Abstract
Background:
The ultimate goal of endodontic therapy is to eliminate all microorganisms present inside root canal and thereby sealing all the possible communicating pathways between pulpal and periradicular tissues, which prevents all the factors that cause recontamination and reinfection of the root canal system. If endodontic treatment fails, next approach is surgical endodontics. Bioceramics are recently introduced materials specifically designed for their potential use in medical field and dentistry.
Aim:
To evaluate and compare the push-out bond strength of mineral trioxide aggregate (MTA) by adding titanium dioxide (TiO2), silver, and silicon dioxide nanoparticles.
Materials and Methods:
Totally, 60 single-rooted human teeth were used. Middle third of the root was sectioned to obtain 2-mm thick root section. Acrylic was adapted to the section to obtain disks of 5 mm diameter and 2 mm thickness. Canal was prepared by GG Drill. Samples were divided into four groups of 15 each (n = 15): • Group I (control): MTA • Group II: MTA + TiO2 nanoparticles. • Group III: MTA + silver nanoparticles. • Group IV: MTA + silicon dioxide nanoparticles. The cement mixture was compacted into the canal. Samples were subjected to push-out bond strength using universal testing machine.
Statistical Analysis Used:
The data were analyzed statistically by analysis of variance and post hoc comparison by Tukey's t-test.
Results:
The highest push-out bond strength was shown by Group II (MTA with TiO2 nanoparticles), followed by Group III (MTA with silver nanoparticles) and Group I (MTA control group). The lowest push-out bond strength was shown by Group III (MTA with silicon dioxide nanoparticles).
Conclusions:
TiO2 and silver nanoparticles when added into MTA lead to an increase in push-out bond strength of MTA.
Keywords: Mineral trioxide aggregate, push-out bond strength, silicon dioxide nanoparticles, silver nanoparticles, titanium dioxide nanoparticles
INTRODUCTION
The ultimate goal of endodontic therapy is to eliminate all microorganisms present inside root canal and thereby sealing all the possible communicating pathways between pulpal and periradicular tissues, which prevents all the factors that cause recontamination and reinfection of the root canal system. Adequately filled teeth can fail due to several reasons such as incomplete removal of microorganisms which generally reside at the deeper portions of the dentinal tubules, microleakage from temporary as well as permanent coronal restoration, and presence of recurrent or secondary decay at the restorative margins.[1]
If traditional root canal treatment fails, the next alternative approach should be orthograde retreatment. When all efforts for successful completion of orthograde endodontic treatment are unsuccessful, the final option would be endodontic periapical surgery. Periapical surgery usually comprises exposure of the involved area, periapical curettage, root end resection, and root end preparation followed by an insertion of a root end repair material (RERM).[2] The three-dimensional hermetic barrier provided by RERMs at resected root end interface determines the success of periapical surgery.
Numerous materials have been aided as RERMs, including amalgam, gutta percha, zinc oxide–eugenol cements, zinc polycarboxylate, Diaket, Cavit, calcium hydroxide, glass ionomer cement, Compomer, composites, calcium silicate based, or bioceramic and biomimetic materials such as Biodentine, Bioaggregate, and mineral trioxide aggregate (MTA).[3] Thus, as the existing RERMs currently used in endodontics are lacking and do not possess all these desired characteristics, there was a need for introduction of newer repair materials.
Bioceramics are one such recently introduced material, which have become quite popular. Bioceramics are a group of ceramic materials specifically designed for their potential use in medical field and dentistry. These materials are mainly alumina, glass ceramics, bioactive glass, composites, zirconia, various hydroxyapatite, and calcium phosphates. Alumina and zirconia are used in prosthetic devices. Bioceramic materials used in endodontics are as follows:[4]
Calcium silicate-based materials, which comprise various cements such as MTA, Portland cement, Biodentine, and various sealers such as Modified Portland cement (CPM) Endo-Sealer, MTA Fill apex, Bio Root Root canal sealer (RCS) sealer, and TechBio sealer
Calcium phosphates/hydroxyapatite-based materials/tricalcium phosphate
Calcium silicates and calcium phosphates (mixed), which contain iRoot FS, iRoot BP, iRoot BP Plus, Ceramicrete, EndoSequence BC Sealer, Total Fill, and Bioaggregate.
After the introduction of bioceramic materials into clinical endodontics, MTA, introduced by Mahmoud Torabinejad (1992), is considered the gold standard material containing various components such as fine hydrophilic powder particles of tricalcium aluminate, tricalcium silicate, tricalcium oxide, calcium sulfate dihydrate, silicate oxide, tetra calcium aluminoferrite, and small amounts of mineral oxides (bismuth oxide).[5]
Bond strength is an important property of endodontic repair material and it describes how strongly the material is attached to the dentine. Bond strength of a RERM or a biomimetic material should be sufficient enough to resist the compaction forces of the repair material. Bond strength is an important property of endodontic repair material and it describes how strongly the material is attached to the dentine which includes strength tests such as shear, tensile, and push-out strength tests. For investigating this property in vitro, the push-out bond strength test was shown to be effective and reliable as the test conditions were comparable to the clinical situation, in which the tested sample materials are placed directly into prepared canals having a natural canal shape and tubule arrangement.[6] For investigating this property in vitro, the push-out bond strength test was shown to be effective and reliable as the test conditions were comparable to the clinical situation, in which the tested sample materials are placed directly into prepared canals having a natural canal shape and tubule arrangement. Some of the efforts to enhance the properties of MTA include incorporation of some nanoparticles such as silver, zinc oxide, silver zeolite, and titanium dioxide (TiO2), which have drawn attention in the dental field.
MATERIALS AND METHODS
The Institutional Ethics Committee of Krishna Institute of Medical Sciences, Karad, approved the present study under the reference number: KIMSDU/IEC/07/2019.
For this in vitro study, 60 intact permanent single-rooted extracted teeth were collected irrespective of age and sex of the patients. Teeth extracted for orthodontic and periodontal reasons were included in this study. Grossly decayed or carious teeth, fractured teeth. Fractured teeth, root canal-treated teeth, and teeth with resorbed roots were not included in the study. All the samples were decoronated to obtain a standardized length. Following decoronation, all teeth were stored in 0.5% chloramine-T solution until the initiation of the study procedures. Then, 2-mm-thick root slices were prepared from the middle third of the root canals, using a diamond disk. Metal molds of 1.5 cm diameter and 2 mm thickness were made and acrylic was adapted to section to obtain the disks of 1.5 cm diameter and 2 mm thickness. These disks were mounted onto the Metal Jig and the canals were prepared by three passes of GG Drill no. 5 to obtain a 1.3 mm internal diameter. Sections were immersed in 17% ethylenediaminetetraacetic acid for 3 min and then in 3% sodium hypochlorite for 3 min and finally were washed with distilled water and dried.
All the samples were divided into four groups of 15 each (n = 15). Group I: MTA (Angelus, Londrina, PR, Brazil); Group II: MTA with TiO2 nanoparticles (mean particle size: 30 nm); Group III: MTA with Silver nanoparticles (mean particle size: 30 nm); Group IV: MTA with silicon dioxide nanoparticles (mean particle size: 30 nm).
For Group I, the MTA was mixed with spatula on mixing pad according to the manufacturer's instructions and the cement mixture was compacted with plugger into canal. For Group II, TiO2 nanoparticles in powder form were added to MTA powder at a ratio of 1% by weight of MTA powder. For Group III, silver nanoparticles in powder form were added to MTA powder at a ratio of 1% by weight of MTA powder. For Group IV, silicon dioxide nanoparticles in powder form were added to MTA powder at a ratio of 1% by weight of MTA powder. After adding nanoparticles in all groups, powder was mixed with distilled water on a mixing pad according to the manufacturer's instructions and the cement mixture was compacted with plugger into canal [Figure 1]. For storage of samples, they were wrapped in pieces of gauge soaked in synthetic tissue fluid with pH 7.4. Samples were kept in an incubator at 37°C for 72 h.
Figure 1.
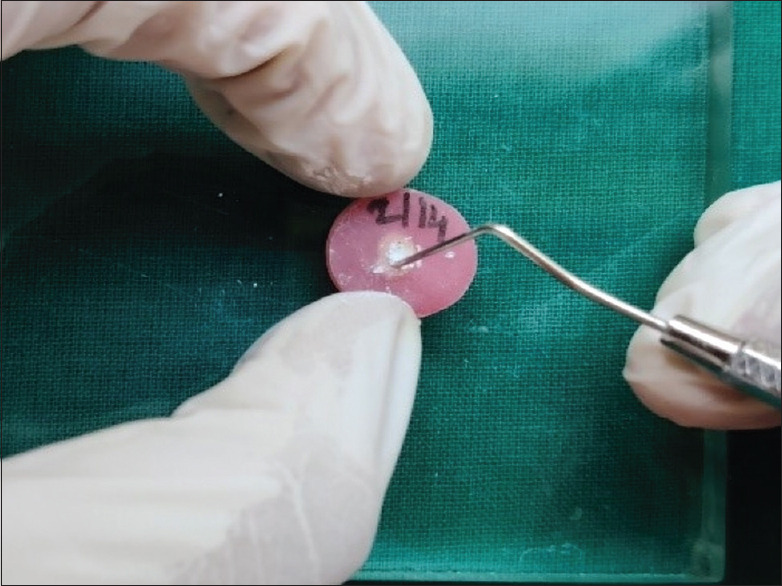
Acrylic disk of 1.5 cm diameter and 2 mm thickness in dimensions, Canal prepared to obtain 1.3 mm diameter following which Cement mixture (MTA with or without nanoparticles) compacted inside the canals. MTA: Mineral trioxide aggregate
Measuring of push-out bond strength
Samples were subjected to push-out bond strength using universal testing machine. They were placed on metal slab with central hole to allow free motion of plunger (diameter of 1 mm) at the cross-head speed of 1 mm/min and the load applied at the time of dislodgment of material was recorded in Newtons [Figure 2].
Figure 2.
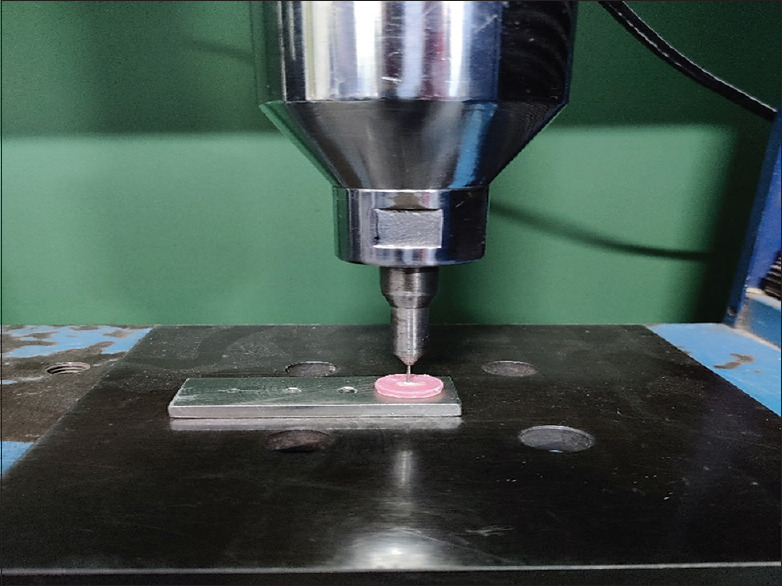
Sample testing under Universal testing machine. Samples were placed on metal slab with central hole to allow free motion of plunger (diameter of 1 mm) at the cross-head speed of 1 mm/min and the load applied at time of dislodgment of material was recorded in Newtons
Statistical analysis
All statistical analysis was performed using the IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp SPSS 13 Chicago INC statistical software version. statistical software version. The data were analyzed statistically using one-way analysis of variance (ANOVA) and post hoc Tukey's t-test.
RESULTS
The mean push-out bond strength values and the standard deviations in MPa are presented in [Table 1]. The one-way ANOVA showed that there were significant differences in the mean push-out bond strength values among the four experimental groups with P < 0.001 [Table 2].
Table 1.
Descriptive statistics for push-out bond strength among four groups
| Descriptive statistics | |||||
|---|---|---|---|---|---|
| Group | n | Minimum | Maximum | Mean | SD |
| Group I MTA | 15 | 1.59 | 5.93 | 3.7113 | 1.20914 |
| Group II MTA with titanium dioxide nanoparticles | 15 | 2.15 | 8.19 | 6.1633 | 1.63761 |
| Group III MTA with silver nanoparticles | 15 | 2.12 | 6.75 | 5.1453 | 1.10572 |
| Group IV MTA with silicon dioxide nanoparticles | 15 | 1.97 | 4.36 | 3.1480 | 0.66035 |
MTA: Mineral trioxide aggregate, SD: Standard deviation
Table 2.
Comparison of mean push-out bond strength among four groups by analysis of variance
| ANOVA (push-out bond strength) | |||||
|---|---|---|---|---|---|
| Sum of squares | Degree of freedom | Mean square | F | Significant (P) | |
| Between groups | 84.390 | 3 | 28.130 | 19.392 | <0.001* |
| Within groups | 81.234 | 56 | 1.451 | ||
| Total | 165.624 | 59 | |||
ANOVA: Analysis of variance. *P<0.05> : Statistically significant.
The mean push-out bond strength was highest for Group II followed by Group III.
There was a statistically significant difference between Group I and II, Group I and III, Group I and IV, and Group II and IV. The lowest value was seen for Group IV, but there was no significant difference between Group I and IV. Similarly, there was an insignificant difference between Group II and III [Table 3].
Table 3.
Comparison of mean push out bond strength among four groups by Tukey's post hoc test
| Multiple comparisons Dependent variable: Push-out bond strength (Tukey's HSD) | |||||||
|---|---|---|---|---|---|---|---|
| (I) Group | (J) Group | Mean difference (I-J) | SE | Significant (P) | 95% CI |
||
| Lower bound | Upper bound | ||||||
| Group I MTA | Group II MTA with titanium dioxide nanoparticle | −2.45200* | 0.43979 | <0.001* | −3.6165 | −1.2875 | |
| Group I MTA | Group III MTA with silver nanoparticles | −1.43400* | 0.43979 | 0.010 | −2.5985 | −0.2695 | |
| Group I MTA | Group IV MTA with silicon dioxide nanoparticles | 0.56333 | 0.43979 | 0.579 | −0.6012 | 1.7278 | |
| Group II MTA with titanium dioxide nanoparticle | Group III MTA with silver nanoparticles | 1.01800 | 0.43979 | 0.107 | −0.1465 | 2.1825 | |
| Group II MTA with titanium dioxide nanoparticle | Group IV MTA with silicon dioxide nanoparticles | 3.01533* | 0.43979 | <0.001* | 1.8508 | 4.1798 | |
| MTA with silver nanoparticles | MTA with silicon dioxide nanoparticles | 1.99733* | 0.43979 | <0.001* | 0.8328 | 3.1618 | |
*The mean difference is significant at the 0.05 level. MTA: Mineral trioxide aggregate, SE: Standard error, HSD: Honestly significant difference, SE: Standard error, CI: Confidence interval
DISCUSSION
The utmost goal of any endodontic treatment is getting a good clinical prognosis and hence when choosing an appropriate RERM, it should have sufficient physiochemical properties and it should fulfill the criteria of biocompatibility; cytotoxicity; apical seal and marginal adaptation which is critical for ensuring a favorable outcome of endodontic treatment.[7] Among the physiochemical properties, adhesiveness to intraradicular dentin is very important. These materials should remain adapted to the dentin canal walls when the teeth are exposed to external mechanical forces occurring during function.
Traditionally, calcium hydroxide has been used as a RERM of choice. Apexification with calcium hydroxide involves a repeated placement of nonsetting calcium hydroxide until the formation of an apical barrier. There are various disadvantages associated with usage of calcium hydroxide dressing such as poor patient compliance, susceptibility to root fracture, and need for multiple appointments.[8] Thus, because of various drawbacks associated with the use of calcium hydroxide, there was a need for introduction of newer and better materials, which could overcome the shortcomings of traditional materials.
The field of endodontics is constantly emerging and advancing due to introduction of newer techniques and technological advances. The field of endodontics has grown exponentially due to various advances in the endodontic material sciences.[9] Bioceramics are considered among the recently introduced materials in the field of endodontics that have prominently changed the face of endodontics. There are various advantages of bioceramic materials:[10]
Exceptional biocompatibility properties due to their likeness with biological hydroxyapatite
Intrinsic osteoinductive capacity because of their potential to absorb osteoinductive substances if there is a bone healing process nearby
Capacity to work as a regenerative scaffold of resorbable lattices which provide a framework which is in due course dissolved as the body rebuilds tissue
Potential to achieve exceptional hermetic seal and form a chemical bond with the tooth structure and have good radiopacity
Antibacterial properties due to precipitation in situ after setting, a mechanism that leads to bacterial sequestration.
The first bioceramic material successfully to be used and introduced in endodontics was the MTA cement, founded by Dr. Torabinejad in 1993. When MTA powder is mixed with water, hydration takes place. Due to hydration of the powder, there is a formation of a colloidal gel which undergoes solidification into a hard structure that consists of discrete crystals in an amorphous matrix. The crystals are made up of calcium oxide and the amorphous region composed of phosphate, calcium, chloride, carbon, and silica. Average size of each particle is about 10 μm. The range of particle size is from 0.1 μm to 100 μm. The tricalcium oxide component in MTA reacts with tissue fluid and forms calcium hydroxide leading to hard tissue formation.[11] MTA has a few excellent properties such as superior compressive strength, ability to set in presence of moisture, effective marginal marginal adaptation, and sealing. In spite of this, MTA has several drawbacks associated with it such as relatively longer setting time, workability issues, potential to cause discoloration of tooth, and lesser bond strength.[12]
The ongoing research in the field of nanotechnology world is due to the extravagant properties that nanoparticles possess. The kind of force that nanoparticles have in the field of dentistry and especially in conservative dentistry and endodontics for the treatment of various oral problems and diseases is swiftly progressing with every passing year. These nanoparticles can be incorporated in a sealer, obturating material, intracanal medicament, and irrigating solutions to provide the desired results.[13]
In the present study, it was observed that there was a statistically significant difference among all the four groups with regard to push-out bond strength. It can be inferred that all the nanoparticles incorporated in MTA used in this study can be used in root practice as a RERM. The highest push-out bond strength was shown by Group II (MTA with TiO2 nanoparticles), followed by Group III (MTA with silver) and Group I (MTA control group). The lowest push-out bond strength was shown by Group IV (MTA with silicon dioxide nanoparticles).
This increase in push-out bond strength on incorporation of TiO2 nanoparticles in MTA is because of its unique property, i.e., “pozzolanic activity.” Due to pozzolanic activity, the highly active TiO2 nanoparticles quickly consume calcium hydroxide, which is a product of cement hydration. This reaction is favorable to form a denser structure of hydroxyapatite. Hence, basically two events take place in this reaction. First, the amount of free calcium hydroxide is eventually decreased. Second, when this reaction is taking place, there is an increase in the formation of calcium silicate hydrate and calcium aluminate hydrate. These hydration products are effective in increasing the overall strength of the material. Because of this, hydration of cement is expedited and as a result greater volumes of the reaction products are eventually formed.[14] Furthermore, on the other hand, TiO2 nanoparticles would fill spaces between cement particles, producing smaller pores to increase the physical strength. Therefore, it is confirmed that the addition of nanoparticles to cement mortars improved their strength characteristics.[15] This in accordance with a study performed by Mohammad Samiei et al., where they concluded that addition of TiO2 nanoparticles in 1% weight ratio of MTA leads to an increase in push-out bond strength, compressive strength, working time, and setting time.[16]
In our study, the second highest value for the push-out bond strength was seen for Group III (MTA with silver nanoparticles). Enhancement of physical properties and strength on incorporation of silver nanoparticles is due to water-based characteristics of silver nanoparticles; they may provide an increase in surface tension of dentin substrate which might improve the penetration of MTA particles through the dentin. Furthermore, silver nanoparticles possess strong ionic interatomic bonding giving rise to a desirable and improved mechanical property.[17] This is in accordance with the study done by Zahra Jowkar et al. where they investigated effect of intraradicular dentin pretreatment with silver nanoparticles and found that best results were obtained for silver nanoparticle pretreated group.[18] Furthermore, Zhang et al. concluded that addition of silver nanoparticles into composite resin improves its mechanical properties.[19]
There was a decrease in push-out bond strength in Group IV (MTA with silicon dioxide nanoparticles). This might be due to the reason that incorporation of silicon dioxide nanoparticles causes these particles to agglomerate and aggregate. The agglomerated compounds can have the ability to act as stress concentrating centers in the matrix and can adversely affect the mechanical properties of MTA. Furthermore, when these nanoparticles are added at higher concentrations, they may act as impurities that may interfere with polymerization thus decreasing the bond strength.[20]
In this study, MTA incorporated with nanoparticles were filled in the canals in 1% of MTA powder.[21] This was in accordance with a study performed by Khataee et al., where they evaluated the mechanical properties of modified white cement with nanostructured TiO2 where it was concluded that strength increases with increasing the amount of TiO2 nanoparticles up to 1%, but above it, the compressive strength decreases. Thus wt.% of nanoparticles that was incorporated was 1% of the MTA powder.[22]
Limitations of the study
In this study, only the effect on nanoparticles on only one physical property, i.e., push-out bond strength of MTA, has been taken into consideration. Hence, further studies are needed to evaluate the effect of nanoparticle incorporation in MTA on push-out bond strength.
CONCLUSIONS
MTA modified with nanoparticles can be used as an alternative over MTA as a RERM
TiO2 and silver nanoparticles when incorporated into MTA lead to an increase in push-out bond strength
Silicon dioxide nanoparticles when incorporated into MTA had no significance difference on the push-out bond strength
As we have not evaluated other physical properties, further studies are needed to evaluate the effect of nanoparticles incorporation in MTA on other physical properties.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sivakumar L. Comparative Evaluation of Push-Out Bond Strength of Two Different Root End Repair Material (Doctoral Dissertation), Best Dental Science College, Madurai. 2018.
- 2.von Arx T. Failed root canals: The case for apicoectomy (periradicular surgery) J Oral Maxillofac Surg. 2005;63:832–7. doi: 10.1016/j.joms.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Chong BS, Pitt Ford TR. Root-end filling materials: Rationale and tissue response. Endod Top. 2005;11:114–30. [Google Scholar]
- 4.Raghavendra SS, Jadhav GR, Gathani KM, Kotadia P. Bioceramics in endodontics – A review. J Istanb Univ Fac Dent. 2017;51:S128–37. doi: 10.17096/jiufd.63659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri J. The chemical composition of mineral trioxide aggregate. J Conserv Dent. 2008;11:141–3. doi: 10.4103/0972-0707.48834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huffman BP, Mai S, Pinna L, Weller RN, Primus CM, Gutmann JL, et al. Dislocation resistance of ProRoot Endo Sealer, a calcium silicate-based root canal sealer, from radicular dentine. Int Endod J. 2009;42:34–46. doi: 10.1111/j.1365-2591.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 7.Wadhwa H, Mahajan P, Monga P, Mukheja A, Dhillon J, Bajaj N. Comparative evaluation of push-out bond strength of root-end filling materials in root-end cavities prepared by laser or ultrasonic technique: An in vitro study. J Conserv Dent. 2019;22:396–400. doi: 10.4103/JCD.JCD_545_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 9.Shenoy A, Shenoy N. Dental ceramics: An update. J Conserv Dent. 2010;13:195–203. doi: 10.4103/0972-0707.73379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomer AK, Kumari S, Rastogi D, Cecilia LL. The rise of bioceramics in endodontics: A review. Int J Appl Dent Sci. 2020;6:588–94. [Google Scholar]
- 11.Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev. 2008;108:4551–627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur M, Singh H, Dhillon JS, Batra M, Saini M. MTA versus biodentine: Review of literature with a comparative analysis. J Clin Diagn Res. 2017;11:ZG01–5. doi: 10.7860/JCDR/2017/25840.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grodzinski P, Silver M, Molnar LK. Nanotechnology for cancer diagnostics: Promises and challenges. Expert Rev Mol Diagn. 2006;6:307–18. doi: 10.1586/14737159.6.3.307. [DOI] [PubMed] [Google Scholar]
- 14.Khataee R, Heydari V, Moradkhannejhad L, Safarpour M, Joo SW. Self-cleaning and mechanical properties of modified white cement with nanostructured TiO2. J Nanosci Nanotechnol. 2013;13:5109–14. doi: 10.1166/jnn.2013.7586. [DOI] [PubMed] [Google Scholar]
- 15.Hosseini P, Booshehrian A, Farshchi S. Influence of nano-SiO2 addition on microstructure and mechanical properties of cement mortars for ferrocement. Transp Res Record. 2010;2141:15–20. [Google Scholar]
- 16.Samiei M, Janani M, Asl-Aminabadi N, Ghasemi N, Divband B, Shirazi S, et al. Effect of the TiO2 nanoparticles on the selected physical properties of mineral trioxide aggregate. J Clin Exp Dent. 2017;9:e191–5. doi: 10.4317/jced.53166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jowkar Z, Farpour N, Koohpeima F, Mokhtari MJ, Shafiei F. Effect of silver nanoparticles, zinc oxide nanoparticles and titanium dioxide nanoparticles on microshear bond strength to enamel and dentin. J Contemp Dent Pract. 2018;19:1404–11. [PubMed] [Google Scholar]
- 18.Jowkar Z, Fattah Z, Ghanbarian S, Shafiei F. The effects of silver, zinc oxide, and titanium dioxide nanoparticles used as dentin pretreatments on the microshear bond strength of a conventional glass ionomer cement to dentin. Int J Nanomedicine. 2020;15:4755–62. doi: 10.2147/IJN.S262664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater. 2012;28:842–52. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Y, Kiat-Amnuay S, Powers JM, Zhao Y. Effect of nano-silicon dioxide concentration on the mechanical properties of a maxillofacial silicone elastomer. J Prosthet Dent. 2008;100:465–73. doi: 10.1016/S0022-3913(08)60266-8. [DOI] [PubMed] [Google Scholar]
- 21.Uppal M, Arora G. Evaluation of the push-out bond strength of mineral trioxide aggregate mixed with silver zeolite: An in vitro study. Int J Oral Care Res. 2017;5:286–9. [Google Scholar]
- 22.Elsaka SE, Hamouda IM, Swain MV. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J Dent. 2011;39:589–98. doi: 10.1016/j.jdent.2011.05.006. [DOI] [PubMed] [Google Scholar]


