Abstract
Cancer recurrence and metastasis are still common causes of postsurgery death in patients with solid tumors, suggesting that additional consolidation therapeutic strategies are necessary. We have previously found that oxaliplatin (OXA) treatment causes further up-regulation of CD155, which is abundantly expressed in tumors for resulting in increased sensitivity of cancer to anti-CD155 therapy. Here, we report O-TPNVs, which are TIGIT-expressing cell membrane and platelet cell membrane fusion nanovesicles (TPNVs) loaded with OXA. Platelet-derived membrane components enable O-TPNVs to target postsurgery wounds and interact with circulating tumor cells (CTCs). OXA directly kills residual tumor cells and CTCs, induces immunogenic cell death, and activates the immune system. TPNVs bind to CD155 on tumor cells, block the CD155/TIGIT pathway, and restore CD8+ T cell activity. In vivo analyses reveal that O-TPNVs achieve synergistic chemotherapeutic and immunotherapeutic effects, effectively inhibiting the recurrence and metastasis of triple-negative breast cancer (4T1) after surgery.
Drug-loaded cellular membrane vesicles promote synergistic therapy for inhibiting post-surgery tumor recurrence and metastasis.
INTRODUCTION
Surgical resection is the main treatment for solid tumors (1). However, postsurgery tumor recurrence and metastasis result in poor recovery and low 5-year survival rates (2–6); accordingly, additional therapies are essential for patients after surgery (7–10). Oxaliplatin (OXA) is a widely used chemotherapy drug in clinical practice and can induce immunogenic cell death (ICD) in tumors, exerting synergistic effects with the immune system (11–15). CD155 is abundantly expressed on the surface of tumor cells and can inhibit the immune system by binding to its co-inhibitory receptor, T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT), on the surface of immune cells, enabling immune escape (16–20). Immunosuppressive signals, such as the CD155/TIGIT pathway, may contribute to the low efficacy of OXA monotherapy (12, 15, 21). We have previously shown that OXA treatment further up-regulates CD155 expression in tumor cells, making tumors more sensitive to anti-CD155 therapy. These findings suggest that the combination of OXA and CD155/TIGIT blocking can exert synergistic therapeutic effects in cancer.
Platelets can target postsurgical wounds and circulating tumor cells (CTCs), effectively improving drug bioavailability and reducing drug side effects (22–26). Therefore, platelets and platelet-derived membrane vesicles are candidates for drug delivery in postsurgery cancer therapy. Many platelet-based drugs have been developed for tumor therapy after surgery (9, 27–30).
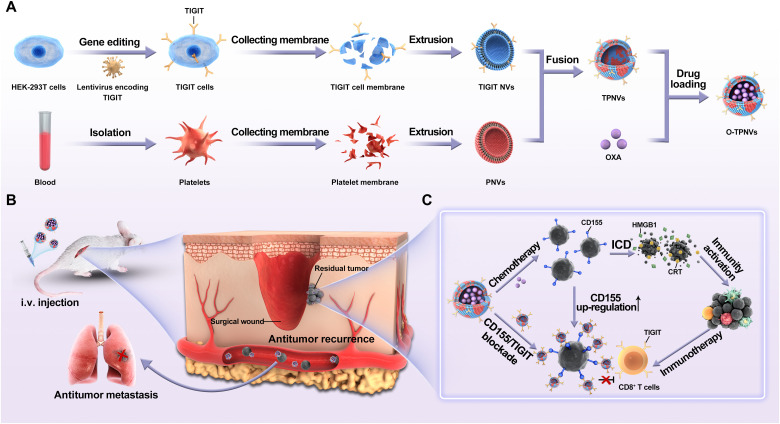
In this study, we report the use of O-TPNVs, which are TIGIT-expressing cell membrane and platelet cell membrane fusion nanovesicles (TPNVs) loaded with OXA, as a synergistic treatment for postsurgery tumor recurrence and metastasis. TIGIT-expressing human embryonic kidney (HEK) 293T cells (TIGIT cells) were established by gene editing. Cell membranes were isolated from TIGIT cells or platelets to prepare TIGIT-expressing cell membrane nanovesicles (TIGIT NVs) or platelet cell membrane NVs (PNVs), respectively. TPNVs were formed by the fusion of TIGIT NVs and PNVs, and OXA-loaded TPNVs (O-TPNVs) were then obtained (Fig. 1A). O-TPNVs target postsurgery wounds and CTCs, and OXA released from O-TPNVs directly kills residual tumor cells and CTCs, induces ICD, and activates the immune system. Acting synergistically with OXA, TPNVs bind to CD155, block the CD155/TIGIT pathway, and enhance the antitumor response of the tumor-infiltrating CD8+ T cells (Fig. 1, B and C). The ability of O-TPNVs to inhibit tumor cell growth, activate the immune system, and target postsurgery wounds was assessed in the present study. We further evaluated the efficacy of O-TPNVs on 4T1 tumor recurrence, metastasis, and survival in postsurgery mouse models; moreover, we discussed the potential use of O-TPNV potential for future clinical application.
Fig. 1. Schematic of O-TPNVs for cancer immunotherapy.
(A) Schematic for the preparation of O-TPNVs. (B and C) Schematic for antitumor effects, including effects against tumor recurrence and metastasis after surgery, of O-TPNVs in vivo. i.v., intravenous.
RESULTS
Up-regulation of CD155 in tumor cells after OXA treatment
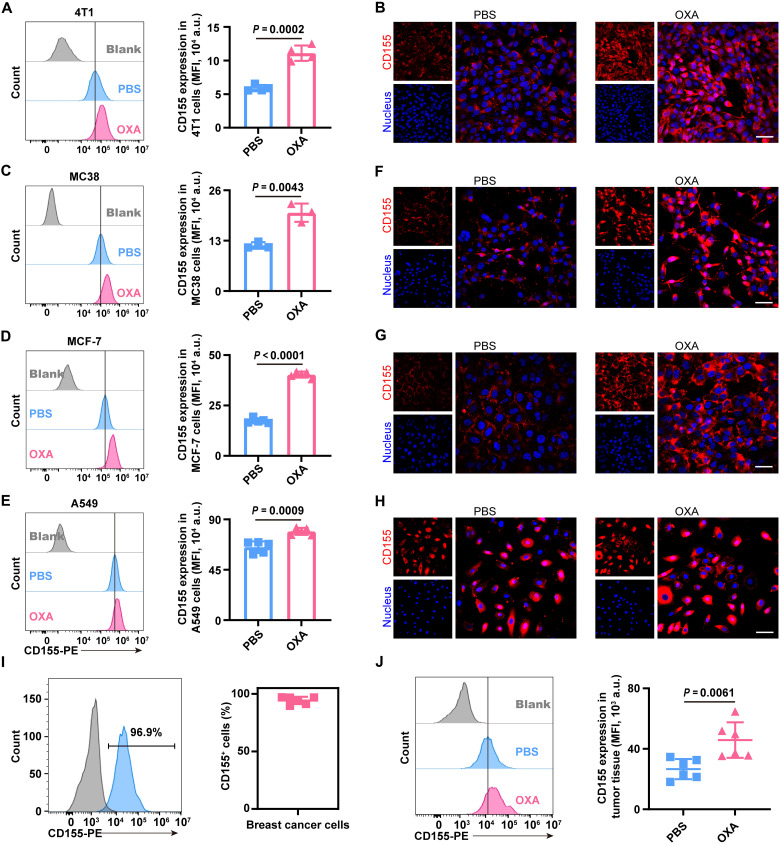
In our previous work, we observed an interesting phenomenon that CD155 expression is up-regulated in tumor cells after OXA treatment. Murine 4T1 breast cancer cells (4T1 cells) were treated with OXA (20 μg/ml) for 24 hours, while cells treated with an equal volume of phosphate-buffered saline (PBS) were used as a control. Up-regulation of CD155 expression in 4T1 cells was revealed by flow cytometry (Fig. 2A, left, and fig. S1A). Compared to that in PBS-treated 4T1 cells, the mean fluorescence intensity (MFI) representing CD155 expression was 1.88-fold higher in OXA-treated 4T1 cells (Fig. 2A, right). The up-regulation of CD155 expression in 4T1 cells after OXA treatment was qualitatively revealed by an immunofluorescence assay (Fig. 2B). This phenomenon was further observed in other tumor cells, including murine MC38 colon carcinoma cells (MC38 cells), human MCF-7 breast cancer cells (MCF-7 cells), and human A549 pulmonary carcinoma cells (A549 cells). After OXA treatment, CD155 expression levels in MC38 cells (P = 0.0043), MCF-7 cells (P < 0.0001), or A549 cells (P = 0.0009) were obviously up-regulated compared to that in PBS-treated cells (Fig. 2, C to E, and fig. S1, B to D). The MFI values representing CD155 expression in OXA-treated MC38 cells, MCF-7 cells, or A549 cells were 1.72-fold, 2.32-fold, or 1.22-fold higher than those in PBS-treated MC38 cells, MCF-7 cells, or A549 cells, respectively. Consistently, the up-regulation of CD155 in MC38 cells, MCF-7 cells, or A549 cells after OXA treatment was also supported by an immunofluorescence analysis (Fig. 2, F to H).
Fig. 2. Up-regulation of CD155 in tumor cells after OXA treatment.
(A and B) Expression of CD155 in 4T1 cells after OXA treatment. (A) Representative flow cytometry image (left) of 4T1 cells treated with PBS or OXA and the corresponding quantification (right) of CD155 MFI (n = 4 biological replicates per group). (B) Representative confocal images of 4T1 cells treated with PBS or OXA (red, CD155; blue, nuclei). Scale bar, 50 μm. (C to E) Expression of CD155 in MC38 cells (C), MCF-7 cells (D), or A549 cells (E) after OXA treatment. Representative flow cytometry image (left) and the corresponding quantification (right) of CD155 MFI. n = 3 biological replicates per group for (C), and n = 5 biological replicates per group for (D) and (E). (F to H) Representative confocal images of MC38 cells (F), MCF-7 cells (G), or A549 cells (H) treated with PBS or OXA (red, CD155; blue, nuclei). Scale bars, 50 μm. (I) In vivo CD155 expression on tumor cells in 4T1 tumor-bearing mice. Representative flow cytometry image (left) and frequency (right) of CD155+ tumor cells in 4T1 tumors (n = 6 biologically independent mice per group). (J) In vivo CD155 expression on tumor cells in 4T1 tumor-bearing mice after treatment with PBS or OXA. Representative flow cytometry image (left) and the quantification of CD155 expressed as MFI (right) (n = 6 biologically independent mice per group). Statistical significance was calculated by unpaired two-tailed t test for (A), (C) to (E), and (J). a.u., arbitrary units.
CD155 expression in tumors was further studied in vivo. Flow cytometry analyses of murine 4T1 tumors indicated the robust expression of the CD155 protein in tumor cells. The frequency of EpCAM+CD155+ tumor cells in murine 4T1 tumors was greater than 90% (Fig. 2I and fig. S2). Consistent with results of in vitro analyses, CD155 expression in EpCAM+ 4T1 tumor cells was further up-regulated (P = 0.0061) after OXA treatment (Fig. 2J). The MFI representing CD155 expression in EpCAM+ 4T1 tumor cells from OXA-treated tumor-bearing mice was 1.72-fold higher than that in EpCAM+ 4T1 tumor cells from PBS-treated tumor-bearing mice.
Preparation and characterization of O-TPNVs
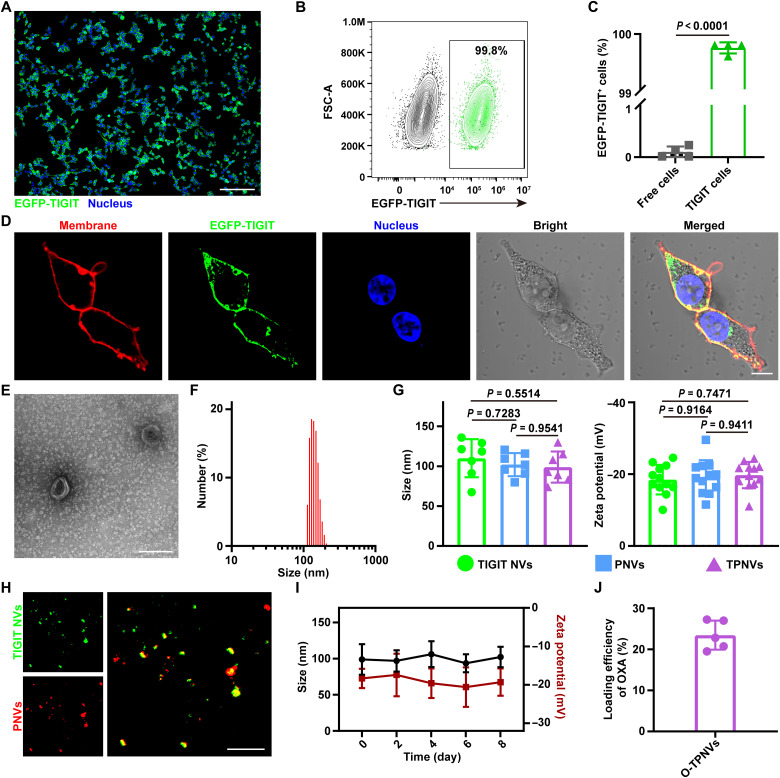
HEK-293T cells (free cells) were infected by a lentivirus encoding the TIGIT gene with an enhanced green fluorescent protein (EGFP) tag fused at the C-terminal region. Puromycin selection was performed to obtain EGFP-TIGIT–expressing HEK-293T cells (EGFP-TIGIT cells). In a wide field of vision, obvious green fluorescence was observed on almost all cells by confocal laser scanning microscopy (CLSM) (Fig. 3A and fig. S3). EGFP-TIGIT–positive cells accounted for more than 99% of cells analyzed by flow cytometry (Fig. 3, B and C). TIGIT was mainly expressed and localized on the cell membrane, as revealed by the good colocalization of green fluorescence from EGFP-TIGIT and red fluorescence from 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) (Fig. 3D and fig. S4). Using the same method, HEK-293T cells (free cells) were infected with a lentivirus encoding the TIGIT gene to establish TIGIT cells for subsequent research.
Fig. 3. Characterization of O-TPNVs.
(A) Confocal images of the stable cell line expressing TIGIT protein (green, EGFP-TIGIT; blue, nuclei). Scale bar, 200 μm. (B and C) Expression of TIGIT on stable cells analyzed by flow cytometry. Representative flow cytometry image (B) and frequency of TIGIT+ cells (C) (n = 4 biological replicates per group). (D) Confocal images of stable cells expressing TIGIT protein on the cell membrane (red, plasma membranes stained with DiI; green, EGFP-TIGIT; blue, nuclei stained with DAPI). Scale bar, 10 μm. (E) TEM image of TPNVs. Scale bar, 200 nm. (F) Particle size distribution of TPNVs analyzed by DLS. (G) Size (left) and zeta potential (right) of TIGIT NVs, PNVs, and TPNVs (n = 7 biologically independent samples for size, and n = 12 biologically independent samples for zeta potential). (H) Confocal images of TPNVs (green, TIGIT NVs labeled with DiO; red, PNVs labeled with DiI). Scale bar, 10 μm. (I) Stability of TPNVs. Changes in size and zeta potential of TPNVs in PBS over a specified period of time (n = 5 biologically independent samples for size, and n = 6 biologically independent samples for zeta potential). (J) Loading efficiency of OXA in TPNVs (n = 5 biological replicates per group). Statistical significance was calculated by unpaired two-tailed t test for (C) or one-way analysis of variance (ANOVA) with a Tukey post hoc test for (G).
The cell membranes derived from TIGIT cells were collected to prepare TIGIT NVs, and the cell membranes derived from platelets were collected to prepare PNVs in a similar way. After TIGIT NVs and PNVs were mixed, sonicated, and extruded, TPNVs were obtained. TPNVs were ellipsoid, as observed by transmission electron microscopy (TEM) (Fig. 3E). A dynamic light scattering (DLS) analysis showed that TPNVs are NVs with a size of about 100 nm and zeta potential of about −20 mV; there were no obvious differences in size and zeta potential among TIGIT NVs, PNVs, and TPNVs (Fig. 3, F and G, and fig. S5). A notable overlap of green fluorescence from 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO)–labeled TIGIT NVs and red fluorescence from DiI-labeled PNVs was observed by CLSM, indicating the successful preparation of TPNVs by the fusion of TIGIT NVs and PNVs (Fig. 3H). In addition, there were no obvious changes in the size and zeta potential of TPNVs, indicating that stability in PBS or 10% serum was high for at least 1 week (Fig. 3I and fig. S6). O-TPNVs were formed by sonication, and the drug loading efficiency of OXA was approximately 23.47% (Fig. 3J). Free cell membrane and platelet cell membrane fusion NVs (FPNVs) and OXA-loaded FPNVs (O-FPNVs) were prepared as controls for later experiments following the same methods.
In vitro biological effects and in vivo wound targeting
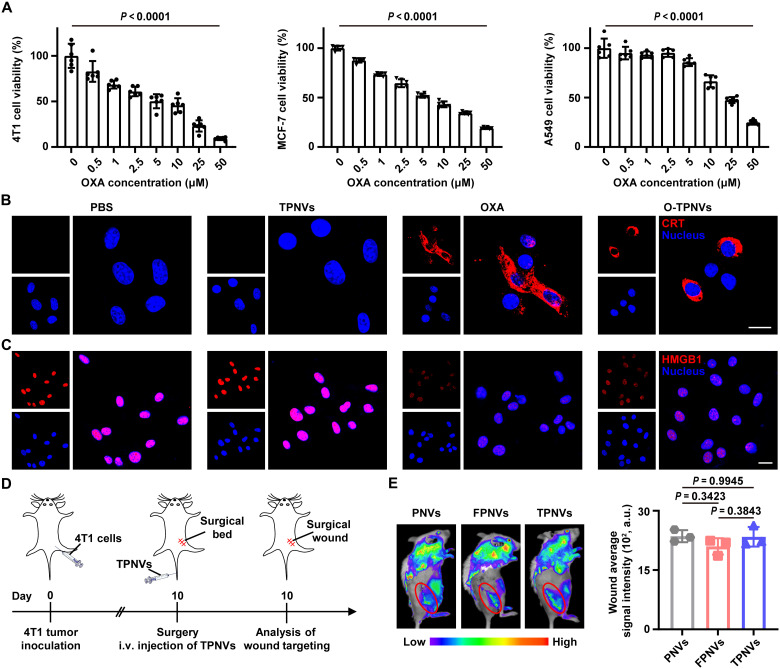
To test the cytotoxic ability of O-TPNVs against tumor cells, 4T1, MC38, B16F10, MCF-7, A549, or HeLa cells were treated with O-TPNVs at different concentrations. O-TPNVs remarkably (P < 0.0001 between 0 μM and 50 μM groups) inhibited the activity and growth of all mouse and human tumor cells examined (Fig. 4A and fig. S7). 4T1 cells were then treated with PBS, TPNVs, OXA, or O-TPNVs (20 μg of TPNVs and 20 μM OXA) to evaluate the induction of ICD. The CRT protein was obviously expressed on the surface of OXA- or O-TPNV–treated 4T1 cells and was expressed at very low levels on the surface of PBS- or TPNV-treated 4T1 cells (Fig. 4B). High HMGB1 protein levels were detected in the nuclei of PBS- or TPNV-treated 4T1 cells, whereas only low levels were detected in the nuclei of OXA- or O-TPNV–treated 4T1 cells (Fig. 4C). The CRT expression on the cell surface and HMGB1 release from the cell nuclei indicated the ability of O-TPNVs and OXA to induce ICD in vitro.
Fig. 4. In vitro biological effects and in vivo targeting.
(A) Viabilities of different tumor cells (4T1, MCF-7, or A549 cells) after 48 hours of incubation with O-TPNVs, with different OXA concentrations (0, 0.5, 1, 2.5, 5, 10, 25, or 50 μM) (n = 6 biological replicates per group). (B) Immunofluorescence images of CRT exposure on the surface of 4T1 cells after different treatments as indicated (red, CRT; blue, nuclei). Scale bar, 20 μm. (C) Immunofluorescence images showing HMGB1 release in 4T1 cells after different treatments as indicated (red, HMGB1; blue, nuclei). Scale bar, 20 μm. (D) Schematic showing the wound targeting analysis schedule for postsurgical mice. (E) Fluorescence imaging (left) and the corresponding fluorescence intensity of wound sites (right) at 2 hours after intravenous injection of PNVs, FPNVs, or TPNVs labeled with Cy5.5-NHS (n = 3 biologically independent mice per group). Statistical significance was calculated by one-way ANOVA with a Tukey post hoc test for (A) and (E).
Platelets and PNVs could effectively target postsurgery wounds (9, 22, 23, 27). To test whether TPNVs exhibit effective wound targeting in vivo, we conducted experiments using 4T1 tumor-bearing mice. After surgery, the mice were intravenously injected with Cy5.5-NHS–labeled PNVs, FPNVs, or TPNVs and detected 2 hours after injection using a NightOWL imaging system (Fig. 4D). TPNVs and FPNVs, like PNVs, effectively accumulated in postsurgery wounds (Fig. 4E, left). There were no obvious differences (P = 0.3423 between PNV- and FPNV-treated mice, P = 0.9945 between PNV- and TPNV-treated mice, and P = 0.3843 between FPNV- and TPNV-treated mice) in the fluorescence intensity at the wound sites among PNV-, FPNV-, or TPNV-treated mice, indicating that the levels of FPNV and TPNV accumulation in wound sites are similar to those of PNVs (Fig. 4E, right). These findings suggested that not only PNVs are effective for targeted delivery after surgery but also membrane fusion technology and the formed hybrid cell membrane NVs (HNVs) have wide applications by combining the original properties of various components.
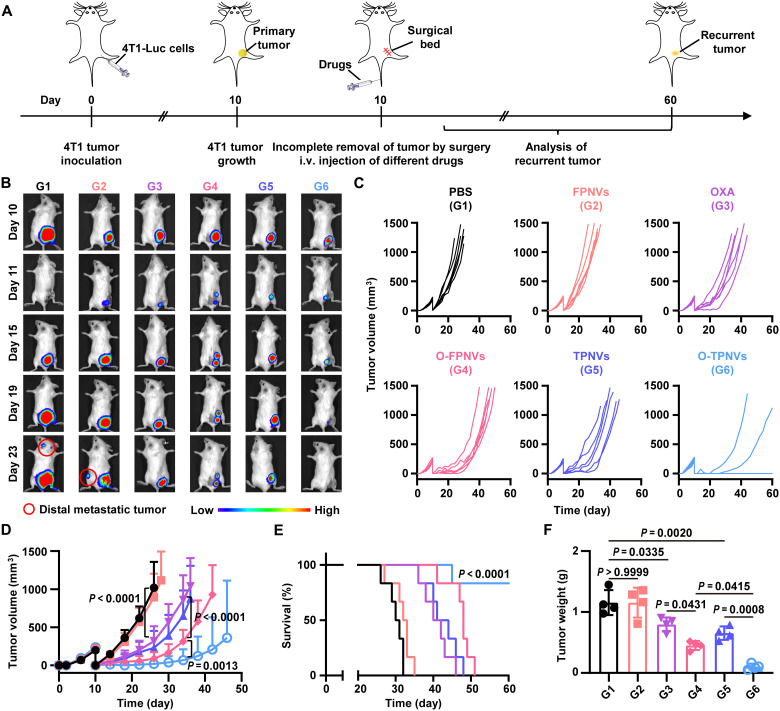
In vivo study on inhibiting postsurgery tumor recurrence
We evaluated the therapeutic effects of O-TPNVs on inhibition of tumor recurrence after surgery. Mice were subcutaneously injected with 4T1-Luc cells, and ~99% of the primary tumors were removed on day 10. After surgery, the mice were treated with PBS, FPNVs, OXA, O-FPNVs, TPNVs, or O-TPNVs on days 10, 13, 16, and 19 as per the experimental schedule (Fig. 5A). Tumor recurrence was monitored by bioluminescence signals from 4T1-Luc tumor cells and recurrent tumor size. The bioluminescence signals and growth curves showed that tumor recurrence was fastest in mice treated with PBS or FPNVs, followed by mice treated with O-FPNVs or TPNVs, and was slowest in mice treated with O-TPNVs (Fig. 5, B to D). O-FPNVs and TPNVs showed limited inhibitory effects on tumor recurrence, while O-TPNVs effectively inhibited tumor recurrence, with no obvious recurrence detected in four of six mice after surgery. Notably, O-FPNVs showed better therapeutic efficacy than that of OXA, further confirming the role of platelet-derived membrane vesicles in targeted drug delivery and enhancing drug efficacy. Obvious distal metastases were observed in PBS- and FPNV-treated mice, whereas no metastases were observed in O-TPNV–treated mice (Fig. 5B). In addition, mouse survival, which correlated with the size of the recurrent tumor, was also recorded. Because of the inhibition of postsurgery tumor recurrence, the survival of OXA-, O-FPNV–, TPNV-, or O-TPNV–treated mice was effectively improved compared with that of PBS-treated mice (Fig. 5E); among them, O-TPNV–treated mice had the longest survival time, with a survival rate of 83.3% on day 60.
Fig. 5. In vivo study on inhibiting tumor recurrence after surgery.
(A) Schematic representation of the therapy schedule in a 4T1 tumor recurrence mouse model. (B) Representative bioluminescence images of 4T1 tumor recurrence in mice. (C and D) Individual (C) and average (D) recurrent tumor growth kinetics in different treatment groups as indicated (n = 6 biologically independent mice per group). (E) Survival curves for mice (n = 6 biologically independent mice per group). (F) Weight of recurrent tumors collected from mice (n = 4 biologically independent mice per group). Statistical significance was calculated by two-way ANOVA with a Tukey post hoc test for (D), log-rank (Mantel-Cox) test for (E), or one-way ANOVA with a Tukey post hoc test for (F).
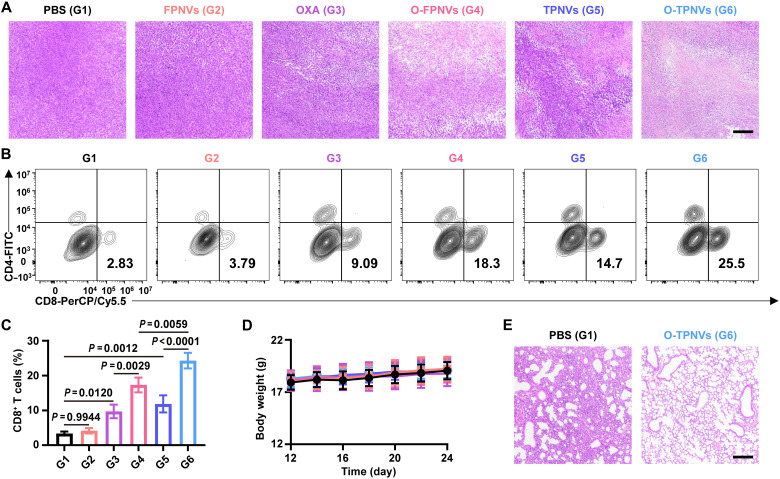
Recurrent tumors from mice in different treatment groups were collected for further analysis. Consistently, the intuitive size and weight of the tumors also indicated that O-TPNVs effectively inhibit postsurgery tumor recurrence, and supported that the targeted delivery and increased efficacy were caused by platelet-derived membrane vesicles (Fig. 5F and fig. S8). Hematoxylin and eosin (H&E) staining of tumor tissues visually showed the mass death of tumor cells after O-TPNV treatment (Fig. 6A). CD8+ T cell infiltration in the tumor microenvironment was also analyzed to assess the immune response in mice after different treatments. The frequency of CD8+ T cells was substantially higher after treatment with O-TPNVs than with O-FPNVs or TPNVs (P = 0.0059 or P < 0.0001, respectively) (Fig. 6, B and C, and fig. S9), suggesting that the combination therapy of OXA and CD155/TIGIT blockade could effectively enhance the antitumor immune response, further confirming the synergistic effect of chemotherapy and immunotherapy.
Fig. 6. In vivo antitumor immune response of O-TPNVs.
(A) Representative H&E staining of recurrent tumors collected from the mice in different groups. Scale bar, 100 μm. (B and C) Representative flow cytometric analysis images (B) and relative quantification (C) of infiltrating CD8+ T cells in recurrent tumors (n = 3 biologically independent mice per group). (D) Body weights of mice in different groups (n = 6 biologically independent mice per group). (E) H&E staining of lungs collected from the mice treated with PBS and O-TPNVs. Scale bar, 100 μm. Statistical significance was calculated by one-way ANOVA with a Tukey post hoc test for (C).
In addition, the in vivo safety of O-TPNVs was investigated. There was no notable difference in mouse weight after treatment (Fig. 6D and fig. S10). No obvious damage was found in major organs by histological analyses (fig. S11). Notably, consistent with the results shown in Fig. 5B, H&E staining of lung tissues showed obvious and severe lung metastases in PBS-treated mice but not in mice treated with O-TPNVs (Fig. 6E).
In vivo study of protective effects against tumor metastasis
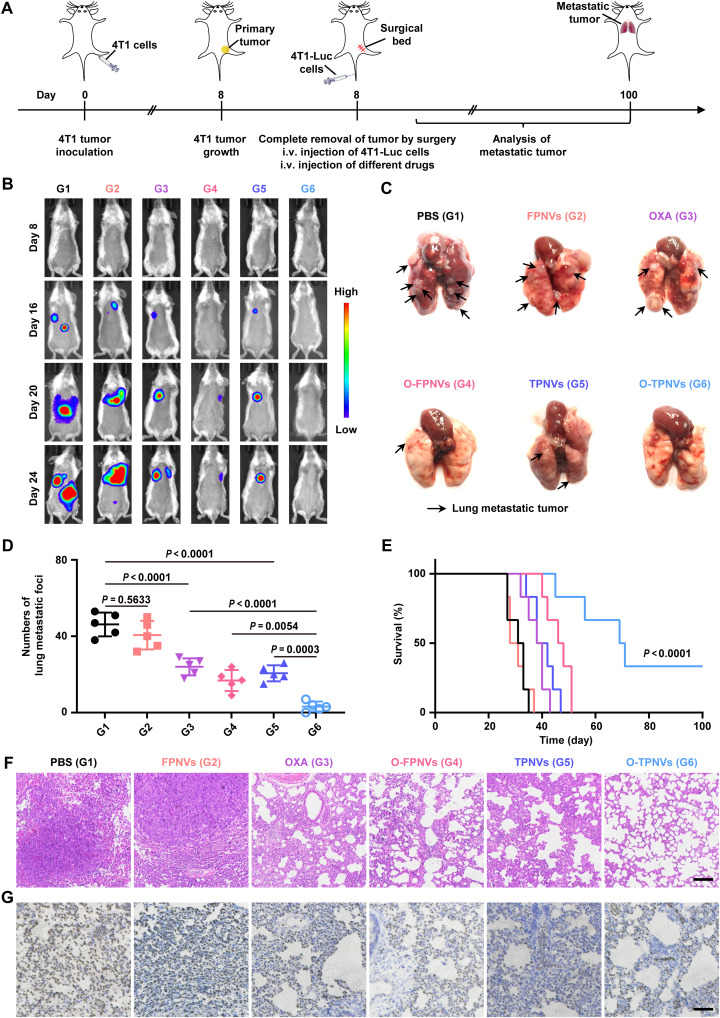
Breast cancer is a highly metastatic cancer (31, 32), and surgery may increase the risk of metastasis (1, 2, 5). In the above work, metastasis to distal and lung tissues was observed in postsurgical mice, whereas O-TPNV–treated mice showed no observable tumor metastasis. To further evaluate the effect of O-TPNVs against tumor metastasis after surgery, we conducted experiments in a mouse model. Mice were subcutaneously injected with 4T1 cells; primary tumors were surgically removed completely on day 8, and the mice were then injected with 4T1-Luc cells via the tail vein to imitate the metastasis process of CTCs after surgery. The mice received intravenous injections of PBS, FPNVs, OXA, O-FPNVs, TPNVs, or O-TPNVs on days 8, 11, 14, and 17 according to the experimental schedule (Fig. 7A). The process of tumor metastasis was monitored by bioluminescence signals from 4T1-Luc tumor cells (Fig. 7B). Lung metastasis was severe in PBS- and FPNV-treated mice; mild in OXA-, O-FPNV–, and TPNV-treated mice; and lowest in O-TPNV-treated mice, with no obvious lung metastasis observed in some mice in this group (Fig. 7, C and D). The survival status, which correlated with the degree of lung metastasis, was also monitored. Compared with the short survival time of PBS-treated mice, the survival time of O-TPNV–treated mice was prolonged (P < 0.0001), and this can be attributed to the effective antitumor metastasis effect of O-TPNVs (Fig. 7E). The survival rates of O-TPNV–treated mice were 66.7 and 33.3% on days 60 and 100, respectively; in contrast, no mice in the other groups survived past day 51. In addition, H&E and Ki-67 staining of mouse lungs further demonstrated the limited effects of O-FPNVs and TPNVs and the substantial antitumor metastasis effect of O-TPNVs (Fig. 7, F and G).
Fig. 7. In vivo study on the effect of antitumor metastasis.
(A) Schematic representation of the therapy schedule in a 4T1 tumor metastasis mouse model. (B) Representative bioluminescence images of 4T1 tumor metastasis in mice. (C) Representative lung images in different groups as indicated. The black arrows indicate the lung metastasis tumors. (D) Numbers of lung metastatic foci of mice (n = 5 biologically independent mice per group). (E) Survival curves for mice (n = 6 biologically independent mice per group). (F and G) Representative images of lung tissue stained with H&E (F) and Ki-67 (G). Scale bars, 500 μm. Statistical significance was calculated by one-way ANOVA with a Tukey post hoc test for (D) or log-rank (Mantel-Cox) test for (E).
DISCUSSION
In summary, we developed a targeted delivery system for synergistic chemotherapy and immunotherapy as a therapeutic strategy for postsurgery tumor recurrence and metastasis. Platelet membrane components enabled O-TPNVs to effectively target postsurgery wounds and CTCs. At the target sites, OXA killed tumor cells and caused ICD, resulting in immune system activation. CD155, which was abundant in tumor cells, was further up-regulated after OXA treatment. TPNVs blocked the CD155/TIGIT pathway, and reverted tumor-infiltrating CD8+ T cells from the exhausted state to eradicate residual tumor cells and CTCs, demonstrating a synergistic effect with OXA. Our results showed that O-TPNVs effectively inhibit the recurrence and metastasis of 4T1 tumors in mice and improve the survival rate of mice after surgery.
However, several challenges in the clinical application of O-TPNVs remain (33, 34). The first and most important issue is the safety of the nanodrug (33, 35–38). Our study was limited to preliminary and basic in vivo toxicity tests, and a large number of systematic toxicological tests are needed to further verify the safety of O-TPNVs (35–37, 39, 40). In addition, quality control of drugs is essential. Stability studies of multiple batches of drugs are needed to guarantee the stability of efficacy (34, 36). A large number of cell membranes can be obtained by in vitro culture of gene-edited cells, providing a stable source for the mass preparation of TIGIT NVs and a basis for further industrialization (27, 34, 36). However, various issues need to be considered. For example, variation in TIGIT expression among batches of cells in different culture environments may lead to differences in the potency of the resulting TPNVs and unpredictable effects. Notably, ensuring an adequate source of platelets is also an urgent problem. Therefore, in the future, careful quality monitoring and control of O-TPNVs in the industrialization process are needed, including strictly ensuring the stable and consistent cell culture conditions (medium composition, temperature and gas composition, etc.), ensuring the stability and consistency of extruder and other equipment parameters during the preparation of TPNVs, and improving methods for monitoring drug quality. Roche pushed tiragolumab, a monoclonal antibody against TIGIT, into phase 3 clinical trials based on promising results in phase 1 and 2 clinical trials (41–43). Although the phase 3 trial of tiragolumab and Tecentriq (atezolizumab, a monoclonal antibody against PD-L1) in non–small cell lung cancer was not promising, it was not a complete failure, as it showed a notable improvement over placebo in phase 1 and 2 clinical trials (42–44). In addition, a nonideal therapeutic effect in non–small cell lung cancer does not also mean a poor therapeutic effect in other cancers. It may also suggest that the simple addition of different immune checkpoint inhibitors is not the key to a breakthrough in cancer treatment. In contrast, the combination of immunotherapy (CD155/TIGIT blocking) and chemotherapy (OXA) in this study seems to be more attractive because of their effective synergies. Currently, many other drugs targeting CD155/TIGIT are in various stages of clinical trials, and the important role of this pathway in tumor immunity makes researchers very optimistic about its prospects (16–18, 45, 46).
Despite these challenges, O-TPNVs and its derivatives have promising applications. HNVs prepared by the fusion of multiple cell membranes retain the properties of the original membrane components and have great potential for use as a drug delivery platform with a wide range of applications (27, 47–49). On the basis of our analyses of postsurgery mouse models, we believe that O-TPNVs have great potential in postsurgery cancer therapy and that HNVs will be an important complementary therapeutic strategy for cancer and other diseases in the future.
MATERIALS AND METHODS
Supplementary materials and methods are shown in the Supplementary Materials.
Study design
In this research work, the main aim is to investigate the antitumor recurrence and metastasis effects of drug-loaded HNVs, O-TPNVs, in the mouse postsurgery models. O-TPNVs were prepared by fusing gene-edited TIGIT-expressing cell membranes with platelet membranes and loading OXA. First, the physicochemical properties (morphology, size, zeta potential, and drug loading efficiency) and stability of TPNVs were characterized. In vitro, the cytotoxicity of O-TPNVs against various tumor cells and their ability to induce ICD in 4T1 tumor cells were analyzed. The ability of TPNVs to target postsurgical wounds was evaluated in 4T1 tumor–resected models. Subsequently, we evaluated the efficacy of O-TPNVs against recurrence in a 4T1 incomplete tumor resection mouse model: in vivo bioluminescence imaging monitoring of tumor recurrence, recording the size of recurrent tumors, and monitoring the survival status of mice. The immune responses induced by O-TPNVs were analyzed by detecting the infiltration of CD8+ T cells in the recurrent tumors. H&E staining of lung tissue and in vivo bioluminescence imaging of tumors were used to preliminarily analyze the ability of O-TPNVs against tumor pulmonary and distal metastasis after surgery. In vivo safety was evaluated by weight change and histopathology analysis of major organs in mice. Last, the antimetastasis effects of O-TPNVs were specifically evaluated in a 4T1 complete tumor resection mouse model, and lung metastasis was further analyzed, including in vivo bioluminescence imaging observation of lung metastasis, counting the number of lung metastases, and H&E and Ki-67 staining of lung tissues. Sample sizes and replication, which were determined according to previously published reports, were represented in detail in the corresponding figure legends. Mice were randomly divided into different groups before treatment begin in animal studies. For all experiments, we report summary results from multiple experiments or display data corresponding to a representative experiment of at least three experiments. No formal blinding was used in experiments.
In vitro detection of CD155 expression in tumor cells
To examine CD155 by flow cytometry, 4T1, MC38, MCF-7, or A549 tumor cells were treated with PBS or OXA (20 μg/ml). After 24 hours, the cells were collected, washed with PBS, and stained with anti-mouse or anti-human CD155-PE (phycoerythrin) antibodies. The cells were then detected using a flow cytometer (CytoFLEX, Beckman Coulter, Brea, CA, USA).
To examine CD155 by immunofluorescence, the tumor cells were also treated with PBS or OXA (20 μg/ml) for 24 hours. Then, the cells were immobilized by 4% paraformaldehyde for 15 min and blocked by 3% bovine serum albumin solution for at least 30 min. The cells were incubated with anti-CD155 antibodies and followed by DY647-conjugated secondary antibodies. 4′,6-Diamidino-2-phenylindole (DAPI) solution was added to stain the nuclei. Last, the cells were detected by CLSM (LSM880, Zeiss, Oberkochen, Germany).
In vivo detection of CD155 expression in tumors
The 4T1 tumor-bearing mice were randomly divided into two groups and treated with PBS or OXA (5 mg/kg). The tumors were collected 24 hours after treatment and prepared into single-cell suspensions. After blocking with anti-mouse CD16/32 antibodies, the cells were stained with the anti-mouse EpCAM-FITC (fluorescein isothiocyanate) and anti-mouse CD155-PE antibodies according to the manufacturer’s instructions. CD155 expression in 4T1 tumors was then detected using the flow cytometer.
Establishment and characterization of TIGIT cells
HEK-293T cells (free cells) were infected with a lentivirus encoding the murine TIGIT gene fused at the C-terminal region with EGFP tag and then cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and puromycin (2 μg/ml) to select cells stably expressing EGFP-TIGIT for about 2 weeks. Established EGFP-TIGIT cells were maintained in complete medium containing puromycin (1 to 2 μg/ml). The EGFP-TIGIT cells were detected by flow cytometry and CLSM. Similarly, HEK-293T cells (free cells) were infected with a lentivirus encoding the murine TIGIT gene for the establishment of TIGIT cells.
Preparation and characterization of TPNVs
First, TIGIT NVs and PNVs were prepared. For TIGIT NVs, TIGIT cells were collected. The TIGIT-expressing cell membranes derived from TIGIT cells were isolated by multistep density gradient ultracentrifugation. The collected membranes were sonicated, followed by extrusion through 1-, 0.4-, 0.2-, and 0.1-μm polycarbonate porous membrane filters using a mini extruder (Avanti Polar Lipids, Alabaster, AL, USA). The suspension was further centrifuged at 100,000g for 90 to 120 min to collect TIGIT NVs. The TIGIT NVs were then resuspended and further purified by washing with PBS. Analogously, the free cell membrane NVs (free NVs) were prepared in the same way using HEK-293T cells (free cells) without gene editing.
For PNVs, the platelets were first isolated from whole blood according to previous reports (27, 28). Next, platelet membranes were acquired from the platelets by a freeze-thaw process. The platelet membranes were also sonicated and extruded through 1-, 0.4-, 0.2-, and 0.1-μm polycarbonate porous membrane filters to prepare PNVs. The PNVs were resuspended and further purified by washing with PBS.
After obtaining the two single-cell membrane NVs, TPNVs were prepared as previously reported, with slight modifications (27, 50, 51). Briefly, the TIGIT NVs and PNVs were mixed at a ratio of 4:1 (protein weight), sonicated, and then extruded through 0.1-μm polycarbonate porous membrane filters using the mini extruder. The TPNVs could be further purified by anti-CD14 antibody–modified magnetic beads. Analogously, FPNVs were prepared as a control following the same methods using free NVs and PNVs. The TPNVs were detected and analyzed by TEM (JEM-1400, JEOL, Tokyo, Japan), DLS (90Plus PALS, Brookhaven, Holtsville, NY, USA), and CLSM.
Animal use and care
BALB/c female mice were obtained from the Laboratory Animal Center of Sun Yat-sen University (Guangzhou, China). The mice were about 4 to 6 weeks old when they were obtained. The animal study followed the guidelines for Laboratory Animal Care and was approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University (SYSU-IACUC-2022-000199).
In vivo wound targeting
To evaluate the wound targeting effects of TPNVs, mice were subcutaneously injected with 5 × 105 4T1 cells into the right flank. On day 10, the primary tumors were resected, retaining about 1% tumors for imitating the residual microtumors in the surgical bed. Immediately, the mice were intravenously injected with 100 μg of Cy5.5-NHS–labeled PNVs, FPNVs, or TPNVs. In vivo fluorescence images and intensities were recorded by using a NightOWL imaging system (LB983, Berthold, Wildbad, Germany) 2 hours after injection.
Postsurgery tumor recurrence model
First, 5 × 105 4T1-Luc cells were subcutaneously injected into the right flank of each BALB/c mouse. On day 10, the primary tumors were resected, retaining about 1% tumors to imitate the residual microtumors in the surgical bed. The mice were then randomly divided into six groups and received intravenous injections of PBS, FPNVs, OXA, O-FPNVs, TPNVs, or O-TPNVs (25 mg of FPNVs or TPNVs and 5 mg of OXA per kilogram body weight) every 3 days for four cycles. Recurrent tumor development was observed and analyzed within 60 days. The in vivo bioluminescence images of recurrent tumors were obtained using an IVIS spectrum system (PerkinElmer). Recurrent tumor sizes were measured using a digital caliper. When mice exhibited signs of impaired health or the tumor size exceeded 1500 mm3, individuals were euthanized. In addition, recurrent tumors were collected and further examined by flow cytometry and histological analyses.
In vivo evaluation of antitumor metastasis
Each BALB/c mouse was first subcutaneously injected with 5 × 105 4T1 cells into the right flank. The primary tumors were completely resected by surgery on day 8. Each mouse was then intravenously injected with 3 × 105 4T1-Luc cells to imitate CTCs escaping from the primary tumor into the circulatory system. Then, the mice were treated with PBS, FPNV, OXA, O-FPNV, TIGIT PNV, or O-TPNV treatment on days 8, 11, 14, and 17. The development of tumor metastasis was observed and analyzed within 100 days. The in vivo bioluminescence images of metastatic tumors were performed using the IVIS spectrum system. In addition, for further analyses, the lungs of mice with different treatments were harvested and then stained with H&E or Ki-67.
Statistics
All results are shown in the legend and presented as means ± SD. Animal survival curves were assessed by a log-rank (Mantel-Cox) test. An unpaired two-tailed t test was used for two-group comparisons, and one-way or two-way analysis of variance (ANOVA) with a Tukey post hoc test was used for multiple group comparisons (more than two groups). All statistical analyses were carried out using GraphPad Prism 9 software (GraphPad Prism Inc., San Diego, CA, USA).
Acknowledgments
We wish to thank Y.-L.Yu (HUST) and L.Bao (NCNST) for assistance with bioluminescence imaging and discussion of some results. We also acknowledge the use of a series of instruments at Sun Yat-sen University.
Funding: This work was supported by the National Natural Science Foundation of China (31922042 and 82272154), the Shenzhen Science and Technology Program (GXWD20201231165807008 and 20200825175848001), the Fundamental Research Funds for the Central Universities (2021-RC310-005 and 2020-RC320-002), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-1-058), and the Singapore National Research Foundation under its Competitive Research Programme (NRF-CRP26-2021-0002).
Author contributions: Y.Y., Q.C., X.Z., Y.Z., and L.M. initiated and designed the research. Y.Y. and Q.C. performed the experiments and analyzed the data. Y.Y., Q.C., X.Z., Y.Z., and L.M. wrote the manuscript. X.Z., Y.Z., and L.M. contributed materials and analysis tools. All authors contributed to the writing of the manuscript, discussion of the results and implications, and editing of the manuscript at all stages. All authors have given approval to the final version of the manuscript.
Competing interests: L.M. and Y.Y. are inventors on a pending patent related to the technology described here, filed by the China National Intellectual Property Administration (CN202210827720.X, 14 July 2022). The authors declare no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Materials and Methods
Figs. S1 to S11
REFERENCES AND NOTES
- 1.L. Wyld, R. A. Audisio, G. J. Poston,The evolution of cancer surgery and future perspectives. Nat. Rev. Clin. Oncol. 12,115–124 (2015). [DOI] [PubMed] [Google Scholar]
- 2.C. L. Chaffer, R. A. Weinberg,A perspective on cancer cell metastasis. Science 331,1559–1564 (2011). [DOI] [PubMed] [Google Scholar]
- 3.R. L. Siegel, K. D. Miller, A. Jemal,Cancer statistics, 2020. CA Cancer J. Clin. 70,7–30 (2020). [DOI] [PubMed] [Google Scholar]
- 4.D. G. Baker, T. M. Masterson, R. Pace, W. C. Constable, H. Wanebo,The influence of the surgical wound on local tumor recurrence. Surgery 106,525–532 (1989). [PubMed] [Google Scholar]
- 5.S. Tohme, R. L. Simmons, A. Tsung,Surgery for cancer: A trigger for metastases. Cancer Res. 77,1548–1552 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.H. Uramoto, F. Tanaka,Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 3,242–249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.E. Y. Lukianova-Hleb, Y. S. Kim, I. Belatsarkouski, A. M. Gillenwater, B. E. O’Neill, D. O. Lapotko,Intraoperative diagnostics and elimination of residual microtumours with plasmonic nanobubbles. Nat. Nanotechnol. 11,525–532 (2016). [DOI] [PubMed] [Google Scholar]
- 8.S. B. Stephan, A. M. Taber, I. Jileaeva, E. P. Pegues, C. L. Sentman, M. T. Stephan,Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 33,97–101 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.C. Wang, W. Sun, Y. Ye, Q. Hu, H. N. Bomba, Z. Gu,In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 1,0011 (2017). [Google Scholar]
- 10.Q. Chen, C. Wang, X. Zhang, G. Chen, Q. Hu, H. Li, J. Wang, D. Wen, Y. Zhang, Y. Lu, G. Yang, C. Jiang, J. Wang, G. Dotti, Z. Gu,In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 14,89–97 (2019). [DOI] [PubMed] [Google Scholar]
- 11.D. Wang, S. J. Lippard,Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 4,307–320 (2005). [DOI] [PubMed] [Google Scholar]
- 12.S. Rottenberg, C. Disler, P. Perego,The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 21,37–50 (2021). [DOI] [PubMed] [Google Scholar]
- 13.L. Zitvogel, L. Apetoh, F. Ghiringhelli, G. Kroemer,Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 8,59–73 (2008). [DOI] [PubMed] [Google Scholar]
- 14.G. Kroemer, L. Galluzzi, O. Kepp, L. Zitvogel,Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31,51–72 (2013). [DOI] [PubMed] [Google Scholar]
- 15.F. Zhou, B. Feng, H. Yu, D. Wang, T. Wang, Y. Ma, S. Wang, Y. Li,Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv. Mater. 31,e1805888 (2019). [DOI] [PubMed] [Google Scholar]
- 16.X. He, C. Xu,Immune checkpoint signaling and cancer immunotherapy. Cell Res. 30,660–669 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A. C. Anderson, N. Joller, V. K. Kuchroo,Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 44,989–1004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R. J. Johnston, L. Comps-Agrar, J. Hackney, X. Yu, M. Huseni, Y. Yang, S. Park, V. Javinal, H. Chiu, B. Irving, D. L. Eaton, J. L. Grogan,The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell 26,923–937 (2014). [DOI] [PubMed] [Google Scholar]
- 19.D. Ostroumov, S. Duong, J. Wingerath, N. Woller, M. P. Manns, K. Timrott, M. Kleine, W. Ramackers, S. Roessler, S. Nahnsen, S. Czemmel, O. Dittrich-Breiholz, T. Eggert, F. Kühnel, T. C. Wirth,Transcriptome profiling identifies TIGIT as a marker of T-cell exhaustion in liver cancer. Hepatology 73,1399–1418 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Y. Kong, L. Zhu, T. D. Schell, J. Zhang, D. F. Claxton, W. C. Ehmann, W. B. Rybka, M. R. George, H. Zeng, H. Zheng,T-cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T-cell exhaustion and poor clinical outcome in AML patients. Clin. Cancer Res. 22,3057–3066 (2016). [DOI] [PubMed] [Google Scholar]
- 21.S. J. Turley, V. Cremasco, J. L. Astarita,Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 15,669–682 (2015). [DOI] [PubMed] [Google Scholar]
- 22.C. M. Hu, R. H. Fang, K. C. Wang, B. T. Luk, S. Thamphiwatana, D. Dehaini, P. Nguyen, P. Angsantikul, C. H. Wen, A. V. Kroll, C. Carpenter, M. Ramesh, V. Qu, S. H. Patel, J. Zhu, W. Shi, F. M. Hofman, T. C. Chen, W. Gao, K. Zhang, S. Chien, L. Zhang,Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526,118–121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A. T. Nurden, P. Nurden, M. Sanchez, I. Andia, E. Anitua,Platelets and wound healing. Front. Biosci. 13,3532–3548 (2008). [DOI] [PubMed] [Google Scholar]
- 24.E. M. Golebiewska, A. W. Poole,Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 29,153–162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.G. F. Nash, L. F. Turner, M. F. Scully, A. K. Kakkar,Platelets and cancer. Lancet Oncol. 3,425–430 (2002). [DOI] [PubMed] [Google Scholar]
- 26.L. J. Gay, B. Felding-Habermann,Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11,123–134 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.L. Rao, L. Wu, Z. Liu, R. Tian, G. Yu, Z. Zhou, K. Yang, H. G. Xiong, A. Zhang, G. T. Yu, W. Sun, H. Xu, J. Guo, A. Li, H. Chen, Z. J. Sun, Y. X. Fu, X. Chen,Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat. Commun. 11,4909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Q. Hu, W. Sun, C. Qian, C. Wang, H. N. Bomba, Z. Gu,Anticancer platelet-mimicking nanovehicles. Adv. Mater. 27,7043–7050 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Y. Lv, F. Li, S. Wang, G. Lu, W. Bao, Y. Wang, Z. Tian, W. Wei, G. Ma,Near-infrared light–triggered platelet arsenal for combined photothermal-immunotherapy against cancer. Sci. Adv. 7,eabd7614 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.X. Zhang, J. Wang, Z. Chen, Q. Hu, C. Wang, J. Yan, G. Dotti, P. Huang, Z. Gu,Engineering PD-1-presenting platelets for cancer immunotherapy. Nano Lett. 18,5716–5725 (2018). [DOI] [PubMed] [Google Scholar]
- 31.H. Hosseini, M. M. S. Obradović, M. Hoffmann, K. L. Harper, M. S. Sosa, M. Werner-Klein, L. K. Nanduri, C. Werno, C. Ehrl, M. Maneck, N. Patwary, G. Haunschild, M. Gužvić, C. Reimelt, M. Grauvogl, N. Eichner, F. Weber, A. D. Hartkopf, F. A. Taran, S. Y. Brucker, T. Fehm, B. Rack, S. Buchholz, R. Spang, G. Meister, J. A. Aguirre-Ghiso, C. A. Klein,Early dissemination seeds metastasis in breast cancer. Nature 540,552–558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.X. Bai, J. Ni, J. Beretov, P. Graham, Y. Li,Triple-negative breast cancer therapeutic resistance: Where is the Achilles’ heel? Cancer Lett. 497,100–111 (2021). [DOI] [PubMed] [Google Scholar]
- 33.J. Shi, P. W. Kantoff, R. Wooster, O. C. Farokhzad,Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 17,20–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R. Xu, A. Rai, M. Chen, W. Suwakulsiri, D. W. Greening, R. J. Simpson,Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 15,617–638 (2018). [DOI] [PubMed] [Google Scholar]
- 35.K. W. Powers, S. C. Brown, V. B. Krishna, S. C. Wasdo, B. M. Moudgil, S. M. Roberts,Research strategies for safety evaluation of nanomaterials. Part VI. Characterization of nanoscale particles for toxicological evaluation. Toxicol. Sci. 90,296–303 (2006). [DOI] [PubMed] [Google Scholar]
- 36.H. Chen, Z. Gu, H. An, C. Chen, J. Chen, R. Cui, S. Chen, W. Chen, X. Chen, X. Chen, Z. Chen, B. Ding, Q. Dong, Q. Fan, T. Fu, D. Hou, Q. Jiang, H. Ke, X. Jiang, G. Liu, S. Li, T. Li, Z. Liu, G. Nie, M. Ovais, D. Pang, N. Qiu, Y. Shen, H. Tian, C. Wang, H. Wang, Z. Wang, H. Xu, J. F. Xu, X. Yang, S. Zhu, X. Zheng, X. Zhang, Y. Zhao, W. Tan, X. Zhang, Y. Zhao,Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 61,1503–1552 (2018). [Google Scholar]
- 37.X. Liu, I. Tang, Z. A. Wainberg, H. Meng,Safety considerations of cancer nanomedicine-a key step toward translation. Small 16,e2000673 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.S. Zhu, L. Li, Z. Gu, C. Chen, Y. Zhao,15 years of small: Research trends in nanosafety. Small 16,e2000980 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Z. Magdolenova, A. Collins, A. Kumar, A. Dhawan, V. Stone, M. Dusinska,Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 8,233–278 (2014). [DOI] [PubMed] [Google Scholar]
- 40.L. Yan, F. Zhao, J. Wang, Y. Zu, Z. Gu, Y. Zhao,A safe-by-design strategy towards safer nanomaterials in nanomedicines. Adv. Mater. 31,1805391 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Roche, “Roche’s novel anti-TIGIT tiragolumab granted FDA breakthrough therapy designation in combination with Tecentriq for PD-L1-high non-small cell lung cancer,” news release, 5 January 2021; http://bit.ly/2LoNrj2.
- 42.B. C. Cho, D. Rodriguez-Abreu, M. Hussein, M. Cobo, A. Patel, N. Secen, G. Gerstner, D. W. Kim, Y. G. Lee, W. C. Su, E. Huang, N. Patil, M. Huang, Z. Zhang, X. Wen, D. Mendus, T. Hoang, R. Meng, M. L. Johnson,LBA2 Updated analysis and patient-reported outcomes (PROs) from CITYSCAPE: A randomised, double-blind, phase II study of the anti-TIGIT antibody tiragolumab + atezolizumab (TA) versus placebo + atezolizumab (PA) as first-line treatment for PD-L1+ NSCLC. Ann. Oncol. 32,S1428 (2021). [Google Scholar]
- 43.B. C. Cho, D. R. Abreu, M. Hussein, M. Cobo, A. J. Patel, N. Secen, K. H. Lee, B. Massuti, S. Hiret, J. C. H. Yang, F. Barlesi, D. H. Lee, L. P. Ares, R. W. Hsieh, N. S. Patil, P. Twomey, X. Yang, R. Meng, M. L. Johnson,Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 23,781–792 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Roche, “Roche reports interim results for phase III SKYSCRAPER-01 study in PD-L1–high metastatic non-small cell lung cancer,” news release, 11 May 2022; https://bit.ly/3yuFijz.
- 45.J.-M. Chauvin, H. M. Zarour,TIGIT in cancer immunotherapy. J. Immunother. Cancer 8,e000957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.A. Rotte, S. Sahasranaman, N. Budha,Targeting TIGIT for immunotherapy of cancer: Update on clinical development. Biomedicine 9,1277 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.L. Chen, H. Qin, R. Zhao, X. Zhao, L. Lin, Y. Chen, Y. Lin, Y. Li, Y. Qin, Y. Li, S. Liu, K. Cheng, H. Chen, J. Shi, G. J. Anderson, Y. Wu, Y. Zhao, G. Nie,Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci. Transl. Med. 13,eabc2816 (2021). [DOI] [PubMed] [Google Scholar]
- 48.X. Han, S. Shen, Q. Fan, G. Chen, E. Archibong, G. Dotti, Z. Liu, Z. Gu, C. Wang,Red blood cell–derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci. Adv. 5,eaaw6870 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Q. Jiang, Y. Liu, R. Guo, X. Yao, S. Sung, Z. Pang, W. Yang,Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 192,292–308 (2019). [DOI] [PubMed] [Google Scholar]
- 50.L. Rao, Q. F. Meng, Q. Huang, Z. Wang, G. T. Yu, A. Li, W. Ma, N. Zhang, S. S. Guo, X. Z. Zhao, K. Liu, Y. Yuan, W. Liu,Platelet-leukocyte hybrid membrane-coated immunomagnetic beads for highly efficient and highly specific isolation of circulating tumor cells. Adv. Funct. Mater. 28,1803531 (2018). [Google Scholar]
- 51.L. L. Bu, L. Rao, G. T. Yu, L. Chen, W. W. Deng, J. F. Liu, H. Wu, Q. F. Meng, S. S. Guo, X. Z. Zhao, W. F. Zhang, G. Chen, Z. Gu, W. Liu, Z. J. Sun,Cancer stem cell-platelet hybrid membrane-coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma. Adv. Funct. Mater. 29,1807733 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Figs. S1 to S11