Abstract
Emerging perfluoroalkyl and polyfluoroalkyl substances contaminate waters at trace concentrations, thus rapid and selective adsorbents are pivotal to mitigate the consequent energy-intensive and time-consuming issues in remediation. In this study, coal combustion residuals-fly ash was modified (FA-SCA) to overcome the universal trade-off between high adsorption capacity and fast kinetics. FA-SCA presented rapid adsorption (teq = 2 min) of PFOX (perfluorooctanoic acid and perfluorooctanesulfonic acid, collectively), where the dynamic adsorption capacity (qdyn = qm/teq) was 2–3 orders of magnitude higher than that of benchmark activated carbons and anion-exchange resins. Investigated by advanced characterization and kinetic models, the fast kinetics and superior qdyn are attributed to (1) elevated external diffusion driven by the submicron particle size; (2) enhanced intraparticle diffusion caused by the developed mesoporous structure (Vmeso/Vmicro = 8.1); (3) numerous quaternary ammonium anion-exchange sites (840 μmol/g), and (4) appropriate adsorption affinity (0.031 L/μmol for PFOS, and 0.023 L/μmol for PFOA). Since the adsorption was proven to be a synergistic process of electrostatic and hydrophobic interactions, effective adsorption ([PFOX]ini = 1.21 μM, concentration levels of highly-contaminant-sites) was obtained at conventional natural water chemistries. High selectivity (>85.4% removal) was also achieved with organic/inorganic competitors, especially compounds with partly similar molecular structures to PFOX. In addition, >90% PFOX was removed consistently during five cycles in mild regeneration conditions (pH 12 and 50 °C). Overall, FA-SCA showed no leaching issues of toxic metals and exhibits great potential in both single-adsorption processes and treatment train systems.
Keywords: PFAS removal, Rapid adsorption, Fly ash, Co-existing competitors, Quaternary ammonium
1. Introduction
Emerging perfluoroalkyl and polyfluoroalkyl substances (PFAS) are groups of ca. 4000 synthetic chemicals characterized by their highly fluorinated carbon chain, and have large-scale demands and industrial applications [1]. The adverse health effects of PFAS have triggered great concerns since the first environmental-related report in 2001 [2], which are exaggerated due to their persistence, widespread exposure, and bioaccumulation [3]. As the most ubiquitous PFAS, perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), named as PFOX collectively, have been monitored at concentrations from low ng/L in natural waters to thousands of μg/L in highly contaminated sites [4-6]. More important, the drinking water supplies for nearly 6 million U.S. residents was reported to exceed the US Environmental Protection Agency’s drinking water advisory level for combined PFOX (70 ng/L) [7]. The rigid regulations and severe situation require the development of effective treatment methods for migration control and sites remediation urgently [8].
Due to the strong C-F bond dissociation energy (ca. 513 kJ/mol) [9], the conventional biodegradation and chemical degradation methods, in wastewater treatment plants (WWTPs), presented ineffective remediation of PFAS, and hence adsorption is the preferred method globally [1,10]. Although advanced degradation methods, such as photocatalysis and UV assisted-advanced oxidation/reduction, have been established to breakdown C-F bond effectively [11-13], individual degradation methods suffer from being energy-intensive with high-costs and extreme operation conditions, especially toward trace PFAS concentrations [14]. Through merging adsorption and advanced degradation methods as a treatment train system [14,15], nearly 90% pre-adsorbed PFOA was degraded in 240 min under UV irradiation [16] since the adsorption process greatly enhanced the local concentration of PFAS for catalysis process. The advantages of combined process also highlight the importance of adsorption methods. As the most studied adsorbents of PFAS remediation, activated carbons (both Powdered activated carbon (PAC) and Granular activated carbon (GAC)) and Anion-exchange resins (AE resins) exhibit merits in effective removal performance and ease of use, however, the slow adsorption kinetics (equilibrium time teq range from 720 to 1440 min) were also observed due to their micro-scale particle size and severe intraparticle diffusion resistance for PFAS molecules [17,18]. Therefore, the fast adsorption kinetic would save the operation time to reach equilibria faster in a batch adsorption process or enhance the overall degradation rates in the combined adsorption/degradation treatment train systems.
A new class of amine-modified adsorbents, including amines/quaternary ammonium-modified hydrogels [19], clays [8] and Covalent organic frameworks (COFs) [20], have been designed for the rapid removal of PFAS. Regarding to the surfactant-like structure of PFAS (negatively charged head group and hydrophobic tail), the major drivers for adsorption are generally electrostatic and hydrophobic interactions [21,22]. Thus, the introduction of cationic center via amine-functionalization could strength the ionic interaction, enabling the rapid dynamic adsorption (teq = 30–120 min). However, unstable adsorption dynamics were observed using amine-modified COFs: up to 67% PFAS were desorbed simultaneously when the experimental time was increased [23]. Since the molecular length of PFOA and PFOS were reported as ca. 1.0 nm and 1.2 nm, respectively [24], the small pore-size (2.6–3.4 nm via BET analysis) would cause major PFAS adsorption on the exterior surface rather than diffusing into the interior pores, resulting in the enhanced desorption tendency. More importantly, the use of advanced amine-modified adsorbents is further limited by the trade-off between fast adsorption dynamic and high equilibrium adsorption capacity, as well as the limited selectivity in the presence of inorganic and organic competitors from water matrices [4,25,26]. Hence, further optimization among adsorption incentives, dynamic adsorption capacity, and sorbent selectivity, were required for effective PFAS removal [27].
In this study, industrial waste coal fly ash (FA) was modified to address beforementioned limitations on PFAS removal (PFOX as model compounds). To overcome the bottleneck of combined high adsorption capacity and fast kinetics, modification methods, such as hydrothermal and silane coupling, were applied to develop mesoporous structure and to introduce quaternary ammonium groups (FA-SCA), respectively. As discussed in recent review articles of PFAS removal [25,28], considerable studies were limited on deionized water background and above-realistic PFAS concentrations (105-108 higher than environmentally relevant concentrations), where the studies of adsorption mechanism were based on unrealistic conditions and the significant influences of co-existing organic matters and inorganic ions was ignored. Hence, as suggested, the developed FA-SCA was tested, with initial PFOX concentration of highly-contaminated-sites level (1.21 μM), in a synthetic lake water (pH 7.5, conductivity: 642 μS/cm). The ionic background could control the simultaneous effect between ionic strength and co-existing competitors, leading to a better evaluation of water matrices as well as the selectivity of designed FA-SCA on PFOX removal. Furthermore, advanced characterization and kinetic models were applied to elucidate the mechanisms of combined fast kinetic and high adsorption capacity.
2. Material and methods
2.1. Materials
FA was obtained from a power plant owned by the East Kentucky Cooperative. The trimethyl-3-(trimethoxysilyl) propylammonium chloride (TTPAC), cetrimonium bromide (CTAB), Sodium dodecyl sulfate (SDS), octanoic acid, sodium gluconate, 3-(perfluorobutyl)propanol and inorganic salts; as well as the feed stocks and analytical standards of PFOA, potassium perfluorooctanesulfonate, perfluorobutanoic acid (PFBA), perfluoropentanoic acid (PFPeA), and hexafluoropropylene oxide dimer acid (HFPO-DA) were purchased from Shanghai Macklin Biochemical Co., Ltd. The benchmark adsorbents powdered activated carbon (C829813, 400 mesh), and anion-exchange resin (Amberlite IRA400 chloride form), with polystyrene matrix, were obtained from Shanghai Macklin Biochemical Co., Ltd., and Shanghai Energy Chemical Co., Ltd., respectively. Suwannee river humic acid (3S101H) and fulvic acid (2S101F) were obtained from the International Humic Substances Society. The metal standard solutions (ICTM 100–1 & HP100034-2) were purchased from the Environmental Express Inc.
2.2. Modification and characterization
Raw FA was firstly modified by ball milling (FA-I) followed by the NaOH hydrothermal (130 °C) and H2SO4 etching processes (FA-II). Quaternary ammonium groups were then introduced by either physically covering the ash with cationic surfactants CTAB (FA-CSC) or silane coupling methods with TTPAC (FA-SCA). Various dosage of TTPAC were evaluated such as 30 wt% and 100 wt% of the modified FA (denoted as FA-SCA30 and FA-SCA100, respectively). Details of modification methods were included in SI section 1.
Surface morphology was characterized using a scanning electron microscope (SEM, ZEISS GeminiSEM 500). The introduced quaternary ammonium was validated using an attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR, PerkinElmer). The Brunauer–Emmett–Teller surface area (SBET) and pore volume (Vpore) were analyzed by a BET analyzer (MicrotracBEL, Belsorp max). The size distribution (via dynamic light scattering, DLS) and zeta potential were carried out using a Zetasizer (Malvern, Nano ZSE) with the refractive index of 1.45. The percentile values (D10, D50 and D90) were measured to indicate the cumulative 10%, 50% and 90% point of diameter in volume distribution, respectively. The volume mean diameter, as well as the span ((D90-D10)/D50), were calculated for a better description of particle size and distribution.
2.3. Analytical methods
A liquid chromatograph mass spectrometer (Shimadzu LCMS-8050) was used for PFAS measurement. A linear dynamic range of 0.5–150 μg/L was achieved (R2 = 0.997) based on eight calibration points. The details of the modified LC-MS/MS method [29,30] can be found in SI section 2. Since the loss of PFAS via syringe filter (pore size: 0.22 μm) was higher than 10%, all the samples were centrifuged at 12,000 rpm for 2 min and the supernatant was then taken for analysis.
The major oxides of FA were measured using an X-ray fluorescence analyzer (XRF, Bruker AXS S4 Pioneer) following ASTM D4326-13 method (2013). The composition of FA was analyzed using an elemental analyzer (ELEMENTAR, VARIO ELIII), an energy dispersive X-ray spectroscopy (EDS, EDAX Falion 60S), and an inductively coupled plasma optical emission spectroscopy (ICP-OES, VARIAN). An inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7800) was used to measure the toxic metals in leaching study based on the previously reported method [31]. Details of these analytical methods were summarized in SI section 3.
2.4. Adsorption and regeneration
The batch adsorption tests of various adsorbents were conducted in a water bath/magnetic stirrer system with the agitation speed of 300 rpm. The synthetic lake water (1000 μM NaH2PO4 buffer, pH 7.5; and 5000 μM NaNO3 ionic strength adjuster, 640 μS/cm) was used as background solution unless noted otherwise. An initial PFOX concentrations of 1.21 μM were selected to mimic highly-contaminated-sites level. The dosage of adsorbents (including FA based and benchmark adsorbents) was chosen as 25 mg/L to mimic the conditions in WWTPs (dosage ranging from 25 to125 mg/L) [25]. Adsorbents were pre-treated before tests: the FA based adsorbents were washed with deionized water and dried in the oven at 50 °C overnight; PAC was washed with deionized water three times prior to drying at 105 °C for 48 h; and AE resin (Amberlite IRA400 chloride form) was soaked into 1 M NaCl solution overnight and then washed with deionized water several times.
Isotherm studies were conducted with [PFOX] from 1.21 to 97 μM and the kinetics were conducted for 240 min. Since a centrifugation process is required to separate adsorbents for LC-MS tests (the loss of PFOX after syringe filter was tested higher than 10%), samples were taken individually from the separate vials under identical experimental conditions, for those timepoints within first 10 min in kinetic studies, to increase the accuracy of the time-resolved sampling. The adsorption time, in this study, was defined as the time prior to the centrifugation process, which was standard (2 min centrifugation) for every individual sample, regardless of the specific timepoints. Various pH (1.6–12.0) and temperature (5–50 °C) were also evaluated. The ionic strength was indirectly reflected via conductivity, which was adjusted with NaNO3 to simulate lake water (642 μS/cm), domestic wastewater (2160 μS/cm), and industrial wastewater (9221 μS/cm). Co-existing Organic matter (OM) or inorganic ions (counterions are Na+ for anions and NO3− for cations) were pre-mixed with the PFOX solution, as well as the octanoic acid, 3-(perfluorobutyl)propanol, and sodium gluconate. For sorbent regeneration, spent FA-SCA was centrifugated at 12,000 rpm and then soaked into 5 vol% methanol with 100,000 μM NaNO3 at pH 12 and 50 °C.
2.5. Data analysis
Triplicate experiments were carried out for the evaluation of water conditions and co-existing competitors, as well as the regeneration process. The removal efficiency of PFOX (1-Cfinal/Cini) were expressed as mean values. The mean values were further evaluated by the analysis of variance (ANOVA), carried out using Microsoft Excel, to illustrate the statical significance on the influence of various water conditions and competitors. A level of significance p < 0.05 was considered as significant differences among the mean values (95% confidence level).
3. Results and discussion
3.1. Composition and leaching potential
The premise of reusing industrial waste is to prevent additional pollution. The analysis of FA composition (Table 1 and Table S1) demonstrated that SiO2 (50.9%) and Al2O3 (28.9%) were major oxides, where toxic metals, 0.033 wt% As, 0.014 wt% Pb, etc., were also observed. Thus, the leaching potential of toxic metals was deliberately tested with enlarged FA dose (48-fold greater, 1200 mg/L) and extended operation time (42-fold longer, 168 h) (SI section 4). As shown in Fig. 1a, target hazardous metals, including Hg, As, Cd, Cr, Pb and Se (identified by the Resource Conservation and Recovery Act, RCRA), and heavy metals (Ni and Mo) met the more stringent drinking water standards (Table S2). Thus, FA sorbents are safe in use for environmental application without leaching issues of toxic metals, and the applications in wastewater treatment has also been reported [32].
Table 1.
The metal oxide composition of raw FA via XRF analysis (unit: %).
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | P2O5 | TiO2 |
|---|---|---|---|---|---|---|---|---|
| 50.9 | 28.9 | 11.1 | 1.8 | 1.2 | 0.3 | 2.4 | 0.9 | 1.5 |
Fig. 1.
(a) Leaching potential of toxic metals from raw FA with enlarged dosage and operation time. (b) schematic of FA modification routes (details in Table 2). SEM imaging of (c) raw FA, (d) FA-II, (e) FA-SCA30; and (f) EDS mapping of FA-SCA30.
3.2. Modification and characterization
Raw FA is negatively charged at neutral pH (pHpzc = 4.5, Figure S1), resulting in an electrostatic repulsion with ionized PFOX. Therefore, modifications were applied to enhance adsorption and diffusion efficiency (Fig. 1b). As shown in Table 2, raw FA (Fig. 1c) was fractured via ball milling (BM), followed by hydrothermal (HT) and acid etching (AE) methods to form a porous structure (Fig. 1d). The largely increased SBET and Vpore enhance adsorption capacity q(PFOA) by 18-fold (BET isotherm in Figure S2). However, the q(PFOA) per SBET of FA-II, after the porous-forming process, is less than that of raw FA, which indicates the formation of inaccessible pores for PFOX molecules. Thus, SBET alone is insufficient to predict the adsorption capacity. Quaternary ammonium was then introduced to enable anion-exchange functionality for further improvement in adsorption.
Table 2.
Evaluation of FA modification methods (enlarged [PFOA]ini as 72 μM for a better comparison, FA dose 25 mg/L, 22 °C in synthetic lake water).
| Sorbent ID | Modification | SBET m2/g | Vpore cm3/g | Volume mean diameter, nm | Span* | q(PFOA) μmol/g | q(PFOA) per SBET μmol/m2 |
|---|---|---|---|---|---|---|---|
| raw FA | N/A | 11.2 | 0.018 | 6746 | 2.3 | 8.70E + 00 | 7.77E − 01 |
| FA-I | BM | 20.7 | 0.038 | 756 | 1.6 | 1.67E + 01 | 8.07E − 01 |
| FA-II | BM-HT-AE | 279 | 0.689 | 583 | 1.7 | 1.64E + 02 | 5.88E − 01 |
| FA-CSC | BM-HT-AECSC, 100 wt% | 246 | 0.428 | 702 | 2.0 | 3.61E + 02 | 1.47E + 00 |
| FA-SCA100 | BM-HT-AESCA, 100 wt% | 30.1 | 0.023 | 527 | 1.8 | 7.62E + 02 | 2.53E + 01 |
| FA-SCA30 | BM-HT-AE SCA, 30 wt% | 51.6 | 0.136 | 549 | 1.7 | 1.57E + 03 | 3.04E + 01 |
The span was calculated by ((D90-D10)/D50), the percentile values were summarized in Table S3.
Rather than the physically entanglement of cationic surfactants (CSC), the silane coupling reaction (SCA) occurred inside the pores, resulting in 89% and 97% diminishment of SBET and Vpore, respectively. Thus, FA-SCA presented a smoother surface with less porous structure (Fig. 1e). According to ATR-FTIR analysis (Figure S3), the formed Si─O─Si bond (stretching vibration at 1065 cm−1) of SCA method made the introduced quaternary ammonium (bending vibration at 1478 cm−1) more stable compared to physically coverage of CSC method [33], resulting in a 111% increase in q(PFOA) with the same dosage of quaternary ammonium. Since the SCA process could potentially block pores and impede diffusion, an optimized dose was then investigated as 30 wt % of FA (Fig. 1f), which presented a 180-fold increase in q(PFOA) compared to that of raw FA (Figure S4). Hence, FA-SCA30 was selected for the following studies of adsorption mechanisms and influences of water matrices.
3.3. Adsorption mechanism
3.3.1. Adsorption isotherm
The isotherm results displayed a better fit by the Langmuir model compared to the Freundlich model (Fig. 2a and Table S4). The maximum adsorption capacity in a single monolayer (qm) was derived as 1740 μmol/g for PFOA (R2 = 0.977) and 3220 μmol/g for PFOS (R2 = 0.924). The Langmuir model assumes that only monolayer adsorption occurs on a surface with adsorption sites of equal adsorption energy, and the model was generally applied for chemisorption process i.e., ion-exchange process in this study [34]. However, other major driving force, besides electrostatic interaction, should exist because (1) both the observed qm are greater than theoretical maximum anion-exchange capacity (quaternary ammonium density: 840 μmol/g, measured via elemental analyzer in Table 3); and (2) the significant discrepancy in qm of monoacid PFOA and PFOS, which should be comparable with similar ion-exchange sites [18]. Furthermore, when the hydrophobicity was evaluated (indirectly reflect by the octanol–water partition coefficient, Kow) with other monoacid short-chain PFAS (Table S5), the adsorption capacity was positively correlated with the log Kow (Fig. 2b). This correlation demonstrates the pivotal role of hydrophobic interaction in PFOX adsorption, which was also reported when using granular activated carbon column [21]. Hence, PFOX adsorption by FA-SCA was proven to be a synergistic process controlled by electrostatic and hydrophobic interactions. The removal efficiency of short-chain PFAS (PFBA, PFPeA and HFPO-DA) was significantly decreased after the addition of the background anions (Figure S5), whereas the high removal of long-chain PFOX remained with the deionized water and synthetic water conditions. Since the main objective of this study is the utilization of waste material for rapid removal of PFOX, the modifications of developed adsorbents (introduced quaternary ammonium and enhanced hydrophobicity) were made to enhance the capacity and selectivity towards the long-chain PFOX. However, the elucidation of the adsorptive removal mechanisms in section 3.3.3 could guide the design of novel adsorbents towards the effective removal of short-chain PFAS.
Fig. 2.
(a) The isotherm studies with the Langmuir and Freundlich fitting. (b) The correlation between adsorption capacity and log Kow (enlarged [PFAS]ini as 72 μM for a better comparison).
Table 3.
The measurement of C, H, N elemental composition of FA based adsorbents. The introduced quaternary ammonium is the only N source of the modification processes.
| Sample | C (%) | N (%) | H (%) |
|---|---|---|---|
| raw FA | 6.00 ± 0.33 | <0.01 | 0.51 ± 0.01 |
| FA-SCA30 | 18.05 ± 0.05 | 1.18 ± 0.02 | 2.74 ± 0.05 |
| RC-1* | 17.85 ± 0.08 | 1.13 ± 0.01 | 2.57 ± 0.03 |
| RC-5** | 17.94 ± 0.16 | 0.97 ± 0.03 | 2.49 ± 0.02 |
RC-1 refers the spent FA-SCA after the first regeneration cycle
RC-5 refers the spent FA-SCA after the fifth regeneration cycles
3.3.2. Rapid adsorption kinetics
As shown in Fig. 3a, both rapid PFOX adsorption (teq = 2 min) and higher removal efficiency were achieved against benchmark PAC (teq ca. 420 min) and AE resins (teq ca. 3000 min) (Fig. 3b). The stable adsorption equilibrium in FA-SCA was also observed for 240 min. At the initial 1.5-min, the strong fit of the Pseudo–first-order model (PFO) suggested that the adsorption occurs immediately after PFOX diffusion through the liquid-sorbent interface without the major hinderance of intraparticle diffusion inside sorbent particles [35]. This statement was further validated using the Weber-Morris intraparticle diffusion model (Fig. 3c), which assumes that the intraparticle diffusion is the rate-limiting step when the fitting curve is a linear line and passes through the origin [36]. Due to the low R2 of linearity and high y-axis intercept, the intraparticle diffusion was not considered to be the rate-limiting step in PFOX adsorption using FA-SCA, where the conclusion was also supported by the fitting of Boyd model (Figure S6). The lower transfer hindrance of intraparticle diffusion in FA-SCA could enhance the adsorption kinetic, and mechanisms for the observed rapid adsorption were discussed in section 3.3.3, collectively. In addition, details of kinetic models were summarized in SI section 7.
Fig. 3.
The kinetic studies of PFOX adsorption in FA-SCA ([PFOX]ini = 1.21 μM, FA dose 25 mg/L, pH 7.5 and 22 °C in synthetic lake water) (a) with PFO model; (b) using benchmark PAC and AE resins (dose 25 mg/L); and (c) fitted by the Weber-Morris intraparticle diffusion. (d) Zeta potential measurements before and after 2-min adsorption test of PFOS (enlarged [PFOS]ini as 72 μM for a clear observation).
The rapid adsorption was further confirmed via Zeta potential (ζ) and DLS analysis. The moderate ζ potential made FA-SCA well-dispersed initially with the hydrodynamic diameter (dhydro) equals to 712 nm. However, ζ potential was immediately converted from + 19.1 mV to −9.8 mV after the 2-min adsorption test (Fig. 3d). This conversion could be attributed to the screening-effect of adsorbed negatively charged PFOX. In addition, the decreased ζ potential in absolute value triggered agglomeration (Figure S7). Compared to the minor agglomeration observed in the 240-min blank control groups (0.6-fold increased dhydro), the significant agglomeration occurred after 2-min adsorption (10-fold increased dhydro) also demonstrated the rapid adsorption process.
3.3.3. Merits and mechanisms
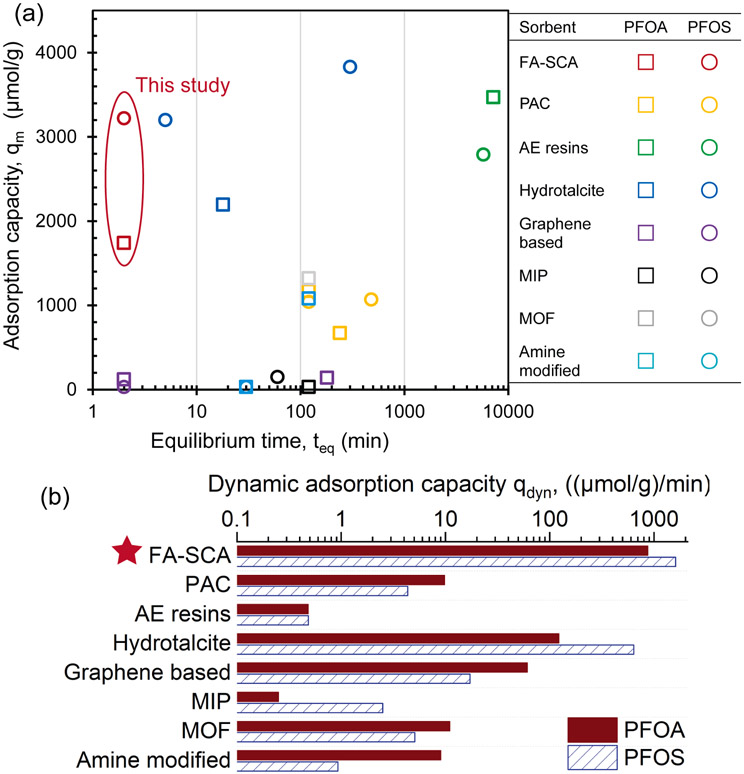
Benchmark and state-of-art sorbents, such as PAC, Metal organic frameworks (MOF) and amine-modified materials, were selected for a comparison of adsorption capacity and adsorption kinetic (Fig. 4a and Table S8). The qm values from Langmuir isotherm model were used in the comparison to eliminate the discrepancy caused by different experimental conditions. FA-SCA exhibited high qm (1740 μmol/g for PFOA and 3220 μmol/g for PFOS), which comparable to that of benchmark PAC and AE resins. Due to the small particle size (D90 < 1 μm), compared to the benchmark adsorbents (about 100 μm), FA-SCA showed an accelerated adsorption process (teq decreased from 120 to 7200 min to 2 min), resulting in a 2–3 orders of magnitude higher dynamic adsorption capacity qdyn (Eq (1)). More important, FA-SCA also showed a superior qdyn among other nanoscale state-of-art sorbents (Fig. 4b). Even though advanced fluorographene sorbents showed rapid adsorption via the fast formation of hydrogen bonding [37], the qm(PFOA) and qm(PFOS) are 13-fold and 76-fold lower than that of FA-SCA, respectively.
Fig. 4.
Comparison with representative sorbents in (a) teq and Langmuir maximum adsorption capacity, qm; and in (b) dynamic adsorption capacity, qdyn = qm/teq. Sorbents include PAC [36,48,49], AE resins [18], hydrotalcite [50,51], graphene-based [37,52], MIP [43,44], MOF [53,54], and amine-modified [34,39]. Adsorption tests were operated at room temperature and pH range from 3.3 to 9.8 (Details in Table S8).
Dynamic adsorption capacity:
| (1) |
In addition to qm and adsorption rate, adsorption affinity is a key criterion since the environmentally relevant concentrations of PFAS are generally in the low μg/L level. The adsorption affinity of different adsorbents was evaluated through the Langmuir affinity coefficient (KL) [38,39]. The common unit of KL was mass-based (L/g or L/mg) [40], however, the molar-based KL (L/μmol) was used in this study for unit consistency in the isotherm and kinetic studies, as well as for the ease of comparison between two types of adsorbates (PFOA and PFOS). These two units of KL were convertible, where the molar-based KL equals to the mass-based KL times the molar weight of the adsorbates. As shown in Table S8, FA-SCA presented higher KL of PFOS (0.031 L/μmol) than that of PFOA (0.023 L/μmol), and the adsorption affinity towards PFOX, collectively, is in between that of PAC (0.004 to 0.008 L/μmol) [41,42] and AE resins (0.178–0.416 L/μmol) [18]. Higher KL (0.112 to 2.380 L/μmol) towards PFOX adsorption was also observed on the adsorbents with numerous cationic centers, including AE resins, molecularly imprinted polymer (MIP), and amine-modified materials [34,39,43,44]. However, higher adsorption affinity might require additional efforts in desorption process during regeneration, such as elevated temperature or strict conditions.
The mechanisms of rapid adsorption and superior qdyn of PFOX using FA-SCA can be elucidated by the submicron particle size, the developed mesoporous structures, and the numerous quaternary ammonium sites. Firstly, the 100-fold smaller particle size of FA-SCA (via DLS and SEM analysis), compared to micro-scale benchmark adsorbents, enhanced the external diffusion of PFOX. The developed porous stucture also increased specific surface area for more adsorption sites. Regarding to the molecular length of PFOA and PFOS (ca. 1.0 nm and 1.2 nm, respectively) [24], however, SBET alone is a poor indicator for adsorption capacity since the existence of inaccessible micropores (as disscussed in section 3.2). The formation of PFOX hemi-micelle, at the concentration of 0.001–0.01 of the critical micelle concentrations (i.e., 25000 μM for PFOA and 8000 μM for PFOS) [45], could further block the micropores [36]. In addition, narrow micropores could hinder internal diffusion of PFOX molecules, and the intraparticle diffusion controlled kninetics are often reported when using micropores-dominanted sorbents [17,46]. Hence, enlarged pore-size could enhance both the adsorpiton capacity and the internal diffusion [47]. Accordingly, the high fraction of mesopores (Vmeso/Vmicro = 8.1, Table S9) and large average pore size (ca. 10.6 nm) of FA-SCA, found in this study, would greatly enhance internal diffusion of PFOX, where the intraparticle diffusion was confirmed not to be the rate-limiting step in beforementioned kinetic studies. Furthermore, since the PFOX adsorption by FA-SCA was driven by electrostatic and hydrophobic interactions (proven in section 3.3.1), the high loading density of quaternary ammonium (840 μmol/g) provide considerable ion-exchange sites for effective PFOX adsorption. Once diffusing through the liquid-sorbent interface, PFOX molecules, with enhanced intraparticle diffusion caused by mesoporous structure, could immediately interact with quaternary ammonium ion-exchange sites inside the mesopores. Meanwhile, the mesoporous structure made the interior ion-exchange sites more accessible, which explains that FA-SCA presented much higher adsorption capacity qm compared to the reported amine-modified materials (Fig. 4a). Overall, the mesoporous structure and numerous adsorption sites of FA-SCA concurrently triggered the fast adsorption dynamic and superior qdyn among benchmark and state-of-art sorbents.
3.4. Water conditions and matrices
Above 91% PFOX removal were achieved consistently (statistically non-significant, p > 0.05) at pH ranging from 2.5 to 8.2 (Fig. 5a). However, PFOX removal was greatly impaired when pH increased from 8.2 to 12.0: nearly only 2.1 % and 10.1% PFOA and PFOS were removed at pH 12, respectively. The obtained isoelectric point (pHiep ~ 11.1) (Fig. 3d) indirectly indicates that positively charged FA-SCA would gradually convert to negatively charged when pH > pHiep, leading to electrostatic repulsion interaction with ionized PFOX. Although the increased pH would deionize amine groups (pH > pKa) and impede PFOX removal when using polyamine AE resins [55], quaternary ammonium is regarded as a “permanent cation”, and the quaternary ammonium AE resins were reported to be effective at strong base condition [18]. Thus, the conversion of surface charge at pH 12 would be attributed to the non-quaternary-ammonium functionalized area of mineral matrix of FA-SCA, since the pHpzc of raw FA was only 4.5 (Figure. S1). The negatively charged quaternary-ammonium hydrogel was also reported at base condition [19], since the pHpzc of the hydrogel was tested as 11.8.
Fig. 5.
The evaluation of water conditions (a) pH, (b) temperature, and (c) ionic strength on PFOX removal efficiency ([PFOX]ini = 1.21 μM, FA dose 25 mg/L, pH 7.5 and 22 °C in synthetic lake water if not particularly indicated).
3.5. Water conditions and matrices
Above 91% PFOX removal were achieved consistently (statistically non-significant, p > 0.05) at pH ranging from 2.5 to 8.2 (Fig. 5a). However, PFOX removal was greatly impaired when pH increased from 8.2 to 12.0: nearly only 2.1 % and 10.1% PFOA and PFOS were removed at pH 12, respectively. The obtained isoelectric point (pHiep ~ 11.1) (Fig. 3d) indirectly indicates that positively charged FA-SCA would gradually convert to negatively charged when pH > pHiep, leading to electrostatic repulsion interaction with ionized PFOX. Although the increased pH would deionize amine groups (pH > pKa) and impede PFOX removal when using polyamine AE resins [55], quaternary ammonium is regarded as a “permanent cation”, and the quaternary ammonium AE resins were reported to be effective at strong base condition [18]. Thus, the conversion of surface charge at pH 12 would be attributed to the non-quaternary-ammonium functionalized area of mineral matrix of FA-SCA, since the pHpzc of raw FA was only 4.5 (Figure. S1). The negatively charged quaternary-ammonium hydrogel was also reported at base condition [19], since the pHpzc of the hydrogel was tested as 11.8.
As shown in Fig. 5b, PFOX removal was consistent from 5 to 22 °C, but a negative impact was observed with increased temperature from 22 to 50 °C (p < 0.05). When T > lower critical solution temperature, temperature-responsive poly(N-isopropylacrylamide) would collapse and become more hydrophobic, due to the decreased entropy caused by the reorientation of water molecules to form hydrogen bonding [29]. However, the quaternary ammonium is not able to form hydrogen bonding with either itself or water molecules. Hence, the negative contribution of increased temperature could be explained by an accelerated desorption process and the increases of PFOX solubility.
Only 35.8% and 22.6% decreases in the removal efficiency of PFOA and PFOS were observed, respectively, when conductivity increased by 113-fold from synthetic lake water to synthetic industrial wastewater (Fig. 5c). Theoretically, increased ionic strength should allow inorganic anions to dominate competitive adsorption because: (1) their much smaller size results in a faster diffusion in the pores of sorbents (Stokes-Einstein equation, Eq (2)); and (2) the much greater concentration (3 to 6 orders of magnitude higher than PFOX). However, the intraparticle diffusion of PFOX, in this study, is enhanced by the developed mesoporous structure of FA-SCA. Furthermore, the screening effect of anions could only impart the electrostatic interactions rather than important hydrophobic interactions between PFOX and FA-SCA, resulting in the limited negative influence with the increased ionic strength.
| (2) |
where diffusion coefficient D is negatively correlated with the multiple of dynamic viscosity η and particle size r.
Additional experiments with higher [PFOX]ini/adsorbent ratio ([PFOX]ini deliberately increased by 60-fold to 72 μM) were carried out to validate that the effective PFOX removal at various water conditions was not attributed to the excess dosage of adsorbents. As shown in Figure S8, a consistent PFOX removal was also achieved, with higher [PFOX]ini, in a broad range of water conditions, where the significant hindrance in PFOX adsorption was also observed in alkaline condition (pH 10.6) and elevated temperature (50 °C).
3.6. Selectivity towards Co-existing competitors
Since the tests were conducted in the synthetic lake water, the evaluation of co-existing organic and inorganic competitors is more accurate with the background pH buffer and ionic strength. For Organic matter (OM), both Natural organic matter (NOM), including fulvic acid (FVA) and Humic acid (HA) macromolecules [56], and charged chain-like surfactants were investigated at the environmentally relevant concentrations ([OM] = 3000 μg/L). OM was reported to largely impart the PFOX removal using PAC [57], however, in this study, negatively charged FVA, HA, and SDS showed weak hindrance in PFOA removal (88% removal compared to 96% removal initially, p < 0.05), where negligible influences were observed on PFOS removal (p > 0.05) (Fig. 6a). This observation could be explained by the competitive adsorption and the differences in hydrophobicity among PFOX molecules: (1) parts of available quaternary ammonium ion-exchange sites would be occupied by these negatively charged OM competitors; (2) the adsorbed OM competitors could cause the electrostatic repulsion because of the negatively charged groups but enhance the hydrophobic interaction with PFOX molecules via their hydrophobic moiety [58]; (3) PFOS exhibits high Kow than PFOA, the adsorbed OM could show higher affinity toward PFOS via hydrophobic interaction [21,59], which in turn offsets the weakened electrostatic attraction. On the contrary, positively-charged CTAB surfactants, once accumulating on FA-SCA, could provide positive center for electrostatic attraction, resulting in the slightly enhancement of PFOX removal. Hence, the influence of OM was highly dependent on adsorption interactions and charge properties of OM.
Fig. 6.
Highly selective PFOX removal with (a) co-existing OM or inorganic ions ([OM] = 3000 μg/L, [inorganic ions] = 500 μM); and (b) structure-similar compounds ([species] = 3000 μg/L), separately or collectively (“all included” group indicating the mixture of octanoic acid, 3-(perfluorobutyl)propanol, and sodium gluconate). All the removal tests were conducted with [PFOX]ini = 1.21 μM and FA-SCA dose 25 mg/L (22 °C in synthetic lake water).
Inorganic ions were also evaluated at environmentally relevant concentrations ([inorganic ions] = 500 μM, [inorganic ions]/[PFOX] = 413). Anions, particularly divalent anions, are considered to be severe competitors for ion-exchange sites, but the salting-out effect could enhance the hydrophobic interaction and compensate the negative influences on PFOX removal [18,55]. With the background ionic strength in this study (5000 μM NaNO3), the negligible influences of both monovalent and divalent anions (Fig. 6a) can not be simply attributed to the salting-out effect. In addition, a slight enhancement of PFOX removal was observed with co-existing divalent and trivalent cations, which could be explained by a bridging-effect between the accumulated multivalent cations and the anionic center of PFOX [60]. Therefore, the adverse influences, caused by anions and multivalent cations, further validated that the adsorption of PFOX on FA-SCA is a synergistic process of electrostatic and hydrophobic interactions.
In addition to the ubiquitous organic and inorganic competitors, the structure-similar chain-like compounds of PFOX, including octanoic acid, 3-(perfluorobutyl)propanol, and sodium gluconate, were selected ([species] = 3000 μg/L) to further investigate the selectivity of FA-SCA sorbents. With the similar carbon chain length of PFOX (8-carbon), octanoic acid (8-carbon) and 3-(perfluorobutyl)propanol (7-carbon) exhibit either the anionic functional groups or the hydrophobic perfluoro groups, exclusively. As shown in Fig. 6b, octanoic acid, with the less hydrophobic C─H bond compared to C─F bond, only showed hindrance on PFOA removal (statistically significant, p < 0.05) instead of on PFOS removal, whereas hydrophobic 3-(perfluorobutyl)propanol only impaired the removal of PFOS (p < 0.05). Therefore, molecules with negatively charged functional groups and highly hydrophobic parts would strengthen the competitive adsorption with PFOX molecules in FA-SCA sorbents. The statement can also be used to illustrate that the sodium gluconate, with a carboxylate group and multiple hydrophilic hydroxyl groups per molecule, had insignificant influences on PFOX removal (p > 0.05). The competitive adsorption became severe when the octanoic acid, 3-(perfluorobutyl)propanol, and sodium gluconate were all included, however, >85.4% removal of PFOX was achieved with highly effective FA-SCA adsorbents.
3.7. Regeneration
Previous discussions demonstrated that increased pH and elevated temperature are unfavorable for PFOX adsorption. Thus, mild regeneration conditions were selected as: 5% methanol with 1/5 vol (Vdes) as that in adsorption tests (Vads) at pH 12 and 50 °C. A consistent PFOX removal (>90%) was achieved during five regeneration cycles (p > 0.05) (Fig. 7a). The effective desorption led to a 5-fold concentration of PFOX, and the concentration ratio could be increased to 25-fold when the methanol ratio increased to 50 vol% (Fig. 7b). The significantly increased concentration ratio could largely reduce the Vdes of regeneration process, and the concentrated PFOX after regeneration could be eventually destroyed via novel thermal or chemical measures, such as microwave thermal and advanced reduction/oxidation methods [61,62], resulting in a more efficient treatment process. In addition to the adsorption performance, the stability of FA-SCA after regeneration was also evaluated since the siloxane bond has the potential to hydrolyze in alkaline condition. A negligible change in carbon content (<1.5%) was observed after five regeneration cycles (Table 3), and>96.6% and 82.2% of nitrogen also remained, respectively, after the first (RC-1) and the fifth regeneration cycles (RC-5). Furthermore, the consistence of the removal efficiency within regeneration cycles also indicated the stability of FA-SCA. Hence, compared to the harsh environments required in the regeneration of strong base AE resins [25], the regeneration of FA-SCA was achieved in a mild conditions with less chemical assumption and less Vdes production. Furthermore, the spent methanol could be recycled through a small distillation unit since the PFOX molecules are not volatile.
Fig. 7.
The regeneration tests of FA-SCA (a) with five cycles using 5 vol% methanol (M); and (b) with different Vdes compared to the Vads at PFOS adsorption process (regeneration processes were conducted at pH 12 and 50 °C).
4. Conclusion
Industrial solid waste fly ash, with developed mesoporous structure and functionalized quaternary ammonium groups, was proven as an effective and selective sorbent in PFOX removal, where the fast adsorption kinetic and high qdyn were achieved concurrently in a synthetic lake water. The key conclusions of this study were summarized as below:
Though SBET was largely enhanced after pore formation, the decreased q(PFOA)/SBET, compared to raw FA, demonstrated that SBET is a poor indicator for adsorption capacity since the inaccessible area and diffusion resistance caused by the micropores.
Adsorption mechanism of PFOX on FA-SCA was considered as a synergistic process of electrostatic and hydrophobic interactions, since (1) the q(PFOA) was increased by 9.6-fold after introducing quaternary ammonium ion-exchange sites; and (2) a positive correlation between the logarithm of octanol/water coefficient Kow (hydrophobicity) and adsorption capacity was established among five different PFAS species.
The external diffusion, intraparticle diffusion and interaction with adsorption sites were enhanced via the developed submicron particle size, formed mesoporous structure and appropriate adsorption affinity, respectively. Kinetic models also showed that the intraparticle diffusion was not the rate-limiting step in this study. In addition, the introduced quaternary ammonium groups further improved the adsorption capacity and affinity.
With the unique properties of FA-SCA, selective PFOX removal (>85.4%) was achieved in the presence of ubiquitous competitors (OM, Ca2+, Cl−, etc.), as well as the structure-similar compounds of PFOX (carbon-chain with either carboxyl or perfluoro groups). Anionic or hydrophobic compounds showed weak influences on PFOX removal, exclusively.
Under a mild regeneration condition (5 vol% methanol at pH 12 and 50 °C), above 93% PFOX was removed consistently within five regeneration cycles. [PFOS] could be concentrated 5 to 25-fold, after regeneration, to decrease the produced Vdes of regeneration waste.
With superior qdyn, high selectivity and safety in use, FA-SCA has the potential to additionally serve as a support material to enable the integration of catalyst/FA-SCA, which could largely promote local concentration to overcome the issues along with the trace concentrations of PFAS at natural sources.
Supplementary Material
Acknowledgements
This work was supported by China Postdoctoral Science Foundation [2020M681204]; the National Natural Science Foundation of China [21908054 & 22075076]; and NIEHS-SRP grant [P42ES007380]. We highly appreciate the collaborations with the UK superfund center and the UK CAER
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cej.2021.133271.
References
- [1].Lenka SP, Kah M, Padhye LP, A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants, Water Res. 199 (2021), 117187, 10.1016/j.watres.2021.117187. [DOI] [PubMed] [Google Scholar]
- [2].Giesy JP, Kannan K, Global distribution of perfluorooctane sulfonate in wildlife, Environ. Sci. Technol 35 (7) (2001) 1339–1342, 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- [3].Brown JB, Conder JM, Arblaster JA, Higgins CP, Assessing human health risks from per- and polyfluoroalkyl substance (pfas)-impacted vegetable consumption: a tiered modeling approach, Environ. Sci. Technol 54 (23) (2020) 15202–15214, 10.1021/acs.est.0c0341110.1021/acs.est.0c03411.s001. [DOI] [PubMed] [Google Scholar]
- [4].Ateia M, Alsbaiee A, Karanfil T, Dichtel W, Efficient PFAS removal by amine-functionalized sorbents: critical review of the current literature, Environ. Sci. Technol. Lett 6 (12) (2019) 688–695, 10.1021/acs.estlett.9b0065910.1021/acs.esdett.9b00659.s001. [DOI] [Google Scholar]
- [5].Tenorio R, Liu J, Xiao X, Maizel A, Higgins CP, Schaefer CE, Strathmann TJ, Destruction of per- and polyfluoroalkyl substances (pfass) in aqueous film-forming foam (afff) with uv-sulfite photoreductive treatment, Environ. Sci. Technol 54 (11) (2020) 6957–6967, 10.1021/acs.est.0c0096110.1021/acs.est.0c00961.s001. [DOI] [PubMed] [Google Scholar]
- [6].Liu Y, Li X, Wang X, Qiao X, Hao S, Lu J, Duan X, Dionysiou DD, Zheng B, Contamination profiles of perfluoroalkyl substances (pfas) in groundwater in the alluvial-pluvial plain of hutuo river, China Water 11 (11) (2019) 2316, 10.3390/w11112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM, Detection of poly- and perfluoroalkyl substances (pfass) in u.s. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants, Environ. Sci. Technol. Lett 3 (10) (2016) 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yan B, Munoz G, Sauvé S, Liu J, Molecular mechanisms of per- and polyfluoroalkyl substances on a modified clay: a combined experimental and molecular simulation study, Water Res. 184 (2020), 116166, 10.1016/j.watres.2020.116166. [DOI] [PubMed] [Google Scholar]
- [9].Rodríguez-Varela M, Durán-Alvarez JC, Jiménez-Cisneros B, Zamora O, Prado B, Occurrence of perfluorinated carboxylic acids in Mexico City’s wastewater: A monitoring study in the sewerage and a mega wastewater treatment plant, Sci. Total Environ 774 (2021), 145060, 10.1016/j.scitotenv.2021.145060. [DOI] [PubMed] [Google Scholar]
- [10].Wang X, Yu N, Qian Y, Shi W, Zhang X, Geng J, Yu H, Wei S, Non-target and suspect screening of per- and polyfluoroalkyl substances in Chinese municipal wastewater treatment plants, Water Res. 183 (2020), 115989, 10.1016/j.watres.2020.115989. [DOI] [PubMed] [Google Scholar]
- [11].He X.i., Zheng N, Hu R, Hu Z, Yu JC, Hydrothermal and pyrolytic conversion of biomasses into catalysts for advanced oxidation treatments, Adv. Funct. Mater 31 (7) (2021) 2006505, 10.1002/adfm.v31.710.1002/adfm.202006505. [DOI] [Google Scholar]
- [12].Huang S, Jaffé PR, Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium sp. Strain A6, Environ. Sci. Technol 53 (19) (2019) 11410–11419, 10.1021/acs.est.9b04047. [DOI] [PubMed] [Google Scholar]
- [13].Qian Y, Guo X, Zhang Y, Peng Y, Sun P, Huang C-H, Niu J, Zhou X, Crittenden JC, Perfluorooctanoic acid degradation using uv–persulfate process: modeling of the degradation and chlorate formation, Environ. Sci. Technol 50 (2) (2016) 772–781, 10.1021/acs.est.5b0371510.1021/acs.est.5b03715.s001. [DOI] [PubMed] [Google Scholar]
- [14].Lu D, Sha S, Luo J, Huang Z, Zhang Jackie X, Treatment train approaches for the remediation of per- and polyfluoroalkyl substances (PFAS): A critical review, J. Hazar. Mater 386 (2020), 121963, 10.1016/j.jhazmat.2019.121963. [DOI] [PubMed] [Google Scholar]
- [15].Lee C-G, Javed H, Zhang D, Kim J-H, Westerhoff P, Li Q, Alvarez PJJ, Porous electrospun fibers embedding tio2 for adsorption and photocatalytic degradation of water pollutants, Environ. Sci. Technol 52 (7) (2018) 4285–4293, 10.1021/acs.est.7b0650810.1021/acs.est.7b06508.s001. [DOI] [PubMed] [Google Scholar]
- [16].Li F, Wei Z, He K, Blaney L, Cheng X, Xu T, Liu W, Zhao D, A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst, Water Res. 185 (2020), 116219, 10.1016/j.watres.2020.116219. [DOI] [PubMed] [Google Scholar]
- [17].Xiao X, Ulrich BA, Chen B, Higgins CP, Sorption of poly-and perfluoroalkyl substances (PFASs) relevant to aqueous film-forming foam (AFFF)-impacted groundwater by biochars and activated carbon, Environ. Sci. Technol 51 (11) (2017) 6342–6351. [DOI] [PubMed] [Google Scholar]
- [18].Maimaiti A, Deng S, Meng P, Wang W, Wang B, Huang J, Wang Y, Yu G, Competitive adsorption of perfluoroalkyl substances on anion exchange resins in simulated AFFF-impacted groundwater, Chem. Eng. J 348 (2018) 494–502. [Google Scholar]
- [19].Ateia M, Arifuzzaman M, Pellizzeri S, Attia MF, Tharayil N, Anker JN, Karanfil T, Cationic polymer for selective removal of GenX and short-chain PFAS from surface waters and wastewaters at ng/L levels, Water Res. 163 (2019), 114874, 10.1016/j.watres.2019.114874. [DOI] [PubMed] [Google Scholar]
- [20].Klemes MJ, Ling Y, Ching C, Wu C, Xiao L, Helbling DE, Dichtel WR, Reduction of a tetrafluoroterephthalonitrile-β-cyclodextrin polymer to remove anionic micropollutants and perfluorinated alkyl substances from water, Angew. Chem 131 (35) (2019) 12177–12181. [DOI] [PubMed] [Google Scholar]
- [21].Park M, Wu S, Lopez IJ, Chang JY, Karanfil T, Snyder SA, Adsorption of perfluoroalkyl substances (PFAS) in groundwater by granular activated carbons: Roles of hydrophobicity of PFAS and carbon characteristics, Water Res. 170 (2020), 115364. [DOI] [PubMed] [Google Scholar]
- [22].Zeng C, Atkinson A, Sharma N, Ashani H, Hjelmstad A, Venkatesh K, Westerhoff P, Removing per- and polyfluoroalkyl substances from groundwaters using activated carbon and ion exchange resin packed columns, AWWA Water Sci. 2 (1) (2020), e1172, 10.1002/aws2.1172. [DOI] [Google Scholar]
- [23].Ji W, Xiao L, Ling Y, Ching C, Matsumoto M, Bisbey RP, Helbling DE, Dichtel WR, Removal of genx and perfluorinated alkyl substances from water by amine-functionalized covalent organic frameworks, J. Am. Chem. Soc 140 (40) (2018) 12677–12681, 10.1021/jacs.8b0695810.1021/jacs.8b06958.s001. [DOI] [PubMed] [Google Scholar]
- [24].Xiao F, Davidsavor KJ, Park S, Nakayama M, Phillips BR, Batch and column study: Sorption of perfluorinated surfactants from water and cosolvent systems by Amberlite XAD resins, J. Colloid Interface Sci 368 (1) (2012) 505–511, 10.1016/j.jcis.2011.11.011. [DOI] [PubMed] [Google Scholar]
- [25].Gagliano E, Sgroi M, Falciglia PP, Vagliasindi FG, Roccaro P, Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration, Water Res. 171 (2020), 115381. [DOI] [PubMed] [Google Scholar]
- [26].Vu CT, Wu T, Recent progress in adsorptive removal of per-and poly-fluoroalkyl substances (PFAS) from water/wastewater, Critical Review. Environ. Sci. Technol (2020) 1–40. [Google Scholar]
- [27].Liu L, Liu Y, Gao B, Ji R, Li C, Wang S, Removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from water by carbonaceous nanomaterials: A review, Critical Review. Environ. Sci. Technol 50 (22) (2020) 2379–2414, 10.1080/10643389.2019.1700751. [DOI] [Google Scholar]
- [28].Ateia M, Alsbaiee A, Karanfil T, Dichtel WJES, T. Letters, Efficient PFAS removal by amine-functionalized sorbents: critical review of the current literature 6 (12) (2019) 688–695. [Google Scholar]
- [29].Saad A, Mills R, Wan H, Mottaleb MA, Ormsbee L, Bhattacharyya D, Thermo-responsive adsorption-desorption of perfluoroorganics from water using PNIPAm hydrogels and pore functionalized membranes, J. Membr. Sci 599 (2020), 117821, 10.1016/j.memsci.2020.117821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu G, Li C, Stewart BA, Liu L, Zhang M, Yang M, Lin K, Enhanced thermal activation of peroxymonosulfate by activated carbon for efficient removal of perfluorooctanoic acid, Chem. Eng. J 399 (2020), 125722. [Google Scholar]
- [31].Wan H, Islam MS, Qian D, Ormsbee L, Bhattacharyya D, Reductive degradation of CCl4 by sulfidized Fe and Pd-Fe nanoparticles: Kinetics, longevity, and morphology aspects, Chem. Eng. J 394 (2020), 125013, 10.1016/j.cej.2020.125013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Koshy N, Singh DN, Fly ash zeolites for water treatment applications, J. Environ. Chem. Eng 4 (2) (2016) 1460–1472. [Google Scholar]
- [33].Liu G, Tsen W-C, Jang S-C, Hu F, Zhong F, Zhang B, Wang J, Liu H, Wang G, Wen S, Composite membranes from quaternized chitosan reinforced with surface-functionalized PVDF electrospun nanofibers for alkaline direct methanol fuel cells, J. Membr. Sci 611 (2020), 118242. [Google Scholar]
- [34].Xing DY, Chen Y, Zhu J, Liu T, Fabrication of hydrolytically stable magnetic core-shell aminosilane nanocomposite for the adsorption of PFOS and PFOA, Chemosphere 251 (2020), 126384, 10.1016/j.chemosphere.2020.126384. [DOI] [PubMed] [Google Scholar]
- [35].Sahoo TR, Prelot B, Chapter 7 - Adsorption processes for the removal of contaminants from wastewater: the perspective role of nanomaterials and nanotechnology, in: Bonelli B, Freyria FS, Rossetti I, Sethi R (Eds.), Nanomaterials for the Detection and Removal of Wastewater Pollutants, Elsevier; 2020, pp. 161–222. https://doi.org/ 10.1016/B978-0-12-818489-9.00007-4. [DOI] [Google Scholar]
- [36].Yu Q, Zhang R, Deng S, Huang J, Yu G, Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study, Water Res. 43 (4) (2009) 1150–1158. [DOI] [PubMed] [Google Scholar]
- [37].Wang W, Xu Z, Zhang X, Wimmer A, Shi E.n., Qin Y, Zhao X, Zhou B, Li L, Rapid and efficient removal of organic micropollutants from environmental water using a magnetic nanoparticles-attached fluorographene-based sorbent, Chem. Eng. J 343 (2018) 61–68. [Google Scholar]
- [38].Ou X, Liu X, Liu W, Rong W, Li J, Lin Z, Surface defects enhance the adsorption affinity and selectivity of Mg(OH)2 towards As(v) and Cr(vi) oxyanions: a combined theoretical and experimental study, Environ. Sci. Nano 5 (11) (2018) 2570–2578, 10.1039/C8EN00654G. [DOI] [Google Scholar]
- [39].Yang A, Ching C, Easier M, Helbling DE, Dichtel WR, Cyclodextrin polymers with nitrogen-containing tripodal crosslinkers for efficient pfas adsorption, ACS Mater. Lett 2 (9) (2020) 1240–1245. [Google Scholar]
- [40].Tran HN, You S-J, Hosseini-Bandegharaei A, Chao H-P, Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review, Water Res. 120 (2017) 88–116, 10.1016/j.watres.2017.04.014. [DOI] [PubMed] [Google Scholar]
- [41].Meng P, Fang X, Maimaiti A, Yu G, Deng S, Efficient removal of perfluorinated compounds from water using a regenerable magnetic activated carbon, Chemosphere 224 (2019) 187–194, 10.1016/j.chemosphere.2019.02.132. [DOI] [PubMed] [Google Scholar]
- [42].Xu J, Liu Z, Zhao D, Gao N, Fu X, Enhanced adsorption of perfluorooctanoic acid (PFOA) from water by granular activated carbon supported magnetite nanoparticles, Sci. Total Environ 723 (2020), 137757. [DOI] [PubMed] [Google Scholar]
- [43].Guo H, Liu Y.u., Ma W, Yan L, Li K, Lin S, Surface molecular imprinting on carbon microspheres for fast and selective adsorption of perfluorooctane sulfonate, J. Hazard. Mater 348 (2018) 29–38. [DOI] [PubMed] [Google Scholar]
- [44].Cao F, Wang L, Yao Y, Wu F, Sun H, Lu S, Synthesis and application of a highly selective molecularly imprinted adsorbent based on multi-walled carbon nanotubes for selective removal of perfluorooctanoic acid, Environ. Sci. Water Res. Technol 4 (5) (2018) 689–700, 10.1039/C7EW00443E. [DOI] [Google Scholar]
- [45].Shih K, Wang F, Adsorption behavior of perfluorochemicals (pfcs) on boehmite: influence of solution chemistry, Procedia Environ. Sci 18 (2013) 106–113, 10.1016/j.proenv.2013.04.015. [DOI] [Google Scholar]
- [46].Wan H, Briot NJ, Saad A, Ormsbee L, Bhattacharyya D, Pore functionalized PVDF membranes with in-situ synthesized metal nanoparticles: Material characterization, and toxic organic degradation, J. Membr. Sci 530 (2017) 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Deng S, Nie Y, Du Z, Huang Q, Meng P, Wang B, Huang J, Yu G, Enhanced adsorption of perfluorooctane sulfonate and perfluorooctanoate by bamboo-derived granular activated carbon, J. Hazard. Mater 282 (2015) 150–157. [DOI] [PubMed] [Google Scholar]
- [48].Chen W, Zhang X, Mamadiev M, Wang Z, Sorption of perfluorooctane sulfonate and perfluorooctanoate on polyacrylonitrile fiber-derived activated carbon fibers: in comparison with activated carbon, RSC Adv. 7 (2) (2017) 927–938, 10.1039/C6RA25230C. [DOI] [Google Scholar]
- [49].Li J, Li Q, Li L-S, Xu L, Removal of perfluorooctanoic acid from water with economical mesoporous melamine-formaldehyde resin microsphere, Chem. Eng. J 320 (2017) 501–509, 10.1016/j.cej.2017.03.073. [DOI] [Google Scholar]
- [50].Chang P-H, Jiang W-T, Li Z, Removal of perfluorooctanoic acid from water using calcined hydrotalcite–A mechanistic study, J. Hazard. Mater 368 (2019) 487–495. [DOI] [PubMed] [Google Scholar]
- [51].Alonso-de-Linaje V, Mangayayam MC, Tobler DJ, Rives V, Espinosa R, Dalby KN, Enhanced sorption of perfluorooctane sulfonate and perfluorooctanoate by hydrotalcites, Environ. Technol. Innovation 21 (2021), 101231. [Google Scholar]
- [52].Ali M, Meaney SP, Giles LW, Holt P, Majumder M, Tabor RF, Capture of perfluorooctanoic acid using oil-filled graphene oxide-silica hybrid capsules, Environ. Sci. Technol 54 (6) (2020) 3549–3558, 10.1021/acs.est.9b0546910.1021/acs.est.9b05469.s001. [DOI] [PubMed] [Google Scholar]
- [53].Yang Y, Zheng Z, Ji W, Xu J, Zhang X, Insights to perfluorooctanoic acid adsorption micro-mechanism over Fe-based metal organic frameworks: Combining computational calculation with response surface methodology, J. Hazard. Mater 395 (2020), 122686. [DOI] [PubMed] [Google Scholar]
- [54].Zhao C, Xu Y, Xiao F, Ma J, Zou Y, Tang W, Perfluorooctane sulfonate removal by metal-organic frameworks (MOFs): Insights into the effect and mechanism of metal nodes and organic ligands, Chem. Eng. J 406 (2021), 126852, 10.1016/j.cej.2020.126852. [DOI] [Google Scholar]
- [55].Du Z, Deng S, Chen Y, Wang B, Huang J, Wang Y, Yu G, Removal of perfluorinated carboxylates from washing wastewater of perfluorooctanesulfonyl fluoride using activated carbons and resins, J. Hazard. Mater 286 (2015) 136–143. [DOI] [PubMed] [Google Scholar]
- [56].Rajaei F, Taheri E, Hadi S, Fatehizadeh A, Amin MM, Rafei N, Fadaei S, Aminabhavi TM, Enhanced removal of humic acid from aqueous solution by combined alternating current electrocoagulation and sulfate radical, Environ. Pollut 277 (2021), 116632, 10.1016/j.envpol.2021.116632. [DOI] [PubMed] [Google Scholar]
- [57].Yu J, Lv L, Lan P, Zhang S, Pan B, Zhang W, Effect of effluent organic matter on the adsorption of perfluorinated compounds onto activated carbon, J. Hazard. Mater 225–226 (2012) 99–106, 10.1016/j.jhazmat.2012.04.073. [DOI] [PubMed] [Google Scholar]
- [58].Du Z, Deng S, Bei Y, Huang Q, Wang B, Huang J, Yu G, Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review, J. Hazard. Mater 274 (2014) 443–454, 10.1016/j.jhazmat.2014.04.038. [DOI] [PubMed] [Google Scholar]
- [59].Deng S, Bei Y, Lu X, Du Z, Wang B, Wang Y, Huang J, Yu G, Effect of co-existing organic compounds on adsorption of perfluorinated compounds onto carbon nanotubes, Front. Environ. Sci. Eng 9 (5) (2015) 784–792. [Google Scholar]
- [60].Chen R, Zhuang Y, Yu Y, Shi B, Enhanced perfluorooctanoic acid (PFOA) accumulation by combination with in-situ formed Mn oxides under drinking water conditions, Water Res. 190 (2021), 116660, 10.1016/j.watres.2020.116660. [DOI] [PubMed] [Google Scholar]
- [61].Gagliano E, Falciglia PP, Zaker Y, Karanfil T, Roccaro P, Microwave regeneration of granular activated carbon saturated with PFAS, Water Res. 198 (2021), 117121, 10.1016/j.watres.2021.117121. [DOI] [PubMed] [Google Scholar]
- [62].Trojanowicz M, Bojanowska-Czajka A, Bartosiewicz I, Kulisa K, Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) – A review of recent advances, Chem. Eng. J 336 (2018) 170–199, 10.1016/j.cej.2017.10.153. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.