Abstract
Numerous studies have demonstrated that assisted reproductive technology (ART: defined here as including only in vitro fertilization and related technologies) is associated with increased adverse pregnancy, neonatal, and childhood developmental outcomes, even in singletons. The comparison group for many had often been a fertile population that conceived without assistance. The Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART) was initiated to define a subfertile population with which to compare ART outcomes. Over more than 10 years, we have used the MOSART database to study pregnancy abnormalities and delivery complications but also to evaluate ongoing health of women, infants, and children. This article will review studies from MOSART in the context of how they compare with those of other investigations. We will present MOSART studies that identified the influence of ART and subfertility/infertility on adverse pregnancy (pregnancy hypertensive disorder, gestational diabetes, placental abnormality) and delivery (preterm birth, low birthweight) outcomes as well as on maternal and child hospitalizations. We will provide evidence that although subfertility/infertility increases the risk of adverse outcomes, there is additional risk associated with the use of ART. Studies exploring the contribution of placental abnormalities as one factor adding to this increased ART-associated risk will be described.
Keywords: assisted reproductive technology (ART), preterm birth, maternal health, child health, placental abnormalities
Introduction
It is now well established that assisted reproductive technology (ART) is associated with increased morbidity for women and children even among singleton pregnancies (1–12). For the purpose of this review ART is defined as techniques that involve manipulation of eggs and embryos and it includes in vitro fertilization, intracytoplasmic sperm injection, and related technologies but not intrauterine insemination (IUI) or controlled ovulation hyperstimulation (COH). In singleton deliveries, ART increases the risk of prematurity and low birthweight when compared with deliveries to women in the fertile population (1, 4, 5, 7). However, ART is used for patients who have been unable to conceive on their own and thus the underlying medical conditions that led to this inability to easily conceive must be considered. Underlying conditions include infertility diagnoses as well as health conditions such as greater body mass index (BMI), chronic hypertension, diabetes, and thyroid problems - which could either be associated with the infertility diagnosis or themselves contribute to fertility problems.
Quality research into ART outcomes requires appropriate comparison groups that account for underlying health conditions. Comparison groups have included deliveries to patients using non-ART fertility treatments, such as COH and IUI, or patients with a diagnosis of infertility who received no treatment to conceive. Unfortunately, many studies that used scientifically sound comparison groups came from a single clinic or medical facility and thus, the sample size was too small to adequately assess rare outcomes or was underpowered to identify small effects. Many studies also have had limited information on outcomes occurring subsequent to delivery.
This article will present information on data that we have analyzed for over 10 years to compare health outcomes in women and children of ART-conceived pregnancies to those of patients with indicators of infertility, as well as to a fertile population with no known infertility. The database we developed, the Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART), has strengths and weaknesses, but it has been used effectively to analyze delivery outcomes as well as the health of women and children over time. MOSART uses a US population, which is important since practice patterns in the US have been different than those in other countries. It is not our intention in this review to provide a comprehensive overview of all outcome studies on ART, nor to present a systematic review of all literature on the topic of ART outcome. Rather, we present the evolution of our findings since establishment of the MOSART database and the central hypotheses that have emerged from these studies, while noting the strengths and limitations of MOSART data. This review will place the MOSART data and study results in the context of similar studies that have advanced our understanding of these topics. While initial results suggested that infertility itself was the major factor driving adverse outcomes of ART (13), our continued work has led us to further hypothesize that ART adds additional risk over that contributed by infertility itself. While we have performed studies that differentiated outcomes according to ART methods and procedures, we will concentrate on the observed differences in the ART-treated as compared to both the fertile group and to women with indicators of infertility, with minimal review of the intra-ART distinctions.
The MOSART database
Study of ART outcomes requires methods for capturing data on both treatment specifics and health. In contrast to other countries, some of which have national, integrated, comprehensive, longitudinal data collection of medical assessment, treatment, and health outcomes, collection of data in the US is piecemeal, incomplete, and rarely designed for research. US birth certificate data are collected individually by states using methods that vary in scope and definitions. States report on an agreed upon subset of variables that they send to the Centers for Disease Control and Prevention (CDC). Longitudinal health data are collected by states separate from birth data, or not at all. This section describes the establishment of a collaboration among public health practitioners, clinicians, and scientists who combined expertise to harness the unique situation in Massachusetts that allowed us to merge the most comprehensive ART data collection system in the world – the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS)—with a unique population-level, multifactor database, the Pregnancy to Early Life Longitudinal (PELL) data system, to create a comprehensive system for the study of ART outcomes.
Structure of MOSART
SART CORS was developed in the early 1990s in response to the need for transparency about success rates at ART clinics. The database was formalized in 1992 under the Fertility Clinic Success Rate and Certification Act of 1992 (Public Law 102–493) SART CORS is a robust ART database that collects patient demographics, reproductive history, cycle-specific treatment data, and outcome information on > 80% of ART clinics and >90% of US ART cycles and it includes all ART cycles in Massachusetts. SART CORS was particularly useful in early years to keep track of high rates of multiple gestation pregnancies following ART treatment. Nevertheless, SART CORS lacks robust information on essential outcomes, including pregnancy and delivery complications, and long-term health for women and children.
In 2009 a group of SART researchers began discussions with investigators at Boston University who were working with a Massachusetts database they had constructed linking birth and fetal death certificates with hospitalization records. The PELL data system was developed under a collaboration among Boston University, the Massachusetts Department of Public Health (MDPH) and the CDC to study exposures and their outcomes during pregnancy. PELL linked data from several Massachusetts datasets from 1998 onwards, including the Birth Defects Registry, the Death Index, and the Early Intervention (EI) Program (Figure 1). PELL documented factors not collected in SART CORS. First, it added information on deliveries not conceived by ART, an essential comparison group to ART deliveries. Second, it added critical information on chronic medical conditions in women with live born or stillborn deliveries as well as medical conditions experienced during pregnancy and delivery. Third, it added a wealth of information on hospitalizations experienced by women and their children during and after delivery. Finally, PELL added information from other Massachusetts datasets. The leap forward for this initiative was linking the SART CORS and PELL databases. The working group for this SART/PELL collaboration named the newly constructed combined database, MOSART. Details of the linkage have been described elsewhere (14). Currently, the MOSART database includes just over one million deliveries and covers the years 2004 through 2017.
Figure 1:
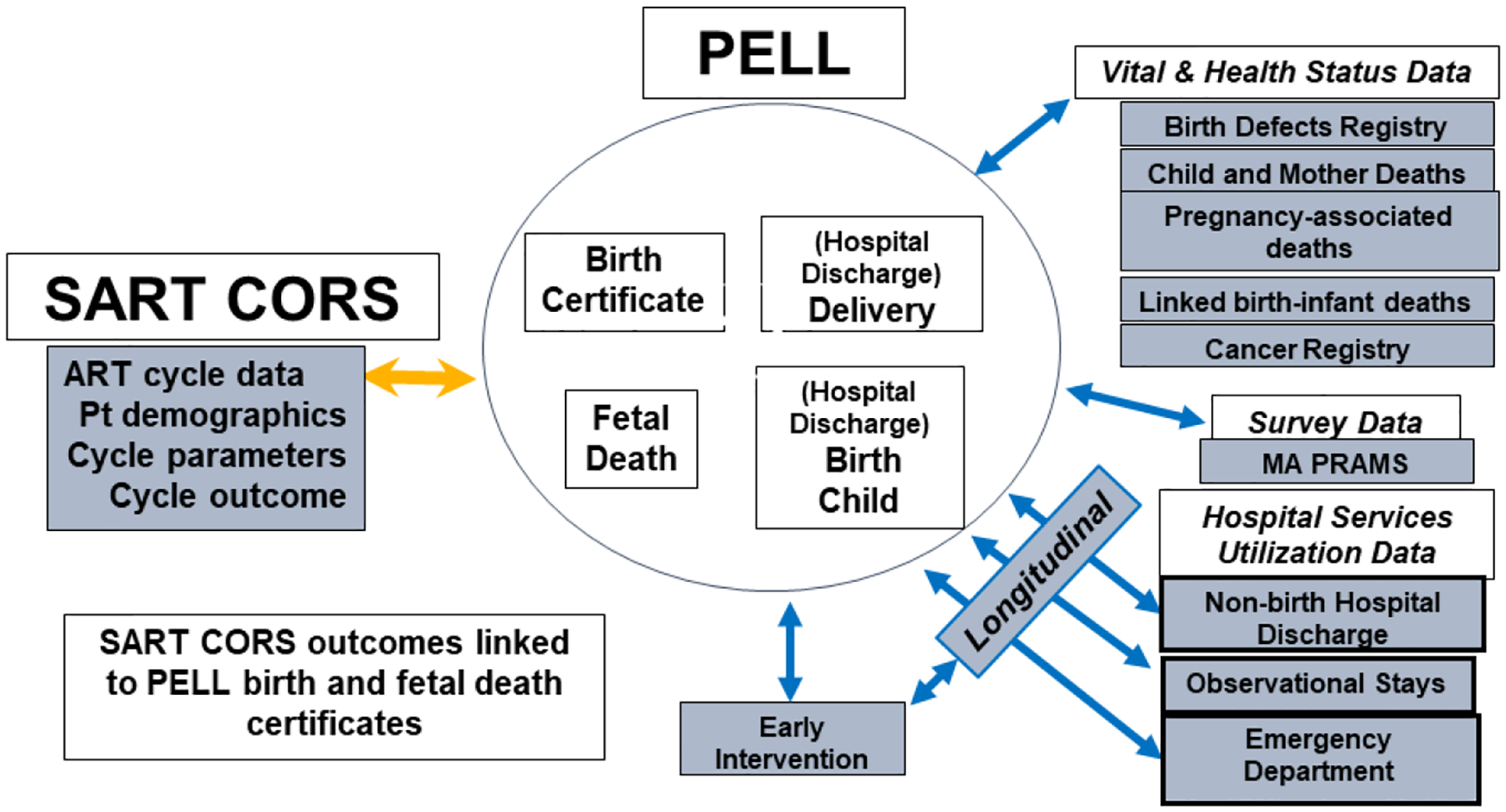
Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART) Schema
More recently, MOSART has been further linked to the Massachusetts Cancer Registry and to the Massachusetts All Payers Claims Database (APCD), a database of inpatient and outpatient insurance claims data. APCD has allowed us to redefine patient medical and fertility history more precisely.
Comparisons with other datasets
There are other datasets – national or research initiative-specific, which are more or less complete than MOSART. Countries including Norway (15,16) Australia (6,17), France (18), and Denmark (19,20) have a variety of birth and ongoing health registries. Some of these datasets have advantages over MOSART for study of longitudinal health since they contain outpatient as well as inpatient data. Other US databases include the CDC’s States Monitoring ART (SMART) surveillance system, which combines birth certificate, national ART-treatment, and some longitudinal health data in 4 states (21), and a linkage of cancer registries and SART CORS data for 14 states (22). These datasets, though excellent, either do not have the wealth of information found in SART CORS (23) or do not contain the additional birth, childhood or women’s health outcomes captured in our contributing datasets. Moreover, other datasets never developed efficacious infertile comparison groups of the sort developed in MOSART.
Despite considerable strengths, MOSART has some distinct limitations. The database is restricted to live and stillborn deliveries at ≥20 weeks gestation or >350 grams. Thus, information on early pregnancies, including miscarriages and fetal reduction, is lacking and we cannot explore questions about outcomes per pregnancy attempt or include women without delivery as a comparison group. We have no information on time-to-pregnancy. Added APCD data are limited in that not all insurance companies submit claims, our inability to obtain Medicaid data, and in the inability to parse outcomes over time. Finally, MOSART data are from one US state, potentially limiting generalizability. Despite these limitations, this is still the most complete data system for the study of ART deliveries and subsequent mother and child health in the US.
Defining subfertile and infertile comparison groups
Retrospectively defining an infertile population can be challenging since historic information and clinical details may not be readily available. This is particularly because no state or national-level database distinguishes among those attempting to conceive, those contracepting, those who are sexually active but not contracepting, and those who are not sexually active. In defining what came to be called our “subfertile” population in MOSART, we had access to the following information on the index delivery of women who did not have ART to conceive: 1) whether the patient had fertility treatment indicated on the birth or fetal death record of the index or a prior delivery (54.8% of the subfertile group), 2) whether the patient had a hospitalization in the 5 years (chosen for practical reasons) prior to delivery with an International Classification of Diseases (ICD9) code for infertility (14.2% of the subfertile group), and 3) whether the patient had prior ART recorded in SART CORS (17.3% of the subfertile group), or 4) a combination of these (13.7%) (24). Based on these definitions, we did not have a defined diagnosis of infertility for every woman in the cohort, and we therefore called this group “subfertile” rather “infertile”. The subfertile group was smaller than the ART group, bolstering our belief that we had not identified every infertile couple hence it was a conservative estimate of the infertile population. We accepted this conservative definition given that the fertile group was considerably larger, and having some infertile women misclassified into the fertile group was not likely to significantly affect comparisons, whereas incorrectly assigning women to the subfertile group could have more effect within that smaller sample. The subfertile group was more similar to the ART group than the fertile group in terms of age (women <25years old 23.5% fertile; 1.6% subfertile, 0.3% ART), race/ethnicity (non-Hispanic white, 66.8% fertile, 85.1% subfertile, 86.5% ART), education (college or graduate degree 40.4% fertile, 69.7% subfertile, 73.6% ART), and insurance coverage at delivery (private 58.2% fertile, 92.0% subfertile, 96.6% ART) (25).
In some later studies, we divided the subfertile group into those who had non-ART infertility treatment and those who did not (26–29). The treated group, which we called the non-ART medically assisted reproduction group (non-ART MAR) and the unassisted subfertile group (those without treatment for the index delivery but having other indicators of infertility) both had characteristics that fell between the fertile and ART groups. More recently, we developed a comparison group of women diagnosed with infertility in the outpatient clinic as well as the hospital (deliveries between 2013–2017), utilizing ICD9 and ICD10 codes in the APCD (30.). This comparison group, that we called “infertile” because all women had a diagnosis of infertility, was nearly three times as large as the subfertile group. Even this more inclusively defined infertile group was missing those who experienced infertility but never presented to a healthcare provider for evaluation. Demographic and reproductive history characteristics of the infertile group were similar to those of the subfertile group and again, fell between those of the fertile and ART-treated groups (30).
Underlying conditions and delivery outcomes
Fertile, subfertile, infertile and ART-treated groups
A major early contribution of MOSART was analysis of delivery outcomes of the three fertility groups: fertile, subfertile, and ART-treated. As shown in Table 1, both the subfertile and the ART-treated compared to the fertile groups had elevated risk of low birth weight (LBW) and preterm birth (PTB), but not of infants born small for gestational age (SGA) (25,31). The ART-treated group had increased risk over that of the subfertile group. Our analyses repeatedly suggested that the subfertile and infertile groups had an elevated risk for LBW and PTB, which led us to hypothesize that adverse outcomes were related to underlying infertility rather than the ART treatment (13). However, even in early studies it was clear that risk in ART deliveries was somewhat higher than in subfertile deliveries. More recently, we analyzed outcomes in non-ART MAR and unassisted subfertile subgroups (26) and in the infertile group linked to APCD (30) and found again that these had characteristics between the other two.
Table 1:
Adjusted odds ratios in singleton live birth deliveries for preterm delivery, low birthweight and small for gestational age in subfertile and ART groups compared to fertile as reported by Declercq et al (25)
| Subfertile | ART | ||
|---|---|---|---|
| Category | AOR (95% CI) | AOR (95% CI)3 | |
| Reference Fertile | Reference Fertile | Reference Subfertile | |
| Preterm (<37 weeks) | 1.24 (1.12–1.38) | 1.53 (1.40–1.67) | 1.23 (1.08–1.41) |
| Low Birthweight (<2,500gms) | 1.20 (1.06–1.36) | 1.51 (1.37–1.67) | 1.26 (1.08–1.47) |
| Small for Gestational Age | 0.95 (0.85–1.06) | 1.05 (0.96–1.16) | 1.10 (0.96–1.27) |
Models adjusted for maternal age, race/ethnicity, marital status, maternal education, payer for delivery, smoking, prenatal care, parity, chronic and pregnancy associated hypertension, other fertility related condition, and infant gender
Studies on ART outcome in singletons from other investigators have also suggested that risk of adverse outcomes is greater for ART-exposed compared to the fertile population (1–12) but that subfertility, IUI, or IUI/(ovulation induction (OI) as well as ART increase these risks (32–42). In a 2013 metanalysis, Pinborg et al. (36) concluded that subfertility, with and without ART was significantly associated with risk of PTB compared to spontaneous conception. Poon and Lian found an OI/IUI effect on PTB between that of ART-treated and a spontaneous conception (37). There are also studies comparing siblings conceived with and without ART treatment. Romundstat et al (43) studied children born with and without assisted fertilization (defined as IVF or ICSI) and found that sequential deliveries to the same woman had comparable rates of PTB and LBW regardless of treatment. However, in a different study, they found an increase in placenta previa in sibling pregnancies conceived with ART compared with those not conceived with ART (16). Dhalwani et al (44) found differences in LBW and PTB comparing ART and non-ART in the same woman. In another intriguing study, Woo et al (45) found that when compared with a prior pregnancy in the same woman, a subsequent pregnancy conceived through gestational surrogacy had more pregnancy complications, including pregnancy hypertension, gestational diabetes, placenta previa, and more PTB and LBW. Using MOSART, we were able to distinguish deliveries of siblings from women who at different points in time were designated as fertile, subfertile, and ART-treated (46). These data revealed that sibling risk for LBW and PTB followed the pattern of lowest in the fertile, more pronounced in the subfertile, and greatest when ART-treated regardless of whether the ART treatment was before or after a delivery designated as subfertile.
Infertility diagnoses
Underlying causes of infertility include diagnoses of endometriosis, PCOS, other ovulatory disorders, uterine factor, tubal factor, male factor, and diminished ovarian reserve (DOR) and some of these influence delivery outcomes. For example, Zullo et al in 2017 (47) conducted a metanalysis that demonstrated increased risk of PTB in women with endometriosis. Farland et al (48) found increased risk for both LBW (independent of gestational age) and PTB in endometriosis patients in the Nurses’ Health Study II cohort. However, Kobayashi (49) suggested that the literature varied on this subject. In another example, PCOS was found in some, but not all studies, to be associated with increased PTB and LBW (50,51).
Using MOSART, we evaluated infertility diagnoses among ART deliveries and found that among ART-treated pregnancies when compared with male factor infertility, ovulatory disorders were associated with gestational diabetes as well as PTB and tubal and uterine factors were associated with more prenatal hospitalization (52). We also evaluated both endometriosis and PCOS and found greater risks for PTB and LBW (53,54). We performed two MOSART studies that looked at obstetric outcomes within diagnostic groups conceived with and without ART treatment. In the first (55) we found elevations in risk of PTB and LBW in ART-treated deliveries with diagnoses of male factor, endometriosis, ovulatory disorder, tubal factor, and inflammation, but not in endometriosis patients who did not undergo ART. Recently (56), we directly compared ART-treated to non-ART treated patients whose diagnoses of tubal factor, PCOS, other ovulatory disorder, and endometriosis were determined from APCD. We found increases in risk for ART-treated pregnancies and deliveries compared with the non-ART counterparts including risk for diabetes and hypertensive disorders of pregnancy as well as in PTB and LBW (Table 2). Of particular interest was the increased risk for placental abnormalities in each of the diagnostic groups when ART treatment was used. We will return to this observation later in this review.
Table 2:
Risk ratios for outcomes of singleton deliveries to women with ART treatment having diagnoses of tubal, PCOS, other ovulatory conditions, or endometriosis compared with fertile women and those with the same diagnosis and no ART treatment as reported by Stern et al (56)1
| Tubal1 | PCOS1 | Endometriosis1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ART compared to Fertile (ref)2 | ART compared to non-ART (ref) 2 | ART compared to Fertile (ref)2 | ART compared to non-ART (ref)2 | ART compared to Fertile (ref)2 | ART compared to non-ART (ref)2 | |||||||
| ARR2,3 | 95% CI | ARR2,3 | 95% CI | ARR2,3 | 95% CI | ARR2,3 | 95% CI | ARR2,3 | 95% CI | ARR2,3 | 95% CI | |
| Pregnancy complications | ||||||||||||
| Pregnancy Hypertension | 0.84 | 0.67–1.06 | 0.83 | 0.61–1.13 | 1.50 | 1.28–1.75 | 1.36 | 1.12–1.65 | 1.44 | 1.13–1.83 | 1.39 | 1.00–1.92 |
| Eclampsia/Preeclampsia | 1.23 | 0.97–1.57 | 0.98 | 0.70–1.38 | 1.53 | 1.25–1.88 | 1.17 | 0.91–1.51 | 1.64 | 1.24–2.17 | 1.32 | 0.90–1.95 |
| Pregnancy Diabetes | 1.37 | 1.16–1.63 | 1.19 | 0.89–1.59 | 1.62 | 1.39–1.89 | 0.99 | 0.83–1.19 | 1.11 | 0.85–1.45 | 1.18 | 0.84–1.64 |
| Placental Problems | 2.38 | 1.86–3.04 | 1.72 | 1.18–2.51 | 1.59 | 1.19–2.12 | 1.49 | 1.02–2.17 | 3.42 | 2.64–4.44 | 2.86 | 1.79–4.58 |
| Delivery complications | ||||||||||||
| Birthweight | ||||||||||||
| Low (<2500) | 1.51 | 1.21–1.89 | 1.61 | 1.13–2.31 | 1.70 | 1.38–2.09 | 1.20 | 0.93–1.54 | 1.33 | 0.97–1.83 | 1.01 | 0.67–1.53 |
| Prematurity | ||||||||||||
| Preterm (<37 weeks) | 1.69 | 1.38–2.05 | 1.37 | 1.02–1.83 | 1.86 | 1.55–2.23 | 1.40 | 1.12–1.76 | 1.75 | 1.35–2.25 | 1.37 | 0.98–1.93 |
Includes live births and fetal deaths for the years 2013–2017.
Modified Poisson regression (GEE with Poisson distribution, exchangeable correlation structure) was used to account for multiple deliveries to the same woman.
Adjusted for mother’s age (< 30, 31–34, ≥ 35), race/ethnicity (NHW, Others, unknown), education (HS or < HS, some college/college, post college, unknown), chronic diabetes (Yes, No), chronic hypertension (Yes, No), parity (1, >=2), BMI (<18.5, 18.5–24.9, 25–29.9, ≥ 30, missing).
Women with subfertility/infertility or ART treatment exposures often also have underlying health conditions beyond the diagnoses to which infertility is commonly attributed. These include higher rates of chronic hypertension and diabetes mellitus and thyroid problems that persist even after accounting for age (24–26, 57)..
Risks to women’s health
Pregnancy-associated risks
We have studied pregnancy associated hypertension, preeclampsia/eclampsia, gestational diabetes, placental problems, and uterine bleeding. Each of these conditions were elevated in ART-treated pregnancies over those in the fertile group but also elevated over those in the subfertile group (31,46,57). For example, using the fertile group as reference, pregnancy hypertension had adjusted odds (AOR) of 1.22 (95% CI 1.15–1.28) in ART-treated patients and 1.12 (95% CI 1.05–1.20) in subfertile patients. Risk in the ART-treated group was marginally greater than the subfertile group with AOR of 1.08 (95% CI 1.00–1.18) (31). AOR of pregnancy-associated uterine bleeding were 3.80 (95%CI 3.31–4.36) for ART-treated and 1.67 (95%CI 1.33–2.09) for subfertile deliveries. These results are consistent with other literature (58–62).
ART pregnancies also have increased rates of cesarean delivery. We found, for example that AORs for singleton cesarean delivery compared to fertile were 1.27 (1.19–1.36) for ART, 1.26 (1.07–1.47) for unassisted subfertile, and 1.09 (0.96–1.24) for non-ART MAR groups, and that underlying medical conditions or prior procedures, particularly, uterine surgery prior to the index pregnancy, associated with increased risk in the ART-treated and subfertile groups (63). Increased rates of cesarean delivery for ART-treated pregnancies have been shown previously (58–60).
Postpartum risks
A standard method for determining health risks in women following delivery is severe maternal morbidity (SMM). SMM is defined by the CDC as having one or more of ICD9/ICD10 codes for 25 maternal morbidities (64). Using MOSART data, we determined that women with an ART-treated delivery are at increased risk for SMM (65). Receipt of blood transfusion was the most common indicator of SMM. Women with singleton ART-treated deliveries had AORs of SMM of 2.27 (95%CI 1.78–2.88) compared with the fertile group. The rates of SMM were not significantly increased in the subfertile group and thus the rate of SMM was significantly higher in the ART-treated than the subfertile group. Increased risk of SMM following ART has since been shown previously (66–68).
Hospitalizations in women
Hospitalization after ART has only rarely been studied and then, has often involved cycle-related outcomes such as ovarian hyperstimulation syndrome (OHSS) or complications of retrievals (69–71). Whether long-term risk of hospitalization is increased following infertility and ART treatment, has received less attention. The MOSART contribution to this question included several papers on hospitalization in women following delivery. In a 2019 paper (26), we found that, in the year post-delivery, adjusting for plurality at birth, there were significantly more hospital discharges and observational stays compared to the fertile group for those treated with ART (AOR 1.19, 95%CI 1.05–1.34) and those with unassisted subfertility (1.59, 1.23–2.07) but not those in the non-ART MAR group. In this case, the unassisted subfertile group appeared to have more risk than the ART group but this could have been an artifact of this group being considerably smaller and the confidence interval wider or the fact that the unassisted subfertile group was largely identified as a group with prior hospitalizations for infertility thus affecting subsequent hospitalization. In another study, we compared hospitalizations among ART-treated women with and without pregnancy or delivery and found no difference in hospitalization (72) suggesting that hospitalization was related more to underlying conditions in these ART-treated women than to ART treatment factors.
In another more recent study, we extended the timeframe for analysis of hospital discharges and observational stays to 8 years post-delivery hospitalization (29). Again, both the non-ART MAR and unassisted subfertile deliveries as well as the ART-treated group had higher rates of overall hospitalization than the fertile group. The increase was apparent in every year post-delivery with adjusted relative risk (ARR) at 8 years ranging from 1.18 (1.12–1.25) for unassisted subfertile, to 1.20 (1.13–1.27) in non-ART MAR, and 1.29 (1.25–1.34) in ART-treated women. Hospitalizations were increased for all subfertile groups over the fertile group at 8 years post-delivery for cardiovascular disease, overweight and obesity, reproductive tract disorders, digestive tract diseases, thyroid conditions (approximately doubled in the subfertile groups), respiratory diseases including asthma, breast diseases, diabetes, and other chronic diseases. Women with ART-treated deliveries had increased hospitalization for non-reproductive infection, anemia, and cancer-related conditions.
Several other recent studies investigated hospitalization in women after ART treatment. Lemardeley et al (18) studied hospitalizations of ART-treated patients in the 2 years before and after oocyte retrieval. They found an increased rate of hospitalizations directly related to ART treatment, for example, due to OHSS or ovarian torsion. Ongoing long-term health was not evaluated. Bungum et al (20) followed women for 9 years to understand the risk of cardiovascular disease but found no association with ART treatment. Despite increased risk of cancer hospitalizations in the above MOSART study (29), we could not clearly distinguish whether ART or subfertility increased the rate of cancers. In part, this is because the study timeframe was not sufficient to identify all cancer onset and because our study sample, large as it was, lacked the power to identify cancers occurring at low rates. Luke et al have studied cancer risk in women using a dataset from 3 states (New York, Texas, and Illinois) with risk compared to the general population. They found no overall increase in cancer risk over the 5 years following ART treatment (73). Nevertheless, a retrospective cohort with 10 years follow up in Norway did demonstrate an increased risk of breast cancer (hazard ratio 1.35 (95 % CI 1.07–1.71)) and thus follow up time may be particularly important for this outcome (15). Despite being unable to follow risk of cancer in our population over time, we evaluated the risk for adverse obstetric outcomes in women with a history of cancer prior to treatment (74). We found a higher risk of PTB and LBW in women with a history of cancer, and among those women who were subfertile or ART-treated there was a higher risk for SGA than for fertile women. We have also found that prior cancer was associated with ovarian stimulation response and pregnancy outcome rates among those receiving ART treatment (75).
Mortality in women
Using MOSART, we evaluated mortality in women over an average follow-up time of 5.6–6.0 years (76). In contrast to many of our other studies where the risk was ART-treated greater than subfertile which was greater than the fertile group, we found that when considering all deaths, women in the fertile group were at greatest risk. Removing women who died of external causes (including accident or misadventure) there was no difference among groups. Although numbers in their study were low, Sabban et al (62) reported a similar reduced rate of mortality in women with ART treatment when compared with fertile women. Using a larger Belgian cohort, Vassard et al (77) also found that the ART population had lower mortality rates.
Neonatal and child health
Health of infants at delivery
To understand the health effects for newborns, we evaluated birth hospitalizations of 351,692 singleton infants in the fertile, subfertile, and ART-treated groups to determine incidence of adverse health conditions (27). Infants born to subfertile or ART treated women had higher odds of infectious disease, respiratory conditions, and gastrointestinal conditions, than the fertile group during the birth hospitalization (Table 3). Risk of neonatal mortality was not elevated. We also studied length of birth hospitalizations and the costs of care incurred (78) and found that, gestational age was an important factor in inter fertility-group differences. In stratified analyses the most pronounced interfertility group differences were among the infants born at >37 weeks gestation.
Table 3:
Selected infant outcomes for delivery hospitalizations in liveborn singleton infants born at ≥23 weeks according to parental subfertility or ART treatment as reported by Hwang et al (27)
| Subfertile | ART | ||
|---|---|---|---|
| aOR (95% CI) | aOR (95% CI)3 | ||
| Reference Fertile | Reference Fertile | Reference Subfertile | |
| Preterm Birth (<37 weeks) | 1.39 (1.26–1.54) | 1.72 (1.60–1.85) | 1.23 (1.09–1.40) |
| Infectious Disease | 1.07 (0.83–1.39) | 1.21 (1.00–1.45) | 1.12 (0.82–1.53) |
| Respiratory Conditions | 1.12 (1.02–1.24) | 1.18 (1.09–1.27) | 1.05 (0.93–1.18) |
| Gastrointestinal Conditions | 1.18 (1.01–1.38) | 1.15 (1.03–1.29) | 0.97 (0.81–1.17) |
| Cardiovascular Conditions | 1.19 (0.98–1.45) | 1.07 (0.92–1.25) | 0.90 (0.70–1.15) |
| Neurologic Conditions | 1.04 (0.89–1.21) | 0.96 (0.85–1.08) | 0.92 (0.76–1.12) |
| Hematologic Conditions | 1.10 (0.95–1.27) | 1.04 (0.93–1.16) | 0.94 (0.79–1.12) |
| Neonatal Mortality | 1.23 (0.67–2.25) | 0.98 (0.61–1.57) | 0.80 (0.38–1.67) |
| Chromosomal Abnormalities | 1.21 (0.79–1.85) | 0.62 (0.41–0.96) | 0.52 (0.29–0.92) |
Models are adjusted for maternal age, race, education, payer (private, public, or self-pay), preexisting diabetes, preexisting hypertension, pregnancy-induced hypertension, gestational diabetes, parity, sex, birth year, and GA (continuous variable).
We further evaluated whether infant health conditions for singleton deliveries varied according to infant sex and found that although differences persisted among the fertility groups, and although males tended to have greater risk than females for many conditions, the magnitude of the male to female differences within each fertility group did not differ (79). Further, when we evaluated outcomes for singleton ART infants among ART deliveries in fresh compared with frozen embryo transfer (80) we found that although infants born from frozen embryo transfer had higher birthweight, they also had increased odds of being large for gestational age, and of infectious disease, hematologic conditions, respiratory disease, and neurologic abnormalities.
There has also been considerable speculation on whether ART increases birth defects, with previous studies differing on the conclusions reached (6,81–84). In a study in 2017 using the Massachusetts Birth Defects Registry, evaluated birth defects in a combined population of singleton and multiple births (85) and found that although multiple pregnancy was the major risk factor for birth defects in ART-treated deliveries, subfertility was the second most important factor. In addition, in the above-mentioned study by Hwang et al (27), found that the risk of chromosomal abnormalities was lower in the ART treated group than in either the fertile or subfertile groups. We could not determine whether ART pregnancies had a higher risk of loss due to chromosomal abnormalities in the fetus, which could have accounted for this difference. Non-chromosomal birth defect rates did not differ.
Child health over 4 years
Studies on long-term health outcomes for children of ART have yielded mixed results (86,87). Certainly, multiple gestation, PTB, and LBW increase the risk for adverse outcomes, but even singletons born at term with normal birthweight after ART have been shown to have altered growth patterns and increased cardiovascular problems compared to children born to mothers without infertility (86,88–90).
To assess longitudinal child health, we analyzed hospitalizations of children over the 4 years after birth (28). Compared to the fertile group, we observed an increase in healthcare utilization for singleton offspring born to women with subfertility. ARR for hospital stays for children in the ART-treated and non-ART MAR groups were minimally though significantly increased for hospital discharge, observational stay, and emergency room (ER) visits. The unassisted subfertile group when compared to the fertile group, had increased rates of observational stays and ER visits. Hospital utilization in all groups was influenced by both gestational age at birth and birthweight. Children born at term with normal birthweight showed a more consistent difference in usage among the subfertile groups with and without treatment compared to the fertile group. Overall, the ART-treated group had the most pronounced differences from the fertile group although direct comparisons among the different subfertile groups were not calculated.
Early intervention and autism
According to the CDC (What is “Early Intervention”? | CDC), Early Intervention (EI) comprises “services and supports that are available to babies and young children with developmental delays and disabilities and their families.” EI programs include speech and physical therapy that support child development. Many studies have shown that the need for EI increases in children born premature (91–93) and can be influenced by socioeconomic factors (94). Given the increased incidence of PTB and LBW in children born of ART treatment and subfertility, we used MOSART to study the association of these factors and EI enrollment.
Our paper (95) was the first to study the likelihood of EI enrollment among singletons in ART-treated, subfertile, and fertile deliveries. Among 318,305 children, both ART and subfertility increased EI enrollment (enrollment= 18.7% ART; 17.4% subfertile; 16.5% fertile: P<0.001). Rates were much higher among those with PTB compared to term birth in all fertility groups. Using mediation analysis to model PTB as the mediator between ART or subfertility and EI enrollment, we found that the Natural Direct Effect of ART on EI enrollment was 1.27 (1.19 – 1.36) for ART vs fertile and 1.20 (1.12 – 1.29) for subfertile vs fertile groups leading us to conclude that despite the influence of PTB, ART and subfertile groups still had independent higher rates of EI enrollment (by 27% and 20% respectively) than the fertile group.
In addition to questions about EI enrollment, there has also been debate about whether autism spectrum disorders (ASD) are increased in children following ART treatment and infertility (96–98). As with EI enrollment, PTB, LBW and SGA status have been shown to be associated with ASD (99). We examined the risk of early ASD diagnosis among singleton children aged 0–3 years comparing children of ART-treated, subfertile, and fertile groups, to assess the direct, indirect (through preterm birth), and total effects of ART and subfertility on early ASD (100). We also analyzed the subgroups of IVF (insemination) and ICSI. Our study was the first population-based study in the US to use preterm birth as a mediator to better estimate the effect of subfertility or ART on ASD. Only the unadjusted numbers in the ART-treated group showed a small increase in the prevalence of ASD (1.2%) when compared with the fertile group. AORs for ART versus fertile were not statistically significant and mediation analysis showed no Natural Direct Effect (100). Although ICSI alone had previously been demonstrated to increase ASD (97), it was not shown to do so in our study.
ART and placental abnormalities
Placentation hypothesis
We have seen higher rates of PTB, LBW, and some maternal and child complications that is greater after ART treatment than after subfertility/infertility alone. To better understand this issue, we preformed several studies that looked at one outcome, PTB, using fertility groups and the wealth of information in MOSART on chronic conditions, pregnancy conditions, and delivery complications. In the first of two papers (101), we analyzed demographic factors (maternal and paternal age, race/ethnicity, education), maternal health (hypertension, diabetes, prior uterine surgery, thyroid conditions, and bleeding disorders), pregnancy conditions (gestational diabetes and hypertensive disorder, placental problems, and bleeding disorders), and infant sex. Through backwards elimination of these factors in multivariate models across fertility groups, we determined that the strongest drivers of both early and late PTB were placental problems (including abruptio placenta, placenta previa, vasa previa, and placenta accreta) and hypertensive disorders. Using mediation analysis, we determined that the percent mediation for placental problems for the ART-treatment effect on PTB was 16% for late PTB and 32% for early PTB. The percent mediation for unassisted subfertility was lower: 7% for late PTB and 12% for early PTB and for MAR, 7% and 12%. In a second paper (102) we found both placental problems and pregnancy hypertensive disorders to be mediators in the association between ART and PTB with mediation differentially affecting outcome depending on ART treatment type (fresh/frozen, IVF/ICSI, autologous/donor egg).
These studies are consistent with the previously demonstrated increased incidence of placental abnormalities in ART-treated pregnancies (16,103–111). Abnormal placental methylation has also emerged as a possible factor in adverse ART outcomes. (112–114). MOSART studies also consistently show increased placental abnormalities in ART-treated and subfertile compared to fertile groups with a greater increase after ART. Luke et al found a 3-fold difference in risk of placental problems in ART-treated compared to fertile and a 2-fold increase in subfertile compared to fertile deliveries in sibling analyses (46). An increase in placental problems in ART-treated pregnancies was particularly obvious in our study of underlying diagnoses with and without ART-treatment (56). These observations are consistent with the hypothesis that abnormal placentation after ART treatment contributes to the increase in PTB, LBW and potentially with longer-term health outcomes over that of subfertility. We are currently evaluating maternal, ART cycle-specific, and laboratory factors that contribute to placenta previa and find that risk factors in the ART population differ from those of the general population (115) but that the condition of the uterus at transfer and the process of embryo transfer itself, may be involved.
Summary and conclusions
Summary
Using the MOSART database we have added to existing studies on the risks of ART pregnancies and deliveries including a comparison of these risks to those of subfertile/infertile women whose pregnancies were conceived without ART. Figure 2a summarizes our findings for the subfertile/infertile groups as compared with the fertile group. We have consistently shown greater risk of adverse outcomes for subfertility/infertility and ART treatment for pregnancy, PTB, LBW, risks of women’s hospitalization, and risks to child health including of specific medical conditions. Figure 2b shows that our studies also demonstrated risks to ART-treated women as compared with the non-ART subfertile/infertile women, to be increased during pregnancy (particularly for placental problems) after delivery (risk of SMM), and for some hospitalizations, and for children to be increased for PTB and LBW as well as some hospitalizations and EI enrollment. While the increased risk during pregnancy and delivery was consistently greater in the ART group, differences were less pronounced or absent when looking at subsequent ongoing health in women and children. We acknowledge that it is possible that some of this increased risk with ART arises from ART patients having more severe infertility, however, on the basis of sibling studies as well as observations of placental abnormalities, we believe our studies demonstrate that this does not explain all of the increase in risk, and we propose that ART treatment itself is a contributing factor.
Figure 2a:
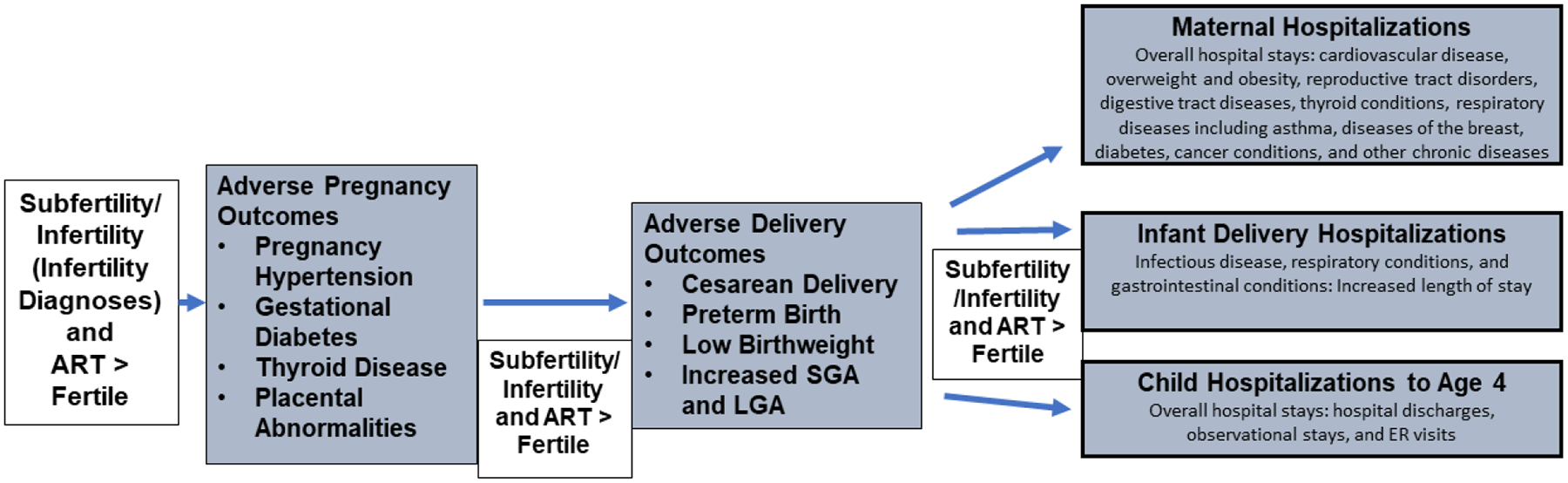
Adverse Outcomes Increased in Subfertile/Infertile and ART Groups
Figure 2b:
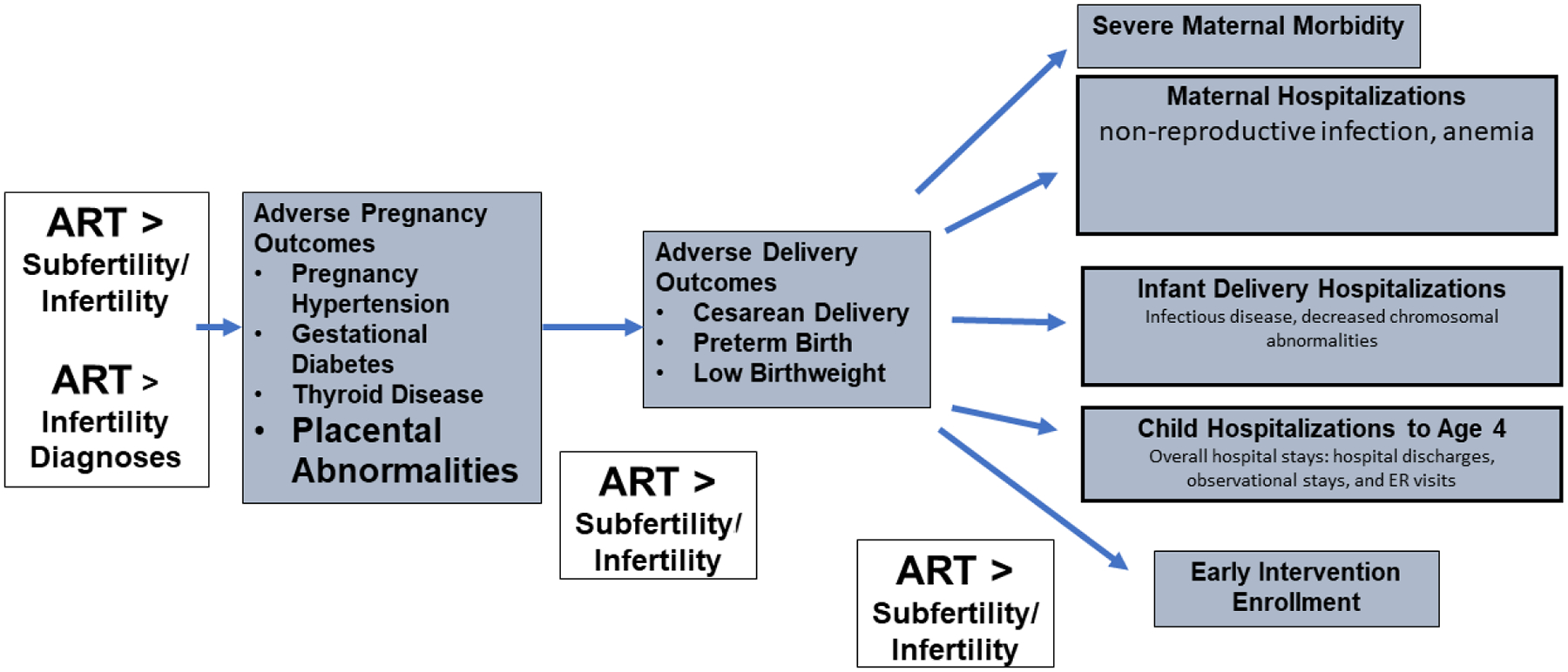
Adverse Outcomes Increased in ART over Subfertile/Infertile Groups
Conclusions
The MOSART dataset has been a unique resource that was developed with the expertise and assistance of multiple collaborating researchers and organizations. This unique dataset has allowed us to contribute a wealth of information to the field of ART outcomes research and to explore the influence of underlying subfertility/infertility to the elevated risks seen following ART treatment. Through use of these data, we have been able to address some of the more pressing questions about the health risk of these procedures and have found that while subfertility/infertility increases risks during and after pregnancy for both mothers and children, ART treatment further increases some of these risks, with the possibility of abnormal placentation contributing to this increased risk. Future studies should further explore this possibility. Our results suggest that patients should be counseled on specific risks they might expect given their infertility diagnoses and/or use of ART as well as be encouraged to inform their obstetricians, primary care physicians, and pediatricians of this medical background so that their pregnancies, deliveries and ongoing health and the health of their children be monitored more closely. Moreover, our MOSART analyses highlight the importance of, and continued need for, population-based monitoring of perinatal health outcomes for this group at state- and national-levels.
Highlights:
The Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART) is a unique data system comprised of cycle-based ART data linked to birth certificates, fetal death certificates, hospital utilization, and other health datasets in the state.
Studies using MOSART data have demonstrated increased risk of adverse outcome in pregnancies and deliveries as a function of subfertility/infertility and, to a greater extent, ART when compared with pregnancies and deliveries to fertile women.
Studies using MOSART data have demonstrated increased risk of adverse health outcomes for women, infants, and young children in the subfertle/infertile and ART groups when compared with those in the fertile group.
Placental abnormalities may be particularly important in the increased risk for preterm birth following ART treatment even as compared with subfertile/infertile groups.
Acknowledgments:
The authors would like to thank other members of our MOSART team including Chia-Ling Liu, RN, MPH, ScD, Daksha Gopal, MPH, Susan Gershman, MS, MPH, PhD, Richard Knowlton MS, Qi Yu, MS, and Emily Lu, MPH who contributed time and expertise to the MOSART studies. We also thank the many PELL collaborators and staff including who worked on creating and maintaining the PELL data system. Further, we thank all SART members for submitting data to SART CORS. Without the efforts of SART, these studies would not have been possible.
Financial Support:
This project was supported by NIH grants HD67270 and HD064595.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: All authors’ institutions received support and travel funds from one or both grants. There are no other conflicts of interest.
References
- 1.Schieve LA, Ferre C, Peterson HB, Macaluso M, Reynolds MA, Wright VC. Perinatal Outcome Among Singleton Infants Conceived Through Assisted Reproductive Technology in the United States. Obstet. Gynecol 2004;103:1144–1153, doi: 10.1097/01.aog.0000127037.12652.76. [DOI] [PubMed] [Google Scholar]
- 2.Dunietz GL, Holzman C, McKane P, Li C, Boulet SL, Todem D, Kissin DM, Copeland G, Bernson D, Sappenfield WM, Diamond MP. Assisted reproductive technology and the risk of preterm birth among primiparas. Fertil Steril. 2015;103:974–979.e1, doi: 10.1016/j.fertnstert.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stojnic J, Radunovic N, Jeremic K, Kotlica BK, Mitrovic M, Tulic I. Perinatal outcome of singleton pregnancies following in vitro fertilization. Clin Exp Obstet Gynecol. 2013;40(2):277–83. [PubMed] [Google Scholar]
- 4.Helmerhorst FM, Perquin DAM, Donker D, Keirse JNC. Perinatal outcome of singletons and twins after assisted conception: A systematic review of controlled studies. BMJ 2004;328:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henningsen AKA, Pinborg A, Lidegaard Ø, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertility and Sterility 2011;95:959–63. [DOI] [PubMed] [Google Scholar]
- 6.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725–30. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 7.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: A meta-analysis. Obstet Gynecol 2004;103:551–63. [DOI] [PubMed] [Google Scholar]
- 8.Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA, and the National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod 2009;24:360–6. [DOI] [PubMed] [Google Scholar]
- 9.Chambers GM, Chapman MG, Grayson N, Shanahan M, Sullivan EA. Babies born after ART treatment cost more than non-ART babies: A cost analysis of inpatient birth-admission costs of singleton and multiple gestation pregnancies. Hum Reprod 2007;22:3108–15. [DOI] [PubMed] [Google Scholar]
- 10.El-Chaar D, Yang Q, Gao J, Bottomley J, Leader A, Wen SW, Walker M. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertility and Sterility 2009;92:1557–61. [DOI] [PubMed] [Google Scholar]
- 11.Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet 2004;21:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavoretto P, Candiani M, Giorgione V, Inversetti A, Abu-Saba MM, Tiberio F, Sigismondi C, Farina A. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: meta-analysis of cohort studies. Ultrasound Obstet Gynecol. 2018;51(1):43–53. doi: 10.1002/uog.18930. [DOI] [PubMed] [Google Scholar]
- 13.Luke B, Stern JE, Hornstein MD, Kotelchuck M, Diop H, Cabral H, Declercq ER. Is the wrong question being asked in infertility research? JARG. 2016;33:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotelchuck M, Hoang L, Stern JE et al. The MOSART Database: Linking the SART CORS Clinical Database to the Population-Based Massachusetts PELL Reproductive Public Health Data System. Matern Child Health J 2014;18:2167–2178). 10.1007/s10995-014-1465-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reigstad MM, Larsen IK, Myklebust TA, Robsahm TE, Oldereid NB, Omland AK, Vagen S, Brinton LA, Storeng R. Risk of breast cancer following fertility treatment–a registry based cohort study of parous women in Norway. Int J Cancer 2015;136:1140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romundstad LB, Romundstad PR, Sunde A, von D€uring V, Skjærven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI: a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod 2006;21:2353–8. [DOI] [PubMed] [Google Scholar]
- 17.Chambers GM, Hoang VP, Lee E, Hansen M, Sullivan EA, Bower C, Chapman M. Hospital costs of multiple-birth and singleton-birth children during the first 5 years of life and the role of assisted reproductive technology. JAMA Pediatr. 2014. Nov;168(11):1045–53. doi: 10.1001/jamapediatrics.2014.1357. [DOI] [PubMed] [Google Scholar]
- 18.Lemardeley G, Pirrello O, Dieterlé S, Zebina A, Astrugue C, Jonveaux P, Lucas-Samuel S, Couchoud C. Overview of hospitalizations in women undergoing oocyte retrieval for ART in the French national health data system. Hum Reprod. 2021;36(10):2769–2781. doi: 10.1093/humrep/deab147. [DOI] [PubMed] [Google Scholar]
- 19.Pinborg A, Loft A, Schmidt L, Andersen AN. Morbidity in a Danish national cohort of 472 IVF/ICSI twins, 1132 non-IVF/ICSI twins and 634 IVF/ICSI singletons: health-related and social implications for the children and their families. Hum Reprod. 2003. Jun;18(6):1234–43. doi: 10.1093/humrep/deg257. [DOI] [PubMed] [Google Scholar]
- 20.Bungum AB, Glazer CH, Arendt LH, Schmidt L, Pinborg A, Bonde JP, Tøttenborg SS. Risk of hospitalization for early onset of cardiovascular disease among infertile women: a register-based cohort study. Hum Reprod. 2019;34(11):2274–2281. doi: 10.1093/humrep/dez154. [DOI] [PubMed] [Google Scholar]
- 21.Mneimneh AS, Boulet SL, Sunderam S, Zhang Y, Jamieson DJ, Crawford S, McKane P, Copeland G, Mersol-Barg M, Grigorescu V, Cohen B, Steele J, Sappenfield W, Diop H, Kirby RS, Kissin DM; States Monitoring ART (SMART) Collaborative. States Monitoring Assisted Reproductive Technology (SMART) Collaborative: data collection, linkage, dissemination, and use. J Womens Health (Larchmt). 2013;22(7):571–7. doi: 10.1089/jwh.2013.4452. [DOI] [PubMed] [Google Scholar]
- 22.Spector LG, Brown MB, Wantman E, Letterie GS, Toner JP, Doody K, Ginsburg E, Williams M, Koch L, Schymura MJ, Luke B. Association of In Vitro Fertilization With Childhood Cancer in the United States. JAMA Pediatr. 2019;173(6):e190392. doi: 10.1001/jamapediatrics.2019.0392. Epub 2019 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers GM, Dyer S, Zegers-Hochschild F, de Mouzon J, Ishihara O, Banker M, Mansour R, Kupka MS, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology, 2014, Human Reprod 2021;36:2921–2934, 10.1093/humrep/deab198 [DOI] [PubMed] [Google Scholar]
- 24.Declercq ER, Belanoff C, Diop H, Gopal D, Hornstein MD, Kotelchuck M, Luke B, Stern JE. Identifying women with indicators of subfertility in a statewide population database: operationalizing the missing link in assisted reproductive technology research. Fertil Steril 2014;101(2):463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Declercq E, Luke B, Belanoff C, Cabral H, Diop H, Gopal D, Hoang L, Kotelchuck M, Stern JE, Hornstein MD. Perinatal Outcomes Associated with Assisted Reproductive Technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). Fertility and Sterility 2015;103:888895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern JE, Liu CL, Cabral H, Gopal D, Harvey E, Missmer SA, Diop H, Coddington CC. Hospitalization before and after delivery in fertile, subfertile, and ART-treated women. JARG 2019; 36:1989–1997; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang SS. Dukhovny D, Gopal D, Cabral H, Missmer SA, Diop H, Declercq E, Stern JE. Health of infants following ART-treated, subfertile and fertile deliveries in Massachusetts. Pediatrics 2018:142(2);e20174069; DOI: 10.1542/peds.2017-4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dukhovny D, Hwang S, Gopal D, Cabral H, Diop H, Stern JE. Association of maternal fertility and fertility treatment with healthcare utilization in infants up to age four. J Perinatol. 2021. Mar 1:10.1038/s41372-021-01003-y. doi: 10.1038/s41372-021-01003-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farland LV, Liu CL, Diop H, Cabral H, Missmer SA, Coddington CC, Hwang SS, Stern JE. Hospitalizations up to 8 years following delivery in ART-treated and subfertile women; Fertil Steril 2019;36(10):1989–1997 DOI: 10.1007/s10815-019-01562-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern JE, Liu CL, Cui X, Gopal D, Cabral HJ, Coddington CC, Missmer SA, Hwang S, Farland LV, Dukhovny D, Diop H Optimizing the control group for evaluating ART outcomes: Can outpatient claims data yield a better control group? JARG 2021:38(5):1089–1100: Open access 10.1007/s10815-021-02111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luke B, Gopal D, Cabral H, Stern JE, Diop H. Pregnancy, birth, and infant outcomes by maternal fertility status: the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol 2017;217:327 e321–327 e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudoin M, Dobbie R, Finlayson A, Chalmers J, Cameron IT, Fleming R. Ovulation induction/intrauterine insemination in infertile couples is associated with low-birth-weight infants. Am J Obstet Gynecol 2003;188(3):611–6. doi: 10.1067/mob.2003.5 [DOI] [PubMed] [Google Scholar]
- 33.Zhu JLP, Obel CP, Hammer Bech BP, Olsen JP, Basso OP. Infertility, Infertility Treatment, and Fetal Growth Restriction. Obstetrics & Gynecology 2007;110:1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basso O, Baird DD. Infertility and preterm delivery, birthweight, and Caesarean section: a study within the Danish National Birth Cohort. Human Reproduction 2003;18:2478–84 [DOI] [PubMed] [Google Scholar]
- 35.Raatikainen K; Kuivasaari-Pirinen P; Hippeläinen M; Heinonen S Comparison of the pregnancy outcomes of subfertile women after infertility treatment and in naturally conceived pregnancies. Hum. Reprod 2012, 27, 1162–1169, doi: 10.1093/humrep/des015. [DOI] [PubMed] [Google Scholar]
- 36.Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Söderström-Anttila V, Nygren KG, Hazekamp J, Bergh C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19(2):87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 37.Poon WB, Lian WB. Perinatal outcomes of intrauterine insemination/clomiphene pregnancies represent an intermediate risk group compared with in vitro fertilisation/intracytoplasmic sperm injection and naturally conceived pregnancies. J Paed and Child Health.2013;49:733–740. [DOI] [PubMed] [Google Scholar]
- 38.Basso O, Olsen J. Subfecundity and neonatal mortality: longitudinal study within the Danish national birth cohort. BMJ 2005;330:393–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YA, Sullivan EA, Black D, Dean J, Bryant J, Chapman M. Preterm birth and low birth weight after assisted reproductive technology-related pregnancy in Australia between 1996 and 2000. Fertil Steril 2005;83:1650–8. [DOI] [PubMed] [Google Scholar]
- 40.McElrath TF, Wise PH. Fertility therapy and the risk of very low birth weight. Obstet Gynecol 1997;90:600–5. [DOI] [PubMed] [Google Scholar]
- 41.Brink Henriksen T, Day Baird D, Olsen J, Hedegaard M, Jørgen Secher N, Wilcox AJ. Time to pregnancy and preterm delivery. Obstetrics & Gynecology 1997;89:594–9. [DOI] [PubMed] [Google Scholar]
- 42.Draper E, Kurinczuk JJ, Abrams K, Clarke M. Assessment of separate contributions to perinatal mortality of infertility history and treatment: a case-control analysis. Lancet 1999;353:1746–9. [DOI] [PubMed] [Google Scholar]
- 43.Romundstad LB, Romundstad PR, Sunde A, et al. Effects of technology or maternal factors on perinatal outcome after assisted fertilization: a population-based cohort study. Lancet 2008;372:737–43. [DOI] [PubMed] [Google Scholar]
- 44.Dhalwani NN, Boulet SL, Kissin DM, Zhang Y, McKane P, Bailey MA, et al. Assisted reproductive technology and perinatal outcomes: conventional versus discordant-sibling design. Fertil Steril 2016;106:710–6. [DOI] [PubMed] [Google Scholar]
- 45.Woo I, Hindoyan R, Landay M, Ho J, Ingles SA, McGinnis LK, Paulson RJ, Chung K. Perinatal outcomes after natural conception versus in vitro fertilization (IVF) in gestational surrogates: a model to evaluate IVF treatment versus maternal effects. Fertil Steril. 2017;108(6):993–998. doi: 10.1016/j.fertnstert.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Luke B, Gopal D, Diop H, Stern JE. Perinatal outcomes of singleton siblings: the effects of maternal fertility status and ART treatment. J Assist Reprod Genet 2016;33: 1203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zullo F, Spagnolo E, Saccone G, Acunzo M, Xodo S, Ceccaroni M, Berghella V. Endometriosis and obstetrics complications: a systematic review and meta-analysis. Fertil Steril. 2017;108(4):667–672.e5. doi: 10.1016/j.fertnstert.2017.07.019. Epub 2017 Sep 2. [DOI] [PubMed] [Google Scholar]
- 48.Farland LV, Prescott J, Sasamoto N, Tobias DK, Gaskins AJ, Stuart JJ, Carusi DA, Chavarro JE, Horne AW, Rich-Edwards JW, Missmer SA. Endometriosis and risk of adverse pregnancy outcomes. Obstet Gynecol. 2019;134(3):527–536. doi: 10.1097/AOG.0000000000003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi H, Kawahara N, Ogawa K, Yoshimoto C. A Relationship between endometriosis and obstetric complications. Reprod Sci. 2020;27(3):771–778. doi: 10.1007/s43032-019-00118-0. Epub 2020 Jan 6. [DOI] [PubMed] [Google Scholar]
- 50.Mills G, Badeghiesh A, Suarthana E, Baghlaf H, Dahan MH. Associations between polycystic ovary syndrome and adverse obstetric and neonatal outcomes: a population study of 9.1 million births. Hum Reprod. 2020;35(8):1914–1921. doi: 10.1093/humrep/deaa144. [DOI] [PubMed] [Google Scholar]
- 51.Sadrzadeh S, Hui EVH, Schoonmade LJ, Painter RC, Lambalk CB. Birthweight and PCOS: systematic review and meta-analysis. Hum Reprod Open. 2017. Aug 19;2017(2):hox010. doi: 10.1093/hropen/hox010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luke B, Stern JE, Kotelchuck M, Declercq E, Cohen B, Diop H. Birth outcomes by infertility diagnosis: Analyses of the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). Journal of Reproductive Medicine 2015;60:480–490. [PMC free article] [PubMed] [Google Scholar]
- 53.Farland LV, Stern JE, Liu CL, Cabral HJ, Coddington CC, Diop H, Dukhovny D, Hwang S, Missmer SA. Pregnancy outcomes among women with endometriosis and fibroids: registry linkage study in Massachusetts; AJOG: 17 Jan 2022, DOI: 10.1007/s10815-021-02376-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farland LV, Stern JE, Liu CL, Cabral H, Coddington CC, Dukhovny D, Diop H, Hwang S, Missmer SA. Polycystic ovary syndrome and risk of adverse pregnancy outcomes: a registry linkage study from Massachusetts. Human Repro 2022-submitted [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stern JE, Luke B, Tobias M, Gopal D, Hornstein MD, Diop H. Adverse pregnancy and birth outcomes by infertility diagnoses with and without ART treatment. Fertility and Sterility 2015;103:1438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stern JE, Lui CL, Cui X, Cabral HJ, Farland LV, Coddington CC, Diop H. Assisted reproductive technology (ART) treatment increases obstetric and neonatal risk over that of the underlying infertility diagnosis. Fertil Steril. 2022. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stern JE, Liu CL, Cabral HJ, Richards EG, Coddington CC, Hwang S, Dukhovny D, Diop H, Missmer SA. Birth outcomes of singleton vaginal deliveries to ART-treated, subfertile, and fertile primiparous women. J Assist Reprod Genet. 2018;35(9):1585–1593. doi: 10.1007/s10815-018-1238-x. Epub 2018 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan EA, Chapman MG, Wang YA, Adamson GD. Population-based study of cesarean section after in vitro fertilization in Australia. Birth 2010;3:184–91. [DOI] [PubMed] [Google Scholar]
- 59.Romundstad LB, Romundstad PR, Sunde A, von During V, Skjaerven R, Vatten LJ. Assisted fertilization and breech delivery: risks and obstetric management. Hum Reprod 2009;24:3205–10. [DOI] [PubMed] [Google Scholar]
- 60.Kallen B, Finnstrom O, Nygren KG, Olausson PO, Wennerholm UB. In vitro fertilisation in Sweden: obstetric characteristics, maternal morbidity and mortality. BJOG 2005;112:1529–35. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi M, Nakai A, Satoh S, Matsuda Y. Adverse obstetric and perinatal outcomes of singleton pregnancies may be related to maternal factors associated with infertility rather than the type of assisted reproductive technology procedure used. Fertil Steril 2012;98:922–8. [DOI] [PubMed] [Google Scholar]
- 62.Sabban H, Zakhari A, Patenaude V et al. Obstetrical and perinatal morbidity and mortality among in-vitro fertilization pregnancies: a population-based study. Arch Gynecol Obstet;296:107–113 (2017). 10.1007/s00404-017-4379-8 [DOI] [PubMed] [Google Scholar]
- 63.Stern JE, Liu CL, Cabral HJ, Richards EG, Coddington CC, Missmer SA, Diop H Factors associated with the increased odds of cesarean delivery in ART pregnancies. Fertil Steril 2018:110(3);429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine, Kilpatrick SK, Ecker JL. Severe maternal morbidity: screening and review. Am J Obstet Gynecol. 2016. Sep;215(3):B17–22. doi: 10.1016/j.ajog.2016.07.050. Epub 2016 Aug 22. [DOI] [PubMed] [Google Scholar]
- 65.Belanoff C, Declercq ER, Diop H, Gopal D, Kotelchuck M, Luke B, Nguyen T, Stern JE. Severe maternal morbidity and the use of assisted reproductive technology. Obstetrics and Gynecology 2016;127:527–534. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Ray C, Pelage L, Seco A, Bouvier-Colle MH, Chantry AA, Deneux-Tharaux C; Epimoms Study Group. Risk of severe maternal morbidity associated with in vitro fertilisation: a population-based study. BJOG. 2019;126(8):1033–1041. doi: 10.1111/1471-0528.15668. Epub 2019 Mar 27. [DOI] [PubMed] [Google Scholar]
- 67.Dayan N, Fell DB, Guo Y, Wang H, Velez MP, Spitzer K, Laskin CA. Severe maternal morbidity in women with high BMI in IVF and unassisted singleton pregnancies. Hum Reprod. 2018;33(8):1548–1556. doi: 10.1093/humrep/dey224. [DOI] [PubMed] [Google Scholar]
- 68.Wang ET, Ozimek JA, Greene N, Ramos L, Vyas N, Kilpatrick SJ, Pisarska MD. Impact of fertility treatment on severe maternal morbidity. Fertil Steril. 2016. Aug;106(2):423–6. doi: 10.1016/j.fertnstert.2016.03.039. Epub 2016 Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jahromi BN, Parsanezhad ME, Shomali Z, Bakhshai P, Alborzi M, Vaziri NM, Anvar Z. Ovarian Hyperstimulation Syndrome: A Narrative Review of Its Pathophysiology, Risk Factors, Prevention, Classification, and Management. IJMS. 2018;43(3):248–260. [PMC free article] [PubMed] [Google Scholar]
- 70.Levi-Setti PE; Cirillo F; Scolaro V; Morenghi E; Heilbron F; Girardello D; Zannoni E; Patrizio P. Appraisal of clinical complications after 23,827 oocyte retrievals in a large assisted reproductive technology program. Fertil Steril. 2018:109(6):1038–1043.e1 [DOI] [PubMed] [Google Scholar]
- 71.Kawwass JF, Kissin DM, Kulkarni AD, Creanga AA, Session DR, Callaghan WM, Jamieson DJ. National ART Surveillance System (NASS) Group. Safety of assisted reproductive technology in the United States, 2000–2011. JAMA. 2015;313(1):88–90. doi: 10.1001/jama.2014.14488. [DOI] [PubMed] [Google Scholar]
- 72.Stern JE. Gopal D, Diop H, Missmer SA, Coddington CC, Luke B. Inpatient hospitalizations in women with and without assisted reproductive technology live birth. J Assist Reprod Genet. 2017. 2017;34(8):1043–1049.ePub June 1. doi: 10.1007/s10815-017-0961-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luke B, Brown MB, Spector LG, Missmer SA, Leach RE, Williams M, Koch L, Smith Y, Stern JE, Ball GD, Schymura MJ. Cancer in women after assisted reproductive technology. Fertil Steril. 2015;104(5):1218–26. doi: 10.1016/j.fertnstert.2015.07.1135. Epub 2015 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farland LV, Stern JE, Hwang SS, et al. Early-life cancer, infertility, and risk of adverse pregnancy outcomes: a registry linkage study in Massachusetts. Cancer Causes & Control : CCC. 2021;32(2):169–180. DOI: 10.1007/s10552-020-01371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farland LV, Stern JE, Hwang SS et al. History of cancer and fertility treatment outcomes: a registry linkage study in Massachusetts. J Assist Reprod Genet (2022). 10.1007/s10815021-02376-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coddington CC, Gopal D, Cui X, Cabral H, Diop H, Stern JE. Influence of subfertility and ART treatment on mortality of women after delivery. Fert.Steril 2020;113(3):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vassard D, Schmidt L, Pinborg A, Lindved Petersen G, Forman JL, Hageman I, Glazer CH & Kamper-Jørgensen M Mortality in women treated with assisted reproductive technology treatment: addressing the healthy patient effect, Amer Journal Epidemiol 2018:bind 187, nr. 9, s. 1889–1895. 10.1093/aje/kwy085 [DOI] [PubMed] [Google Scholar]
- 78.Dukhovny D, Hwang SS, Gopal D, Cabral H, Missmer S, Diop H, Declercq E, Stern JE. Length of stay and cost of birth hospitalization: effects of subfertility and ART. JPerinatol: 2018;38(11):1457–1465 doi.org/ 10.1038/s41372-018-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hwang SS, Dukhovny D, Gopal D, Cabral H, Farland LV, Stern JE. Sex differences in infant health following ART-treated, subfertile, and fertile deliveries. JARG. 2020. 10.1007/s10815020-02004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hwang S, Dukhovny D, Gopal D, Cabral H, Diop H, Coddington CC, Stern JE. Infant health and fresh versus frozen embryo transfer Fertility and Sterility. Fert. Steril 2019;112(5):900–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luke B, Brown MB, Wantman E, Forestieri NE, Browne ML, Fisher SC, Yazdy MM, Ethen MK, Canfield MA, Watkins S, Nichols HB, Farland LV, Oehninger S, Doody KJ, Eisenberg ML, Baker VL. The risk of birth defects with conception by ART. Hum Reprod. 2021;36(1):116–129. doi: 10.1093/humrep/deaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. 2013;19(4):330–353 [DOI] [PubMed] [Google Scholar]
- 83.Davies MJ, Rumbold AR, Marino JL, et al. Maternal factors and the risk of birth defects after IVF and ICSI: a whole of population cohort study. BJOG. 2017;124(10):1537–1544 [DOI] [PubMed] [Google Scholar]
- 84.Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A. Reproductive technologies and the risk of birth defects. N Engl J Med 2012;366(19):1803–1813 [DOI] [PubMed] [Google Scholar]
- 85.Liberman RF, Getz KD, Heinke D, Luke B, Stern JE, Declercq ER, Chen X, Lin AE, Anderka M. Assisted Reproductive Technology and Birth Defects: Effects of Subfertility and Multiple Births. Birth Defects Research. 2017;109(14):1144–1153. DOI: 10.1002/bdr2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen M, Heilbronn LK. The health outcomes of human offspring conceived by assisted reproductive technologies (ART). J Dev Orig Health Dis. 2017;8:388–402. [DOI] [PubMed] [Google Scholar]
- 87.Yeung EH, Sundaram R, Bell EM, Druschel C, Kus C, Xie Y, Buck Louis GM. Infertility treatment and children’s longitudinal growth between birth and 3 years of age. Hum Reprod 2016;31(7):1621–1628. DOI: 10.1093/humrep/dew106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bergh C, Wennerholm UB. Long-term health of children conceived after assisted reproductive technology. J Med Sci. 2020;125:252–257.. 10.1080/03009734.2020.1729904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ceelen M, van Weissenbruch MM, Prein J, Smit JJ, Vermeiden JPW, Spreeuwenberg M, van Leeuwen FE, V Delemarre-van de Waal HA. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8–18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod. 2009;24:2788–95. https://academic.oup.com/humrep/article/24/11/2788/627120. [DOI] [PubMed] [Google Scholar]
- 90.Hart R, Norman RJ. The longer-term health outcomes for children born as a result of IVF treatment: part I–General health outcomes. Hum Reprod Update 2013;19:232–43. https://academic.oup.com/humupd/article/19/3/232/727781. [DOI] [PubMed] [Google Scholar]
- 91.Shapiro-Mendoza C, Kotelchuck M, Barfield W, Davin CA, Diop H, Silver M, Manning SE. Enrollment in early intervention programs among infants born late preterm, early term, and term. Pediatrics. 2013;132(1):e61–69. doi: 10.1542/peds.2012-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barfield WD, Clements KM, Lee KG, Kotelchuck M, Wilber N, Wise PH. Using linked data to assess patterns of early intervention (EI) referral among very low birth weight infants. Matern Child Health J. 2008;12(1):24–33 [DOI] [PubMed] [Google Scholar]
- 93.Clements KM, Barfi eld WD, Kotelchuck M, Lee KG, Wilber N. Birth characteristics associated with early intervention referral, evaluation for eligibility, and program eligibility in the first year of life. Matern Child Health J. 2006;10(5):433–441 [DOI] [PubMed] [Google Scholar]
- 94.Clements KM, Barfi eld WD, Kotelchuck M, Wilber N. Maternal socio-economic and race/ethnic characteristics associated with early intervention participation. Matern Child Health J. 2008;12(6):708–717 [DOI] [PubMed] [Google Scholar]
- 95.Diop H, Gopal D, Cabral H, Belanoff C, Declercq ER, Kotelchuck M, Luke B, Stern JE. Assisted Reproductive Technology and Early Intervention Program Enrollment. Pediatrics. 2016. Mar;137(3):e20152007. doi: 10.1542/peds.2015-2007. Epub 2016 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu L, Gao J, He X, Cai Y, Wang L, Fan X. Association between assisted reproductive technology and the risk of autism spectrum disorders in the offspring: a meta-analysis. Sci Rep. 2017;7:46207. Published 2017 Apr 7. doi: 10.1038/srep46207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kissin DM, Zhang Y, Boulet SL, Fountain C, Bearman P, Schieve L, Yeargin-Allsopp M, Jamieson D. Association of assisted reproductive technology (ART) treatment and parental infertility diagnosis with autism in ART-conceived children. Hum Reprod. 2015;30(2):454–465. doi: 10.1093/humrep/deu338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grether JK, Qian Y Croughan MS, Wu YW, Schembri M, Camarano L Croen LA. Is infertility associated with childhood autism? Journal of Autism and Developmental Disorders 2013;43(3):663–672. [DOI] [PubMed] [Google Scholar]
- 99.Schieve LA, Tian LH, Rankin K,Kogan MD, Yeargin-Allsopp M, Visser S, Rosenberg D. Population impact of preterm birth and low birth weight on developmental disabilities in US children. Ann Epidemiol. 2016;26(4):267–274. doi: 10.1016/j.annepidem.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diop H, Cabral H, Gopal D, Cui X, Stern JE, Kotelchuck M Early Autism Spectrum Disorders in Children Born to Fertile, Subfertile, and ART-Treated Women. Mat Child Health Journal. 2019. June 20, 2019: 10.1007/s10995-019-02770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stern JE, Liu CL, Hwang SS, Dukhovny D, Diop H, Cabral H. Contributions to prematurity of maternal health conditions, subfertility, and assisted reproductive technology. Fertil Steril. 2020;114(4):828–836. doi: 10.1016/j.fertnstert.2020114(4): 828–836. doi: 10.1016/j.fertnstert.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stern JE, Liu CL, Hwang S, Dukhovny D, Farland LV, Coddington CC, Cabral HJ. Influence of placental abnormalities and pregnancy induced hypertension in prematurity associated with assisted reproductive technology techniques. J. Clin. Med 2021;10(8);1681 JCM | Free Full-Text | Influence of Placental Abnormalities and Pregnancy-Induced Hypertension in Prematurity Associated with Various Assisted Reproductive Technology Techniques (mdpi.com). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schachter M, Tovbin Y, Arieli S, Friedler S, Ron-El R, Sherman D. In vitro fertilization is a risk factor for vasa previa. Fertil Steril 2002;78:642–3. [DOI] [PubMed] [Google Scholar]
- 104.Luke B, Brown MB, Wantman E, Seifer DB, Sparks AT, Lin PC, et al. Risk of prematurity and infant morbidity and mortality by maternal fertility status and plurality. J Assist Repro Genet 2019;36:121–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Esh-Broder E, Ariel I, Abas-Bashir N, Bdolah Y, Celnikier DH. Placenta accreta is associated with IVF pregnancies: a retrospective chart review. BJOG. 2011;118(9):1084–9. doi: 10.1111/j.14710528.2011.02976.x. Epub 2011 May 18. [DOI] [PubMed] [Google Scholar]
- 106.Xiang M, Chen S, Zhang X, Ma Y. Placental diseases associated with assisted reproductive technology. Reprod Biol. 2021;21(2):100505. doi: 10.1016/j.repbio.2021.100505, 10.1016/j.repbio.2021.100505 [DOI] [PubMed] [Google Scholar]
- 107.Tanaka H, Tanaka K, Osato K, Kusaka H, Maegawa Y, Taniguchi H, Ikeda T. Evaluation of Maternal and Neonatal Outcomes of Assisted Reproduction Technology: A Retrospective Cohort Study. Medicina (Kaunas). 2020;56(1):32. doi: 10.3390/medicina56010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kyozuka H, Yamaguchi A, Suzuki D, et al. Risk factors for placenta accreta spectrum: findings from the Japan environment and Children’s study. BMC Pregnancy Childbirth. 2019;19(1):447. Published 2019 Nov 27. doi: 10.1186/s12884-019-2608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagata C, Yang L, Yamamoto-Hanada K, Mezawa H, Ayabe T, Ishizuka K, Konishi M, Ohya Y, Saito H, Sago H; Japan Environment & Children’s Study Group. Complications and adverse outcomes in pregnancy and childbirth among women who conceived by assisted reproductive technologies: a nationwide birth cohort study of Japan environment and children’s study. BMC Pregnancy Childbirth. 2019;19(1):77. doi: 10.1186/s12884-019-2213-y, 10.1186/s12884-019-2213-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ganer Herman H, Mizrachi Y, Farhadian Y, Shevach Alon A, Gluck O, Bar J, Kovo M, Raziel A. Placental disorders of pregnancy in subsequent IVF pregnancies - a sibling cohort. Reprod Biomed Online. 2021;42(3):620–626. doi: 10.1016/j.rbmo.2020.11.018, 10.1016/j.rbmo.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 111.Daniel Y, Schreiber L, Geva E, Amit A, Pausner D, Kupferminc MJ, Lessing JB. Do placentae of term singleton pregnancies obtained by assisted reproductive technologies differ from those of spontaneously conceived pregnancies? Hum Reprod. 1999. Apr;14(4):1107–10. doi: 10.1093/humrep/14.4.1107. [DOI] [PubMed] [Google Scholar]
- 112.Wang YX, Yue LF, Zhang JW, Xiong YW, Hu JJ, Wang LL, Li Z, Liu Y, Yang L, Sun LJ. Expression and DNA Methylation Status of the Imprinted Genes PEG10 and L3MBTL1 in the Umbilical Cord Blood and Placenta of the Offspring of Assisted Reproductive Technology. Reprod Sci. 2021;28(4):1133–1141. doi: 10.1007/s43032-020-00417-x, 10.1007/s43032-020-00417-x [DOI] [PubMed] [Google Scholar]
- 113.Vincent RN, Gooding LD, Louie K, Chan Wong E, Ma S. Altered DNA methylation and expression of PLAGL1 in cord blood from assisted reproductive technology pregnancies compared with natural conceptions. Fertil Steril. 2016;106(3):739–748.e3. doi: 10.1016/j.fertnstert.2016.04.036, 10.1016/j.fertnstert.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 114.Marjonen H, Auvinen P, Kahila H, Tšuiko O, Kõks S, Tiirats A, Viltrop T, Tuuri T, Söderström-Anttila V, Suikkari AM, Salumets A, Tiitinen A, Kaminen-Ahola N. rs10732516 polymorphism at the IGF2/H19 locus associates with genotype-specific effects on placental DNA methylation and birth weight of newborns conceived by assisted reproductive technology. Clin Epigenetics. 2018;10:80. doi: 10.1186/s13148-018-0511-2, 10.1186/s13148-018-0511-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carusi DA, Gopal D, Cabral HJ, Bormann CL, Racowsky CR, Stern JE. A unique placenta previa risk factor profile for pregnancies conceived with assisted reproductive technologies. Fertil Steril submitted [DOI] [PubMed] [Google Scholar]


