Abstract
Tsukushi (TSK), a leucine-rich peptidoglycan in the extracellular compartment, mediates multiple signaling pathways that are critical for development and metabolism. TSK regulates signaling pathways that eventually control cellular communication, proliferation, and cell fate determination. Research on TSK has become more sophisticated in recent years, illustrating its involvement in the physiology and pathophysiology of neural, genetic, and metabolic diseases. In a recent study, we showed that TSK therapy reversed the pathophysiological abnormalities of the hydrocephalic (a neurological disorder) brain in mice. This review summarizes the roles of TSK in key signaling processes in the mammalian development, disorders, and evaluating its possible therapeutic and diagnostic potential.
Keywords: Tsukushi, SLRP, Wnt signaling, Mammalian development, Hydrocephalus, Therapy, Biomarker
Introduction
Small leucine-rich proteoglycans (SLRPs) play a crucial role in extracellular matrix maintenance, usually through collagen fibrillogenesis. Studies have demonstrated that they play an integral role in multiple critical cellular processes, such as proliferation, differentiation, inflammation, immunity, metabolism, and development (Hocking et al. 1998; Hsieh et al. 2017; Li et al. 2019; Nio et al. 2017; Xu et al. 2019). It is also well known that SLRPs are essential for regulating many critical pathways in the digestive, endocrine, and cardiovascular systems, as well as in the bone marrow. In addition, several studies have shown that SLRPs participate in many pathophysiological processes through their interactions with signaling pathways. For example, the SLRP proteins keratocan and fibromodulan contribute to fibrosis. Lumican contributes to hypertension caused by atheromatous lesions, decorin and biglycan contribute to the innate and adaptive immune system, and docorine and podocin are involved in diabetes-related kidney disease (Bolton et al. 2012; Li et al. 2019; Moreth et al. 2010; Zhang et al. 2019). As an integral part of collagen fibrillogenesis, SLRP mutations lead to abnormal collagen architecture, which can cause disease conditions (Matsushima et al. 2019). For example, genetic mutations in the SLRPs (decorin, lucan, keratocan, nyctalopin) genes can cause inherited eye disorders (Matsushima et al. 2021; Schaefer and Iozzo 2008). Additionally, evidence suggests that SLRPs can prevent cancer metastasis (decorin, biglycan, lumican) (Schaefer and Iozzo 2008). TSK, a protein from the class IV SLRP family, was recently discovered to be mutated (human) or absent (mice) in the host of the hydrocephalus phenotype (Ito et al. 2021). Incredibly, when administered at the right time, TSK can reverse the hydrocephalic morphology in the developing mouse brain. TSK has been implicated in neurogenesis, anterior commissure formation, eye development, inner ear development, bone growth and liver homeostasis (Ahmad et al. 2020; Ito et al. 2021; Miwa et al. 2020; Ohta et al. 2011; Wang et al. 2019; Yano et al. 2017). However, only in recent years, has its importance in pathophysiology and therapy been highlighted. This review explains the modulatory role played by TSK in multiple signaling pathways throughout the body involved in development and disorders. In addition, the therapeutic potential of TSK in the brain, and its diagnostic properties in metabolic disorders are also addressed.
Composition and structure of Tsukushi
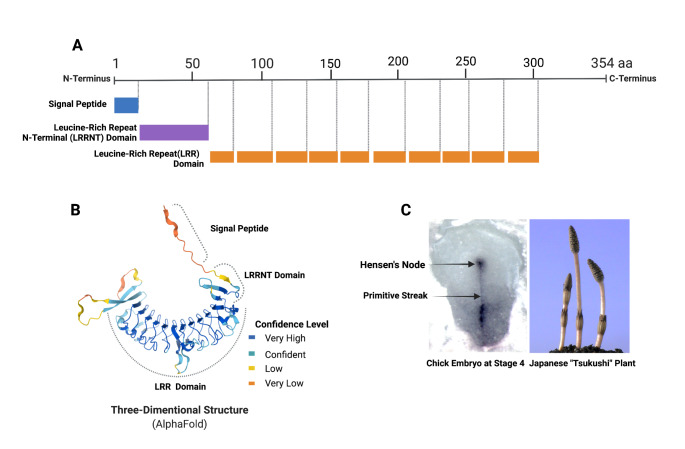
Tsukushi is a class IV SLRP protein discovered in the chick lens as a BMP antagonist. The protein was named after a Japanese horsetail plant of the same name because of its similarity to its expression in chick embryos (Ohta et al. 2004). There are eleven leucine-rich repeat domains on TSK in mice, with a length of 354 amino acids (353 amino acids in humans) and a molecular weight of 38,362 kDa (37,807 kDa in humans). The N-terminal region of this protein includes a signal peptide and a leucin-rich repeat N-terminal domain (LRRNT) with three cysteines, followed by leucine-rich repeats. It shares a C-terminus with the SLRPs nyctaloping and chondoadherin. In comparison to other SLRPs, Tsukushi lacks glycosaminoglycan side chains (GAG) (Matsushima et al. 2021). According to Alphafold structural prediction, Beta sheets are mainly found in the signal peptide region during tertiary structure formation, whereas alpha helices dominate in repeat regions (Fig. 1) (Jumper et al. 2021; Varadi et al. 2022). However, crystal structure for TSK has not been determined. The TSK protein in mice is encoded by a gene located on chromosome 7, called Tsku. The human paralog of Tsku is TSKU, located on the positive strand of chromosome 11. The Tsku gene is conserved in many vertebrates and has 23 paralogs and 204 orthologs. In this review, the term TSK/Tsukushi refers to mouse Tsukushi protein unless the host animal is first mentioned.
Fig. 1.
Composition, structure, and naming origin of Tsukushi; A (Compositional Domains; each box indicates a domain), B (AlphaFold prediction of the tertiary structure, C (Shape similarity in In situ mRNA expression in chick embryo and Tsukushi plant); This figures was Created with BioRender.com
Tsukushi expresses from the embryo to adult stages
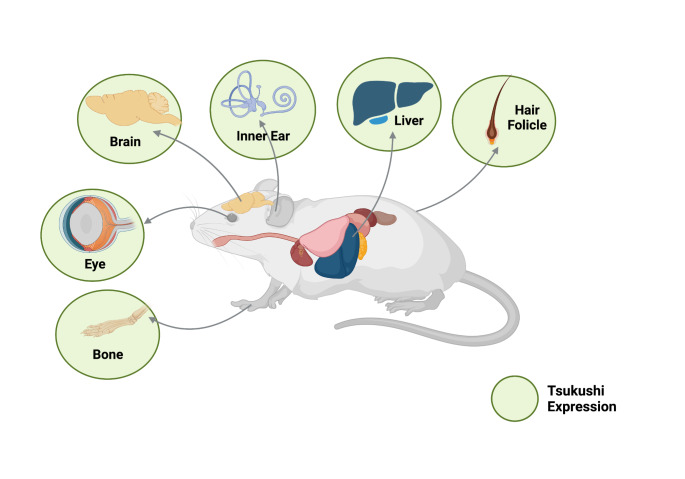
TSK is expressed during the early developmental stages and persists into adulthood, whereas its function varies based on the expressing organ. Studies have shown that several organs and tissues (brain, eye, ear, bone, liver, hair, and blood) predominantly express TSK. Gene expression databases (such as GeneCards and Mouse genome informatics) also enlisted Tsku and TSKU expression in many other unstudied organs (such as heart, thymus, stomach, pancreas, ovary, prostate etc.) as well. However, a comprehensive study of TSK expression in all organs and ages is yet to be conducted. Therefore, we will concentrate on reports that have already been published.
TSK expression was observed in the brain from the early embryonic to adult stages. TSK is expressed in nearly all brain-specific regions during the embryonic stage, but primarily in the anterior commissure, glial wedge, anterior olfactory nucleus, olfactory bulb, piriform cortex, midline zipper glia, and indusium griseum; at the postnatal stage P0, TSK is expressed in the lateral ventricle, hippocampus (it fades after postnatal stage P15), and cortex, which later disappears and is restricted to the subventricular zone (SVZ) (Ahmad et al. 2020; Hossain et al. 2013; Ito et al. 2010). Embryonic stages also express TSK in the inner ear non-prosensory region (early embryonic stages) and prosensory region (late embryonic stages), which later confines in Corti, spiral ganglion cells, and the stria vascularis in adults (Miwa et al. 2020). In the early postnatal stage P21, TSK is found in almost all bones (Yano et al. 2017). TSK is expressed in the developing optic cup of the eye and the adult retinal layer and lens epithelium, primarily in the inner non-pigmented layer (Ohta et al. 2011). During hair morphogenesis and hair cycle (embryonic stage E12 - postnatal stage P30), TSK is expressed in hair follicles (Niimori et al. 2012). In response to an inflamed wound, TSK is also expressed by macrophages and epidermal tissues. (Niimori et al. 2014). Liver hepatocytes produce TSK as a hepatokine (adult stages), which is induced by endoplasmic reticulum stress, inflammation, and a high-fat diet (Fig. 2) (Mouchiroud et al. 2019; Wang et al. 2019; Xiong et al. 2018).
Fig. 2.
TSK expression in the multiple organs of the mouse; age-based variation is ignored; This figure was Created with BioRender.com
Tsukushi is a multi-signaling modulator
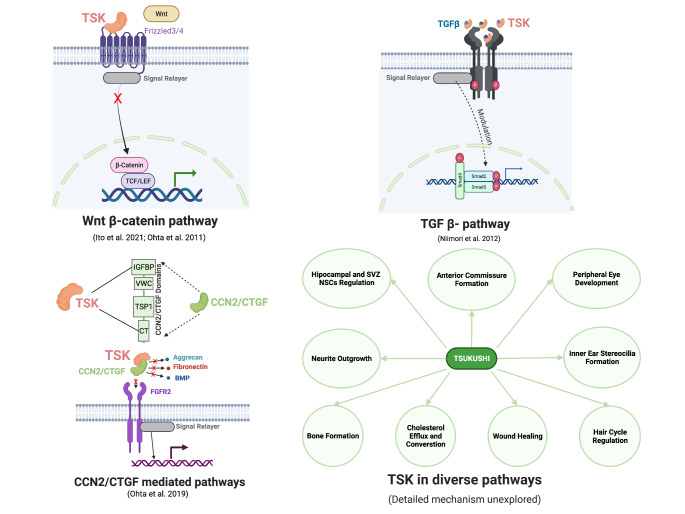
TSK interacts with multiple signaling pathways of cellular processes involved in the development, homeostasis, proliferation, differentiation, and immune response. TSK has been found to influence Wnt, Transcription growth factor beta (TGF-β), cell communication network factor CCN2/CTGF, and netrin signaling pathways in mice by directly binding to the key molecule or its receptor. TSK functions as a Wnt signaling inhibitor and fine-tuning the development process to ensure proper development. TSK competes with Wnt molecule WNT2B to bind to the Wnt receptor Frizzled4 (FZD4), which is required for proper peripheral eye development (Ohta et al. 2011). TSK also binds to frizzled 3 and inhibit Wnt signaling to maintain SVZ neurogenesis (Ito et al. 2021). TSK can play a binary role in TGF-β signaling (increase or suppress) depending on the context of the tissue microenvironment. TSK positively regulates TGF-β1 signaling in the hair cycle by binding with TGF-β1 and increase the expression of TGF-β1 and downstream phosphorylated Smad2/3 (Niimori et al. 2012). On the other hand, TSK control of macrophage TGF-β1 expression which aids wound healing and prevents myofibroblast differentiation. This control also maintains inflammatory cells in a state of dormancy (Niimori et al. 2014). The binary nature of TSK in TGF-β signaling is not properly understood. Furthermore, the structure of the TSK-FDZ4/3 and TSK-TGF-1 complexes needs to be investigated. The only known binding detail for TSK is the interaction between TSK and CCN2. TSK binds to the IGFBP and CT domains of CCN2/CTGF, a CCN family molecule. By binding with BMP2, fibronectin, aggrecan, FGFR2, and LRP6, CCN2/CTGF can regulate bone and cartilage formation, angiogenesis, wound repair and cancer progression implying the possible modulatory role of TSK in these molecules and their relevant pathways (Fig. 3) Aoyama et al. 2012; Ohta et al. 2019, p. 2; Yeger and Perbal 2021). However, whether TSK modulates these pathways positively or negatively remains to be determined.
Fig. 3.
Key pathways regulated by TSK; red “x” sign indicates negative regulation, “modulation” means up or downregulation; This figure is Created with BioRender.com
TSK has also been shown to function as a regulator of neurite outgrowth, hippocampal neural stem cell terminal differentiation, bone elongation, inner ear stereocilia formation, cholesterol efflux, and conversion, although the details of TSK-based regulation in these processes have not been studied (Ahmad et al. 2020; Ito et al. 2010; Miwa et al. 2020; Mouchiroud et al. 2019; Yano et al. 2017).TSK interacts with a variety of pathways to regulate these processes. TSK participates in anterior commissure formation and is expressed in the strategic regions of the developing mouse brain (anterior olfactory nucleus, anterior commissure surrounding, and midline). These regions also express axon guidance molecules such as ephrin receptors Eph, fibroblast growth factor (FGF) receptors, netrin, semaphorin, and draxin, and their absence interferes with proper development, implying that TSK interacts with them and modulates their activity (Hossain et al. 2013; Ito et al. 2010; Lindwall et al. 2007). Finally, TSK controls neural stem cell differentiation in the brain hippocampal neural stem cell niche. This niche is regulated by the Wnt, Bone morphogenic protein (BMP), Notch, Sonic hedgehog (Shh), and insulin-like growth factors (IGF) pathways, implying that TSK interacts with these pathways as well (Ahmad et al. 2020; Nicola et al. 2015; Sueda and Kageyama 2020). TSK appears to interact with a pathway that regulates SOX2 and BMP4 and maintains proper stereocilia formation during inner ear development (Miwa et al. 2020). TSK regulates the differentiation of growth plate chondrocytes, bone elongation, mass, and overall body size during bone growth by modulating the expression of SRY-Box transcription factor SOX9 and Runt-related transcription factor RUNX2 (Yano et al. 2017). SOX9 expression in bone morphogenesis is primarily regulated by the Wnt, BMP, TGF, and FGF pathways, implying that TSK may interact with these molecules or their receptors to regulate SOX9 expression (Fig. 3) (Blitz et al. 2013; Day et al. 2005; Hill et al. 2005; Yoon et al. 2005).
Tsukushi impairment causes disruptive phenotypes
The involvement of TSK in numerous developmental and physiological processes makes it a key molecule that ensure the proper maintenance of some critical molecular functions. Therefore, TSK deficiency impairs the regulation of development and results in abnormal phenotypes. In the brain, TSK deficiency leads to three morphotypes: abnormal anterior commissure, abnormal corpus callosum, and enlarged LV. TSK deficiency causes axons of the anterior and posterior-anterior commissures to fail to cross the midline, resulting in an almost complete absence of anterior commissures in adults. In addition, the hippocampus and dentate gyrus shrink. At the same time, the number of neural stem cells (NSCs) increases at p15, altering the ratio of quiescent NSCs and causing abnormal terminal differentiation (Ahmad et al. 2020; Hossain et al. 2013; Ito et al. 2010). TSK deficiency causes expansion of the ciliary body of the developing peripheral eye due to unregulated Wnt2b-Fzd4 expression, which delays the hair cycle by downregulating TGF-β1 throughout the process (Niimori et al. 2012; Ohta et al. 2011).
Furthermore, reduced bone length, weight loss, and short stature were also observed in TSK-deficient mice as well as morphologically abnormal growth plates (Yano et al. 2017). In addition, in TSK-deficient mice, the stereocilia of the inner hair cells become dislocated and short, resulting in hearing loss (Miwa et al. 2020). TSK deficiency also impairs immunity and causes excessive wound inflammation due to the upregulated of TGF-β1, Stat3, and Il6 (Niimori et al. 2014).
Tsukushi as a potential biomarker and therapy
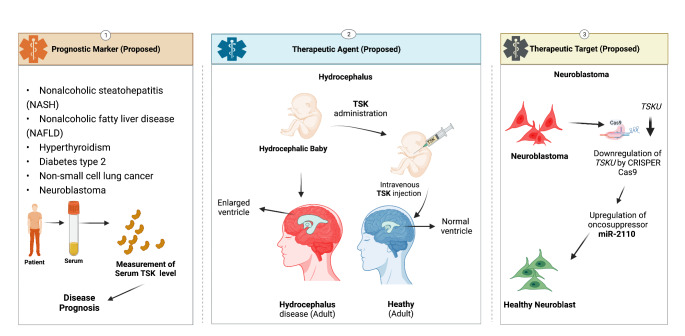
The expression and function of TSK as a critical regulator of physiological processes throughout the body make it a good candidate for future investigation into its role in diseases. So far, research on the activity of TSK as a hepatokine and cell cycle regulation in some cancer cells supports its potential as a biomarker. A study of metabolic disease non-alcoholic steatohepatitis (NASH) indicated that TSK expression as a hepatokine is higher in the plasma of diet-induced NASH-positive animals than in healthy mice, supporting NASH pathogenesis. Significantly, modifying the diet of these NASH animals can reverse the diseases by lowering circulatory TSK levels, showing that TSK is a therapeutic target for NASH pathology (Xiong et al., 2019). TSK levels have been linked to nonalcoholic fatty liver disease (NAFLD), which is associated with dyslipidemia, atherosclerosis, and obesity. According to one study, endoplasmic reticulum stress and inflammation enhanced hepatic TSK in mice (Mouchiroud et al. 2019). TSK functions as a cholesterol homeostasis maintenance molecule by lowering circulating HDL cholesterol, decreasing the cholesterol export capability of the liver, and decreasing cholesterol-to-bile acid conversion. TSK levels were higher in patients with acetaminophen-induced acute liver failure (ALF), a type of hepatic inflammation. Statistics showed that higher TSK levels in the blood were related to poor transplant-free survival at hospital discharge, suggesting that TSK can be utilized as a prognostic tool. These findings suggest that hepatokine TSK levels in the blood can be used as biomarkers for liver stress in NAFLD, a precursor to atherogenic dyslipidemia and atherosclerosis. In another study, an elevation in TSK in the blood has been linked as a prominent risk factor for hyperthyroidism in people (Liu et al. 2020). TSK levels decreased when thyroid condition of the patients was improved by thionamide therapy. The study emphasized the potential of TSK as an early biomarker while taking other risk factors such as age, gender, smoking, BMI, fasting glucose, LDL-cholesterol, and insulin resistance into account. A Study on type 2 diabetes found that serum TSK increase is independently related to the disease compared to standard glucose tolerance (Li et al. 2021).
TSK overexpression has been linked to cancer (lung cancer and neuroblastoma). TSK upregulation was found to influence epithelial to mesenchymal transition while promoting cell proliferation in a study on lung cancer tissue and cell lines (H1975) (Yamada et al. 2019). A later study by another group found that TSK levels were upregulated in non-small cell lung cancer (NSCLC) patients, which correlated with a low level of infiltration of B cells and poor survival (Huang et al. 2021). TSK overexpression in the brain has been linked to severe neuroblastoma, which frequently results in poor patient survival (Zhao et al. 2018). miR-2110, a suppressor of the neuroblastoma, targets TSK for cell differentiation and survival inhibition. A low level of mir-2110 resulted in a high level of TSK on neuroblastoma. According to these cancer studies, TSK appears to be a potential prognostic biomarker for NSCLC and therapeutic target for neuroblastoma. Perhaps a CrisprCas9-based gene editing method will be one option for neuroblastoma treatment (Fig. 4).
Fig. 4.
Proposed application of TSK in prognosis and therapy; downward arrow sign indicates downregulation of the gene; This figure was Created with BioRender.com
Our recent study showed that a lack of TSK in the mouse brain results in severe phenotypes, one of which is directly associated with communicating hydrocephalus disease (Ito et al. 2021). Hydrocephalus is a potentially lethal condition caused by lateral ventricle (LV) expansion and elevated intracranial pressure (Rekate 2008). Our studies showed that a lack of TSK alters the NSC niche regulation of the SVZ towards the development of the hydrocephalic phenotype. In healthy mice, TSK is expressed in the ependymal cells of the SVZ and cerebrospinal fluid, which maintains the proper coordination and differentiation of NSCs by maintaining Wnt signaling, leading to appropriate SVZ development. In contrast, a lack of TSK dysregulates Wnt signaling and impairs NSC differentiation and survival of the progeny cells, leading to LV expansion. We also discovered that TSK exonic mutations are linked to hydrocephalus disease in humans. While simulating these hydrocephalic mutations, we found that mutant TSK does not bind to FZD3, the Wnt receptor, resulting in Wnt regulation failure. This is because the mutations impair the structural integrity of TSK. Significantly, TSK administration (not mutant TSK) in the early postnatal TSK knockout mouse brain prevented the development of the hydrocephalic phenotype, whereas mutant TSK did not. Mouse TSK shares 85% protein similarity with human TSK and has structural domains that are similar. As a result, as an SVZ niche preserver and repairer, TSK is a strong candidate for hydrocephalus therapy in humans (Ito et al. 2021). TSK has already been detected in human blood, implying that intravenous administration of human TSK could be effective to therapy. We believe that giving TSK intravenously to a hydrocephalic baby during infancy could help cure the condition (Fig. 4). However, it should be thoroughly investigated whether TSK injected intravenously remain functional and can cross the blood-brain barrier. Further research on TSK administration in higher primate models is required.
Conclusions
It is clear from the preceding discussion that TSK is a critical molecule for proper development and cellular homeostasis. In this context, TSK is a critical fine tuner of multiple signaling cascades. TSK deficiency causes a variety of phenotypic abnormalities, while its overexpression is linked to metabolic diseases, and its mutation causes hydrocephalus. As a result, it can be used as a biomarker or a therapeutic target to improve disease condition such as liver conditions, cancer and neuroblastoma. However, a detailed mechanism is missing in most of the development and metabolic studies which could have offer more clearer picture of TSK modulation effect in diverse disease microenvironments. Nonetheless, TSK function as a Wnt inhibitor makes it a good candidate for neuro-disorder therapy which was reflected when TSK administration could reverse the hydrocephalus pathophysiology. Research on TSK appears to be progressing over other SLRP family proteins in diagnostic and therapeutic approaches. However, more analysis in these directions is critical, as they will undoubtedly benefit human health.
Acknowledgements
This work was supported by JST SPRING: Grant Number JPMJSP2127, Kumamoto University; Advanced Research Project Stem Cell-Based Tissue Regeneration, Research and Education Unit; Program for Leading Graduate Schools “HIGO Program” in Kumamoto University (AI).
Abbreviations
- ALF
Acute liver failure
- BMP
Bone morphogenic protein
- CCN2
Cell communication network factor 2
- CT
Cystine-Knot conformation
- CTGF
Connective tissue growth factor
- Eph
Ephrin binding receptor
- FGF
Fibroblast growth factors
- FGFR
Fibroblast growth factor receptor
- FZD
Frizzled class receptors
- HDL
High density lipoproteins
- IGFBP
Insulin-like growth factor-binding proteins
- IGF
Insulin like growth factor
- IL
Interleukin
- LDL
Low density lipoproteins
- LRP
LDL receptor-related protein
- LRR
Leucine-rich repeats
- LRRNT
Leucine-rich repeat N-terminal domain
- LV
Lateral ventricle
- miR
Micro RNA
- NASH
Non-alcoholic steatohepatitis
- NAFLD
Non-alcoholic fatty liver disease
- NSC
Neural stem cell
- NSCLC
Non-small cell lung cancer
- RUNX
Runt-related transcription factor
- Shh
Sonic hedgehog protein
- SLRP
Small leucine-rich proteoglycans
- SOX
SRY-Box transcription factor
- STAT
Signal transducer and activator of transcription
- SVZ
Subventricular zone
- TSK
Tsukushi protein
- Tsku
Mouse Tsukushi encoding gene
- TSKU
Human Tsukushi encoding gene
- TGF-β
Transcription growth factor beta
- Wnt
Wnt family member proteins
Author contribution
AI and KO defined the aim and structure of the review. AI wrote the manuscript draft and prepared the figures. KO critically revised the manuscript. All authors read and approved the final manuscript draft.
Conflict of interest
The authors declare that there is no potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad SAI, Anam MB, Istiaq A, Ito N, Ohta K. Tsukushi is essential for proper maintenance and terminal differentiation of mouse hippocampal neural stem cells. Dev Growth Differ. 2020;62:108–117. doi: 10.1111/dgd.12649. [DOI] [PubMed] [Google Scholar]
- Aoyama E, Kubota S, Takigawa M. CCN2/CTGF binds to fibroblast growth factor receptor 2 and modulates its signaling. FEBS Lett. 2012;586:4270–4275. doi: 10.1016/j.febslet.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development. 2013;140:2680–2690. doi: 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- Bolton K, Segal D, Walder K. The small leucine-rich proteoglycan, biglycan, is highly expressed in adipose tissue of Psammomys obesus and is associated with obesity and type 2 diabetes. Biologics. 2012;6:67–72. doi: 10.2147/BTT.S27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/β-Catenin Signaling Prevents Osteoblasts from Differentiating into Chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. doi: 10.1016/S0945-053X(98)90121-4. [DOI] [PubMed] [Google Scholar]
- Hossain M, Ahmed G, Naser IB, Shinmyo Y, Ito A, Riyadh MA, Felemban A, Song X, Ohta K, Tanaka H. The combinatorial guidance activities of draxin and Tsukushi are essential for forebrain commissure formation. Dev Biol. 2013;374:58–70. doi: 10.1016/j.ydbio.2012.11.029. [DOI] [PubMed] [Google Scholar]
- Hsieh LT-H, Nastase M-V, Roedig H, Zeng-Brouwers J, Poluzzi C, Schwalm S, Fork C, Tredup C, Brandes RP, Wygrecka M, Huwiler A, Pfeilschifter J, Schaefer L. Biglycan- and Sphingosine Kinase-1 Signaling Crosstalk Regulates the Synthesis of Macrophage Chemoattractants. Int J Mol Sci. 2017;18:595. doi: 10.3390/ijms18030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang D, Fu J, Zhao L, Li D, Sun H, Liu X, Xu J, Tian T, Zhang L, Liu Y, Zhang Y, Zhao Y. Tsukushi is a novel prognostic biomarker and correlates with tumor-infiltrating B cells in non-small cell lung cancer. Aging. 2021;13:4428–4451. doi: 10.18632/aging.202403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Shinmyo Y, Abe T, Oshima N, Tanaka H, Ohta K. Tsukushi is required for anterior commissure formation in mouse brain. Biochem Biophys Res Commun. 2010;402:813–818. doi: 10.1016/j.bbrc.2010.10.127. [DOI] [PubMed] [Google Scholar]
- Ito N, Riyadh MA, Ahmad SAI, Hattori S, Kanemura Y, Kiyonari H, Abe T, Furuta Y, Shinmyo Y, Kaneko N, Hirota Y, Lupo G, Hatakeyama J, Abdulhaleem M, Anam FA, Yamaguchi MB, Takeo M, Takebayashi T, Takebayashi H, Oike M, Nakagata Y, Shimamura N, Holtzman K, Takahashi MJ, Guillemot Y, Miyakawa F, Sawamoto T, Ohta K. Dysfunction of the proteoglycan Tsukushi causes hydrocephalus through altered neurogenesis in the subventricular zone in mice. Sci Transl Med. 2021;13:eaay7896. doi: 10.1126/scitranslmed.aay7896. [DOI] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu D, Fu Y, Zhang C, Tong H, Li, Shufeng, Yan Y. Podocan Promotes Differentiation of Bovine Skeletal Muscle Satellite Cells by Regulating the Wnt4-β-Catenin Signaling Pathway. Front Physiol. 2019;10:1010. doi: 10.3389/fphys.2019.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-Y, Wu X-N, Deng X, Zhang P-P, Li H-X, Chen K, Wu D-P, Gu T, Wang C-X, Zhao L, Wang D, Yang L, Yuan G-Y. Serum Tsukushi levels are elevated in newly diagnosed type 2 diabetic patients. Diabetes Res Clin Pract. 2021;178:108987. doi: 10.1016/j.diabres.2021.108987. [DOI] [PubMed] [Google Scholar]
- Lindwall C, Fothergill T, Richards LJ. Commissure formation in the mammalian forebrain. Curr Opin Neurobiol Dev. 2007;17:3–14. doi: 10.1016/j.conb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhang P, Wei X, Deng Y, Liu W, Guo D, Liu J, Xu B, Huang C, Huang J, Lin J, Liu S, Xue Y, Zhang H. Elevated Serum Tsukushi Levels in Patients With Hyperthyroidism. Front Endocrinol (Lausanne) 2020;11:580097. doi: 10.3389/fendo.2020.580097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima N, Takatsuka S, Miyashita H, Kretsinger RH. Leucine Rich Repeat Proteins: Sequences, Mutations, Structures and Diseases. Protein Pept Lett. 2019;26:108–131. doi: 10.2174/0929866526666181208170027. [DOI] [PubMed] [Google Scholar]
- Matsushima N, Miyashita H, Kretsinger RH. Sequence features, structure, ligand interaction, and diseases in small leucine rich repeat proteoglycans. J Cell Commun Signal. 2021;15:519–531. doi: 10.1007/s12079-021-00616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T, Ohta K, Ito N, Hattori S, Miyakawa T, Takeo T, Nakagata N, Song W-J, Minoda R. Tsukushi is essential for the development of the inner ear. Mol Brain. 2020;13:29. doi: 10.1186/s13041-020-00570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreth K, Brodbeck R, Babelova A, Gretz N, Spieker T, Zeng-Brouwers J, Pfeilschifter J, Young MF, Schaefer RM, Schaefer L. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest. 2010;120:4251–4272. doi: 10.1172/JCI42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud M, Camiré É, Aldow M, Caron A, Jubinville É, Turcotte L, Kaci I, Beaulieu M-J, Roy C, Labbé SM, Varin TV, Gélinas Y, Lamothe J, Trottier J, Mitchell PL, Guénard F, Festuccia WT, Joubert P, Rose CF, Karvellas CJ, Barbier O, Morissette MC, Marette A, Laplante M. The hepatokine Tsukushi is released in response to NAFLD and impacts cholesterol homeostasis. JCI Insight. 2019;4:129492. doi: 10.1172/jci.insight.129492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola Z, Fabel K, Kempermann G. Development of the adult neurogenic niche in the hippocampus of mice. Front Neuroanat. 2015;9:53. doi: 10.3389/fnana.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimori D, Kawano R, Felemban A, Niimori-Kita K, Tanaka H, Ihn H, Ohta K. Tsukushi controls the hair cycle by regulating TGF-β1 signaling. Dev Biol. 2012;372:81–87. doi: 10.1016/j.ydbio.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Niimori D, Kawano R, Niimori-Kita K, Ihn H, Ohta K. Tsukushi is involved in the wound healing by regulating the expression of cytokines and growth factors. J Cell Commun Signal. 2014;8:173–177. doi: 10.1007/s12079-014-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nio Y, Okawara M, Okuda S, Matsuo T, Furuyama N. Podocan Is Expressed in Blood and Adipose Tissue and Correlates Negatively With the Induction of Diabetic Nephropathy. J Endocr Soc. 2017;1:772–786. doi: 10.1210/js.2017-00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Lupo G, Kuriyama S, Keynes R, Holt CE, Harris WA, Tanaka H, Ohnuma S-I. Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev Cell. 2004;7:347–358. doi: 10.1016/j.devcel.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Ito A, Kuriyama S, Lupo G, Kosaka M, Ohnuma S, Nakagawa S, Tanaka H. Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4. Proc Natl Acad Sci U S A. 2011;108:14962–14967. doi: 10.1073/pnas.1100513108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Aoyama E, Ahmad SAI, Ito N, Anam MB, Kubota S, Takigawa M. CCN2/CTGF binds the small leucine rich proteoglycan protein Tsukushi. J Cell Commun Signal. 2019;13:113–118. doi: 10.1007/s12079-018-0487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekate HL. The definition and classification of hydrocephalus: a personal recommendation to stimulate debate. Cerebrospinal Fluid Res. 2008;5:2. doi: 10.1186/1743-8454-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Biological Functions of the Small Leucine-rich Proteoglycans: From Genetics to Signal Transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueda R, Kageyama R. Regulation of active and quiescent somatic stem cells by Notch signaling. Dev Growth Differ. 2020;62:59–66. doi: 10.1111/dgd.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, Žídek A, Green T, Tunyasuvunakool K, Petersen S, Jumper J, Clancy E, Green R, Vora A, Lutfi M, Figurnov M, Cowie A, Hobbs N, Kohli P, Kleywegt G, Birney E, Hassabis D, Velankar S. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sharma VP, Shen H, Xiao Y, Zhu Q, Xiong X, Guo L, Jiang L, Ohta K, Li S, Shi H, Rui L, Lin JD. The hepatokine Tsukushi gates energy expenditure via brown fat sympathetic innervation. Nat Metab. 2019;1:251–260. doi: 10.1038/s42255-018-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Wang Q, Wang S, Zhang J, Liu T, Guo L, Yu Y, Lin JD. Mapping the molecular signatures of diet-induced NASH and its regulation by the hepatokine Tsukushi. Mol Metab. 2018;20:128–137. doi: 10.1016/j.molmet.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Zhang R, Dong M, Zhang Z, Li H, Zhan C, Li X. Osteoglycin (OGN) Inhibits Cell Proliferation and Invasiveness in Breast Cancer via PI3K/Akt/mTOR Signaling Pathway. Onco Targets Ther. 2019;12:10639–10650. doi: 10.2147/OTT.S222967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Ohta K, Motooka Y, Fujino K, Kudoh S, Tenjin Y, Sato Y, Matsuo A, Ikeda K, Suzuki M, Ito T. Significance of Tsukushi in lung cancer. Lung Cancer. 2019;131:104–111. doi: 10.1016/j.lungcan.2019.03.024. [DOI] [PubMed] [Google Scholar]
- Yano K, Washio K, Tsumanuma Y, Yamato M, Ohta K, Okano T, Izumi Y. The role of Tsukushi (TSK), a small leucine-rich repeat proteoglycan, in bone growth. Regen Ther. 2017;7:98–107. doi: 10.1016/j.reth.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeger H, Perbal B. The CCN axis in cancer development and progression. J Cell Commun Signal. 2021;15:491–517. doi: 10.1007/s12079-021-00618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. PNAS. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li H, Zhao Y, Guo Q, Yu Y, Zhu S, Zhang S, Min L, Li P. Asporin promotes cell proliferation via interacting with PSMD2 in gastric cancer. Front Biosci (Landmark Ed) 2019;24:1178–1189. doi: 10.2741/4774. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Partridge V, Sousares M, Shelton SD, Holland CL, Pertsemlidis A, Du L. microRNA-2110 functions as an onco-suppressor in neuroblastoma by directly targeting Tsukushi. PLoS ONE. 2018;13:e0208777. doi: 10.1371/journal.pone.0208777. [DOI] [PMC free article] [PubMed] [Google Scholar]