997) to obtain the taxonomic annotation and species abundance. Then, non-metric multidimensional scaling (NMDS) based on Bray–Curtis dissimilarity matrices at the species level was performed to provide an overview of the differences between the two groups. Also, linear discriminant analysis (LDA) with effect size (LEfSe) was performed to identify the important differential microbes between the two groups. The non-redundant gene set sequences were annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and BLAST (version 2.2.28+) with an e-value of 1e−5 (Xie et al., 2011). In addition, carbohydrate-active enzymes (CAZy) annotation was performed using the hmmscan tool. The metagenomic datasets are available in the NCBI database under the accession number PRJNA853881.
Metabolite profiles and metabolic pathways analysis
A total of 50 mg of cecal content sample and 500 µL pre-cooled methanol/water (70%, v/v) with 1 µg/mL 2-chlorobenzene alanine as an internal standard were vortexed for 3 min and then centrifuged at 16,260 × g at 4 °C for 10 min. The supernatant was collected and analyzed using ultra-performance liquid chromatography (UPLC) and tandem mass spectrometry.
The T3 UPLC conditions were as follows: the chromatographic column was a Waters ACQUITY UPLC HSS T3 C18 (1.8 µm, 2.1 mm × 100 mm); the mobile phase consisted of water (0.1% formic acid) and acetonitrile (0.1% formic acid); and the gradient elution was 95:5 V/V at 0 min, 10:90 V/V at 11.0 min, 10:90 V/V at 12.0 min, 95:5 V/V at 12.1 min, and 95:5 V/V at 14.0 min. The amide UPLC conditions included: the chromatographic column was a Waters ACQUITY UPLC BEH amide (1.7 µm, 2.1 mm × 100 mm); the mobile phase consisted of water (25 mM ammonium formate/0.4% ammonia) and acetonitrile; and the gradient elution was 10:90 V/V at 0 min, 40:60 V/V at 9.0 min, 60:40 V/V at 10.0 min, 60:40 V/V at 11.0 min, 10:90 V/V at 11.1 min, and 10:90 V/V at 15.0 min.
T3 and amide had the same mass spectrometry parameters, including the electrospray ionization source temperature at 500 °C; ion spray voltage at 5,500 V (positive) and −4,500 V (negative); ion source gas I, gas II, and curtain gas were set at 55, 60, and 25.0 psi, respectively; and collision-activated dissociation parameter was set as high. In triple quadrupole-linear ion trap mass spectrometry, each ion pair is scanned and detected according to the optimized declustering potential and collision energy. Finally, a specific set of MRM transitions was monitored for each period, based on the metabolites that were eluted within that particular period.
Statistical analysis
The GraphPad Prism version 9.0 software was used to generate a bar chart. The data on the growth performance, serum parameters, and digestive enzymes were statistically analyzed using independent sample t-tests in SPSS (version 26.0). The metagenomics statistic data were presented using the Wilcoxon rank-sum test bar chart. The metabolome statistics data were analyzed based on the retention time and ion current strength using the MultiQuant software to calculate the content of each compound (Zhang et al., 2021). In addition, Spearman’s correlation coefficient analysis was conducted for the growth performance, serum parameters, digestive enzymes, and cecal microbiota and metabolites (peak area transformed as Log10). The Spearman’s correlation coefficient was analyzed using SPSS (version 26.0), and a heatmap of the Spearman’s correlation analysis was made using Heml 1.0: Heatmap Illustrator software (Deng et al., 2014). A value of P < 0.05 was considered statistically significant and the P values in the figures are denoted using asterisks (* P < 0.05; ** P < 0.01).
Results
Growth performance and serum hormones
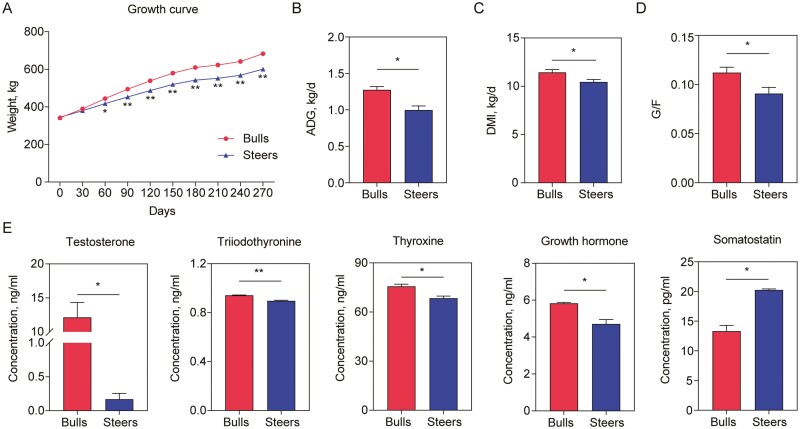
The body weights of the two groups were not significantly different until 30 d of the experimental period, after which until the end of the study, the body weights were significantly decreased in the steer group when compared to the bull group (Figure 1A). Compared to the bull group, the ADG, DMI, and G/F were significantly decreased in the steer group (P < 0.05; Figure 1B–D). The concentrations of serum testosterone, triiodothyronine, thyroxine, and growth hormone were also significantly decreased (P < 0.05), whereas the somatostatin concentration was significantly increased (P < 0.05) in the steer group when compared to the bull group before slaughter (Figure 1E).
Figure 1.
The effects of castration on the growth performance and serum parameters: (A) a reduced body weight after 60 d, (B) a decreased average daily gain, (C) a decreased dry matter intake, (D) a decreased feed efficiency (G/F), and (E) the serum hormone concentrations. n = 9 individuals/group; * P < 0.05; ** P < 0.01.
pH, short-chain fatty acids, and digestive enzyme activities of the cecal contents
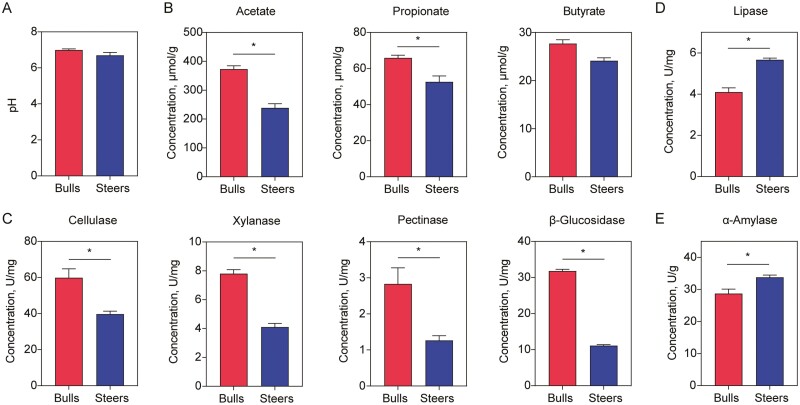
The pH value of the cecal contents was not significantly different between the two groups (Figure 2A). The concentrations of acetate and propionate were significantly lower in the steer group than those in the bull group (Figure 2B). Moreover, the cellulase, xylanase, pectinase, and β-glucosidase activities in the steer group were significantly lower than those in the bull group (Figure 2C). However, the lipase and α-amylase activities were significantly increased in the steer group (Figure 2D, E).
Figure 2.
Effects of castration on the pH, short-chain fatty acids, and digestive enzyme activities in the cecal contents. (A) Cecal contents pH value. (B) Decreased concentrations of acetate and propionate. (C) Decreased concentrations of cellulase, xylanase, pectinase, and β-glucosidase. (D) Increased concentrations of lipase and (E) α-amylase. n = 6 individuals/group; * P < 0.05; ** P < 0.01.
Composition of the cecal microbiota
In total, 787,115,284 reads were generated with 65,592,940 reads per sample. A total of 619,629,424 reads were retained, with 51,635,785 reads per sample after quality control and removing host genes. Therefore, a total of 6,750,990 contigs with an average N50 length of 1157 bp per sample were assembled after de novo assembly. A total of 9,947,081 ORFs were predicted, with an average ORF length of 520 bp.
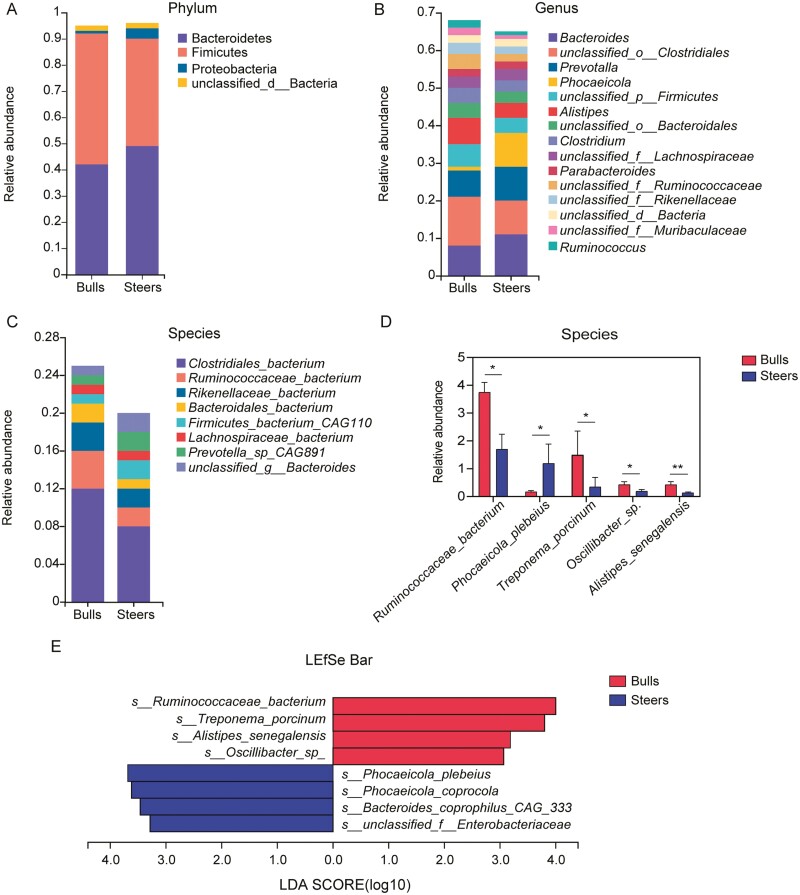
In addition, NMDS at the species level was well represented in both groups (stress = 0.026; Supplementary Figure S1). At the phylum level, the cecal microbiota was dominated by Firmicutes (bull: 49.66%, steer: 41.11%) and Bacteroidetes (bull: 41.52%, steer: 48.98%) in the two groups (Figure 3A). At the genus level, the dominant genera were Bacteroides (bull: 10.74%, steer: 7.83%), unclassified_o__Clostridiales (bull: 13.28%, steer: 9.44%), and Prevotella (bull: 6.70%, steer: 9.30%; Figure 3B). At the species level, the dominant bacteria were Clostridiales_bacterium (bull: 11.73%, steer: 8.04%), Ruminococcaceae_bacterium (bull: 3.76%, steer: 1.71), and Rikenellaceae_bacterium (bull: 2.79%, steer: 1.97; Figure 3C). Compared to the bull group, the relative abundance of Phocaeicola_plebeius was significantly increased in the steer group at the species level, and the relative abundances of Ruminococcaceae_bacterium, Treponema_porcinum, Oscillibacter_sp., and Alistipes_senegalensis were significantly decreased (Figure 3D). In addition, the LEfSe analysis was performed to determine the discrepant microbes between the two groups; Ruminococcaceae_bacterium, Treponema_porcinum, Alistipes_senegalensis, and Oscillibacter_sp. were significantly enriched in the bull group, whereas Phocaeicola_plebeius, Phocaeicola_coprocola, and Bacteroides_coprophilus_CAG:333 were significantly enriched in the steer group (LDA > 3.0, P < 0.05; Figure 3E).
Figure 3.
Castration changed the cecal microbiota composition between the bull and steer groups. The composition of the cecal microbiota of cattle is shown at the phylum (A), genus (B), and species (C) levels. Microbial taxa with an average relative abundance > 1% in each group are included. (D) Bar chart showing the differences in the taxonomic groups at the species level. The statistical method that was used was the Wilcoxon rank-sum test. Microbial taxa with an average relative abundance >0.1% in each group are included. (E) The total difference in the bacteria at the species level analyzed using the LEfSe method (LDA score > 3). n = 6 individuals/group; * P < 0.05; ** P < 0.01.
Functional profiles of the cecal microbiota
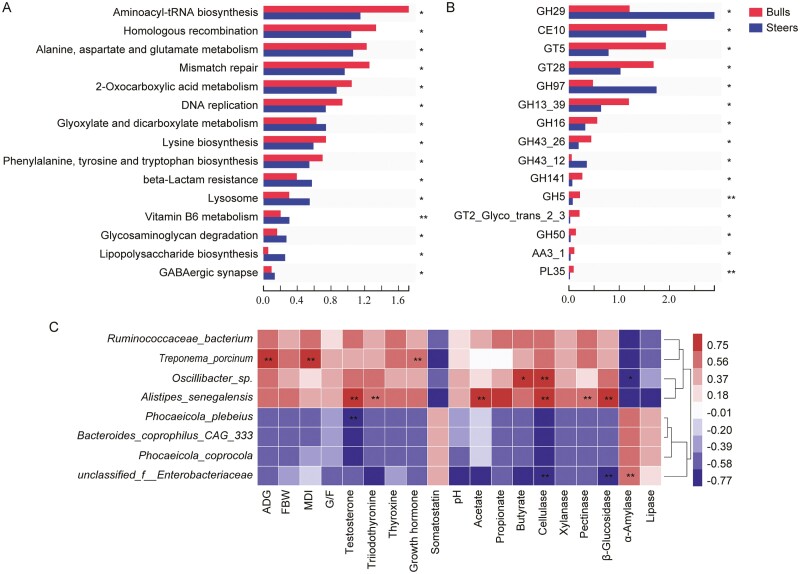
The top 15 different KEGG functional potentials were analyzed. We found that the genes involved in “glyoxylate and dicarboxylate metabolism,” “vitamin B6 metabolism,” “glycosaminoglycan degradation,” and “lipopolysaccharide biosynthesis” were highly enriched in the steer group, while those involved in “aminoacyl-tRNA biosynthesis,” “alanine, aspartate, and glutamate metabolism,” “2-oxocarboxylic acid metabolism,” “lysine biosynthesis,” and “phenylalanine, tyrosine, and tryptophan biosynthesis” were enriched in the bull group (P < 0.05; Figure 4A). Furthermore, we determined the top 15 CAZyme profiles, including nine glycoside hydrolases (GHs), three glycosyltransferases (GTs), one auxiliary activity, one carbohydrate esterase, and one polysaccharide lyase. Therefore, the genes encoding the GHs were the most dominant. The GH29 and GH97 families, which are involved in glycogen and starch degradation, were more abundant in the steer group than in the bull group (P < 0.05). Also, the GH16 and GH5 families were lower in the steer group than in the bull group (P < 0.05) and are usually involved in plant cellulose, hemicellulose, and xylan degradation (Figure 4B).
Figure 4.
Castration changed the cecal microbiota functions between the bull and steer groups. (A) The top 15 different KEGG metabolic pathways. (B) The top 15 different CAZyme profiles. (C) The Spearman correlation heatmap between the growth performance, serum parameters, digestive enzymes, and cecal differential microbiota (|r| > 0.7). n = 6 individuals/group; * P < 0.05; ** P < 0.01. ADG, average daily gain; FBW, final body weight; DMI, dry matter intake; G/F, feed efficiency.
Correlation analysis between the growth performance, serum hormones, digestive enzymes, and microbiota
The Spearman’s correlation coefficient analysis identified correlations among the growth performance, serum parameters, digestive enzymes, and significantly different microbiota (|r| > 0.7, P < 0.05; Figure 4C). The relative abundance of Treponema_porcinum was positively correlated with the ADG, DMI, and growth hormones (P < 0.01). Oscillibacter_sp. was positively correlated with butyrate (P < 0.05) and cellulase (P < 0.01) and negatively correlated with α-amylase (P < 0.05). Alistipes_senegalensis was positively correlated with the testosterone, triiodothyronine, acetate, cellulase, pectinase, and β-glucosidase levels (P < 0.01), and the testosterone levels were negatively correlated with Phocaeicola_plebeius. In addition, the relative abundance of unclassified_f__Enterobacteriaceae was negatively correlated with cellulase and β-glucosidase and positively correlated with α-amylase.
Cecal metabolome
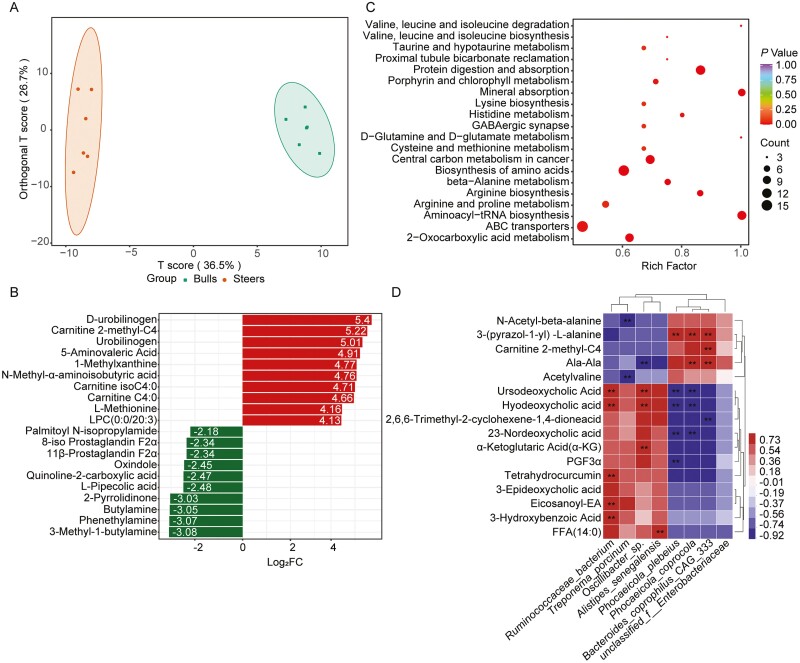
A total of 798 metabolites were identified in the cecal contents. The orthogonal projections to latent structures discriminant analysis score plots showed that the two groups had good separation regarding the cecal metabolites. Also, the random permutation test indicated satisfactory accuracy of the model (R2X = 0.706, R2Y = 0.995, Q2 = 0.856; Figure 5A). Variable importance in projection (VIP) and fold change (FC) were used to select differential metabolites, and metabolites satisfying both VIP ≥ 1 and FC ≥ 2 or ≤ 0.5 were considered differential metabolites. A total of 225 differential metabolites were selected, and 174 differential metabolites were higher in the steer group and 51 differential metabolites were higher in the bull group (Figure 5B, Supplementary Table S2). The top 20 enriched pathways were then identified by the metabolic pathway analysis based on these 225 significantly distinct cecal metabolites. “Protein digestion and absorption,” “aminoacyl-tRNA biosynthesis,” “mineral absorption,” “valine, leucine, and isoleucine degradation,” “beta-alanine metabolism,” “porphyrin and chlorophyll metabolism,” “arginine biosynthesis,” “biosynthesis of amino acids,” and “2-oxocarboxylic acid metabolism” were highly enriched in the steer group than the bull group (P < 0.05, rich factor > 0.5; Figure 5C).
Figure 5.
Castration changed the cecal content metabolome between the bull and steer groups. (A) The orthogonal projections to latent structures discriminant analysis (OPLS-DA) of the cecal content metabolites of the bull and steer groups. (B) The top 20 different metabolites. (C) The pathway analysis of the differential metabolites. (D) The correlation analysis of the differential microbiota and metabolites in the cecal contents (|r| > 0.8). n = 6 individuals/group; * P < 0.05; ** P < 0.01.
Microbiota-related metabolites in the cecum
The Spearman’s correlation coefficient analysis identified potential associations between the differential microbiota and differential metabolites in the cecum (|r| > 0.8, P < 0.05; Figure 5D). The relative abundance of Ruminococcaceae_bacterium was positively correlated with ursodeoxycholic acid, hyodeoxycholic acid, tetrahydrocurcumin, 3-hydroxybenzoic acid, and eicosanoyl-EA (P < 0.01). α-Ketoglutaric acid was negatively correlated with Oscillibacter_sp. (P < 0.01). Furthermore, Treponema_porcinum was negatively correlated with N-acetyl-beta-alanine and N-acetylvaline (P < 0.01), and 3-(pyrazol-1-yl)-L-alanine was positively correlated Phocaeicola_plebeius, Phocaeicola_coprocola, and Bacteroides_coprophilus_CAG:333. However, the ursodeoxycholic acid, hyodeoxycholic acid, and 23-nordeoxycholic acid levels were negatively correlated with Phocaeicola_plebeius and Phocaeicola_coprocola.
Discussion
Generally, it is believed that the castration of cattle results in reduced growth performance (Lee et al., 1990). Our results supported this, showing a significant decrease in the body weight, ADG, DMI, and G/F after castration. Marti et al. (2013) showed that Holstein calves castrated at 3 or 8 months of age had a lower ADG, DMI, and feed efficiency than intact bulls fed a high-concentrate diet. This finding is consistent with our results. However, Pang et al. (2006) reported that the cattle ADG did not differ with or without castration when the cattle were fed forage-based diets. This may be due to the age of castration and the type of diet.
Cattle castration can change hormone concentrations, especially testosterone levels. In this study, castration of cattle caused a 98% decrease in testosterone levels. Testosterone can increase aggression and promote muscle growth by encouraging nitrogen deposition (Galbraith et al., 1978). In addition, testosterone stimulates skeletal muscle protein synthesis and inhibits breakdown, resulting in higher body weights in bulls than in steers (Urban, 2011). Steen (1995) reported that testosterone resulted in a 19% higher ADG and 3% higher average daily feed intake in bulls than in steers. This results in a higher growth rate in bulls. In addition, an increased testosterone concentration induces a higher growth hormone concentration (Galbraith et al., 1978), and the action of the growth hormone is similar to that of the testosterone hormone, which promotes protein synthesis in the skeletal muscle (Urban, 2011). Thus, reduced testosterone and growth hormones inhibit protein synthesis in the skeletal muscle, reducing the growth performance of steers. Also, somatostatin serves as an inhibitory neurotransmitter in the brain (Hajdu et al., 2003) and was found to decrease feed intake in mice (Cummings et al., 1998). Elevated somatostatin levels may also result in a lower DMI in steers. Changes in the testosterone, growth hormone, and somatostatin levels were consistent with the results of Guo et al. (2017). Then, triiodothyronine and thyroxine are secreted by the thyroid gland, control tissue development, cellular growth, and metabolism, and promote animal growth (Bianco et al., 2002). Triiodothyronine and thyroxine levels were found to be lower in steers, which can translate to inhibited growth. This result is consistent with the findings of Du et al. (2016). Therefore, our results support the findings of these studies and suggest that these hormonal changes may result in slower skeletal muscle protein synthesis and reduced feed intake in steers, resulting in lower body weight and ADG.
Many studies have shown that DMI levels affect ruminal passage rate, and lower intakes frequently resulted in slower transit rates of digesta in steers (Montgomery et al., 2004; Klein et al., 2015; Freetly et al., 2020). In contrast, the faster the diets left the rumen as DMI increased in bulls, the more of the diet escaped ruminal fermentation and was later degraded and fermented in the large intestine (Van Kessel et al., 2002; Montgomery et al., 2004); this might alter the nutrients and organisms reaching the cecum. Acetate, propionate, and butyrate are three major SCFAs produced by gut fermentation and are the main source of energy for ruminants, providing approximately 70% of the energy requirements (Bergman, 1990). Acetate plays an important regulatory role in body weight control by affecting energy intake and expenditure (Hernandez et al., 2019). Acetate and propionate produced in the cecum and proximal colon can provide an important source of energy and glucose precursors for the growing lamb (DeGregorio et al., 1984). In the rat study, acetate production in the gut can cross the blood-brain barrier by monocarboxylate transporters and increase acetate levels in the brain leading to the activation of the parasympathetic nervous system, which further increases glucose-stimulated insulin secretion, ghrelin secretion, and collectively promoted hyperphagia and energy retention that ultimately caused weight gain (Vijay and Morris, 2014; Perry et al., 2016). Subcutaneous injections of propionate and butyric acid can stimulate insulin secretion, which in turn raises muscle energy expenditure in cattle by enhancing the transfer of amino acids and protein synthesis (Richardson et al., 2004; Hu et al., 2018). In this study, we observed decreased acetate and propionate levels in the cecal contents of the steers. Lower SCFA levels may result in reduced energy, which could lead to lower body weights at slaughter. Li et al. (2022) reported that castration resulted in decreased growth performance, and SCFAs were significantly reduced in the rumen of the cattle. We observed the same changes in the cecum of the steers in this study. Thus, reduced SCFA content in the cecal contents may be another reason for decreased growth performance in steers.
With the faster passage rate in bulls, the cecum may have received more potentially biodegradable cellulose, providing cellulolytic organisms with additional opportunities. The activity of digestive enzymes is closely related to the breakdown of nutrients. Cellobiose is created when cellulase hydrolyzes the cellulose present in the plant cell wall. Afterward, β-glucanase converts cellobiose into glucose molecules (de Souza and Kawaguti, 2021). Lactational performance, intake, and digestibility of whole-plant faba bean silage for dairy cows were improved by adding a mixture of xylanase and cellulase (Yang et al., 2022). Pectinase hydrolyzes the pectic, which is a common component of plant cell walls, to release nutrients and turn polysaccharides into soluble sugars that can be used as energy by ruminants (Azzaz et al., 2021). Furthermore, Miorin et al. (2022) showed that xylanase improved the growth performance of bulls. In contrast, in this study, the activity of these digestive enzymes was reduced in steers, which may explain their slow growth. Interestingly, the activities of lipase and α-amylase were significantly higher in steers than those in bulls, indicating an increased ability of steers to digest starch and fat. Currently, there is controversy regarding the effect of lipase and α-amylase on animal growth performance (Rojo et al., 2005; Brenes et al., 2008; Wang et al., 2020b). This effect may be caused by the animal breed, diet, management, and other factors, and requires further research for confirmation.
To better elucidate the differences in the cecal microbiota between bulls and steers, metagenome analysis was used. At the species level, Ruminococcaceae_bacterium, Treponema_porcinum, Alistipes_senegalensis, and Oscillibacter_sp. were significantly enriched in the bull group, whereas Phocaeicola_plebeius, Phocaeicola_coprocola, Bacteroides_coprophilus_CAG:333, and unclassified_f_Enterobacteriaceae were significantly enriched in the steer group as determined by the LefSe analysis. Ruminococcaceae can degrade cellulose and hemicellulose components in plant material, producing SCFAs that can be absorbed by the host (Biddle et al., 2013; Xie et al., 2022). Our results showed that castration could reduce the relative abundance of Ruminococcaceae; therefore, it may decrease SCFAs in steers. Treponema_porcinum belongs to the genus Treponema, which was associated with the concentration of acetate in the rumen fluid of adult sheep (Wang et al., 2020a). Conversely, a reduction in Treponema_porcinum may lead to a lower acetate in the cecum of the steers. It was also positively correlated with growth hormone levels. Thus, decreased growth hormone concentrations may reduce the relative abundance of Treponema_porcinum. Oscillibacter_sp. was determined to be a significant component of the rat cecal microbiota (Li et al., 2019). Also, Oscillibacter_sp. play an important role in starch degradation and complex sugar fermentation (Lin et al., 2021). The correlation analysis showed that Oscillibacter_sp. was positively correlated with butyrate and cellulase and negatively correlated with α-amylase. This suggests that a decrease in Oscillibacter_sp. may result in an increase in α-amylase and decrease in cellulase, leading to a decrease in butyrate in steers. Alistipes_senegalensis occurs in circular colonies (0.2–0.3 mm) that produce pigment. It can hydrolyze tryptophan to indole and ferment mannose (Mishra and Gimenez et al., 2012; Mishra and Lagier et al., 2012). In this study, the correlation analysis showed that Alistipes_senegalensis was positively correlated with testosterone, triiodothyronine, acetate, cellulase, pectinase, and β-glucosidase. It also indicated that decreased testosterone and triiodothyronine concentrations may reduce the relative abundance of Alistipes_senegalensis, thereby inhibiting the acetate, pectinase, and β-glucosidase concentrations in steers. Despite reports that Alistipes could be examined as a potential SCFA producer (Parker et al., 2020), these findings require further confirmation. Phocaeicola_plebeius and Phocaeicola_coprocola belong to the genus Phocaeicola. Phocaeicola_plebeius (formerly Bacteroides_plebeius) (Miyazaki et al., 2022), increased in abundance in a study on feed restriction (at 80% of the feed intake) in bearded chickens; this improved the lipid metabolism and enhanced meat quality and flavor (Ye et al., 2022). This study showed a negative correlation between testosterone levels and Phocaeicola_plebeius. Lower testosterone may improve the meat quality of steers because of an increase in the relative abundance of Phocaeicola_plebeius. Further research is necessary, though, to corroborate this. Interestingly, we found unclassified_f__Enterobacteriaceae in our study, and the relative abundance was negatively correlated with cellulase and β-glucosidase and positively correlated with α-amylase. Thus, they may regulate the concentrations of cellulase, β-glucosidase, and α-amylase. Enterobacteriaceae, belonging to the phylum Proteobacteria, is a major family of gram-negative bacteria (Zhang et al., 2020a). Li et al. (2020a) suggested that the phylum Proteobacteria can degrade nutrients in the diet and provide a suitable environment for symbiotic bacteria. Therefore, the function of unclassified_f__Enterobacteriacea requires further investigation. Results from our unpublished study showed that being fed on a daily diet supplemented with oregano essential oils and antimicrobial peptides could improve the growth performance of steers and increase the relative abundance of Ruminococcaceae_bacterium and Oscillibacter_sp. in the cecum (unpublished). This confirmed that the reduced relative abundance of Ruminococcaceae_bacterium and Oscillibacter_sp. inhibited the growth performance of steers. This also suggests that the cecal microbiota have an important impact on the growth performance of cattle.
The KEGG functional taxonomy showed that amino acid metabolism, including “alanine, aspartate, and glutamate metabolism,” “lysine biosynthesis,” “phenylalanine, tyrosine, and tryptophan biosynthesis,” and “2-oxocarboxylic acid metabolism” were significantly enriched in the bull group. A previous study reported that nitrogen metabolism contributes to differential feed efficiency in beef cattle and promotes bull growth (Li et al., 2017). An important function, “vitamin B6 metabolism” was extremely enriched in the steer group. Vitamin B6 is an essential functional substance in biosomes that plays an important role in animal health. It can be synthesized by ruminal bacteria and participate in protein metabolism in cattle (Lambert et al., 2004; Castagnino et al., 2016). Vitamin B6, a water-soluble vitamin, regulates fatty acid metabolism (Bender, 1999). A previous study showed that vitamin B6 supplementation in feed-restricted diets changed the contents of unsaturated fatty acids, including linoleic, α-linolenic, arachidonic, docosahexaenoic, and eicosapentaenoic acids in the serum and liver of rats (Bertrandt et al., 2010). Furthermore, the enrichment of genes from microbes encoding CAZymes is involved in carbohydrate deconstruction. In this study, we found that the GH29 and GH97 families were enriched in the steer group. The GH29 family of enzymes encodes α-L-fucosidase (EC 3.2.1.51) and α-1,3/1,4-L-fucosidase (EC 3.2.1.111). The GH29 family hydrolyzes fucose to produce oligosaccharides (You et al., 2019). Then, GH97 encodes glucoamylase (EC 3.2.1.3), α-glucosidase (EC 3.2.1.20), and α-galactosidase (EC 3.2.1.22). The GH97 family can hydrolyze starch and catalyze the hydrolysis of α-glucosidic linkages of maltooligosaccharides (Kitamura et al., 2008). Using Rapid ID 32A, Phocaeicola_plebeius and Phocaeicola_coprocola were positive for α-galactosidase and α-glucosidase (Kitahara et al., 2005). This suggests that the increased relative abundance of Phocaeicola_plebeius and Phocaeicola_coprocola might promote the expression of genes encoding α-galactosidase and α-glucosidase. Another important finding is that the GH5 family encodes endo-β-1,4-glucanase/cellulase (EC 3.2.1.4), β-mannosidase (EC 3.2.1.25), endo-β-1,4-xylanase (EC 3.2.1.8), and β-glucosidase (EC 3.2.1.21). The GH5 family is responsible for the degradation of cellulose, hemicellulose, and oligosaccharides (Su et al., 2022). This suggests that bulls are better at digesting cellulose and hemicellulose than steers. These results are similar to the results of this study on cecal digestive enzymes.
Castration regulates cecal metabolites and metabolic pathways. These pathways are implicated in amino acid metabolism (including “valine, leucine, and isoleucine degradation,” “arginine biosynthesis,” and “beta-alanine metabolism”), 2-oxocarboxylic acid metabolism, and metabolism of cofactors and vitamins (including “porphyrin and chlorophyll metabolism”). Notably, the microbiota has also been shown to be involved in the 2-oxocarboxylate metabolic pathway. This study showed that L-valine, L-phenylalanine, L-aspartic acid, L-isoleucine, L-lysine, methionine, L-glutamic acid, L-leucine, α-ketoglutaric acid, and N-acetylornithine were enriched in this pathway. Valine, isoleucine, and leucine are the most abundant branched-chain amino acids (BCAAs), which are essential amino acids that are synthesized by gut microbiota (Agus et al., 2021). Diet may be the only significant source of BCAAs in the gut, with a minor contribution from BCAAs synthesized by gut microbiota (Neinast et al., 2019). BCAAs that escape the small intestine or are produced in the large intestine are digested further in the large intestine by bacterial enzymes and the surviving peptidases, producing several metabolites such as SCFAs, ammonia, and amines (Gibson et al., 1989; Nyangale et al., 2012). These amino acids are mainly absorbed in the small intestine, and whether they are absorbed in the large intestine is still the subject of debate (Bröer 2008; van der Wielen et al., 2017). The Spearman correlation showed that α-ketoglutaric acid was strongly positively correlated with Oscillibacter_sp. and strongly negatively correlated with Phocaeicola_plebeius, Phocaeicola_coprocola, and Bacteroides_coprophilus_CAG:333. Previous results have shown that testosterone is negatively correlated with Phocaeicola_plebeius. This suggests that testosterone may regulate Phocaeicola_plebeius, further altering the concentration of α-ketoglutaric acid. α-Ketoglutaric acid, a crucial intermediate of amino acid metabolism, has been shown to perform various physiological functions, such as regulating energy and amino acid metabolism and promoting gut development (Liu et al., 2018). α-Ketoglutaric acid is also essential for the oxidation of fatty acids and amino acids (Kim et al., 2015). The final concentrations of acetate, propionate, and butyrate increased linearly with the α-ketoglutaric acid concentration during in vitro ruminal fermentation of wheat straw (Zhang et al., 2011). In this study, a deficiency in α-ketoglutaric acid may lead to a decrease in the concentrations of SCFAs in the cecum of the steers. Agans et al. (2018) showed that the human gut microbiota can utilize dietary fatty acids to sustain growth, and the abundance of Alistipes increased the dietary fats remaining in the medium. Our finding that an FFA (14:0), myristic acid, was strongly negatively correlated with Alistipes_senegalensis suggests that they may degrade and utilize FFAs (14:0). However, the causal relationship between the microbiota and metabolites still requires further research.
Conclusions
In conclusion, castration reduced the concentration of testosterone and growth hormones, altered cecal microbial composition and function, and decreased the relative abundance of Ruminococcaceae_bacterium and Oscillibacter_sp. These changes in the cecal microbiota might decrease the activities of cellulase, xylanase, pectinase, and β-glucosidase and decrease the levels of the metabolite α-ketoglutaric acid, thus, inhibiting growth performance in steers. Accordingly, these findings provide new insights into the relationship between the cecal microbiota and growth performance and provide a theoretical and practical basis for improving the growth performance of steers.
Supplementary Material
Acknowledgment
This study was supported by the Education Science and Technology Innovation Project of Gansu Province (GSSYLXM-02), the Gansu Beef Cattle Quality Fattening Project (GSA-XMLZ-2021-01), the Industry Support Project in Gansu Province (2020C-08), and the Agricultural Special Project of Gansu Province (GSSLCSX-2020-1)
Glossary
Abbreviations
- ADG
average daily gain
- DMI
dry matter intake; G/F, feed efficiency
- SCFA
short-chain fatty acid
- CV
coefficient of variation
- ORF
open reading frame
- NMDS
non-metric multidimensional scaling
- LDA
linear discriminant analysis
- LEfSe
linear discriminant analysis effect size
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- CAZy
carbohydrate-active enzymes
- UPLC
ultra-performance liquid chromatography
- GHs
glycoside hydrolases
- GTs
glycosyltransferases
- VIP
variable importance in projection
- FC
fold change
- BCAAs
branched-chain amino acids
Contributor Information
Zemin Li, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China.
Jinping Shi, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China.
Yu Lei, Key Laboratory of Animal Genetics, Breeding and Reproduction of Shaanxi Province, College of Animal Science and Technology, Northwest A&F University, Yangling, China.
Jianping Wu, Institute of Rural Development, Northwest Normal University, Lanzhou, China.
Rui Zhang, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China.
Xiao Zhang, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China.
Li Jia, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China.
Ying Wang, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China.
Yue Ma, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China.
Pengjia He, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China.
Yannan Ma, Institute of Rural Development, Northwest Normal University, Lanzhou, China.
Qiang Cheng, Jingchuan Xukang Food Co., Ltd, Pingliang, China.
Zhao Zhang, Gansu Huarui Agriculture Co., Ltd, Zhangye, China.
Ke Zhang, Key Laboratory of Animal Genetics, Breeding and Reproduction of Shaanxi Province, College of Animal Science and Technology, Northwest A&F University, Yangling, China.
Zhaomin Lei, College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Literature Cited
- Agans, R., Gordon A., Kramer D. L., Perez-Burillo S., and Paliy O.. . 2018. Dietary fatty acids sustain growth of human gut microbiota. Appl. Environ. Microbiol. 84:e01525–e01518. doi: 10.1128/AEM.01525-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agus, A., Clément K., and Sokol H.. . 2021. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70:1174–1182. doi: 10.1136/gutjnl-2020-323071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul, S. F., Madden T. L., Schffer A. A., Jinghui Z., Zheng Z., Webb M., and Lipman D. J.. . 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Rodríguez, J., Albertí P., Ripoll G., Blasco I., and Sanz A.. . 2017. Effect of castration at 10 months of age on growth physiology and behavior of male feral beef cattle. Anim. Sci. J. 88:991–998. doi: 10.1111/asj.12728. [DOI] [PubMed] [Google Scholar]
- Azzaz, H. H., Kholif A. E., Murad H. A., El-Bordeny N. E., Ebeid H. M., Hassaan N. A., and Anele U. Y.. . 2021. A new pectinase produced from compared with a commercial pectinase enhanced feed digestion, milk production and milk fatty acid profile of Damascus goats fed pectin-rich diet. Ann. Anim. Sci. 21:639–656. doi: 10.2478/aoas-2020-0083. [DOI] [Google Scholar]
- Bender, D. A. 1999. Non-nutritional uses of vitamin B6. Br. J. Nutr. 81:7–20. doi: 10.1017/S0007114599000082. [DOI] [PubMed] [Google Scholar]
- Bergman, E. N. 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Bertrandt, J., Klos A., and Debski B.. . 2010. Content of polyunsaturated fatty acids (PUFAs) in serum and liver of rats fed restricted diets supplemented with vitamins B2, B6 and folic acid. Biofactors 22:189–192. doi: 10.1002/biof.5520220139. [DOI] [PubMed] [Google Scholar]
- Bianco, A. C., Salvatore D., Gereben B., Berry M. J., and Larsen P. R.. . 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Biddle, A., Stewart L., Blanchard J., and Leschine S.. . 2013. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 5:627–640. doi: 10.3390/d5030627. [DOI] [Google Scholar]
- Bong, J. J., Jeong J. Y., Rajasekar P., Cho Y. M., Kwon E. G., Kim H. C., Paek B. H., and Baik M.. . 2012. Differential expression of genes associated with lipid metabolism in longissimus dorsi of Korean bulls and steers. Meat Sci. 91:284–293. doi: 10.1016/j.meatsci.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Brenes, A., Centeno C., Viveros A., and Arija I.. . 2008. Effect of enzyme addition on the nutritive value of high oleic acid sunflower seeds in chicken diets. Poult. Sci. 87:2300–2310. doi: 10.3382/ps.2008-00130. [DOI] [PubMed] [Google Scholar]
- Bröer, S. 2008. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Castagnino, D. S., K. L.Kammes.,M. S.Allen.,Gervais R., P. Y.Chouinard., and Girard C. L.. . 2016. Particle length of silages affects apparent ruminal synthesis of B vitamins in lactating dairy cows. J. Dairy. Sci. 99:6229–6236. doi: 10.3168/jds.2016-11274. [DOI] [PubMed] [Google Scholar]
- Chen, S., Zhou Y., Chen Y., and Gu J.. . 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, S. L., Truong B. G., and Gietzen D. W.. . 1998. Neuropeptide Y and somatostatin in the anterior piriform cortex alter intake of amino acid-deficient diets. Peptides 19:527–535. doi: 10.1016/s0196-9781(97)00468-3. [DOI] [PubMed] [Google Scholar]
- DeGregorio, R. M., R. E.Tucker, Jr, Mitchell G. E., and Gill W. W.. . 1984. Acetate and propionate production in the cecum and proximal colon of lambs. J. Anim. Sci 58:203–207. doi: 10.2527/jas1984.581203x. [DOI] [PubMed] [Google Scholar]
- Deng, W., Wang Y. B., Liu Z. X., Cheng H., and Xue Y.. . 2014. HemI: A Toolkit for Illustrating Heatmaps. PLoS One 9:e111988. doi: 10.1371/journal.pone.0111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, L. L., Li Q. F., Li Y., Cao Y. F., Yu C. Q., Li X. M., and Wang X. L.. . 2016. Effect of free stall barn-feeding and castration on fattening performance of Holstein Bulls. Scientia Agr. Sin. 17:3443–3452. doi: 10.3864/j.issn.0578-1752.2016.17.017. [DOI] [Google Scholar]
- Freetly, H. C., Dickey A., Lindholm-Perry A. K., Thallman R. M., Keele J. W., Foote A. P., and Wells J. E.. . 2020. Digestive tract microbiota of beef cattle that differed in feed efficiency. J. Anim. Sci. 98:skaa008. doi: 10.1093/jas/skaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, L., Niu B., Zhu Z., Wu S., and Li W.. . 2012. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, H., Dempster D. G., and Miller T. B.. . 1978. A note on the effect of castration on the growth performance and concentrations of some blood metabolites and hormones in British Friesian male cattle. Animals 26:339–342. doi: 10.1017/S0003356100040964. [DOI] [Google Scholar]
- Gibson, S. A., McFarlan C., Hay S., and MacFarlane G. T.. . 1989. Significance of microflora in proteolysis in the colon. Appl. Environ. Microb. 55:679–683. doi: 10.1128/aem.55.3.679-683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressley, T. F., Hall M. B., and Armentano L. E.. . 2011. Ruminant Nutrition Symposium: productivity, digestion, and health responses to hindgut acidosis in ruminants. J. Anim. Sci. 89:1120–1130. doi: 10.2527/jas.2010-3460. [DOI] [PubMed] [Google Scholar]
- Guo, T. J., Zhang C. J., Zhang L. Q., Wang L. Q., Sang D. J., and Yu X.. . 2017. Effects of different castration on hormone concentration in serum of Simmental cattle aged from 17 to 21 mouths. Acta Agr. Boreali-occidentalis Sin. 8:1111–1117. doi: 10.7606/j.issn.1004-1389.2017.08.001. [DOI] [Google Scholar]
- Hajdu, I., Szentirmai E., Obal F., and Krueger J. M.. . 2003. Different brain structures mediate drinking and sleep suppression elicited by the somatostatin analog, octreotide, in rats. Brain Res. 994:115–123. doi: 10.1016/j.brainres.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Hernandez, M. A. G., Canfora E. E., Jocken J. W. E., and Blaak E. E.. . 2019. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 11:1943. doi: 10.3390/nu11081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover, W. H. 1978. Digestion and absorption in the hindgut of ruminants. J. Anim. Sci. 46:1789–1799. doi: 10.2527/jas1978.4661789x. [DOI] [PubMed] [Google Scholar]
- Hu, J., Lin S., Zheng B., and Cheung P. C. K.. . 2018. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. 8:1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- Kim, S. W., Nyachoti C. M., Yin Y., Wu G., Kang Y., Xu Z., and He L.. . 2015. The physiological basis and nutritional function of alpha-ketoglutarate. Curr. Protein Pept. Sci. 16:567–581. doi: 10.2174/1389203716666150630140157. [DOI] [PubMed] [Google Scholar]
- Kirat, D., and Kato S.. . 2010. Monocarboxylate transporter 1 (MCT1) mediates transport of short-chain fatty acids in bovine caecum. Exp. Physiol. 91:835–844. doi: 10.1113/expphysiol.2006.033837. [DOI] [PubMed] [Google Scholar]
- Kitahara, M., Sakamoto M., Ike M., Sakata S., and Benno Y.. . 2005. Bacteroides plebeius sp nov and Bacteroides coprocola sp nov., isolated from human faeces. Int. J. Syst. Evol. Micr. 55:2143–2147. doi: 10.1099/ijs.0.63788-0. [DOI] [PubMed] [Google Scholar]
- Kitamura, M., Okuyama M., Tanzawa F., Mori H., Kitago Y., Watanabe N., Kimura A., Tanaka I., and Yao M.. . 2008. Structural and functional analysis of a glycoside hydrolase family 97 enzyme from Bacteroides thetaiotaomicron. J. Biol. Chem. 283:36328–36337. doi: 10.1074/jbc.M806115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, S. I., Larson Q. P., Bauer M. L., Caton J. S., and Dahlen C. R.. . 2015. Effects of alternate day feeding of dried distiller’s grains plus solubles in forage-fed steers on intake, ruminal fermentation and passage rates, and serum nonesterified fatty acid. J. Anim. Sci. 93:3959–3968. doi: 10.2527/jas.2015-9070. [DOI] [PubMed] [Google Scholar]
- Krause, T. R., Lourenco J. M., Welch C. B., Rothrock M. J., Callaway T. R., and Dean P. T.. . 2020. The relationship between the rumen microbiome and carcass merit in Angus steers. J. Anim. Sci. 98:skaa287. doi: 10.1093/jas/skaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, B. D., Titgemeyer E. C., Löest C. A., and Johnson D. E.. . 2004. Effect of glycine and vitamin supplementation on sulphur amino acid utilization by growing cattle. J. Anim. Physiol. An. N 88:288–300. doi: 10.1111/j.1439-0396.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- Lee, C. Y., Henricks D. M., Skelley G. C., and Grimes L. W.. . 1990. Growth and hormonal response of intact and castrate male cattle to trenbolone acetate and estradiol. J. Anim. Sci. 68:2682–2689. doi: 10.2527/1990.6892682x. [DOI] [PubMed] [Google Scholar]
- Li, H., and Durbin R.. . 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R., Li Y., Kristiansen K., and Wang J.. . 2008. SOAP: short oligonucleotide alignment program. Bioinformatics 24:713–714. doi: 10.1093/bioinformatics/btn025. [DOI] [PubMed] [Google Scholar]
- Li, D., Liu C., Luo R., Sadakane K., and Lam T.. . 2015. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- Li, F., Guan L. L., and McBain A. J.. . 2017. Metatranscriptomic profiling reveals linkages between the active rumen microbiome and feed efficiency in beef cattle. Appl. Environ. Microb. 83:e00061–e00017. doi: 10.1128/AEM.00061-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Shah A. M., Wang Z. S., Hu R., Li G. Y., Yao X. H., Wang Y. Y., Liang X. R., Zhang Q. Q., Zou H. W., Peng Q. H., Xue B., and Wang L. Z.. . 2022. Effects of different castration degrees on growth performance, serum indexes and rumen fermentation of Junlian cattle. Chin. J. Anim Nutr. 4:2457–2466. doi: 10.3969/j.issn.1006-267x.2022.04.040. [DOI] [Google Scholar]
- Li, X., Zeng F., Huang Y., and Liu B.. . 2019. The positive effects of Grifola frondosa heteropolysaccharide on NAFLD and regulation of the gut microbiota. Int. J. Mol. Sci. 20:p.5302. doi: 10.3390/ijms20215302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Yan C., Liu T., Xu C., Wen K., Liu L., Zhao M., Zhang J., Geng T., and Gong D.. . 2020a. Research Note: increase of bad bacteria and decrease of good bacteria in the gut of layers with vs. without hepatic steatosis. Poult. Sci. 99:5074–5078. doi: 10.1016/j.psj.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Guo B., Wu Z., Wang W., and Cai H.. . 2020b. Effects of fermented soybean meal supplementation on the growth performance and cecal microbiota community of broiler chickens. Animals 10:p1098. doi: 10.3390/ani10061098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L., Trabi E. B., Xie F., and Mao S.. . 2021. Comparison of the fermentation and bacterial community in the colon of Hu sheep fed a low-grain, non-pelleted, or pelleted high-grain diet. Appl. Microbiol. Biot. 105:2071–2080. doi: 10.1007/s00253-021-11158-5. [DOI] [PubMed] [Google Scholar]
- Liu, S., He L., Jiang Q., Duraipandiyan V., Dhabi N. A. A., Liu G., Yao K., and Yin Y.. . 2018. Effect of dietary α‐ketoglutarate and allicin supplementation on the composition and diversity of the cecal microbial community in growing pigs. J. Sci. Food Agr. 98:5816–5821. doi: 10.1002/jsfa.9131. [DOI] [PubMed] [Google Scholar]
- Marti, S., Realini C. E., Bach A., Pérez-Juan M., and Devant M.. . 2013. Effect of castration and slaughter age on performance, carcass, and meat quality traits of Holstein calves fed a high-concentrate diet. J. Anim. Sci. 91:1129–1140. doi: 10.2527/jas.2012-5717. [DOI] [PubMed] [Google Scholar]
- Miorin, R. L., Batista L. H. C., Nascimento F. A., Costa E Silva L. F., Koontz A., Pettigrew J. E., Resende F. D., and Siqueira G. R.. . 2022. Effect of supplementation strategies and the use of exogenous xylanase enzyme on ruminal fermentation, digestibility, animal performance, and carcass characteristics of Nellore bulls grazing during dry season. Anim. Feed Sci. Technol. 290:115373. doi: 10.1016/j.anifeedsci.2022.115373. [DOI] [Google Scholar]
- Mishra, A. K., Gimenez G., Lagier J., Robert C., Raoult D., and Fournier P.. . 2012a. Genome sequence and description of Alistipes senegalensis sp. nov. Stand. Genomic Sci. 6:304–314. doi: 10.4056/sigs.2625821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, A. K., Lagier J. C., Robert C., Raoult D., and Fournier P. E.. . 2012b. Non-contiguous finished genome sequence and description of Alistipes senegalensis sp. nov. Stand. Genomic Sci. 6:304–314. doi: 10.4056/sigs.2625821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, T., Ikegaya M., and Alonso-Gil S.. . 2022. Structural and mechanistic insights into the substrate specificity and hydrolysis of GH31 α-N-acetylgalactosaminidase. Biochimie 195:90–99. doi: 10.1016/j.biochi.2021.11.007. [DOI] [PubMed] [Google Scholar]
- Montgomery, S. P., Drouillard J. S., Titgemeyer E. C., Sindt J. J., Farran T. B., Pike J. N., Coetzer C. M., Trater A. M., and Higgins J. J.. . 2004. Effects of wet corn gluten feed and intake level on diet digestibility and ruminal passage rate in steers. J. Anim. Sci. 82:3526–3536. doi: 10.2527/2004.82123526x. [DOI] [PubMed] [Google Scholar]
- Myer, P. R., Wells J. E., Smith T., Kuehn L. A., and Freetly H. C.. . 2015. Cecum microbial communities from steers differing in feed efficiency. J. Anim. Sci. 93:5327. doi: 10.2527/jas.2015-9415. [DOI] [PubMed] [Google Scholar]
- Neinast, M., Murashige D., and Arany Z.. . 2019. Branched chain amino acids. Annu. Rev. Physiol. 81:139–164. doi: 10.1146/annurev-physiol-020518-114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, H., Park J., and Takagi T.. . 2006. MetaGene: prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 34:5623–5630. doi: 10.1093/nar/gkl723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. 2016. Nutrient requirements of beef cattle. 8th rev. ed. Washington, DC: Natl. Acad. Press. [Google Scholar]
- Nyangale, E. P., Mottram D. S., and Gibson G. R.. . 2012. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J. Proteome Res. 11:5573–5585. doi: 10.1021/pr300637d. [DOI] [PubMed] [Google Scholar]
- Pang, W. Y., Earley B., Sweeney T., and Crowe M. A.. . 2006. Effect of carprofen administration during banding or burdizzo castration of bulls on plasma cortisol, in vitro interferon-γ production, acute-phase proteins, feed intake, and growth. J. Anim. Sci. 84:351–359. doi: 10.2527/2006.842351x. [DOI] [PubMed] [Google Scholar]
- Parker, B. J., Wearsch P. A., Veloo A. C. M., and Rodriguez-Palacios A.. . 2020. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 11:906. doi: 10.3389/fimmu.2020.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, R. J., Peng L., Barry N. A., Cline G. W., Zhang D., Cardone R. L., Petersen K. F., Kibbey R. G., Goodman A. L., and Shulman G. I.. . 2016. Acetate mediates a microbiome–brain-β-cell axis to promote metabolic syndrome. Nature 534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, E., Herd R., Archer J., and Arthur P.. . 2004. Metabolic differences in Angus steers divergently selected for residual feed intake. Anim. Prod. Sci. 44:441–452. doi: 10.1071/EA02219. [DOI] [Google Scholar]
- Rojo, R., Mendoza G. D., González S. S., Landois L., Bárcena R., and Crosby M. M.. . 2005. Effects of exogenous amylases from Bacillus licheniformis and Aspergillus niger on ruminal starch digestion and lamb performance. Anim. Feed Sci. Technol. 123:655–665. doi: 10.1016/j.anifeedsci.2005.04.053. [DOI] [Google Scholar]
- Siciliano-Jones, J., and Murphy M. R.. . 1989. Production of volatile fatty acids in the rumen and cecum-colon of steers as affected by forage:concentrate and forage physical form. J. Dairy Sci. 72:485–492. doi: 10.3168/jds.S0022-0302(89)79130-X. [DOI] [PubMed] [Google Scholar]
- de Souza, T. S. P., and Kawaguti H. Y.. . 2021. Cellulases, hemicellulases, and pectinases: applications in the food and beverage industry. Food Bioprocess Technol 14:1446–1477. doi: 10.1007/s11947-021-02678-z. [DOI] [Google Scholar]
- Steen, R. W. J. 1995. The effect of plane of nutrition and slaughter weight on growth and food efficiency in bulls, steers and heifers of three breed crosses. Livest. Sci. 42:1–11. doi: 10.1016/0301-6226(95)00002-3. [DOI] [Google Scholar]
- Su, M., Hao Z., Shi H., Li T., Wang H., Li Q., Zhang Y., and Ma Y.. . 2022. Metagenomic analysis revealed differences in composition and function between liquidassociated and solid-associated microorganisms of sheep rumen. Front. Microbiol. 13:851567. doi: 10.3389/fmicb.2022.851567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban, R. J. 2011. Growth hormone and testosterone: anabolic effects on muscle. Horm. Res. Paediat. 76:81–83. doi: 10.1159/000329184. [DOI] [PubMed] [Google Scholar]
- Van Kessel, J. S., Nedoluha P. C., Williams-Campbell A., Baldwin R. L. V., and McLeod K. R.. . 2002. Effects of ruminal and postruminal infusion of starch hydrolysate or glucose on the microbial ecology of the gastrointestinal tract in growing steers. J. Anim. Sci. 80:3027–3034. doi: 10.2527/2002.80113027x. [DOI] [PubMed] [Google Scholar]
- Vijay, N., and Morris E. M.. . 2014. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 20:1487–1498. doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Li Y., MaiTiSaiYiDi T., Yang H., and Yang K.. . 2020a. Effect of dietary gossypol supplement on fermentation characteristics and bacterial diversity in the rumen of sheep. PLoS One 15:e0234378. doi: 10.1371/journal.pone.0234378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Wang Y., Lin X., Gou Z., Fan Q., Ye J., and Jiang S.. . 2020b. Potential effects of acidifier and amylase as substitutes for antibiotic on the growth performance, nutrient digestion and gut microbiota in yellow-feathered broilers. Animals 10:1858. doi: 10.3390/ani10101858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whon, T. W., Kim H. S., Shin N. R., Jung E. S., Tak E. J., Sung H., Jung M. J., Jeong Y. S., Hyun D. W., Kim P. S., . et al. 2021. Male castration increases adiposity via small intestinal microbial alterations. EMBO Rep. 22:e50663. doi: 10.15252/embr.202050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wielen, N., Moughan P. J., and Mensink M.. . 2017. Amino acid absorption in the large intestine of humans and porcine models. J. Nutr. 147:1493–1498. doi: 10.3945/jn.117.248187. [DOI] [PubMed] [Google Scholar]
- Xie, C., Mao X., Huang J., Ding Y., Wu J., Dong S., Kong L., Gao G., Li C., and Wei L.. . 2011. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 39:W316–W322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J., Li L., Dai T., Qi X., Wang Y., Zheng T., Gao X., Zhang Y., Ai Y., Ma L., . et al. 2022. Short-Chain fatty acids produced by ruminococcaceae mediate α-Linolenic acid promote intestinal stem cells proliferation. Mol. Nut. Food Res. 66:2100408. doi: 10.1002/mnfr.202100408. [DOI] [PubMed] [Google Scholar]
- Xu, H., Cao J., Li X., Lu X., Xia Y., Fan D., Zhao H., Ju D., and Xiao C.. . 2020. Regional differences in the gut microbiota and gut-associated immunologic factors in the ileum and cecum of rats with collagen-induced arthritis. Front. Pharmacol. 11:587534. doi: 10.3389/fphar.2020.587534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., Refat B., Guevara-Oquendo V. H., and Yu P.. . 2022. Lactational performance, feeding behavior, ruminal fermentation and nutrient digestibility in dairy cows fed whole-plant faba bean silage-based diet with fibrolytic enzyme. Animal 16:100606. doi: 10.1016/j.animal.2022.100606. [DOI] [PubMed] [Google Scholar]
- Ye, J., Jiang S., Cheng Z., Ding F., Fan Q., Lin X., Wang Y., and Gou Z.. . 2022. Feed restriction improves lipid metabolism by changing the structure of the cecal microbial community and enhances the meat quality and flavor of bearded chickens. Animals 12:970. doi: 10.3390/ani12080970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H., and Huang J.. . 2016. Effects of soybean meal replacement with fermented alfalfa meal on the growth performance, serum antioxidant functions, digestive enzyme activities, and cecal microflora of geese. J. Integr. Agr. 15:2077–2086. doi: 10.1016/S2095-3119(15)61198-4. [DOI] [Google Scholar]
- You, J., Lin S. J., and Jiang T.. . 2019. Origins and evolution of the α-L-fucosidases: from bacteria to metazoans. Front. Microbiol. 10:1756. doi: 10.3389/fmicb.2019.01756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Ji S., Chen Y., Yang B., Xu X., and Hu C.. . 2011. Effect of α-ketoglutaric acid on in vitro gas production, ruminal fermentation, and bacterial diversity. Anim. Feed Sci. Technol. 170:291–296. doi: 10.1016/j.anifeedsci.2011.09.012. [DOI] [Google Scholar]
- Zhang, H., Liu J., Lv Y., Jiang Y., Pan J., Zhu Y., Huang M., and Zhang S.. . 2020a. Changes in intestinal microbiota of type 2 diabetes in mice in response to dietary supplementation with instant tea or matcha. Can. J. Diab. 44:44–52. doi: 10.1016/j.jcjd.2019.04.021. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Li Z., Zhao H., Chen X., and Jia G.. . 2020b. Effects of drinking water temperature and flow rate during cold season on growth performance, nutrient digestibility and cecum microflora of weaned piglets. Animals 10:1048. doi: 10.3390/ani10061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R., Wu J., Lei Y., Bai Y., Jia L., Li Z., Liu T., Xu Y., Sun J., Wang Y., . et al. 2021. Oregano essential oils promote rumen digestive ability by modulating epithelial development and microbiota composition in beef cattle. Front. Nutr. 8:722557. doi: 10.3389/fnut.2021.722557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.