Abstract
Certain bacteria, known as probiotics, have had a vastly beneficial effect on people's health; considering their benefits they have been mixed with a wide variety of foods for several decades now. The ability of probiotics to modify the immunological response of the host, antagonize pathogenic microbes, or compete for adhesion sites with pathogenic microorganisms is related to the action of probiotics against microorganisms. Infections of the digestive tract, irritable bowel, lactose intolerance, allergies, infections of the urogenital tract, cystic fibrosis, and various cancers can all be prevented and treated with the use of probiotics. They can reduce the side effects of various antibodies. In the field of oral health, dental caries, periodontal disease, and bad breath can be prevented and treated with the use of probiotics. The findings of several of these clinical studies indicate that probiotics may be beneficial in the treatment and prevention of various diseases and health issues. Validation of a significant number of these clinical investigations is necessary before the results can be applied to the clinical setting. Clinical studies play an important part in such investigations, and in the not-too-distant future, the outcomes of such trials will determine whether or not probiotics are effective in the treatment of disease. This article will attempt to provide a summary of the available literature on the benefits that these probiotics have with regard to health and disease.
Probiotics are foods and/or supplements that contain non-pathogenic microbes such as bacteria and yeast that colonize the gut and can potentially yield a variety of health benefits. Research into the various ways in which probiotic bacteria could be used in the treatment of intestinal disorders is ongoing. Thanks to clinical studies and laboratory experiments, we now know more about how probiotics affect gut microbiome disorders. Studies can prove that probiotics can alleviate a variety of gastrointestinal ailments and improve overall health. This article concentrates on probiotics and commensal microbes, as well as their potential role in gut microbiome-related illnesses. In this section, we mark certain areas that need further work and studies so as to enhance our understanding of how probiotics help in the treatment and reduction of chances of gastrointestinal diseases.
Keywords: lactobacilli, immunomodulation, probiotics, intestinal disease, gut microflora
Introduction and background
Human beings consume a significant number of pathogens every day, primarily bacteria. For several decades, probiotic microorganisms have been utilized in several diets due to their positive effects on human health [1,2]. The Agriculture Organization of the United States and the World Health Organization [3] defined probiotics in 2001 as bacteria that, when given to a host at adequate levels, improve their health. Regarding probiotics, Lactobacillus and Bifidobacterium are the two most frequently used genera. These bacteria are generally thought of as harmless due to their ability to survive in the body to cure and prevent diseases, unlike the usual pathogens. Along with that, these microorganisms have played a vital role in the process of fermenting milk and food preservation for many years [4]. Numerous randomly selected clinical trials have shown that probiotic strains are safe and effective in providing users with their benefits. These benefits include the prevention of acute diarrhoea, Crohn's disease, cardiovascular and urogenital infections, cancer, lactose intolerance, cystic fibrosis, dental caries, and oral diseases. Bacteria may also be beneficial in preventing tooth decay, treating periodontal disease, and reducing oral malodor. Probiotics' beneficial role in preventing inflammatory disorders is an ever-expanding list [5]. Over time, scientists have become highly inquisitive about discovering, analyzing, and studying species with probiotic properties. The role of probiotics in health and illness is summarized in this article and is derived from reviewed studies. Human intestinal health can be improved with the use of probiotics. A mixture of prebiotics has also been proposed. Consequently, the probiotics market has grown rapidly, trying to make probiotics a part of food and supplements [6]. The use of probiotics has been recommended to improve immunity and general health. It is now understood that the commensal human gut microbes may contribute to the onset of metabolic illnesses such as obesity, diabetes, and inflammatory bowel disease. Interestingly, it has been seen that the consumption of probiotics significantly increases the prognosis and management of such disorders. A concise explanation of the idea, purpose, and connection between gut microbiome-related disorders and probiotics is given in this review.
Review
How probiotics work in our body
There are various ways the probiotics can work inside our body: Interacting and stimulating the growth of the body's good commensal microbes, and inhibiting the growth of pathogens inside the human body. They sometimes also increase the host's antigenic response time, which in turn increases the synthesis of antimicrobial compounds and can also block the site where the pathogen might bind. Furthermore, probiotics exhibit adherence and (at least temporary) colonization of the human body, increasing the duration of retention and leading to sustained probiotic function [5-12]. Several probiotics used as food materials in today's world have been listed in the table below (Table 1).
Table 1. Probiotics used in various products in the world.
| Probiotic bacteria | Product | Country |
| 1. Bifidobacterium bifidum | Infant formula | Turkey |
| 2. B. breve | Drink | Japan |
| 3. B. lactis | Infant formula | Israel |
| 4. Lactobacillus acidophilus | Yogurt | UK |
| 5. L. casei Shirota | Drink | Argentina, Australia, Belgium, Brazil, China, Germany, Japan, Indonesia, UK, USA, Taiwan |
| 6. L. casei | Yogurt | France, Colorado/Arizona (USA) |
| 7. L. lactis L1A | Yogurt | Sweden |
| 8. L. plantarum 299v | Fruit drink, Ice cream, Recovery drink, Oat mixture | Sweden |
Probiotics and their benefits
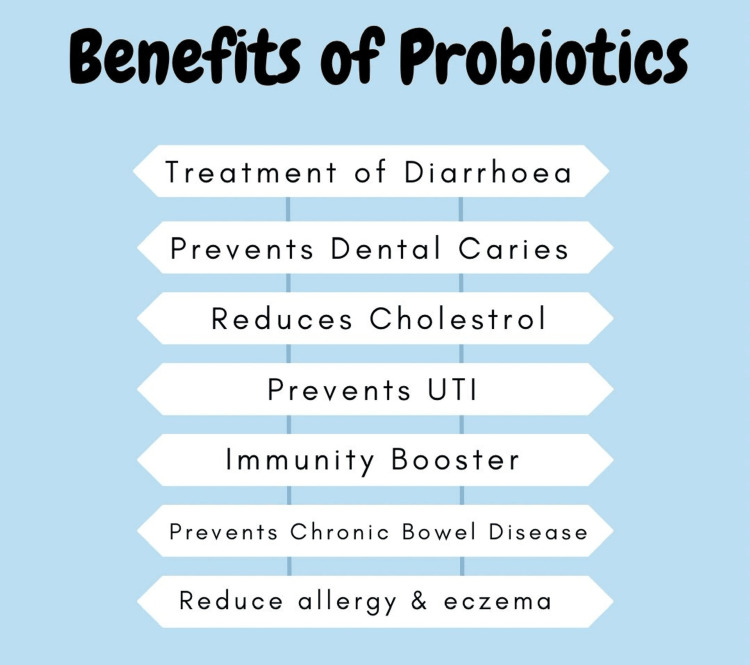
Figure 1 lists a few benefits of probiotics for a healthy human life. These include a large variety of uses not just for a particular part but almost everywhere in our body. In the gastrointestinal tract, lies their well-known benefit of improving our digestion and reducing the amount of cholesterol. Their other uses involve the treatment of diarrhea and the prevention of inflammatory bowel diseases. Furthermore, the benefits will include preventing dental caries and strengthening our immune system, especially during allergic conditions. The constant growth of harmful microorganisms can also be inhibited by probiotics to an extent. Bifidobacterium creates glutamine, which preserves the integrity of the mucosa and improves the mucosal barrier's defenses [13-14]. Many studies have proved that probiotics help deal with different kinds of diarrhea, such as travelers' diarrhea, antibiotic-induced diarrhea, and rotavirus-related diarrhea in small children [15,16]. Irritable bowel syndrome has a poorly understood cause, and hence treatment for such people is challenging. However, introduction to the Enterococcus strain PR88 as an oral probiotic showed clinical improvement in patients. Research has persisted for the use of probiotics and led to some intriguing results of probiotics and their utilization in complex conditions [17].
Figure 1. Benefits of Probiotics.
This illustration has been created by the author of this study.
UTI: Urinary tract infection
The positive effects of probiotics on patients with food allergies are a result of their combined impact on the gut's non-immunologic and immunologic defense barriers. The natural dietary protein's immunomodulatory characteristics are altered by lactobacilli. Probiotics affect the immune system by stimulating the gastrointestinal lymphoid tissue's lymphoid cells [18-21]. Probiotics' effect on atopic dermatitis has been studied in depth. The intensity of eczema and the number of different processes in the given population decreased with the consumption of living and heat-neutralized probiotic bacteria [22]. Lactobacilli protects women from developing urinary tract infections (UTIs) [23]. Using the distal urethral isolates L. rhamnosus GR-1 and L. fermentum B-54 with RC-14 as a substitute for anti-gram-positive bacilli activities and hydrogen peroxide production, studies have shown that the two-strain combination works. For a year, researchers found that using single or multiple two capsules a week vaginally can help prevent the recurrence of UTIs [23-26]. Human probiotics reduce cholesterol. Probiotics cause direct digestion of lipids, which influences cholesterol production. No research showed statistically significant changes in cholesterol levels [27].
Dental caries, a multi-factor bacterial illness, is characterized by acid decalcification of the outer layer of our teeth. To be effective in preventing dental caries, a probiotic must be able to attach itself to teeth coatings and mix into the microbial flora that composes the dental biofilm. To halt the spread of cariogenic bacteria, it must also go to fight and repel them. Research has found that the cariogenic species Streptococcus sobrinus could not grow on a hydroxyapatite surface because only Streptococcus thermophilus and Lactobacillus lactis ssp. lactis could form a biofilm there. The ability of W. cibaria isolates to inhibit S. mutans' capacity to create microbes both in vitro and in vivo, and furthermore to prevent this microbe from proliferating, has been demonstrated recently [28-30]. Periodontal diseases are classified into two categories: gingivitis and periodontitis. Gum disease, unlike periodontitis, is a short-lived, non-debilitating inflammation of the gums that affects only the unattached gingiva and not the alveolar bone [31]. Most efforts to prevent periodontal disease and make sure a full recovery focus on getting rid of pathogens and strengthening the outer boundary, which also makes a person less likely to get sick. Periodontal health may be improved if these good bacteria find a way to establish themselves in dental biofilm and inhibit harmful bacterial growth and metabolism then and there. A wide range of data analysis has found probiotic bacteria to be safe. Few clinical studies have been published on how these probiotics could be beneficial in the treatment of periodontal disease [32,33]. Laboratory research is therefore the key source of data on probiotics that deliberately address periodontal tissues. Gum or lozenges containing probiotics improved the periodontal health of patients with periodontal disease. Many people have foetor ex ore or bad breath, which is commonly referred to as halitosis. Volatile sulfur compounds (VSCs) are primarily produced by Gram-negative anaerobes in dental plaque and on the surface of the tongue and are the main reason for bad breath in the oral cavity. This ailment has also been shown to respond well to bacterial therapy. Probiotic strains of bacteria from healthy people's native oral microbiota could be used to start replacing the bacteria allegedly involved in halitosis as an adjunct for prevention and treatment [34].
How probiotics affect oral health
The oral effects of probiotics are unknown. There is a slight reduction in gum disease with the use of probiotics. Substances made by lactic acid bacteria cause inflammation. Most studies show that probiotics can eliminate infections by outcompeting bacteria for bonding surfaces and nutrients [35,36]. None of the reviewed research read by the author indicated the harmful effects of bacteriotherapy [37-43]. Taipale et al. reported that two probiotic intervention group patients and two placebo control group patients were eliminated due to gastrointestinal discomfort and atopic eczema, respectively. Bacteriotherapy has proven to be helpful in strengthening the immune system in people with HIV and cancer. This review focuses on periodontal disease and dental caries (each of which has been described separately).
Caries and probiotics
Caries has several causes. Pathogenesis includes the host's immune system, microbiota (mainly S. mutans), and diet. Microbiological reasons are the predominant cause of tooth caries, though all of these factors must be present. Only 19 of 725 articles on PUBMED met the inclusion criteria for this review. Only two studies (systematic review) looked explored the effects of probiotic use on dental caries. The authors' research was hampered by having to compare the colony-forming unit (CFU) counts of S. mutans and Lactobacilli earlier and now when they use probiotics. S. mutans CFU levels decreased significantly in-between bacteriotherapy, but not Lactobacilli. S. mutans CFU levels in the intervention group were lower (105 CFU/ml) and less elevated (>106 CFU/ml) than in the comparison group or when Lactobacilli CFU counts were compared. Study design, treatment duration, probiotic strains and concentrations, and follow-up period have varying methodologies in the published studies.
Probiotics role in human gut-associated microbiome diseases
Human Gut Microbiota Dysbiosis
Microbial dysbiosis is a term used to describe an unbalance in the structure and function of intestinal microorganisms [44]. Antibiotic use, bacterial infections, and changes in the diet all contribute to the problem, which has become more prevalent in the modern era. Irritable bowel syndrome (IBS), celiac disease, and other intestinal illnesses are associated with a lack of useful bacteria in the gut. Beneficial probiotics in the gastrointestinal tract inhibit pathogenic microbes from trying to infiltrate and grow by competing for space and resources [45]. Re-establishing healthy commensal microbes and trying to prevent infections in patients following antibiotic therapy is among the most vital uses of probiotics. They are usually used for curing antibiotic-associated diarrhea (AAD), which occurs whenever the microbial community is disrupted. Carbapenem-resistant Clostridioides difficile (previously Clostridium difficile), a disease-causing bacterium, is one of the major causes of AAD. Previous reviews and meta-analyses have shown that probiotics when used in conjunction with other treatments, can help avoid AAD in patients of any age group [46]. Probiotics help stop diarrhea induced by C. difficile both in adults and children [47,48]. There was no perceivable increased likelihood of side effects in this study's findings that probiotic diagnosis may reduce AAD incidence by 51%. Furthermore, a study on this topic has shown that Lactobacillus rhamnosus and Saccharomyces boulardii are seen to be highly effective in protecting AAD.
Chronic Bowel Disease (Inflammatory Bowel Disease)
Inflammatory bowel disease (IBD) is a chronic inflammatory condition that affects the digestive system. IBD includes Crohn's disease (CD), ulcerative colitis (UC), and indeterminate colitis (IC), which are distinguishable based on where the GI tract inflammation is located [49]. IBD is assumed to be caused by an aberrant immune system response; while the exact etiology is still unclear, stress and an unbalanced diet are claimed to be the potential causes. According to some studies, IBD pathogenesis may account for the presence of gut pathogens. Furthermore, numerous investigations have demonstrated that the microbiome of IBD patients differs from that of healthy individuals. Additionally, it has been proposed that preserving the balance of the gut microbes may be crucial for preventing IBD [50]. Probiotics have drawn a lot of attention recently as a potential treatment to alter the microbe's positive effects on IBD. Probiotics, for instance, have been utilized to treat ulcerative colitis and induce remission [51-52]. However, a recent study found that probiotic supplementation appears to be a workable adjuvant therapy for UC and not for CD in individuals with inflammatory bowel disease. As a reason, there is presently insufficient knowledge concerning probiotics' efficacy to make broad recommendations for treatment in CD patients.
Crohn's disease (CD), an irritable bowel disorder that involves losing weight, constipation, temperature, lethargy, and abdominal pain, affects the entire gastrointestinal tract (GIT). The onset of CD is influenced by several factors, including genetic, environmental, and microbial [52], although the exact cause is still unknown. CD's symptoms can be treated with various medications, however, the disease has no known cure. Lower immune system function and intestinal inflammation can be treated with immunosuppressants and steroid medications [53]. Similarly, probiotics may offer an additional technique to standard therapy. It has been proved that early therapy was preferable to postoperative probiotic feeding in CD patients. A more recent study, however, found that performing adjuvant multi-strain probiotic treatment had no appreciable impact on inflammatory responses in CD patients. Additional study is required to clarify the function of probiotics due to discrepancies in the findings of clinical trials employing probiotic supplements to treat CD.
Sources of probiotics
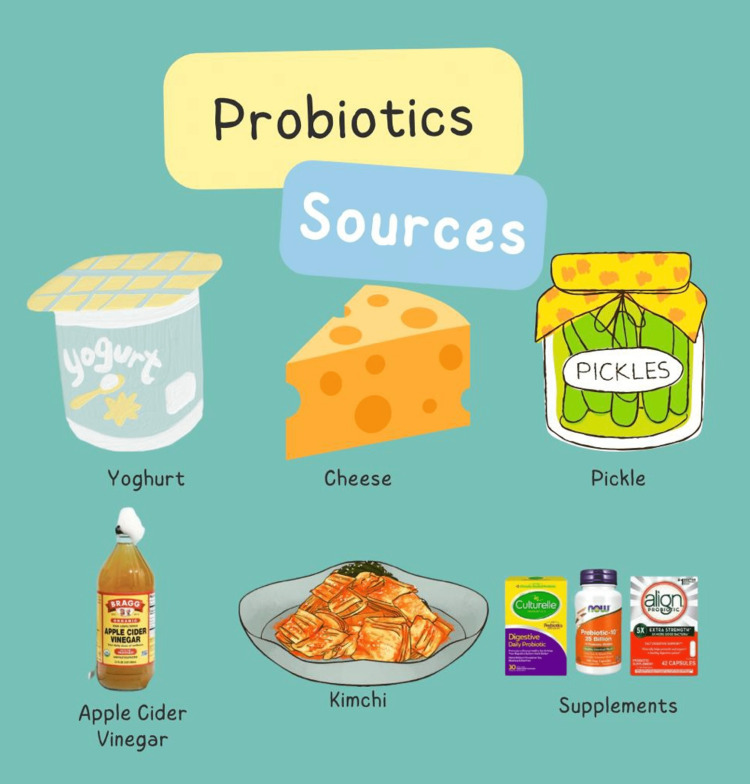
Live bacteria and yeasts are known as probiotics, and they may be good for one's health. They can be found in some meals and supplements as well as in the human digestive system. Probiotic bacteria are beneficial. They are found all over the body, although most people only think of the stomach and intestines when they think of them. Fermented foods such as yogurts and kimchi are sources of probiotics. Additionally, probiotic supplements are also available (Figure 2).
Figure 2. Sources of Probiotics.
This illustration has been created by the author of this study.
Conclusions
The study of the intricate connections between diet and health, as it relates to probiotics, is a relatively recent area of medical research. Although preliminary evidence from multiple research labs has been encouraging. This research will make it possible to identify the probiotics that are most suitable for a given application and the optimum delivery systems, such as food items (cheese, milk, and yogurt) or supplements (chewing gum, lozenges). Because these bacteria have the benefit of being completely adapted to the human oral ecology, the presence of probiotics in the native human microflora should be studied. Previous studies regarding the benefits of probiotics on the human intestinal microbiome have shown to have promising findings for the cure of gut-related disorders. Additionally, in recent years there has been an increase in the number of studies that investigate how microorganisms contribute to patients' intestinal illnesses. Although much more research that is hypothesis-driven is required, there is a possibility that probiotics could be utilized as therapeutic options or prevention measures for diseases that are related to the individual gut flora.
The rapid advancement of next-generation sequencing (NGS) technologies has made it possible to gain a deeper comprehension of the roles that specific probiotics play in the protection and enhancement of human health. It is quite likely that these probiotics are related to the equilibrium of the bacteria in the intestines, as well as the regulatory control of intestinal disorders. These various omics technologies, such as microbial genome sequencing, transcriptomics, metagenomics, and sometimes even metabolomics, have increased our knowledge of the isolation and recruitment of suitable probiotic strains, the confirmation of their genuine probiotic effect as well as the mechanisms involved, and the evaluation of an individual's health effects in in vitro and in vivo experiments. This has led to a greater depth of knowledge regarding these aspects of probiotic research. Therefore, to build innovative probiotic strains and maybe pharmabiotic treatments, up-to-date information on bacteria and their effects on humans at an omics level, which has only recently begun towards becoming available, is required. The incorrect use of certain microbes, such as L. rhamnosus, has indeed been linked to the development of septicemia, septic shock, or endocarditis in individuals who exhibit symptoms of inflammation of the digestive organs. For this reason, it is essential, while treating particular patients, to make use of the appropriate quantity of probiotics.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Probiotics for oral health: myth or reality? Bonifait L, Chandad F, Grenier D. https://pubmed.ncbi.nlm.nih.gov/19840501/ J Can Dent Assoc. 2009;75:585–590. [PubMed] [Google Scholar]
- 2.Probiotics and their fermented food products are beneficial for health. Parvez S. J Appl Microbiol. 2006;100:1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- 3.Cordoba: Argentina. 2001; [ Nov; 2022 ]. 2001. Probiotic in food: health and nutritional properties and guidelines for evaluation. [Google Scholar]
- 4.Fuller R. In: Probiotics. Dordrecht: Springer; 1992. History and development of probiotics; pp. 1–8. [Google Scholar]
- 5.Potential uses of probiotics in clinical practice. Reid G, Jass J, Sebulsky MT, McCormick JK. Clin Microbiol Rev. 2003;16:658–672. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The unregulated probiotic market. de Simone C. https://www.sciencedirect.com/science/article/pii/S1542356518300843. Clin Gastroenterol Hepatol. 2019;17:809–817. doi: 10.1016/j.cgh.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. Choi S, Hwang YJ, Shin MJ, Yi H. J Microbiol Biotechnol. 2017;27:2228–2236. doi: 10.4014/jmb.1710.10001. [DOI] [PubMed] [Google Scholar]
- 8.A human gut microbial gene catalogue established by metagenomic sequencing. Qin J, Li R, Raes J, et al. https://www.nature.com/articles/nature08821. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semi-rational screening of probiotics from the fecal flora of healthy adults against DSS-induced colitis mice by enhancing anti-inflammatory activity and modulating the gut microbiota. Wang W, Xing W, Wei S, et al. J Microbiol Biotechnol. 2019;29:1478–1487. doi: 10.4014/jmb.1807.06061. [DOI] [PubMed] [Google Scholar]
- 10.The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. Fedorak RN, Feagan BG, Hotte N, et al. Clin Gastroenterol Hepatol. 2015;13:928–935. doi: 10.1016/j.cgh.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 11.New methods for selecting and evaluating probiotics. Gueimonde M, Salminen S. Dig Liver Dis. 2006;38:242–247. doi: 10.1016/S1590-8658(07)60003-6. [DOI] [PubMed] [Google Scholar]
- 12.Probiotic immunomodulation in health and disease. Erickson KL, Hubbard NE. J Nutr. 2000;130:403–409. doi: 10.1093/jn/130.2.403S. [DOI] [PubMed] [Google Scholar]
- 13.Inhibition of growth of some enteropathogenic strains in mixed cultures of Streptococcus faecalis and Clostridium butyricum. Seo G, Shimizu K, Sasatsu M, Kono M. https://www.semanticscholar.org/paper/Inhibition-of-growth-of-some-enteropathogenic-in-of-Seo-Shimizu/0a1d577128561321b54bbe54a03b7da976d1f9c0 Microbios Letter. 1989;40:151–160. [Google Scholar]
- 14.Relative expression of wild-type and activated KI-RAS2 oncogene in colorectal carcinomas. Kotsinas A, Spandidos D, Romanowski P, Wyllie A. Int J Oncol. 1993;3:841–845. doi: 10.3892/ijo.3.5.841. [DOI] [PubMed] [Google Scholar]
- 15.Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Saavedra JM, Bauman NA, Perman JA, Yolken RH, Saavedra JM, Bauman NA, Oung I. Lancet. 1994;344:1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- 16.Black FT, Andersen PL, Ørskov J, Ørskov F, Gaarslev K, Laulund S. In: Travel Medicine. Berlin, Heidelberg: Springer; 1989. Prophylactic efficacy of lactobacilli on traveler's diarrhea. [Google Scholar]
- 17.New possibility in the treatment of irritable bowel syndrome: probiotics as a modification of the microflora of the colon. Niedzielin K, Kordecki H, Kosik R. https://www.semanticscholar.org/paper/New-possibility-in-the-treatment-of-irritable-bowel-Niedzielin-Kordecki/df361a72216345772bbfd113fdffed1b83647097 Gastroenterology. 1998;114:402. [Google Scholar]
- 18.The influence of chronic yogurt consumption on population of young and elderly adults. Trapp CL, Chang CC, Halpern G, Keen C, Gershwin M. https://www.semanticscholar.org/paper/The-influence-of-chronic-yogurt-consumption-on-of-Trapp-Chang/4a8371874d1032b26f68c074029824dc8eff35ec Int J Imunother. 1993;9:53–64. [Google Scholar]
- 19.Detection of bifidobacterium strains that induce large quantities of IgA. Yasui H, Nagaoka N, Mike A, Hayakawa K, Ohwaki M. Microb Ecol Health Dis. 1992;5:155–162. [Google Scholar]
- 20.Down-regulation of anti-CD3 antibody-induced IL-4 production by bovine caseins hydrolysed with Lactobacillus GG-derived enzymes. Sütas Y, Hurme M, Isolauri E. Scand J Immunol. 1996;43:687–689. doi: 10.1046/j.1365-3083.1996.d01-258.x. [DOI] [PubMed] [Google Scholar]
- 21.Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. Kirjavainen PV, Salminen SJ, Isolauri E. J Pediatr Gastroenterol Nutr. 2003;36:223–227. doi: 10.1097/00005176-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Improved immunogenecity of oral Dx RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Vaccine. 1995;13:310–312. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- 23.Recurrent urethritis in women. Bruce AW, Chadwick P, Hassan A, VanCott GF. https://pubmed.ncbi.nlm.nih.gov/4633489/ Can Med Assoc J. 1973;108:973–976. [PMC free article] [PubMed] [Google Scholar]
- 24.Oral use of Lactobacillus rhamnosus GR-1 and L. Fermentum RC-14 significantly alters vaginal flora: randomised placebo-controlled trial in 64 healthy women. Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, Bruce AW. FEMS Immunol Med Microbiol. 2003;35:131–134. doi: 10.1016/S0928-8244(02)00465-0. [DOI] [PubMed] [Google Scholar]
- 25.Oral probiotics can resolve urogenital infections. Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B. FEMS Immunol Med Microbiol. 2001;30:49–52. doi: 10.1111/j.1574-695X.2001.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 26.Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections. Reid G, Bruce AW, Taylor M. https://www.scienceopen.com/document?vid=496f67cb-83ec-4bcb-8f78-073ecc9eac75 Microecol Therapy. 1995;23:32–45. [Google Scholar]
- 27.Studies of a surfactant and cholesteremia in the Maasai. Mann GV, Spoerry A. Am J Clin Nutr. 1974;27:464–469. doi: 10.1093/ajcn/27.5.464. [DOI] [PubMed] [Google Scholar]
- 28.Dental caries. Selwitz RH, Ismail AI, Pitts NB. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 29.Selection of dairy bacterial strains as probiotics for oral health. Comelli EM, Guggenheim B, Stingele F, Neeser JR. Eur J Oral Sci. 2002;110:218–224. doi: 10.1034/j.1600-0447.2002.21216.x. [DOI] [PubMed] [Google Scholar]
- 30.Effect of Weissella cibaria isolates on the formation of Streptococcus mutans biofilm. Kang MS, Chung J, Kim SM, Yang KH, Oh JS. Caries Res. 2006;40:418–425. doi: 10.1159/000094288. [DOI] [PubMed] [Google Scholar]
- 31.Current concepts in periodontal pathogenesis. Preshaw PM, Seymour RA, Heasman PA. Dent Update. 2004;31:570-572, 574-578. doi: 10.12968/denu.2004.31.10.570. [DOI] [PubMed] [Google Scholar]
- 32.Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Riccia DN, Bizzini F, Perilli MG, Polimeni A, Trinchieri V, Amicosante G, Cifone MG. Oral Dis. 2007;13:376–385. doi: 10.1111/j.1601-0825.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 33.Intake of dairy products and periodontal disease: the Hisayama Study. Shimazaki Y, Shirota T, Uchida K, et al. J Periodontol. 2008;79:131–137. doi: 10.1902/jop.2008.070202. [DOI] [PubMed] [Google Scholar]
- 34.Halitosis (breath odor) Scully C, Greenman J. Periodontol 2000. 2008;48:66–75. doi: 10.1111/j.1600-0757.2008.00266.x. [DOI] [PubMed] [Google Scholar]
- 35.An assessment of adhesion, aggregation and surface charges of Lactobacillus strains derived from the human oral cavity. Piwat S, Sophatha B, Teanpaisan R. Lett Appl Microbiol. 2015;61:98–105. doi: 10.1111/lam.12434. [DOI] [PubMed] [Google Scholar]
- 36.Oral microbial habitat a dynamic entity. Faran Ali SM, Tanwir F. J Oral Biol Craniofac Res. 2012;2:181–187. doi: 10.1016/j.jobcr.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The effect of a streptococci containing probiotic in periodontal therapy: a randomized controlled trial. Laleman I, Yilmaz E, Ozcelik O, et al. J Clin Periodontol. 2015;42:1032–1041. doi: 10.1111/jcpe.12464. [DOI] [PubMed] [Google Scholar]
- 38.Clinical effects of Lactobacillus rhamnosus in non-surgical treatment of chronic periodontitis: a randomized placebo-controlled trial with 1-year follow-up. Morales A, Carvajal P, Silva N, et al. J Periodontol. 2016;87:944–952. doi: 10.1902/jop.2016.150665. [DOI] [PubMed] [Google Scholar]
- 39.Enhancement of salivary human neutrophil peptide 1-3 levels by probiotic supplementation. Wattanarat O, Makeudom A, Sastraruji T, Piwat S, Tianviwat S, Teanpaisan R, Krisanaprakornkit S. BMC Oral Health. 2015;15:19. doi: 10.1186/s12903-015-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Effect of probiotic containing ice-cream on Salivary Mutans Streptococci (SMS) levels in children of 6-12 years of age: a randomized controlled double blind study with six-months follow up. Ashwin D, Ke V, Taranath M, Ramagoni NK, Nara A, Sarpangala M. J Clin Diagn Res. 2015;9:6–9. doi: 10.7860/JCDR/2015/10942.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.A comparative evaluation of probiotics on salivary mutans streptococci counts in Indian children. Jindal G, Pandey RK, Agarwal J, Singh M. Eur Arch Paediatr Dent. 2011;12:211–215. doi: 10.1007/BF03262809. [DOI] [PubMed] [Google Scholar]
- 42.Effect of chocobar ice cream containing Bifidobacterium on salivary Streptococcus mutans and Lactobacilli: a randomized controlled trial. Nagarajappa R, Daryani H, Sharda AJ, Asawa K, Batra M, Sanadhya S, Ramesh G. Oral Health Prev Dent. 2015;13:213–218. doi: 10.3290/j.ohpd.a32673. [DOI] [PubMed] [Google Scholar]
- 43.Bifidobacterium animalis subsp. lactis BB-12 administration in early childhood: a randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic. Taipale T, Pienihäkkinen K, Salminen S, Jokela J, Söderling E. Caries Res. 2012;46:69–77. doi: 10.1159/000335567. [DOI] [PubMed] [Google Scholar]
- 44.Dysbiosis of the gut microbiota in disease. Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4315779/ Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adhesion of inactivated probiotic strains to intestinal mucus. Ouwehand AC, Tölkkö S, Kulmala J, Salminen S, Salminen E. Lett Appl Microbiol. 2000;31:82–86. doi: 10.1046/j.1472-765x.2000.00773.x. [DOI] [PubMed] [Google Scholar]
- 46.Probiotics for the prevention of antibiotic-associated diarrhea in outpatients-A systematic review and meta-analysis. Blaabjerg S, Artzi DM, Aabenhus R. Antibiotics (Basel) 2017;6:21. doi: 10.3390/antibiotics6040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Does eating yogurt prevent antibiotic-associated diarrhoea? A placebo-controlled randomised controlled trial in general practice. Conway S, Hart A, Clark A, Harvey I. https://pubmed.ncbi.nlm.nih.gov/18252070/ Br J Gen Pract. 2007;57:953–959. doi: 10.3399/096016407782604811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. J Pediatr. 1999;135:564–568. doi: 10.1016/s0022-3476(99)70053-3. [DOI] [PubMed] [Google Scholar]
- 49.A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn's disease. Bjarnason I, Sission G, Hayee B. Inflammopharmacology. 2019;27:465–473. doi: 10.1007/s10787-019-00595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Effects of probiotics on gut microbiota in patients with inflammatory bowel disease: a double-blind, placebo-controlled clinical trial. Shadnoush M, Hosseini RS, Khalilnezhad A, Navai L, Goudarzi H, Vaezjalali M. Korean J Gastroenterol. 2015;65:215–221. doi: 10.4166/kjg.2015.65.4.215. [DOI] [PubMed] [Google Scholar]
- 51.The association of diet, gut microbiota and colorectal cancer: what we eat may imply what we get. Yang J, Yu J. Protein Cell. 2018;9:474–487. doi: 10.1007/s13238-018-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O'neil DA, Macfarlane GT. https://pubmed.ncbi.nlm.nih.gov/15647189/ Gut. 2005;54:242–249. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inflammatory bowel disease: cause and immunobiology. Baumgart DC, Carding SR. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]