Abstract
Killing of intracellular Penicillium marneffei conidia is demonstrated in gamma interferon-lipopolysaccharide-activated human THP1 and mouse J774 cells. Iron overload significantly reduces the antifungal activity of macrophages. Likewise, exogenous iron enhances and iron chelators inhibit the extracellular growth of P. marneffei. These results suggest that iron availability critically affects immunity to and the pathogenicity of P. marneffei.
Iron is essential for living cells, including pathogenic microorganisms. As a defense against microbes, the human body withholds iron through sequestration by high-affinity iron-binding proteins, such as transferrin and ferritin (16). Conversely, invading pathogens compete with host iron by (i) secreting siderophores, (ii) reducing insoluble Fe(III) to soluble Fe(II), and (iii) up-regulating surface receptors for iron-containing proteins (5, 9, 16). Altered iron availability is often a key component in the host-microorganisms interplay. Excess iron inhibits the transcription of the inducible nitric oxide synthase (iNOS), impairing nitric oxide (NO) synthesis (18). This may result in the inhibition of the microbicidal activity of macrophages (19).
Penicillium marneffei is a dimorphic fungus endemic in southeast Asia, recently associated with life-threatening infections in AIDS patients. It grows in vitro at 25°C as mold and in vivo as yeast inside phagocytes (4, 13). Macrophages and T lymphocytes play a central role in host resistance to P. marneffei (7). We and others recently reproduced in vitro at 37°C the intracellular growth of P. marneffei as yeast and showed that activated J774 macrophages inhibit yeast transformation of P. marneffei conidia by the secretion of NO (3, 8). In view of the interplay between NO and iron, we studied the role of iron on (i) the in vitro growth requirement of P. marneffei and (ii) the fungicidal activity of activated human and mouse macrophages.
Conidia of P. marneffei IUM 885346 were cultured on yeast morphology agar (YMA) (Difco) (15). P. marneffei cultures in axenic minimal essential medium (MEM; with 20 mM HEPES buffer, pH 7.2; GIBCO-BRL) were seeded in microtiter plates at 5 × 103 conidia/well plus or minus fetal bovine serum (FBS) (Euroclone, Celbio, Italy), FeCl3 · 6H2O, hemin chloride, or deferoxamine (DFO) (Sigma, Milan, Italy). P. marneffei growth after 48 h was evaluated by the 3-(4,5-dimethylthiozol-2,5-diphenyl)tetrazolium bromide (MTT) assay (14). Murine J774 and human THP1 monocytic-macrophage cell lines were maintained in complete medium (MEM plus 10% FBS; Pen/Strept) in 5% CO2 at 37°C (17). J774 cells or THP1 cells differentiated by 0.3 μM phorbol myristate acetate (PMA) for 72 h were seeded into chamber slides (Nunc) at 105 cells/well in complete medium. After P. marneffei phagocytosis (2 h at 2 × 105 conidia/well), monolayers were washed and stimulated with different doses of recombinant gamma interferon (rIFN-γ) (Genzyme) and 1 μg of lipopolysaccharide (LPS) (Sigma) per ml for 24 to 48 h. P. marneffei yeasts were quantified both microscopically on p-aminosalicylic acid-stained smears and as CFU in YMA for 48 h after macrophage lysis. The percent yeasts was calculated as follows: (number of intracellular yeasts/number of intracellular conidia) × 100. Supernatants were assayed for the presence of nitrite with the Griess reagent (14).
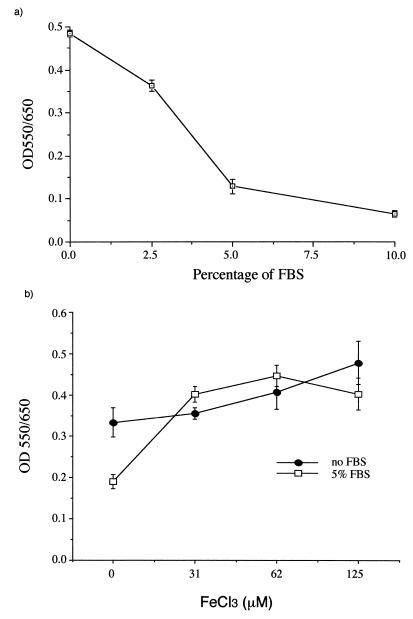
Effect of serum, iron, and DFO on P. marneffei growth in axenic medium.
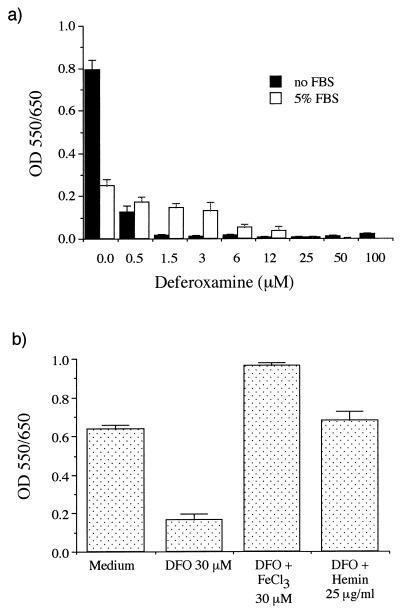
In axenic medium, FBS induced a dose-dependent inhibition of P. marneffei growth, with a >80% reduction at 10% FBS (Fig. 1a). Iron supplementation as FeCl3 abrogated the inhibitory effect of FBS (Fig. 1b). Moreover, the iron chelator DFO strongly inhibited fungal growth in a dose-dependent manner (Fig. 2a), and this inhibition was reversed by adding inorganic (FeCl3) or organic (hemin) iron (Fig. 2b). Therefore, enhancing or limiting iron availability had opposite effects on the growth of P. marneffei in axenic medium. The P. marneffei iron acquisition mechanism is presently unknown, even though hydroxamate siderophore production in several Penicillium species has been reported (6). The fact that DFO inhibits P. marneffei growth indicates that the fungus cannot utilize this exogenous siderophore in its ferrated form, as is the case with several Zygomycetes species (1).
FIG. 1.
Effect of different concentrations of FBS (a) and iron (FeCl3) (b) on P. marneffei growth in axenic medium. The assays were performed with MEM containing 20 mM HEPES buffer, in microtiter wells, in triplicate, and with 5 × 103 conidia/well for 48 h at 37°C. Fungal growth was assessed by MTT reduction. Data are expressed as the mean ± standard deviation of a representative experiment (n = 4). OD, optical density.
FIG. 2.
Effect of DFO with or without FBS (a) or iron (FeCl3 or hemin) (b) on P. marneffei growth in axenic medium. Methods are the same as for Fig. 1. OD, optical density.
Effect of NO and NO donors on intracellular P. marneffei survival.
For the first time in a human model, we observed that P. marneffei conidia were readily phagocytosed by THP1 cells (41% ± 7% of the cells had internalized one or more conidia after 2 h) and showed the characteristic dimorphism (yeast transformation) after 24 h of coculture (Table 1). Fungal viability (CFU after 48 h) was significantly reduced in PMA-differentiated, IFN-γ-plus-LPS-stimulated cells, compared to unstimulated THP1 cells. The percentage of yeasts was also decreased (60.0% versus 75.6%). As expected, no nitrite was detected in the supernatant. Although the effect of IFN-γ plus LPS is apparently not NO mediated, the NO donor sodium nitroprusside (SNP) inhibited the yeast transformation of phagocytosed P. marneffei conidia (60.4% versus 75.6%) and caused a 53.4% reduction in CFU.
TABLE 1.
Effects of immunological activation and of NO on the survival of P. marneffei conidia phagocytosed by human or mouse macrophages
| Treatment | Effect of treatment on antifungal activity of:
|
|||||
|---|---|---|---|---|---|---|
| Human THP1 cells
|
Mouse J774 cells
|
|||||
| CFU (102)a | % Yeasts | Nitrite (μM)b | CFU (102)a | % Yeasts | Nitrite (μM)b | |
| None (medium only) | 96.7 ± 12 | 75.6 | 0 | 207 ± 11 | 57.0 | 0 |
| SNP (200 μM) | 45.1 ± 6c | 60.4 | 8.9 ± 0.3 | 88 ± 10d | 27.2 | 12.3 ± 0.5 |
| IFN-γ + LPS (1 μg/ml)e | 53.6 ± 15c | 60.0 | 0 | 74 ± 9d | 36.5 | 19.6 ± 0.5 |
| IFN-γ + LPS + l-NMMA | ND | ND | ND | 179 ± 13 | 42.4 | 0 |
CFU in YMA after a 48-h culture. The results are expressed as the mean ± standard deviation of triplicate experiments.
Nitrite levels by the Griess reagent in 48-h supernatants.
P < 0.05, compared to control medium (by analysis of variance).
P < 0.01, compared to control medium (by analysis of variance).
The doses of IFN-γ were 50 and 500 U/ml for J774 and THP1 cells, respectively.
Like in THP1 cells, killing of intracellular yeasts occurred in J774 cells after SNP or IFN-γ-plus-LPS treatment. As expected with J774 cells, both treatments resulted in increased nitrite levels. Conversely, with N-mono-methyl-l-arginine (l-NMMA), an inhibitor of iNOS, CFU and the yeast percentage returned to control values. Thus, the fungicidal activity of both mouse and human macrophages was up-regulated by IFN-γ plus LPS. This effect was more clearly related to the induction of iNOS in the former cells than that in the latter cells. However, in both cases, it could be mimicked by SNP addition, confirming the important anti-P. marneffei activity of reactive nitrogen intermediates (3, 8).
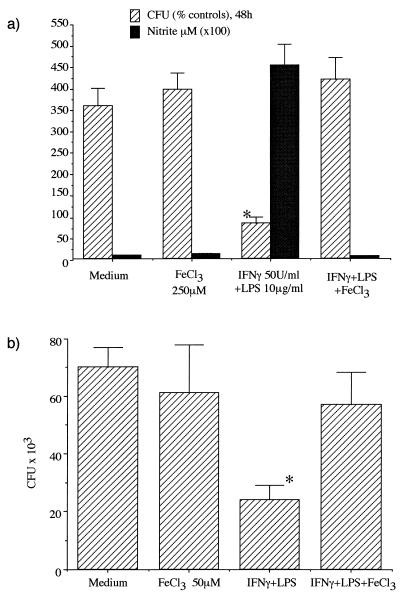
Effect of iron overload on yeast transformation and survival of P. marneffei.
Treatment with FeCl3 alone did not alter J774 and THP1 cell viability nor their capacity to phagocytose P. marneffei conidia (data not shown). Similarly, iron treatment did not change the percentage of yeasts in unstimulated J774 or THP1 cells (data not shown). However, iron loading significantly impaired the fungicidal activity of IFN-γ-plus-LPS-stimulated mouse (Fig. 3a) as well as human (Fig. 3b) macrophages, and it increased the number of recovered CFU. Although iron loading inhibits IFN-γ-plus-LPS-mediated effects and impairs anti-P. marneffei activity in both macrophage types, the prevailing mechanism may differ according to the cell type. In J774 macrophages, the increase in CFU is associated with a reduced NO production, while the iron-dependent reduction in microbicidal activity observed in THP1 cells cannot be ascribed to a down-regulation in the nitrite production, as NO production is not elicited after IFN-γ stimulation in THP1 cells. The decreased antifungal activity may be linked to the profound alterations of IFN-γ signaling reported in iron-loaded THP1 cells (17). This extends previous observations of the permissive role of iron on the intramacrophagic growth of other yeasts, such as Cryptococcus neoformans or Histoplasma capsulatum (10–12).
FIG. 3.
Antifungal activity of FeCl3-loaded J774 (a) and THP1 (b) cells, activated by IFN-γ plus LPS. P. marneffei conidia at a ratio of 1:2 were phagocytosed by J774- or PMA-differentiated THP1 cells for 2 h. After removal of nonphagocytosed conidia, cultures were incubated for a further 48 h (37°C, 5% CO2) and stimulated with IFN-γ plus LPS. Cells were then washed and lysed, and live fungi were plated in YMA for 48 h. The results are expressed as percent CFU (mean + standard deviation) compared to the controls at 2 h (a) and as mean CFU ± standard deviation (b). Supernatants of J774 cells were assayed for nitrite content with the Griess reagent. ∗, P < 0.05, compared to control medium (by analysis of variance).
In human immunodeficiency virus infection, iron is reported to accumulate in the mononuclear-phagocyte system, mainly as a result of the long-lasting inflammatory process (2). Together with depressed T-cell numbers and function, such a progressive iron loading may contribute to a defective immune response of phagocytes toward several pathogens, including P. marneffei, as evidenced by the present work. This suggests that, for the prevention and treatment of P. marneffei infections, pharmacological strategies of either limiting iron availability or delivering NO at the specific site of intracellular fungal multiplication should be explored. If successful, such strategies may also be applied to other intracellular pathogens.
Acknowledgments
We thank A. M. Viviani for providing the P. marneffei strain.
This work was supported by the I.S.S., National Program for Research on AIDS, Contract No. 50A.033 and 50B.037, Rome, Italy.
REFERENCES
- 1.Boelaert J R, De Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt H, Schneider Y. Mucormycosis during deferoxamine tharapy is a siderophore-mediated infection: in vitro and in vivo animal studies. J Clin Investig. 1993;91:1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boelaert J R, Weinberg G A, Weinberg E. Altered iron metabolism in HIV infection: mechanisms, possible consequences, and proposals for management. Infect Agents Dis. 1996;5:36–46. [PubMed] [Google Scholar]
- 3.Cogliati M, Roverselli A, Boelaert J R, Taramelli D, Lombardi L, Viviani A M. Development of an in vitro macrophage system to assess Penicillium marneffei growth and susceptibility to nitric oxide. Infect Immun. 1997;65:279–284. doi: 10.1128/iai.65.1.279-284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper C. From bamboo rats to humans: odyssey of Penicillium marneffei. ASM News. 1998;64:390–397. [Google Scholar]
- 5.Jacobson E, Goodner A, Nyhus K. Ferrous iron uptake by Cryptococcus neoformans. Infect Immun. 1998;66:4169–4175. doi: 10.1128/iai.66.9.4169-4175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konetschny-Rapp S, Huschka H G, Winkelmann G, Jung G. High-performance liquid chromatography of siderophores from fungi. Biol Metals. 1998;1:9–17. doi: 10.1007/BF01128012. [DOI] [PubMed] [Google Scholar]
- 7.Kudeken N, Kawakami K, Kusano N, Saito A. Cell-mediated immunity in host resistance against infection caused by Penicillium marneffei. J Med Vet Mycol. 1996;34:371–378. doi: 10.1080/02681219680000671. [DOI] [PubMed] [Google Scholar]
- 8.Kudeken N, Kawakami K, Saito A. Different susceptibilities of yeasts and conidia of Penicillium marneffei to nitric oxide (NO)-mediated fungicidal activity of murine macrophages. Clin Exp Immunol. 1998;112:287–293. doi: 10.1046/j.1365-2249.1998.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neilands J. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- 10.Newman S. Macrophage in host defense against Histoplasma capsulatum. Trends Microbiol. 1999;7:67–71. doi: 10.1016/s0966-842x(98)01431-0. [DOI] [PubMed] [Google Scholar]
- 11.Newman S, Gootee L, Brunner G, Deepe J G. Chloroquine induces human macrophage killing of Histoplasma capsulatum by limiting the availability of intracellular iron and is therapeutic in a murine model of histoplasmosis. J Clin Investig. 1994;93:1422–1429. doi: 10.1172/JCI117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleppico S, Boelaert J R, Omodeo Salè F, Mazzolla R, Morucci P, Bistoni F, Blasi E. Differential effects of iron load on basal and interferon-gamma plus lipopolysaccharide enhanced anticryptococcal activity by the murine microglial cell line BV-2. J Neuroimmunol. 1999;93:102–107. doi: 10.1016/s0165-5728(98)00206-9. [DOI] [PubMed] [Google Scholar]
- 13.Supparatpinyo K, Khamwan C, Baosoung V, Nelson K, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 14.Taramelli D, Basilico N, Pagani E, Grande R, Monti D, Ghione M, Olliaro P. The heme moiety of malaria pigment (β-hematin) mediates the inhibition of nitric oxide and tumor necrosis factor-α production by lipopolysaccharide-stimulated macrophages. Exp Parasitol. 1995;81:501–511. doi: 10.1006/expr.1995.1143. [DOI] [PubMed] [Google Scholar]
- 15.Viviani M A, Hill J O, Dixon D M. Penicillium marneffei: dimorphism and treatment. In: van den Bossche H, Odds F, Kerridge D, editors. Dimorphic fungi in biology and medicine. New York, N.Y: Plenum Press; 1993. pp. 413–422. [Google Scholar]
- 16.Weinberg D E, Weinberg G A. The role of iron in infection. Curr Opin Infect Dis. 1995;8:164–169. [Google Scholar]
- 17.Weiss G, Fuchs D, Hausen A, Reibnegger G, Wezner E, Werner-Felmayer G, Watcher H. Iron modulates interferon-gamma effect in the human myelomonocytic cell line THP-1. Exp Hematol. 1992;20:605–610. [PubMed] [Google Scholar]
- 18.Weiss G, Fuchs D, Hausen A, Reibnegger G, Werner E, Werner-Felmayer G, Watcher H. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med. 1994;180:969–976. doi: 10.1084/jem.180.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Crichton R, Boelaert J, Jorens P, Hermans A, Ward R, Lallemand F, De Witte P. Decreased release of nitric oxide (NO) by alveolar macrophages after in vivo loading of rats with either iron or ethanol. Biochem Pharmacol. 1998;55:21–25. doi: 10.1016/s0006-2952(97)00382-1. [DOI] [PubMed] [Google Scholar]