Abstract
Context:
Vehicles combined with calcium hydroxide, as an intracanal medicament, play a key factor in affecting antibacterial, calcium release, and pH.
Aims:
The aim of this study is to investigate the effect of three vehicles (glycerin, chlorhexidine gluconate/CHX, and chitosan nanoparticle) combined with calcium hydroxide as an intracanal medicament on antibacterial efficacy against Enterococcus faecalis, calcium ion release, and pH of at different interval times of 7 and 14 days.
Settings and Design:
The research was experimental laboratory.
Materials and Methods:
Each study used 24 samples of eight teeth each and was randomly divided into three groups based on the vehicle of calcium hydroxide: group 1: glycerin, group 2: CHX, and group 3: chitosan nanoparticles. Each vehicle group was then further divided into two subgroups of four teeth based on the interval times (group A: 7 days and group B: 14 days). The antibacterial efficacy was determined using an agar diffusion method. Calcium ion release was analyzed with atomic absorption spectrometry, and pH was measured using a pH meter.
Statistical Analysis Used:
Data from each study were analyzed by two-way ANOVA, followed by Tukey's test with a significance level of 95%.
Results:
The results exhibited that chitosan nanoparticles had the highest antibacterial efficacy against E. faecalis, calcium ion release, and pH, while the lowest was glycerin at 7 and 14 days (P < 0.05).
Conclusion:
Calcium hydroxide combined with chitosan nanoparticle as an intracanal medicament produced the highest antibacterial efficacy against E. faecalis, calcium ion release, and pH than glycerin and CHX at intervals of 7 and 14 days.
Keywords: Antibacterial, calcium hydroxide, calcium ion release, intracanal medicament, pH
INTRODUCTION
The prerequisite for successful root canal treatment is the achievement of an endodontic triad which includes biomechanical preparation (cleaning and shaping), chemical cleaning of the root canal using irrigation solutions, sterilization of the root canal with intracanal medicaments, and obturation with root canal filling materials.[1] The goal of root canal treatment is to eliminate bacteria and their products from the root canal. Although the biomechanical preparation of root canals effectively reduces bacterial colonies, the complicated anatomy of the root canal can serve as a proliferation field for bacteria, resulting in root canal treatment failure. To overcome this condition, the application of intracanal medicaments as root canal sterilization materials can increase the success of root canal treatment.[2]
An intracanal medicament is used in root canal treatment to disinfect or sterilize root canals between visits. The requirement for an intracanal medicament is that it is stable for a certain period, is harmless to the body, and is bactericidal.[3] Calcium hydroxide is the most commonly used intracanal medicament in root canal treatment. Calcium hydroxide is available in a variety of preparations. One of them is a powder form, which will be combined with a vehicle that has an essential role in the decomposition rate of calcium hydroxide into calcium and hydroxyl ions, resulting in an increase in pH to 12.5 at the surrounding area.[4] Hydroxyl ions can cause the pH of the environment to become alkaline; therefore, it has an antibacterial effect and inhibits bacterial growth, while calcium ions can improve root canal repair, inhibits tooth resorption, and hard tissue formation.[4,5]
Previous studies have shown that the type of vehicles of calcium hydroxide directly relates to the concentration and rate of ion release and antibacterial efficacy.[6,7] Glycerin is a vehicle of calcium hydroxide powder that is often used and is the golden standard for clinical use because it is easy to apply to root canals.[5] The disadvantage of glycerin is that it is a thick solution; hence, when combined with calcium hydroxide powder, it takes a long time to release calcium and hydroxyl ions. Its solubility is also low because it has a high-molecular weight. In addition, it has low antibacterial efficacy against microorganisms, especially Enterococcus faecalis.[8]
Chlorhexidine gluconate (CHX) is employed in endodontics as an irrigant solution or as an intracanal medicament mixed with calcium hydroxide powder. The properties of CHX are biocompatible, high substantive, and have broad antibacterial properties. This material is low toxicity and is effective against Gram-positive and Gram-negative bacteria,[9] especially against microorganisms resistant to calcium hydroxide, such as E. faecalis.[10]
Currently, chitosan has been widely studied in dentistry, especially in the field of endodontics, because it has high-antibacterial properties. Chitosan is derived from the deacetylation of chitin, a natural polymer derived from the exoskeleton of the crustacean group. Chitosan contains copolymers of glucosamine and N-acetyl glucosamine.[11] Previous studies reported that chitosan has high-antibacterial properties against E. faecalis, and this antibacterial activity persists for a long time.[12] The antibacterial efficacy of chitosan is induced by the interaction of the positive charge of chitosan and the negative charge of the bacterial cell, thereby changing the permeability of the bacterial cell and resulting in leakage of intracellular components.[13] Chitosan is stable to release ions in a controlled and slow manner when utilized as an intracanal medicament.[11] The nanoparticles shape of chitosan has an advantage as antibacterial agents due to a higher surface area and charge density that enables chitosan to react with the negative charge surface of bacterial cells, leading to bacterial cell death.[13]
E. faecalis is anaerobic, facultative Gram-positive bacterial species. These bacteria are resistant to calcium hydroxide because they have the ability to penetrate into the dentinal tubules and their branches and are resistant to alkaline pH. E. faecalis is also the most common bacteria found in failed root canal treatment.[2] Since E. faecalis is resistant to calcium hydroxide, an effective vehicle is needed to dissolve calcium hydroxide powder into calcium and hydroxyl ions, enhancing intracanal medicaments to have high-antibacterial activity against E. faecalis.
The contact period of the intracanal medicament on the root canal wall may also determine its effectiveness. Barekatain et al.[6] reported that intracanal medicaments worked optimally as root canal sterilization agents for at least 2 weeks. Another study showed that intracanal medicaments with calcium hydroxide were kept in the root canal for a minimum of 1 week and a maximum of 2 weeks because more prolonged contact with calcium hydroxide resulted in teeth being susceptible to fracture.[4]
The beneficial properties of chitosan are interesting to study as a vehicle for calcium hydroxide as intracanal medicaments to increase its antibacterial efficacy, especially against E. faecalis. It is expected that vehicles combined with calcium hydroxide powder do not change the release of calcium and hydroxyl ions. Therefore, vehicles used with calcium hydroxide have been required to enhance the antibacterial effect but not disrupt the physicochemical properties (such as calcium ion release and pH).
However, no previous study has investigated the chitosan nanoparticles combined with calcium hydroxide are able to increase their antibacterial efficacy against E. faecalis, calcium ion release, and pH without disturbing other properties. Thus, the aim of this study was to investigate the effect of three vehicles, namely glycerin, CHX, and chitosan nanoparticles of calcium hydroxide as intracanal medicament at a time interval of 7 and 14 days on the antibacterial efficacy against E. faecalis, calcium ions release, and pH. The null hypotheses tests were: (i) there would be no difference of glycerin, CHX, and chitosan nanoparticles as vehicles combined with calcium hydroxide on antibacterial efficacy against E. faecalis, calcium ion release, and pH and (ii) there would be no difference in the interval times between 7 and 14 days in terms of antibacterial efficacy against E. faecalis, calcium ion release, and pH.
MATERIALS AND METHODS
The research protocol has been approved by the Institutional Ethics Committee No. 00696/KKEP/FKG-UGM/EC/2021.
Preparation of intracanal medicament
One gram of pure calcium hydroxide powder as measured by a digital scale was dissolved with 1.5 ml of glycerin and stirred with a stainless steel spatula until homogeneous (Group 1). One gram of pure calcium hydroxide powder as measured by a digital scale was dissolved with 1.5 ml of 2% CHX (Brasseler, Savannah, GA, USA) and stirred with a stainless steel spatula until homogeneous (Group 2). Chitosan nanoparticles used in this study were in the form of a gel (NHI, Tangerang, Indonesia) which will be diluted into a solution to obtain chitosan nanoparticles solution with a concentration of 3%. The dilution process of chitosan nanoparticles was carried out by dissolving 3 g of chitosan nanoparticles into 100 ml of 0.1% acetic acid and then stirring with an automatic stirrer until homogeneous. Furthermore, 1 g of pure calcium hydroxide powder was measured with a digital scale dissolved with 1.5 ml of 3% chitosan nanoparticles solution and stirred until it became a homogeneous paste (Group 3).[14]
Antibacterial efficacy against Enterococcus faecalis
The antibacterial activity was tested using bacterial colonies of E. faecalis ATCC 29212 pure stock containing 107–108 CFU/ml bacteria, grown on Mueller Hinton Agar (MHA) media, and incubated for 24 h at 37°C. Furthermore, the bacterial colonies were taken using a sterile tube, dissolved in Brain Heart Infusion (BHI) media in a test tube, and incubated for 24 h at 37°C. Following visible turbidity, the suspension was standardized according to the Brown II standard containing 103–104 CFU/ml bacteria by diluting it using sterile NaCl three times in a dilution ratio of 1:9, resulting in the concentration of bacteria was 104 CFU/ml. After dilution, the bacteria planted could be spread evenly in the Petri dish.
A sterile cotton swab was dipped in the microorganism suspension and then smeared on the surface of the agar on a Petri dish evenly and waited to dry. This work was performed on seven Petri dishes. The evaluation was carried out by the diffusion method. In the diffusion method, four wells with a diameter of 6 mm were made using a perforator in each Petri. Each well was marked according to its group. Group 1A: glycerin at an interval time of 7 days. Group 1B: glycerin at 14 days. Group 2A: CHX at 7 days. Group 2B: CHX at 14 days. Group 3A: chitosan nanoparticles at 7 days. Group 3B: Chitosan nanoparticles at 14 days. Then all the Petri dishes were incubated at 37°C for 24 h. After incubation, the zones of microbial growth inhibition, which indicate antibacterial efficacy against E. faecalis, were assessed at the radical zone using a sliding caliper on a millimeter-scale with a precision of 0.02 mm, based on Figure 1 and the formula developed by Levinson.[15] The measurement of the radical zone of the well:
Figure 1.
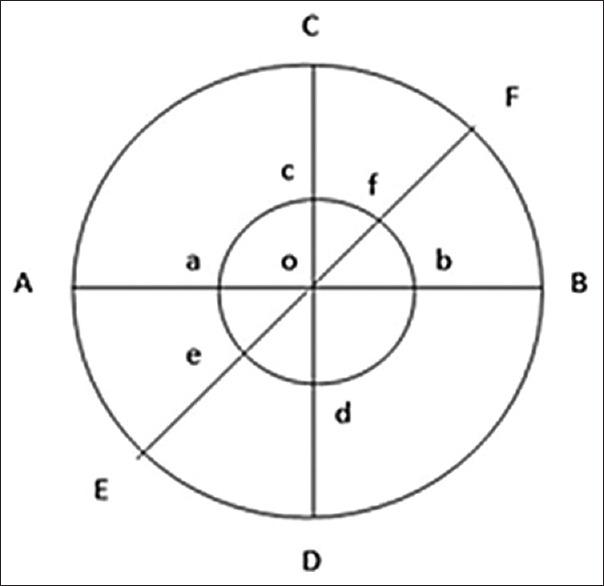
The measurement of inhibition zone; Point O: Center point of the well; Line AB, CD, and EF: Diameter of the radical zone; Line ab, cd and ef: Diameter of the well (6 mm)
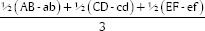
Preparation of samples for evaluation of calcium ion release and pH
Twenty-four single root premolars, straight rooted, intact, and free of caries, and the foramen apical diameter was standardized with a size 20 K-file (Dentsply, Maillefer, Ballaigues, Switzerland) was cut between the crown and the root to obtain a length of 12 mm using an IsoMetTM (Buehler Ltda, Lake Bluff, IL, USA). All teeth were subjected to root canal exploration and debridement using barbed broach (Dentsply, Maillefer). The working length was created 1 mm short of the apical foramen, and then the crown-down technique was performed using a rotary file (ProTaper Next, Dentsply) up to the #F3 file. Each file alteration was irrigated with 2 mL of 2.5% NaOCl (Golden Falcon, Dubai, UAE) with a continuous irrigation technique for 1 min. Final irrigation was performed at 2 mL in each root canal using 17% EDTA (PULPDENT, Watertown, MA, USA), then rinsed with 5 mL distilled water. Subsequently, the root canals were dried with paper points, and intracanal medicament was applied using a Lentulo spiral (Dentsply, Maillefer). The orifice of the root canal was then sealed with resin-modified glass ionomer cement (Fuji II LC, GC, Bunkyo-ku, Tokyo, Japan). All teeth were randomly assigned into groups based on the vehicles and time intervals described above. The tooth was then suspended in the small glass vial containing 10 ml distilled water with the aid of molding wax, and only the apical third of the roots were submerged in distilled water. All samples were then stored in an incubator at 37°C for 7 and 14 days.
Measurement of calcium ion release
The distilled water solution of 3 ml was taken periodically at interval times of 7 and 14 days from glass vial bottles. The distilled water was replaced with fresh distilled water each time taken. The distilled water solution taken was then analyzed by atomic absorption spectrometry (3110-PerkinElmer Inc., Waltham, MA, USA).
pH measurement
Measurement of pH was performed in media containing distilled water, which was used to immerse the apical third of the tooth, using a pH meter (DKK-TOA Corporation, Takadanobaba, Shinjuku-ku, Tokyo, Japan). Initially, a baseline was determined at the beginning of the immersion, and then pH was measured at interval times of 7 and 14 days.
Statistical analysis
Data obtained from each evaluation were assessed separately using the Shapiro–Wilk normality test for normal data distribution and Levene's test for homogeneity of variances. Then, data of each evaluation were analyzed using ANOVA followed by Tukey's test with a probability value (P value) <0.05 was considered statistically significant. All data were processed and analyzed using the Statistical Package for the Social Sciences software version 23 (IBM Inc., Chicago, IL, USA).
RESULTS
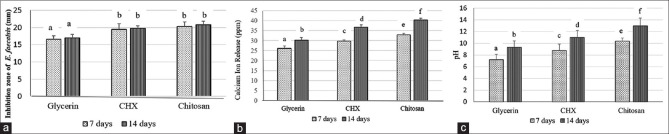
Figure 2a shows the antibacterial efficacy of glycerin, CHX, and chitosan nanoparticles as vehicles to calcium hydroxide against E. faecalis. It can be seen that chitosan nanoparticles had a significantly higher antibacterial efficacy compared to glycerin (P < 0.05), but an insignificant difference occurred between chitosan and CHX (P > 0.05).
Figure 2.
The mean and standard deviation. (a) Inhibition zone of glycerin, CHX, and chitosan nanoparticles as vehicles for calcium hydroxide, (b) Calcium ion release of glycerin, CHX, and chitosan nanoparticles as vehicles for calcium hydroxide, (c) pH of glycerin, CHX, and chitosan nanoparticles as vehicles for calcium hydroxide. Different letters indicate statistically significant differences
Figure 2b exhibits that chitosan nanoparticles produced the significantly greatest calcium ion release compared to glycerin and CHX (P < 0.05). Figure 2c also demonstrates that chitosan nanoparticles generated the highest pH than glycerin and CHX (P < 0.05). The interval time of 7 days exhibited significantly lower calcium ion release and pH in all vehicles compared to 14 days (P < 0.05), except for the antibacterial efficacy against E. faecalis (P > 0.05).
DISCUSSION
Intracanal medicaments are used to eradicate the microorganisms from the root canals following the biomechanical stage during root canal treatment. Calcium hydroxide has been used widely as an intracanal medicament; however, it has inadequate to remove microorganisms' cells within dentinal tubules. It is because calcium hydroxide has low solubility, diffusibility, and dentin-buffering capacity, which reduces its antimicrobial influence.[16] Therefore, calcium hydroxide powder requires vehicles to be combined to enhance its antibacterial efficacy and create a paste form, which can be applied easily into the root canal system.[17]
The purpose of using vehicles combined with calcium hydroxide powder was to produce a deliberation of Ca2+ and OH− ionic release, allow slow diffusion in the tissues with low solubility in tissue fluids, and have no adverse effect on the stimulation of hard tissue deposition.[18] The dissociation of calcium hydroxide into Ca2+ and OH− ions produces the calcium hydroxide's antibacterial effect. It is due to the hydroxyl ion (OH−) promoting an alkaline pH and interaction with the microbial cell wall and inactivating microbial by-products such as endotoxin. Therefore, the present studies focused on evaluating the vehicles mixed with calcium hydroxide, which are vital factors to induce dissociation of calcium ions, pH, and antibacterial effect of calcium hydroxide as an intracanal medicament.[19]
Since calcium hydroxide has deficient handling properties, the spreading throughout the canal is hard to accomplish uniformly. Accordingly, the consistency of vehicles combine with calcium hydroxide plays an important factor in enhancing clinical handling and reducing the rate of hydroxyl ion release, permitting intracanal medicament to the root canal system less frequently.[5]
Previous investigators reported that vehicles to be combined with calcium hydroxide must preserve the paste in consistency form, which does not set or harden, to enhance flow, sustain the high pH of calcium hydroxide, increase radiopacity, and not to change the biological properties of calcium hydroxide.[20] The study by Athanassiadis and Walsh[18] also showed that the vehicle type directly correlates with concentration, rate of ionic discharge, and antibacterial effect. In addition, the choice of the vehicle is related to increasing the removal of microorganisms within root canals, particularly E. faecalis, which is recognized as the most resistant endodontic microorganism, since E. faecalis has the capability to penetrate deep within the dentinal tubules, to create a biofilm that withstands with the alkaline pH of calcium hydroxide.[2]
This study used the agar diffusion method to evaluate antibacterial efficacy against E. faecalis since this technique has been widely used to evaluate the antibacterial activity of endodontic materials and offers many advantages, such as simplicity, low cost, the ability to test enormous numbers of microorganisms and antimicrobial agents, and the ease to interpret results provided.[21] In this study, the null hypothesis was rejected. The results showed that chitosan nanoparticles are one of the vehicles used in this study that produced the greatest antibacterial efficacy against E. faecalis compared to glycerin and CHX. These results are in agreement with the previous studies done by Shaik et al.[22] The efficacy of chitosan to eliminate microorganisms can be explained by two mechanisms. The first mechanism is that chitosan nanoparticles bind to the target cell membrane through electrostatic forces resulting in changes in the membrane, depolarization, and loss of membrane integrity. The important cell functions of the bacteria are disrupted, such as respiration, nutrient transport, and energy transduction resulting in bacterial cell death. The second mechanism is the production of free radicals such as reactive oxygen species, which can affect the resistance of bacterial cells by inhibiting protein function and damaging DNA.[23] Besides, chitosan nanoparticle is categorized as aqueous vehicles, which have rapid hydroxyl ions dissociation, achieving high pH values in the initial periods. In other words, aqueous suspension permits a more efficient release of hydroxyl ions. The results of this study are also in accordance with the findings of Grover and Shetty,[5] which showed that the highest calcium ion release and pH occurred in chitosan nanoparticles compared to the other two vehicles.
CXH, which is classified as an aqueous vehicle, produced the antibacterial efficacy against E. faecalis insignificantly compared to chitosan nanoparticles. The antibacterial property of CHX is ascribed to its cationic molecule, which adsorbed to the negatively charged inner cell membrane, inducing the leakage of intracellular elements, and resulting in cell death.[10,13] As an aqueous vehicle, CHX has a high degree of solubility when paste remains a direct contact with tissue and tissue fluids, but unfortunately, the handling property of CHX combined with calcium hydroxide powder is poor. Consequently, the distribution of intracanal medicament throughout the root canal is not uniform.[20] This condition may elucidate why the calcium ion release and pH were lower than the chitosan nanoparticles in this study.
Glycerin, which is classified as a viscous vehicle, comprises water-soluble substances. This property generates the release of calcium and hydroxyl ions more gradually for prolonged periods. Its solubility and diffusion are also low because it has a high-molecular weight.[10] This property may explain that glycerin has the lowest antibacterial efficacy against E. faecalis than other vehicles used in this study. The benefit of using glycerin is the ease of handling and applying of intracanal medicament to the root canal.[18] According to Grover and Shetty,[5] chitosan nanoparticles as a vehicle combined with calcium hydroxide produced intracanal medicament that makes handling and applying to root canal is better than CHX but lower than using glycerin.
This study showed that antibacterial efficacy against E. faecalis had similar results at 7 and 14 days, although calcium ion release and pH increased from 7 to 14 days. Other factors probably influence the antibacterial effect of calcium hydroxides, such as the level of hydrosolubility of the vehicle used, differences in viscosity, acid–base characteristics, dentin permeability, and the level of calcification present in the root canal system.[18] Other factors, including a substrate, bacterial species, biofilm models, assessment protocols, concentration, and interaction time may also influence the antibacterial effect of calcium hydroxide.[24]
Calcium ions act as an antibacterial effect due to the removing carbon dioxide used by bacteria respiration, particularly anaerobe bacteria, such as E. faecalis. Calcium ions also directly affect the mineralization of the extracellular matrix, hence inducing healing and repairing at the periapical area.[16] The number of calcium ions in root canals can activate Adenosine triphosphate (ATP), which plays an essential role in mineralization.[25] Chitosan nanoparticles also have chelating properties in the existence of calcium ions, which could enhance stability due to calcium ion attachment to the surface of chitosan nanoparticles.[3]
In the present study, 3% chitosan nanoparticles were used as a calcium hydroxide vehicle. Chitosan is insoluble in water and also at neutral, alkaline pH values, but it is soluble in organic acids such as acetic acid. A previous study reported that acetic acid prevents the growth of microorganisms at a concentration of 1% and higher; therefore, the lowest concentration used in this study was 0.1% to avoid any damaging or hindering effect on the viability of E. faecalis. Hence, in this study, chitosan gel was mixed with 0.1% acetic acid to attain a 3% chitosan solution.[14] In this study, Lentulo spiral instruments were employed to compactly fill the root canal spaces with intracanal medicaments since it is essential to condense homogeneous filling of intracanal medicament in the root canal space to amplify the antibacterial properties of intracanal medicaments.[26]
Chitosan nanoparticles in this study had the highest increased pH from 7 to 14 days, it is proved that chitosan has a better-continued release of hydroxyl ions compared to other vehicles.[9] Consequently, chitosan has the potential as a vehicle for calcium hydroxide in endodontic cases necessitating long-term intracanal medication. However, the results of this study are not in accordance with Del Carpio-Perochena et al.[3] who reported that the antibacterial efficacy of chitosan was not significantly different between 17 days and less period contact. These different results might be due to the method used in that study.
CONCLUSION
Calcium hydroxide combined with chitosan nanoparticle as an intracanal medicament produced the highest antibacterial efficacy against E. faecalis, calcium ion release, and pH than glycerin and CHX at intervals of 7 and 14 days. Thus, chitosan nanoparticle has the potential to be vehicle combined with calcium hydroxide in future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Garg A, Garg N. New Delhi: Jaypee Brothers Medical Publisher; 2014. Textbook of Endodontic. 3th ed. [Google Scholar]
- 2.Hussein MM, Abdallah AM, Mokhles NA, Meheissen MA. An ex vivo study to determine the antibacterial efficacy of chitosan nanoparticles, calcium hydroxide and double antibiotic paste as intracanal medicaments against Entrococcus faecalis biofilm. ENDO. 2019;13:255–63. [Google Scholar]
- 3.Del Carpio-Perochena A, Kishen A, Felitti R, Bhagirath AY, Medapati MR, Lai C, et al. Antibacterial properties of chitosan nanoparticles and propolis associated with calcium hydroxide against single- and multispecies biofilms: An in vitro and in situ study. J Endod. 2017;43:1332–6. doi: 10.1016/j.joen.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Tamanna S, Iftekhar H. Intracanal medicaments-their use in modern endodontics. A narrative review. J Oral Res Rev. 2019;11:89–94. [Google Scholar]
- 5.Grover C, Shetty N. Evaluation of calcium ion release and change in pH on combining calcium hydroxide with different vehicles. Contemp Clin Dent. 2014;5:434–9. doi: 10.4103/0976-237X.142803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barekatain B, Hasheminia SM, Shadmehr E, Attary Z. The effect of calcium hydroxide placement on pH and calcium concentration in periapical environment: An in vitro study. Indian J Dent Res. 2012;23:226–9. doi: 10.4103/0970-9290.100431. [DOI] [PubMed] [Google Scholar]
- 7.Attia DA, Farag AM, Afifi LK, Darrag AM. Antimicrobial effect of different intracanal medications on various microorganisms. Tanta Dent J. 2014;12:41–7. [Google Scholar]
- 8.Nalawade TM, Bhat K, Sogi SH. Bactericidal activity of propylene glycol, glycerin, polyethylene glycol 400, and polyethylene glycol 1000 against selected microorganisms. J Int Soc. 2015;5:114–9. doi: 10.4103/2231-0762.155736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anggani HS, Rusli V, Bachtiar EW. Chitosan gel prevents the growth of Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola in mini-implant during orthodontic treatment. Saudi Dent J. 2021;33:1024–8. doi: 10.1016/j.sdentj.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saatchi M, Navaei H, Maracy MR, Shojaei H. Antibacterial effect of calcium hydroxide combined with chlorhexidine on Enterococcus faecalis: A systemic review and meta-analysis. J Appl Oral Sci. 2014;22:356–65. doi: 10.1590/1678-775720140032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shetty C, Shetty A, Shetty S, Kaur G, Hedge MN, Nidhi L. Applications of chitosan in dentistry. Indian J Public Health Res Dev. 2020;11:89–95. [Google Scholar]
- 12.Del Carpio-Perochena A, Bramante CM, Duarte MA, de Moura MR, Aouada FA, Kishen A. Chelating and antibacterial properties of chitosan nanoparticles on dentin. Restor Dent Endod. 2015;40:195–201. doi: 10.5395/rde.2015.40.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal N, Dakshita JS, Udai PS, Kanwardeep S, Urja AJ, Shivika G. Evaluation of antibacterial effcacy of chitosan, chlorhexidine, propolis and sodium hypochlorite on Enterococcus faecalis bioflm: An in vitro study. J Clin Exp Dent. 2017;9:e1066–74. doi: 10.4317/jced.53777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikumar GP, Kumar RS, Bardia S, Geojan NE, Nishad G, Bhagat P. Antifungal effectiveness of various intracanal medicaments against Candida albicans: An in vitro study. J Contemp Dent Pract. 2020;21:1042–7. [PubMed] [Google Scholar]
- 15.Levinson W. New York: McGraw Hill; 2006. Review of Medical Microbiology and Immunology. 9th ed. [Google Scholar]
- 16.Abbott PV. Endodontics – Current and future. J Conserv Dent. 2012;15:202–5. doi: 10.4103/0972-0707.97935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinha N, Patil S, Dodwad PK, Patil AC, Singh B. Evaluation of antimicrobial efficacy of calcium hydroxide paste, chlorhexidine gel, and a combination of both as intracanal medicament: An in vivo comparative study. J Conserv Dent. 2013;16:65–70. doi: 10.4103/0972-0707.105302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Athanassiadis B, Walsh L. Aspects of solvent chemistry for calcium hydroxide medicament. Materials. 2017;10:1219–27. doi: 10.3390/ma10101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanomaru JM, Agular AP, Andrade GM, Bernardi MI, Filho MT. Antibacterial activity of intracanal medications based on calcium hydroxide and zinc oxide micro-or nanoparticles: An ex vivo study. Rev Odontol Unesp. 2017;46:153–7. [Google Scholar]
- 20.Kazemipoor M, Tabrizizadeh M, Dastani M, Hakimian R. The effect of retreatment procedure on the pH changes at the surface of root dentin using two different calcium hydroxide pastes. J Conserv Dent. 2012;15:346–50. doi: 10.4103/0972-0707.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6:71–9. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaik J, Garlapati R, Nagesh B, Sujana V, Jayaprakash T, Naidu S. Comparative evaluation of antimicrobial efficacy of triple antibiotic paste and calcium hydroxide using chitosan as carrier against Candida albicans and Enterococcus faecalis: An in vitro study. J Conserv Dent. 2014;17:335–9. doi: 10.4103/0972-0707.136444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soliman AY, Roshdy NB, Lutfy RA. Assessment of the effect of chitosan nanoparticles on the ultra-structure of dentinal wall: A comparative in vitro study. Acta Sci Dent. 2020;4:32–8. [Google Scholar]
- 24.Shresta A, Kishen A. Antibacterial nanoparticles in endodontics: A review. J Endod. 2016;42:1417–26. doi: 10.1016/j.joen.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Farhad A, Mohammadi Z. Calcium hydroxide: A review. Int Dent J. 2005;55:293–301. doi: 10.1111/j.1875-595x.2005.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 26.Ranga Reddy DS, Shankar NG, Venkatesan SM. Qualitative and quantitative analysis of intracanal delivery systems of calcium hydroxide: An in vitro study. J Sci Dent. 2013;3:8–14. [Google Scholar]