Abstract
Matricellular proteins are responsible for regulating the microenvironment, the behaviors of surrounding cells, and the homeostasis of tissues. Periostin (POSTN), a non-structural matricellular protein, can bind to many extracellular matrix proteins through its different domains. POSTN usually presents at low levels in most adult tissues but is highly expressed in pathological sites such as in tumors and inflamed organs. POSTN can bind to diverse integrins to interact with multiple signaling pathways within cells, which is one of its core biological functions. Increasing evidence shows that POSTN can activate the TGF-β, the PI3K/Akt, the Wnt, the RhoA/ROCK, the NF-κB, the MAPK and the JAK pathways to promote the occurrence and development of many diseases, especially cancer and inflammatory diseases. Furthermore, POSTN can interact with some pathways in an upstream and downstream relationship, forming complicated crosstalk. This article focuses on the interactions between POSTN and different signaling pathways in diverse diseases, attempting to explain the mechanisms of interaction and provide novel guidelines for the development of targeted therapies.
Keywords: Periostin, Extracellular matrix, Integrins, Signaling pathways, Crosstalk
Introduction
The extracellular matrix (ECM) is a dynamic structural network that can regulate the activity of extracellular signaling molecules, such as growth factors, cytokines, chemokines, and extracellular enzymes, as well as cell function by directly activating signaling pathways through binding to receptors on the cell surface, which is an essential part of the tissue microenvironment (Bedore et al. 2014). Matricellular proteins, originally classified by Bornstein (Bornstein 1995), can regulate the microenvironment, the behavior of surrounding cells, and the homeostasis of tissues. Increasing evidence shows that matricellular proteins play a vital role in the occurrence and development of tumors and inflammatory diseases (Chiodoni et al. 2010). Therefore, for the homeostasis of normal organs including wound healing and tissue repair, it is important to strictly control the homeostasis and remodeling of the ECM (Wu and Ouyang 2014). As a non-structural matricellular protein, POSTN, also called osteoblast-specific factor-2 (OSF-2), was originally cloned from the mouse preosteogenic cell line MC3T3-E1, without the fibroblast cell line NIH3T3 (Horiuchi et al. 1999; Takeshita et al. 1993). With a molecular weight of 90 kDa, POSTN contains an N-terminal secretory signal peptide, followed by a cysteine-rich domain (EMI domain), four tandem repeats of FAS1 domains, and a hydrophilic carboxy-terminal structure domain (CTD). Having been proven to bind to a variety of proteins, including ECM proteins, these different domains have diverse biological functions (Kudo 2017, 2019a). Although POSTN does not directly participate in the formation of the ECM, it plays an important role in the interaction between the cell and the surrounding microenvironment due to its fine composition structure.
POSTN, usually present in low levels, becomes highly expressed in injured or inflamed tissues of adult organisms (Wu and Ouyang 2014). Its role in promoting the occurrence and development of various diseases is to activate different signaling pathways by combining with integrins, thereby stimulating or inhibiting the downstream molecules and exerting its biological functions. In recent years, a large number of studies have revealed that the high expression of POSTN observed in different types of tumors is related to metastasis and tumor progression. For example, Yue et al. (2021) and Yu et al. (2018) confirmed that POSTN derived from cancer-associated fibroblasts (CAFs) can promote the migration and invasion of ovarian cancer cells through the TGF-β pathway, while also promoting human head and neck squamous cell carcinoma by interacting with PTK7 through the Wnt pathway, respectively. Additionally, POSTN can accelerate the occurrence and development of colorectal cancer (Ma et al. 2020). Furthermore, POSTN, through the activation of different pathways, also plays a key role in diverse inflammatory diseases such as asthma and allergic illnesses (Izuhara et al. 2016). This article focuses on the interaction between POSTN and different signaling pathways in diverse diseases and attempts to explain the mechanisms of interaction.
The structure of POSTN
As a member of the elastin microfibrillar interface protein (EMILIN) family, the EMI domain, consisting of about 80 amino acid residues including 6 highly conserved cysteine residues (Doliana et al. 2000), has previously been demonstrated to interact with fibronectin and collagen fibers. Kii et al. (2016) proved that there is an interaction between POSTN and fibronectin through Co-immunoprecipitation and in situ proximity ligation. Norris et al. (Norris et al. 2007) showed that POSTN can directly interact with collagen I through immunoprecipitation and immunogold transmission electron microscopy experiments. Moreover, the EMI domain is essential for the multimerization of POSTN and can promote the cross-linking of collagen by forming a network structure with fibronectin and tenascin C (Zhu et al. 2021). Because of this, POSTN promotes the infiltration and contraction of the ECM (Kudo 2019a). The four-repeat fasciclin-like (FAS1) domain, a tandem repeat of four FAS1 domains, was originally a neural cell adhesion molecule found in insects (Clout and Hohenester 2003; Clout et al. 2003). This domain has been shown to bind to tenascin C, bone morphogenetic protein 1 (BMP-1), and cellular communication network factor 3 (CCN3) (Kii et al. 2010; Hwang et al. 2014; Takayama et al. 2017) and, therefore, may act as an anchor point to mediate the interaction with the EMI domain and other ECM components (Zhu et al. 2021). Through the four-repeat FAS1 domain, POSTN can bind to the integrins αvβ3, αvβ5, and α6β4 on the surface of target cells to activate multiple signaling pathways in the cells (Gonzalez-Gonzalez and Alonso 2018), one of POSTN's primary biological functions. The C-terminal domain (CTD) contains an arginine-rich heparin binding site and is responsible for binding to proteoglycans. Heparin, a highly acidic polysaccharide including sulfate groups that binds to transmembrane proteins and secreted proteins, is called a heparin sulfate proteoglycan (Kii and Ito 2017). Such a combination may regulate cellular processes such as cell migration and growth factor signaling (Ratajczak-Wielgomas and Dziegiel 2015). The structure of POSTN and a schematic diagram of its different domains interacting with other ECM proteins are shown in Fig. 1.
Fig. 1.
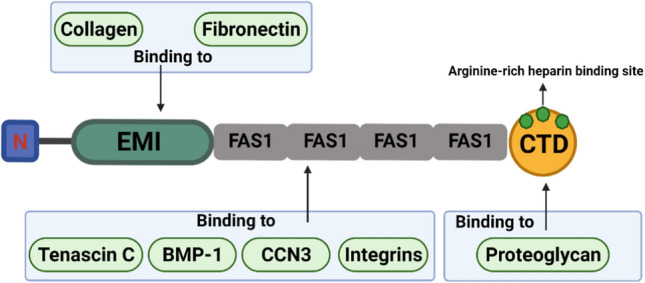
POSTN contains an N-terminal secretory signal peptide followed by an EMI domain, the four-repeat FAS1 domain, and a CTD. Collagen I and fibronectin can bind to the EMI domain; the four-repeat FAS1 domain can interact with tenascin C, BMP-1, CCN3, and integrins; CTD contains an arginine-rich heparin binding site, which can bind to proteoglycans. POSTN can play an important role between the cell and the surrounding microenvironment due to its fine composition structure. The image is adapted from Zhu et al. (2021)
POSTN and the TGF-β pathway
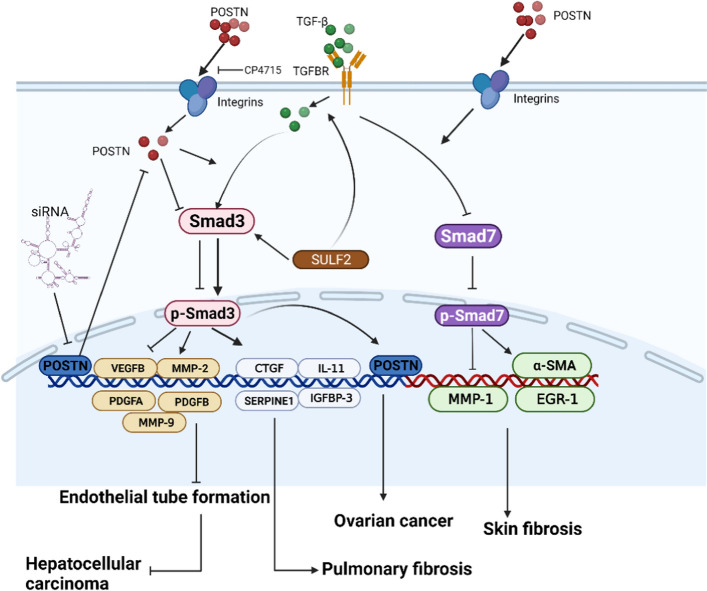
TGF-β is a multifunctional cytokine with a variety of pathophysiological functions. TGF-β comprises a family of three subtypes in mammals, namely TGF-β1, TGF-β2, and TGF-β3, all of which have different biological activities (Tang et al. 2018a; Voisin et al. 2020), however, the research matter on TGF-β1 is the most abundant and in-depth (Gonzalez-Sanchez et al. 2021). Only the promoter region of TGF-β1 can be directly activated by different trans-activating proteins such as reactive oxygen species, plasmin, and acids, which is why it can play key roles in fibrogenesis, carcinogenesis, immune regulation, cell proliferation, and differentiation (Prud'homme 2007). TGF-β signals cause different downstream behaviors in a context-dependent manner, particularly in cancer (Chung et al. 2021). The main signal transduction mediator of the TGF-β family is the Smad family of proteins, which transfers signals into the nucleus to activate different target genes related to proliferation, differentiation, chemotaxis, and immune regulation (Sabbadini et al. 2021; Massague 1998). Smad3 is pathogenic, while Smad2 and Smad7 are protective (Tang et al. 2018a). Studies have shown that the TGF-β/Smad3 pathway can regulate non-coding RNA at the transcription level and transform miRNA from an anti-fibrotic to a pro-fibrotic state (Tang et al. 2018a). In recent years, surmounting evidence has indicated that there is an upstream and downstream relationship between POSTN and the TGF- β pathway and, in some cases, they can interact and promote each other's secretion to execute specific biological functions.
Crosstalk between POSTN and the TGF-β pathway was seen in pulmonary fibrosis in a study by Nanri et al. (2020). Furthermore, TGF-β, which can promote fibroblasts to differentiate into myofibroblasts, synthesize ECM protein, and promote epithelial to mesenchymal transformation (EMT), has been highlighted as a central mediator in the pathophysiological mechanisms of pulmonary fibrosis (Chanda et al. 2019; Meng et al. 2016). Nanri et al. used an overexpression plasmid encoding POSTN, transferring it into cells and stimulating them with a TGF-β treatment. They found that POSTN upregulates the expression of pulmonary fibrosis-specific molecules such as SERPINE1, CTGF, IGFBP-3, and IL-11 at the protein and mRNA levels. Then, to test whether Smad3 was affected by POSTN, they knocked out POSTN and found that the phosphorylation of Smad3 decreased significantly, whereas POSTN overexpression significantly increased its phosphorylation. The results suggest that the crosstalk between TGF-β and POSTN merges upstream of Smad3 and that POSTN signaling affects the activation of Smad3. Moreover, this process of promoting pulmonary fibrosis is caused by the integrins αvβ3 and αvβ5 that transfer POSTN into Smad3. Integrin αvβ3 is the main receptor of POSTN, while αvβ5 acts as a complementary receptor in keratinocytes (Masuoka et al. 2012). Previous studies by this team confirmed that CP4715, an inhibitor of integrin αvβ3, can alleviate pulmonary fibrosis in mice by inhibiting the TGF-β pathway (Yoshihara et al. 2020). Therefore, this crosstalk is very important for the TGF-β pathway and its downstream effect molecules in the regulation of pulmonary fibrosis. In addition to pulmonary fibrosis, the interaction between POSTN and the TGF-β pathway is also involved in the development of systemic sclerosis. Kanaoka et al. (2018) found that, although recombinant POSTN (rPOSTN) itself is not enough to cause significant changes in the ECM, rPOSTN can stimulate dermal fibroblasts to form a higher level of collagen I in the presence of TGF-β. Therefore, they transduced a retrovirus expressing human POSTN into dermal fibroblasts to overexpress POSTN. Western blots revealed that, after stimulation with TGF-β, the expression of α-smooth muscle actin (α-SMA) and early growth response-1 (EGR-1) were upregulated, while the level and activity of MMP-1 decreased. In addition, western blots and immunofluorescence also showed that the level of Smad7 in POSTN-silenced dermal fibroblasts was higher than that of the control group, thus, it is suggested that POSTN may contribute to skin fibrosis by downregulating the level of protective Smad7 and that this decrease in Smad7 expression is, in part, due to the regulation of integrin αv. In this regard, the observed interaction between POSTN and Smad7 may contribute to the fibrotic response of early systemic sclerosis, because the expression of POSTN increases significantly in the early stages of this disease (Yamaguchi et al. 2013).
In addition to mediating fibrosis, the interaction between POSTN and the TGF-β pathway also plays a corresponding role in the tumor environment. Chen et al. (2017) transfected POSTN siRNA into cells expressing Sulfatase2 (SULF2), which promotes cell proliferation, adhesion, chemotaxis, and endothelial tube formation in a paracrine manner. They found a decrease in the mRNA expression of angiogenic factors, such as VEGFB, matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9), and PDGFA and B. Subsequently, they treated the cells with low or high expression of SULF2 with TGF-β1 and found that the phosphorylation of Smad3 as well as the expression of POSTN, induced by TGF-β1, were elevated. Moreover, they determined that TGFBR3 is the target for SULF2, where SULF2 promotes the release of TGF-β1 from TGFBR3, activating the TGF-β pathway and enhancing the expression of POSTN. In turn, Smad3 is phosphorylated and upregulates the expression of angiogenic factors, which induce the occurrence of hepatocellular carcinoma. In addition, through the production of TGF-β, macrophages can promote ovarian cancer cells to produce large amounts of POSTN, elevating its concentration in the tumor microenvironment (Tang et al. 2018b). In another study on the migration and invasion of ovarian cancer (Yue et al. 2021), it was observed that the mRNA expression of POSTN and cancer-associated fibroblast (CAF) markers in human ovarian fibroblasts treated with TGF-β1 was increased, suggesting that the overexpression of POSTN in ovarian cancer stroma may be caused by TGF-β1-mediated CAF activation. Additionally, many studies have demonstrated the TGF-β/POSTN interaction in the tumor environment, for example, the axis promotes the metastasis of head and neck cancer (Qin et al. 2016) and the EMT of prostate cancer and U-87MG human glioblastoma cells (Ouanouki et al. 2018). The above afore-mentioned studies show that TGF-β1 can enhance the expression of POSTN, however, whether or not this increased expression activates the secretion of TGF-β1, will be explained by the following experiments. Chen et al. (2021) found that TGF-β1 is stored in the ECM in the form of latent TGF-β binding protein and forms a complex with αvβ3. Highly expressed POSTN can bind to and activate αvβ3 on the cell surface, promoting the release of TGF-β1 into the tumor microenvironment in its active form (Wipff and Hinz 2008). TGF-β1 binds to the receptors on the cell surface and promotes the expression and secretion of POSTN. Therefore, a positive feedback loop is formed between POSTN and the TGF-β pathway, which promotes the transformation and maintenance of cancer cell stem cells.
In addition, Seki and colleagues (2019) demonstrated that inhibiting the TGF-β-induced POSTN overexpression in the pulmonary artery can prevent pulmonary hypertension. In this study, under hypoxia, the activation of Smad3 decreased in POSTN expressing cells of PN-Cre/Tgfbr1fl/flc mice. Compared with the control group, the right ventricular pressure and pulmonary artery muscularization of mice were significantly decreased in the POSTN expressing group. These results suggest that Smad3-mediated effects on the TGF-β pathway play an important role in the pathogenesis of pulmonary hypertension.
In summary, POSTN can be used as a downstream molecule secreted by the TGF- β pathway. Its predominant biological function is through the participation of integrins to affect the expression of the Smad family proteins, especially Smad3 and Smad7, which play different roles in fibrosis and tumor dynamics. In addition, this effect also plays an important role in myogenesis in Myotonic dystrophy type 1 (Shen et al. 2021). Therefore, the TGF-β/POSTN axis is expected to be used as a targeted therapy in the future and have greater clinical applications. The possible mechanisms of interaction between POSTN and the TGF-β pathway are shown in Fig. 2.
Fig. 2.
POSTN can activate the TGF-β/Smad pathway to accelerate the occurrence and development of fibrosis and cancer. (1) POSTN can induce the phosphorylation of Smad3 with the stimulation of TGF-β, upregulating the expression of SERPINE1, CTGF, IGFBP-3, and IL-11 to promote pulmonary fibrosis. This process can be prevented by an inhibitor of integrin αvβ3, CP4715. (2) POSTN can decrease the level of protective Smad7, subsequently upregulating the expression of α-SMA and EGR-1 and downregulating the level of MMP-1 to promote skin fibrosis. (3) POSTN siRNA is transfected into the cells expressing SULF2 and it was found that the expression of VEGFB, MMP-2, MMP-9, PDGFA, and PDGFB decreased through the TGF-β/Smad pathway. This process can be inhibited by SULF2, which eventually induces the occurrence of hepatocellular carcinoma. (4) POSTN can promote migration and invasion of ovarian cancer by activating the TGF-β/Smad pathway
POSTN and the PI3K/Akt pathway
The PI3K/Akt pathway can be activated in many diseases and is considered to be a very important therapeutic target (Aoki and Fujishita 2017). According to its structure and substrate specificity, PI3K can be divided into three types of molecules (I, II, and III), among which PI3KI is the most studied and exhibits a variety of biological activities (Vanhaesebroeck et al. 1997; Fruman et al. 1998; Thorpe et al. 2015), Akt is an important messenger in the PI3K signaling pathway (Sun et al. 2020). More than 100 proteins have been identified as participants in this pathway (Jafari et al. 2019) and it has important functions in many cellular processes, including the regulation of cell cycle, cell survival, inflammation, metabolism, and apoptosis (Tang et al. 2018c). In recent years, studies have revealed an array of functions for POSTN in diseases through the PI3K/Akt pathway.
Current evidence shows that the main purpose of POSTN and PI3K/Akt pathway interaction is to promote the proliferation and migration of different cells. High expression of POSTN can increase the rates of cell survival and proliferation in different tissues, including the skin (Nishiyama et al. 2011) and heart (Polizzotti et al. 2012), which is mediated by integrin αvβ1, αvβ3, and αvβ5 (Bao et al. 2004). A recent study by Molina et al. (2014), through Ki-67 immunofluorescence assay and migration analysis, found that POSTN, secreted by periodontal ligament fibroblasts, can increase the migration and proliferation of these fibroblasts when they are attacked by inflammatory and bacterial toxic factors. Through integrin αvβ1, αvβ3 and αvβ5, it activates mTOR, downstream of the PI3K/Akt pathway, then induces cell proliferation and migration, thus maintaining periodontal ligament integrity and promoting regeneration. Periodontal ligament fibroblastic POSTN is highly specific and is essential to the integrity of periodontal tissue as well as the stability of its internal environment, which is important in the development and eruption of teeth (Ma et al. 2011; Rios et al. 2005). In another study, POSTN secreted by these fibroblasts promoted the migration of human mesenchymal stem cells through the αvβ3/FAK/PI3K/Akt pathway (Matsuzawa et al. 2015), which suggests that POSTN may help to maintain rapid periodontal ligament turnover, high reconstruction ability, and high regeneration potential. In this experiment, rPOSTN (100 ng/ml) was used to stimulate human mesenchymal stem cells and the expression of p-FAK and p-Akt was detected at 0, 10, 15, 20, 30, and 60 min. The results showed that the expression of p-FAK increased at 15 min after POSTN stimulation and that p-Akt increased at 20 min after stimulation, thus there was a time lag in the reaction between the two, displaying that FAK is the upstream molecule of Akt. In one of the earliest studies of POSTN for cardiac healing after acute myocardial infarction, Shimazaki et al. (2008) found that inhibition of FAK blocked POSTN-mediated recruitment of fibroblasts. POSTN also reverses the osteogenesis of periodontal ligament stem cells that were inhibited by high glucose through the Akt pathway (Chen et al. 2021). In addition to participating in the function of the periodontal ligament, Qin et al. (2015) indicated that POSTN and the integrin β1/FAK/PI3K/Akt pathways are also involved in the survival and migration of adipose-derived stem cells, providing new ideas for the treatment of critical limb ischemia. In this experiment, they found that eNOS, the downstream cascade of the PI3K/Akt pathway, was activated.
Therefore, the interaction between highly expressed POSTN and the PI3K/Akt pathway is key to promoting proliferation and migration in smooth muscle cells, stimulating periodontal ligament cells and stem cells, and altering the occurrence and development of cancer in tumor cells (Wang and Ouyang 2012). A study by Chu et al. showed that POSTN secreted by CAFs rather than cancer cells themselves (Choi et al. 2011; Karlan et al. 2014) can reduce the cisplatin (DDP)-induced apoptosis in ovarian cancer cells (Chu et al. 2020). They used LY294002, a specific inhibitor of the PI3K/Akt pathway, to identify whether POSTN works through this pathway. Flow cytometry showed that apoptosis in the DDP + POSTN + LY294002 group was significantly higher than that in the DDP + POSTN group, which indicated that LY294002 could reverse the DDP resistance induced by POSTN. Furthermore, the change of the Bcl-2/BAX ratio in the western blots also supported the conclusion that POSTN can promote DDP resistance of ovarian cancer through the PI3K/Akt pathway. Moreover, POSTN can promote the invasion of cholangiocarcinoma cells through the PI3K/Akt pathway (Utispan et al. 2012). POSTN binds to and activates integrins αvβ3, αvβ5 and αvβ1, respectively, and phosphorylates intracellular Akt in the two processes mentioned above. It has been reported that POSTN promotes tumor progression by phosphorylating Akt in a variety of tumors (Liu et al. 2018a; Xiao et al. 2015; Yang et al. 2012). Therefore, the molecular role of the Akt pathway has proven to be an important determinant of POSTN in tumor progression (Ouyang et al. 2009).
In addition, Kumar et al. (2018) found that POSTN can promote hepatic fibrosis by upregulating the expression levels and activities of lysyl oxidase (LOX) and lysyl oxidase-like (LOXL) isoforms 1–3, as well as by increasing the expression of collagen I and fibronectin in hepatic stellate cells. They also found that POSTN induces Smad3 phosphorylation through integrin αvβ3. Interestingly, the PI3K pathway is involved in this process, independent of the TGF-β pathway, which may indicate that POSTN has the potential to function independently of this pathway.
In summary, POSTN, as the upstream molecule of the PI3K/Akt pathway, activates the pathway by binding to and activating different integrins, which then activates downstream cascades and exerts the biological functions of promoting cell proliferation, migration, and tumor occurrence and development, accordingly. This suggests that the POSTN/PI3K/Akt axis may be a potential target for the treatment of tumors, especially the inhibition of drug resistance (Chu et al. 2020). It has also been reported that bronchial smooth muscle cells can produce POSTN through the PI3K/Akt pathway after IL-13 stimulation (Makita et al. 2018), which indicates that POSTN not only has crosstalk but also a secretory relationship with the pathway. Therefore, understanding the mechanism of POSTN production in different cells may be helpful to clarify the pathogenesis of corresponding diseases. The mechanisms by which POSTN interacts with the PI3K/Akt pathway are shown in Fig. 3.
Fig. 3.
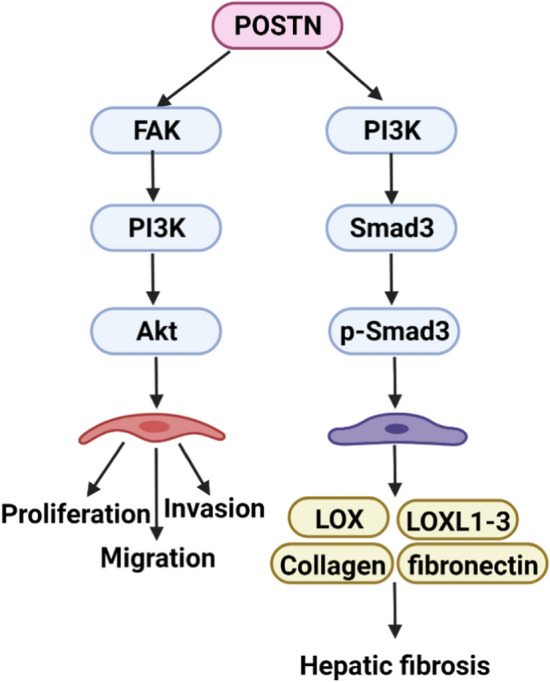
The mechanisms of POSTN interacting with the PI3K/Akt pathway. (1) POSTN can promote cell proliferation, invasion, and migration by activating the FAK/PI3K/Akt pathway. (2) POSTN can increase the expression of LOX, LOXL1-3, collagen, and fibronectin by inducing Smad3 phosphorylation, which is dependent on PI3K
POSTN and the NF-κB pathway
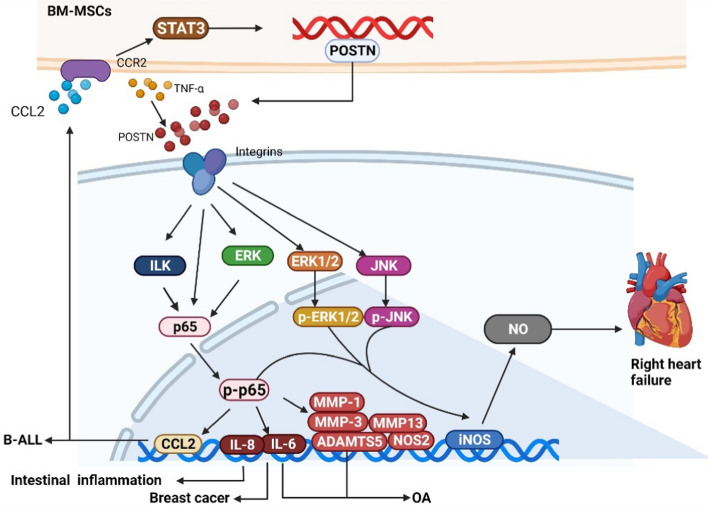
NF-κB, first discovered by Sen et al. in 1986 (Zhang et al. 2021), is a transcription factor that specifically binds to the enhancer region of the immunoglobulin κ light chain gene (Jimi and Ghosh 2005). This gene was considered to be a regulator of B-cell differentiation and function at first but was later proved to be a ubiquitous transcription factor with a key regulatory role in the immune response, the inflammatory response, and tumorigenesis (Hayden and Ghosh 2012). In previous experiments, POSTN was shown to activate the NF-κB pathway in different cells and then promote the production of corresponding cytokines and chemokines (Masuoka et al. 2012). The activation of the NF-κB pathway is identified by the nuclear translocation of p65 (Shinji et al. 2016).
Some studies have revealed that POSTN deposition can lead to chronic inflammation (Parulekar et al. 2014; Bentley et al. 2014) and that the NF-κB pathway is a downstream signaling pathway induced by POSTN (Nishiyama et al. 2011; Taniguchi et al. 2014). Chijimatsu et al. (2015) treated chondrocytes with rPOSTN (0, 3, 10, 30 μg/ml) and/or BAY117082 and found that POSTN can promote the expression of MMP-1, MMP-3, MMP-13, IL-6, IL-8, and NOS2, where BAY117082, an inhibitor of the NF-κB pathway through suppressed IκB phosphorylation, can inhibit POSTN-induced gene expression. They also found that nuclear translocation of p65 occurred in chondrocytes and suggested that POSTN could promote the development of osteoarthritis (OA) by activating NF-κB signaling. Similarly, in a study on temporomandibular joint osteoarthritis, Fan et al. (2020) concluded that POSTN caused the nuclear translocation of p65, subsequently affecting the expression of ADAMTS5 in a time- and dose-dependent manner. They also saw an increase in total IκB and a downregulation of p-IκB by BAY110782. There is no surgical treatment for OA, especially temporomandibular joint osteoarthritis, therefore the POSTN/NF-κB axis may be useful as an alternative treatment target for cartilage degeneration. Unfortunately, the interaction between POSTN, the NF-κB pathway, and integrins in OA has not been studied. In addition to bone tissue, crosstalk between these factors can also promote inflammation in other tissues. Jia et al. (2020) confirmed that the activated NF-κB pathway is upstream of POSTN-mediated cell migration and adhesion, where curcumol was shown to alleviate liver fibrosis by disrupting this process. Koh and colleagues (2016) found that TNF-α could increase the expression of POSTN in intestinal epithelial cells. Through the upregulation of POSTN and TNF-α, the mRNA level of IL-8 and the activity of NF-κB were significantly increased and this effect could be inhibited by anti-integrin αv CD51. In addition, Imoto et al. (2018), by injecting rPOSTN (100–1000 ng/mL) into non-treated normal right ventricular fibroblasts (RVFbs) of rats with the same time (1 h), found POSTN could increase the phosphorylation of ERK1/2, JNK, and NF-κB in RVFbs in a dose-dependent manner, where the maximum effect of POSTN was observed at 1000 ng/ml. Then, they injected rPOSTN (1000 ng/ml) into rats’ RVFbs with 24 h and found that rPOSTN (1000 ng/mL) significantly increased iNOS expression and subsequent NO production, playing a role in the inflammatory cytokine-induced systolic dysfunction in hearts, which could be prevented by inhibitors of the ERK1/2, JNK, and NF-κB pathways. Furthermore, they also demonstrated that POSTN, which activates these three pathways, inhibits the influx of Ca2+ in the L-type Ca2+ channel of H9c2 cardiomyocytes by NO, usually, this process results in right heart failure. Exactly how POSTN activates the three pathways demonstrated in this experiment requires further study.
The interaction between POSTN and the NF-κB pathway not only regulates the progression of inflammation but also plays an important role in cancer. Ma et al. (2017) showed that POSTN deficiency can significantly reduce the growth and transmission of mouse B-cell acute lymphoblastic leukemia (B-ALL) cells. A follow-up study by his team (Ma et al. 2019) proved that POSTN, secreted by bone marrow-derived mesenchymal stromal cells (BM-MSCs), can activate the integrin/integrin-linked kinase (ILK)/NF-κB pathway to promote the B-ALL cells’ expression of CCL2, which is a member of the C–C motif chemokine ligand-2 chemokine family. CCL2 plays an important role in recruiting monocytes and macrophages to inflammatory sites and tumors by binding to its homologous receptor, CCR2 (Yoshimura 2017). In turn, increased CCL2 upregulated the POSTN level of bone marrow mesenchymal stromal cells by activating STAT3, thus promoting the proliferation of leukemia cells and their adhesion to bone marrow mesenchymal stem cells, which eventually led to the progression of B-ALL. Coincidentally, in a previous study, Lambert et al. (2016) found that POSTN can regulate the transcription of IL-6 and IL-8, which is mediated by the NF-κB pathway, subsequently controlling the activation of STAT3 to maintain the tumor microenvironment of breast cancer stem cells. Interestingly, the ERK pathway acts upstream of NF-κB. All of this evidence shows that the POSTN/NF-κB axis forms crosstalk with other pathways and promotes tumorigenesis. The crosstalk formed by these multiple pathways may become the focus of future research, targeting the regulation of tumor progression.
In summary, the NF-κB pathway is key to the regulation of the inflammatory response and tumorigenesis, while having an additional important role, in combination with POSTN, in the progression of inflammatory diseases and a variety of cancers. The POSTN/NF-κB axis primarily activates the nuclear translocation of p65, up- or downregulating downstream molecules for a pro-inflammatory effect or forming crosstalk with other pathways to maintain the tumor microenvironment. In addition, this axis can affect intracerebral hemorrhage in rats (Chen et al. 2020) and participate in regulating the dynamic balance between osteogenesis and osteoclasts (Cai et al. 2021), indicating a need for further exploration into the mechanisms of the POSTN/NF-κB axis. The mechanisms by which POSTN activates the NF-κB pathway to engage in disease progression are shown in Fig. 4.
Fig. 4.
POSTN can activate the NF-κB pathway to participate in cancer and inflammatory diseases. (1) rPOSTN can upregulate the expression of MMP-1, MMP-3, MMP-13, IL-6, IL-8, NOS2, and ADAMTS5 by inducing the phosphorylation of p65 to promote OA. (2) TNF-α can increase the expression of POSTN in intestinal epithelial cells, while the mRNA level of IL-8 and the activity of NF-κB were significantly increased under the upregulation of POSTN and TNF-α. (3) rPOSTN can increase the phosphorylation of ERK1/2, JNK, and NF-κB in RVFbs, where increased iNOS expression and subsequent NO production eventually induced right heart failure. (4) POSTN secreted by BM-MSCs can activate the integrin/ILK/NF-κB pathway to promote B-ALL cells’ expression of CCL2, which, in turn, upregulates the POSTN level of BM-MSCs by activating STAT3 through CCR2 binding, eventually leading to the progression of B-ALL. (5) POSTN can increase the level of IL-6 and IL-8, mediated by the NF-κB pathway while maintaining the tumor microenvironment of breast cancer stem cells, among which the ERK pathway acts upstream of NF-κB
POSTN and the Wnt pathway
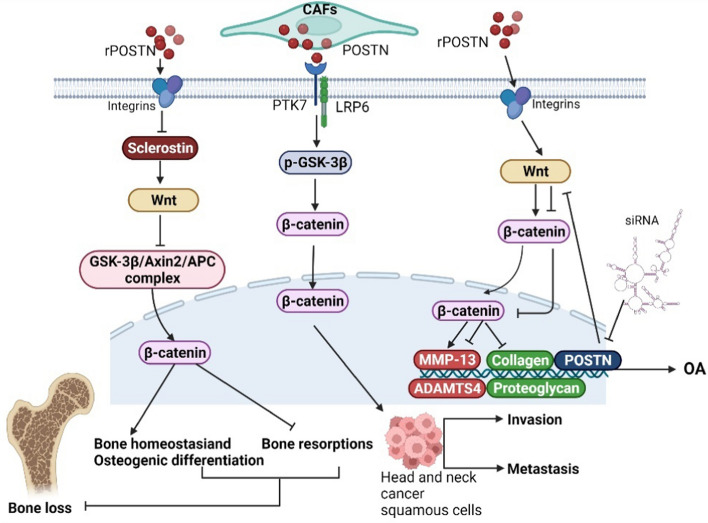
The Wnt proteins are a class of secretory glycoproteins that are highly conserved among vertebrates sharing overall sequence identity and gene structure but are slightly less conserved among other vertebrates and invertebrates (Miller 2001). They are a family of 19 members in humans, activating a multitude of intracellular signaling cascades and participating in a variety of cellular functions, including proliferation, differentiation, apoptosis, migration, and polarity (Kikuchi et al. 2012). It is well known that Wnt proteins act along two signaling pathways, the classical and the non-classical Wnt pathway (Miller 2001). The characteristics of the classical Wnt/β-catenin pathway, with a key molecule β-catenin, have been deeply studied and are known to be associated with the development of several cancers (Moon et al. 2004; Polakis 2007). This pathway is activated by the interaction of Wnt with the seven-channel transmembrane receptor Frizzled (FZD) and the single-channel transmembrane coreceptor LRP6 (Wu et al. 2009), the connection of the related receptor complexes allows for β-catenin to accumulate and for nuclear translocation to occur in the cytoplasm (Lories et al. 2013).
Many previous reports confirmed that POSTN is the upstream promoter of Wnt/β-catenin signaling in tumor-associated diseases (Zhang et al. 2017a; Zhou et al. 2015). A study on head and neck cancer (Yu et al. 2018) found that POSTN, secreted by CAFs, can bind to protein tyrosine kinase 7 (PTK7) on the cell membrane, which is known to be upregulated in various types of cancer (Liu et al. 2015; Jie et al. 2021), and induce the phosphorylation of glycogen synthase kinase-3β (GSK-3β) and the low phosphorylation of β-catenin through LRP6, the phosphorylation events result in β-catenin accumulation and entry into the nucleus and the subsequent promotion of metastasis and invasion of head and neck cancer squamous cells. The results of new exon and RNA sequencing techniques show that POSTN creates a Wnt-signal active, tumor-supportive, and extracellular environment that promotes the expression of basal breast cancer subtypes (Villani et al. 2021). Additionally, recent studies have shown that the POSTN-Wnt/β-catenin axis regulates bone formation and other bone-related diseases, while also playing a significant role in maintaining bone homeostasis, regulating osteogenic differentiation, and inhibiting bone resorption (Wang et al. 2014; Rossini et al. 2013; Kramer et al. 2010). In the regulation of bone formation, sclerostin, a protein secreted by osteocytes, becomes an important medium for the POSTN-Wnt/β-catenin axis. As an antagonist of the pathway, sclerostin competitively inhibits LRP6, a process that is upregulated during mechanical unloading and downregulated during mechanical stimulation (Lin et al. 2009; Robling et al. 2008). In an experiment on the prevention of bone loss by naringin, Lv et al. (Lv et al. 2015) found that the POSTN-induced downregulation of sclerostin can activate the Wnt/β-catenin pathway and inhibit bone loss. The mechanism may involve the binding of Wnt proteins to FZD and LRP6 to inactivate the cytoplasmic GSK-3β/Axin2/APC complex, allowing for β-catenin to accumulate in the cytoplasm and move into the nucleus, this would promote the expression of specific genes in bone, eventually resulting in the differentiation of osteoblasts and the decrease of osteoclast production (Robinson et al. 2006). Integrin αvβ3 is directly involved in the downregulation of sclerostin induced by POSTN (Tu et al. 2012). In recent studies, POSTN, induced by estrogen, could mediate the osteogenic differentiation of bone marrow stromal cells in ovariectomized rats (Li et al. 2020), could promote the osteogenesis of CTLA4-modified human bone marrow mesenchymal stem cells (Zhang et al. 2017b), and could promote coronal suture fusion in Twist1± mice (Bai et al. 2018). These processes cannot be separated from the classical Wnt/β-catenin pathway. In addition to promoting bone formation and osteogenic differentiation, Attur et al. (2015) found that rPOSTN can result in OA, increasing the expression of MMP-13 and ADAMTS4 in a time- and dose-dependent manner and promoting cartilage degeneration through the degradation of collagen and proteoglycans. When the POSTN gene was inhibited by siRNA transfection, the expression of MMP-13 decreased. This induction of POSTN can be inhibited by CCT031374, an inhibitor of the Wnt/β-catenin pathway, indicating that POSTN promotes OA through the Wnt/β-catenin pathway, they also found that this process selectively activated the Wnt/β-catenin pathway rather than the ERK1/2, JNK, and NF-κB pathways. Other studies have found that the serum levels of POSTN and sclerostin in patients with ankylosing spondylitis are lower than those in the respective control groups and it is speculated that this is related to the Wnt/β-catenin signaling pathway (Solmaz et al. 2018), Wnt-3a may also be involved in this process (Klingberg et al. 2014). Interestingly, a new study found that POSTN promotes spermatogonia proliferation by activating the Wnt/β-catenin pathway (Li et al. 2021), which may provide a direction for the future treatment of male infertility.
The non-classical Wnt pathway is independent of β-catenin and involves Wnt-5a as its key ligand (Kikuchi et al. 2012), this pathway has an important role in the regulation of cell migration and polarity during embryogenesis (Nishita et al. 2010). Hasegawa et al. (2015) found that Wnt-5a promotes collagen synthesis and expression of bone-related genes, including OPN, BSP, OSX, and OCN, in human periodontal ligament cells through the upregulation of POSTN expression, where this was mediated by TGF-β1.
In summary, POSTN can interact with both the classical and the non-classical Wnt pathway and perform corresponding biological functions. The study on the crosstalk formed by POSTN and the classical Wnt pathway is relatively mature, and it describes the main function of the crosstalk, which is to regulate osteogenic differentiation and bone formation. Sclerostin, as an antagonist of the Wnt/β-catenin pathway, also plays an important role, where integrin αvβ3 is directly involved in its mechanism of action. Therefore, in-depth studies of the POSTN-sclerostin-Wnt/β-catenin axis are necessary and could be of great significance to promote osteogenic differentiation and inhibit bone loss in the future. The possible mechanisms of interaction between POSTN and the Wnt pathway are shown in Fig. 5.
Fig. 5.
POSTN can activate Wnt signaling to participate in related diseases. (1) POSTN secreted by CAFs can bind to PTK7 and induce the phosphorylation of GSK-3β and the low phosphorylation of β-catenin through LRP6, resulting in β-catenin accumulation and entry into the nucleus; this promotes the metastasis and invasion of head and neck cancer squamous cells. (2) The downregulation of sclerostin by POSTN can activate the Wnt pathway and inactivate the cytoplasmic GSK-3β/Axin2/APC complex, thus β-catenin can accumulate in the cytoplasm and translocate into the nucleus to inhibit bone loss. (3) rPOSTN can increase the expression of MMP-13 and ADAMTS4 and degrade collagen and proteoglycans through the activation of Wnt/β-catenin signaling, resulting in OA; furthermore, the expression of MMP-13 is decreased when the POSTN gene is inhibited by siRNA transfection
POSTN and the MAPK pathway
The MAPK pathway, a highly conserved signal transduction pathway in eukaryotic cells, is a cascade of protein kinases composed of RAS, RAF, MEK, and ERK (Liu et al. 2018b). As one of the most notable signaling pathways, the MAPK pathway transmits the signals of upstream extracellular growth factors to a variety of downstream effectors located in the nucleus, it also regulates normal cell functions, such as cell proliferation, differentiation, survival, and apoptosis (Dhillon et al. 2007). According to sequence similarity and specific activation signals, the MAPKs are divided into four main subfamilies: ERK, ERK5, JNK, and p38 (Whitaker and Cook 2021). The ERK kinase subfamily is usually activated by extracellular mitogens and growth factors, while JNK and p38 have strong responses to environmental stress and intracellular signals (Westfall et al. 2004). The MAPK pathway can also be divided into three branches: the ERK1/2 MAPK pathway, the JNK/p38 MAPK pathway, and the ERK5 MAPK pathway, of which the ERK1/2 MAPK pathway is the most widely studied (Lake et al. 2016). According to previous reports, bronchial smooth muscle cells can produce POSTN through the PI3K/Akt pathway as well as through the MAPK pathway after IL-13 stimulation (Makita et al. 2018), indicating that POSTN and the MAPK pathway have an upstream and downstream relationship.
Li et al. (2011) found that Angiotensin II (Ang-II) can increase the expression of POSTN in a mouse model through the MAPK pathway. In follow-up studies, it was found that the presence of PD98059 and SB202190, inhibitors of ERK1/2 and p38 MAPK, respectively, significantly inhibited the expression of POSTN, whereas SP600125, a specific JNK inhibitor, showed no inhibition of POSTN expression (Li et al. 2014). This suggests that there may be selective specificity in the interaction between the MAPK pathway and POSTN. Also revealed in this study was that overexpressed POSTN can promote cardiomyocyte senescence through the Ang-II-ERK MAPK pathway. Moreover, POSTN promotes the proliferation of melanoma (Kotobuki et al. 2014) and EMT of hepatoblastoma (Chen et al. 2019) through the ERK1/2 MAPK pathway, with these activation effects being mediated by αvβ3 and αvβ5. Importantly, in addition to the ERK1/2 MAPK pathway, POSTN also interacts with the p38 MAPK pathway to participate in the occurrence and development of inflammatory diseases such as asthma (Mo et al. 2019; Zhang et al. 2018) and renal fibrosis (An et al. 2019). Although there seems to be no obvious upstream or downstream relationship between POSTN and the JNK MAPK pathway in cardiomyocytes, in periodontal ligament stem cells, TNF-α treatment (10 ng/ml) stimulates the inflammatory environment and inhibits scratch closure, alkaline-phosphatase (ALP) activity, and mineralization of periodontal ligament stem cells, while also reducing the expression of several osteogenic markers. After adding recombinant POSTN, it was found that POSTN could significantly reverse the effects of TNF-α. In addition, SP600125 inhibited the scratch closure, ALP activity, and mineralization of periodontal ligament stem cells, all of which were enhanced by POSTN (Tang et al. 2017). This experiment concluded that POSTN promotes the migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the JNK MAPK pathway under inflammatory conditions. POSTN also interacts with the PI3K/Akt pathway to promote the migration and proliferation of periodontal ligament stem cells (Padial-Molina et al. 2014; Matsuzawa et al. 2015). The PI3K/Akt and MAPK pathways often engage in complex crosstalk at many levels (Aksamitiene et al. 2012), which indicates that POSTN could produce complex and delicate crosstalk with at least two signaling pathways when performing some of its functions. A clear understanding of POSTN’s interactions with these pathways and the differences between in vivo and in vitro experiments could provide a strong basis for targeted clinical therapy in the future. The mechanisms of activation between POSTN and the MAPK pathway are shown in Fig. 6.
Fig. 6.
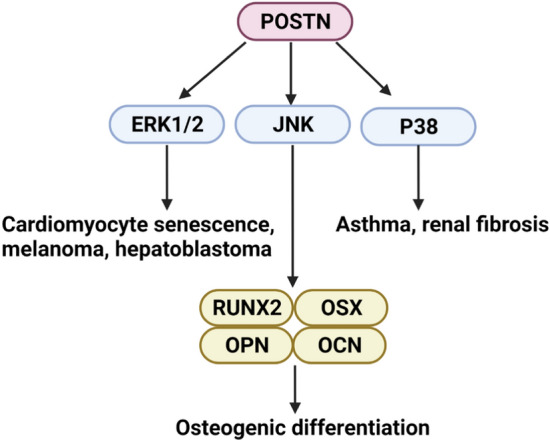
POSTN can activate the MAPK pathway to perform various biological functions. (1) POSTN can promote cardiomyocyte senescence, melanoma, and hepatoblastoma through activation of the ERK1/2 MAPK pathway. (2) POSTN can increase the expression of osteogenesis-related genes, such as RUNX2, OSX, OPN, and OCN through the JNK MAPK pathway, promoting osteogenic differentiation. (3) POSTN can interact with the p38 MAPK pathway to participate in the occurrence and development of asthma and renal fibrosis
POSTN and other pathways
Suzaki and colleagues (2017) have found that leflunomide, an inhibitor of the JAK/STAT6 pathway, can significantly reduce IL-13-stimulated POSTN production at 40 μmol/L in airway epithelial cells, especially goblet cells, this result is similar to that of a previous study where bronchial smooth muscle cells produced POSTN through the PI3K/Akt and MAPK pathways, as well as through JAK/STAT6 (Makita et al. 2018). In a subsequent study, it was found that hypersensitive stimulation could activate keratinocytes in the epidermis to produce thymic stromal lymphopoietin (TLSP), a cytokine involved in inflammatory diseases such as allergic pruritus, asthma, and atopic dermatitis (Cianferoni and Spergel 2014). The released TSLP binds to keratinocytes through autocrine/paracrine mechanisms and induces the secretion of POSTN through the JAK/STAT3 pathway. Secreted POSTN elicits an allergic itch through the interaction with αvβ3 (Mishra et al. 2020). In addition, in abnormal scars, IL-4 and IL-13 prompt the secretion of POSTN, inducing TGF-β1 secretion via the RhoA/ROCK pathway, which further promotes the production and secretion of POSTN. Thus, in combination, POSTN and TGF-β1 stimulate skin fibrosis and form a vicious cycle (Maeda et al. 2019). As a result, the inhibition of IL-4/IL-13 and the RhoA/ROCK pathway may be a potential therapeutic strategy to reduce skin fibrosis and treat abnormal scar formation. Han et al. (2020) found that POSTN interacts with the tyrosine kinase receptor DDR1 and activates its phosphorylation, subsequently leading to the activation of Akt and upregulating the expression of β-catenin, which degrades collagen and proteoglycans in cartilage and upregulates the expression of MMP-13. The use of DDR1 inhibitors alleviated the above effects. Moreover, it has been found that the Notch pathway can regulate the transcription of POSTN in hepatocytes and hepatocellular carcinoma cell lines. Within the range of -5000/ + 5000 bp at the transcriptional initiation site of POSTN, five regions binding to RBPJ, a DNA binding protein partner of Notch1, were found. Chromatin immunoprecipitation analysis confirmed that Notch1 is related to at least two of these regions (Kongkavitoon et al. 2018).
Conclusion
In this article, we review the potential of POSTN to activate different signaling pathways and exert corresponding biological functions. As a non-structural matricellular protein, POSTN is usually expressed at a low level in most adult tissues but can become highly expressed in tissues where adult organisms are affected by stimulating factors, such as inflammatory factors, EMT, angiogenesis, and so on. As shown in Table 1, POSTN can activate TGF-β1/Smad, PI3K/Akt, NF-κB, Wnt, MAPK, JAK, RhoA/ROCK, and other pathways by binding to integrins on the surface of target cells. It can also form crosstalk with important biological functions to participate in the occurrence and development of different diseases. In vivo, POSTN activates signaling pathways, not only promoting angiogenesis, cell migration and invasion, and EMT to result in the occurrence and development of cancer, but also upregulating the expression of inflammatory factors, such as IL-6, IL-8, and NOS2, to promote inflammation and fibrosis in different tissues.
Table 1.
POSTN activates different signaling pathways that are involved indiverse diseases
| Interacting pathway | Function | Diseases | References |
|---|---|---|---|
| TGF-β1 pathway | Induce fibrosis | Pulmonary fibrosis, pulmonary arterial hypertension, systemic sclerosis | Nanri et al. (2020), Yoshihara et al. (2020), Kanaoka et al. (2018), Seki et al. (2019) |
| Induce angiogenesis | Hepatocellular carcinoma | Chen (2017) | |
| Promote migration and invasion | Ovarian cancer, head and neck cancer | Yue et al. (2021), Qin et al. (2016) | |
|
Induce EMT Induce pulmonary hypertension |
Prostatic cancer, glioblastoma |
Ouanouki et al. (2018) Seki et al. (2019) |
|
| PI3K/Akt pathway | Induce fibrosis | Hepatic fibrosis | Kumar et al. (2018) |
| Induce bone formation | Chen et al. (2021), Padial-Molina et al. (2014), Matsuzawa et al. (2015) | ||
| Promote migration and invasion | Ovarian cancer, cholangiocarcinoma | Chu et al. (2020), Utispan et al. (2012) | |
| NF-κB pathway | Upregulate inflammation factors | Osteoarthritis, right ventricular failure, inflammatory bowel disease | Chijimatsu et al. (2015) Fan et al. (2020), Koh et al. (2016), Imoto et al. (2018) |
| Induce fibrosis | Hepatic fibrosis | Jia et al. (2020) | |
| Promote proliferation | B-ALL | Ma et al. (2019) | |
| Maintain tumor microenvironment | Breast cancer | Lambert et al. (2016) | |
| Wnt pathway | Promote migration and invasion | Head and neck cancer, breast cancer | Yu et al. (2018), Villani et al. (2021) |
| Induce bone formation | Robinson et al. (2006), Li et al. (2020), Zhang et al. (2017b), Bai et al. (2018) | ||
| Upregulate inflammation factors | Osteoarthritis | Attur et al. (2015) | |
| MAPK pathway | Induce senescence | Cardiomyocyte senescence | Li et al. (2011) |
| Induce EMT | Hepatoblastoma | Chen et al. (2019) | |
| Promote proliferation | Melanoma | Kotobuki et al. (2014) | |
| Upregulate inflammation factors | Asthma, renal fibrosis | Mo et al. (2019), Zhang et al. (2018), An et al. (2019) | |
| Induce bone formation | Tang et al. (2017) | ||
| Other pathways | |||
| JAK pathway | Upregulate inflammation factors | Asthma | Suzaki et al. (2017) |
| Induce allergy | Skin itch | Mishra et al. (2020) | |
| RhoA/ROCK pathway | Induce fibrosis | Abnormal scar formation | Maeda et al. (2019) |
| Notch pathway | Regulate transcription | Hepatocellular carcinoma and liver bile-duct carcinoma | Kongkavitoon et al. (2018) |
POSTN plays a myriad of roles in the progression of a plethora of diseases, especially cancer and inflammatory diseases. Several studies on preclinical models found that the use of DNA aptamers or antibodies against POSTN effectively inhibited the growth and metastasis of primary tumors (Lee et al. 2013; Zhu et al. 2011; Kyutoku et al. 2011). Thus, blocking POSTN may be an attractive strategy for cancer treatment. Moreover, POSTN has become the main target for understanding the mechanisms of inflammatory diseases such as fibrosis, asthma, and allergic itch, where the clinical treatment of fibrosis through POSTN inhibition has already begun (Kudo 2019b). Therefore, it may be urgent to elucidate the mechanisms of POSTN and different pathways crosstalk formation. In addition, POSTN also plays an important role in promoting osteoblast differentiation. The multiple roles of POSTN in regulating matrix composition and cell function during bone repair and regeneration may be mediated by a variety of subtypes, thus it is necessary to clarify the role of these different subtypes to target POSTN or select suitable POSTN fragments for tissue engineering methods for bone repair and reconstruction (Duchamp de Lageneste 2019). Although the research on POSTN has made remarkable progress in recent years, there are still many problems that need to be solved in basic research and clinical application. Firstly, POSTN has been described in detail in the respiratory system, digestive system, and cardiovascular system, however, little is known about its functions in areas such as the immune system and the nervous system. Secondly, as a biomarker of bone formation, there is a lack of research on POSTN’s role in bone degeneration caused by internal pathological factors or external abnormal stress load, such as spinal degeneration. In a recent study by Zhu et al. (2021), POSTN was considered as an emerging molecule with a potentially important role in degenerative spinal diseases. Finally, it is not clear whether drugs that target POSTN and its associated signaling pathways will have adverse effects on normal tissues. To sum up, we hope that new research will provide an improved and broader understanding of the role of POSTN in the pathogenesis of various diseases, subsequently facilitating the production of useful diagnostic or therapeutic drugs to improve people's quality of life.
Acknowledgements
This work was supported by the Special fund project for doctoral training program of Lanzhou University Second Hospital (YJS-BD-09) and Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2021-QN-A19, CY2020-MS20) and Gansu Natural Science Foundation (No. 20JR10RA717) and Lanzhou Science and Technology Development Guiding Project (No. 2020-ZD-95).
Authors’ contributions
ZW, JA and DZ conceived and wrote the article and had equal contributions to the article. ZW and DZ contributed to making of figures. ZW and JA wrote the manuscript. ZW, HC, AL, JK, WL revised the manuscript. ZW and XK contributed to proofreading of the article. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40:139–146. doi: 10.1042/BST20110609. [DOI] [PubMed] [Google Scholar]
- An JN, Yang SH, Kim YC, Hwang JH, Park JY, Kim DK, Kim JH, Kim DW, Hur DW, Oh YK, Lim CS, Kim YS, Lee JP. Periostin induces kidney fibrosis after acute kidney injury via the p38 MAPK pathway. AM J Physiol Renal Physiol. 2019;316:426–437. doi: 10.1152/ajprenal.00203.2018. [DOI] [PubMed] [Google Scholar]
- Aoki M, Fujishita T. Oncogenic roles of the PI3K/AKT/mTOR axis. Curr Top Microbiol Immunol. 2017;407:153–189. doi: 10.1007/82_2017_6. [DOI] [PubMed] [Google Scholar]
- Attur M, Yang Q, Shimada K, Tachida Y, Nagase H, Mignatti P, Statman L, Palmer G, Kirsch T, Beier F, Abramson SB. Elevated expression of periostin in human osteoarthritic cartilage and its potential role in matrix degradation via matrix metalloproteinase-13. FASEB J. 2015;29:4107–4121. doi: 10.1096/fj.15-272427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, Li D, Xu L, Duan H, Yuan J, Wei M. Recombinant mouse periostin ameliorates coronal sutures fusion in Twist1(+/-) mice. J Transl Med. 2018;16:103. doi: 10.1186/s12967-018-1454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson R, Rich J, Wang X. Periostin potently promotes metastatic growth of colon cancer by augumenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–339. doi: 10.1016/S1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- Bedore J, Leask A, Seguin CA. Targeting the extracellular matrix: matricellular proteins regulate cell-extracellular matrix communication within distinct niches of the intervertebral disc. Matrix Biol. 2014;37:124–130. doi: 10.1016/j.matbio.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Bentley JK, Chen Q, Hong JY, Popova AP, Lei J, Moore BB, Hershenson MB. Periostin is required for maximal airways inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2014;134:1433–1442. doi: 10.1016/j.jaci.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Bio. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Qin H, Yu G. Effect of periostin silencing on Runx2, RANKL and OPG expression in osteoblasts. J Orofac Orthop. 2021;82:82–91. doi: 10.1007/s00056-020-00253-3. [DOI] [PubMed] [Google Scholar]
- Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ. Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. Transcriptional induction of periostin by a sulfatase 2-TGFβ1-SMAD signaling axis mediates tumor angiogenesis in hepatocellular carcinoma. Cancer Res. 2017;77:632–645. doi: 10.1158/0008-5472.CAN-15-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Tian X, Gong W, Sun B, Li G, Liu D, Guo P, He Y, Chen Z, Xia Y, Song T, Guo H. Periostin mediates epithelial-mesenchymal transition through the MAPK/ERK pathway in hepatoblastoma. Cancer Biol Med. 2019;16:89–100. doi: 10.20892/j.issn.2095-3941.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang C, Liu Y, Liang X, Yang C, Zhang X, Li X. Protective effects of medicinal plant breviscapine on postcerebral hemorrhage in rats. J Integr Neurosci. 2020;19:101–109. doi: 10.31083/j.jin.2020.01.1253. [DOI] [PubMed] [Google Scholar]
- Chen G, Wang Y, Zhao X, Xie XZ, Zhao JG, Deng T, Chen ZY, Chen HB, Tong YF, Yang Z, Ding XW, Guo PY, Yu HT, Wu LJ, Zhang SN, Zhu QD, Li JJ, Shan YF, Yu FX, Yu ZP, Xia JL. A positive feedback loop between Periostin and TGFbeta1 induces and maintains the stemness of hepatocellular carcinoma cells via AP-2alpha activation. J Exp Clin Cancer Res. 2021;40:218. doi: 10.1186/s13046-021-02011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijimatsu R, Kunugiza Y, Taniyama Y, Nakamura N, Tomita T, Yoshikawa H. Expression and pathological effects of periostin in human osteoarthritis cartilage. BMC Musculoskelet Disord. 2015;16:215. doi: 10.1186/s12891-015-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010;29:295–307. doi: 10.1007/s10555-010-9221-8. [DOI] [PubMed] [Google Scholar]
- Choi KU, Yun JS, Lee IH, Heo SC, Shin SH, Jeon ES, Choi YJ, Suh DS, Yoon MS, Kim JH. Lysophosphatidic acid-induced expression of periostin in stromal cells: Prognoistic relevance of periostin expression in epithelial ovarian cancer. Int J Cancer. 2011;128:332–342. doi: 10.1002/ijc.25341. [DOI] [PubMed] [Google Scholar]
- Chu L, Wang F, Zhang W, Li HF, Xu J, Tong XW. Periostin secreted by carcinoma-associated fibroblasts promotes ovarian cancer cell platinum resistance through the PI3K/Akt signaling pathway. Technol Cancer Res Treat. 2020;19:1533033820977535. doi: 10.1177/1533033820977535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Chan MK, Li JS, Chan AS, Tang PC, Leung KT, To KF, Lan HY, Tang PM. TGF-beta signaling: from tissue fibrosis to tumor microenvironment. Int J Mol Sci. 2021;22(14):7575. doi: 10.3390/ijms22147575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianferoni A, Spergel J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol. 2014;10:1463–1474. doi: 10.1586/1744666X.2014.967684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clout N, Hohenester E. A model of FAS1 domain 4 of the corneal protein beta(ig)-h3 gives a clearer view on corneal dystrophies. Mol vis. 2003;9:440–448. [PubMed] [Google Scholar]
- Clout N, Tisi D, Hohenester E. Novel fold revealed by the structure of a FAS1 domain oair from the insect cell adhesion molecule fasciclin I. Structure. 2003;11:197–203. doi: 10.1016/S0969-2126(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Doliana R, Bot S, Bonaldo P, Colombatti A. EMI, a novel cysteine-rich domain of EMILINs and other extracellular proteins, interacts with the gC1q domains and participates in multimerization. FEBS Lett. 2000;484:164–168. doi: 10.1016/S0014-5793(00)02140-2. [DOI] [PubMed] [Google Scholar]
- Duchamp de Lageneste O, Colnot C (2019) Periostin in bone regeneration, Periostin, pp 49–61 [DOI] [PubMed]
- Fan B, Liu X, Chen X, Xu W, Zhao H, Yang C, Zhang S. Periostin mediates condylar resorption via the NF-kappaB-ADAMTS5 pathway. Inflammation. 2020;43:455–465. doi: 10.1007/s10753-019-01129-4. [DOI] [PubMed] [Google Scholar]
- Fruman D, Meyers R, Cantley L. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez L, Alonso J. Periostin: a matricellular protein with multiple functions in cancer development and progression. Front Oncol. 2018;8:225. doi: 10.3389/fonc.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sanchez E, Vaquero J, Fernandez-Barrena MG, Lasarte JJ, Avila MA, Sarobe P, Reig M, Calvo M, Fabregat I. The TGF-beta pathway: a pharmacological target in hepatocellular carcinoma? Cancers (basel) 2021;13(13):3248. doi: 10.3390/cancers13133248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T, Mignatti P, Abramson SB, Attur M. Periostin interaction with discoidin domain receptor-1 (DDR1) promotes cartilage degeneration. PLoS ONE. 2020;15:e0231501. doi: 10.1371/journal.pone.0231501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa D, Wada N, Maeda H, Yoshida S, Mitarai H, Tomokiyo A, Monnouchi S, Hamano S, Yuda A, Akamine A. Wnt5a induces collagen production by human periodontal ligament cells through TGFbeta1-mediated upregulation of periostin expression. J Cell Physiol. 2015;230:2647–2660. doi: 10.1002/jcp.24950. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewaid L, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Hwang EY, Jeong MS, Park EK, Kim JH, Jang SB. Structural characterization and interaction of periostin and bone morphogenetic protein for regulation of collagen cross-linking. Biochem Biophys Res Commun. 2014;449:425–431. doi: 10.1016/j.bbrc.2014.05.055. [DOI] [PubMed] [Google Scholar]
- Imoto K, Okada M, Yamawaki H. Periostin mediates right ventricular failure through induction of inducible nitric oxide synthase expression in right ventricular fibroblasts from monocrotaline-induced pulmonary arterial hypertensive rats. Int J Mol Sci. 2018;20(1):61. doi: 10.3390/ijms20010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CT, Matthews JG, Arron JR. Roles of periostin in respiratory disorders. Am J Respir Crit Care Med. 2016;193:949–956. doi: 10.1164/rccm.201510-2032PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari M, Ghadami E, Dadkhah T, Akhavan-Niaki H. PI3k/AKT signaling pathway: erythropoiesis and beyond. J Cell Physiol. 2019;234:2373–2385. doi: 10.1002/jcp.27262. [DOI] [PubMed] [Google Scholar]
- Jia Y, Gao L, Yang X, Zhang F, Chen A, Wang S, Shao J, Tan S, Zheng S. Blockade of periostin-dependent migration and adhesion by curcumol via inhibition of nuclear factor kappa B signaling in hepatic stellate cells. Toxicology. 2020;440:152475. doi: 10.1016/j.tox.2020.152475. [DOI] [PubMed] [Google Scholar]
- Jie Y, Liu G, Feng L, Li Y, Wu L, Li Y, Rong G, Li Y, Wei H, Gu A. PTK7-targeting CAR T-cells for the treatment of lung cancer and other malignancies. Front Immunol. 2021;12:665970. doi: 10.3389/fimmu.2021.665970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi E, Ghosh S. Role of nuclear factor-kappaB in the immune system and bone. Immunol Rev. 2005;208:80–87. doi: 10.1111/j.0105-2896.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- Kanaoka M, Yamaguchi Y, Komitsu N, Feghali-Bostwick CA, Ogawa M, Arima K, Izuhara K, Aihara M. Pro-fibrotic phenotype of human skin fibroblasts induced by periostin via modulating TGF-beta signaling. J Dermatol Sci. 2018;90:199–208. doi: 10.1016/j.jdermsci.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Karlan BY, Dering J, Walsh C, Orsulic S, Lester J, Anderson LA, Ginther CL, Fejzo M, Slamon D. POSTN/TGFBI-associated stromal signature predicts poor prognosis in serous epithelial ovarian cancer. Gynecol Oncol. 2014;132:334–342. doi: 10.1016/j.ygyno.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Kii I, Ito H. Periostin and its interacting proteins in the construction of extracellular architectures. Cell Mol Life Sci. 2017;74:4269–4277. doi: 10.1007/s00018-017-2644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kii I, Nishiyama T, Li M, Matsumoto K-I, Saito M, Amizuka N, Kudo A. Incorporation of Tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem. 2010;285:2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kii I, Nishiyama T, Kudo A. Periostin promotes secretion of fibronectin from the endoplasmic reticulum. Biochem Biophys Res Commun. 2016;470:888–893. doi: 10.1016/j.bbrc.2016.01.139. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (oxf) 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- Klingberg E, Nurkkala M, Carlsten H, Forsblad-d'Elia H. Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol. 2014;41:1349–1356. doi: 10.3899/jrheum.131199. [DOI] [PubMed] [Google Scholar]
- Koh SJ, Choi Y, Kim BG, Lee KL, Kim DW, Kim JH, Kim JW, Kim JS. Matricellular protein periostin mediates intestinal inflammation through the activation of nuclear factor kappaB signaling. PLoS ONE. 2016;11:e0149652. doi: 10.1371/journal.pone.0149652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongkavitoon P, Butta P, Sanpavat A, Bhattarakosol P, Tangtanatakul P, Wongprom B, Tangkijvanich P, Hirankarn N, Palaga T. Regulation of periostin expression by Notch signaling in hepatocytes and liver cancer cell lines. Biochem Biophys Res Commun. 2018;506:739–745. doi: 10.1016/j.bbrc.2018.10.144. [DOI] [PubMed] [Google Scholar]
- Kotobuki Y, Yang L, Serada S, Tanemura A, Yang F, Nomura S, Kudo A, Izuhara K, Murota H, Fujimoto M, Katayama I, Naka T. Periostin accelerates human malignant melanoma progression by modifying the melanoma microenvironment. Pigment Cell Melanoma Res. 2014;27:630–639. doi: 10.1111/pcmr.12245. [DOI] [PubMed] [Google Scholar]
- Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30:3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A. Introductory review: periostin-gene and protein structure. Cell Mol Life Sci. 2017;74:4259–4268. doi: 10.1007/s00018-017-2643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A. The Structure of the Periostin Gene, Its Transcriptional Control and Alternative Splicing, and Protein Expression. Adv Exp Med Biol. 2019;1132:7–20. doi: 10.1007/978-981-13-6657-4_2. [DOI] [PubMed] [Google Scholar]
- Kudo A. Naming, History, Future. Adv Exp Med Biol. 2019;1132:3–4. doi: 10.1007/978-981-13-6657-4_1. [DOI] [PubMed] [Google Scholar]
- Kumar P, Smith T, Raeman R, Chopyk DM, Brink H, Liu Y, Sulchek T, Anania FA. Periostin promotes liver fibrogenesis by activating lysyl oxidase in hepatic stellate cells. J Biol Chem. 2018;293:12781–12792. doi: 10.1074/jbc.RA117.001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyutoku M, Taniyama Y, Katsuragi N, Shimizu H, Kunugiza Y, Lekushi K, Koibuchi N, Sanada F, Oshita Y, Morishita R. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med. 2011;28:181–186. doi: 10.3892/ijmm.2011.712. [DOI] [PubMed] [Google Scholar]
- Lake D, Correa SA, Muller J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol Life Sci. 2016;73:4397–4413. doi: 10.1007/s00018-016-2297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AW, Wong CK, Ozturk S, Papageorgis P, Raghunathan R, Alekseyev Y, Gower AC, Reinhard BM, Abdolmaleky HM, Thiagalingam S. Tumor cell-derived periostin regulates cytokines that maintain breast cancer stem cells. Mol Cancer Res. 2016;14:103–113. doi: 10.1158/1541-7786.MCR-15-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Kim IS, Park SA, Kim Y, Lee JE, Noh DY, Kim KT, Ryu SH, Suh PG. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol Ther. 2013;21:1004–1013. doi: 10.1038/mt.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- Li Q, Liu X, Wei J. Ageing related periostin expression increase from cardiac fibroblasts promotes cardiomyocytes senescent. Biochem Biophys Res Commun. 2014;452:497–502. doi: 10.1016/j.bbrc.2014.08.109. [DOI] [PubMed] [Google Scholar]
- Li C, Li X, Wang X, Miao P, Liu J, Li C, Li D, Zhou W, Jin Z, Cao M. Periostin mediates oestrogen-induced osteogenic differentiation of bone marrow stromal cells in ovariectomised rats. Biomed Res Int. 2020;2020:9405909. doi: 10.1155/2020/9405909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Cheng D, Xu P, Nie H, Zhang T, Pang X. POSTN promotes the proliferation of spermatogonial cells by activating the wnt/beta-catenin signaling pathway. Reprod Sci. 2021;28:2906–2915. doi: 10.1007/s43032-021-00596-1. [DOI] [PubMed] [Google Scholar]
- Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang C, Yuan J, Fu J, Wu M, Su J, Wang X, Yuan X, Jiang W. PTK7 regulates Id1 expression in CD44-high glioma cells. Neuro Oncol. 2015;17:505–515. doi: 10.1093/neuonc/nou227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Feng X, Wang B, Wang X, Wang C, Yu M, Cao G, Wang H. Bone marrow mesenchymal stem cells promote head and neck cancer progression through Periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer Sci. 2018;109:688–698. doi: 10.1111/cas.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Yang X, Geng M, Huang M. Targeting ERK, an Achilles' Heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. 2018;8:552–562. doi: 10.1016/j.apsb.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories RJ, Corr M, Lane NE. To Wnt or not to Wnt: the bone and joint health dilemma. Nat Rev Rheumatol. 2013;9:328–339. doi: 10.1038/nrrheum.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Sun X, Ma J, Ma X, Xing G, Wang Y, Sun L, Wang J, Li F, Li Y. Involvement of periostin-sclerostin-Wnt/beta-catenin signaling pathway in the prevention of neurectomy-induced bone loss by naringin. Biochem Biophys Res Commun. 2015;468:587–593. doi: 10.1016/j.bbrc.2015.10.152. [DOI] [PubMed] [Google Scholar]
- Ma D, Zhang R, Sun Y, Rios HF, Haruyama N, Han X, Kulkarni AB, Qin C, Feng JQ. A novel role of periostin in postnatal tooth formation and mineralization. J Biol Chem. 2011;286:4302–4309. doi: 10.1074/jbc.M110.140202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Zhao X, Huang J, Jia X, Deng M, Cui D, Du Z, Fu G, Ouyang G, Xiao C. A critical role of periostin in B-cell acute lymphoblastic leukemia. Leukemia. 2017;31:1835–1837. doi: 10.1038/leu.2017.149. [DOI] [PubMed] [Google Scholar]
- Ma Z, Zhao X, Deng M, Huang Z, Wang J, Wu Y, Cui D, Liu Y, Liu R, Ouyang G. Bone marrow mesenchymal stromal cell-derived periostin promotes B-ALL progression by modulating CCL2 in leukemia cells. Cell Rep. 2019;26:1533–1543. doi: 10.1016/j.celrep.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Ma H, Wang J, Zhao X, Wu T, Huang Z, Chen D, Liu Y, Ouyang G. Periostin promotes colorectal tumorigenesis through integrin-FAK-Src pathway-mediated YAP/TAZ activation. Cell Rep. 2020;30:793–806. doi: 10.1016/j.celrep.2019.12.075. [DOI] [PubMed] [Google Scholar]
- Maeda D, Kubo T, Kiya K, Kawai K, Matsuzaki S, Kobayashi D, Fujiwara T, Katayama T, Hosokawa K. Periostin is induced by IL-4/IL-13 in dermal fibroblasts and promotes RhoA/ROCK pathway-mediated TGF-beta1 secretion in abnormal scar formation. J Plast Surg Hand Surg. 2019;53:288–294. doi: 10.1080/2000656X.2019.1612752. [DOI] [PubMed] [Google Scholar]
- Makita K, Mikami Y, Matsuzaki H, Miyashita N, Takeshima H, Noguchi S, Horie M, Urushiyama H, Iikura M, Hojo M, Nagase T, Yamauchi Y. Mechanism of periostin production in human bronchial smooth muscle cells. Int Arch Allergy Immunol. 2018;175:26–35. doi: 10.1159/000485892. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, Takeuchi S, Koike K, Ono J, Noshiro H, Furue M, Conway SJ, Narisawa Y, Izuhara K. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa M, Arai C, Nomura Y, Murata T, Yamakoshi Y, Oida S, Hanada N, Nakamura Y. Periostin of human periodontal ligament fibroblasts promotes migration of human mesenchymal stem cell through the alphavbeta3 integrin/FAK/PI3K/Akt pathway. J Periodontal Res. 2015;50:855–863. doi: 10.1111/jre.12277. [DOI] [PubMed] [Google Scholar]
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- Miller JR. The Wnts. Genome Biol. 2001;3:1–15. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Wheeler JJ, Pitake S, Ding H, Jiang C, Fukuyama T, Paps JS, Ralph P, Coyne J, Parkington M, DeBrecht J, Ehrhardt-Humbert LC, Cruse GP, Baumer W, Ji RR, Ko MC, Olivry T. Periostin activation of integrin receptors on sensory neurons induces allergic itch. Cell Rep. 2020;31:107472. doi: 10.1016/j.celrep.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y, Zhang K, Feng Y, Yi L, Liang Y, Wu W, Zhao J, Zhang Z, Hu Q, He J, Zhen G. Epithelial SERPINB10, a novel marker of airway eosinophilia in asthma, contributes to allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2019;316:245–254. doi: 10.1152/ajplung.00362.2017. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Nanri Y, Nunomura S, Terasaki S, Yoshihara T, Hirano Y, Yokosaki Y, Yamaguchi Y, Feghali-Bostwick C, Ajito K, Murakami S, Conway SJ, Izuhara K. The cross-talk between TGF-β and periostin can be targeted for pulmonary fibrosis. Am J Respir Cell Mol Biol. 2020;62:204–216. doi: 10.1165/rcmb.2019-0245OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M, Enomoto M, Yamagata K, Minami Y. Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol. 2010;20:346–354. doi: 10.1016/j.tcb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS ONE. 2011;6:e18410. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouanouki A, Lamy S, Annabi B. Periostin, a signal transduction intermediate in TGF-β-induced EMT in U-87MG human glioblastoma cells, and its inhibition by anthocyanidins. Oncotarget. 2018;9:22023–22037. doi: 10.18632/oncotarget.25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang G, Liu M, Ruan K, Song G, Mao Y, Bao S. Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB pathway. Cancer Lett. 2009;281:213–219. doi: 10.1016/j.canlet.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Padial-Molina M, Volk SL, Rios HF. Periostin increases migration and proliferation of human periodontal ligament fibroblasts challenged by tumor necrosis factor -alpha and Porphyromonas gingivalis lipopolysaccharides. J Periodontal Res. 2014;49:405–414. doi: 10.1111/jre.12120. [DOI] [PubMed] [Google Scholar]
- Parulekar AD, Atik MA, Hanania NA. Periostin, a novel biomarker of TH2-driven asthma. Curr Opin Pulm Med. 2014;20:60–65. doi: 10.1097/MCP.0000000000000005. [DOI] [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Polizzotti BD, Arab S, Kuhn B. Intrapericardial delivery of gelfoam enables the targeted delivery of Periostin peptide after myocardial infarction by inducing fibrin clot formation. PLoS ONE. 2012;7:e36788. doi: 10.1371/journal.pone.0036788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme GJ. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest. 2007;87:1077–1091. doi: 10.1038/labinvest.3700669. [DOI] [PubMed] [Google Scholar]
- Qin J, Yuan F, Peng Z, Ye K, Yang X, Huang L, Jiang M, Lu X. Periostin enhances adipose-derived stem cell adhesion, migration, and therapeutic efficiency in Apo E deficient mice with hind limb ischemia. Stem Cell Res Ther. 2015;6:138. doi: 10.1186/s13287-015-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Yan M, Zhang J, Wang X, Shen Z, Lv Z, Li Z, Wei W, Chen W. TGFbeta3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci Rep. 2016;6:20587. doi: 10.1038/srep20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak-Wielgomas K, Dziegiel P. The role of periostin in neoplastic processes. Folia Histochem Cytobiol. 2015;53:120–132. doi: 10.5603/FHC.a2015.0014. [DOI] [PubMed] [Google Scholar]
- Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1016/S0021-9258(19)84086-3. [DOI] [PubMed] [Google Scholar]
- Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- Rossini M, Gatti D, Adami S. Involvement of WNT/beta-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93:121–132. doi: 10.1007/s00223-013-9749-z. [DOI] [PubMed] [Google Scholar]
- Sabbadini F, Bertolini M, De Matteis S, Mangiameli D, Contarelli S, Pietrobono S, Melisi D. The multifaceted role of TGF-beta in gastrointestinal tumors. Cancers (basel) 2021;13(16):3960. doi: 10.3390/cancers13163960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Furukawa N, Koitabashi N, Obokata M, Conway SJ, Arakawa H, Kurabayashi M. Periostin-expressing cell-specific transforming growth factor-beta inhibition in pulmonary artery prevents pulmonary arterial hypertension. PLoS ONE. 2019;14:e0220795. doi: 10.1371/journal.pone.0220795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Liu Z, Wang C, Xu F, Zhang J, Li M, Lei Y, Wang A, Bi C, Zhu G. Inhibition of postn rescues myogenesis defects in myotonic dystrophy type 1 myoblast model. Front Cell Dev Biol. 2021;9:710112. doi: 10.3389/fcell.2021.710112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinji I, Misako K, Asako Y, Kazuhito A. Suppressive activity of quercetin on periostin functions in vivro. In Vivo. 2016;30:17–25. [PubMed] [Google Scholar]
- Solmaz D, Uslu S, Kozaci D, Karaca N, Bulbul H, Tarhan EF, Ozmen M, Can G, Akar S. Evaluation of periostin and factors associated with new bone formation in ankylosing spondylitis: periostin may be associated with the Wnt pahway. Int J Rheum Dis. 2018;21:502–509. doi: 10.1111/1756-185X.13186. [DOI] [PubMed] [Google Scholar]
- Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthr Cartil. 2020;28:400–409. doi: 10.1016/j.joca.2020.02.027. [DOI] [PubMed] [Google Scholar]
- Suzaki I, Kawano S, Komiya K, Tanabe T, Akaba T, Asano K, Suzaki H, Izuhara K, Rubin BK. Inhibition of IL-13-induced periostin in airway epithelium attenuates cellular protein expression of MUC5AC. Respirology. 2017;22:93–100. doi: 10.1111/resp.12873. [DOI] [PubMed] [Google Scholar]
- Takayama I, Tanabe H, Nishiyama T, Ito H, Amizuka N, Li M, Katsube KI, Kii I, Kudo A. Periostin is required for matricellular localization of CCN3 in periodontal ligament of mice. J Cell Commun Signal. 2017;11:5–13. doi: 10.1007/s12079-016-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fascilin I. Biochen J. 1993;294:291–274. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Liu L, Wang P, Chen D, Wu Z, Tang C. Periostin promotes migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the Jun amino-terminal kinases (JNK) pathway under inflammatory conditions. Cell Prolif. 2017;50(6):e12369. doi: 10.1111/cpr.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang PM, Zhang YY, Mak TS, Tang PC, Huang XR, Lan HY. Transforming growth factor-beta signalling in renal fibrosis: from Smads to non-coding RNAs. J Physiol. 2018;596:3493–3503. doi: 10.1113/JP274492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Liu B, Bu X, Zhao P. Cross-talk between ovarian cancer cells and macrophages through periostin promotes macrophage recruitment. Cancer Sci. 2018;109:1309–1318. doi: 10.1111/cas.13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Wang Y, Hemmings BA, Ruegg C, Xue G. PKB/Akt-dependent regulation of inflammation in cancer. Semin Cancer Biol. 2018;48:62–69. doi: 10.1016/j.semcancer.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Arima K, Masuoka M, Ohta S, Shiraishi H, Ontsuka K, Suzuki S, Inamitsu M, Yamamoto KI, Simmons O, Toda S, Conway SJ, Hamasaki Y, Izuhara K. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1alpha/IL-6 loop. J Invest Dermatol. 2014;134:1295–1304. doi: 10.1038/jid.2013.500. [DOI] [PubMed] [Google Scholar]
- Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utispan K, Sonongbua J, Thuwajit P, Chau-In S, Pairojkul C, Wongkham S, Thuwajit C. Periostin activates integrin alpha5beta1 through a PI3K/AKTdependent pathway in invasion of cholangiocarcinoma. Int J Oncol. 2012;41:1110–1118. doi: 10.3892/ijo.2012.1530. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers S, Panayotou G. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/S0968-0004(97)01061-X. [DOI] [PubMed] [Google Scholar]
- Villani R, Murigneux V, Alexis J, Sim SL, Wagels M, Saunders N, Soyer HP, Parmentier L, Nikolaev S, Fink JL, Roy E, Khosrotehrani K. Subtype-specific analyses reveal infiltrative basal cell carcinomas are highly interactive with their environment. J Invest Dermatol. 2021;141:2380–2390. doi: 10.1016/j.jid.2021.02.760. [DOI] [PubMed] [Google Scholar]
- Voisin A, Damon-Soubeyrand C, Bravard S, Saez F, Drevet JR, Guiton R. Differential expression and localisation of TGF-beta isoforms and receptors in the murine epididymis. Sci Rep. 2020;10:995. doi: 10.1038/s41598-020-57839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ouyang G. Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell. 2012;10:111–112. doi: 10.1016/j.stem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li Y, Paulson C, Shao J, Zhang X, Wu M, Chen W. Wnt and the Wnt signaling pathway in bone development and disease. Landmark ED. 2014;19:379–407. doi: 10.2741/4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall PJ, Ballon DR, Thorner J. When the stress of your environment makes you go HOG wild. Science. 2004;306:1151–1152. doi: 10.1126/science.1104879. [DOI] [PubMed] [Google Scholar]
- Whitaker RH, Cook JG. Stress relief techniques: p38 MAPK determines the balance of cell cycle and apoptosis pathways. Biomolecules. 2021;11(10):1444. doi: 10.3390/biom11101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Wu T, Ouyang G. Matricellular proteins: multifaceted extracellular regulators in tumor dormancy. Protein Cell. 2014;5:249–252. doi: 10.1007/s13238-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Huang H, Abreu JG, He X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE. 2009;4:e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZM, Wang XY, Wang AM. Periostin induces chemoresistance in colon cancer cells through activation of the PI3K/Akt/survivin pathway. Biotechnol Appl Biochem. 2015;62:401–406. doi: 10.1002/bab.1193. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, Aihara M, Takahashi K. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br J Dermatol. 2013;168:717–725. doi: 10.1111/bjd.12117. [DOI] [PubMed] [Google Scholar]
- Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, Matsui S, Kudo A, Naka T, Murota H, Katayama I. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS ONE. 2012;7:e41994. doi: 10.1371/journal.pone.0041994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara T, Nanri Y, Nunomura S, Yamaguchi Y, Feghali-Bostwick C, Ajito K, Murakami S, Mawatari M, Izuhara K. Periostin plays a critical role in the cell cycle in lung fibroblasts. Respir Res. 2020;21:38. doi: 10.1186/s12931-020-1299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T. The production of monocyte chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments. Cytokine. 2017;98:71–78. doi: 10.1016/j.cyto.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Yu B, Wu K, Wang X, Zhang J, Wang L, Jiang Y, Zhu X, Chen W, Yan M. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 2018;9:1082. doi: 10.1038/s41419-018-1116-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yue H, Li W, Chen R, Wang J, Lu X, Li J. Stromal POSTN induced by TGF-beta1 facilitates the migration and invasion of ovarian cancer. Gynecol Oncol. 2021;160:530–538. doi: 10.1016/j.ygyno.2020.11.026. [DOI] [PubMed] [Google Scholar]
- Zhang R, Yao RR, Li JH, Dong G, Ma M, Zheng QD, Gao DM, Cui JF, Ren ZG, Chen RX. Activated hepatic stellate cells secrete periostin to induce stem cell-like phenotype of residual hepatocellular carcinoma cells after heat treatment. Sci Rep. 2017;7:2164. doi: 10.1038/s41598-017-01177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Luo K, Rong Z, Wang Z, Luo F, Zhang Z, Sun D, Dong S, Xu J, Dai F. Periostin upregulates wnt/beta-catenin signaling to promote the osteogenesis of ctla4-modified human bone marrow-mesenchymal stem cells. Sci Rep. 2017;7:41634. doi: 10.1038/srep41634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Liang Y, Feng Y, Wu W, Zhang H, He J, Hu Q, Zhao J, Xu Y, Liu Z, Zhen G. Decreased epithelial and sputum miR-221-3p associates with airway eosinophilic inflammation and CXCL17 expression in asthma. Am J Physiol Lung Cell Mol Physiol. 2018;315:253–264. doi: 10.1152/ajplung.00567.2017. [DOI] [PubMed] [Google Scholar]
- Zhang GZ, Liu MQ, Chen HW, Wu ZL, Gao YC, Ma ZJ, He XG, Kang XW. NF-kappaB signalling pathways in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2021;54:e13057. doi: 10.1111/cpr.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17:170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Saxton RE, Ramos L, Chang DD, Karlan BY, Gasson JC, Slamon DJ. Neutralizing monoclonal antibody to periostin inhibits ovarian tumor growth and metastasis. Mol Cancer Ther. 2011;10:1500–1508. doi: 10.1158/1535-7163.MCT-11-0046. [DOI] [PubMed] [Google Scholar]
- Zhu D, Zhou W, Wang Z, Wang Y, Liu M, Zhang G, Guo X, Kang X. Periostin: an emerging molecule with a potential role in spinal degenerative diseases. Front Med (lausanne) 2021;8:694800. doi: 10.3389/fmed.2021.694800. [DOI] [PMC free article] [PubMed] [Google Scholar]