Abstract
BRASH is an acronym describing the vicious cycle seen in patients taking atrioventricular (AV) nodal blockers who tend to present with bradycardia, renal failure, atrioventricular nodal blockade, shock, and hyperkalemia. Herein, we report the case of an 87-year-old hypertensive patient on verapamil who presented with complaints of fever and shortness of breath. She was found to have bradycardia, hyperkalemia, renal impairment, and borderline hypotension. Differentiating this case from previous case reports on BRASH syndrome, this patient was found to simultaneously have toxic levels of serum verapamil.
Keywords: verapamil mechanism of action, acute kidney injury and brash syndrome, pathophysiology of brash syndrome, brash syndrome, verapamil toxicity
Introduction
The BRASH syndrome is a collective of bradycardia, renal failure, atrioventricular nodal blockade, shock, and hyperkalemia recently surfacing in literature [1]. Frequently, this syndrome presents alongside hyperkalemia-associated arrhythmias and has emerged as a separate entity as the typical mild hyperkalemia findings in patients did not correlate with electrocardiographic findings [2-5]. Ultimately, patients with BRASH syndrome progress to have multi-organ failure. Among these reported cases, contributory factors such as renal impairment have been suggested to worsen the effects of mildly elevated potassium levels. We present a case of verapamil toxicity as a contributory factor to BRASH syndrome and its contribution to the vicious cycle of bradycardia, worsening renal failure, increasing hyperkalemia, and, in turn, increasing serum verapamil levels.
Case presentation
An 87-year-old female, known to have type 2 diabetes mellitus, hypertension, and dyslipidemia, presented to the emergency department (ED) with complaints of fever, shortness of breath, chest pain, and fatigue for one day. Her home medications included sustained-release (SR) verapamil 240 mg, gliclazide 30 mg daily, and atorvastatin 40 mg daily. There was no history of a possible overdose of medication
On presentation, her vital signs revealed bradycardia with a heart rate of 42 beats per minute, blood pressure of 112/36 mmHg, 78% SpO2, respiratory rate of 24 breaths per minute, and temperature of 36°C. The electrocardiography (ECG) revealed a sinus arrest with an escape junctional rhythm and an incomplete right bundle branch block (Figure 1). These were considered new changes as her previous ECG four months ago was apparently normal (Figure 2).
Figure 1. The patient’s ECG on presentation showing a sinus arrest with an escape junctional rhythm and an incomplete right bundle branch block.
ECG: electrocardiography
Figure 2. The patient’s ECG four months prior to presentation indicating a normal ECG.
ECG: electrocardiography
Upon physical examination, the patient was conscious and oriented. She had decreased air entry in bilateral lung bases. The remaining systemic examination, however, was unremarkable. Positive laboratory findings are summarized in Table 1.
Table 1. Positive laboratory findings.
Normal ranges are provided between brackets.
FBC: full blood count, WBC: white blood cell, eGFR: estimated glomerular filtration rate, CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration
| FBC | |
| WBC count | 15.5 × 103/uL (3.6-11 × 103/uL) |
| Neutrophil | 83.9% |
| Lymphocyte | 8.3% |
| C-reactive protein | 227.7 mg/L (<5 mg/L) |
| Procalcitonin | 0.82 ng/mL (<0.05 ng/mL) |
| Creatinine | 1.8 mg/dL (0.7-1.2 mg/dL) |
| eGFR (CKD-EPI) | 26.9 mL/minute/1.73 m2 (>60 mL/minute/1.73 m2) |
| Urea and electrolytes | |
| Potassium | 5.6 mmol/L (3.3-4.8 mmol/L) |
| Urea | 81 mg/dL (12-40 mg/dL) |
| Verapamil level | 1,670+++ ng/mL (20-250 ng/mL) |
These findings were suggestive of an acute kidney injury, hyperkalemia, and an infective process indicated by the elevated inflammatory markers. Serum verapamil level was reported as above laboratory alert level despite being on therapeutic doses. Chest X-ray reportedly showed bilateral pleural effusions with bilateral lower lung zone airspace consolidations signifying pneumonia (Figure 3).
Figure 3. The patient’s chest X-ray indicating pneumonia.
Bedside echocardiography was done, revealing an ejection fraction of 60% with associated bradycardia. During the reassessment of the patient, her heart rate dropped to 30 beats per minute, and 0.6 mg of atropine was administered. Unfortunately, the heart rate failed to improve, and shortly after, she went into cardiac arrest. Cardiopulmonary resuscitation (CPR) was commenced as per the advanced cardiac life support (ACLS) algorithm. The cardiac monitor showed asystole; three cycles of CPR were performed prior to achieving return of spontaneous circulation (ROSC). During the resuscitation, a venous blood gas sample showed hyperkalemia with a potassium level of 6.2 mmol/L. The patient was given 1 g of calcium gluconate IV, insulin, dextrose IV, and a salbutamol nebulizer.
She remained unstable after ROSC with a heart rate ranging from 20 to 30 beats per minute, a palpable central pulse, and an unrecordable blood pressure necessitating the use of transcutaneous pacing along with an epinephrine infusion. Due to hypoventilation and inadequate chest expansion, she was intubated using rapid sequence intubation.
Soon after, her heart rate improved to 60 beats per minute, and her systolic blood pressure picked up to about 110 mmHg. The cardiology team then switched the transcutaneous pacing to transvenous pacing where thereafter the patient remained hemodynamically stable.
Discussion
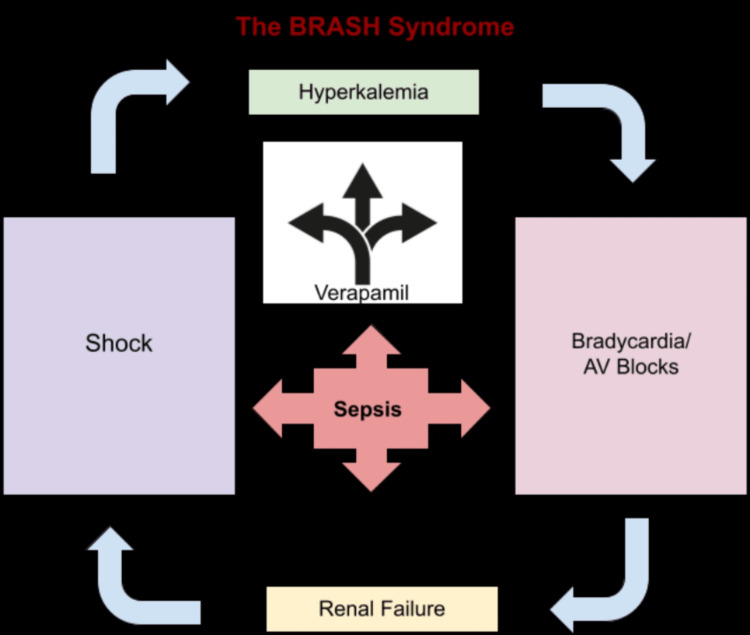
The BRASH syndrome is characterized by the presence of bradycardia, renal failure, atrioventricular nodal blockade medication, shock, and hyperkalemia, whereby each of the named symptoms plays a continuous role in sustaining the vicious cycle of BRASH by magnifying and worsening the effects of each other (Figure 4).
Figure 4. The vicious cycle of BRASH syndrome (based on the findings in the case presented).
BRASH: bradycardia, renal failure, atrioventricular nodal blockade, shock, and hyperkalemia
Verapamil falls under the phenylalkylamines (non-dihydropyridine) subgroup of calcium channel blockers (CCB). It acts by inhibiting voltage-dependent L-type calcium channels, causing relaxation of vascular smooth muscle and in turn resulting in a negative inotropic and chronotropic cardiac effect [6]. Verapamil binds to calcium channels at the level of the sinoatrial (SA) and atrioventricular (AV) nodes and results in a negative chronotropic effect [7].
Verapamil is mainly metabolized in the liver, and approximately 70% of its metabolites are excreted through the urine. Hepatic and renal failure may prolong its half-life [8]. While acute administration of verapamil has been shown to result in an increased hepatic and renal blood flow, this is not seen in chronic administration, which can result in delayed clearance of the drug in those on chronic use [9]. The patient’s creatinine level was elevated (1.8 mg/dL), and she had an acute impaired renal function (eGFR: 26.9 mL/minute), which could have contributed to the toxic levels of serum verapamil.
Toxic levels of non-dihydropyridine CCBs can present with ECG findings of sinus bradycardia and various conduction abnormalities including AV blocks, QT prolongation, and heart block. While the patient had no history of underlying cardiac pathology prior to the events of this case, she did have bradycardia coupled with an escape junctional rhythm, which is likely a result of high levels of serum verapamil [10].
The critical pathophysiological characteristic of this syndrome involves a synergistic effect of mild hyperkalemia and therapeutic doses of AV nodal blocker medications resulting in significant bradycardia [2]. Similarly, in our case, the patient had a potassium level of 5.6 mEq/L and was on therapeutic doses of verapamil 240 mg. In the presence of a systemic infection, she appears to have developed acute renal impairment leading to severe and unstable bradycardia rapidly deteriorating into cardiac arrest. In addition, the development of acute renal failure allowed for the accumulation of serum verapamil leading to toxic levels (Figure 4).
Conclusions
Conclusively, we can say that sepsis contributed to the findings of acute renal failure, metabolic acidosis, and, in turn, the reduced excretion of verapamil. The sepsis was also a potential cause of the hypotension. However, by adding a toxic level of verapamil to the equation, one could also argue that verapamil would have a synergistic effect toward hypotension, bradycardia, metabolic acidosis, and mild hyperkalemia. This could further be supported by the fact that the bradycardia eventually required cardiac pacing to stabilize the patient after her cardiac arrest. Such cases of renal dysfunction, as seen in this case of sepsis, can swiftly potentiate verapamil toxicity. The effects of verapamil toxicity are the same effects gathered under the acronym of the BRASH syndrome.
Acknowledgments
The case was reviewed by Dr. Sara Nourridin Kazim. The electrocardiogram (ECG) was interpreted by Dr. Salah Aldeen Roqia.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.BRASH syndrome: bradycardia, renal failure, AV blockade, shock, and hyperkalemia. Farkas JD, Long B, Koyfman A, Menson K. https://www.sciencedirect.com/science/article/pii/S0736467920303991. J Emerg Med. 2020;59:216–223. doi: 10.1016/j.jemermed.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 2.BRASH syndrome: an emerging emergency. Chothia MY, Davids MR. https://www.clinical-medicine.panafrican-med-journal.com/content/article/4/128/full/ PAMJ Clin Med. 2020;4:128. [Google Scholar]
- 3.BRASH syndrome with hyperkalemia: an under-recognized clinical condition. Arif AW, Khan MS, Masri A, Mba B, Talha Ayub M, Doukky R. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7587309/ Methodist Debakey Cardiovasc J. 2020;16:241–244. doi: 10.14797/mdcj-16-3-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Two cases of BRASH syndrome: a diagnostic challenge. Shah P, Silangruz K, Lee E, Nishimura Y. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9067425/ Eur J Case Rep Intern Med. 2022;9:3314. doi: 10.12890/2022_003314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Junctional bradycardia with verapamil in renal failure--care required even with mild hyperkalaemia. Hegazi MO, Aldabie G, Al-Mutairi S, El Sayed A. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2710.2012.01352.x. J Clin Pharm Ther. 2012;37:726–728. doi: 10.1111/j.1365-2710.2012.01352.x. [DOI] [PubMed] [Google Scholar]
- 6.Pharmacological aspects of calcium channel blockers. Scholz H. https://link.springer.com/article/10.1007/BF00051613. Cardiovasc Drugs Ther. 1997;10 Suppl 3:869–872. doi: 10.1007/BF00051613. [DOI] [PubMed] [Google Scholar]
- 7.The role of existing and newer calcium channel blockers in the treatment of hypertension. Basile J. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1524-6175.2004.03683.x. J Clin Hypertens (Greenwich) 2004;6:621–629. doi: 10.1111/j.1524-6175.2004.03683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Food and Drug Administration: Verapamil. November. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018925s010lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018925s010lbl.pdf
- 9.Verapamil pharmacokinetics and apparent hepatic and renal blood flow. Meredith PA, Elliott HL, Pasanisi F, Kelman AW, Sumner DJ, Reid JL. https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2125.1985.tb05038.x. Br J Clin Pharmacol. 1985;20:101–106. doi: 10.1111/j.1365-2125.1985.tb05038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verapamil intoxication: a literature review of overdoses and discussion of therapeutic options. Hofer CA, Smith JK, Tenholder MF. https://www.sciencedirect.com/user/identity/landing?code=klTbBfZtiefzkzghH2iTfyQ1k_4ErFdUeRkHSsgG&state=retryCounter%3D0%26csrfToken%3D17564dea-9373-4a6f-96a2-d7593ef63b6b%26idpPolicy%3Durn%253Acom%253Aelsevier%253Aidp%253Apolicy%253Aproduct%253Ainst_assoc%26returnUrl%3D%252Fscience%252Farticle%252Fabs%252Fpii%252F000293439390314F%26prompt%3Dlogin%26cid%3Darp-4eed5837-3abf-4882-8992-607458894340. Am J Med. 1993;95:431–438. doi: 10.1016/0002-9343(93)90314-f. [DOI] [PubMed] [Google Scholar]