Abstract
Basement membranes are thin sheets of extracellular matrix with many diverse roles in the body. Those in normal tissue are also highly insoluble and resist attempts to extract and characterize their components. A mouse tumor, the EHS tumor, has provided large amounts of basement membrane material, which has facilitated the structural and functional characterization of its components. An extract of the tumor, known as Matrigel, contains components which reconstitute into a solid gel at 37°. This solid basement membrane matrix has been used in both cell culture and in vivo. Matrigel has been utilized in some 12,000-plus publications for a variety of studies with embryonic, normal, and stem or malignant cells. Evidence presented in this Commentary suggests that Matrigel isolated from tumors grown in diverse hosts could exert unique effects that could be helpful in analyzing the causes of various pathologies and for screening possible therapeutic agents.
Keywords: Matrigel, Basement membrane, Extracellular matrix, Laminin, Invasion, Collagen type IV
The 1960s and 1970 saw a large increase in our understanding of the complexities of extracellular matrices in terms of the variety of components, their structure, and their function. This Editorial will focus on one type of extracellular matrix—the basement membrane—and how our understanding of this acelluar structure was greatly enhanced by a murine tumor-derived basement membrane extract known as Matrigel. Our Laboratory at the NIDCR, NIH, in the 1960s was focused on studying the causes of congenital anomalies involving connective tissue. Collagen was known to be the main structural component in the extracellular matrix but it was not until 1969 when Edward J Miller and Victor Matukas in a group led by Karl A Piez showed that the collagen in cartilage, which they named type II collagen, was identified as genetically distinct from the collagen found in skin, bone, and tendon.
At that time, it was known that various tumors were rich in collagen, but the nature of the collagen was not well established. We received a rat chondrosarcoma isolated by Richard Swarm, then at Hoffman LaRoche, and Barbara D. Smith showed that its major collagen component was type II collagen. The tumor was named the Swarm chondrosarcoma and proved to be an abundant source of not only collagen II but also other cartilage components.
We had also received cells from Dr Swarm which he had obtained from Dr. Engelbreth-Holm who had isolated them from spontaneous mouse tumor (now named the EHS tumor) and classified them as a poorly differentiated chondrosarcoma. Based on electron microscope studies and amino acid analyses, Roslyn W. Orkin, concluded that the tumor was definitely not cartilaginous and more closely resembled authentic basement membranes and contained type IV collagen (reviewed in Kleinman and Martin 2005).
Pamela Gehron Robey, who at that time was doing her thesis research in NICDR, isolated an abundant protein from the EHS tumor using a high salt extraction. Characterization of the protein was carried out collaboratively with Rupert Timpl at the Max Planck Institute for Biochemistry in Munich. These combined studies established that the protein (laminin) isolated by Pamela Gehron Robey was a trimeric molecule, highly immunogenic, and present in other natural basement membranes based on immunostaining.
Based on many studies and particularly those of Marilyn Farquhar at Yale University, it was clear that proteoglycan molecules were also abundant in native basement membranes. Fortunately, the NIDCR lab had a senior scientist, John Hassell, whose specialty was studying proteoglycans, and he confirmed the presence of heparan-sulfate proteoglycans in the EHS tumor matrix, which was also present in authentic basement membranes, using immunostaining with antibody against the tumor proteoglycan.
Matrigel, an extract derived from the EHS tumor, contains all of the known major components of many tissue basement membranes and was first described in the mid 1980s (Kleinman et al. 2003, Kleinman and Martin 2005). It is relatively easy to make. The tumor homogenate is washed free of soluble proteins with repeated saline extractions/washes. The insoluble complexes are extracted with a chaotropic agent, such as either 1 M guanidine or 2 molar urea. After centrifugation and dialysis versus tris buffered saline at Ph7.4 in the cold, a colorless solution which formed a solid gel when warmed to 37° was obtained. John R. Hassell named the extract Matrigel (Kleinman et al. 2003).
Elizabeth D. Hay, a distinguished anatomist and cell biologist who was a scientific advisor to NIDCR learned about Matrigel and asked for some Matrigel for her developmental studies. She put embryonic notochord in 3D Matrigel and improved the explant survival with extensive outgrowth of (stem) cells. Since then, many studies in our laboratory and in other laboratories outside of NIH showed that Matrigel was a very useful substrate for 3D cell culture and for in vivo studies for analyzing differentiation, development, and pathological pathways as well as for testing inhibitors/stimulators of these processes and for drug screening, toxicology testing, disease modeling, etc. Below are summarized some of the applications that have been developed. Many others also exist.
Cell differentiation in vitro
Many cell types undergo significant differentiation when plated on or in Matrigel. One of the first cell types to be studied were cells from the testis which formed acinar-like structures resembling the testis that allowed for spermatogonia differentiation. Such structures could also be implanted back into the rats and survived well. Many additional differentiated acinar-like structures were seen with pancreatic cells, mammary epithelial cells, salivary gland cells, and others when single cells were plated on Matrigel. Such differentiation allows for in vitro modeling. For example, neurospheres of brain cells have been used to study the effects of the Zika virus. Also, the possibility of organ replacement or supplementation with transplanted cells could be further developed. An example would be for boosting pancreatic function in diabetes. Stem cells also differentiate into various structures and organs depending on the culture conditions and cell sources (Arnaoutova et al. 2012).
Angiogenesis assays in vitro and in vivo
In 1988, Tom Lawley at NCI, NIH, showed that endothelial cells will form capillary like structures with a lumen on 3D Matrigel (Fig. 1) (Kubota et al. 1988). Many others confirmed these findings and established the “Matrigel tube assay” as a screening assay for studying factors and genes that either promote or inhibit angiogenesis. Even though first published in 1988, the tube assay is still in active use with many adaptations/ modifications. It has also been shown that tumor cells sometimes behave like endothelial cells forming capillary like structures in a process called “vascular mimicry”. Furthermore, co-culture of endothelial cells with tumor cells result with some tumor cells undergoing perivascular migration referred to EVMM or “extravascular migratory metastasis” (Lugassy et al. 2013). EVMM has been demonstrated in vivo as a mechanism for the spread of various types of tumors, including melanoma.
Fig. 1.
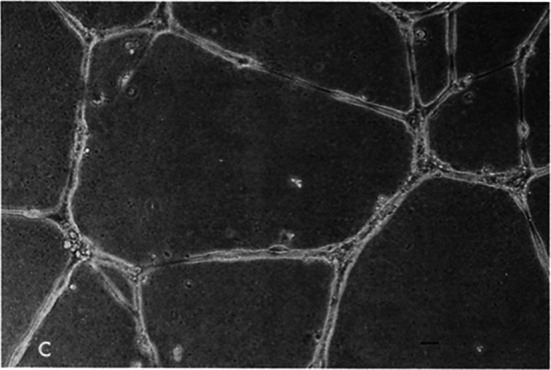
Matrigel Tube Assay HUVEC endothelial cells cultured on Matrigel for 18 h. A complex network of anastomosing cells is observed. Bars, 20 μm. Kubota et al 1988
Subcutaneous implants of Matrigel with added factors (or cells) can be used also to study angiogenesis and factors/cells that regulate this differentiation (Passaniti et al. 1992). This ‘Matrigel plug assay’, first described by Tony Passaniti at the National Institute on Aging, is also widely used as a simple, reliable in vivo angiogenesis assay. Tony Passaniti used this assay to study the angiogenic potential in mice of different ages (Fig. 2). Matrigel, prepared from tumors grown in young mice contains angiogenic factors which stimulate a robust vascular response when implanted subcutaneously in 3–6 month-old mice but a significantly less potent response is observed in older mice (18–26 months of age). This important finding indicates the reduced ability of older animals to initiate a vascular response even to factors known to be highly angiogenic.
Fig. 2.
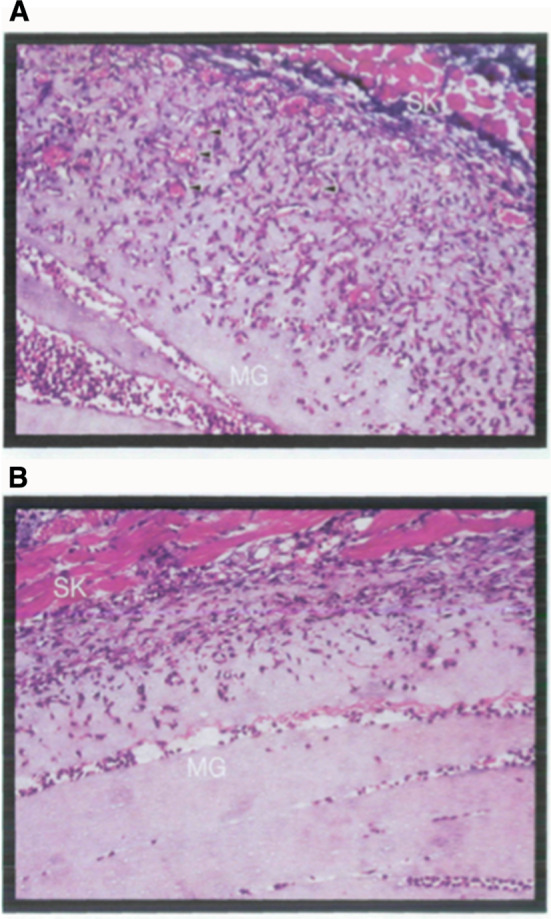
In vivo angiogenesis assay. a Young (3-month) and b old (26-month) C57BL mice were given subcutaneous injections of equal amounts (0.5 mL) of cold (liquid) Matrigel containing 75 ng bFGF. The resultant gels were dissected 6 days after injection and processed for histology. Representative Trichrome-Masson stained sections from mice aged 3 months (a) or 26 months (b) are shown. SK = skin; MG = Matrigel; Arrowheads indicate neovessels with red blood cells within the Matrigel. Pili et al 1994
The original in vivo Matrigel angiogenesis assay was developed to screen for inhibitors of angiogenesis and tumor growth to accelerate drug discovery. Future studies using the basement membrane proteins and growth factors found in Matrigel isolated from mice of different ages could be important tools for drug development and therapy and could be valuable in determining the mechanisms of age-associated vascular dysfunction, drug response, and tumor growth.
Tumor assay in vitro and in vivo: growth, morphology, and invasion assays
In 1987, Adriana Albini and others at NIDCR found that the malignant potential of tumor cells could be readily observed when cultured in 3D on Matrigel. The low invasive cells either grew slowly and in some cases showed rudimentary differentiation while the more malignant cells grew rapidly, appeared highly undifferentiated, and invaded the Matrigel. This morphological assay of tumor malignancy is widely used but is not quantitative. Adriana Albini developed a quantitative invasion/chemoinvasion assay using a well-established migration assay involving Boyden Chambers (Fig. 3; Albini et al. 1987). She coated the filters with Matrigel and was able to show that non-metastatic tumor cells did not invade to the lower surface of the filter whereas the highly malignant cells did invade to the lower surface of the filter. She further showed that if she collected the invaded cells and subjected them to three cycles of selection in the invasion assay and then injected the invaded cells into mice, the selected invaded cells grew faster than the parental cells. The invasion assay is still widely used today to determine tumor cell malignancy, to test for various factors and genes that influence malignancy, to select for more (or less) malignant subpopulations, and to determine the invasive activities in co-culture of different cell types. The assay is also useful to follow the purification of factors affecting malignancy and test tissue and cell extracts for their chemoinvasive activity.
Fig. 3.
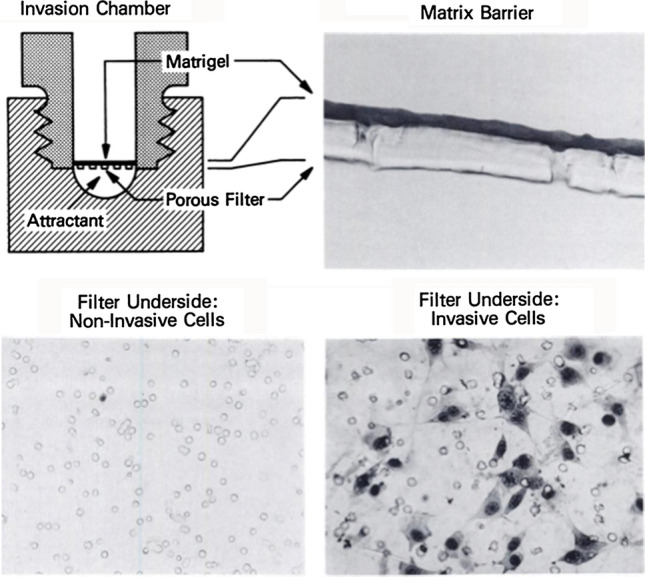
Invasion assay: Boyden chamber assembly used for the invasion assay is shown in the upper left, section of a Matrigel-coated (50 μg protein) filter is shown in the upper right. Lower surfaces of an invasion membrane when poorly invasive NIH3T3 (lower left) and highly invasive T24/3T3 (lower right) are assayed for invasion, shown lower panels. Albini et al 1987
In 1990, Rafi Fridman of NIDCR showed that co injection of tumor cells with Matrigel resulted in increased tumor take and growth (Fig. 4; Fridman et al. 1990)). This landmark finding has led to the improved and faster development of animal models for human cancers. Others showed that as few as one cell plus Matrigel could lead to tumor take and growth, thus paving the way for a proof of principle assay for cancer stem cells. Matrigel is also used in combination with other cells such as fibroblasts to further promote tumor growth in vivo. It is also used for orthotopic injections of tumor cells to create relevant physiological animal models. Such models allow for physiological screening of therapeutics and pathways and genes involved in malignancy. Angiogenic factors in the Matrigel contribute to its ability to promote the take and growth of tumor cells (Kibbey et al. 1992).
Fig. 4.
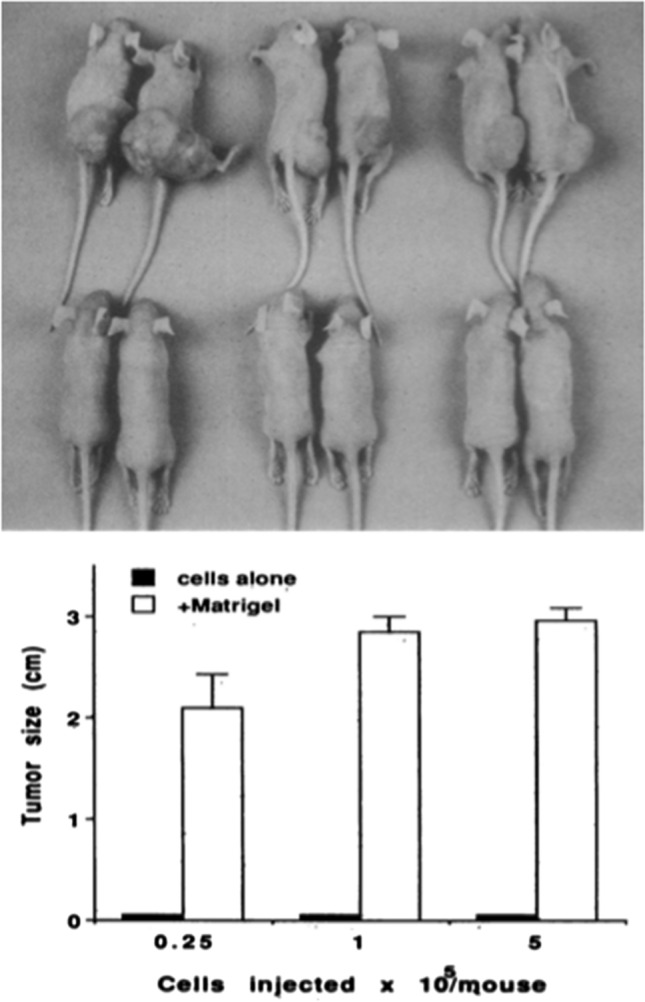
Matrigel tumor growth assay. Representative subcutaneous tumors formed by SCLC cell lines in athymic nude mice inoculated with 5 × 105 cells either alone (bottom) or within (top row) Matrigel. Fridman et al 1990
Growth of tumor cells in a suspension of dilute Matrigel results in micro tumors (tumor spheres) that can be used to study tumor growth, aggressiveness, screen for drugs that block or enhance tumor growth, and identify important genes that regulate tumor growth (Benton et al. 2014, 2015). Such model systems for a variety of tumors are in wide use due to the many applications and for the ability to perform large scale testing without the use of animals (Benton et al. 2011).
Possible future applications
Aging studies
Since Matrigel is generally prepared from young mice, this Matrigel is not useful for studies modeling the response of older patients. Therefore, more types of Matrigel need to be developed. It is well understood that young animals react much differently than old. For example, lung fibrosis model induced by bleomycin resolves in the young but not in the old. Male animals (except for breast cancer studies) are often used to grow tumors as females can provide new litters. As in the case of tumors, the extracellular matrix in the tumor microenvironment (Maman and Witz 2018) and cellular metabolism (Faubert et al. 2020) are critical factors in determining the response to therapy and identifying targets for drug development.
For example, Roberto Pili showed that old mice support the growth of EHS tumors but at a reduced growth rate (Pili et al. 1994). Also by histology, the extracellular matrix area is increased relative to tumor cells in the tumors from old animals. Striking differences were noted when Matrigel, a known angiogenesis-inducer was tested in mice. Matrigel prepared from old mice induced much less of an angiogenic response than Matrigel prepared from tumor grown in young mice. A similar response was observed when these Matrigels were tested in the in vitro capillary assay. In contrast to cells on the Matrigel from young mice which produced many capillary-like structures, on old Matrigel many endothelial cells detached and formed few if any capillary-like structures (Passaniti et al. 1995). It should be noted that fetal calf serum, routinely used in culturing cells reversed some of the differences of the old Matrigel. If old Matrigel is employed, serum from old animals could maintain the “old” microenvironment. These results suggest that Matrigel from tumors grown in old mice may represent an aged microenvironment.
Matrigel prepared from different disease model animals
Some 30% of older Americans are obese. Obesity-related conditions include heart disease, stroke, diabetes, and some cancers. Therefore, it would be of interest to compare cellular function on Matrigel isolated from old and obese mice. Here one could screen various cell- and organ-based assays to assess the effect of these basement membrane microenvironments. Also, a proteomics analysis might identify critical factors driving disease and identify possible therapeutic targets to reduce the risks of obesity in patients, including the elderly. Likewise, Matrigel could be prepared from diabetic animals or from other animal models of human diseases. Interestingly, hyperglycemia and oxidative stress (both hallmarks of diabetes) have a negative impact on endothelial tube formation and angiogenesis (Mochin et al. 2015). Since a considerable amount of Matrigel can be prepared from the EHS tumors, many screening assays would be possible from a limited number of animals.
Additional future uses of Matrigel
Functional tissue replacement
Since Matrigel promotes cell differentiation into functioning structures, such as gland acini, bone canaliculi, and myelin production. it may be possible to replace or support tissue function in vivo with Matrigel differentiated cells in vitro. For example, functioning pancreatic acinar cells cultured on Matrigel have been transplanted in diabetic mice with a reduction in blood sugar for several days thus showing the feasibility of such an approach (unpublished observations). Such studies could be done to restore or boost the levels of certain hormones or factors important for homeostasis.
Wound/tissue healing
Many patients suffer and even die from poor wound healing, especially the elderly and immobilized patients. Some preliminary studies have shown promising results for cardiac and intestinal repair. Matrigel would be expected to repair such conditions more quickly through multiple mechanisms, involving angiogenesis, differentiation, or tissue turnover. It also may have use in facilitating tissue transplants and increasing transplant survival.
Author contributions
All authors contributed equally to the writing and content of this article.
Funding
No research funds were used.
Data availability
This is a review and all data are published.
Code availability
software application or custom code.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable as the figures are from papers published by US government employees and are not subject to copyright.
Ethical approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47(12):3239–3245. [PubMed] [Google Scholar]
- Arnaoutova I, George J, Kleinman HK, Benton G. Basement membrane (BME) has multiple uses with stem cells. St Cell Rep Rev. 2012;8:163–169. doi: 10.1007/s12015-011-9278-y. [DOI] [PubMed] [Google Scholar]
- Benton G, Kleinman HK, George J, Arnaoutova I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with tumor cells. Int J Cancer. 2011;128:1751–1757. doi: 10.1002/ijc.25781. [DOI] [PubMed] [Google Scholar]
- Benton G, Arnaoutova I, George J, Kleinman HK, Koblinski J. Matrigel: from discovery and ECM mimcry to assays and models for cancer research. Adv Drug Discov Rev. 2014;79–80:318. doi: 10.1016/j.addr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Benton G, DeGray G, Kleinman HK, George J, Arnaoutova I. In vitro microtumors provide a physiological predictive tool for breast cancer therapeutic screening. Plos One. 2015;10(4):e0123312. doi: 10.1371/journal.pone.0123312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Solmonson A, DeBernardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368:152–163. doi: 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman R, Giaccone G, Kanemoto T, Martin GR, Gazdar AF, Mulshine JL. Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc Natl Acad Sci USA. 1990;87(17):6698–6702. doi: 10.1073/pnas.87.17.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbey MC, Grant DS, Kleinman HK. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992;84(21):1633–1638. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Martin GRM. Basement membrane extracellular matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Philp D, Hoffman M. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14:526–532. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugassy C, Zadran S, Laurent Bentolila L, Wadehra M, Prakash R, Carmichael ST, Kleinman H, Péault B, Larue L, Barnhill RL. Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: an alternative to Intravascular cancer dissemination. Cancer Microenviron. 2013;6:19–29. doi: 10.1007/s12307-012-0128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maman S, Witz IP. A history of exploring cancer in context. Nat Rev. 2018;18:359–376. doi: 10.1038/s41568-018-0006-7. [DOI] [PubMed] [Google Scholar]
- Mochin MT, Underwood KF, Pierce AD, Cooper B, McLenithan JC, Nalvarte C, MacKerell AD, Arbiser J, Karlsson AI, Moise RA, Moskovitz J, Passaniti A. A Role for methionine sulfoxide reductase-A in redox regulation of RUNX2 DNA-binding activity. Microvasc Res. 2015;97:55–64. doi: 10.1016/j.mvr.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67(4):519–528. [PubMed] [Google Scholar]
- Passaniti A, Pili R, Yang C (1995) The effect of aging on endothelial cell differentiation, angiogenesis, and tumor growth. In: Proc Base Mem Symp NIDDK pp 193–204
- Pili R, Guo Y, Chang J, Nakanishi H, Martin GR, Passaniti A. Altered angiogenesis underlying age-dependent changes in tumor growth. J Natl Cancer Inst. 1994;86:1303–1314. doi: 10.1093/jnci/86.17.1303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review and all data are published.
software application or custom code.


