Abstract
Aims
Studies with implantable cardiac monitors (ICMs) show that one-third of patients with cryptogenic stroke/transient ischaemic attack (TIA) have episodes of subclinical atrial fibrillation (SCAF) and benefit switching from antiplatelet- to anticoagulant therapy. However, ICMs are costly and resource demanding. We aimed to build a score based on participant’s baseline characteristics that could assess individual risk of SCAF.
Methods and results
In a prospective study, 236 eligible patients with a final diagnosis of cryptogenic stroke/TIA had an ICM implantated during the index hospitalization. Pre-specified evaluated variables were: CHA2DS2-VASc, P-wave duration, P-wave morphology, premature atrial beats (PAC)/24 h, supraventricular tachycardia/24 h, left atrial end-systolic volume index (LAVI), Troponin-T, NT-proBNP, and D-dimer. SCAF was detected in 84 patients (36%). All pre-specified variables were significantly associated with SCAF detection in univariate analysis. P-wave duration, followed by PAC/24 h, NT-proBNP, and LAVI, had the largest ratio of SCAF prevalence between its upper and lower quartiles (3.3, vs. 3.2, vs. 3.1 vs. 2.8, respectively). However, in a multivariate analysis, only PAC/24t, P-wave duration, P-wave morphology, and LAVIs remained significant predictors and were included in the PROACTIA score. Subclinical atrial fibrillation prevalence was 75% in the highest vs. 10% in the lowest quartile of the PROACTIA score with a 10-fold higher number of patients with an atrial fibrillation burden >6 h in the highest vs. the lowest quartile.
Conclusion
The PROACTIA score can identify patients with cryptogenic stroke/TIA at risk of subsequent SCAF detection. The large difference in SCAF prevalence between groups may provide a basis for future tailored therapy.
Clinical trial registration
Clinical Trial Registration: ClinicalTrials.gov; NCT02725944.
Keywords: Embolic stroke of undetermined source, Subclinical atrial fibrillation, Implantable loop recorder, Implantable cardiac monitors, Secondary stroke prevention
What’s new?
The most comprehensive prospective evaluation of predictors for device detected subclinical atrial fibrillation (SCAF) in patients with cryptogenic stroke or transient ischaemic attack (TIA).
In univariate analysis CHA2DS2-VASc, P-wave duration, P-wave morphology, premature atrial beats/24 h, supraventricular tachycardia/24 h, left atrial end-systolic volume index, Troponin-T, NT-proBNP, and D-dimer were significantly associated with SCAF.
In multivariate analysis, only premature atrial contractions /24 h, P-wave duration, P-wave morphology, and LAVIs remained significant predictors and were included in the PROACTIA score.
The highest vs. the lowest quartile of PROACTIA score had a seven times higher prevalence of SCAF and a 10-fold higher number of patients with an atrial fibrillation burden >6 h.
The PROACTIA score based on easily available baseline variables can provide a basis for individually tailored therapy in patients with cryptogenic stroke or TIA.
Introduction
About one-third of all stroke cases are cryptogenic, i.e. strokes that remain without an identifiable cause even after a comprehensive evaluation.1 However, 20–34% of cryptogenic stroke cases have episodes of subclinical atrial fibrillation [SCAF—i.e. asymptomatic episodes of atrial fibrillation (AF) detected by monitoring devices] during extended monitoring and follow-up, with higher detection rates the longer the monitoring time.2 Implantable cardiac monitors (ICMs) with validated algorithms for AF detection3 have demonstrated a superior ability to detect SCAF compared to other rhythm monitoring strategies.4 Furthermore, modern ICM have home-monitoring capabilities that enable timely initiation of oral anticoagulation (OAC) after detection of SCAF. This strategy is associated with significant reductions in stroke recurrence.5 However, available resources and costs are currently limiting the widespread use of this technology.
The main objective of this study was thus to measure a broad set of baseline variables and prospectively evaluate their ability to predict the risk of SCAF upon follow-up in patients with cryptogenic stroke or transient ischaemic attack (TIA). We hypothesized that from these baseline variables a scoring system could be built to enable a reliable prediction of the risk of underlying SCAF in patients with cryptogenic stroke/TIA that could later provide a basis for individualized treatment and follow-up.
Methods
Study design and population
The PROACTIA study is an event-driven prospective single centre cohort study.
Patients hospitalized for first-time stroke or TIA, were screened for study inclusion with the registration of medical history, carotid Doppler ultrasound, 12-lead electrocardiogram (ECG), 24 h-Holter ECG registration and verification of ischaemic stroke by cerebral computed tomography, and/or magnetic resonance imagingas a part of the routine examinations. Eligible patients with embolic stroke of undetermined source (ESUS, i.e. a non-lacunar stroke without proximal arterial stenosis or known cardio-embolic source6) were invited to the study and were examined with echocardiography. After verifying the stroke or TIA as cryptogenic according to the TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria,7 the patients were enrolled in the study. All study participants had an ICM (Reveal Linq; Medtronic, Inc., Minneapolis, MN, USA) implanted prior to discharge. Stroke recurrence during follow-up was defined as a new clinical event with a corresponding new area of brain infarct. Patients with known or newly detected AF, non-AF indications for OAC or contraindications for OAC, were excluded. Those unable to sign the informed consent or with a life expectancy of <2 years were also excluded.
The study was conducted according to the Declaration of Helsinki and was approved by the Regional Committees for Medical and Health Research Ethics with reference number 2014/1260. All study participants provided written informed consent before study inclusion. The trial is registered at ClinicalTrials.gov (No. NCT02725944).
Data sources/measurement
The following variables were pre-specified to be evaluated as possible predictors of the individual risk for underlying SCAF:
CHA2DS2-VASc score,
Number of supraventricular tachycardia (SVT)/24 h and premature atrial contractions (PAC)/24 h in baseline 24 h Holter ECG,
P-wave duration and P-wave morphology in baseline 12-lead ECG,
Left atrial systolic volume index (LAVIs) in baseline echocardiography,
Biomarkers: D-dimer, high-sensitivity cardiac Troponin-T, NT-proBNP.
12-lead resting ECG was recorded on paper at a speed of 50 mm/s, and P-wave duration and P-wave morphology were measured manually. P-wave duration was measured as the longest P-wave on 12-lead ECG. P-wave morphology was considered positive if the P-wave was biphasic in the inferior leads.8
The 24 h Holter ECG was registered using OxyHolter® Recorder (Maynard, MA, USA). Episodes of irregular ventricular rhythm without detectable sinus P-waves lasting more than 30 s were considered as AF episodes, and the patients were treated with oral anticoagulants (OACs), primarily direct oral anticoagulants (DOACs), and not included in the study. Supraventricular tachycardia was defined as three or more consecutive PAC beats. The total number of SVTs and PACs were calculated automatically by OxyHolter® Recorder software and reviewed manually to exclude artefacts. The total numbers were divided by monitoring time and given as SVT/24 h and PAC/24 h.
Transthoracic echocardiographic images were obtained using GE Vivid E9 (GE Healthcare, Horten, Norway), with M5S probe, and stored electronically for later off-line analysis with ComPACS (v.10.6. MediMatic, Genova, Italy). Left atrial volume was determined by the modified Simpson’s biplane model and indexed using body surface area. The analyses were performed according to current guidelines.9
The ICM was implanted subcutaneously under local anaesthesia according to the vendor´s recommendation. No prophylactic antibiotics were used. Of the 251 implanted monitors only one adverse event was observed (pocket infection within 1 week after the implantation). The monitor was programmed for AF only and atrial tachycardia with a tachycardia alert just above the estimated maximal heart rate level according to the formula, ‘220 minus age’. Atrial fibrillation was defined as an episode of irregular heart rhythm, without detectable P-waves, lasting more than 30 s, and adjudicated by two cardiologists. Details of the AF detection algorithm have been reported previously.3 The home-monitoring analyses were performed once weekly. Current medication was registered from the patient’s charts upon discharge.
Antiplatelet therapy was switched to OAC (primarily DOACs) once AF was detected.
Missing data
Initial ECG data were lost for two patients and were, therefore, extracted from the ECG registered during the control visit after the 1 year follow-up. The NT-proBNP and D-dimer analyses from the admission blood sampling were not available in 10 and 27 patients, respectively. They were substituted with values obtained from blood sampling upon study inclusion. Testing in additional patients did not reveal any systematic variance between admission and inclusion values (data not shown).
Bias
All ECG and echocardiographic analyses were performed at the time of inclusion without knowledge of later SCAF detection status to eliminate the risk of bias. Furthermore, the principal investigator (LSS) interpreted the ICM recordings without access to the clinical background data or results, and ICM-detected AF episodes were adjudicated by a second cardiologist (HK) blinded to the baseline data. In cases of disagreement, a third blinded cardiologist determined the result. Data analyses were performed according to the original plan.
Ethical approval
Ethical approval was obtained from the Regional Committees for Medical and Health Research Ethics with reference number 2014/1260.
Informed consent
All patients provided informed written consent.
Statistical methods
Continuous values are expressed as mean and standard deviation (SD), or median and interquartile range (IQR) when non-normally distributed. Nominal variables are presented as counts and percentages. Non-detectable values for Troponin-T <0.05 and for D-dimer <0.3 were given as 0.04 and 0.2, respectively. A two-sided t-test was used for comparison of normally distributed variables and the non-parametric Kruskal–Wallis test for non-normally distributed values. Categorical data were analysed using two-tailed χ2 statistics with Yates’s correction. Univariate- and multivariate regression analyses were performed to estimate the risk for SCAF. To be considered for inclusion in a multivariate risk model, baseline variables were required to be recorded in ≥90% of patients and found to be significant (P ≤ 0.05) in a univariate logistic regression model. Data for PAC/24 h, SVT/24 h, Tnt, proBNP were log-transformed prior to regression analysis due to non-normal distribution. To avoid non-computable values in patients with 0 PAC/24 h or 0 SVT/24 h the following transformation were used: log(1 + PAC/24 h) and log(1 + SVT/24 h). Receiver operator characteristic (ROC) curves and area under the curve (AUC) were calculated for the estimation of incremental prognostic information on SCAF prediction. Variable selection for the logistic regression model was based on AUC scores calculated using leave-one-out cross-validation (LOOCV). The AUC score is the area under the ROC curve, or equivalently the probability that the model scores a random positive case higher than a random negative one. Leave-one-out cross-validation works by leaving one data point out of the data set at a time and estimating a separate model instance for each, which is used to score the left-out data point. This procedure is designed to eliminate the bias that follows from estimating and evaluating a model on the same data set. The variable selection procedure used backwards steps, starting with the full set of 10 variables and removing the one, that results in the highest AUC increase until no further improvement was possible.10
A significance level of 0.05 was adopted. Statistical analyses were performed using IBM SPSS Statistics, Version 26, and statistical analysis program R, version 3.6.
Results
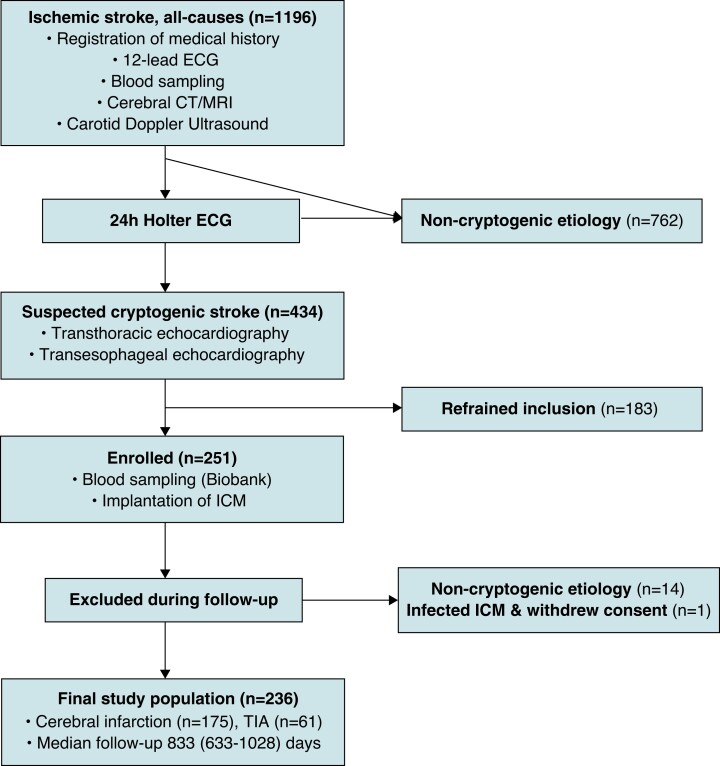
The inclusion process is presented in Figure 1. The demographics and clinical characteristics of the 236 study participants are presented in Table 1.
Figure 1.
Flowchart describing the screening and enrolment process. ICM, implantable cardiac monitor; TIA, transient ischaemic attack.
Table 1.
Demographics and clinical characteristics of study participants
| Total n = 236 | Non-AF n = 152 | SCAF n = 84 | P-value | |
|---|---|---|---|---|
| Female, n (%) | 90 (38) | 59 (39) | 31 (37) | 0.726 |
| Male, n (%) | 146 (62) | 93 (61) | 53 (63) | |
| TIA, n (%) | 61 (26) | 44 (29) | 17 (20) | 0.151 |
| Stroke, n (%) | 175 (74) | 109 (71) | 67 (80) | |
| Age, years, mean (SD) | 68.6 (12.5) | 66.7 (13.0) | 72.1 (10.7) | 0.001 |
| BMI, kg/m2, mean (SD) | 27.2 (4.5) | 27.0 (4.3) | 27.6 (4.8) | 0.316 |
| Current smoker, n (%) | 28 (12) | 18 (12) | 10 (12) | 0.854 |
| CHA2DS2-VASc, median (IQR) | 4 (3–5) | 4 (3–5) | 5 (4–6) | 0.014 |
| HT, n (%) | 150 (64) | 87 (57) | 63 (75) | 0.007 |
| Vascular disease, n (%) | 40 (17) | 25 (16) | 15 (18) | 0.782 |
| CHF, n (%) | 8 (3) | 3 (2) | 5 (6) | 0.106 |
| DM, n (%) | 30 (13) | 17 (11) | 13 (16) | 0.343 |
| DVT/PE, n (%) | 6 (3) | 2 (1) | 4 (5) | 0.105 |
| P-duration, ms, mean (SD) | 109.6 (18.7) | 106.1 (16.7) | 116 (20.5) | 0.026 |
| P-morphology bimodal, n (%) | 122 (51.7) | 67 (44) | 56 (67) | 0.002 |
| PAC/24-h ECG, median (IQR) | 90 (35–460) | 66 (28–206) | 347 (59–1917) | <0.001 |
| SVT/24-h ECG, median (IQR) | 1 (0–5) | 1 (0–3) | 3 (1–27) | <0.001 |
| D-dimer, mg/L, median (IQR) | 0.4 (0.2–0.7) | 0.3 (0.2–0.6) | 0.5 (0.3–0.9) | <0.001 |
| TnT, ng/L, median (IQR) | 11.5 (7–18) | 10 (7–16) | 14.5 (9.3–26.8) | <0.001 |
| NT-proBNP, ng/L, median (IQR) | 143 (59–387) | 104.5 (51–283) | 245 (102–774) | <0.001 |
| LAVI, mL/m2, mean (SD) | 37 (11) | 35 (9) | 42 (12) | <0.001 |
| Beta-blockers, n (%) | 61 (26) | 34 (22) | 27 (32) | 0.095 |
| ACEI/ARB, n (%) | 104 (44) | 59 (39) | 45 (54) | 0.026 |
| Ca-channels blockers, n (%) | 59 (25) | 33 (22) | 26 (31) | 0.11 |
| Diuretics, n (%) | 39 (17) | 23 (27) | 16 (11) | 0.001 |
| Statins, n (%) | 206 (87) | 134 (88) | 72 (86) | 0.297 |
| Ezetimib, n (%) | 10 (4) | 8 (5) | 2 (2) | |
| ASA, n (%) | 27 (11) | 16 (11) | 11 (13) | 0.541 |
| Antiplatelet therapy other than ASA, n (%) | 212 (90) | 136 (90) | 76 (91) | 0.014 |
SCAF, subclinical atrial fibrillation; TIA, transient ischaemic attack; SD, standard deviation; IQR, interquartile range; 25th percentile and 75th percentile values; BMI, body mass index; HT, hypertension; CHF, congestive heart failure; DM, diabetes mellitus; DVT, deep venous embolism; PE, pulmonary embolism; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers (angiotensin subtypes I and II: AT1 and AT2); PAC, atrial premature beat; LAVI, left atrial volume index.
Subclinical atrial fibrillation was detected in 84 patients (36%) after a median of 113 days (25–336). Antiplatelet therapy was switched to OAC five (2–14) days after SCAF detection. Cumulative SCAF burden was <6 min in 16 patients (19.5%) and longer than 6 h in 41 patients (50%). Participants with SCAF were older and had a higher CHA2DS2-VASc score, more hypertension, longer P-wave duration, higher prevalence of bimodal P-wave morphology on ECG, higher number of SVTs and PACs during 24-h Holter ECG monitoring, larger left atrial volumes and higher levels of D-dimer, TnT, and NT-proBNP as compared to those without SCAF. Eleven recurrent strokes (2.0/100 patient year) were observed, of which five occurred on antiplatelet therapy and six on ongoing OAC therapy. None of the patients with recurrent stroke on antiplatelet therapy had episodes of SCAF detected after their stroke recurrence. A total of five bleeding episodes were observed (0.9/100 patient year): two were intracerebral bleedings (both on DOAC treatment) and three were gastrointestinal bleedings (one on DOAC treatment, one on antiplatelet therapy and one on DOAC and antiplatelet therapy due to diagnosed acute coronary syndrome during follow-up).
All the pre-specified variables were significantly associated with SCAF detection in univariate analyses. The univariate results are presented together with the prevalence of SCAF detection on follow-up for each quartile of the individual pre-specified variables in Table 2.
Table 2.
Predictive value and individual risk score components for subclinical atrial fibrillation, unadjusted effect estimates
| Clinical risk factor | OR (95% CI) | % SCAF prevalence per quartile | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Age | 1.04 (1.01–1.06)** | 24 | 31 | 39 | 49 |
| CHA2DS2-VASc | 1.30 (1.08–1.56)** | 24 | 32 | 44 | 42 |
| P-duration, ms | 1.03 (1.02–1.05)*** | 17 | 31 | 39 | 56 |
| P-morphology, bimodal | 2.41 (1.39–4.18)** | 25 | 45 | ||
| log(1 + PAC/24-h ECG) | 2.50 (1.75–3.57)*** | 20 | 27 | 31 | 64 |
| log(1 + SVT/24-h ECG) | 3.87 (2.26–6.63)*** | 22 | 29 | 31 | 61 |
| D-dimer, mg/L | 1.57 (1.07–2.32)* | 20 | 31 | 44 | 47 |
| logTNT, ng/L | 4.18 (1.86–9.45)** | 24 | 31 | 34 | 54 |
| logNT-proBNP, ng/L | 2.49 (1.58–3.95)*** | 19 | 29 | 37 | 58 |
| LAVI, mL/m2 | 1.07 (1.04–1.10)*** | 20 | 32 | 34 | 56 |
SD, standard deviation; IQR, interquartile range; 25th percentile and 75th percentile values; PAC, atrial premature beat; SVT, supraventricular tachycardia; LAVI, left atrial volume index; *P < 0.05, **P < 0.01, ***P < 0.001.
Of the pre-specified variables, only PAC/24t, P-wave duration, P-wave morphology, and LAVIs remained as significant predictors in the multivariate analysis, and the predictive power was not improved by adding other variables to the equation. The PROACTIA score was, therefore, based on the formula derived from the multivariate analysis:
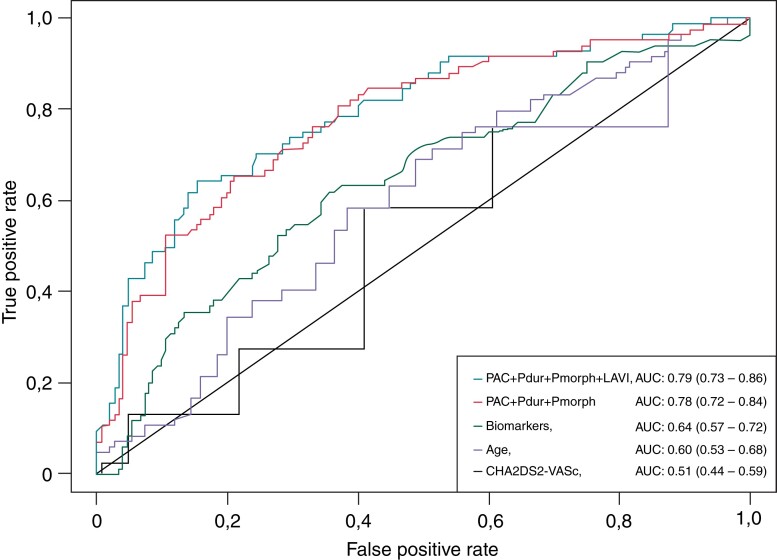
Receiver operator characteristic-curve characteristics of different prediction models with their respective AUC values and confidence intervals are presented in Figure 2.
Figure 2.
ROC-curves of different prediction models of subclinical atrial fibrillation with their respective AUC values and corresponding confidence intervals. PAC, premature atrial contraction; Pdur, P-wave duration; Pmorph, P-wave morphology (biphasic); LAVI, left atrial volume index; TnT, Troponin-T; NT-proBNP, N-terminal proBrain Naturetic Peptide.
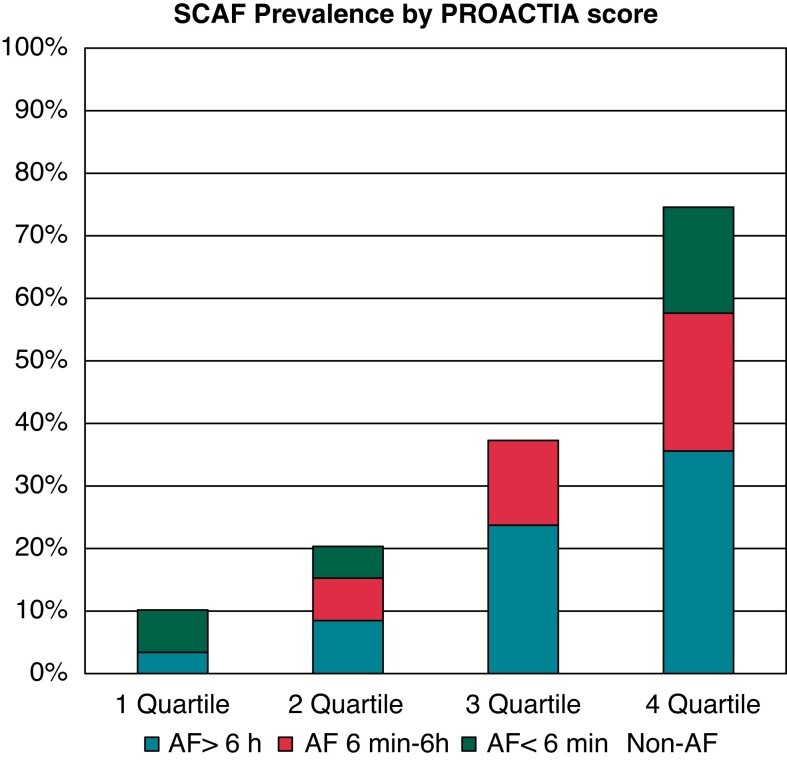
Demographics and clinical characteristics of study participants divided by quartiles of PROACTIA score are described in Table 3. The SCAF prevalence in the highest quartile was 7.3 times higher than the SCAF prevalence observed in the lowest quartile (75% vs. 10%) (Figure 3). Furthermore, the AF burden within the lowest PROACTIA score quartile was significantly lower than the corresponding values observed in the higher quartiles (Figure 3).
Table 3.
Demographics and clinical characteristics of study participants (n = 236) divided by quartiles of PROACTIA score
| PROACTIA score quartiles | |||||
|---|---|---|---|---|---|
| Quartile 1 n = 59 | Quartile 2 n = 59 | Quartile 3 n = 59 | Quartile 4 n = 59 | P-value | |
| SCAF detected, n (%) | 6 (10) | 12 (20%) | 22 (37%) | 44 (75%) | <0.001 |
| SCAF burden, <6 min, n (%) | 4 (7) | 3 (5) | 0 | 10 (17) | <0.001 |
| SCAF burden, 6 min–6 h, n (%) | 0 | 4 (7) | 8 (13) | 13 (22) | <0.001 |
| SCAF burden, >6 h, n (%) | 2 (3) | 5 (8) | 14 (24) | 21 (36) | <0.001 |
| Index stroke, n (%) | 39 (66%) | 42 (72%) | 48 (81%) | 46 (78%) | 0.230 |
| Index TIA, n (%) | 20 (34%) | 17 (28%) | 11 (19%) | 13 (22%) | |
| Age, years, mean (SD) | 62.5 (11.9) | 67.4 (13) | 69.6 (11.4) | 75.1 (10.3) | <0.001 |
| Women (%) | 24 (41%) | 24 (41%) | 23 (39%) | 19 (32%) | 0.748 |
| CHA2DS2-VASc, median (IQR) | 3 (3–5) | 4 (3–6) | 5 (3–5) | 5 (4–6) | <0.001 |
| BMI kg/m2, mean (SD) | 28 (4) | 28 (5) | 27 (5) | 27 (5) | 0.838 |
| Hypertension, n (%) | 25 (42%) | 38 (64%) | 40 (68%) | 47 (80%) | <0.001 |
| Vascular disease, n (%) | 9 (15%) | 10 (17%) | 8 (14%) | 14 (24%) | 0.484 |
| Heart failure, n (%) | 1 (2%) | 0 (0%) | 1 (1%) | 6 (10%) | 0.01 |
| Diabetes mellitus, n, (%) | 7 (12%) | 6 (10%) | 8 (14%) | 9 (15%) | 0.858 |
| PE/Venous thrombosis, n (%) | 1 (2%) | 1 (2%) | 1 (2%) | 3 (5%) | 0.562 |
| Pdur, ms, mean (SD) | 100.8 (14) | 104.7 (14.1) | 110.7 (15.8) | 122.5 (22.4) | <0.001 |
| P-morphology bimodal, n, (%) | 14 (24%) | 27 (46%) | 36 (61%) | 45 (76%) | <0.001 |
| PAC/24ECG, median (IQR) | 35 (11–70) | 61 (29–195) | 186 (62–864) | 738 (204–3546) | <0.001 |
| SVT/24ECG, median (IQR) | 0 (0–1) | 1 (0–3) | 2 (1–7) | 4 (1–19) | <0.001 |
| D-dimer, mg/L, median (IQR) | 0.3 (0.2–0.5) | 0.3 (0.2–0.6) | 0.3 (0.2–0.7) | 0.7 (0.4–1) | 0.047 |
| Troponin-T, ng/L, median (IQR) | 8 (6–14) | 10 (6–16) | 11 (8–17) | 18 (12–34) | <0.001 |
| NT-proBNP, ng/L, median (IQR) | 61 (30–141) | 93 (51–293) | 152 (76–470) | 361 (177–812) | 0.036 |
| LAVI, mL/m2 (SD) | 29 (6) | 35 (9) | 38 (7) | 48 (12) | <0.001 |
SCAF, subclinical atrial fibrillation; TIA, transient ischaemic attack; SD, standard deviation; IQR, interquartile range; 25th percentile and 75th percentile values; BMI, body mass index; HT, hypertension; CHF, congestive heart failure; DM, diabetes mellitus; DVT, deep venous embolism; PE, pulmonary embolism; Pdur, P-wave duration of 12-leads ECG; PAC, atrial premature beat; SVT, supraventricular tachycardia; LAVI, left atrial volume index.
Figure 3.
SCAF by Proactia score quartiles in the study population.
Discussion
The PROACTIA study detected SCAF in 36% of patients which is in line with previous reports using ICM in cryptogenic stroke patients as summarized in a recent meta-analysis.11 All patients with detected SCAF were switched to OAC within days of detection. With this individualized strategy, we observed a very low rate of recurrent stroke (2/100 patient years) combined with a low bleeding incidence (0.9/100 patient year). This is in line with the meta-analysis by Tsivgoulis et al.11 that demonstrated a decreased risk ratio of recurrent stroke of 0.45 (95% CI, 0.21–0.97) in cryptogenic stroke/ESUS patients who underwent prolonged rhythm monitoring with the initiation of OAC upon detection of SCAF episodes >30 s versus standard of care without prolonged rhythm monitoring. In the 2021 Guideline for the prevention of stroke in patients with stroke and TIA long-term rhythm monitoring is recommended for cryptogenic stroke patients with the initiation of OAC regardless of the time spent in AF because of the high risk of recurrent stroke in these patients even with brief subclinical episodes of AF.12 With the high prevalence of SCAF in cryptogenic stroke/TIA patients, the effect of DOAC treatment in unselected cryptogenic stroke/TIA patients was tested in NAVIGATE ESUS13 and RESPECT ESUS.14 Neither showed a benefit of DOAC treatment in unselected cryptogenic stroke/TIA patients. However, a subgroup analysis of the NAVIGATE-ESUS study, selecting patients with increased left atrium diameter (9% of the total study population), demonstrated a marked reduction in yearly stroke recurrence among those treated with rivaroxaban as compared to aspirin (1.7% vs. 6.7%).15 This result supports the notion that individually tailored therapy, based on baseline characteristics, may be feasible for cryptogenic stroke and TIA patients.
Patients in PROACTIA received individually tailored therapy during follow-up by means of ICMs. However, as stated initially, available resources and costs are currently limiting the use of ICM technology in patients with cryptogenic stroke and TIA. Hence, there is an urgent need for novel measures enabling reliable risk stratification based on baseline data in these patients. Therefore, we aimed to build such a risk stratifying model. We found SCAF to be associated with all the pre-specified risk factors in univariate analysis. However, in multivariate analysis only PAC/24t, P-wave duration, P-wave morphology, and LAVIs remained as significant predictors of SCAF, and they remained significant after cross-validation. No further predictive power was gained by adding any of the other variables to the model. We, therefore, composed the PROACTIA score from these variables.
Several studies have evaluated scoring systems to predict AF, but most have relied on non-invasive methods for AF detection with a lower AF detection rate than provided by modern ICM technology,4 or were not restricted to a cryptogenic stroke/TIA population thus complicating comparison with our study. We have, therefore, focused on studies that have utilized ICM for SCAF detection in patients with cryptogenic stroke or TIA. The largest reported study was by Xu et al., who retrospectively reviewed 389 patients followed with an ICM implanted after cryptogenic stroke with a SCAF detection rate of 26%. They found age and left atrial enlargement to be independently predictive of SCAF detection, while CHA2DS2-VASc and co-morbidities such as hypertension, diabetes, obstructive sleep apnoea, and coronary artery disease were not. However, no Holter ECG data, ECG measures, or biomarkers were reported. A history of SVT/PAC was twice as frequent in patients with SCAF, but was not statistically significant in their study.16
However, concordant to our findings, other ICM-based studies that did report Holter ECG data found significant associations between frequency of PAC on 24 h Holter and SCAF detection.17,18 Furthermore, ECG indices of atrial myopathy such as P-wave duration18 and morphology,19 have also been found associated with SCAF detection by ICM.
The CHA2DS2-VASc score has been a cornerstone in the evaluation of AF patients with respect to the risk of stroke and the need for OAC. However, the association between CHA2DS2-VASc score and SCAF detection is not well documented and was not found in the meta-analysis from Tsivgoulis et al.2
In a substudy of CRYSTAL AF Zhao et al.20 evaluated both CHADS2 and the clinically based HAVOC score with points for age (≥75), obesity, congestive heart failure, hypertension, coronary artery disease, peripheral vascular disease, and valve disease, and found higher SCAF prevalence with increasing CHADS2 and HAVOC score with the best prediction achieved by the HAVOC score. However, SCAF prevalence in their high-risk group was only 3 times higher than in their low-risk group (33% vs. 11%) as compared to 7.3 with the PROACTIA score. Furthermore, of the individual HAVOC score components—age was the only significant univariate predictor of SCAF. Concordantly, in PROACTIA, age alone was a more powerful predictor than CHA2DS2-VASc, but neither age nor CHA2DS2-VASc or any of the other clinical variables measured in PROACTIA remained significant as independent predictors in our final multivariate model, nor did they add predictive power to our risk score when LAVIs or PAC/24 h were included in the analysis. Similarly, even though the biomarkers were increased in patients with subsequent SCAF detection, they did not improve the predictive power conferred by the ECG markers and LAVIs.
Limitations of the study
Even though PROACTIA is one of the largest ICM-based studies, the number of events were limited to 8.4 events for each pre-specified predictor. However, the final model contains only four predictors, which gives more than 20 events per predictor. The LOOCV was applied in the model selection process in order to counteract any risk of bias, which can indeed otherwise be a risk for datasets of limited size. The given AUC performance was also calculated using LOOCV, which effectively eliminates the ‘double use of data problem’ that arises when a model is estimated and evaluated on the same data set. However, the results should be confirmed in an independent validation cohort.
Atrial fibrillation is associated with multiple risk factors on the population level. On the individual level, not all risk factors may be present. However, those risk factors that are present may contribute synergistically in a dose-dependent manner.15,17 In the PROACTIA score the dose–response relationship of the risk factors is respected, as the score sum is calculated based on the exact values of the individual parameters from each patient, instead of cut-offs. We, therefore, believe this enables a more robust and precise individual risk prediction of SCAF. The mathematical expression composing the PROACTIA is complex. However, with the aid of an app-based risk calculator, clinicians will easily be able to estimate individual risk in their daily practice.
Clinical implications
The PROACTIA score enables the identification of cryptogenic stroke/TIA patients with low, intermediate and a high risk of subsequent SCAF detection. This may provide the basis for a prospective multi-centre study of tailored therapy in which low-risk patients receive neither prolonged rhythm monitoring nor OAC, intermediate-risk patients receive prolonged rhythm monitoring, preferably by ICM, and high-risk patients receive OAC and not antiplatelet medication as the primary treatment.
Conclusions
The study results support the primary hypothesis that the PROACTIA score built on easily available baseline ECG, 24 h Holter, and echocardiographic left atrium parameters, can identify patients with cryptogenic stroke/TIA at risk of subsequent SCAF detection. The prevalence of SCAF was 7.3 times higher in patients with the highest vs. the lowest quartile of PROACTIA score. The large difference in SCAF prevalence between groups may provide a basis for future tailored therapy in this large and resource-demanding patient population.
Supplementary Material
Contributor Information
Loreta Skrebelyte-Strøm, Akershus University Hospital, Lørenskog, Norway; University of Oslo, Oslo, Norway.
Ole Morten Rønning, Akershus University Hospital, Lørenskog, Norway; University of Oslo, Oslo, Norway.
Fredrik A Dahl, Akershus University Hospital, Lørenskog, Norway; Norwegian Computing Center, Oslo, Norway.
Kjetil Steine, Akershus University Hospital, Lørenskog, Norway; University of Oslo, Oslo, Norway.
Harald Kjekshus, Akershus University Hospital, Lørenskog, Norway.
Funding
This work was supported by the Trust Stiftelsen Dam (L.S.-S.; grant number 2016/FO82036, 2016) and Akershus University Hospital (K.S.; grant number 296908, 2016 and 2017).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Yaghi S, Bernstein RA, Passman R, Okin PM, Furie KL. Cryptogenic stroke: research and practice. Circ Res 2017;120:527–40. [DOI] [PubMed] [Google Scholar]
- 2. Tsivgoulis G, Katsanos AH, Kohrmann M, Caso V, Perren F, Palaiodimou L, et al. Duration of implantable cardiac monitoring and detection of atrial fibrillation in ischemic stroke patients: a systematic review and meta-analysis. J Stroke 2019;21:302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hindricks G, Pokushalov E, Urban L, Taborsky M, Kuck KH, Lebedev D, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the xpect trial. Circ Arrhythm Electrophysiol 2010;3:141–7. [DOI] [PubMed] [Google Scholar]
- 4. Choe WC, Passman RS, Brachmann J, Morillo CA, Sanna T, Bernstein RA, et al. A comparison of atrial fibrillation monitoring strategies after cryptogenic stroke (from the cryptogenic stroke and underlying af trial). Am J Cardiol 2015;116:889–93. [DOI] [PubMed] [Google Scholar]
- 5. Triantafyllou S, Katsanos AH, Dilaveris P, Giannopoulos G, Kossyvakis C, Adreanides E, et al. Implantable cardiac monitoring in the secondary prevention of cryptogenic stroke. Ann Neurol 2020;88:946–55. [DOI] [PubMed] [Google Scholar]
- 6. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–38. [DOI] [PubMed] [Google Scholar]
- 7. Adams HPJ, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 8. Hayashi H, Horie M. Biphasic p wave in inferior leads and the development of atrial fibrillation. J Arrhythm 2015;31:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 10. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. New York: Springer; 2009. [Google Scholar]
- 11. Tsivgoulis G, Katsanos AH, Grory BM, Kohrmann M, Ricci BA, Tsioufis K, et al. Prolonged cardiac rhythm monitoring and secondary stroke prevention in patients with cryptogenic cerebral ischemia. Stroke 2019;50:2175–80. [DOI] [PubMed] [Google Scholar]
- 12. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke 2021;52:e364–467. [DOI] [PubMed] [Google Scholar]
- 13. Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018;378:2191–201. [DOI] [PubMed] [Google Scholar]
- 14. Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med 2019;380:1906–17. [DOI] [PubMed] [Google Scholar]
- 15. Healey JS, Gladstone DJ, Swaminathan B, Eckstein J, Mundl H, Epstein AE, et al. Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: secondary analysis of the navigate esus randomized clinical trial. JAMA Neurol 2019;76:764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu J, Sethi P, Biby S, Allred J, Seiler A, Sabir R. Predictors of atrial fibrillation detection and features of recurrent strokes in patients after cryptogenic stroke. J Stroke Cerebrovasc Dis 2020;29:104934. [DOI] [PubMed] [Google Scholar]
- 17. Victor CU, Carolina PE, Jorge TR, Joaquin CR, Manuel SG, Marta CM, et al. Incidence and predictive factors of hidden atrial fibrillation detected by implantable loop recorder after an embolic stroke of undetermined source. J Atr Fibrillation 2018;11:2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013;80:1546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Acampa M, Lazzerini PE, Guideri F, Tassi R, Andreini I, Domenichelli C, et al. Electrocardiographic predictors of silent atrial fibrillation in cryptogenic stroke. Heart Lung Circ 2019;28:1664–9. [DOI] [PubMed] [Google Scholar]
- 20. Zhao SX, Ziegler PD, Crawford MH, Kwong C, Koehler JL, Passman RS. Evaluation of a clinical score for predicting atrial fibrillation in cryptogenic stroke patients with insertable cardiac monitors: results from the crystal af study. Ther Adv Neurol Disord 2019;12:1756286419842698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.