Abstract
Fermentation capacity of microbial ecosystems intrinsically depends on substrate supply and the ability of a microbial community to deliver monomers for fermentation. In established microbial ecosystems, the microbial community is adapted to efficiently degrade and ferment available biopolymers which is often concurrently reflected in the richness of the microbial community and its functional potential. During the first year of life, the human gut microbial environment is a rather dynamic system that is characterized by a change in physiological conditions (e.g. from aerobic to anaerobic conditions, physical growth of the gastrointestinal tract, development of the intestinal immune system) but also by a change in nutrient supply from a compositionally limited liquid to a diverse solid diet, which demands major compositional and functional changes of the intestinal microbiota. How these transitions link to intestinal microbial fermentation capacity has gained comparatively little interest so far. This mini‐review aims to collect evidence that already after birth, there is seeding of a hidden population of various fermentation organisms which remain present at low abundance until the cessation of breastfeeding removes nutritional restrictions of a liquid milk‐based diet. The introduction of solid food containing plant and animal material is accompanied by an altering microbiota. The concurrent increases in the abundance of degraders and fermenters lead to higher intestinal fermentation capacity indicated by increased faecal levels of the final fermentation metabolites propionate and butyrate. Recent reports indicate that the development of fermentation capacity is an important step during gut microbiota development, as chronic disorders such as allergy and atopic dermatitis have been linked to lower degradation and fermentation capacity indicated by reduced levels of final fermentation metabolites at 1 year of age.
Breastfeeding has a major impact on infant gut microbiota composition and fermentation activity during the first months of life. Yet infants are also colonized by low abundant populations of butyrate and propionate producers who increase in abundance once the dietary constraints of breastfeeding have been lifted leading to an increase in intestinal microbial fermentation capacity.

GASTROINTESTINAL FERMENTATION
Microbial fermentative systems occur in natural (e.g. soil, wetlands, gastrointestinal tract) and artificial (e.g. anaerobic digester, industrially produced fermented food) environments and are often coupled to methanogenesis. A complete degradation of carbohydrates through combined fermentation and methanogenesis theoretically mainly yields the gases CH4 and CO2 (Symons & Buswell, 1933; Tarvin & Buswell, 1934). Such optimal systems are dependent on factors such as carbohydrate supply and fermentation time and rarely occur in nature.
In the mammalian gastrointestinal tract, intestinal microbial degraders, which are predominantly bacteria accounting for 1010–1011 cells g−1 faeces and to a much lesser extent a fluctuating, low diversity population yeast and fungi (Nash et al., 2017; Raimondi et al., 2019), release mono‐, di‐, and oligosaccharides from undigested complex carbohydrates (e.g. plant derived and possibly technologically processed [resistant] starches, cellulose, hemicellulose, arabinoxylan, pectin) through the activity of carbohydrate active enzymes (CAZY) including glycosyl hydrolases (GH), glycosyl transferases (GT), polysaccharide lyases (PL) and carbohydrate esterases (CE) (Wardman et al., 2022).
Fermenting bacteria, yeast and fungi produce propionate or butyrate, or the fermentation intermediates lactate, acetate, succinate, formate and 1,2‐propanediol which can be metabolized by fermentation specialists to propionate and butyrate (Figure 1) (Louis & Flint, 2017). Alternative metabolic pathways lead to the formation of propionate from lactate (acrylate pathway), succinate (succinate pathway) and 1,2‐propanediol (propanediol pathway, Reichardt et al., 2014); some specialists are capable of the conversion of lactate and acetate to butyrate (Duncan et al., 2004). Methanogenic archaea use fermentation derived gases H2 and the terminal electron acceptors CO2, acetate or formate to produce CH4 (Figure 1) (Gaci et al., 2014). In the human gastrointestinal tract, microbes not only compete for substrates but also rely on trophic interactions, that is, a nutrient‐related relationship, to gain energy. Cross‐feeding networks are thus an essential component of fermentative systems. Fermentation capacity is tightly linked not only to external factors, such as the supply of carbohydrates, but also to intrinsic factors including the metabolic cross‐feeding potential of the microbial ecosystem.
FIGURE 1.

Scheme indicating the succession of microbial degradation, fermentation and methanogenesis of dietary carbohydrates. Microbial communities in anaerobic ecosystems consisting of degraders, fermenters and methanogens interact (i) in releasing fermentable monosaccharides from complex carbohydrates, (ii) fermenting and cross‐feeding on fermentation metabolites and (iii) delivering substrates for methanogens. Overall fermentation capacity, that is, the potential to form C02 and CH4 from carbohydrates, is determined by microbial community composition and functional diversity and fermentation potential. In infants, fermentation capacity increases during weaning.
The mammalian gastrointestinal tract is a dynamic system with intermittent and compositionally changing nutrient supplies (Pereira & Berry, 2017). Nutrient alterations are especially severe during the first year of life, when a physiologically developing gut system hosting a community of intestinal microbes is exposed to a dietary switch from a liquid breast milk or formula‐based diet to solid food that gradually increases in compositional complexity. Yet, these transitions occur in most cases without obvious delay, and without major apparent adverse impact on the infant. The longitudinal development of the human gut microbiota during the first year(s) of life can thus act as a model system to study how changing of environmental conditions including alterations in dietary supply lead to shifts in microbial taxonomic community composition while concurrently increasing intestinal fermentation capacity. While the focus of this review is set on the role of carbohydrate‐based nutrients, short chain fatty acids (SCFAs) can also be produced during fermentation of amino acids, but that likely constitutes only a minor proportion of overall fermentation activity (Vital et al., 2017).
BREASTFEEDING AND HUMAN MILK OLIGOSACCHARIDES DRIVE MICROBIAL COMMUNITY COMPOSITION BEFORE WEANING
During recent years, several longitudinal cohort studies not only using 16S rRNA gene sequencing or metagenomics but also (targeted) cultivation have largely contributed to the investigation of the infant gut microbiota development (Bäckhed et al., 2015; Ferreti et al., 2018; Pham et al., 2016; Stewart et al., 2018). The infant gut microbiota evolves from an initially diverse and heterogeneous composition during the first days, to a simpler, and finally a complex adult like community at around 2 to 3 years of age (Ferretti et al., 2018; Tsukuda et al., 2021). This non‐random and sequential transition is driven by nutrient availability and composition, the establishment of an anaerobic environment and microbial interactions (Bäckhed et al., 2015). First, colonizers are transmitted vertically during birth or breastfeeding through skin contact or milk, or are obtained from the hospital environment (Ferretti et al., 2018). Immediately after birth, facultative anaerobes, mainly Enterococcus, Staphylococcus, Streptococcus spp. and members of the family Enterobacteriaceae reach high densities (Bäckhed et al., 2015; Obermajer et al., 2017), which and mainly use mono‐ and disaccharides for growth. Initial colonization and concurrent oxygen depletion allow for the growth of strict anaerobic bacteria including Bifidobacteriaceae (Bäckhed et al., 2015; Obermajer et al., 2017).
Concurrently, breastfeeding provides a strong nutritive selective factor due to its content in human milk oligosaccharides (HMOs, 5–15 g L−1), that is, complex glycans not digested by the host that can be degraded and metabolized by a group of highly adapted intestinal bacteria. HMOs are composed of the five monomeric building blocks, d‐glucose (Glc), d‐galactose (Gal), N‐acetyl‐d‐glucosamine (Glc‐NAc), l‐fucose (Fuc) and sialic acid (Sia) (Figure 2) (Bode & Jantscher‐Krenn, 2012). All HMOs contain lactose (Galβ1–4Glc) at the reducing end. Lactose can be elongated by the addition of the disaccharides lacto‐N‐biose (Galβ1–3GlcNAc) or N‐acetyllactosamine (Galβ1–4GlcNAc). Di‐ and oligosaccharides can be modified with Fuc in α1–2‐, α1–3‐, or α1–4‐linkages, or with Sia in α2–3‐ or α2–6‐linkages (Bode & Jantscher‐Krenn, 2012). HMO content and composition change during lactation phases (Plows et al., 2021). Two‐ and 3‐fucosyllactose (2‐FL and 3‐FL) are usually among the most abundant HMOs (Plows et al., 2021). Milk of mothers that lack the fucosyltransferase 2 encoding gene (fut2) do not secrete α1–2 fucosylated HMO and might have overall lower HMO content (Azad et al., 2018). Around 95% of HMOs reach the colonic environment. Microbial degradation and fermentation of these structurally and compositionally diverse HMOs asks for a specialized repertoire of GH enzymes and for additional metabolic pathways to ferment the HMO monomers l‐fucose (Bunesova et al., 2016; Tsukuda et al., 2021) and sialic acid (Egan et al., 2014). Specialization for HMO degradation and fermentation is widespread among infant‐associated Bifidobacterium species such as Bifidobacterium bifidum, Bifidobacterium breve and Bifidobacterium longum subsp. infantis (Lawson et al., 2020; Tsukuda et al., 2021). In contrast to B. longum subsp. infantis, strains of B. bifidum possess extracellular GH and release HMO monomers to the intestinal microbial community for cross‐feeding (Garrido et al., 2012). Intra‐genus cross‐feeding of Bifidobacterium species in the presence of sialyllactose and fucosyllactose has been reported (Egan et al., 2014; Lawson et al., 2020; Schwab et al., 2017, Tsukuda et al., 2021).
FIGURE 2.

Overview of major dietary carbohydrates before (A) and after (B) weaning. Undigested dietary carbohydrates of breastfed infants are mainly human milk oligosaccharides composed of five different monomers (A). For comparison, examples of structurally and compositionally diverse plant‐polysaccharides are shown which are introduced to the food plan during the weaning phase (B).
The dominance of Bifidobacterium spp. in the infant gut might explain the lower overall microbial richness of breastfed infant gut microbiota compared with that of formula‐fed infants observed in some studies (Ferretti et al., 2018; Stewart et al., 2018). HMOs are likely supplied in surplus as spent HMOs and fucose were recovered in faeces with concentrations decreasing during the first months of breastfeeding (Borewicz et al., 2020; Sasaki et al., 2022). The faecal presence of HMOs and fucose suggests that the carbohydrate‐dependent energy needs of the Bifidobacterium‐dominated intestinal microbiota are fulfilled.
HMO UTILIZATION BY BIFIDOBACTERIA DEFINES FAECAL FERMENTATION METABOLITE PROFILES
Microbial composition and fermentation activity of major microbial groups during the breastfeeding phase is tightly linked to faecal fermentation profiles. Bifidobacterium spp. produce mainly acetate and lactate via the bifid shunt, and/or form formate and ethanol from pyruvate as alternative to lactate (de Vuyst et al., 2013). Accordingly, acetate is SCFA produced immediately after birth constituting up to 94% of the total major SCFAs (acetate, propionate and butyrate) in faeces until 3 months of age (Table 1).
TABLE 1.
Levels of major SCFAs acetate, propionate and butyrate in infant faeces during the first year of life
| Age | Acetate | Propionate | Butyrate | Total SCFA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Levels (μmol/g) a | Proportion of Total SCFAs (%) | Occurrence (%) | Levels (μmol/g) a | Proportion of Total SCFAs (%) | Occurrence (%) | Levels (μmol/g) a | Proportion of Total SCFAs (%) | Occurrence (%) | Levels (μmol/g) a | |
| 0–2 weeks | 80–89 | 86–98 | 100 | 0.9–1.7 | 1–11 | 54–84 | 0.2–1.3 | 0–3 | 26–92 | 82–92 |
| 1 month | 69–130 | 87–98 | 100 | 6–7 | 2–10 | 74–92 | 1.9–2 | 1–3 | 46–90 | 79–139 |
| 3 months | 35–110 | 71–94 | 100 | 4–9 | 5–18 | 58–100 | 1–5 | 0–10 | 35–100 | 49–106 |
| 6 months | 37–110 | 64–95 | 100 | 7–20 | 5–23 | 88–100 | 3–7 | 0–13 | 63–100 | 48–133 |
| 12 months | 32–100 | 59–75 | 100 | 9–25 | 1.6–22 | 99–100 | 1.6–22 | 3–24 | 69–100 | 54–137 |
The fermentation intermediates lactate and formate were regularly detected in faecal samples collected from infants during the first year of life (Pham et al., 2016; Tsukuda et al., 2021). Levels of lactate and formate were higher before than after weaning (Tsukuda et al., 2021). In addition, 1,2‐propanediol, a metabolite of Bifidobacterium l‐fucose metabolism (Bunesova et al., 2016), accumulated in faeces of breast milk‐fed infants (He et al., 2019) suggesting that bifidobacteria were driving HMO degradation and fermentation to acetate, lactate, 1,2‐propanediol and formate. The faecal accumulation of fermentation metabolites lactate, succinate and formate indicates the limited overall fermentation capacity of the infant gut microbiota during the breastfeeding period.
CAN EARLY OCCURRING INTESTINAL PROPIONATE AND BUTYRATE PRODUCERS UTILIZE HMOS OR HMOS DEGRADATION PRODUCTS?
Fermentation of breast milk oligosaccharides selects for a Bifidobacterium‐dominated profile with mainly acetate, lactate, formate and 1,2‐propanediol recovered as faecal fermentation metabolites. Utilization of acetate and lactate, or 1,2‐propanediol would allow specialized cross‐feeders to produce butyrate (Duncan et al., 2004) or propionate (Schwab et al., 2017), respectively, and indeed several longitudinal studies repeatedly observed faecal propionate and butyrate in meconium or as early as 1–2 weeks after birth (Appert et al., 2020, Gio‐Batta et al., 2020, Nilsen et al., 2020; Norin et al., 2004; Pham et al., 2016; Tsukuda et al., 2021; Table 1). Before weaning, levels of propionate and butyrate were usually significantly lower than of acetate only representing a maximum of 10–18% of total SCFAs. Until 6 months of age, the concentration of propionate was generally higher than of butyrate (Table 1). While not all infants were identified as producers of propionate and butyrate based on faecal analysis (Table 1), the reliable and repeated recovery of low levels of propionate and butyrate from infant faeces gives strong indication that butyrate and propionate producers are a metabolically active part of the infant gut microbiota already days and weeks after birth.
Among the early detected succinate/propionate producers were members of the Bacteroidota (Bäckhed et al., 2015). This phylum includes taxa that are specialized in degrading complex plant polysaccharides or the endogenous mucin (Salyers et al., 1977), a heavily glycosylated protein that is secreted by the goblet cells of the intestinal epithelium (Pelaseyed et al., 2014). In addition, the Bacteroides species B. fragilis and B. vulgatus were capable of metabolizing HMOs, and were able to degrade a broad range of HMOs in comparison to Bifidobacterium, which preferred short chain HMOs (Marcobal et al., 2010). Succinate, a metabolite of Bacteroidota fermentation, was observed in faeces of breastfed and formula‐fed infants and thus both in the presence and absence of HMOs (Bridgman et al., 2017; He et al., 2019) suggesting that the occurrence of Bacteroidota does not rely on HMO. Nonetheless, in the presence of HMOs, Bifidobacterium spp. might be more competitive in vivo (Marcobal et al., 2011). Human milk oligosaccharides degradation has also been reported for the mucin degrading propionate producer Akkermansia muciniphila and B. bifidum. Similar to Bacteroides, A. muciniphila and B. bifidum employ the same enzymes for mucus and for HMOs degradation (Kostopoulos et al., 2020; Marcobal et al., 2011; Turroni et al., 2010). Occurrence and abundance of A. muciniphila was nevertheless reported as low at 3 and 6 months (Sasaki et al., 2022).
Gut intestinal butyrate producers encompass a taxonomically and functionally diverse group mainly within the phylum Bacillota and the families Lachnospiraceae, Ruminococcocaceae, Clostridiaceae and Peptostreptococcaceae. There are two major pathways that use either butyryl‐CoA: acetate CoA‐transferase (ButCoAT) or butyrate kinase (Buk) in the final step of the butyrate formation pathway (Louis & Flint, 2017). Endospore‐forming members of the Clostridiaceae and Peptostreptococcaceae, which rely on Buk, are likely the butyrate forming pioneers within the infant gut microbiota (Appert et al., 2020; Rada et al., 2008; Tsukuda et al., 2021; Vital et al., 2017). Members of both families are anaerobes but can tolerate oxygen to various extent (Browne et al., 2016), and are often saccharolytic and proteolytic with access to a wide variety of substrates (Wiegel et al., 2006). Strains of Clostridiaceae (e.g. Clostridium perfringens) and Peptostreptococcaceae (e.g. Clostridoides difficile) showed an overall reduced efficiency to produce butyrate from glucose when compared with ButCoAT‐depending Faecalibacterium prausnitzii (Ruminococcaceae), Eubacterium rectale /Roseburia spp. and Anaerobutyricum hallii (Lachnospiraceae) during growth in yeast extract‐casitone‐fatty acid medium with glucose as the only carbohydrate source (Appert et al., 2020). Nevertheless, Clostridiaceae were more abundant in faeces of infants before weaning and might account for the rather low faecal butyrate levels that were observed (Appert et al., 2020). In addition, the occurrence of Clostridiaceae and Peptostreptococcaceae might be inversely related to breastfeeding and the presence of Bifidobacterium (Bäckhed et al., 2015; Rada et al., 2008). Strains of C. perfringens and C. difficile did not, or only poorly grew in the presence of 2‐fucosyllactose (Salli et al., 2021) indicating that HMOs are an unlikely carbohydrate source. Some strains of C. perfringens grew in the presence of fucose indicating the potential for cross‐feeding (Salli et al., 2021).
Human milk oligosaccharides degradation and utilization has been reported for butyrate producing Roseburia hominis or Roseburia inulivorans (Pichler et al., 2020), yet such species were only present at low abundance before weaning constituting around 3–4 log cells g−1 faeces (Appert et al., 2020). Similarly, the butyrate‐producing F. prausnitzii was only detectable at low abundance (Appert et al., 2020), and was not able to utilize HMOs or to cross‐feed on bifidobacteria HMOs degradation metabolites in vitro (Cheng et al., 2020). The cross‐feeding specialist A. hallii, which formed butyrate and propionate in co‐cultures with bifidobacteria and 2‐FL (Schwab et al., 2017) in vitro, was only detected at low abundance and not in all infants at 3 and 6 months (Appert et al., 2020) but could contribute to SCFA formation.
Taken together, these observations show that propionate and butyrate producers with the potential for HMO utilization and cross‐feeding are present during the first months. The ability to use HMOs as alternative or secondary substrate might support the colonization or seeding of mucin utilizing propionate producers such as the genera Bacteroides and Akkermansia or of primary degraders and butyrate producers including R. hominis, R. inulivorans and F. prausnitzii (Lopez‐Siles et al., 2012; Scott et al., 2014) during infant age. Such an early colonization might prime the intestinal microbial community for the development of degradation and fermentation capacity during and after weaning. The presence of such hidden functional populations seems not to be limited to degrading and fermenting microorganisms but might also include methanogens. Hudson and Roberts (1993) identified 20% of 1‐week old infants as carriers of methanogens using a cultivation dependent‐enrichment procedure with a detection limit of >102 cells g−1.
THE DIET CHANGE DURING WEANING LEADS TO HIGHER FERMENTATION CAPACITY
With the introduction of solid food, the diversity and complexity of dietary carbohydrates increases (Figures 2 and 3). In addition, the omission of the selective pressure of a HMO containing breast milk diet slowly reduces the competitive advantage of HMOs utilizers. Studies reported either a decreasing or a stable abundance of Bifidobacterium during or after weaning, possibly due to continuation of supplementary breastfeeding. Concurrently, the abundance of degraders of complex carbohydrates increased in the months after the switch to solid feeding (Appert et al., 2020; Nilsen et al., 2020; Roger & McCartney, 2010). Plant‐derived carbohydrates introduce novel dietary hexoses (e.g. d‐mannose, d‐fructose) and pentoses (e.g. l‐arabinose, d‐xylose), and sugar acids (d‐glucuronic and d‐galacturonic acid) that are connected through a variety of α‐ and β‐type linkages (Figure 2). Additionally, plant carbohydrates contain a variety of non‐carbohydrate components such as acetyl and methyl groups, hydroxycinnamic acid or borate (Figure 2). The efficient degradation of these polymers asks for a more complex CAZY machinery including GH, PL and CE that are hosted by specialist microbial degraders, and is connected to a change in faecal fermentation profiles.
FIGURE 3.
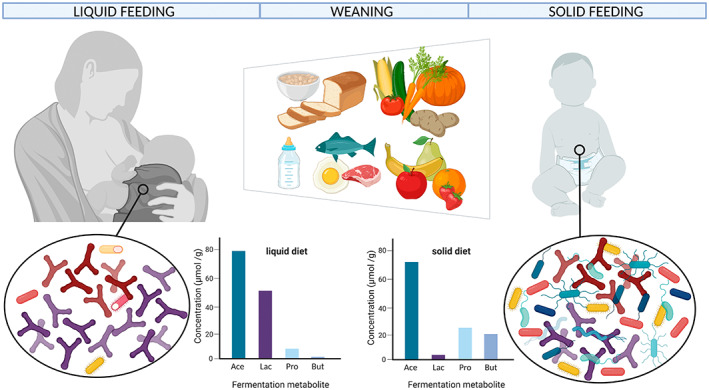
Scheme depicting the changes of microbial communities and faecal fermentation profiles that occur with the introduction of solid food. The intestinal microbiota changes from a Bifidobacterium dominated microbiota that produces mostly acetate and lactate to compositionally and functionally more diverse microbial communities with the introduction of solid food. This transformation leads to increasing faecal levels of propionate and butyrate. Figure was prepared using biorender. Ace, acetate; but, butyrate; lac, lactate; pro, propionate.
The transition process that leads to increased fermentation capacity after weaning takes several months. Infant age at the beginning of complementary feeding of any solids or non‐water liquids might affect microbial fermentative response. In a previous study, an early start of complementary feeding (≤3 months) was associated with higher faecal butyrate and propionate levels at 12 months compared with introduction of complementary feeding at 3 months of age (Differding et al., 2020). There was no detectable difference of SCFA levels at 3 months shortly after the start of complementary feeding (Differding et al., 2020) indicating the time‐consuming response of the infant gut microbiota.
The faecal SCFA with most dramatic higher levels after the introduction of solid food is butyrate together with major butyrate producers such as E. rectale/Roseburia spp., F. prausnitzii, and A. hallii becoming more abundant (Appert et al., 2020; Nilsen et al., 2020; Vital et al., 2017). Strains of E. rectale and Roseburia are able to hydrolyze and ferment structurally and compositionally different carbohydrates while F. prausnitzii can use pectin (Lopez‐Siles et al., 2012; Scott et al., 2014). The ability of A. hallii to utilize complex dietary carbohydrates is limited, but members of this species can cross‐feed on fermentation intermediates (Schwab et al., 2017).
The change of diet during weaning is tightly linked to alterations of the faecal microbial community and of fermentation profiles. With the introduction of solid food, the α‐diversity of faecal microbiota increases (Appert et al., 2020; Bäckhed et al., 2015). In infants from Western countries, a lower β‐diversity of weaned infants indicated the development of a more homogenous microbiota (Bäckhed et al., 2015).
THE IMPACT OF BIRTH MODE AND BREASTFEEDING ON FERMENTATION CAPACITY
It is likely that any disturbances at the beginning of life that impact the seeding of microbes, or dietary alternatives to breastfeeding affect the development of fermentation capacity. Seeding of the infant gut microbiota is affected by birth mode, which also impacts microbiota development during the first year of life (Bäckhed et al., 2015; Reyman et al., 2019). However, breastfeeding is a confounding factor, as frequently a lower proportion of caesarean born infants is breastfed, and for a reduced duration (Reyman et al., 2019). A study that compared faecal SCFA profiles in exclusively (30%), partly (42%) and non‐breastfed (28%) infants (n = 158) at 4 months found significant differences depending on breastfeeding status but no association with birth mode (Bridgman et al., 2017). Infants that were still exclusively breastfed at 4 months had lower levels of propionate and butyrate, and higher levels of lactate compared with non‐breastfed infants. In contrast, faecal levels of butyrate were higher in caesarean‐born compared with vaginally delivered infants in a study with 70 participants and infants that were breastfed for a median of around 1 month only (Mueller et al., 2021).
Then again, differences in faecal microbiota composition and fermentation metabolites between non, partly and exclusively breastfed infants indicate that, as soon as the nutritional pressure of breastfeeding and HMOs consumption is reduced or stopped, fermentation capacity increased indicated by higher propionate and butyrate, and lower lactate levels, and that this might already happen before weaning. Concurrently, in exclusively formula fed infants, faecal microbial α‐diversity was higher compared with breastfed infants (Bäckhed et al., 2015; Bridgman et al., 2017; Ho et al., 2018), preceding observations made during and after weaning.
ALTERATIONS OR DISTURBANCES IN FERMENTATION CAPACITY DEVELOPMENT MIGHT BE LINKED TO HEALTH OUTCOMES LATER IN LIFE
While the comparatively limited fermentation capacity of the infant microbiota during the breastfeeding period might not be detrimental to health outcomes, the potential for butyrate formation after weaning seems beneficial for health in later life and has been negatively linked to the occurrence of chronic diseases such as allergy and atopic dermatitis (AD) (Cait et al., 2019; Roduit et al., 2019). Sandin et al. (2009) reported that lower levels of butyrate at 1 year were associated with food allergy at 4 years. While the cause has not been completely clarified, the risk of developing AD was lower in infants with higher faecal butyrate at 1 year of age (Roduit et al., 2019).
Recent studies observed that lower butyrate formation capacity of infants with AD might be linked to primary degradation potential, as there was lower abundance in CAZY related to HMO degradation in infants with AD at 3 months and of CAZY associated with plant‐cell wall, animal and fungal carbohydrates at 1 year (Cait et al., 2019). These differences in CAZY profiles might be indicative of a disrupted carbohydrate degradation and cross‐feeding scheme which might impact the development of fermentation capacity. In another study, the abundance of the starch‐degrader Ruminococcus bromii and of the mucing‐utilizing A. muciniphila was lower at 1 year (Sasaki et al., 2022) in infants AD compared with infants without AD, which again links health status to alterations in carbohydrate degradation capacity.
CONCLUSION
This mini‐review aims to provide a perspective on how the gastrointestinal fermentation system develops from a comparatively limited fermentation state that is characterized by the accumulation of fermentation intermediates to a more complex fermentation system. The surplus supplies of HMOs during breastfeeding not only provide competitive advantage for HMO degrading specialists but also limit fermentation pathways. Seeding of a low abundant population of more diverse fermentation specialists even before the introduction of solid food prepares the microbial community for the diet change that occurs during weaning. The origin of these strict anaerobes has not been completely clarified. Recent metagenome studies reliably identified strains of Bifidobacterium, Bacteroides and Enterobacteriaceae including E. coli (Asnicar et al., 2017; Ferretti et al., 2018; Li et al., 2021) as being vertically transmitted during the first weeks after birth but did rarely report the transmission of any butyrate producers. This gap might be due to low faecal abundance of taxa such as Faecalibacterium, Roseburia and A. hallii in infant faeces, which interferes with their detectability in shotgun metagenome sequencing based analysis. Novel targeted cultivation approaches might enable to isolate and characterize such low abundant community members (Watterson et al., 2021). A second possibility is the transfer of endospore formers. Browne et al. (2016) estimated that up to 50% of taxa present in adult humans are able to form endospores. As the occurrence of chronic diseases has been linked to alterations in fermentation capacity compared with healthy control infants, future efforts can be directed towards a targeted seeding of degraders and fermenters, for example as probiotics, to ensure the development of a functional intestinal microbial fermentation system.
AUTHOR CONTRIBUTIONS
clarissa schwab: Conceptualization (lead); writing – original draft (lead); writing – review and editing (lead).
FUNDING INFORMATION
No funding information provided.
CONFLICT OF INTEREST
There is no conflict of interest to declare.
Schwab, C. (2022) The development of human gut microbiota fermentation capacity during the first year of life. Microbial Biotechnology, 15, 2865–2874. Available from: 10.1111/1751-7915.14165
REFERENCES
- Appert, O. , Ramirez‐Garcia, A. , Frei, R. , Roduit, C. , Constancias, F. , Neuzil‐Bunesova, V. et al. (2020) Initial butyrate producers during infant gut microbiota development are endospore formers. Environmental Microbiology, 22, 3909–3921. [DOI] [PubMed] [Google Scholar]
- Asnicar, F. , Manara, S. , Zolfo, M. , Truong, D.T. , Scholz, M. , Armanini, F. et al. (2017) Studying vertical microbiome transmission from mothers to infants by strain‐level metagenomic profiling. mSystems, 2, e00164–e00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad, M.B. , Robertson, B. , Atakora, F. , Becker, A.B. , Subbarao, P. , Moraes, T.J. , et al. (2018) Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. Journal of Nutrition, 148(11), 1733–1742. Available from: 10.1093/jn/nxy175 [DOI] [PubMed] [Google Scholar]
- Bäckhed, F. , Roswall, J. , Peng, Y. , Feng, Q. , Jia, H. , Kovatcheva‐Datchary, P. et al. (2015) Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host & Microbe, 17, 690–703. [DOI] [PubMed] [Google Scholar]
- Bode, L. & Jantscher‐Krenn, E. (2012) Structure‐function relationships of human milk oligosaccharides. Advances in Nutrition, 3, 383 S–391 S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borewicz, K. , Gu, F. , Saccenti, E. , Hechler, C. , Beijers, R. , de Weerth, C. et al. (2020) The association between breastmilk oligosaccharides and faecal microbiota in healthy breastfed infants at two, six, and twelve weeks of age. Scientific Reports, 10, 4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman, S.L. , Azad, M.B. , Field, C.J. , Haqq, A.M. , Becker, A.B. , Mandhane, P.J. et al. (2017) Fecal short‐chain fatty acid variations by breastfeeding status in infants at 4 months: differences in relative versus absolute concentrations. Frontiers in Nutrition, 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, H.P. , Forster, S.C. , Anonye, B.O. , Kumar, N. , Neville, B.A. , Stares, M.D. et al. (2016) Culturing of ‘unculturable'human microbiota reveals novel taxa and extensive sporulation. Nature, 533, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunesova, V. , Lacroix, C. & Schwab, C. (2016) Fucosyllactose and L‐fucose utilization of infant Bifidobacterium longum and Bifidobacterium kashiwanohense . BMC Microbiology, 16, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cait, A. , Cardenas, E. , Dimitriu, P.A. , Amenyogbe, N. , Dai, D. , Cait, J. et al. (2019) Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. The Journal of Allergy and Clinical Immunology, 14, 1638–1647. [DOI] [PubMed] [Google Scholar]
- Cheng, L. , Kiewiet, M.B.G. , Logtenberg, M. , Groeneveld, A. , Nauta, A. & Schols, H.A. (2020) Effects of different human milk oligosaccharides on growth of bifidobacteria in monoculture and co‐culture with Faecalibacterium prausnitzii . Frontiers in Microbiology, 11, 569700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst, L. , Moens, F. , Selak, M. , Rivière, A. & Leroy, F. (2013) Summer meeting 2013: growth and physiology of bifidobacteria. Journal of Applied Microbiology, 116, 477–491. [DOI] [PubMed] [Google Scholar]
- Differding, M.K. , Benjamin‐Neelon, S.E. , Hoyo, C. , Østbye, T. & Mueller, N.T. (2020) Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiology, 20, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, S.H. , Louis, P. & Flint, H.J. (2004) Lactate‐utilizing bacteria, isolated from human feces, that produce butyrate as major fermentation product. Applied and Environmental Microbiology, 70, 5810–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan, M. , O'Connell Motherway, M. , Ventura, M. & van Sinderen, D. (2014) Metabolism of sialic acid by Bifidobacterium breve UCC2003. Applied and Environmental Microbiology, 80, 4414–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti, P. , Pasolli, E. , Tett, A. , Asnicar, F. , Gorfer, V. , Fedi, S. et al. (2018) Mother‐to‐infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host & Microbe, 11, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaci, N. , Borrel, G. , Tottey, W. , O'Toole, P.W. & Brugère, J.F. (2014) Archaea and the human gut: new beginning of an old story. World Journal of Gastroenterology, 20, 16062–16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, D. , Barile, D. & Mills, D.A. (2012) A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Advances in Nutrition, 3, 415 S–421 S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gio‐Batta, M. , Sjöberg, F. , Jonsson, K. , Barman, M. , Lundell, A.‐C. , Adlerberth, I. et al. (2020) Fecal short chain fatty acids in children living on farms and a link between valeric acid and protection from eczema. Scientific Reports, 10, 22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Parenti, M. , Grip, T. , Lönnerdahl, B. , Timby, N. , Domellöf, M. et al. (2019) Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast‐fed infants as reference: a randomized controlled trial. Scientific Reports, 9, 11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, N.T. , Li, F. , Lee‐Sarwar, K.A. , Tun, H.M. , Brown, B.P. , Pannaraj, P.S. et al. (2018) Meta‐analaysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nature Communications, 9, 4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, M.J. & Roberts, A.K. (1993) Establishment of methanogens in the infant intestine. Microbial Ecology in Health and Disease, 6, 301–308. [Google Scholar]
- Kostopoulos, I. , Elzinga, J. , Ottman, N. , Klievink, J.T. , Blijenberg, B. , Aalvink, S. et al. (2020) Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro . Scientific Reports, 10, 14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, M.A.E. , O'Neill, I.J. , Kujawska, M. , Javvadi, S.G. , Wijeyesekera, A. , Flegg, Z. et al. (2020) Breast milk‐derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. The ISME Journal, 14, 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Tapiainen, T. , Brinkac, L. , Lorenzi, H.A. , Moncera, K. , Tejesvi, M.V. et al. (2021) Vertical transmission of gut microbiome and antimicrobial resistance genes in infants exposed to antibiotics at birth. The Journal of Infectious Diseases, 224, 1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Siles, M. , Khan, T.M. , Duncan, S.H. , Harmsen, H.J.M. , Garcia‐Gil, L.J. & Flint, H.J. (2012) Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host‐derived substrates for growth. Applied and Environmental Microbiology, 78, 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, P. & Flint, H.J. (2017) Formation of propionate and butyrate by the human colonic microbiota. Environmental Microbiology, 19, 29–41. [DOI] [PubMed] [Google Scholar]
- Marcobal, A. , Barboza, M. , Froehlich, J.W. , Block, D.E. , Bruce German, J. , Lebrilla, C.B. et al. (2010) Consumption of human milk oligosaccharides by gut‐related microbes. Journal of Agricultural and Food Chemistry, 58, 5334–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal, A. , Barboza, M. , Sonnenburg, E.D. , Pudlo, N. , Martens, E.C. , Desai, P. et al. (2011) Bacteroides in the infant gut consume milk oligosaccharides via mucus‐utilization pathways. Cell Host and Microbe, 10, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, N.T. , Differding, M.K. , Østbye, T. , Hoyo, C. & Benjamin‐Neelon, S.E. (2021) Association of birth mode of delivery with infant faecal microbiota, potential pathobionts, and short chain fatty acids: a longitudinal study over the first year of life. BJOG, 128, 1293–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash, A.K. , Auchtung, T.A. , Wong, M.C. , Smith, D.P. , Jonathan, R.G. , Rose, M.C. et al. (2017) The gut mycobiome of the human microbiome project healthy cohort. Microbiome, 5, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen, M. , Saunders, C.M. , Angell, I.L. , Arntzen, M.Ø. , Lødrup Carlsen, K.C. , Carlsen, K.‐H. et al. (2020) Butyrate levels in the transition from an infant to an adult‐like gut microbiota correlate with bacterial networks associated with Eubacterium rectale and Ruminococcus gnavus . Genes, 11, 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norin, E. , Midtvedt, T. & Björkstén, B. (2004) Development of faecal short‐chain fatty acid pattern during the first year of life in Estonian and Swedish infants. Microbial Ecology in Health and Disease, 16, 8–12. [Google Scholar]
- Obermajer, T. , Grabnar, I. , Benedik, E. , Tušar, T. , Robič Pikler, T. , Fidler Mis, N. et al. (2017) Microbes in infant gut development: placing abundance within environmental, clinical and growth parameters. Scientific Reports, 7, 11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaseyed, T. , Bergström, J.H. , Gustafsson, J.K. , Ermund, A. , Birchenough, G.M.H. , Schütte, A. et al. (2014) The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunological Reviews, 260, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, F. & Berry, D. (2017) Microbial nutrient niches in the gut. Environmental Microbiology, 19, 1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, V.T. , Lacroix, C. , Braegger, C.P. & Chassard, C. (2016) Early colonization of functional groups of microbes in the infant gut. Environmental Microbiology, 18, 2246–2258. [DOI] [PubMed] [Google Scholar]
- Pichler, M.J. , Yamada, C. , Shuoker, B. , Alvarez‐Silva, C. , Gotoh, A. , Leth, M.L. et al. (2020) Butyrate producing colonic Clostridiales metabolise human milk oligosaccharides and cross‐feed on mucin via conserved pathways. Nature Communications, 11, 3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plows, J.F. , Berger, P.K. , Jones, R.B. , Alderete, T. , Yonemitsu, C. , Najera, J.A. et al. (2021) Longitudinal changes in human milk oligosaccharides (HMOs) over the course of 24 months of lactation. The Journal of Nutrition, 8, 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada, V. , Nevoral, J. , Trojanová, I. , Tománková, E. , Šmehilova, M. & Killer, J. (2008) Growth of infant faecal bifidobacteria and clostridia on prebiotic oligosaccharides in in vitro conditions. Anaerobe, 14, 205–208. [DOI] [PubMed] [Google Scholar]
- Raimondi, S. , Amaretti, A. , Gozzoli, C. , Simone, M. , Righini, L. , Candeliere, F. et al. (2019) Longitudinal survey of fungi in the human gut: its profiling, phenotyping, and colonization. Frontiers in Microbiology, 10, 1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt, N. , Duncan, S.H. , Young, P. , Belenguer, A. , McWilliam Leitch, C. , Scott, K.P. et al. (2014) Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. The ISME Journal, 8, 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyman, M. , van Houten, M.A. , van Baarle, D. , Bosch, A.A.T.M. , Man, W.H. , Chu, M.L.J.M. et al. (2019) Impact of delivery mode‐associated gut microbiota dynamics on health in the first year of life. Nature Communications, 10, 4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roduit, C. , Frei, R. , Ferstl, R. , Loeliger, S. , Westermann, P. , Rhyner, C. et al. (2019) High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy, 74, 799–809. [DOI] [PubMed] [Google Scholar]
- Roger, L.C. & McCartney, A.L. (2010) Longitudinal investigation of the faecal microbiota of healthy full‐term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology, 156, 3317–3328. [DOI] [PubMed] [Google Scholar]
- Salli, K. , Hirvonen, J. , Siitonen, J. , Ahonen, I. , Anglenius, H. & Maukonen, J. (2021) Selective utilization of the human milk oligosaccharides 2′‐fucosyllactose, 3‐fucosyllactose, and difucosyllactose by various probiotic and pathogenic bacteria. Journal of Agricultural and Food Chemistry, 69, 170–182. [DOI] [PubMed] [Google Scholar]
- Salyers, A.A. , Vercellotti, J.R. , West, S.E. & Wilkins, T.D. (1977) Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Applied and Environmental Microbiology, 33, 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin, A. , Braback, L. , Norin, E. & Bjorksten, B. (2009) Faecal short chain fatty acid pattern and allergy in early childhood. Acta Paediatrica, 98, 823–827. [DOI] [PubMed] [Google Scholar]
- Sasaki, M. , Schwab, C. , Ramirez Garcia, A. , Li, Q. , Ferstl, R. , Bersuch, E. et al. (2022) The abundance of Ruminococcus bromii is associated with faecal butyrate levels and atopic dermatitis in infancy. Allergy available as e‐paper . [DOI] [PMC free article] [PubMed]
- Schwab, C. , Ruscheweyh, H.‐J. , Bunesova, V. , Pham, V.T. , Beerenwinkel, N. & Lacroix, C. (2017) Trophic interactions of infant bifidobacteria and Eubacterium hallii during L‐fucose and fucosyllactose degradation. Frontiers in Microbiology, 8, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, K.P. , Martin, J.C. , Duncan, S.H. & Flint, H.J. (2014) Prebiotic stimulation of human colonic butyrate‐producing bacteria and bifidobacteria, in vitro . FEMS Microbiology Ecology, 87, 30–40. [DOI] [PubMed] [Google Scholar]
- Stewart, J. , Ajami, N.J. , O'Brien, J.L. , Hutchinson, D.S. , Smith, D.P. , Wong, M.C. et al. (2018) Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature, 562, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons, G.E. & Buswell, A.M. (1933) The methane fermentation of carbohydrates. Journal of the American Chemical Society, 55, 2028–2036. [Google Scholar]
- Tarvin, D. & Buswell, A.M. (1934) The methane fermentation of organic acids and carbohydrates. Journal of the American Chemical Society, 56, 1751–1755. [Google Scholar]
- Tsukuda, N. , Yahagi, K. , Hara, T. , Watanabe, Y. , Matsumoto, H. , Mori, H. et al. (2021) Key bacteria taxa and metabolic pathways affecting gut short‐chain fatty acid profiles in early life. The ISME Journal, 15, 2574–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni, F. , Bottacini, F. , Foroni, E. , Mulder, I. , Kim, J.‐H. , Zomer, A. et al. (2010) Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host derived glycan foraging. PNAS, 107, 19514–19519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital, M. , Karch, A. & Pieper, D.H. (2017) Colonic butyrate‐producing communities in humans: an overview using omics data. mSystems, 2, e00130–e00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman, J.F. , Bains, R.K. , Rahfeld, P. & Withers, S.G. (2022) Carbohydrate‐active enzymes (CAZymes) in the human gut microbiome. Nature Reviews. Microbiology, 20, 542–556. [DOI] [PubMed] [Google Scholar]
- Watterson, W.J. , Tanyeri, M. , Watson, A.R. , Cham, C.M. , Shan, Y. et al. (2021) Droplet‐based high‐throughput cultivation for accurate screening of antibiotic resistant gut microbes. eLife Sciences, 9, e56998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegel, J. , Tanner, R. & Rainey, F.A. (2006) An introduction to the family Clostridiaceae. In: Falkow, S. , Rosenberg, E. , Schleifer, K.‐H. & Stackebrandt, E. (Eds.) The prokaryotes. New York, NY: Springer, pp. 654–678. [Google Scholar]


