Abstract
Objective
Asthma in obese patients represents a specific phenotype that is associated with increased symptoms, more frequent and severe exacerbations, reduced responsiveness to treatment, and decreased quality of life. Marketing and placebos have been shown to alter subjective responses to interventions in both asthma and obesity. We evaluated obesity as a potential treatment effect modifier of the effects enhanced drug messaging or placebos on subjective asthma outcomes.
Methods
We conducted a secondary analysis of a multicenter, randomized clinical trial that studied the effect of messaging and placebos on asthma outcomes. A total of 601 participants were randomized (1:1:1:1:1) to one of 5 groups: enhanced messaging with montelukast or placebo, neutral messaging with montelukast or placebo, or usual care and followed for 4 weeks after randomization. We compared baseline characteristics by obesity status for 600 participants with data on body weight. Obesity was evaluated as an effect modifier for enhanced messaging (versus neutral messaging) and on placebo effects (versus usual care) in 362 participants assigned to a placebo group or usual care for three asthma questionnaires: Asthma Control Questionnaire, Asthma Quality of Life Questionnaire and Asthma Symptoms Utility Index.
Results
Overall, 227 (37%) of participants were obese. Obese participants were older (mean age 41 vs 34), more likely female (82% vs 67%) and self-identified as Black (44% vs 25%) than non-obese participants. As previously published, enhanced messaging was associated with improvements in patient-reported asthma scores, but there was no evidence for a placebo effect. Obesity status did not influence the message effects nor did it modify responses to placebo.
Conclusion
Obesity has been shown to be an important factor associated with asthma outcomes and an effect modifier of drug treatment effects. We conducted a post hoc, subgroup analysis of data from a multicenter randomized trial of enhanced messaging and placebo associated with drug treatment on asthma outcomes. Our findings suggest that observed differences in treatment effects between obese and non-obese patients sometimes seen in trials of asthma treatments are unlikely to be due to different “placebo” effects of treatment and may reflect differential physiologic effects of active agents.
Keywords: obesity, asthma, asthma questionnaires, messaging
Introduction
Asthma associated with obesity is associated with adverse asthma outcomes including poorer quality of life, more severe disease, increased daily symptoms, and more frequent exacerbations2–6 when compared to non-obese asthmatics. It is also well recognized that obese patients with asthma respond differently to treatment interventions,5,6 and that subjective and objective asthma measures in obese patients often do not correlate.7–9
We conducted a multicenter randomized study to evaluate the effects of placebos and enhanced messaging on responses to oral drugs (montelukast or placebo) in poorly controlled asthmatics. The primary outcomes were patient-reported outcomes, questionnaire scores; secondary outcomes were based on diary cards and spirometric measures of lung function. Overall, we found small, inconsistent placebo effects on asthma questionnaire scores and consistent effects of positive messaging on these outcomes in participants assigned to placebo. There were no placebo or messaging effects on lung function outcomes.
In this analysis, we evaluated obesity as a potential effect modifier. We did not include specific messaging targeting body weight or image in the positive messaging intervention for this trial, recognizing that ads emphasizing negative health consequences elicit stronger cognitive and emotional responses from overweight and obese adults and may have unintended impacts.34 We used images of a slender, active adult, which could produce differential responses to the contextual marketing cues (eg, appearance, activities, branding, labeling, price of a product) even if the cues related to obesity were unintentional. Contextual marketing cues can alter a patient’s subjective experience of benefit or harm of a medication—including asthma medications.1 Contextual marketing cues (eg, appearance, branding, labeling, price of a product) combined with placebo effects has been referred to as “marketing placebo effect”10,11 and response to marketing cues may be influenced by personal characteristics, like obesity.
The marketing placebo effect is thought to operate through the mesolimbic reward circuit, specifically the ventral tegmental area (VTA) where neurons are thought to play distinct roles in positive and negative reinforcement, decision-making, working memory, incentive salience, and aversion. These are the same brain regions implicated in overeating in the obese.12,33 The effective characteristics of marketing advertisements in obese patients, particularly those with asthma, is not well understood, but there is expanding evidence to suggest that marketing strategies can affect food intake and be a risk factor for body mass index.37 In both phenomenon, the stimulus (eg, branding, appearance, consumption of highly palatable food) stimulates dopamine activity and creates the feeling of pleasure and satisfaction.13–16 Placebos are reported to improve subjective and objective outcomes in up to 30–40% of patients in many clinical conditions, including asthma, hypertension, myocardial infarction, insomnia, and depression.17–19 In asthma, placebo effects have been observed to improve both patient-reported and physiologic outcomes; however, the magnitude of effect has been variable15,20–23 and little is known about the role of marketing placebo effects in patients with asthma and how it may differentially affect the response to asthma medication in obese and non-obese patients with asthma. Improving our understanding of the role of obesity and the marketing placebo effect can potentially provide insight into novel approaches to improving asthma outcomes in this hard to treat asthma population.
In this study, we aimed to quantify the effects of obesity on the relationship between enhanced drug messaging (ie, contextual marketing) and subjective asthma outcomes. We conducted a secondary analysis of the Trial of Asthma Patient Education (TAPE) trial1 that studied enhanced messaging and its effect on asthma outcomes.
Methods
The 20 centers of the American Lung Association Asthma Clinical Research Centers (ALA-ACRC) conducted the trial from December 2003 to December 2005. The ALA-ACRC Coordinating Center collected and analyzed the data. The trial was sponsored by the American Lung Association and NIH. All centers obtained and maintained IRB approval throughout the study. The trial complied with the Declaration of Helsinki. The trial was registered at ClinicalTrials.gov, NCT00148408 under the acronym Trial of Asthma Patient Education (TAPE).
Study Population
We conducted a secondary analysis of the TAPE trial1 including participants who had data on weight. The TAPE trial was a multicenter, randomized clinical trial conducted by the American Lung Association Asthma Clinical Research Centers from December 2003 to December 2005 at 20 sites across the United States. The methods and results were previously published.1 Briefly, the TAPE trial enrolled nonsmokers age 15 years or older with a history of physician diagnosed asthma and regular use of asthma medication, post-bronchodilator FEV1 of greater than 75% of predicted value, and at least one of the following indicators of poor asthma control: Asthma Control Questionnaire (ACQ) score >1.5, use of ß-agonists for asthma symptoms > twice per week, or more than one nocturnal awakening per week.
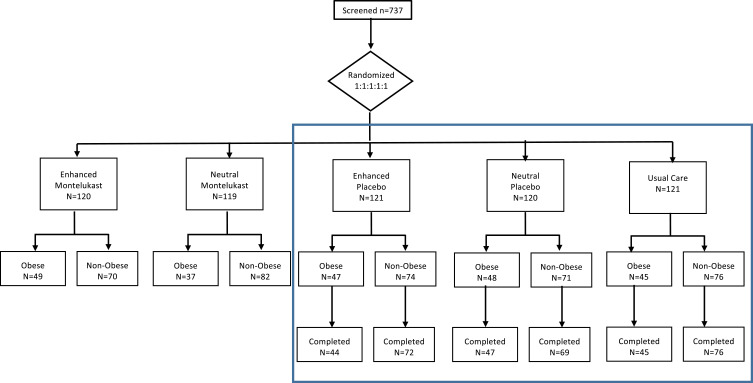
Participants were randomized to one of five groups: enhanced-message and montelukast 10mg, enhanced-message and placebo, neutral-message and montelukast 10mg, neutral-message and placebo, and usual care (no study drug or messaging intervention) (Figure 1). The usual care group received a NIH pamphlet on controlling your asthma. All participants had a two-week run in period for collection of baseline data and during which they documented asthma symptoms in a diary. At the randomization visit, participants, except for those assigned to usual care, viewed an informational computer-based presentation with enhanced drug messaging or neutral drug messaging. The drug messaging was repeated two weeks later. Outcomes were measured at two and four weeks after randomization.
Figure 1.
Consort diagram. Data from intervention groups included in blue box were included in the analysis of change in patient-reported outcome.
Messaging Intervention
The message intervention included 3 components: a scripted message, a computer presentation, and the appearance of the study drug capsule containing montelukast or placebo. The enhanced message script and computer presentation emphasized the benefits of the treatment and the likelihood of improved asthma symptoms. The value and potency of the study drug treatment was emphasized by including a television commercial for montelukast. In the enhanced presentation, the study drug was referred to using its brand name, Singulair®. The neutral script and computer presentation were similar to the enhanced but did not have the positive messages about the expected benefits of montelukast, expressed uncertainty about improvement, and did not contain the television commercial. In the neutral presentation study drug was referred to by the generic name, montelukast. The groups also received different colored capsules. The enhanced groups received a two-tone blue capsule containing montelukast or placebo while the neutral group received a monotone off-white capsule containing montelukast or placebo.
Baseline Variables
In this study, baseline BMI (basal metabolic index) >30 kg/m2 was used to define obesity and BMI <30 kg/m2 was used to define the non-obese participants.32 Baseline variables included demographic characteristics (ie, age, self-reported race and ethnicity, gender), body mass index, smoking history, socioeconomic factors (ie, educational attainment, employment status) and asthma characteristics (ie, age of asthma onset, frequency of short-acting beta-agonist use, frequency of inhaled corticosteroid inhaler use).
Outcomes
The primary outcomes for these analyses were change from baseline for three patient-reported asthma outcomes measured at baseline and two and four weeks after randomization: the Asthma Control Questionnaire (ACQ),24 the Asthma Symptom Utility Index (ASUI),25,26 and the Asthma Quality of Life Questionnaire (AQLQ).27–29 All questionnaires were previously validated and widely used in asthma research. ACQ assesses asthma control over one week; AQLQ and ASUI have a 2 week recall.24–29 The 6 item version of the ACQ score that does not include percent predicted FEV1 was calculated for this analysis.
Statistical Analysis
All trial participants were included in the comparison of baseline characteristics by obesity status (Figure 1). We used descriptive statistics to summarize participants baseline characteristics and Chi-square and Kruskal–Wallis tests to evaluate differences in characteristics between obese and non-obese participants.
Data from participants assigned to placebo with either an enhanced or neutral message were analyzed to evaluate whether obesity was a treatment modifier of message effects on patient-reported outcomes (Figure 1, blue box). Study outcomes were analyzed as change from baseline values at two visits (2 and 4 weeks post randomization) in linear regression, mixed effects models. Participant ID was modeled as a random effect in all models; the intervention (neutral versus enhanced), obesity and visit were fixed effects in unadjusted models; age, gender and race were included as fixed effects in adjusted models. Effect modification was evaluated by inclusion of interaction terms for intervention assignment and obesity status at follow-up visits.1
In the TAPE trial, the “placebo effect” was estimated by comparing those assigned to placebo with a neutral message versus the usual care group (Figure 1, blue box). Data from participants assigned to placebo with a neutral message or usual care were compared to evaluate obesity as a treatment modifier of the placebo effect on patient-reported outcomes. Mixed effects models as described previously were used to evaluate obesity as a treatment modifier of the placebo effect. In these models, the intervention was defined as neutral-placebo or usual care. Analyses were performed with SAS V9.
Results
A total of 601 study participants were enrolled in the trial between December 2003 and December 2005, 600 with data on weight at baseline were included in this analysis (Figure 1). Baseline characteristics are shown in Table 1. Overall 37% of participants were obese. Obese participants were on average older with higher proportions of Blacks and females compared to the non-obese participants, who were more likely to be students. Obese participants had worse scores on asthma questionnaires and a lower percent predicted FVC.
Table 1.
Baseline Characteristics
| Characteristic | Total (N= 600) | Non-Obese (N= 373) | Obese (N= 227) | P-value* |
|---|---|---|---|---|
| Age, Median(IQR) | 37 (26, 48) | 34 (24, 47) | 41 (30, 50) | <0.001 |
| Male, N (%) | 164 (27%) | 123 (33%) | 41 (18%) | <0.001 |
| Race or ethnic group, N (%) | ||||
| White | 349 (58%) | 242 (65%) | 107 (47%) | <0.001 |
| Black | 196 (33%) | 95 (25%) | 101 (44%) | |
| Hispanic | 37 (6%) | 23 (6%) | 14 (6%) | |
| Other | 18 (3%) | 13 (3%) | 5 (2%) | |
| BMI, Median(IQR) | 27 (24, 34) | 24 (22, 27) | 36 (33, 41) | <0.001 |
| Former smoker, N(%) | 88 (15%) | 45 (12%) | 43 (19%) | 0.021 |
| Socioeconomic | ||||
| Education, N(%) | ||||
| <High school | 49 (8%) | 28 (8%) | 21 (9%) | <0.001 |
| High school or equivalent | 86 (14%) | 42 (11%) | 44 (19%) | |
| Some college | 260 (43%) | 148 (40%) | 112 (49%) | |
| Bachelor’s Degree | 113 (19%) | 86 (23%) | 27 (12%) | |
| Some post graduate studies | 36 (6%) | 25 (7%) | 11 (5%) | |
| Post graduate degree | 56 (9%) | 44 (12%) | 12 (5%) | |
| Employment, N(%) | ||||
| Student | 99 (17%) | 81 (22%) | 18 (8%) | <0.001 |
| Not working outside the home | 62 (10%) | 32 (9%) | 30 (13%) | |
| Currently employed full time | 294 (49%) | 184 (49%) | 110 (48%) | |
| Currently employed part time | 70 (12%) | 42 (11%) | 28 (12%) | |
| Retired | 29 (5%) | 18 (5%) | 11 (5%) | |
| Disabled, unable to work | 24 (4%) | 6 (2%) | 18 (8%) | |
| Other | 22 (4%) | 10 (3%) | 12 (5%) | |
| Combined household income | ||||
| <$20,000 | 127 (21%) | 71 (19%) | 56 (25%) | 0.389 |
| $20,000-$50,000 | 184 (31%) | 110 (29%) | 74 (33%) | |
| >$50,000-$75,000 | 68 (11%) | 45 (12%) | 23 (10%) | |
| >$75,000 | 66 (11%) | 46 (12%) | 20 (9%) | |
| Declined to answer | 114 (19%) | 74 (20%) | 40 (18%) | |
| Unknown | 41 (7%) | 27 (7%) | 14 (6%) | |
| Asthma Characteristics | ||||
| Age of asthma onset, Median (IQR) | 13 (4, 29) | 12 (4, 25) | 18 (5, 35) | 0.007 |
| Use inhaled short-acting beta-agonist ≥2x per week | 505 (84%) | 319 (86%) | 186 (82%) | 0.243 |
| Daily use of ICS or ICS/LABA | 325 (54%) | 192 (51%) | 133 (59%) | 0.090 |
| Asthma Questionnaires, Median (IQR) | ||||
| ACQ6 Score (↓ range: 0–6) | 1.5 (0.8, 2.0) | 1.3 (0.8, 1.8) | 1.7 (1.0, 2.3) | <0.001 |
| AQLQ Score (↑ range: 0–7) | 5.1 (4.1, 5.9) | 5.4 (4.4, 6.0) | 4.7 (3.9, 5.4) | <0.001 |
| ASUI Score (↑ range: 0–1) | 0.80 (0.69, 0.88) | 0.83 (0.73, 0.89) | 0.77 (0.64, 0.84) | <0.001 |
| Spirometry, Median(IQR) | ||||
| Pre-BD FEV1‡ | 2.68 (2.23, 3.19) | 2.82 (2.40, 3.33) | 2.41 (2.00, 2.91) | <0.001 |
| Pre-BD %Predicted FEV1‡ | 86 (77, 94) | 86 (78, 95) | 86 (75, 93) | 0.172 |
| Post-BD FEV1‡ | 2.90 (2.43, 3.42) | 3.07 (2.61, 3.59) | 2.59 (2.18, 3.06) | <0.001 |
| Post-BD % Predicted FEV1‡ | 92 (84, 101) | 93 (85, 102) | 91 (81, 99) | 0.004 |
| % change FEV1 from BD‡ | 6.2 (3.0, 12.5) | 6.5 (3.4, 12.8) | 5.9 (2.2, 11.6) | 0.057 |
| Pre-BD FVC‡ | 3.55 (2.92, 4.27) | 3.75 (3.15, 4.53) | 3.19 (2.69, 3.84) | <0.001 |
| Pre-BD % Predicted FVC‡ | 94 (85, 103) | 95 (87, 105) | 91 (83, 101) | <0.001 |
| Post-BD FVC‡ | 3.65 (3.07, 4.43) | 3.87 (3.24, 4.61) | 3.29 (2.78, 3.99) | <0.001 |
| Post-BD % Predicted FVC‡ | 96 (89, 106) | 98 (90, 107) | 94 (86, 103) | <0.001 |
| % change FVC from BD‡ | 2.1 (−0.5, 5.3) | 1.8 (−0.2, 4.8) | 2.4 (−0.7, 6.5) | 0.602 |
| Pre-BD Peak Flow‡ | 380 (330, 450) | 390 (340, 460) | 370 (320, 430) | 0.023 |
| Pre-BD FEV1/FVC ratio‡ | 0.76 (0.69, 0.81) | 0.75 (0.69, 0.81) | 0.77 (0.71, 0.82) | 0.069 |
| Post-BD FEV1/FVC ratio‡ | 0.79 (0.74, 0.85) | 0.79 (0.73, 0.85) | 0.80 (0.75, 0.84) | 0.859 |
| % change from BD FEV1/FVC ratio‡ | 4.16 (1.63, 7.94) | 4.89 (2.01, 8.84) | 3.22 (1.34, 5.97) | <0.001 |
Notes: *P-values are based upon Chi-squared and Kruskal–Wallis tests for categorical and continuous characteristics, respectively. ↑ High scores indicate better health. ↓ Low scores indicate better health. ‡Predicted values for FEV1 and FVC are taken from: Hankinson JL et al.22
Abbreviations: SD, standard deviation; DI, metered dose inhaler; Neb, nebulizer; ICS, inhaled corticosteroids; LABA, long-acting beta-agonist; ACQ6, Asthma Control Score; ASUI, Asthma Symptom Utility Index; AQLQ, Asthma Quality of Life; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BD, bronchodilator.
Participants assigned to placebo with a neutral message (N = 120) or to placebo with an enhanced message (N = 121) were evaluated to determine whether obesity modified the effect of message intervention. Participants assigned to montelukast with neutral or enhanced messages were not included in this analysis because the effects of montelukast on these outcomes regardless of assigned message were significant,1 so the potential for effect modification related to obesity was fundamentally different in the groups receiving active drug versus placebo groups.
All participants assigned to placebo, obese and non-obese, had improvements in asthma scores during follow-up regardless of the message type. As reported for the TAPE trial, overall the ACQ6 score improvement in the enhanced message-placebo group was larger than in the neutral message-placebo group.1 The mean changes in the AQLQ and ASUI were also larger in the enhanced message-placebo group compared to improvements in the neutral message-placebo group, although the effects of message type on there two outcomes did not reach statistical significance of <0.05.1
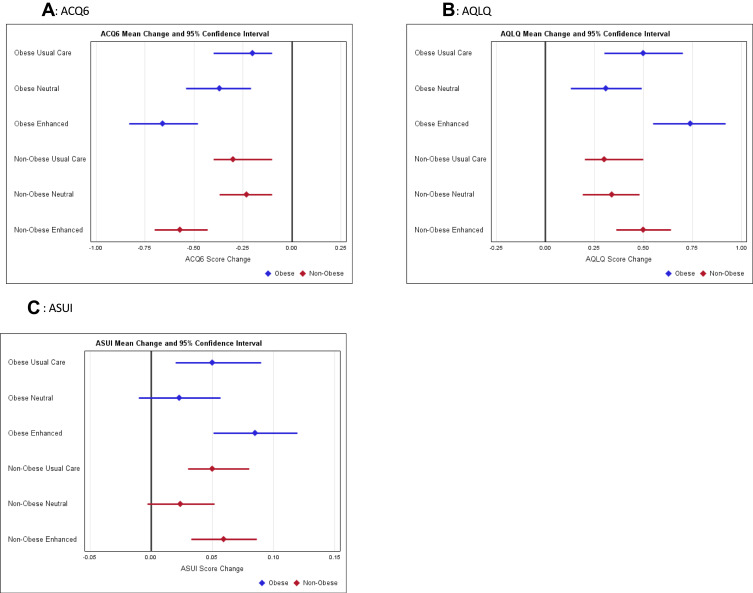
Similar proportions of participants were classified as obese in the enhanced message-placebo group (47 [40%]) as were in the neutral message-placebo group (47 [39%]) (Figure 1, blue box). In obese participants, the mean change in ACQ6 scores adjusted for age, gender and race was −0.6 (95% CI −0.8, −0.5) in enhanced message-placebo group versus −0.4 (95% CI −0.6, −0.2) in the neutral message-placebo group. In non-obese participants, the adjusted mean changes were −0.6 (95% CI −0.7, −0.4) in participants assigned to enhanced messaging with placebo versus −0.2 (95% CI −0.4, −0.1) in those assigned to the neutral message (Figure 2A and Supplemental Table 1). So, although there was an overall effect of messaging on change in ACQ6 scores (enhanced verus neutral message p-value=0.005) which exceeded the MCID of 0.5,36 there was no evidence of a difference in that effect by obesity status (interaction p-value=0.76). There also was no evidence to support the hypotheses that obesity status influenced the message effect on changes in AQLQ or ASUI scores (Figures 2B and C, and Supplemental Table 1).
Figure 2.
Change in scores by intervention group and obesity status, mean and 95% confidence intervals by messaging and obesity for (A) ACQ6, (B) AQLQ and (C) ASUI. Estimates of change from baseline values at two visits (2 and 4 weeks post randomization) from linear regression, mixed effects models.
In the TAPE trial, participants assigned to placebo with a neutral message (N = 120) were compared to those assigned to usual care (N = 121) to evaluate the placebo effect. Overall, there was no evidence to support a placebo effect on these patient-reported outcomes.1 Approximately equal proportions of participants were obese in the neutral message group (47 [39%]), as were in the usual care group (45 [37%]) (Figure 1). The adjusted mean changes in ACQ6 scores for obese participants were −0.3 (95% CI −0.5, −0.1) for participants assigned to the neutral message-placebo group versus −0.2 (95% CI −0.4, −0.0) in the usual care group. In non-obese participants, the adjusted mean changes were even more similar, −0.2 (95% CI −0.4, −0.1) in the neutral message-placebo group versus −0.2 (95% CI −0.3, −0.0) in the usual care group (Figure 2A and Supplemental Table 2). There was no evidence supporting effect that obesity status modified placebo effects on ACQ6, AQLQ nor ASUI scores (Figure 2A–C, Supplemental Table 2).
Discussion
In this secondary analysis of the TAPE trial,1 we confirmed findings of other researchers indicating obesity is associated with worse asthma scores and some measures of the lung function.2–6 The majority of obese participants in our study were Black, female, had late onset asthma, and a lower %predicted FVC than non-obese participants. Regardless, the effects of enhanced messaging on patient-reported asthma outcomes were similar in obese and non-obese participants. Neither obese nor non-obese participants showed evidence for placebo effects on these outcomes.
Consistent with earlier reports describing asthma in the obese population, our patients tended to have lower lung function and had higher asthma symptom scores than their non-obese counterparts.5,30,31 Despite these differences, obesity did not influence the placebo or message effects on patient-reported outcomes. There was no evidence to support a placebo effect on subjective asthma outcomes in either obese or non-obese patients. Nor was the effect of the enhanced message different in obese versus non-obese participants; both obese and non-obese participants assigned to the enhanced message had improved scores.
Our results seem to be in contrast to work by Peters-Golden in which 3073 individuals with moderate asthma enrolled in four studies were pooled. They demonstrated that although all individuals assigned to placebo improved in a pre-post comparison, the magnitude of the responses (asthma control days) in the placebo groups correlated negatively with increasing BMI.6 We also showed that obese and non-obese participants improved during follow-up regardless of treatment assignment, however we did not see evidence of a greater response in obese participants. Our analysis was more conservative than Peter-Golden since we used a dichotomous classification of obesity rather evaluate correlations between continuous measures. In addition, their study included relatively fewer obese patients (16%), fewer Black patients (3% overall and 6% of obese group), and almost half of study participants were non-USA citizens (45% overall and 58% of obese group). Another difference is the relatively short follow-up in our study.
However, they also demonstrated that placebo effects were more likely to modify the treatment effect of inhaled beclomethasone than of oral montelukast,6 which is consistent with our results of no intrinsic effect of obesity on treatment response. Hence, the need to consider obesity when designing controlled trials for asthma treatments or selecting treatments for asthmatics may depend more on the physiologic effects of the agents than systematic differences in treatment response due to obesity.
Additional limitations to this study include the post-hoc nature of the analysis and relatively small numbers as this was an exploratory secondary analysis. In addition, our sample only included asthmatics with mild airway obstruction and poorly controlled symptoms, and therefore, our findings may not be generalizable to the overall asthma population. However, this ensured we used strict criteria to define our patient population. Our follow-up period was short, 4 weeks, but we did observe improvements in asthma scores in all groups. We do not think that additional exposure to an enhanced message or placebo would have increased the observed effects. In a sub-study of 99 participants followed with electronic monitoring, enhanced messaging had a negative effect on treatment adherence when paired with an ineffective treatment (placebo) indicating that participants are less likely to continue ineffective treatments.35
In conclusion, our findings found that obesity did not influence the susceptibility to the effects of enhanced drug messaging on asthma-related symptoms and quality of life. Furthermore, we found no evidence to support a placebo effect on subjective asthma outcomes regardless of obesity status. Our findings suggest that addressing treatment needs for asthma patients with obesity—a hard to treat asthma phenotype—and highlight the need for future investigation.
Ethics and Consent Statement
The trial protocol, individual interventions and informed consent statements were reviewed and approved by local IRBs at all participating sites, including the Data Coordinating Center. No deception was used in the conduct of the trial. The informed consent document stated that “The purpose of this research study is to investigate the way that educational approaches and presentation of a drug may affect the response to montelukast and placebo (an inactive medication) in subjects with asthma. You are being asked to join this research study because you have asthma that causes you symptoms with your current treatment.”
Disclosure
Dr Anne M Mathews was affiliated with Duke University during the conduct of the study and is now affiliated with CU-Anschutz. Dr Robert A Wise reports personal fees from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers, Chemerx, Chiesi, Puretech, Contrafect, FSD Pharma, Merck, GSK, Savara, Vaxart, Galderma; grants and/or personal fees from Verona, Sanofi, GSK, 4Dx, AbbVie, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Wise RA, Bartlett SJ, Brown ED, et al.; American Lung Association Asthma Clinical Research Centers. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association asthma clinical research centers. J Allergy Clin Immunol. 2009;124(3):436–44, 444e1–8. PMID: 19632710; PMCID: PMC2948850. doi: 10.1016/j.jaci.2009.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rönmark E, Andersson C, Nyström L, Forsberg B, Järvholm B, Lundbäck B. Obesity increases the risk of incident asthma among adults. Eur Respir J. 2005;25(2):282–288. PMID: 15684292. doi: 10.1183/09031936.05.00054304 [DOI] [PubMed] [Google Scholar]
- 3.Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol. 2010;108(3):729–734. PMID: 19875708; PMCID: PMC2838637. doi: 10.1152/japplphysiol.00845.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosen DM, Schatz M, Magid DJ, Camargo CA Jr. The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122(3):507–11.e6. PMID: 18774387. doi: 10.1016/j.jaci.2008.06.024 [DOI] [PubMed] [Google Scholar]
- 5.Dixon AE, Shade DM, Cohen RI, et al.; American Lung Association-Asthma Clinical Research Centers. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma. 2006;43(7):553–558. PMID: 16939998. doi: 10.1080/02770900600859123 [DOI] [PubMed] [Google Scholar]
- 6.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27(3):495–503. PMID: 16507848. doi: 10.1183/09031936.06.00077205 [DOI] [PubMed] [Google Scholar]
- 7.Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the Normative Aging Study. Thorax. 2002;57(7):581–585. PMID: 12096199; PMCID: PMC1746377. doi: 10.1136/thorax.57.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56(1):4–8. PMID: 11120896; PMCID: PMC1745919. doi: 10.1136/thorax.56.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sin DD, Stafinski T, Ng YC, Bell NR, Jacobs P. The impact of chronic obstructive pulmonary disease on work loss in the United States. Am J Respir Crit Care Med. 2002;165(5):704–707. PMID: 11874818. doi: 10.1164/ajrccm.165.5.2104055 [DOI] [PubMed] [Google Scholar]
- 10.Enax L, Weber B. Marketing placebo effects – from behavioral effects to behavior change. J Agriculture & Food Ind Org. 2015;13(1):15–31. doi: 10.1515/jafio-2015-0015 [DOI] [Google Scholar]
- 11.Enax L, Hu Y, Trautner P, Weber B. Nutrition labels influence value computation of food products in the ventromedial prefrontal cortex. Obesity. 2015;23(4):786–792. PMID: 25755174. doi: 10.1002/oby.21027 [DOI] [PubMed] [Google Scholar]
- 12.Barry D, Clarke M, Petry NM. Obesity and its relationship to addictions: is overeating a form of addictive behavior? Am J Addict. 2009;18(6):439–451. PMID: 19874165; PMCID: PMC2910406. doi: 10.3109/10550490903205579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutile S, Kaptchuk TJ, Wechsler ME. The placebo effect in asthma. Curr Allergy Asthma Rep. 2014;14(8):456. PMID: 24951239; PMCID: PMC4351653. doi: 10.1007/s11882-014-0456-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenberg SA, Lehrer PM, Hochron S. The effects of suggestion and emotional arousal on pulmonary function in asthma: a review and a hypothesis regarding vagal mediation. Psychosom Med. 1992;54(2):192–216. PMID: 1565756. doi: 10.1097/00006842-199203000-00006 [DOI] [PubMed] [Google Scholar]
- 15.Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg-Smith SM, Kline JN. Placebo response in asthma: a robust and objective phenomenon. J Allergy Clin Immunol. 2007;119(6):1375–1381. PMID: 17451796. doi: 10.1016/j.jaci.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 16.Kaptchuk TJ, Kelley JM, Deykin A, et al. Do “placebo responders” exist? Contemp Clin Trials. 2008;29(4):587–595. PMID: 18378192. doi: 10.1016/j.cct.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 17.Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159(17):1602–1606. PMID: 13271123. doi: 10.1001/jama.1955.02960340022006 [DOI] [PubMed] [Google Scholar]
- 18.Brown WA. The placebo effect. Sci Am. 1998;278(1):90–95. PMID: 9418301. doi: 10.1038/scientificamerican0198-90 [DOI] [PubMed] [Google Scholar]
- 19.Lasagna L. The placebo effect. J Allergy Clin Immunol. 1986;78(1 Pt 2):161–165. PMID: 3722643. doi: 10.1016/0091-6749(86)90008-4 [DOI] [PubMed] [Google Scholar]
- 20.Joyce DP, Jackevicius C, Chapman KR, McIvor RA, Kesten S. The placebo effect in asthma drug therapy trials: a meta-analysis. J Asthma. 2000;37(4):303–318. PMID: 10883741. doi: 10.3109/02770900009055454 [DOI] [PubMed] [Google Scholar]
- 21.Spector SL. Asthma and bronchitis. Compr Ther. 1976;2(3):26–35. PMID: 767046. [PubMed] [Google Scholar]
- 22.McFadden ER Jr, Luparello T, Lyons HA, Bleecker E. The mechanism of action of suggestion in the induction of acute asthma attacks. Psychosom Med. 1969;31(2):134–143. PMID: 5784000. doi: 10.1097/00006842-196903000-00007 [DOI] [PubMed] [Google Scholar]
- 23.Luparello T, Lyons HA, Bleecker ER, McFadden ER Jr. Influences of suggestion on airway reactivity in asthmatic subjects. Psychosom Med. 1968;30(6):819–825. PMID: 5726052. doi: 10.1097/00006842-196811000-00002 [DOI] [PubMed] [Google Scholar]
- 24.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. PMID: 10573240. doi: 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- 25.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma symptom utility index. Chest. 1998;114(4):998–1007. PMID: 9792568. doi: 10.1378/chest.114.4.998 [DOI] [PubMed] [Google Scholar]
- 26.Bime C, Nguyen J, Wise RA. Measures of asthma control. Curr Opin Pulm Med. 2012;18(1):48–56. PMID: 22081089; PMCID: PMC7274081. doi: 10.1097/MCP.0b013e32834db0f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32–38. PMID: 10489826. doi: 10.1034/j.1399-3003.1999.14a08.x [DOI] [PubMed] [Google Scholar]
- 28.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47(2):76–83. PMID: 1549827; PMCID: PMC463574. doi: 10.1136/thx.47.2.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis. 1993;147(4):832–838. PMID: 8466117. doi: 10.1164/ajrccm/147.4.832 [DOI] [PubMed] [Google Scholar]
- 30.Teague WG, Phillips BR, Fahy JV, et al. Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6(2):545–554.e4; PMID: 28866107; PMCID: PMC5832534. doi: 10.1016/j.jaip.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang M, Henderson RJ, Holbrook JT, et al. Does obesity increase respiratory tract infections in patients with asthma? J Allergy Clin Immunol Pract. 2019;7(3):954–961.e6. PMID: 30312805; PMCID: PMC6609096. doi: 10.1016/j.jaip.2018.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization: Obesity and overweight. Fact sheet; 2021. Available from:http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed April 26, 2021.
- 33.Yohn SE, Galbraith J, Calipari ES, Conn PJ. Shared behavioral and neurocircuitry disruptions in drug addiction, obesity, and binge eating disorder: focus on group I mGluRs in the mesolimbic dopamine pathway. ACS Chem Neurosci. 2019;10(5):2125–2143. PMID: 30933466; PMCID: PMC7898461. doi: 10.1021/acschemneuro.8b00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon H, Scully M, Durkin S, et al. Finding the keys to successful adult-targeted advertisements on obesity prevention: an experimental audience testing study. BMC Public Health. 2015;15:804. PMID: 26290169; PMCID: PMC4546051. doi: 10.1186/s12889-015-2159-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clerisme-Beaty EM, Bartlett SJ, Teague WG, et al. The Madison Avenue effect: how drug presentation style influences adherence and outcome in patients with asthma. J Allergy Clin Immunol. 2011;127(2):406–411. PMID: 21281871; PMCID: PMC3050545. doi: 10.1016/j.jaci.2010.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. PMID: 15823451. doi: 10.1016/j.rmed.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 37.Cohen DA, Collins R, Hunter G, Ghosh-Dastidar B, Dubowitz T. Store impulse marketing strategies and body mass index. Am J Public Health. 2015;105(7):1446–1452. PMID: 25521881; PMCID: PMC4463383. doi: 10.2105/AJPH.2014.302220 [DOI] [PMC free article] [PubMed] [Google Scholar]