Abstract
We previously identified 18 stimulatory Chlamydia trachomatis major outer membrane protein (MOMP) peptides containing at least 23 epitopes presented with various HLA class II allotypes. Only one peptide contained an epitope localized in a variable segment (VS2). Continued studies reported here identified a total of five VS peptides containing T-cell epitopes that are distributed among MOMPs VS1, VS2, and VS4. Only MOMP-primed T-cell cultures from subjects infected with serovar E responded to the serovar E VS peptides, while the response of such cultures to constant-segment peptides was independent of the infecting serovar. Furthermore, MOMP-primed T cells proliferated in response only to the VS peptides encoded in serovar E but not to the corresponding peptides derived from serovar F, I, or J, confirming that these responses were serovar specific.
Chlamydia trachomatis causes ocular and genital tract infections worldwide. In the United States, the annual cost of treating sexually transmitted diseases caused by C. trachomatis is billions of dollars (9). Effective preventive measures against C. trachomatis infections are not yet available, due in part to insufficient understanding of human immune responses to C. trachomatis. Evidence for protective immunity against C. trachomatis is scant and inconclusive. However, earlier studies have suggested that short-lived immunity develops after an episode of chlamydial infection (1, 10, 12) and that protection may be serovar specific (reviewed in reference 8); while reinfection with C. trachomatis is common, it is usually not with serovars of preceding infections. Consequently, many immunological studies of human genital tract infections have been focused on the major outer membrane protein (MOMP) because C. trachomatis serovars are defined by serological variation in this protein. In humans, MOMP elicits C. trachomatis-neutralizing antibodies that recognize its surface-exposed variable segments (VSs), where amino acid sequences vary substantially among serovars (4). Therefore, some have speculated that B-cell responses directed toward serovar-specific epitopes located in VSs of MOMP are a critical component of protective immunity (16–18).
Because of the role of T helper (Th) cells in priming and maintaining B-cell responses, we have investigated HLA class II-restricted Th cell responses to MOMP in humans genitally infected with C. trachomatis, focusing on commonly involved serovar E. The main results of our first study (14) were that (i) in vitro priming of peripheral blood mononuclear cells (PBMC) with MOMP yielded MOMP-specific Th cell cultures from 83% of serovar E-infected subjects; (ii) proliferative responses of T-MOMP′ cultures to synthetic peptides localized at least 23 Th cell epitopes, and T-MOMP′ cultures from most subjects responded to multiple epitopes; (iii) multiple HLA-DR and other HLA class II molecules were used to present the MOMP epitopes to the Th cells; and (iv) remarkably, 22 of the 23 epitopes were distributed among the five MOMP constant segments (CS) and only 1 epitope was clearly located in a VS. The CS epitopes activated MOMP′ T cells regardless of the serovar infecting the donors, but the epitope localized in VS2-overlapping peptide 145-163 activated cells from only serovar E-infected subjects.
The potential importance of MOMP VSs in protective immunity prompted us to investigate further the puzzling apparent scarcity of VS Th cell epitopes. We report here that Th cell responses to MOMP VSs are at least fairly frequent in C. trachomatis-infected subjects and that Th cell epitopes are located in VS1, -2, and -4, being undetected only in VS3.
The methodology used to locate the VS epitopes was based closely on that detailed in reference 14. Male and female subjects who had confirmed genital tract infections with C. trachomatis were identified at the University of Wisconsin Student Health Clinic (Madison) and at the Indiana University Medical School (Indianapolis). C. trachomatis serovars were determined at the Indiana University Medical School (20).
Heparinized venous blood (30 ml) was used for HLA class II typing (14) and for isolation of PBMC by centrifugation over Ficoll-Hypaque (5). A portion of the PBMC was transformed with the Epstein-Barr virus (19) to produce HLA class II-matched lymphoblastoid cell lines (LCLs) that could be used as antigen-presenting cells (APCs). The remaining PBMC were primed in vitro at 2 to 3 μg/ml with serovar E MOMP that had been isolated from C. trachomatis serovar E UW/5 elementary bodies (3). After 7 to 9 days of T-cell activation, human recombinant interleukin-2 was added at 50 U/ml. Additional recombinant interleukin-2 medium was added in accordance with cell growth (14). The resultant cultures (T-MOMP′) were tested for their response to 10 μM MOMP synthetic peptides in a standard proliferation assay using gamma ray-irradiated HLA class II-matched LCL APCs and quantifying the incorporation of [3H]thymidine (14). All determinations were made in triplicate, and the statistical significance of differences was evaluated with Student's t test corrected for small samples and unequal variances (15).
In the present study, we obtained T-MOMP′ cultures from 23 subjects and tested those from 13 serovar E and 8 non-E subjects with the same peptides used previously (14). The results obtained with T-MOMP′ cells from the present and previous (14) studies are summarized in Table 1. As previously observed, a great majority of the independent T-MOMP′ populations tested were stimulated by peptides located in MOMP CSs, regardless of the infecting serovars. Peptide 145-163, overlapping VS2, was confirmed to be Th cell stimulatory. Furthermore, four additional peptides located in three of the four MOMP VSs were found to contain Th cell epitopes.
TABLE 1.
Summary of serovar E MOMP peptides found to be Th cell stimulatory in our present and previous (14) studiesa
| Serovar E MOMP peptide | Previous study
|
Present study
|
Total no. of subjects
|
|||
|---|---|---|---|---|---|---|
| E | Non-E | E | Non-E | E | Non-E | |
| 34-53 | 3/6 | 1/4 | 4/10 | 5/7 | 7/16 | 6/11 |
| 47-67 | 3/6 | 0/4 | 3/10 | 1/7 | 6/16 | 1/17 |
| 60-80 | 0/6 | 0/4 | 0/10 | 0/7 | 0/16 | 0/11 |
| 75-92 | 0/5 | 0/3 | 1/10 | 0/6 | 1/15 | 0/9 |
| 89-105 | 4/6 | 2/4 | 2/10 | 0/9 | 6/16 | 2/13 |
| 102-121 | 0/5 | 0/4 | 1/11 | 0/2 | 1/16 | 0/6 |
| 117-135 | 2/6 | 0/4 | 2/8 | 0/2 | 4/14 | 0/6 |
| 132-151 | 0/5 | 0/4 | 1/9 | 0/8 | 1/14 | 0/16 |
| 145-163 | 1/6 | 0/5 | 2/10 | 0/8 | 3/16 | 0/12 |
| 157-175 | 1/6 | 2/3 | 1/8 | 0/3 | 2/14 | 2/6 |
| 168-187 | 3/6 | 2/3 | 3/9 | 1/3 | 6/15 | 3/6 |
| 180-199 | 1/6 | 1/3 | 2/7 | 1/3 | 3/13 | 2/6 |
| 191-211 | 0/6 | 2/3 | 3/8 | 1/3 | 3/14 | 3/6 |
| 206-225 | 2/7 | 1/5 | 2/9 | 0/2 | 4/16 | 1/7 |
| 218-233 | 0/6 | 0/4 | 0/6 | 0/7 | 0/12 | 0/11 |
| 227-243 | 0/6 | 0/4 | 0/6 | 0/7 | 0/12 | 0/11 |
| 238-254 | 2/6 | 1/4 | 1/9 | 1/2 | 3/15 | 2/6 |
| 249-265 | 6/7 | 3/4 | 8/11 | 4/7 | 14/18 | 7/11 |
| 260-276 | 2/7 | 0/4 | 2/9 | 0/2 | 4/16 | 0/6 |
| 271-287 | 2/7 | 0/4 | 1/8 | 0/2 | 3/15 | 0/6 |
| 282-298 | 0/6 | 0/4 | 0/6 | 0/7 | 0/12 | 0/11 |
| 293-307 | 0/6 | 0/4 | 0/6 | 0/7 | 0/12 | 0/11 |
| 300-318 | NT | NT | 6/11 | 0/9 | 6/11 | 0/9 |
| 308-324 | 1/6 | 0/4 | 0/9 | 0/7 | 1/15 | 0/11 |
| 319-336 | 2/6 | 1/4 | 3/8 | 1/2 | 5/14 | 2/6 |
| 332-348 | 1/6 | 1/4 | 3/8 | 1/2 | 4/14 | 2/6 |
| 344-359 | 1/7 | 2/3 | 3/10 | 1/2 | 4/17 | 3/5 |
| 355-371 | 1/7 | 1/3 | 1/9 | 2/2 | 2/16 | 3/5 |
The fractions shown are the proportions of infected subjects whose T-MOMP′ cultures responded to the indicated serovar E MOMP peptides. For several subjects, multiple T-MOMP′ cultures were obtained and concordant results were obtained each time. In those cases, the response of the first T-MOMP′ culture tested was used as a representative response. Because of T-cell availability and other considerations, not all of the T-MOMP′ cells were tested with all of the peptides. Stimulatory peptides are in boldface, and peptides that overlap VS sequences by more than seven amino acids are underlined. E, infected with serovar E; Non-E, infected with a serovar other than E or an unidentified serovar; NT, not tested.
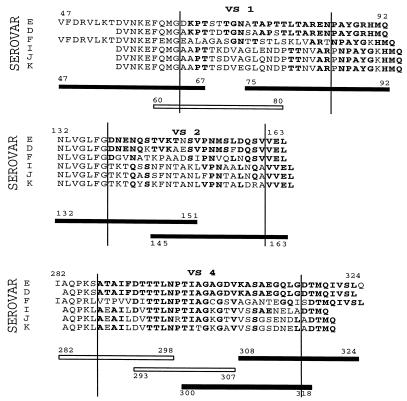
Synthetic peptides spanning the VSs of serovar E MOMP that were tested are described in Fig. 1. The VS sequences are delimited with vertical lines, and Th cell-stimulatory peptides are shown as black bars. The corresponding amino acid sequences of serovars D, F, I, J, and K, all of which were represented in our subject population, are shown for comparison. Stimulatory peptide 47-67 overlaps VS1 by only four amino acids and was recognized by some serovar E-infected subjects, as well as by one non-E-infected subject (Table 1). Further mapping with shorter peptides localized a Th cell epitope within the CS portion of this peptide (data not shown). In contrast, the other five stimulatory peptides overlap VSs by at least seven amino acids and were recognized by only serovar E-infected subjects (Table 1), suggesting that these five peptides contain serovar-specific epitopes.
FIG. 1.
Sequences of VS-spanning synthetic peptides tested in this study. Synthetic peptides spanning three of the four MOMP VSs and used in our experiments are shown. VSs as described by Kaltenboeck et al. (11) are delimited by vertical bars. Amino acids shared by serovar E and other serovars are in boldface. Peptides that stimulated Th cells primed with the serovar E MOMP are shown as filled bars, and nonstimulatory peptides are shown as unfilled bars. VSs of different serovars exhibit substantial differences in amino acid composition while containing short segments of conserved amino acids (boldface). In addition, insertions of one or two amino acids within VSs of some non-E serovars have the effect of displacing segments of conserved amino acids in the C-terminal direction relative to the serovar E sequence. The stimulatory epitope of peptide 47-67 is within CS1 and is not VS specific, while the other five stimulatory peptides contain VS-specific epitopes (see text).
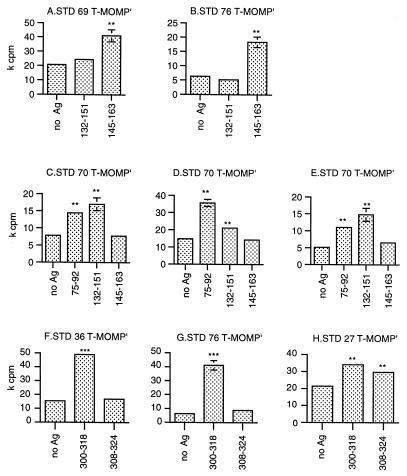
Figure 2 shows the proliferation of T-MOMP′ cells obtained from five different serovar E-infected subjects in response to the five peptides located in VSs of serovar E MOMP. T-MOMP′ cultures from two serovar E-infected subjects (STD 69 and STD 76) tested for the first time in the present study clearly responded to peptide 145-163, residues 145 to 160 of which are present in VS2 (Fig. 2A and B). We confirmed our previous identification of peptide 145-163 as a VS-overlapping stimulatory peptide (14); although the amino acid sequences of the serovar E and D MOMPs are almost identical at residues 145 to 160 (Fig. 1), only five or six residues are shared by other serovars, making it highly likely that this peptide contains a serovar E-specific epitope (see below).
FIG. 2.
Identification of Th cell-stimulatory peptides located in MOMP VSs. Proliferation of T-MOMP′ cells in response to synthetic peptides that overlap MOMP VS segments by at least seven amino acids (Fig. 1) is shown. T-MOMP′ cells primed in vitro with the C. trachomatis serovar E MOMP were tested in proliferation assays as previously described (14). Data shown for STD 70 were obtained with T-MOMP′ cells prepared from three independent blood samples (C, D, and E). HLA class II-matched LCLs derived from the following C. trachomatis-infected subjects were used as APCs as follows: A, STD 32; B and G, STD 76; F, STD 36; G, STD 76; H, STD 27. In addition, an LCL from uninfected control subject STD 64 was used as APCs for C, D, and E. The statistical significance of differences in [3H]thymidine incorporation in the presence and absence of peptides was evaluated with Student's t test corrected for small sample numbers and unequal variances. ∗, 0.025 < P < 0.05; ∗∗, 0.01 < P < 0.025; ∗∗∗, P < 0.01. Absence of error bars indicates variances too small to show on the scale used. Ag, antigen.
A second VS2 Th cell epitope may be located in MOMP peptide 132-151, which reproducibly stimulated T-MOMP′ cultures prepared from three different blood samples of serovar E-infected subject STD 70 (Fig. 2C, D, and E). Residues 139 to 151 of this peptide lie within VS2 and vary extensively among different serovars (Fig. 1). The facts that STD 70 T-MOMP′ cells responded to peptide 132-151 but not to peptide 145-163 (Fig. 2C, D, and E) and that none of the T-MOMP′ cells derived from the subjects who responded to peptide 145-163 recognized peptide 132-151 (Fig. 2A and B) strongly indicate that overlapping peptides 145-163 and 132-151 contain distinct serovar E-specific epitopes.
Peptide 75-92 includes residues 75 to 83 in the C-terminal portion of VS1 and stimulated proliferation of T-MOMP′ cells derived from three different blood samples of STD 70 (Fig. 2C, D, and E). The Th cell epitope was localized in the VS1 portion of peptide 75-92; peptide 75-88, containing most of the variable amino acids, stimulated proliferation of STD 70 T-MOMP′ cells as well as did peptide 75-92, while peptide 79-92, containing most of the conserved amino acids, did not stimulate significant proliferation of the same T-MOMP′ cells (data not shown).
Two additional Th cell-stimulatory peptides, 300-318 and 308-324, overlapped VS4. Peptide 300-318 stimulated T-MOMP′ cells from 6 of 11 serovar E-infected subjects (54%) and none of 7 non-E-infected subjects; representative positive data are shown in Fig. 2F, G, and H. Peptide 308-324 was recognized by T-MOMP′ cells from only 1 out of the 15 serovar E-infected subjects tested so far (Fig. 2H). However, the small experimental variances obtained with several subjects indicate significant peptide-dependent proliferation (e.g., Fig. 2F and G), suggesting that responses to this peptide are more common than is currently apparent.
The identification of five Th cell-stimulatory VS peptides provides only a minimum estimate of the number of serovar E VS T-cell epitopes. As we have shown (14), a single CS 17-mer (MOMP peptide 249-265) could contain at least six Th cell epitopes that were presented with diverse HLA class II allotypes. Fine epitope mapping and restriction element analysis, as performed previously (14), are necessary to determine the true number of epitopes in each peptide and whether different subjects were responding to distinct epitopes present within a stimulatory VS peptide.
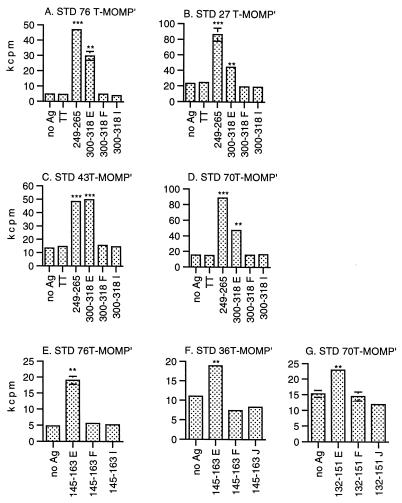
Although the VS peptides derived from serovar E MOMP were exclusively recognized by serovar E-infected subjects (Table 1), it was possible that the T-cell responses we observed were toward the segments of amino acid identity within the VSs and thus are not truly serovar specific. Therefore, we tested whether T-MOMP′ cells obtained from serovar E-infected subjects and primed in vitro with serovar E MOMP could proliferate in response to corresponding VS peptides derived from different serovars. MOMP peptides from serovars E, F, I, and J were chosen because these serovars were represented among STD subjects whose PBMC yielded T-MOMP′ cultures after in vitro priming with the serovar E MOMP. Availability of T-MOMP′ cells prompted us to focus on peptides 132-151, 145-163, and 300-318, the latter two of which are recognized by T-MOMP′ cells from a relatively large fraction of our subjects (Table 1). Representative data are shown in Fig. 3.
FIG. 3.
Serovar-specific responses of T-MOMP′ cells to MOMP VS peptides. T-MOMP′ cells were primed in vitro with serovar E MOMP, and proliferation assays were performed as described in the legend to Fig. 2. The responses to the serovar E peptides were compared with those to the corresponding peptides derived from serovars F, I, and J. Tetanus toxoid (TT) was used as a negative control for antigen (Ag) specificity, and MOMP CS peptide 249-265 was used as a positive control, to which a great majority of infected subjects respond regardless of the infecting serovar. HLA class II-matched LCLs derived from the following subjects were used as APCs: A and E, STD76; B, STD27; C, STD43; D and G, STD70; F, STD36. Absence of error bars indicates standard deviations too small to show on the scale used. ∗, 0.01 < P < 0.025; ∗∗, 0.001 < P < 0.01; ∗∗∗, P < 0.001.
Serovar E peptide 300-318 (300-318E) stimulated significant proliferation in all of the T-MOMP′ cultures tested in this experiment, while its serovar F and I versions did not (Fig. 3A through D). Similarly, peptide 145-163E stimulated proliferation in two of the T-MOMP′ cultures tested while its F and J versions did not (Fig. 3E and F). This pattern of proliferation in specific response to serovar E versions of VS peptides was repeated with peptide 132-151 (Fig. 3G). While STD 70 is, so far, the only available subject whose T cells responded to peptide 132-151, completely concordant results were obtained with T-MOMP′ cells from three different blood samples collected over a 120-day interval (data not shown). Similarly, STD 27 T-MOMP′ cells produced from one additional blood sample yielded concordant results with peptide 300-318 and two additional STD 76 T-MOMP′ populations yielded concordant results with peptides 300-318 and 145-163 (data not shown).
In summary, all of the T-MOMP′ populations obtained from five serovar E-infected subjects and primed with serovar E MOMP proliferated in response to serovar E MOMP VS peptide 132-151, 145-163, or 300-318 but not to the corresponding peptide derived from serovar F, I, or J. These reproducible results establish the presence of serovar E-specific Th cell epitopes within these three serovar E VS peptides. Peptides 75-92 and 308-324 have not yet been tested in this way because of the unavailability of T-MOMP′ cells. However, the amino acid sequences of serovar E peptides 75-92 and 308-324 differ from those of other serovars at numerous positions (Fig. 1), making it highly likely that they also contain serovar E-specific epitopes.
The present study confirms our previous report of Th cell-stimulatory peptide 145-163 in VS2 (14) and newly localizes four stimulatory peptides to VS1 (75-92), VS2 (132-151), and VS4 (300-318 and 308-324). During the revision of this report, similar localization of VS Th cell epitopes using different methodology was reported (2). We show here, as in reference 2, that serovar E VS peptides were exclusively recognized by serovar E-infected subjects but not by subjects infected with non-E serovars (Table 1). Beyond reference 2, we directly demonstrate that the T-MOMP′ cultures responding to the VS peptides indeed recognize serovar E-specific epitopes; T cells obtained from subjects infected with serovar E proliferated in response to serovar E VS peptides but not in response to the corresponding peptides derived from serovar F, I, or J (Fig. 3). Therefore, our present study provides solid evidence for the presence of VS-specific Th cell responses in many C. trachomatis-infected humans.
The frequency of observed T-MOMP′ responses to serovar E VSs among all of the C. trachomatis-infected subjects we studied (Table 1) was expectedly limited because (i) serovar E-specific responses were not observed in the 38% of our subjects infected with non-E serovars and (ii) some serovar E-infected subjects whose T-MOMP′ cultures responded to CS epitopes but not to VS epitopes may have lacked HLA class II allotypes used to present the VS epitopes. Few, if any, T-cell epitopes may prove to be in VS3 because it is only 14 amino acids long. While VS-responsive T-cell clones may sometimes have been outgrown and become undetectable during in vitro expansion of T-MOMP′ cultures, the methodology we used readily identified five MOMP peptides (75-92, 132-151, 145-163, 300-318, and 308-324) that contain serovar E VS-specific epitopes. Of the seven serovar E-infected subjects studied in detail, one responded to one VS epitope, three responded to two epitopes, and one responded to three epitopes. While observed responses to some VS epitopes were infrequent, it is noteworthy that frequencies of responses to VS peptides 145-163 and 300-318 were about 43 and 85%, respectively. It is likely that similar studies with subjects infected with non-E serovars and with synthetic MOMP VS peptides corresponding to those serovars will reveal additional VS epitopes.
Because the peptide-specific T-cell proliferation was observed after priming of T cells with exogenous intact protein antigen, it is likely that the observed responses are, at least in part, to VS epitopes presented in association with HLA class II molecules, as we have shown for numerous CS epitopes (14). We have also recently localized an HLA-A2 (i.e., class I)-restricted cytotoxic T-lymphocyte (CTL) epitope, MOMP peptide 155-163, in MOMP VS2 (13). This CTL epitope is recognized only by subjects infected with serovar E and, interestingly, is included in Th cell-stimulatory VS2 MOMP peptide 145-163. Both peptides overlap MOMP peptide 139-160, which may possess a human B-cell epitope (2a). The colocation of B-cell, Th cell, and CTL epitopes in peptide 145-163 is noteworthy because a short peptide containing all three epitopes might be used in a subunit vaccine to elicit both humoral and cellular responses to C. trachomatis.
Certain point mutations within the overlapping sequence described above might destroy all three epitopes, allowing C. trachomatis to escape from potentially protective host immune responses. Until now, within-serovar sequence variations in MOMP VSs were interpreted exclusively in relation to possible mechanisms of escaping serovar-specific antibody responses of infected humans. The localization of VS T-cell epitopes (2 and this study) raises the possibility that mutations altering such epitopes could also contribute to immune response evasion by C. trachomatis. Such escape mutants might have unaltered serovar B-cell epitopes but altered T-cell epitopes. Indeed, relatively frequent amino acid variations were observed in MOMP VS2 when the entire MOMP gene from clinical C. trachomatis isolates immunotyped as serovar E was sequenced (6). Remarkably, one of the amino acid substitutions present in several independent C. trachomatis isolates had occurred at the second residue of CTL MOMP epitope 155-163, which is required for binding to HLA-A2, suggesting that those variants of serovar E have been selected by escaping from the host CTL response. This particular amino acid change might also affect the generation and/or the recognition of a VS2-localized Th cell epitope contained in peptide 145-163. Point mutations were also observed in VS1 sequence-containing MOMP peptide 75-92 (6), in which we localized a Th cell epitope.
The point mutations occurring within the T-cell epitopes described above raise the possibility that within-serovar variants harboring such mutations have been selected for by evasion of potentially protective Th cell and CTL responses of the host against MOMP VSs (7). But this possibility must be extended to mutations affecting the CSs too: different C. trachomatis isolates serotyped as serovar E had many CS amino acid variations between isolates, many of which resulted from point mutations at the same sites in different isolates (6). Remarkably, some of the recurrent CS point mutations had occurred in peptide sequences 206 to 225, 238 to 254, 271 to 287, and 345 to 359, in which we had localized possibly protective Th cell epitopes (14).
Thus, the localization of Th cell and CTL epitopes in CSs and VSs of MOMP, combined with the discovery of some recurrent mutations in some of those epitopes, may contribute to an understanding of two puzzling facts, i.e., (i) that recurrent genital tract infections in humans are common despite the high frequencies of Th cell and CTL responses to CSs and VSs and (ii) that apparent serovar specificity of (limited) protective immunity may be based in part on T-cell epitopes in the same VSs that define serovars.
Acknowledgments
This work was supported by National Institutes of Health grants AI 34617 and AI 15486.
Footnotes
This is paper 3491 from the Laboratory of Genetics, University of Wisconsin-Madison.
REFERENCES
- 1.Alani M D, Darougar S, Burns D C, Thin R N, Dunn H. Isolation of Chlamydia trachomatis from the male urethra. Br J Vener Dis. 1977;53:88–92. doi: 10.1136/sti.53.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arno J N, Xie C, Jones R B, VanDerPol B. Identification of T cells that respond to serovar-specific regions of the Chlamydia trachomatis major outer membrane protein in persons with serovar E infection. J Infect Dis. 1998;178:1713–1718. doi: 10.1086/314478. [DOI] [PubMed] [Google Scholar]
- 2a.Batteiger B E, Lin P-M. Human antibodies to linear B cell epitopes in variable sequence regions of the major outer membrane protein of Chlamydia trachomatis. In: Stephens R S, Byrne G I, Christiansen G, Grayston J T, Rank R G, Ridgway G L, Saikku P, Schachter J, Stamm W E, editors. Chlamydial Infections: Proceedings of the Ninth International Symposium on Human Chlamydial Infection, Napa, Calif. Cambridge, England: Cambridge University Press; 1998. pp. 462–465. [Google Scholar]
- 3.Bavoil P, Ohlin A, Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984;44:479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne G I, Stephens R S, Ada G, Caldwell H D, Su H, Morrison R P, Van der Pol B, Bavoil P, Bobo L, Everson S, et al. Workshop on in vitro neutralization of Chlamydia trachomatis: summary of proceedings. J Infect Dis. 1993;168:415–420. doi: 10.1093/infdis/168.2.415. [DOI] [PubMed] [Google Scholar]
- 5.Chen B P, DeMars R, Sondel P. Presentation of soluble antigen to human cells by products of multiple HLA-linked loci: analysis of antigen presentation by a panel of cloned autologous HLA-mutant Epstein-Barr virus-transformed lymphoblastoid cell lines. Hum Immunol. 1987;18:75–91. doi: 10.1016/0198-8859(87)90114-5. [DOI] [PubMed] [Google Scholar]
- 6.Dean D, Millman K. Molecular and mutation trends analyses of omp 1 alleles for serovar E of Chlamydia trachomatis. J Clin Investig. 1997;99:475–483. doi: 10.1172/JCI119182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans D T, O'Connor D H, Jing P, Dzuris J L, Sidney J, DaSilva J, Allen T M, Horton H, Rudersdorf R A, Vogel T, Pauza C D, Bontrop R E, DeMars R, Sette A, Hughes A L, Watkins D I. Virus-specific cytotoxic T-lymphocyte responses select for amino acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 8.Grayston J T, Wang S P. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–77. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Gunn R A, Rolfs R T, Greenspan J R, Seidman R L, Wasserheit J N. The changing paradigm of sexually transmitted disease control in the era of managed health care. JAMA. 1998;279:680–684. doi: 10.1001/jama.279.9.680. [DOI] [PubMed] [Google Scholar]
- 10.Jones R B, Batteiger B E. Human immune responses to Chlamydia trachomatis infections. In: Oriel J, Ridgeway G, Schachter J, Taylor-Robinson D, Ward M, editors. Chlamydial Infections. London: Cambridge University Press; 1986. pp. 423–432. [Google Scholar]
- 11.Kaltenboeck B, Kousoulas K G, Storz J. Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. J Bacteriol. 1993;175:487–502. doi: 10.1128/jb.175.2.487-502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz B P, Batteiger B E, Jones R B. Effect of prior sexually transmitted disease on the isolation of Chlamydia trachomatis. Sex Transm Dis. 1987;14:160–164. doi: 10.1097/00007435-198707000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Kim S-K, Angevine M, Demick K, Ortiz L, Rudersdorf R, Watkins D, DeMars R. Induction of HLA class I-restricted CD8+ CTLs specific for the major outer membrane protein of Chlamydia trachomatis in human genital tract infections. J Immunol. 1999;162:6855–6866. [PubMed] [Google Scholar]
- 14.Ortiz L, Demick K P, Petersen J W, Polka M, Rudersdorf R A, Van der Pol B, Jones R, Angevine M, DeMars R I. Chlamydia trachomatis major outer membrane protein (MOMP) epitopes that activate HLA class-II restricted T cells from infected humans. J Immunol. 1996;157:4554–4567. [PubMed] [Google Scholar]
- 15.Snedecor G W, Cochran W G. Statistical methods, sixth ed. Ames: The Iowa State University Press; 1969. [Google Scholar]
- 16.Su H, Caldwell H D. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992;175:227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su H, Caldwell H D. Immunogenicity of a synthetic oligopeptide corresponding to antigenically common T-helper and B-cell neutralizing epitopes of the major outer membrane protein of Chlamydia trachomatis. Vaccine. 1993;11:1159–1166. doi: 10.1016/0264-410x(93)90080-h. [DOI] [PubMed] [Google Scholar]
- 18.Su H, Feilzer K, Caldwell H D, Morrison R P. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tosato G. Generation of Epstein Barr virus (EBV)-immortalized B cell lines. In: Coligan J E, Kruisbeek A M, Marguiles D A, Shevach E M, Strober W, editors. Current protocols in immunology. Vol. 2. New York, N.Y: Wiley Interscience; 1991. p. 7.22.2. [DOI] [PubMed] [Google Scholar]
- 20.VanderPol B J, Jones R B. Comparison of immunotyping of Chlamydia trachomatis by indirect fluorescent-antibody staining and radioimmunoassay. J Clin Microbiol. 1992;30:1014–1015. doi: 10.1128/jcm.30.4.1014-1015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]