Abstract
The Lyme disease spirochete Borrelia burgdorferi expresses diverse subsurface yet antigenically cross-reactive Bdr protein paralogs from distinct circular- and linear-plasmid loci. We assessed the possible effects of in vitro and in vivo growth on bdr locus structure, searching for recombinational events leading to either deletions or insertions of central repeat units or novel amino- and carboxy-terminus combinations. Our data indicate that, apart from plasmid loss during in vitro cultivation, the bdr paralog loci of strain B31 are stable. This suggests that recombinatorial variation of bdr genes is not essential for persistent mammalian infection.
The genomic sequence of the spirochete Borrelia burgdorferi, the etiologic agent of Lyme disease, revealed a host of paralogous gene families (9, 13). In a previous study, we characterized the Bdr (Borrelia direct repeat) protein family (paralogous gene family 80 [9, 13, 40]). bdr paralogs are carried on multiple circular plasmids (cp's) and linear plasmids (lp's) of Borrelia burgdorferi sensu lato isolates (1, 2, 9, 13, 20, 29, 31, 39; see also the Borrelia burgdorferi Genome Database at http://www.tigr.org). Orthologs have been found on lp's and cp's of the causative agents of relapsing fever Borrelia hermsii (B. Stevenson, personal communication) and Borrelia turicatae (8).
B. burgdorferi Bdr proteins are expressed at low levels in a temperature-independent manner and are not surface exposed. Yet they are recognized by the humoral immune response during Lyme disease (40). Protein sizes range from approximately 20 to 30 kDa, depending on the number of short direct repeats in a central “variable number of tandem repeat” (VNTR) (17, 33) domain. Different combinations of N and C termini of Bdrs carried by distinct plasmid loci yield several distinct homology groups. Nevertheless, the proteins give rise to cross-reacting antibodies, likely due to the more conserved VNTR domains. We were therefore able to detect Bdr proteins in a variety of B. burgdorferi sensu lato isolates as well as B. turicatae and B. hermsii using an antibody against B. burgdorferi B31 BdrA. Each of the isolates had a specific reactive protein pattern (40).
On the multiple 32-kb circular plasmids (cp32's) and the related 56-kb linear plasmid lp56 of B. burgdorferi strain B31, bdr paralogs are in two distinct loci (2.9-like [20] and ORF-1-2-C-3-E [10, 38, 39]) separated by approximately 5 kb. Single bdr loci are on lp28-1, lp28-2, lp28-3, lp36, and lp38, some of which carry bdr pseudogenes unlikely to encode functional proteins (9, 13). There is evidence that the different paralog loci have undergone recombination in the past. (i) In two of the 2.9-like cp32 loci, on cp32-1 and cp32-6, a bdr paralog has been replaced by an unrelated rev paralog (20). (ii) The varying numbers of repeats in the bdr VNTR domains appear to indicate that the different paralogs have evolved through deletions and/or insertions of repeat units. This could be the result of recombination between multiple loci or of slipped-strand mispairing in combination with inadequate DNA mismatch repair pathways (33). (iii) Different combinations of N- and C-terminal domains suggest recombination events in the VNTR domain as well (40). However, it is unknown whether these rearrangements are the result of recent recombinatorial events and whether they occur in vivo. To address these questions, we analyzed the effect of prolonged in vitro or in vivo growth on the bdr loci. The assays were designed to detect (i) recombinations within the bdr VNTR domains leading to insertions and/or deletions of repeat units and/or novel N- and C-terminus combinations and (ii) variations in bdr-flanking regions. The results indicate that, in the absence of plasmid loss, bdr loci are stable during both in vitro and in vivo growth.
In vitro and in vivo growth of B. burgdorferi B31 strains.
Three B. burgdorferi strain B31 clones were used. B31-ATCC and B313 are in vitro-passaged clones and have been analyzed in earlier studies (23, 40). Frozen stocks of B31-5A3, a low-passage-number infectious clone (18), and B31-5A3-derived mouse isolates were a gift from S. J. Norris (University of Texas Medical School, Houston). These isolates had been produced in the course of previous studies by Zhang et al. (35–37) and had revealed extensive segmental recombinations in the vlsE locus on lp28-1 during in vivo growth. Briefly, eight immunocompetent C3H/HeN mice were infected with B31-5A3, which had previously undergone no more than three in vitro passages since cloning. One year postinfection, samples of heart, bladder, and skin were inoculated into BSKII medium (4), the B. burgdorferi cultures were grown at 34°C, and aliquots were frozen in 15% glycerol at −70°C. A total of 17 isolates were obtained: 8 heart isolates (5A3-1487 to 5A3-1494), 7 bladder isolates (5A3-1495 to 5A3-1501) and 2 skin isolates (5A3-1502 and 5A3-1503) (35–37). After thawing, the B. burgdorferi isolates were passaged once in BSKII at 34°C and total bacterial DNA was isolated using the DNeasy tissue kit (Qiagen).
Computer predictions of PCR amplicon and restriction fragment length polymorphism (RFLP) fragment sizes.
B. burgdorferi B31 DNA sequences were downloaded from the Institute for Genomic Research B. burgdorferi genome server (http://www.tigr.org). Based on alignments of bdr paralog sequences using ClustalX version 1.62b (32), degenerate and compatible PCR primers were designed with the help of the OLIGO primer analysis software version 4.04 (National Biosciences, Inc.) (22) and amplicon sizes were calculated (Table 1). Restriction endonuclease fragments of individual circular and linear plasmids (Table 1) were predicted using the MacDNASIS Pro program suite version 3.7 (Hitachi Software Engineering).
TABLE 1.
Predicted sizes of B. burgdorferi B31 bdr paralog PCR amplicons and Southern blot restriction fragments
| Paralog | Locus | Locus presence in B31 clonea
|
PCR primerb
|
PCR amplicon size (bp) | XbaI restriction fragment size (bp) | |||
|---|---|---|---|---|---|---|---|---|
| 5A3 | ATCC | B313 | Fwd | Rev | ||||
| bdrK | cp32-6 | + | − | − | A | F | 571 | 8,650 |
| bdrC/Mc | cp32-2/7 | + | + | + | A | F | 475 | 3,048 |
| bdrQ | cp32-9 | + | + | − | A | F | 442 | 9,368 |
| bdrG | cp32-4 | + | + | + | A | F | 289 | 3,074 |
| bdrA | cp32-1 | + | + | + | A | G | 519 | 5,788 |
| bdrE | cp32-3 | − | + | + | A | G | 477 | 8,499 |
| bdrO | cp32-8 | + | − | − | A | G | 465 | 5,946 |
| bdrV | lp56 | − | − | − | A | G | 423 | 7,927 |
| bdrW | lp56 | − | − | − | B | H | 581 | 9,869 |
| bdrF | cp32-3 | − | + | + | B | H | 494 | 5,257 |
| bdrD/Nc | cp32-2/7 | + | + | + | B | H | 473 | 4,762 |
| bdrP | cp32-8 | + | − | − | B | H | 440 | 4,743 |
| bdrR | cp32-9 | + | + | − | B | H | 440 | 4,717 |
| bdrH | cp32-4 | + | + | + | B | H | 386 | 4,659 |
| bdrT | lp28-2 | − | + | − | B | I | 665 | 4,165 |
| bdrU | lp28-3 | + | + | − | B | I | 534 | 8,841 |
| bdrSd | lp28-1 | + | + | − | B | Id | 450d | 1,422 |
| bdrIe | cp32-5 | NDf | + | − | ND | ND | ND | ND |
| bdrJe | cp32-5 | ND | + | − | ND | ND | ND | ND |
+, presence; −, absence. For B31-ATCC and B313, plasmid profiles were determined independently in a previous study using plasmid-specific probes and PCR primers (40). The plasmid profile of B31-5A3 was deduced from the observed bdr PCR amplicon sizes.
As described in the legend to Fig. 1A.
B31-ATCC and B313 carry cp32-2 and not cp32-7 (40). bdrC and bdrD sequences are not in the database, since cp32-2 has not been sequenced in its entirety. The available sequences, however, indicate that cp32-2 and cp32-7 are almost identical (and in fact may be incompatible [28]). We therefore assume that their bdr paralogs are very similar if not identical.
bdrS is considered a nonfunctional pseudogene, since it does not possess a consensus Shine-Dalgarno ribosomal binding site upstream of the start codon (9, 13, 40). bdrS and bdrY, a pseudogene on lp38, were not considered at the point of primer design. Although primer B-fwd is complementary to the bdrS locus, I-rev is complementary for only 7 bases at the 3′ end, making PCR amplification unlikely under the conditions used. bdrS is, however, detected by Southern hybridization (Fig. 2). bdrY and a distant relative of the bdr family, bdrX on lp36, are neither amplified by PCR nor detected by Southern hybridizations.
bdrM and bdrN sequences are not in the database, since cp32-5 was not sequenced in its entirety. Amplicon and fragment sizes can therefore not be predicted. However, PCR amplicons and RFLP fragments likely corresponding to the cp32-5 paralogs can be seen in B31-ATCC (see the text and Fig. 1B and 2).
ND, not done.
Stability of the bdr VNTR domain.
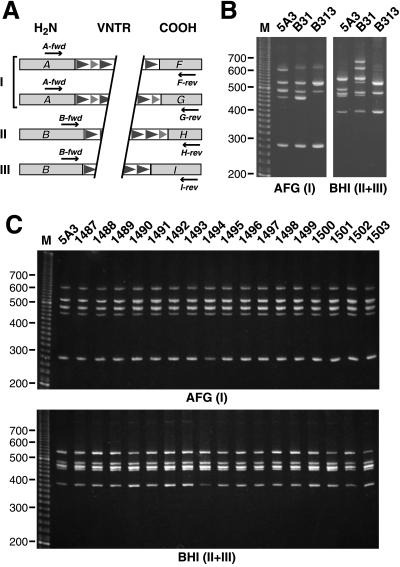
We assessed possible variations in the VNTR domains by multiplex PCR amplification of bdr paralogs followed by separation on high-resolution polyacrylamide gels. As mentioned above, Bdr protein sequences differ mainly in their N and C termini, which has led to their division into several homology groups. B. burgdorferi B31 carries bdr paralogs which belong to homology groups I (N- and C-terminal group A and groups F and G, respectively), II (B and H), and III (B and I) (40). Oligonucleotide primers specific for N- and C-terminal groups A and B and groups F, G, H, and I were designed for the separate or combined amplification of B. burgdorferi B31 bdr homology groups I, II, and III (see above) (Fig. 1A). bdr paralogs were amplified from total bacterial DNA using Taq polymerase (Boehringer Mannheim). PCR conditions in a Hybaid DNA thermal cycler consisted of 30 cycles of 94°C for 15 s, 49°C for 30 s, and 72°C for 45 s. Amplification products were separated in a nondenaturing 5% polyacrylamide gel using 1× Tris-borate-EDTA (TBE) as running buffer and visualized with ethidium bromide (3). This allowed separation of fragments which differ by less than 20 bp, thereby permitting the detection of possible differences in the number of short (21-bp) or long (33-bp) direct repeat units.
FIG. 1.
Multiplex PCR assay indicating stability of the bdr VNTR domains. (A) Amplification scheme. bdr VNTR domains were amplified by using oligonucleotide primers specific for the N- and C-terminal homology groups A or B and F, G, H, or I (40), respectively. Primer sequences (5′ to 3′) are as follows: A-fwd, WRGGRTTTAGYRARGARGCAATAGAT; F-rev, ATTGGRGCAA RYGTTATTCCCATTAT; G-rev, GAATYGCTGCTGTTAWTATCATAAAATGTA; B-fwd, CWCAAGATTTRTCWAAAAGATATTATCACAATGA; H-rev, CCCKGCTATCATTGTKATAGACATTGC; I-rev, ATATAGCARTGGGMACWAYTAYTACTG. Degenerate nucleotides are indicated according to International Union of Pure and Applied Chemistry nomenclature as follows: R = A + G, Y = C + T, W = A + T, K = G + T, M = A + C. (B and C) PCR amplicons obtained with primer combinations A-fwd/F-rev/G-rev (AFG) or B-fwd/H-rev/I-rev (BHI) by using either three B31 clones (B) or 17 isolates obtained 1 year after infection of mice with B31-5A3 (C) were separated on a 5% nondenaturing polyacrylamide gel. A 20-bp ladder (Gibco) served as the size marker.
Plasmid loss during in vitro passage has been observed repeatedly (6, 16, 18, 19, 24, 34) and has led to different plasmid profiles of the three B31 clones used in this work (Table 1). As Fig. 1B shows, there are differences in the amplicon pattern between B31-5A3, B31-ATCC, and B313. Yet the product sizes match those of the expected bdr paralogs on plasmids carried by the individual clone. For example, compared to B31-ATCC, B313 lacks lp28-2 (23) and thus the bdrT amplicon. Please note that the unsequenced cp32-5 paralogs bdrI and bdrJ likely account for two of the additional amplicons seen with B31-ATCC, e.g., the approximately 620-bp B-fwd/H-rev/I-rev product. In contrast, amplicon patterns of the mouse tissue isolates after 1 year of infection were identical to those of the original B31-5A3 clone used for infection (Fig. 1C). This indicates that the bdr VNTR domains are stable after even prolonged in vivo growth.
Assaying all isolates by PCR using B-fwd, F-rev, and G-rev primers, we were also unable to detect intraplasmidic recombinations of the bdr paralogs carried by the two cp32 loci (not shown). This kind of recombinational event would likely be subject to negative selection: it would delete the putative plasmid replication-partitioning gene locus (ORF-1-2-C-3) (10, 28, 38, 39) and might lead to plasmid loss.
Stability of flanking regions.
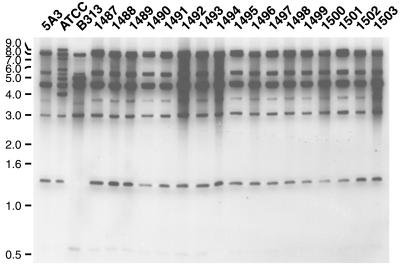
To assess possible variations in bdr-flanking regions we used an RFLP-based approach. Total bacterial DNA was digested separately with HindIII and XbaI, and the resulting fragments were separated on 0.7 to 1.0% agarose gels in 0.5× TBE (3). Subsequently, the DNA was transferred to positively charged nylon membranes (Immobilon-Ny+; Millipore) by alkaline transfer (21). bdr PCR amplicons (see above) were pooled at equivalent concentrations, labeled with horseradish peroxidase by using the ECL direct labeling kit (Amersham Pharmacia), and used as probes. Southern blot hybridizations and subsequent washes were carried out in glass tubes in a Autoblot mini-hybridization oven (Bellco) at 42°C under low-stringency conditions (0.5× SSC in primary wash; 20× SSC is 0.3 M sodium citrate plus 3 M NaCl, pH 7).
Figure 2 shows the results obtained from the XbaI digest. As expected from their different plasmid profiles (Table 1), the three B31 clones obtained before or after prolonged in vitro passage differ in their RFLP pattern. As was the case for the PCR assay described above, the hybridizing restriction fragments match the predicted bdr-carrying restriction fragments. For example, B313 lacks the bdrS (lp28-1) and bdrT (lp28-2) fragments. The additional fragments in B31-ATCC, e.g., a 6-kb fragment, are likely to originate from the cp32-5 paralog(s) that has yet to be sequenced. In contrast, the mouse tissue B31-5A3-derived isolates have an RFLP pattern identical to that of the original B31-5A3 clone. Note that the upper band of 8- to 9-kb doublets apparently missing in the original clone is readily detected in longer film exposures (not shown). Together, these data indicate that the bdr loci and their flanking sequences are stable.
FIG. 2.
RFLP analysis indicating stability of the bdr-flanking regions. Southern blots of total B. burgdorferi DNA digested with HindIII (data not shown) and XbaI were probed with a pool of PCR-derived bdr probes. Three in vitro-cultured B31 clones (5A3, ATCC, and B313) and 17 isolates obtained 1 year after infection of mice with B31-5A3 (1487 to 1503) are shown. A 1-kb ladder (Gibco) served as the size marker.
Potential applications in strain typing.
The fact that Bdr proteins have sequence heterogeneity but also genus-wide antigenic cross-reactivity (40) is remarkable and potentially useful. We have previously speculated that the VNTR domains with their more-conserved direct repeat units are mainly responsible for this property. It is interesting that in another type of plasmid-encoded B. burgdorferi antigen with short direct repeats, VraA (26), the repetitive region has been determined to be the antigenic domain.
Originally used as markers for human genetic mapping (17), VNTR loci have since been identified in a number of bacterial species (33). Recently, they have been used to study the genetic diversity of Mycobacterium tuberculosis (14). Since all bdr paralogs so far identified are plasmid encoded, we surmise that Bdr protein and plasmid profiles will correspond closely. The stability of bdr loci in vivo and the previously shown cross-reactivity of antibodies could make anti-Bdr-based immunoblots or bdr-based PCR assays alternative tools for plasmid profiling and strain typing. They might be especially useful in quickly assessing possible variations of complex Borrelia populations present in vector ticks or mouse reservoirs.
Conclusion.
Several previous studies have shown that B. burgdorferi adapts to different environments by regulating its surface protein expression. The majority of the involved proteins are plasmid encoded. One example of adaptation is the differential expression of outer surface proteins OspA and OspC in the tick vector and the mammalian host (25, 30). The extensive recombinatorial variation of the B. burgdorferi vlsE genes in vivo, notably shown with clones from the isolates used here (35–37), is another example. This phenomenon is similar to the variation of variable major protein gene families (vsp and vlp) in relapsing fever Borrelia spp. (5, 7, 15).
The Bdr protein paralogs appear to play a different role in Borrelia. In an earlier characterization we concluded that the proteins are targeted to the spirochete's cytoplasmic membrane and expressed concurrently at low levels under a variety of conditions (40). This suggested that the Bdr proteins are not under selective pressure during infection of vertebrates. The stability of bdr loci in vivo demonstrated here supports this. In comparison to the extensive recombination in the vlsE expression locus on lp28-1 (35, 36), which can be detected as early as 4 days after infection (37), the bdr loci remain stable over a year in a natural environment. Studies of the Erp proteins, another protein family encoded on the cp32's, have yielded similar results. Although the Erps are antigenic surface lipoproteins (27) and erp loci show evidence of past recombination (28), they were stable during in vivo experiments similar to the ones presented here (12). The biological function of the majority of the cp32-encoded proteins, including the Bdrs, remains unknown. The recent demonstration that the cp32's indeed represent prophage DNA of polyhedral tailed bacteriophages (11) will certainly encourage further studies of these molecules as well as the proteins they encode.
Acknowledgments
We are grateful to Steven J. Norris for providing the B31-5A3 mouse isolates and for comments on the manuscript. We thank Brian Stevenson and Scott Samuels for helpful discussions and Jerri Howell and Hanna Kim for technical assistance.
This work was supported by the Centers for Disease Control and Prevention (Cooperative Agreement U50/CCU914771) and the National Institutes of Health (grant AI24424).
REFERENCES
- 1.Akins D R, Bourell K W, Caimano M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins D R, Caimano M J, Yang X, Cerna F, Norgard M V, Radolf J D. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect Immun. 1999;67:1526–1532. doi: 10.1128/iai.67.3.1526-1532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 4.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour A G. Linear DNA of Borrelia species and antigenic variation. Trends Microbiol. 1993;1:236–239. doi: 10.1016/0966-842x(93)90139-i. [DOI] [PubMed] [Google Scholar]
- 6.Barbour A G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988;26:475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadavid D, Pennington P M, Kerentseva T A, Bergström S, Barbour A G. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–3360. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlyon J A, Marconi R T. Cloning and molecular characterization of a multicopy, linear plasmid-carried, repeat motif-containing gene from Borrelia turicatae, a causative agent of relapsing fever. J Bacteriol. 1998;180:4974–4981. doi: 10.1128/jb.180.18.4974-4981.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. Fraser. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol., in press. [DOI] [PubMed]
- 10.Dunn J J, Buchstein S R, Butler L-L, Fisenne S, Polin D S, Lade B N, Luft B J. Complete nucleotide sequence of a circular plasmid from the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1994;176:2706–2717. doi: 10.1128/jb.176.9.2706-2717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggers C H, Samuels D S. Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J Bacteriol. 1999;181:7308–7313. doi: 10.1128/jb.181.23.7308-7313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Hage N, Lieto L D, Stevenson B. Stability of erp loci during Borrelia burgdorferi infection: recombination is not required for chronic infection of immunocompetent mice. Infect Immun. 1999;67:3146–3150. doi: 10.1128/iai.67.6.3146-3150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 14.Frothingham R, Meeker-O'Connell W A. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998;144:1189–1196. doi: 10.1099/00221287-144-5-1189. [DOI] [PubMed] [Google Scholar]
- 15.Hinnebusch B J, Barbour A G, Restrepo B I, Schwan T G. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect Immun. 1998;66:432–440. doi: 10.1128/iai.66.2.432-440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes C A, Engstrom S M, Coleman L A, Kodner C B, Johnson R C. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect Immun. 1993;61:5115–5122. doi: 10.1128/iai.61.12.5115-5122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura Y, Leppert M, O'Connell P, Wolff R, Holm T, Culver M, Martin C, Fujimoto E, Hoff M, Kumlin E, White R. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987;235:1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- 18.Norris S J, Howell J K, Garza S A, Ferdows M S, Barbour A G. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect Immun. 1995;63:2206–2212. doi: 10.1128/iai.63.6.2206-2212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persing D H, Mathiesen D, Podzorski D, Barthold S W. Genetic stability of Borrelia burgdorferi recovered from chronically infected immunocompetent mice. Infect Immun. 1994;62:3521–3527. doi: 10.1128/iai.62.8.3521-3527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porcella S F, Popova T G, Akins D R, Li M, Radolf J D, Norgard M V. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein family. J Bacteriol. 1996;178:3293–3307. doi: 10.1128/jb.178.11.3293-3307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed K C, Mann D A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985;13:7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rychlik W, Rhoads R E. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res. 1989;17:8543–8551. doi: 10.1093/nar/17.21.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadziene A, Wilske B, Ferdows M S, Barbour A G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993;61:2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skare J T, Foley D M, Hernandez S R, Moore D C, Blanco D R, Miller J N, Lovett M A. Cloning and molecular characterization of plasmid-encoded antigens of Borrelia burgdorferi. Infect Immun. 1999;67:4407–4417. doi: 10.1128/iai.67.9.4407-4417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson B, Casjens S, Rosa P. Evidence of past recombination events among the genes encoding the Erp antigens of Borrelia burgdorferi. Microbiology. 1998;144:1869–1879. doi: 10.1099/00221287-144-7-1869. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson B, Casjens S, van Vugt R, Porcella S F, Tilly K, Bono J L, Rosa P. Characterization of cp18, a naturally truncated member of the cp32 family of Borrelia burgdorferi plasmids. J Bacteriol. 1997;179:4285–4291. doi: 10.1128/jb.179.13.4285-4291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theisen M. Molecular cloning and characterization of nlpH, encoding a novel, surface-exposed, polymorphic, plasmid-encoded 33-kilodalton lipoprotein of Borrelia afzelii. J Bacteriol. 1996;178:6435–6442. doi: 10.1128/jb.178.22.6435-6442.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Belkum A, Scherer S, van Alphen L, Verbrugh H. Short-sequence DNA repeats in prokaryotic genomes. Microbiol Mol Biol Rev. 1998;62:275–293. doi: 10.1128/mmbr.62.2.275-293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Johnson R C. Analysis and comparison of plasmid profiles of Borrelia burgdorferi sensu lato strains. J Clin Microbiol. 1995;33:2679–2685. doi: 10.1128/jcm.33.10.2679-2685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J R, Norris S J. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect Immun. 1998;66:3698–3704. doi: 10.1128/iai.66.8.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J R, Norris S J. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun. 1998;66:3689–3697. doi: 10.1128/iai.66.8.3689-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zückert W R, Filipuzzi-Jenny E, Stålhammar-Carlemalm M, Meister-Turner J, Meyer J. Repeated DNA sequences on circular and linear plasmids of Borrelia burgdorferi sensu lato. In: Axford J S, Rees D H E, editors. Lyme borreliosis. Vol. 260. New York, N.Y: Plenum; 1994. pp. 253–260. [Google Scholar]
- 39.Zückert W R, Meyer J. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J Bacteriol. 1996;178:2287–2298. doi: 10.1128/jb.178.8.2287-2298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zückert W R, Meyer J, Barbour A G. Comparative analysis and immunological characterization of the Borrelia Bdr protein family. Infect Immun. 1999;67:3257–3266. doi: 10.1128/iai.67.7.3257-3266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]