Abstract
Insulin-like growth factor-1 (IGF-1) plays an important role in function and development of the mammary gland. However, high levels of IGF-1 has been associated with an increased risk of breast cancer development. Epithelial–mesenchymal transition (EMT) is a process where epithelial cells lose their epithelial characteristics and acquire a mesenchymal phenotype, which is considered one of the most important mechanisms in cancer initiation and promotion of metastasis. Extracellular vesicles (EVs) are released into the extracellular space by different cell types, which mediate intercellular communication and play an important role in different physiological and pathological processes, such as cancer. In this study, we demonstrate that EVs from MDA-MB-231 breast cancer cells stimulated with IGF-1 (IGF-1 EVs) decrease the levels of E-cadherin, increase the expression of vimentin and N-cadherin and stimulate the secretion of metalloproteinase-9 in mammary non-tumorigenic epithelial cells MCF10A. IGF-1 EVs also induce the expression of Snail1, Twist1 and Sip1, which are transcription factors involved in EMT. Moreover, IGF-1 EVs induce activation of ERK1/2, Akt1 and Akt2, migration and invasion. In summary, we demonstrate, for the first time, that IGF-1 EVs induce an EMT process in mammary non-tumorigenic epithelial cells MCF10A.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-021-00638-y.
Keywords: IGF-1, Extracellular vesicles, EMT, MCF10A, MDA-MB-231, ERK1/2, Akt1/2
Introduction
Breast cancer is the most frequent malignancy among females in developed countries and the leading cause of death related to cancer in women worldwide (Bray et al. 2018). Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer that is defined by the lack of estrogen and progesterone receptors expression and the absence of HER2/neu overexpression and/or amplification (Godone et al. 2018; Wang et al. 2015). TNBC accounts for 10–15% of all breast cancer subtypes and has a higher frequency in younger premenopausal women with a higher rate of early recurrence, distant metastasis and a poorer prognosis (Duffy et al. 2012; Vagia et al. 2020).
Insulin-like growth factor (IGF) family comprises IGF-1, IGF-2, IGF-binding proteins (IGFBPs), and their respective receptors (Gallagher and LeRoith 2010; Hwa et al. 1999). IGFs are polypeptide hormones with a similar tertiary structure to insulin that are synthesized in the majority of body’s tissues (Firth and Baxter 2002). IGF-1 is synthesized mainly in the liver via growth hormone and participates in development and function of the mammary gland, however high circulating levels of IGF-1 in healthy women has been linked to an increased risk of breast cancer development (Christopoulos et al. 2015; Firth and Baxter 2002). Biological effects mediated by IGF-1 are through binding and activation to type 1 IGF receptor (IGF-1R), which trigger intracellular signaling cascades including Shc, Grb2 and insulin receptor substrate (Gross and Yee 2003; Motallebnezhad et al. 2016). IGF-1R is overexpressed in a variety of cancers, including breast cancer, and the loss of tumor suppressor genes, such as BRCA1, p53 and PTEN, promotes an increase of IGF-1R expression in tumors (Anisimov and Bartke 2013; Yakar et al. 2005). Moreover, IGF-1 has been implicated in malign transformation, due to its ability to maintain proliferation, cell survival, resistance to apoptosis, migration, invasion, angiogenesis, metastasis and an epithelial–mesenchymal transition (EMT) process via PI3K/Akt and Ras/Raf/ERK pathways (Christopoulos et al. 2015; Li et al. 2017; Motallebnezhad et al. 2016; Seccareccia and Brodt 2012; Zielinska et al. 2015).
EMT process involves the loss of cell–cell interactions and cell polarity, and the acquisition of mesenchymal properties including migration and invasion, which are regulated by several transcription factors, such as Fox2, Snail1/2, ZEB1/2 and Twist (Thiery et al. 2009). During EMT process occurs a decrease of epithelial markers expression, such as E-cadherin, β-catenin, desmoplakin, cytokeratin-8, cytokeratin-18, and an increase of mesenchymal markers expression including N-cadherin, vimentin, fibronectin, smooth muscle actin (SMA) and secretion of matrix metalloproteinases (MMPs) (Gavert and Ben-Ze'ev 2008; Li et al. 2017; Lu and Kang 2019). EMT process mediates tumor progression, because during invasion and metastasis the cancer cells acquire the ability to migrate, secrete MMPs, degrade basement membrane and invade surrounding tissues (Lu and Kang 2019; Zielinska et al. 2015).
Extracellular vesicles (EVs) are secreted into the extracellular space by different cell types under normal and pathological conditions, and exert their action at short and long distances, providing an alternative mode of intercellular communication (Kalra et al. 2016; Turturici et al. 2014; Vader et al. 2014). EVs are classified into three types according to their biogenesis and size: exosomes (30–100 nm) are a homogeneous group of vesicles with an endocytic origin; microvesicles (100–1000 nm) are a heterogeneous group of vesicles generated directly from plasma membrane; and apoptotic bodies (1–5 μm) derived from apoptotic cells (Colombo et al. 2014; Kalra et al. 2016; Raposo and Stoorvogel 2013). Moreover, EVs mediate a variety of cellular functions in their target cells, which are dependent on cell type of origin and their composition, because these vesicles contain a variety of cargo molecules, including proteins, mRNA, microRNA, lncRNA and lipids (Colombo et al. 2014; Kalra et al. 2016; Raposo and Stoorvogel 2013). In cancer, EVs are crucial in the maintenance and acquisition of cancer-associated hallmarks, because they are able to mediate evasion of growth suppressors, sustained proliferative signaling, evasion of apoptosis and immune destruction, migration, invasion, angiogenesis and EMT (Greening et al. 2015; Kanada et al. 2016; Vader et al. 2014). However, the role of EVs from MDA-MB-231 breast cancer cells stimulated with IGF-1 in the EMT process is still not studied.
In this study, we demonstrate that EVs released from MDA-MB-231 breast cancer cells stimulated with IGF-1 promote a decrease of E-cadherin expression, which is accompanied with an increase of N-cadherin, vimentin and E-cadherin transcriptional repressors (Snail1, Twist1 and Sip1) expression in mammary non-tumorigenic epithelial cells MCF10A. Moreover, EVs also induce activation of ERK1/2, Akt1 and Akt2, as well as an increase of MMP-9 secretion, migration and invasion.
Materials and methods
Antibodies and reagents
IGF-1, epidermal growth factor (EGF), A6730 and tetramethylrhodamine (TRITC)-conjugated phalloidin were from Sigma-Aldrich (Merck KGaA). Hoechst dye, CD9 C-4 antibody (Ab), E-cadherin 67A4 Ab, vimentin V9 Ab, N-cadherin 13A9 Ab, Akt1 B-1 Ab and Akt2 F-7 Ab were from Santa Cruz Biotechnology (Santa Cruz, CA). Flotillin-2 (Flot-2) Ab and basement membrane matrix (BD Matrigel) were from BD Biosciences (Bedford, MA). CD63 MEM-259 Ab was from Abcam (Cambridge, MA). ERK1/2 inhibitor (3-(2-aminoethyl)-5-((4-ethoxyphenyl)methylene)-2,4 thiazolidinedione hydrochloride, HCl) was from Merck Millipore (Rockland, MA). ERK1/2 Ab and phosphospecific Abs to threonine (Thr)-202 and tyrosine (Tyr)-204 of ERK1/2 (anti-p-ERK1/2), and to serine (Ser)-473 and Ser-474 of Akt1 and Akt2 respectively (anti-p-Akt Ab) were from Cell Signaling Technology (Beverly, MA). CellMask™ Orange plasma membrane stain was from ThermoFisher Scientific. Actin Ab was kindly provided by Dr. Jose-Manuel Hernandez (Cinvestav-IPN).
Cell lines and culture
The TNBC MDA-MB-231 cells were cultured in Dulbecco´s modified Eagle’s medium (DMEM) supplemented with 3.7 g/l sodium bicarbonate, 5% fetal bovine serum (FBS) and antibiotics. Human mammary non-tumorigenic epithelial cells MFC10A were cultured in DMEM/F12 medium (3:1) supplemented with 10% FBS, 0.5 µg/ml hydrocortisone, 20 ng/ml EGF, 10 μg/ml insulin and antibiotics. Cells were cultured in a humidified atmosphere containing 5% CO2 and 95% air at 37 °C. MDA-MB-231 cells were FBS-starved in DMEM for 24 h before stimulation with IGF-1, while MCF10A cells were FBS-starved in DMEM/F12 without hydrocortisone, EGF and insulin for 18 h before treatment with inhibitors and/or EVs.
Stimulation of MDA-MB-231 cells with IGF-1
Cultures of MDA-MB-231 cells (8 × 106 cells/100-mm culture plate) were washed twice with phosphate-buffered saline (PBS) and stimulated with 10 nM IGF-1 in DMEM for 48 h at 37 °C. After incubation, conditioned media were collected and EV fractions were obtained (Fig. S1).
Isolation of EVs
Conditioned media were centrifuged twice at 600 × g for 10 min at 4 °C. Supernatants were carefully aspirated and then sequentially centrifuged twice at 2000× g for 15 min, once at 10,000× g for 30 min and once at 110,000× g for 70 min at 4 °C. Supernatants were aspirated and pellets were reconstituted in PBS or FBS-free DMEM (EV fractions). Protein concentration of EV fractions was determined by the micro-Bradford protein assay (Bio-Rad, USA).
Stimulation of MCF10A cells with EV fractions
Cultures of MCF10A cells (1.5 × 106 cells/35-mm culture plate) were washed twice with PBS and treated for different times with EV fractions from MDA-MB-231 cells unstimulated or stimulated with 10 nM IGF-1 for 48 h (80 μg EVs/experimental condition) in FBS-free DMEM/F12 (Fig. S1). After stimulation, media were collected and cells were solubilized in 0.5 ml of ice-cold RIPA buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM sodium orthovanadate, 100 mM NaF, 10 mM sodium pyrophosphate, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, 1.5 mM MgCl2, 0.1% SDS and 1 mM PMSF). Protein concentration of samples was determined by the micro-Bradford protein assay (Bio-Rad, USA).
Transmission electron microscopy (TEM)
One fraction of EVs (80 μg EVs) was suspended in 100 μl PBS, and then an aliquot (30 μl) was adsorbed on carbon-coated copper grids with mesh formvar (0.3%) for 5 min at room temperature. Grids were negatively stained for 30 s with 2% uranyl acetate at room temperature, and excess fluid was removed using filter paper. Grids were air-dried and analyzed using a JEM-1400 transmission electron microscope (JEOL, Japan) operated at 80 kV and coupled with a digital camera Veleta (Olympus SIS, Germany).
Western blot (WB) analysis
Equal amounts of protein were separated using 10% SDS-PAGE separating gels followed by transfer to nitrocellulose membranes. Membranes were blocked for 2 h at room temperature using 5% non-fat dried milk in PBS pH 7.2/0.1% Tween 20 (wash buffer), and incubated overnight at 4 ºC with primary Ab. Membranes were washed three times with wash buffer and incubated with secondary Ab conjugated to horseradish peroxidase for 2 h at room temperature. After washing three times with wash buffer, the immunoreactive bands were visualized using WB luminol reagent and exposition to an autoradiography film. For quantification, autoradiograms were scanned and bands were analyzed using the ImageJ software v 1.52e (NIH, USA).
Immunoprecipitation (IP)
Lysates were clarified by centrifugation at 13,500× g for 10 min at 4 °C. Supernatants were transferred to fresh tubes and equal amounts of protein were immunoprecipitated overnight at 4 °C with protein A-agarose linked to anti-Akt1 Ab or anti-Akt2 Ab. Immunoprecipitates were washed three times with RIPA buffer and proteins were extracted with SDS-PAGE sample buffer by boiling for 5 min and resolved by SDS-PAGE and analyzed by WB.
Quantitative real-time PCR (RT-qPCR)
Total RNA was obtained by using TRIzol reagent (Invitrogen). cDNA was synthesized using SuperScript III reverse transcription system and 1 μg of total RNA as template. For real-time PCR, relative gene expression was determined by using the iQ SYBR Green Supermix (Bio-Rad, USA). Primers are shown in Table 1, and amplification was performed by 45 cycles of sequential denaturation (95 °C, 2 min), annealing (60 °C, 15 s) and extension (72 °C, 20 s). Results were analyzed by using the 2−ΔΔCT method and normalized to β-actin data.
Table 1.
Forward and reverse primer sequences used for real-time quantitative polymerase chain reaction
| Gene | Forward primer 5′ → 3′ | Reverse primer 5′ → 3′ |
|---|---|---|
| Snail1 | GCGAGCTGCAGGACTCTAAT | CCTCTGTCCTCATCTGACA |
| Twist1 | GGAGTCCGCAGTCTTACGAG | TGGAGGACCTGGTAGAGGAA |
| Sip1 | AATGGCAACAGCAACAAGTG | CCCCGTCAGCACATAACTTT |
| E-cadherin | CGACCAACCCAAGAATCTA | AGGCTGTGCCTTCCTACAGA |
| Vimentin | GGTACTCAGTGGACTCCTGCTTT | CTGCCAACCGGAACAATGA |
| β-actin | TCCCTGGAGAAGAGCTACGA | AGCACTGTGTTGGCGTACAG |
Scratch-wound assays
Cultures of MCF10A cells (1.5 × 106 cells/35-mm culture plate) were treated for 2 h with 12 µM mitomycin C to inhibit proliferation during the experiment. Cell cultures were scratched, washed twice with PBS and supplemented with FBS-free DMEM/F12 with or without inhibitors and/or EV fractions (80 μg EVs/experimental condition) for 48 h at 37 °C. Cultures were photographed using an inverted microscope coupled to a camera (FSX100; Olympus Corporation). Images from ≥ 3 fields per experimental condition were acquired and analyzed using the ImageJ software v 1.52e (NIH, USA).
Zymography
Conditioned media were concentrated using 5000 Da Centricon® filters (EDM Millipore). Equal volumes of non-heated conditioned medium and sample buffer (2.5% SDS, 2% sucrose, 4 μg/ml phenol red) were mixed and loaded into 8% polyacrylamide gels copolymerized with gelatin (1 mg/ml). Gels were rinsed twice for 30 min with 2.5% Triton X-100, and incubated in assay buffer (50 mM Tris–HCl pH 7.4, 5 mM CaCl2) for 48 h at 37 °C. After incubation, gels were fixed and stained with a solution of Coomassie Brilliant Blue G-250 (0.25%) dissolved in acetic acid (10%) and methanol (30%). Proteolytic activity was identified as clear bands on a blue background. Controls of MMP-2 and MMP-9 secretions were included, which were prepared by treatment of MDA-MB-231 cells with 400 mg/dl ethanol and 100 ng/ml PDB for 24 h at 37 °C respectively (Ke et al. 2006; Park et al. 2000).
Invasion assays
Inserts of 24-well plates (Costar; Corning, Inc) were covered with 50 µl Matrigel (3 mg /ml) and incubated overnight at 37 °C. Next, MCF10A cells (1 × 105) were plated on Matrigel of each insert (Upper chamber) in fresh FBS-free DMEM/F12. Lower chamber contained 600 µl EV fractions dissolved in FBS-free DMEM/F12 (80 μg EVs/experimental condition). Plates were incubated for 72 h at 37 °C under a humidified atmosphere with 5% CO2 and 95% air. After incubation, cells and Matrigel on the upper surface of membranes were removed with cotton swabs, and the cells on the lower surface of the membranes were washed with PBS and fixed with paraformaldehyde (4%) for 12 min at room temperature. Invaded cells were imaged using an inverted microscope coupled to a camera (FSX100; Olympus Corporation). Quantification of invaded cells was determined by staining of membranes with crystal violet (0.5%) in PBS for 15 min at room temperature and elution of dye with 300 µl of acetic acid (10%). Absorbance of collected solution was measured at 600 nm.
Immunofluorescence confocal microscopy
MCF10A cells were grown on chamber slides (Nunc Lab-Tek® II), washed with PBS, equilibrated in DMEM and treated for 2 h with 12 μM mitomycin C. Cultures were scratched, washed with DMEM and treated with EVs (80 μg EVs/experimental condition) for 48 h at 37 °C. After incubation, cells were fixed with paraformaldehyde (4%) for 20 min, permeabilized with 0.5% Triton X-100 for 20 min and blocked with 10% FBS for 30 min at room temperature. Cells were incubated for 12 h at 4 °C with anti-vimentin Ab (1:100) followed by FITC-labeled anti-mouse secondary Ab for 2 h at 4 °C. Staining of fibrillar actin was performed by incubation of cells for 1 h at room temperature with TRITC-conjugated phalloidin, and cells were counterstained with Hoechst for 20 min at room temperature and mounted on glass slides using Vectashield. Cells were analyzed by confocal microscopy (Model TCS SP2; Leica Microsystems, Inc). Imagens were analyzed by using the ImageJ software v 1.52e (NIH, USA).
EV uptake assays using confocal microscopy
EV fractions (80 μg EVs/experimental condition) were stained with CellMask™ Orange plasma membrane stain (2.5 µg/ml) for 30 min at 4 °C, and then washed with PBS and centrifuged at 110,000× g for 70 min at 4 °C. EVs were reconstituted in 100 µl FBS-free DMEM. MCF10A cells (1.5 × 106 cells/experimental condition) were incubated for 20 min at 37 °C with unstained EVs and stained EVs. After incubation, cells were washed twice with PBS, fixed for 20 min with paraformaldehyde (4%) at room temperature and washed twice with PBS. Next, cells were counterstained with Hoechst for 20 min at room temperature and mounted on glass slides using Vectashield. Preparations were analyzed by confocal microscopy (Model TCS SP2; Leica Microsystems, Inc). Imagens were analyzed by using the ImageJ software v 1.52e (NIH, USA).
Statistical analysis
Data are expressed as mean ± SD of at least three independent experiments, and were analyzed using one-way ANOVA and Turkey’s multiple comparison test. Statistical probability of P < 0.05 was considered significant.
Results
Characterization of EVs from MDA-MB-231 cells unstimulated and stimulated with IGF-1
First, we determined that stimulation of MDA-MB-231 breast cancer cells with 10–50 nM IGF-1 induced maximal migration (Fig. S2). Therefore, we studied whether EVs released from MDA-MB-231 cells stimulated with 10 nM IGF-1 induced an EMT process in mammary non-tumorigenic epithelial cells MCF10A.
EV fractions from MDA-MB-231 cells incubated with FBS-free DMEM for 48 h (Ctrl EVs) and EV fractions from MDA-MB-231 cells stimulated with 10 nM IGF-1 for 48 h (IGF-1 EVs) were characterized by TEM and WB with Abs against Flot-2, CD63 and CD9, because these proteins are molecular markers associated with EVs (Raposo and Stoorvogel 2013). TEM demonstrated the presence of vesicles with sizes between 50 and 500 nm, and WB showed the presence of Flot-2, CD63 and CD9 in Ctrl EVs and IGF-1 EVs (Fig. 1a, b).
Fig. 1.
IGF-1 EVs induce migration in mammary non-tumorigenic epithelial cells MCF10A. a and b Ctrl EVs and IGF-1 EVs were analyzed by TEM and WB with anti-Flot-2 Ab, anti-CD63 Ab and anti-CD9 Ab. One control of culture medium depleted of EVs was included (CM). c and d Migration assays were performed with MCF10A and MDA-MB-231 cells treated with Ctrl EVs and EVs from MDA-MB-231 cells stimulated with 10 nM IGF-1 for 12, 24, 36 and 48 h. One control of MCF10A and MDA-MB-231 cells without treatment with EVs (Basal) and a control of migration (FBS) were included. Graphs represent the mean ± SD and indicate the fold of migration above Ctrl EVs value. *P˂0.05, **P˂0.01, ***P˂0.001
EVs from MDA-MB-231 cells stimulated with IGF-1 induce migration in MCF10A cells
In order to determine whether EVs from MDA-MB-231 cells stimulated with IGF-1 for different periods of time induced migration in MCF10A cells, migration assays were performed with MCF10A cells treated for 48 h with Ctrl EVs and EVs from MDA-MB-231 cells stimulated with 10 nM IGF-1 for 12, 24, 36 and 48 h (Fig. S3). Results demonstrated that EVs from MDA-MB-231 cells stimulated with 10 nM IGF-1 for 12–48 h induced migration, whereas treatment with Ctrl EVs did not induce migration in MCF10A cells (Fig. 1c).Therefore, our experiments were performed with EV fractions from MDA-MB-231 cells stimulated with 10 nM IGF-1 for 48 h.
Next, we determined whether EVs from MDA-MB-231 cells stimulated with IGF-1 for different periods of time induced migration in MDA-MB-231 cells. Migration assays were performed with MDA-MB-231 cells treated with Ctrl EVs and EVs from MDA-MB-231 cells stimulated with 10 nM IGF-1 for 12, 24, 36 and 48 h (Fig. S3). Results demonstrated that EVs from MDA-MB-231 cells stimulated with 10 nM IGF-1 for 12–48 h did not induce migration in MDA-MB-231 cells (Fig. 1d).
IGF-1 EVs regulate E-cadherin and N-cadherin expression
We determined whether IGF-1 EVs induced downregulation of E-cadherin expression and an increase of N-cadherin expression in MCF10A cells. Cultures of MCF10A cells were treated with Ctrl EVs and IGF-1 EVs for various periods of time and then total RNA and cell lysates were obtained. E-cadherin transcripts were analyzed by RT-qPCR and cell lysates were analyzed by WB with anti-E-cadherin Ab and anti-N-cadherin Ab. Findings showed that IGF-1 EVs induced downregulation of E-cadherin transcripts at 9 h of treatment in MCF10A cells (Fig. 2a). Moreover, IGF-1 EVs induced a transient downregulation of E-cadherin expression at 9 h of treatment, and an increase of N-cadherin expression at 9, 12 and 24 h of treatment in MCF10A cells (Fig. 2b, c). In contrast, Ctrl EVs did not induce downregulation of E-cadherin or an increase of N-cadherin expression at 9, 12 and 18 h of treatment in MCF10A cells (Fig. S4).
Fig. 2.
IGF-1 EVs regulate expression of E- and N-cadherin. MCF10A cells were treated for various periods of time with Ctrl EVs and IGF-1 EVs and then total RNA or cell lysates were obtained. a E-cadherin transcripts were analyzed by RT-qPCR. b and c Cell lysates were analyzed by WB with anti-E-cadherin Ab, anti-N-cadherin Ab and anti-actin Ab. One control of MCF10A cells without treatment with EVs was included (Basal). Graphs represent the mean ± SD and indicate the fold of E-cadherin and N-cadherin above Ctrl EVs value. *P˂0.05, **P˂0.01
IGF-1 EVs induce an increase of E-cadherin transcriptional repressors
Transcripts of Snail1, Twist1 and Sip1, which are E-cadherin transcriptional repressors, were analyzed by RT-qPCR using total RNA from MCF10A cells treated for 6 and/or 9 h with Ctrl EVs and IGF-1 EVs. Findings showed that treatment of MCF10A cells with IGF-1 EVs induced an increase of Snail1 transcripts at 6 and 9 h of treatment, and an increase of Twist1 and Sip1 transcripts at 6 h of treatment (Fig. 3a–c).
Fig. 3.
IGF-1 EVs induce an increase of E-cadherin transcriptional repressors. a–c MCF10A cells were treated for various periods of time with Ctrl EVs and IGF-1 EVs and then total RNA was obtained. One control of MCF10A cells without treatment with EVs was included (Basal). Snail1, Twist1 and Sip1 transcripts were analyzed by RT-qPCR. Comparisons were made to cells treated with Ctrl EVs. *P˂0.01, **P˂0.01, ***P˂0.001
IGF-1 EVs enhance vimentin expression
To determine whether treatment with IGF-1 EVs induced an increase of vimentin expression, cultures of MCF10A cells were treated with Ctrl EVs and IGF-1 EVs for various periods of time and then total RNA and cell lysates were obtained. Vimentin expression was analyzed by RT-qPCR and WB with anti-vimentin Ab. Results showed that IGF-1 EVs induced an increase of vimentin transcripts at 6 h and an increase of vimentin expression at 9, 12 and 24 h of treatment in MCF10A cells (Fig. 4a, b).
Fig. 4.
IGF-1 EVs induce an increase of vimentin expression. MCF10A cells were treated for various periods of time with Ctrl EVs and IGF-1 EVs and then total RNA or cell lysates were obtained. a Vimentin transcripts were analyzed by RT-qPCR. Comparisons were made to cells treated with Ctrl EVs. b Cell lysates were analyzed by WB with anti-vimentin Ab and anti-actin Ab. Graph represents the mean ± SD and indicate the fold of vimentin above Ctrl EVs value. c MCF10A cells cultured on coverslips were stimulated for 48 h with Ctrl EVs and IGF-1 EVs. Cells were stained with anti-vimentin Ab, TRITC-conjugated phalloidin and nucleus were stained with Hoechst dye. Cells were analyzed by confocal microscopy. One control of MCF10A cells without treatment with EVs was included (Basal). Scale bar 40 μm. *P˂0.05, **P˂0.01
To further substantiate our findings, we studied vimentin expression by using confocal microscopy in scratch-wound assays of MCF10A cells treated for 48 h with Ctrl EVs and IGF-1 EVs. As illustrated in Fig. 4c, MCF10A cells treated with Ctrl EVs had a little bit of vimentin expression, however treatment with IGF-1 EVs remarkably induced an increase of vimentin expression, and it was mainly localized in migrating cells.
IGF-1 EVs induce an increase of MMP-9 secretion and invasion
To determine whether treatment with IGF-1 EVs induced an increase of MMP-2 and MMP-9 secretion, MCF10A cells were treated for various periods of time with Ctrl EVs and IGF-1 EVs, and then conditioned media were obtained and cells were lysed. MMP-2 and MMP-9 secretion was analyzed by gelatin zymography of conditioned media, and cell lysates were analyzed by WB with anti-actin Ab. Findings showed that IGF-1 EVs only induced an increase of MMP-9 secretion at 24 h of treatment in MCF10A cells. WB of cell lysates with anti-actin Ab confirmed that similar number of MCF10A cells was stimulated with Ctrl EVs and IGF-1 EVs (Fig. 5a).
Fig. 5.
IGF-1 EVs induce an increase of MMP-9 secretion and invasion. a MCF10A cells were treated for various periods of time with Ctrl EVs and IGF-1 EVs and then conditioned media were obtained. MMP-2 and MMP-9 secretion was analyzed by gelatin-substrate gels. Positive controls of MMP-2 (EtOH) and MMP-9 (PDB) secretion were included. One control of MCF10A cells without treatment with EVs was included (Basal). Graph represents the mean ± SD and indicate the fold of MMP-2 and MMP-9 secretion above Ctrl EVs value. Comparisons were made to cells treated with Ctrl EVs and basal values as indicated. b Invasion assays were performed with MCF10A cells treated with Ctrl EVs and IGF-1 EVs for 72 h. One control of MCF10A cells without treatment with EVs (Basal) and a control of invasion (FBS) were included. Graph represents the mean ± SD and indicate the fold of invasion above Ctrl EVs value. **P˂0.01, ***P˂0.001
Invasion assays were performed using MCF10A cells treated with Ctrl EVs and IGF-1 EVs for 72 h. As illustrated in Fig. 5b, treatment with IGF-1 EVs induced invasion in MCF10A cells.
IGF-1 EVs induce migration via ERK1/2
We determined whether IGF-1 EVs induced ERK1/2 activation, which is mediated by their phosphorylation at Thr-202 and Tyr-204 (p-ERK1/2) (Guo et al. 2020). MCF10A cells were treated for various periods of time with Ctrl EVs and IGF-1 EVs and lysed. Cell lysates were analyzed by WB with anti-p-ERK1/2 Ab. Findings showed that IGF-1 EVs induced an increase of ERK1/2 phosphorylation at Thr-202 and Tyr-204, reaching a maximum at 20 min of treatment (Fig. 6a).
Fig. 6.
IGF-1 EVs induce migration via ERK1/2 activity. a MCF10A cells were treated for various periods of time with Ctrl EVs and IGF-1 EVs and lysed. Cell lysates were analyzed by WB with anti-p-ERK1/2 Ab, anti-ERK1/2 Ab and anti-actin Ab. b Migration assays were performed with MCF10A cells pretreated for 1 h with 50 nM ERK1/2 inhibitor and treated with Ctrl EVs and IGF-1 EVs for 48 h. One control of MCF10A cells without treatment with EVs was included (Basal). Graphs represent the mean ± SD and indicate the fold of p-ERK1/2 and migration above Ctrl EVs value. *P˂0.05, ***P˂0.001
To determine the role of ERK1/2 in cell migration induced by IGF-1 EVs, migration assays were performed using MCF10A cells pretreated with 50 nM ERK1/2 inhibitor for 1 h and then treated with Ctrl EVs and IGF-1 EVs for 48 h. Findings showed that treatment with ERK1/2 inhibitor completely inhibited migration induced by IGF-1 EVs in MCF10A cells (Fig. 6b).
IGF-1 EVs induce migration via Akt activity
To determine whether IGF-1 EVs induced Akt1 and Akt2 activation, which is mediated by their phosphorylation at Ser-473 (p-Akt1) and Ser-474 (p-Akt2) of Akt1 and Akt2 respectively (Dillon et al. 2009), lysates from MCF10A cells stimulated for various periods of time with Ctrl EVs and IGF-1 EVs were analyzed by IP with anti-Akt1 Ab and anti-Akt2 Ab, followed by WB with anti-p-Akt Ab. Findings showed that IGF-1 EVs induced an increase of phosphorylation of Akt1 at Ser-473 and Akt2 at Ser-474 in MCF10A cells, reaching a maximum at 20 min of treatment (Fig. 7a, b).
Fig. 7.
IGF-1 EVs induce migration via Akt1/2 activity. a and b MCF10A cells were treated for various periods of time with Ctrl EVs and IGF-1 EVs and lysed. Cell lysates were analyzed by IP with anti-Akt1 Ab and anti-Akt2 Ab followed by WB with anti-p-Akt Ab. Membranes were reprobed with anti-Akt1 Ab and anti-Akt2 Ab. Cell lysates were also analyzed by WB with anti-actin Ab. c Migration assays were performed with MCF10A cells pretreated for 1 h with 2 μM A6730 and treated with Ctrl EVs and IGF-1 EVs for 48 h. One control of MCF10A cells without treatment with EVs was included (Basal). Graphs represent the mean ± SD and indicate the fold of p-Akt1, p-Akt2 and migration above Ctrl EVs value. *P˂0.05, **P˂0.01, ***P˂0.001
To determine the role of Akt1/2 in cell migration induced by IGF-1 EVs, migration assays were performed with MCF10A cells pretreated with 2 μM A6730 (Akt1/2 inhibitor) for 1 h, and then treated with Ctrl EVs and IGF-1 EVs for 48 h. As illustrated in Fig. 7c, treatment with A6730 completely inhibited the migration induced by IGF-1 EVs in MCF10A cells.
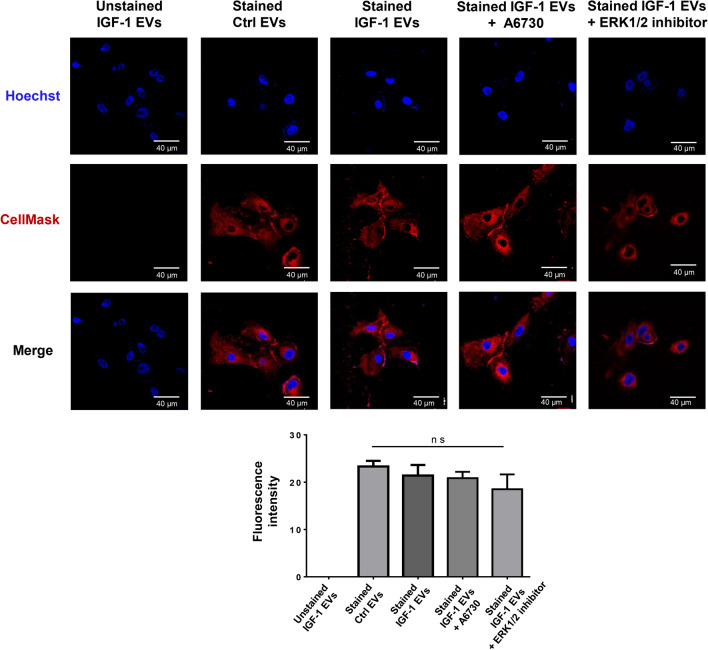
Ctrl EVs and IGF-1 EVs are taken up by MCF10A cells
To determine whether Ctrl EVs and IGF-1 EVs were taken up by MCF10A cells, Ctrl EVs and IGF-1 EVs were stained using CellMask™ orange dye. Next, MCF10A cells were treated with unstained IGF-1 EVs, stained Ctrl EVs and stained IGF-1 EVs for 20 min, and then cells were analyzed by confocal microscopy. Findings showed that MCF10A cells treated with stained Ctrl EVs and stained IGF-1 EVs had a red staining and it was higher compared with cells treated with unstained IGF-1 EVs. Analysis of fluorescence values between stained Ctrl EVs and stained IGF-1 EVs demonstrated that there is not a significant difference between these values. Moreover, the role of Akt1/2 and ERK1/2 activity in the uptake of IGF-1 EVs was studied. MCF10A cells were pretreated with A6730 and ERK1/2 inhibitor and then treated with stained IGF-1 EVs. Results demonstrated that inhibition of ERK1/2 and Akt1/2 activity did not inhibit the uptake of IGF-1 EVs in MCF10A cells (Fig. 8).
Fig. 8.
Ctrl EVs and IGF-1 EVs are taken up by MCF10A cells. Confocal microscopy analysis of MCF10A cells untreated and pretreated with A6730 and ERK1/2 inhibitor and then treated with unstained IGF-1 EVs, stained Ctrl EVs and stained IGF-1 EVs. Nucleus were stained with Hoechst dye. Scale bar 40 μm. Graph represents the mean ± SD and indicate the fold of fluorescence intensity value above Ctrl EVs fluorescence intensity value. ns, not significant
Discussion
IGF system contributes to progression and development of breast cancer through a variety of mechanisms, including overexpression of IGF-1 and IGF-2, changes in the expression of IGF receptors and reduction of circulating IGFBPs levels (Farabaugh et al. 2015; Motallebnezhad et al. 2016). Moreover, IGF-1R is overexpressed in breast cancer, and its expression has been related with an aggressive phenotype, poor clinical outcome and therapy resistance (Christopoulos et al. 2015; Jones et al. 2007; Papa et al. 1993). However, the role of EVs from MDA-MB-231 breast cancer cells stimulated with IGF-1 in the EMT process has not been studied.
In this study, TEM demonstrate that we isolated a heterogeneous population of EVs from MDA-MB-231 breast cancer cells unstimulated and stimulated with IGF-1, while WB demonstrate the presence of specific markers of EVs. We hypothesize that Ctrl EVs and IGF-1 EVs have a variety of specific cargos, and that only IGF-1 EVs are able to induce cellular processes involved in the EMT in mammary non-tumorigenic epithelial cells MCF10A.
Metastasis is mediated by the EMT, because during EMT process the epithelial tumor cells acquire the ability to migrate and invade the surrounding tissues (Thiery et al. 2009). We demonstrate that IGF-1 EVs induce migration in MCF10A cells. However, IGF-1 EVs do not induce migration in MDA-MB-231 cells, whereas IGF-1 induces migration in MDA-MB-231 cells. Our findings demonstrate that IGF-1 EVs are not able to mediate migration via an autocrine and/or paracrine mechanism in MDA-MB-231 cells. We propose that stimulation of MDA-MB-231 cells with IGF-1 mediates specific changes in the cargo of secreted EVs compared with EVs released from unstimulated MDA-MB-231 cells (Ctrl EVs), and then IGF-1 EVs are able to induce activation of signal transduction pathways that mediate migration in MCF10A cells. In contrast, cargos in IGF-1 EVs are not able to activate signal transduction pathways that mediate migration in MDA-MB-231 cells. Supporting our proposal, stimulation of MDA-MB-231 cells with linoleic acid (LA) releases EVs that mediate cellular processes involved with angiogenesis in human umbilical vein endothelial cells (HUVECs), whereas EVs released from unstimulated MDA-MB-231 cells are not able to mediate these cellular processes (Garcia-Hernandez et al. 2020).
EMT process requires that non-motile epithelial cells acquire motile and invasive properties through the disassembly of adherens junctions (Thiery et al. 2009). Particularly, disassembly of adherens junctions involves the loss or reduction of E-cadherin expression and the cytoskeleton reorganization with the assembly of actin stress fibers anchored to focal adhesions (Gumbiner 2005; Lee et al. 2006). Downregulation of E-cadherin expression is mediated by a variety of transcription factors including Snail1, Snail2, Twist1, ZEB2, E47 and Sip1, which directly bind to specific sequences (E-boxes) on E-cadherin gene promoter and downregulate its transcription (Baranwal and Alahari 2009; Cano et al. 2000; Peinado et al. 2004). Our findings demonstrate that IGF-1 EVs induce downregulation of E-cadherin transcripts at 9 h of treatment and a transient downregulation of E-cadherin protein expression in MCF10A cells. Furthermore, IGF-1 EVs induce a transient upregulation of E-cadherin transcriptional repressors including Snail1, Twist1 and Sip1, whereas Ctrl EVs induce downregulation of Twist1 transcripts in MCF10A cells. Our findings support the proposal that treatment of MDA-MB-231 cells with IGF-1 promotes changes in EVs cargos that mediate downregulation of E-cadherin expression in MCF10A cells. Moreover, we propose that Ctrl EVs have cargos that inhibit the expression of Twist1 in MCF10A cells, however it remains to be studied. Supporting our proposal, EVs from PAR-2 activated MDA-MB-231 cells induce downregulation of E-cadherin expression through transfer of microRNA221 to target MCF-7 breast cancer cells (Das et al. 2019).
During EMT process, epithelial cells assume a mesenchymal phenotype through acquisition of mesenchymal markers including N-cadherin, fibronectin, α-SMA, vimentin, and fibroblast-specific-protein1 (Saitoh 2018). Our results demonstrate that IGF-1 EVs induce a transient increases of N-cadherin and vimentin expression at 9, 12 and 24 h of treatment in MCF10A cells. Moreover, IGF-1 EVs induce an increase of vimentin expression mainly in motile cells MCF10A, which are localized at the edges of the scratch. In agreement with our findings, EVs from MDA-MB-231 cells stimulated with LA induce a transient increase of vimentin expression with two peaks of maximal expression, and a transient increase of N –cadherin expression with one peak of maximal expression in MCF10A cells (Galindo-Hernandez et al. 2014). Moreover, LA induces a transient increase of N-cadherin and vimentin expression with one peak of maximal expression in MCF10A cells (Espinosa-Neira et al. 2011). Since, EMT is a transient process used for tumor cells in the invasion and metastasis process (Thiery et al. 2009), we propose that different stimulators of EMT process induce the expression at different times of specific mesenchymal markers, including vimentin and N-cadherin, in MCF10A cells. Our findings support the proposal that IGF-1 EVs induce an EMT process in MCF10A cells and they suggest that IGF-1 EVs play an important role in tumor invasion and metastasis. Supporting our proposal, N-cadherin is involved in the detachment of tumor cells, migration and invasion; whereas the intermediate filament vimentin has been related with migration of epithelial cells and tumor invasion (Gavert and Ben-Ze'ev 2008; Hulit et al. 2007; Thiery 2003).
One of the hallmarks of EMT is the capacity to invade the extracellular matrix, which involves the activation of signal transduction pathways that mediate specific cellular processes including the activation of NFκB and the secretion of MMPs (Gavert and Ben-Ze'ev 2008; Lu and Kang 2019). Particularly, NFκB is involved in the EMT process because it mediates expression of vimentin, MMP-9, Snail1/2, ZEB1/2 and Sip1 (Min et al. 2008). We demonstrate that IGF-1 EVs induce an increase of MMP-9 secretion and invasion in MCF10A cells. Interestingly, Ctrl EVs induce an increase of MMP-9 secretion but they do not induce invasion in MCF10A cells. EMT process involves a variety cell processes, including the loss of cell–cell adhesions, secretion of MMPs, remodeling of actin cytoskeleton, expression of specific integrins and invasion (Thiery et al. 2009; Yilmaz and Christofori 2009). We propose that Ctrl EVs have cargos that induce some cellular processes related with the EMT process, such as secretion of MMP-9, but they do not have all the cargos required to mediate an EMT process in MCF10A cells. Moreover, our results support the proposal that IGF-1 EVs induce an EMT process in MCF10A cells. In agreement with our findings, EVs from MCF-7 and MDA-MB-231 breast cancer cells induce secretion of MMP-9, TGF-β and IL-6 in THP-1 monocytic cells (Redzic et al. 2013). Supporting our proposal, EVs from MDA-MB-231 cells stimulated with LA and EVs from breast cancer patients induce an increase of MMP-2 and MMP-9 secretion, invasion and an EMT process in MCF10A cells (Galindo-Hernandez et al. 2015; Galindo-Hernandez et al. 2014). Moreover, our findings support the proposal that ligands used for stimulation of breast cancer cells induce the release of EVs with a variety of cargos that mediate specific cellular responses in their target cells.
ERK1/2 signaling pathway regulates basic cellular processes including proliferation and differentiation, whereas hyperactivation of ERK1/2 has been implicated in cancer, inflammation and development disorders (Guo et al. 2020). Our findings demonstrate that IGF-1 EVs induce ERK1/2 activation and migration via ERK1/2 activity in MCF10A cells. Since, migration is a hallmark of EMT process (Gavert and Ben-Ze'ev 2008), our results demonstrate that IGF-1 EVs mediate an EMT process through ERK1/2 activation in MCF10A cells. In agreement with our findings, exosomes released from the highly metastatic hepatocellular carcinoma cell line MHCC97H induce an EMT process via ERK1/2 activation in the less aggressive hepatocellular carcinoma cell line HLE (Chen et al. 2018). Moreover, EVs from human umbilical cord mesenchymal stem cells induce migration and invasion via ERK1/2 activity in MCF-7 and MDA-MB-231 breast cancer cells (Zhou et al. 2019). Our findings strongly suggest that EVs from breast cancer cells stimulated with IGF-1 play an important role in tumor invasion and metastasis in breast cancer through an EMT process mediated by ERK1/2 activation.
Mouse models and cell cultures have demonstrated that Akt1 participates in tumor initiation and that Akt1 activation has anti-migratory and anti-metastatic properties independent of breast cancer subtype (Dillon et al. 2009; Hinz and Jucker 2019; Li et al. 2018). In contrast, Akt2 activation mediates migration, invasion and metastasis (Dillon et al. 2009; Hinz and Jucker 2019; Irie et al. 2005). Our findings demonstrate that IGF-1 EVs induce Akt1 and Akt2 activation, and migration via Akt1 and/or Akt2 activity in MCF10A cells. Since, we used an inhibitor of Akt1 and Akt2 (A6730), we were not able to determine whether Akt1 and/or Akt2 mediate the migration induced by IGF1-EVs. Interestingly, EVs from MDA-MB-231 cells stimulated with LA do not induce Akt1 activation, but they induce Akt2 activation and migration via Akt2 activity in MCF10A cells (Leal-Orta et al. 2019). Moreover, exosomes from the highly metastatic hepatocellular carcinoma cell line MHCC97H induce migration in the less aggressive hepatocellular carcinoma cell line HLE via an independent pathway of Akt1/Akt2, whereas EVs from endothelial cells accelerate skin wound healing in diabetic mice and induce Akt1 activation in skin fibroblasts from diabetic patients (Chen et al. 2018; Wei et al. 2020). These findings support the proposal that EVs released from different cell types unstimulated or stimulated with specific ligands induce activation of specific signal transduction pathways that mediate specific cellular responses in their target cells.
Cancer cells communicate with surrounding cells and stroma through release and uptake of EVs, which are composed of a variety of cargos that mediate different cellular responses including angiogenesis, migration and invasion (Jurj et al. 2020; Ramirez-Ricardo et al. 2020). Our findings demonstrate that Ctrl EVs and IGF-1 EVs are taken up for the MCF10A cells. In agreement with our findings, EVs from MDA-MB-231 cells unstimulated and stimulated with LA are taken up for MCF10A cells, and EVs from healthy women and breast cancer patients are taken up for MDA-MB-231 cells (Leal-Orta et al. 2019; Ramirez-Ricardo et al. 2020). Moreover, our findings demonstrate that ERK1/2 and Akt1/2 activities are not required for the uptake of IGF-1 EVs in MCF10A cells. In agreement with our results, EVs from MDA-MB-231 cells stimulated with LA are taken up for MCF10A cells through an independent process of PI3K activity (Leal-Orta et al. 2019). It remains to be studied the role of ERK1/2 and Akt1/2 activities in the uptake of Ctrl EVs in MCF10A cells, and the signal transduction pathways involved in the uptake of Ctrl EVs and IGF-1 EVs in MCF10A cells.
In conclusion, our findings demonstrate that EVs from MDA-MB-231 breast cancer cells stimulated with 10 nM IGF-1 induce an EMT process in mammary epithelial cells MCF10A (Fig. 9). Our findings describe a new role for IGF-1 EVs in the EMT process, and they strongly suggest that IGF-1 plays an important role in the invasion and metastasis process in breast cancer via the release of EVs from breast cancer cells.
Fig. 9.
IGF-1 EVs induce an EMT process in mammary non-tumorigenic epithelial cells MCF10A
Supplementary Information
Below is the link to the electronic supplementary material.
Schematic diagram showing the general experimental procedure. (TIF 1470 KB)
IGF-1 Induces migration in MDA-MB-231 breast cancer cells. Migration assays were performed with MDA-MB-231 cells unstimuated (Basal) and stimulated with 1, 5, 10, 20 and 50 nM IGF-1 for 48. One control of migration was included (FBS). Graph represents the mean ± SD and indicate the fold of migration above basal value. *P˂0.05, ***P˂0.001. (TIF 1845 KB)
Schematic diagram showing the general experimental procedure of stimulation of MDA-MB-231 cells with 10 nM IGF-1 for 12, 24, 36 and 48 h and the isolation of EV fractions, as well as the treatment of MCF10A or MDA-MB-231 cells with the EVs isolated. (TIF 1389 KB)
Ctrl EVs do not regulate the expression of E- and N-cadherin. MCF10A cells were treated for various periods of time with Ctrl EVs and lysed. a and b Cell lysates were analyzed by WB with anti-E-cadherin Ab, anti-N-cadherin Ab and anti-actin Ab. One control of MCF10A cells without treatment with EVs was included (Basal). Graphs represent the mean ± SD and indicate the fold of E-cadherin and N-cadherin above basal value. ns: not significant. (TIF 498 KB)
Acknowledgements
We are grateful to Nora Ruiz and Maria de Lourdes-Rojas (LaNSE, Cinvestav-IPN) for their technical assistance in TEM.
Funding
This research was funded by CONACYT (255429) and CONACYT-FOSISS (Salud 2015-1-261637), Mexico. Grants from CONACYT supported E L-O, J R-R and AG-H.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol/hematol. 2013;87:201–223. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranwal S, Alahari SK. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384:6–11. doi: 10.1016/j.bbrc.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cano A, et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Chen L, et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9:513. doi: 10.1038/s41419-018-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, et al. Triple-negative breast cancer-derived microvesicles transfer microRNA221 to the recipient cells and thereby promote epithelial-to-mesenchymal transition. J Biol Chem. 2019;294:13681–13696. doi: 10.1074/jbc.RA119.008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009;69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MJ, McGowan PM, Crown J. Targeted therapy for triple-negative breast cancer: where are we? Int J Cancer. 2012;131:2471–2477. doi: 10.1002/ijc.27632. [DOI] [PubMed] [Google Scholar]
- Espinosa-Neira R, Mejia-Rangel J, Cortes-Reynosa P, Salazar EP. Linoleic acid induces an EMT-like process in mammary epithelial cells MCF10A. Int J Biochem Cell Biol. 2011;43:1782–1791. doi: 10.1016/j.biocel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Farabaugh SM, Boone DN, Lee AV. Role of IGF1R in breast cancer subtypes stemness, and lineage differentiation. Front Endocrinol. 2015;6:59. doi: 10.3389/fendo.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Galindo-Hernandez O, et al. Extracellular vesicles from women with breast cancer promote an epithelial-mesenchymal transition-like process in mammary epithelial cells MCF10A. Tumour Biol: J Int Soc Oncodev Biol Med. 2015;36:9649–9659. doi: 10.1007/s13277-015-3711-9. [DOI] [PubMed] [Google Scholar]
- Galindo-Hernandez O, Serna-Marquez N, Castillo-Sanchez R, Salazar EP. Extracellular vesicles from MDA-MB-231 breast cancer cells stimulated with linoleic acid promote an EMT-like process in MCF10A cells. Prostaglandins, Leukot, Essential Fatty Acids. 2014;91:299–310. doi: 10.1016/j.plefa.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–618. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hernandez A, Leal-Orta E, Ramirez-Ricardo J, Cortes-Reynosa P, Thompson-Bonilla R, Salazar EP. Linoleic acid induces secretion of extracellular vesicles from MDA-MB-231 breast cancer cells that mediate cellular processes involved with angiogenesis in HUVECs. Prostaglandins Other Lipid Med. 2020;153:106519. doi: 10.1016/j.prostaglandins.2020.106519. [DOI] [PubMed] [Google Scholar]
- Gavert N, Ben-Ze'ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199–209. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Godone RLN, Leitao GM, Araujo NB, Castelletti CHM, Lima-Filho JL, Martins DBG. Clinical and molecular aspects of breast cancer: targets and therapies. Biomed Pharmacother = Biomed Pharmacother. 2018;106:14–34. doi: 10.1016/j.biopha.2018.06.066. [DOI] [PubMed] [Google Scholar]
- Greening DW, Gopal SK, Mathias RA, Liu L, Sheng J, Zhu HJ, Simpson RJ. Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression. Semin Cell Dev Biol. 2015;40:60–71. doi: 10.1016/j.semcdb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Gross JM, Yee D. The type-1 insulin-like growth factor receptor tyrosine kinase and breast cancer: biology and therapeutic relevance. Cancer Metastasis Rev. 2003;22:327–336. doi: 10.1023/a:1023720928680. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz N, Jucker M. Distinct functions of AKT isoforms in breast cancer: a comprehensive review. Cell Commun Signal: CCS. 2019;17:154. doi: 10.1186/s12964-019-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulit J, et al. N-cadherin signaling potentiates mammary tumor metastasis via enhanced extracellular signal-regulated kinase activation. Can Res. 2007;67:3106–3116. doi: 10.1158/0008-5472.CAN-06-3401. [DOI] [PubMed] [Google Scholar]
- Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- Irie HY, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Moorehead RA. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26:1636–1644. doi: 10.1038/sj.onc.1209955. [DOI] [PubMed] [Google Scholar]
- Jurj A, Zanoaga O, Braicu C, Lazar V, Tomuleasa C, Irimie A, Berindan-Neagoe I. A comprehensive picture of extracellular vesicles and their contents. Mol Transf Cancer Cells Cancers. 2020 doi: 10.3390/cancers12020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanada M, Bachmann MH, Contag CH. Signaling by extracellular vesicles advances cancer hallmarks. Trends Cancer. 2016;2:84–94. doi: 10.1016/j.trecan.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Ke Z, et al. MMP-2 mediates ethanol-induced invasion of mammary epithelial cells over-expressing ErbB2. Int J Cancer. 2006;119:8–16. doi: 10.1002/ijc.21769. [DOI] [PubMed] [Google Scholar]
- Leal-Orta E, Ramirez-Ricardo J, Cortes-Reynosa P, Galindo-Hernandez O, Salazar EP. Role of PI3K/Akt on migration and invasion of MCF10A cells treated with extracellular vesicles from MDA-MB-231 cells stimulated with linoleic acid. J Cell Commun Signal. 2019;13:235–244. doi: 10.1007/s12079-018-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Batth IS, Qu X, Xu L, Song N, Wang R, Liu Y. IGF-IR signaling in epithelial to mesenchymal transition and targeting IGF-IR therapy: overview and new insights. Mol Cancer. 2017;16:6. doi: 10.1186/s12943-016-0576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, et al. Akt1 inhibition promotes breast cancer metastasis through EGFR-mediated beta-catenin nuclear accumulation. Cell Commun Signal: CCS. 2018;16:82. doi: 10.1186/s12964-018-0295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49:361–374. doi: 10.1016/j.devcel.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- Motallebnezhad M, Aghebati-Maleki L, Jadidi-Niaragh F, Nickho H, Samadi-Kafil H, Shamsasenjan K, Yousefi M. The insulin-like growth factor-I receptor (IGF-IR) in breast cancer: biology and treatment strategies. Tumour Biol: J Int Soc Oncodev Biol Med. 2016;37:11711–11721. doi: 10.1007/s13277-016-5176-x. [DOI] [PubMed] [Google Scholar]
- Papa V, et al. Insulin-like growth factor-I receptors are overexpressed and predict a low risk in human breast cancer. Can Res. 1993;53:3736–3740. [PubMed] [Google Scholar]
- Park MJ, et al. Protein kinase C activation by phorbol ester increases in vitro invasion through regulation of matrix metalloproteinases/tissue inhibitors of metalloproteinases system in D54 human glioblastoma cells. Neurosci Letters. 2000;290:201–204. doi: 10.1016/s0304-3940(00)01358-6. [DOI] [PubMed] [Google Scholar]
- Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ricardo J, et al. Circulating extracellular vesicles from patients with breast cancer enhance migration and invasion via a Srcdependent pathway in MDAMB231 breast cancer cells. Mol Med Rep. 2020;22:1932–1948. doi: 10.3892/mmr.2020.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzic JS, et al. Extracellular vesicles secreted from cancer cell lines stimulate secretion of MMP-9, IL-6, TGF-beta1 and EMMPRIN. PLoS ONE. 2013;8:e71225. doi: 10.1371/journal.pone.0071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M. Involvement of partial EMT in cancer progression. J Biochem. 2018;164:257–264. doi: 10.1093/jb/mvy047. [DOI] [PubMed] [Google Scholar]
- Seccareccia E, Brodt P. The role of the insulin-like growth factor-i receptor in malignancy: an update. Growth Horm IGF Res: off J Growth Horm Res Soc Int IGF Res Soc. 2012;22:193–199. doi: 10.1016/j.ghir.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. AmJ Physiol Cell Physiol. 2014;306:C621–633. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- Vader P, Breakefield XO, Wood MJ. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014;20:385–393. doi: 10.1016/j.molmed.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagia E, Mahalingam D, Cristofanilli M. The landscape of targeted therapies in TNBC. Cancers. 2020 doi: 10.3390/cancers12040916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cao S, Chen Y. Molecular treatment of different breast cancers. Anticancer Agents Med Chem. 2015;15:701–720. doi: 10.2174/1871520615666150129211901. [DOI] [PubMed] [Google Scholar]
- Wei F, et al. Plasma endothelial cells-derived extracellular vesicles promote wound healing in diabetes through YAP and the PI3K/Akt/mTOR pathway. Aging. 2020;12:12002–12018. doi: 10.18632/aging.103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407–420. doi: 10.1016/j.cytogfr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- Zhou X, et al. Mesenchymal stem cellderived extracellular vesicles promote the in vitro proliferation and migration of breast cancer cells through the activation of the ERK pathway. Int J Oncol. 2019;54:1843–1852. doi: 10.3892/ijo.2019.4747. [DOI] [PubMed] [Google Scholar]
- Zielinska HA, Bahl A, Holly JM, Perks CM. Epithelial-to-mesenchymal transition in breast cancer: a role for insulin-like growth factor I and insulin-like growth factor-binding protein 3? Breast Cancer. 2015;7:9–19. doi: 10.2147/BCTT.S43932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic diagram showing the general experimental procedure. (TIF 1470 KB)
IGF-1 Induces migration in MDA-MB-231 breast cancer cells. Migration assays were performed with MDA-MB-231 cells unstimuated (Basal) and stimulated with 1, 5, 10, 20 and 50 nM IGF-1 for 48. One control of migration was included (FBS). Graph represents the mean ± SD and indicate the fold of migration above basal value. *P˂0.05, ***P˂0.001. (TIF 1845 KB)
Schematic diagram showing the general experimental procedure of stimulation of MDA-MB-231 cells with 10 nM IGF-1 for 12, 24, 36 and 48 h and the isolation of EV fractions, as well as the treatment of MCF10A or MDA-MB-231 cells with the EVs isolated. (TIF 1389 KB)
Ctrl EVs do not regulate the expression of E- and N-cadherin. MCF10A cells were treated for various periods of time with Ctrl EVs and lysed. a and b Cell lysates were analyzed by WB with anti-E-cadherin Ab, anti-N-cadherin Ab and anti-actin Ab. One control of MCF10A cells without treatment with EVs was included (Basal). Graphs represent the mean ± SD and indicate the fold of E-cadherin and N-cadherin above basal value. ns: not significant. (TIF 498 KB)