Abstract
As part of the EHRS-PATHS study examining comorbidities in atrial fibrillation (AF) across Europe, the aim was (i) to evaluate how multimorbidity is currently addressed by clinicians during AF treatment to characterize the treatment structure and (ii) to assess how the interdisciplinary management of multimorbid AF is currently conducted. An online survey was distributed among European Heart Rhythm Association (EHRA) members in Europe that included 21 questions and a free-text option for comments on detection, assessment, and management of AF-related comorbidities. A total of 451 responses were received with 339 responses eligible for inclusion. Of these, 221 were male (66%), 300 (91.5%) were physicians, and 196 (57.8%) were working in academic university teaching hospitals. Half of the respondents managed between 20 and 50 patients per month with multimorbid AF. Varying rates of specialist services and referral to these services were available at each location (e.g. heart failure and diabetes), with a greater number of specialist services available at academic university teaching hospitals compared with non-teaching hospitals [e.g. anticoagulation clinic 92 (47%) vs. 50 (35%), P < 0.03]. Barriers to referring to specialist services for AF comorbidities included lack of integrated care model (n = 174, 51%), organizational or institutional issues (n = 145, 43%), and issues with patient adherence (n = 126, 37%), highlighting the need for organizational restructuring and developing an integrated collaborative evidenced-based approach to multimorbid AF care. The survey and analyses of free-text comments demonstrated the need for systematic, integrated management of AF-related comorbidities, and these results will inform the next phases of the EHRA-PATHS study.
Keywords: Atrial fibrillation, Comorbidity, Integrated care, Management, EHRA survey
What’s new?
To the best of our knowledge, this is the first pan-European survey focused on the management of multimorbid atrial fibrillation (AF).
Of 339 respondents, 81.4% (n = 276) were cardiologists/electrophysiologists, and 51.5% (n = 174) reported between 10 and 30years of specialist experience.
67% (n = 227) of respondents reported that ±40% of patients with multimorbid AF need onwards referral to other specialist clinicians.
Availability of AF specialist services is variable across Europe with only 51% of respondents reporting having specialist AF clinics, and only 42% (n = 142) of respondents reporting having specialist anti-coagulant services locally.
51% (n = 174) of respondents identified that the lack of integrated care impacts negatively on patient outcomes.
Improved access to patient lifestyle modifications; improved organization flexibility to enable innovation; greater access to evidence-based guidelines; and better interdisciplinary collaboration are key to improving patient outcomes in the future.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in adults affecting 2–4% (c. 33 million people) of the global population.1 It is associated with a substantial burden to both patients and health systems globally with growing prevalence in middle- and high-income countries associated with increased detection in the last decade with increased prevalence of predisposing conditions such as hypertension, diabetes, and obesity. Despite this, there are high levels of morbidity and mortality as well as the fiscal and societal burden.1 AF is a complex long-term condition (LTC) and management of AF involves a multifaceted, holistic, and multidisciplinary approach.
Multimorbidity has previously been defined as the presence of two or more diagnosed LTCs in association with an index condition.2 Multimorbidity in association with AF has been identified as a common phenomenon associated with increased all-cause mortality. While there has been growing interest in understanding this phenomenon and the impact it has on both treatment and outcomes, there remains a paucity of data on the pathway-based approaches used in the management of the components of multimorbidity. A previous research study conducted in the UK identified the all-cause mortality rate in AF-confirmed patients as 6.7% (n = 248/3651) after 7 years of observation and follow-up. In this sample, AF with four or more comorbidities had a six-fold higher risk of mortality compared with participants with AF and non-LTC.2
The growing data confirming the strong association between survival in middle-aged to older adults with AF with the degree of multimorbidity demonstrate the need for new interventions to optimize outcomes. It is acknowledged that pathway-driven approaches to care for this patient population are likely to be in place throughout Europe, but they have not been evaluated and reported within the currently available literature. It is therefore important to consider how systematic and standardized care can be provided through a pathway-based approach for patients with multimorbid AF. There is evidence that patient pathway-based interventions may have a positive effect on health outcomes in non-AF population, but these benefits have not been consistently identified across studies and disease processes.3–6
The EHRA-PATHS project is a multi-stage pan-European study coordinated by the European Heart Rhythm Association (EHRA)/European Society of Cardiology (ESC) and uniting 14 research partner organizations from across Europe. The overriding aim of EHRA-PATHS is to develop a new pathway of care for older patients with multimorbid AF through interdisciplinary, patient-centred, and systematic approaches.7 This survey study is a component of ‘work package 2’ within the EHRA-PATHS study, which focuses on undertaking a clinical practice gap analysis and measuring current clinical practices across the region, taking account of both clinicians’ and patients’ experiences. From this, future components of the EHRA-PATHS study will have a greater understanding of the obstacles which are likely to have an impact on the provision of integrated care across European countries for patients with multimorbid AF.7 The EHRA-PATHS study will culminate in the development and evaluation of a new patient pathway-based intervention for the management of multimorbid AF.
This study aimed to increase the understanding of the challenge of multiple comorbidities in patients living with AF throughout Europe. The study aims to:
evaluate how multimorbidity is currently addressed by clinicians during AF treatment to characterize the treatment structure; and
assess how the interdisciplinary management of multimorbid AF is currently conducted.
These aims were achieved through the following objectives:
identify the specific methods used by clinicians to assess, diagnose, manage, and refer multimorbid AF patients throughout Europe;
describe key areas of complexity in the management of multimorbid AF across Europe; and
highlight areas of interprofessional working to optimize health status in patients with multimorbid AF throughout Europe.
Methods
This survey study used a multi-method cross-sectional design employing both quantitative and qualitative approaches. As no previously tested or validated questionnaire was available, the survey was developed through a process of review of the existing literature and stakeholder discussions with members of the wider EHRA-PATHS investigator team. The final survey content was based on a group agreement from the central research steering group prior to the piloting of the survey in a small sample of stakeholders.
After final revisions, the questionnaire was hosted on the Qualtrics Survey Platform as an e-survey. A digital link to the survey was shared among all EHRA members through the regular organizational newsletter and through personal mailing lists within the EHRA membership. The survey was to be opened for a minimum of 4 weeks, and during this time, routine weekly reminders were sent. Reminders were sent through the above-reported channels, and local EHRA-PATHS lead investigators were also encouraged to promote the survey at a regional and national level within each EHRA-PATHS partner country. An a priori decision was made to prolong the data collection period if <250 eligible responses had been received within the initial 4-week data collection period, but the maximum data collection period would not exceed 10 weeks. In total, the survey was live between 1 November 2021 and 12 December 2021 for a total of 6 weeks.
The final survey included 21 questions in total covering the following three key areas: (i) variables relating to the participants (n = 7), (ii) questions relating to local AF referral and management practices (n = 4), and (iii) questions relating to participants’ experiences of managing multimorbid AF (n = 10). Answers were predominately binary or categorical and a number of questions offered a free-text option to expand on the answer. A final qualitative question focusing on what factors influenced the successful management of multimorbid AF-enabled participants to respond freely and participants were encouraged to expand as much as possible.
Target audience and sampling
In this study, a sample of physicians, registered nurses, and allied healthcare professionals who work directly with patients with AF in European countries and are members of the EHRA/ESC were recruited through convenience sampling methods. Respondents from outside European countries and those who were not registered healthcare professionals were excluded from the analysis. From a sampling perspective, a pragmatic approach to sampling was employed. It was expected that c. 10% of EHRA members would respond to the survey (c. n = 350). Based on the eligibility criteria, all responses from outside of the European Union (EU) countries were excluded from the analysis process.
Ethical approval
This study has been registered with King’s College London Research Ethics Committee under the minimal risk registration process (Ref MRA-20/21-25315).
Data analysis
Quantitative data analysis was conducted through the Qualtrics survey platform (2021). Using descriptive statistics, data were presented as counts and percentages. To maximize the inclusion of survey respondents, responses with missing data were not excluded from the analysis process. Rather, a ‘not answered’ category was added within the analysis to highlight missingness within the data set.
Qualitative data analysis of free-text responses was undertaken using conventional content analysis involving both deductive and inductive reasonings.8 The coding process involved the categorization of free-text concepts into content categories. These categories were patterns or themes that were directly expressed within the free-text comments provided by the survey participants. Initially, a table of potential codes was developed prior to commencing data analysis, and the early phase of analysis relied heavily on concepts derived from the author’s medical experiences and concepts identified from the pre-existing literature. As the analysis progressed new concepts emerged from the data. Data saturation was achieved when no new codes were identified across multiple responses (minimum 10 responses).
Analysis was undertaken by E.B. and G.L. who reviewed and categorized codes into larger themes by consensus. Thematic saturation was reached after approximately 200 responses. Qualitative data were managed using NVivo v.11. Rigour was maintained throughout using trustworthiness criteria which were used to demonstrate that data collection and analysis were conducted using precise, consistent, and exhaustive approaches (Nowell et al. 2017).
Mixed method integration of data was undertaken using a triangulation design and a convergence model.9,10 This involved an initial analysis of qualitative and quantitative results in isolation. Following this, results were compared and similarities or contrasting findings were considered together in the final analysis and discussion.
Results
A total of 451 responses were recorded with 376 responses from 29 European countries (including the UK) and 75 responses submitted from clinicians practising outside of the EU. A further 37 responses were submitted with no responses. Both were excluded from analysis as per the study eligibility criteria. This resulted in 339 responses included in the data analysis.
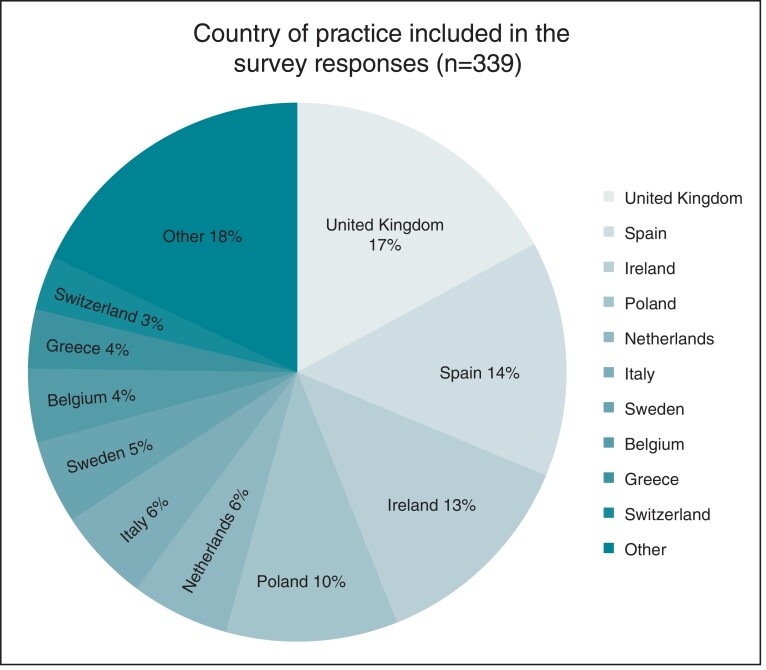
The geographical distribution of the responses is presented in Figure 1. Eighty-two percent (n = 278) of responses were received from the top 10 performing countries with the most responses received from the UK, Spain, and Ireland (17.1%, 14.2% and 12.7%, respectively; Table 1).
Figure 1.
Country of practice from survey respondents (n = 339).
Table 1.
Demographic and clinical characteristics of respondents (n, %)
| Geographical distribution of responses (top 10 responding countries) | |
|---|---|
| United Kingdom | 58 (17.1) |
| Spain | 48 (14.2) |
| Ireland | 43 (12.7) |
| Poland | 35 (10.3) |
| Netherlands | 20 (5.9) |
| Italy | 19 (5.6) |
| Switzerland | 11 (3.2) |
| Greece | 12 (3.5) |
| Sweden | 17 (5.0) |
| Belgium | 15 (4.4) |
| Gender | |
| Male | 224 (66.1) |
| Female | 108 (31.9) |
| Third gender/non-binary | 1 (0.3) |
| Not disclosed | 6 (1.8) |
| Professional group and specialist practice area | |
| Electrophysiologist | 109 (32.2) |
| Cardiologist | 167 (49.3) |
| Physician with speciality other than cardiology | 34 (10.0) |
| Geriatric/Frailty | 10 |
| Acute/Internal Medicine | 10 |
| Primary Care | 7 |
| Stroke Medicine | 2 |
| Renal/Cardiology | 1 |
| Not disclosed | 4 |
| Nurse or allied health professional working in general cardiology | 10 (3.0) |
| Nurse or allied health professional working in electrophysiology/arrhythmias | 16 (4.7) |
| Other | 3 (0.9) |
| Academic/Clinical Academic | 2 |
| Advanced Clinical Practitioner | 1 |
| Respondents’ specialist area of interest in AF management (can choose >1 specialty) | |
| Arrhythmias/electrophysiology and devices | 196 (57.8) |
| General cardiology | 206 (60.8) |
| Heart failure | 129 (38.1) |
| Valvular disease | 46 (13.6) |
| Imaging | 54 (15.9) |
| Interventional cardiology | 35 (10.0) |
| Cardiovascular prevention | 63 (18.6) |
| Congenital heart disease | 19 (5.6) |
| Stroke | 38 (11.2) |
| Other | 15 (4.4) |
| Number of years practising in this speciality | |
| <5 years | 56 (16.5) |
| 5–10 years | 62 (18.3) |
| 10–20 years | 91 (26.8) |
| 20–30 years | 83 (24.5) |
| >30 years | 47 (13.9) |
| Hospital designation | |
| University Hospital/academic teaching hospital | 196 (57.8) |
| Non-academic teaching hospital | 51 (15.0) |
| Community or district hospital | 47 (13.9) |
| Specialized Heart Centre | 18 (5.3) |
| Other settings | 27 (8.0) |
| Nil comments | |
Within this sample, 66% (n = 221) were male, 91.5% (n = 300) were doctors, and 57.8% (n = 196) worked at an academic university teaching hospital. Electrophysiologists consisted of 109 responses (32%), cardiologists (n = 167, 49%) and 10% from non-cardiologist physicians (n = 34, 10%). Respondents’ main specialist areas of AF management were electrophysiology and devices (n = 196, 58%) and general cardiology (206, 61%). Nearly two-thirds of respondents (n = 221, 65.2%) reported >10 years of specialist clinical experience highlighting experienced clinicians responding to the survey.
Regarding current clinical practice for multimorbid AF management, the analysis was undertaken for comparing university teaching hospitals vs. non-university teaching hospitals. No statistical difference was seen in a number of AF patients seen per month by setting P = 0.20 (Table 2). In terms of specialized services available between the two types of hospitals, differences were seen in specialist services for anticoagulation, syncope, and chest pain (all P < 0.05). Reasons for referral rates were primarily related to this being the number that needed to be referred (44%) but others stated that resourcing the services was an issue (18%). Barriers identified in relation to resources included a lack of integrated models of care with a higher percentage citing this in university teaching hospitals compared with non-teaching hospitals (58% vs. 42%, P = 0.003). Non-teaching hospitals also reported organizational and institutional issues being a major barrier that impacted patient outcomes (43% vs. 33%, P = 0.04).
Table 2.
Describing the current multimorbid AF management in Europe comparing university academic hospitals and non-academic hospitals
| n = (%) | Total sample | University Hospital (198) | Non-university hospital (143) | P-value |
|---|---|---|---|---|
| Typical numbers of patients seen with AF per month | ||||
| <20 | 47 (13.9) | 22 (11.1) | 25 (17.5) | |
| 20–50 | 169 (49.9) | 102 (51.5) | 68 (47.6) | |
| 51–100 | 87 (25.7) | 56 (28.3) | 31 (21.7) | 0.20 |
| 101–150 | 19 (5.6) | 9 (4.5) | 11 (7.69) | |
| >150 | 17 (5.0) | 9 (4.5) | 8 (5.59) | |
| What specialized outpatient services are available at your centre? | ||||
| Atrial fibrillation | 174 (51.3) | 107 (54.0) | 67 (46.8) | 0.19 |
| Heart failure | 249 (73.5) | 145 (73.2) | 102 (71.3) | 0.70 |
| Hypertension | 134 (39.5) | 82 (41.4) | 52 (36.4) | 0.33 |
| Diabetes | 177 (52.2) | 107 (54.0) | 70 (49.0) | 0.35 |
| Lipid | 138 (40.7) | 85 (42.9) | 53 (37.1) | 0.28 |
| Anticoagulation | 142 (41.9) | 92 (46.5) | 50 (35.0) | 0.03* |
| Syncope | 106 (31.3) | 70 (35.4) | 36 (25.2) | 0.04* |
| Chest pain | 146 (43.1) | 95 (48.0) | 58 (40.6) | 0.03* |
| Palpitations/arrhythmia/resynchronization | 148 (43.7) | 93 (47.0) | 53 (37.1) | 0.07 |
| Sleep apnoea | 110 (32.4) | 68 (34.3) | 42 (29.4) | 0.33 |
| Comprehensive geriatric assessment (dementia, falls, frailty, etc.) | 116 (34.2) | 71 (36.0) | 43 (30.1) | 0.26 |
| Other | 17 (5.0) | 6 (3.03) | 13 (9.09) | 0.03* |
| Valvular heart disease clinic | 2 | |||
| Cardio-oncology services | 5 | |||
| Private health clinic | 1 | |||
| Cardiogenetic services | 3 | |||
| Cardiac rehabilitation services | 2 | |||
| Cardiac disease in the young | 1 | |||
| Pulmonology/pulmonary hypertension | 2 | |||
| Stroke services | 2 | |||
| Weight loss/Health promotion services | 2 | |||
| Pacemaker service | 1 | |||
| Post-COVID POTS | 1 | |||
| None—no specialized clinics available | 19 (5.6) | |||
| What proportion of patients with comorbidities are referred to other specialty services? | ||||
| >80% | 7 (2.1) | 3 (15.8) | 4 (2.80) | |
| 61–80% | 12 (3.5) | 7 (3.54) | 5 (3.50) | |
| 41–60% | 55 (16.2) | 32 (16.2) | 23 (16.1) | 0.80 |
| 20–40% | 104 (30.7) | 64 (32.3) | 42 (29.4) | |
| <19% | 123 (36.3) | 67 (33.8) | 56 (39.2) | |
| No response | 38 (11.2) | |||
| What is the reason for this referral rate? | ||||
| That is the number that needs referring | 151 (44.5) | 84 (42.4) | 67 (46.9) | |
| Resourcing issue so I need to be selective and prioritize | 61 (18.0) | 34 (17.2) | 27 (18.9) | |
| There is an established process with the relevant specialties | 73 (21.5) | 46 (23.2) | 27 (18.9) | 0.60 |
| Other: | 16 (4.7) | 8 (4.04) | 9 (6.29) | |
| Referral to GP for onwards referral to specialist services | 4 | |||
| Patients don’t want onwards referral (time, money, access) | 1 | |||
| Palliative Care/End of Life Care needs | 1 | |||
| Availability of services | 1 | |||
| Complexity | 2 | |||
| Need for hospitalization | 1 | |||
| Scope of Practice of non-medical providers | 1 | |||
| No response | 38 (11.2) | |||
| What are the barriers within your current practice which potentially impacts on patient outcomes? | ||||
| Lack of integrated model of care for complex patients with AF | 174 (51.3) | 115 (58.0) | 60 (42.0) | 0.003* |
| Lack of evidence-based guidelines | 41 (12.1) | 23 (11.6) | 18 (12.6) | 0.79 |
| Lack of applicability of guidelines to my current practice | 31 (9.1) | 19 (9.60) | 12 (8.39) | 0.70 |
| Lack of time | 123 (36.3) | 73 (36.9) | 50 (35.0) | 0.72 |
| Organizational/Institutional | 145 (42.8) | 87 (43.9) | 58 (40.6) | 0.53 |
| Insurance/financial reasons | 43 (12.7) | 22 (11.1) | 22 (15.4) | 0.25 |
| Patient adherence/compliance | 126 (37.2) | 65 (32.8) | 62 (43.4) | 0.04* |
| Treatment-related adverse events | 36 (10.6) | 20 (10.1) | 16 (11.2) | 0.75 |
| Other | 21 (6.2) | |||
| The results of poor patient health choices | 1 | |||
| Relations between Cardiology and primary care providers | 2 | |||
| Competence of Primary Care providers | 2 | |||
| No changes to clinical practice Access/Availability of complex interventions/diagnostics | 1 | |||
| Patient choice/autonomy | 2 | |||
| Capacity in associated medical specialties | 1 | |||
| Lack of evidence-based guidelines for complexity (e.g. AF in cancer care) | 1 | |||
| DOAC reimbursement issues | 1 | |||
| More specialized AF clinicians | 1 | |||
| No barriers | 7 | |||
AF, atrial fibrillation; DOAC, direct oral anticoagulants; GP, general practitioner; and POTS, postural orthostatic tachycardia syndrome. *p < 0.05.
Six countries had high response rates and across these geographical locations, and most respondents reported seeing 20–50 patients per month highlighting consistency in the pattern of current clinical practice (Table 3), with 74% reporting heart failure outpatient services at their workplace (n = 249, 74%), with half reported access to specialist outpatient AF services (n = 174, 51%) and diabetes (n = 177, 52%), respectively. In terms of onward referrals for management of multimorbid AF, 227 (67%) of respondents reported ≤40% of the patient being referred to other services, suggesting that for many patients, there is no specialist management of other non-cardiac comorbidities. The primary barrier identified were similar to those seen when comparing university teaching hospitals and non-teaching hospitals as shown in Table 2.
Table 3.
Describing the current multimorbid AF management in Europe comparing the top six responding countries and other countries
| n = (%) | Total sample | Top six responding countries (n = 223) | Other countries (n = 118) | P-value |
|---|---|---|---|---|
| Typical numbers of patients seen with AF per month | ||||
| <20 | 47 (13.9) | 27 (12.1) | 20 (16.9) | |
| 20–50 | 169 (49.9) | 119 (53.4) | 51 (43.2) | |
| 51–100 | 87 (25.7) | 54 (24.2) | 33 (28.0) | 0.46 |
| 101–150 | 19 (5.6) | 13 (5.83) | 7 (5.93) | |
| >150 | 17 (5.0) | 10 (4.48) | 7 (5.93) | |
| What specialized outpatient services are available at your centre? | ||||
| Atrial fibrillation | 174 (51.3) | 112 (50.2) | 63 (53.4) | 0.58 |
| Heart failure | 249 (73.5) | 172 (77.1) | 75 (63.6) | 0.008* |
| Hypertension | 134 (39.5) | 90 (40.4) | 44 (37.3) | 0.62 |
| Diabetes | 177 (52.2) | 125 (56.1) | 52 (44.1) | 0.04* |
| Lipid | 138 (40.7) | 88 (39.5) | 50 (42.4) | 0.60 |
| Anticoagulation | 142 (41.9) | 96 (43.0) | 46 (39.0) | 0.47 |
| Syncope | 106 (31.3) | 73 (32.7) | 33 (28.0) | 0.35 |
| Chest pain | 146 (43.1) | 100 (44.8) | 47 (39.8) | 0.37 |
| Palpitations/arrhythmia/resynchronization | 148 (43.7) | 102 (45.7) | 44 (37.3) | 0.13 |
| Sleep apnoea | 110 (32.4) | 76 (34.1) | 34 (28.8) | 0.32 |
| Comprehensive geriatric assessment (dementia, falls, frailty, etc.) | 116 (34.2) | 80 (35.9) | 34 (28.8) | 0.19 |
| Other: | 17 (5.0) | |||
| Valvular heart disease clinic | 2 | |||
| Cardio-oncology services | 5 | |||
| Private health clinic | 1 | |||
| Cardiogenetic services | 3 | |||
| Cardiac rehabilitation services | 2 | |||
| Cardiac disease in the young | 1 | |||
| Pulmonology/pulmonary hypertension | 2 | |||
| Stroke services | 2 | |||
| Weight loss/Health promotion services | 2 | |||
| Pacemaker service | 1 | |||
| Post-COVID POTS | 1 | |||
| None—no specialized clinics available | 19 (5.6) | 7 (3.14) | 12 (10.2) | 0.007* |
| What proportion of patients with comorbidities are referred to other specialty services? | 201 | 102 | ||
| >80% | 7 (2.1) | 4 (1.99) | 3 (2.94) | |
| 61–80% | 12 (3.5) | 5 (2.49) | 7 (5.93) | |
| 41–60% | 55 (16.2) | 30 (13.5) | 25 (24.5) | 0.004* |
| 20–40% | 104 (30.7) | 66 (29.6) | 40 (39.2) | |
| <19% | 123 (36.3) | 96 (47.8) | 27 (26.5) | |
| No response | 38 (11.2) | |||
| What is the reason for this referral rate? | ||||
| That is the number that needs referring | 151 (44.5) | 102 (50.7) | 49 (48.0) | |
| Resourcing issue so I need to be selective and prioritize | 61 (18.0) | 47 (23.3) | 14 (13.7) | 0.05 |
| There is an established process with the relevant specialties | 73 (21.5) | 40 (19.9) | 33 (32.4) | |
| Other: | 16 (4.7) | 11 (5.47) | 6 (5.88) | |
| Referral to GP for onwards referral to specialist services | 4 | |||
| Patients don’t want onwards referral (time, money, access) | 1 | |||
| Palliative Care/End of Life Care needs | 1 | |||
| Availability of services | 1 | |||
| Complexity | 2 | |||
| Need for hospitalization | 1 | |||
| Scope of Practice of non-medical providers | 1 | |||
| No response | 38 (11.2) | |||
| What are the barriers within your current practice which potentially impact patient outcomes? | ||||
| Lack of integrated model of care for complex patients with AF | 174 (51.3) | 122 (54.7) | 53 (44.9) | 0.09 |
| Lack of evidence-based guidelines | 41 (12.1) | 29 (13.0) | 12 (10.2) | 0.44 |
| Lack of applicability of guidelines to my current practice | 31 (9.1) | 20 (8.97) | 11 (9.32) | 0.91 |
| Lack of time | 123 (36.3) | 83 (37.2) | 40 (33.9) | 0.54 |
| Organizational/Institutional | 145 (42.8) | 101 (45.3) | 44 (37.3) | 0.16 |
| Insurance/financial reasons | 43 (12.7) | 33 (14.8) | 11 (9.32) | 0.15 |
| Patient adherence/compliance | 126 (37.2) | 77 (34.5) | 50 (42.4) | 0.15 |
| Treatment-related adverse events | 36 (10.6) | 23 (10.3) | 13 (11.0) | 0.84 |
| Other: | 21 (6.2) | |||
| The results of poor patient health choices | 1 | |||
| Relations between Cardiology and primary care providers | 2 | |||
| Competence of Primary Care providers | 2 | |||
| No changes to clinical practice | 1 | |||
| Access/Availability of complex interventions/diagnostics | 2 | |||
| Patient choice/autonomy | 1 | |||
| Capacity in associated medical specialties | 1 | |||
| Lack of evidence-based guidelines for complexity (e.g. AF in cancer care) | 1 | |||
| DOAC reimbursement issues | 1 | |||
| More specialized AF clinicians | 1 | |||
| No barriers | 7 | |||
AF, atrial fibrillation; DOAC, direct oral anticoagulants; GP, General Practitioner; POTS, postural orthostatic tachycardia syndrome. *p < 0.05.
Qualitative results
As well as completing the online survey, respondents were given the option of adding free-text comments and 229 qualitative responses were completed. Initially, 56 codes were identified which were subsequently condensed down into 38 refined codes. The four themes clearly highlight the lack of integrated comorbid AF management and the four themes identified were as follows:
improving access to lifestyle and health promotion interventions, including the early management of risk factors or comorbidities,
organizational restructuring to enable innovation in care provision,
working towards achieving an evidence-based and integrated approach to multimorbid AF care for all,
aiming for great collaboration and interdisciplinary working.
The following section will provide a narrative summary of the key findings within each of the above themes.
Improving access to lifestyle and health promotion interventions, including early management of risk factors for comorbidities
Within this theme, there were three key sub-themes identified from within the participant responses. These were (i) early lifestyle modification interventions with potential for long-term behaviour change and long-term impact in particular weight loss management, exercise programmes, and smoking cessation; (ii) patient compliance and engagement with treatments with increased compliance including lifestyle changes considered key to improving patient outcomes and greater self-management; and (iii) patient education and risk-factor awareness with greater patient education in relation to AF but more specifically in relation to comorbidities. Self-surveillance for risk factors for comorbidities and multimorbidity was seen as a solution with education potentially leading to earlier diagnosis and optimal management.
Organizational restructuring to enable innovation in care provision
Within this theme, there were three key sub-themes identified from within the participant responses. These were (i) inflexibility in organizational structure and governance in acute care along with the challenges of financing treatments and poor reimbursement for treatments in countries where health insurance is required were cited; (ii) unclear pathway to diagnosis and treatment—the most common change reported by participants was having a specialized AF clinic to allow complex diagnostics, provide patient education and regular patient follow-up could be undertaken—this subtheme highlighted the need for a clear standardized pathway (at the local level) for patients with complex AF and multimorbidity and the third subtheme; (iii) poorly defined essential specialist services and priorities focus on the need to educate non-cardiologists in the management of AF (i.e. general medicine, stroke medicine, and emergency medicine) was identified as a way of sharing the workload across different medical specialties. Some respondents highlighted the challenge of accessing specialist-associated services such as sleep studies and anticoagulation clinics being a barrier to effective management of patients.
Working towards achieving an evidence-based and integrated approach to multimorbid AF care for all
The two key sub-themes identified were (i) achieving consensus on core components of care in the standardized practice approach with most respondents advocating for the integrated model of care as this would be expected to have the greatest impact on patient outcomes; there was also a need expressed for easy-to-use evidence-based/informed guidelines (especially in frail patients where a comprehensive geriatric assessment was recommended) and (ii) building knowledge and skills for clinicians where education across all clinician groups was recommended by several respondents, particularly where this might reduce the number of referrals for patients with less complex AF-related healthcare needs who may be managed by a generalist or primary care clinician.
Aiming for greater collaboration and interdisciplinary working
The following three key sub-themes were identified: (i) working together equals aiming higher where the need for greater interdisciplinary and interprofessional working was acknowledged by many respondents and the need for greater collaboration between cardiologists and primary care/gerontology clinicians; (ii) building the specialist workforce that included increasing the scope of practice for nurses and allied health professionals, particularly those with advanced clinical practice backgrounds and the third subtheme; (iii) working with primary care clinicians reflected the need to work with primary care clinicians to improve the pre-hospital identification, investigation, and management of multimorbid AF. Three participants said they did not know what practice change could improve outcomes for patients with multimorbid AF, which highlights the complexity of the problem currently under investigation.
Discussion
Overall, the findings from this survey demonstrate the variation in the care provision for patients with multimorbid AF across Europe, and the responses have captured the complexity of managing the multimorbid AF patient from the perspective of healthcare professionals. Limited access to other specialist services constraining the potential to optimize health status for patients with multimorbid AF was evident, and differences between university teaching hospitals and non-teaching hospitals were also reported. The management of AF patients with multimorbidity remains a global health challenge because of the need for a shift in focus to patient pathway-based interventions instead of the management of a specific and often single pathophysiological focus. Previous research has highlighted the need for an interdisciplinary, patient-centred approach to multimorbid care which focuses on optimizing determinants of health-related quality of life through shared decision-making and developing self-efficacy through shared health-related goal setting.3–6
Participants’ responses broadly support the need for a systematic approach in the assessment of AF patients’ multimorbidity and its impact on patient health and decision-making. The findings suggest that this approach is the first of multiple steps needed to achieve a sustained improvement of patient health status. Other steps include changes in organizational structures and governance to embed change in clinical practice, facilitate greater interdisciplinary working practices, grow relationships with non-cardiology (and perhaps especially gerontology) and primary care colleagues and the need for greater education for both patients and clinicians.
Within the assessment of the impact of multimorbid AF, the presence and degree of multimorbidity and its impact on general well-being and health status should be monitored in patients a risk.11,12 A hospital-based AF coordination centre, for example, could support both general practitioner and specialists in coordinated AF care.13 Previous pan-European studies investigating the provision of healthcare over geographically diverse areas have shown the potential impact of these variations in health inequality.14,15
It is important that an assessment of multimorbid AF considers how different comorbidities and their treatments interact and impact the patient, including the number and types of medications and potential polypharmacy. Previous research has identified that approximately 20% of patients with two comorbidities are prescribed between four and nine medications. Furthermore, 1% of these patients were prescribed 10 or more medications.16 Primary care physicians have previously highlighted the challenge of managing polypharmacy where medications are commenced by specialty clinicians.17,18 This has been reported to be associated with poor communication and rationale for treatment.17 This provides momentum to the finding of this study that better communication with primary care and a more collaborative approach could be important in improving and optimizing patient care. There is evidence to support the concept of greater interdisciplinary working particularly in relation to the role of geriatricians and pharmacists in reviewing polypharmacy and complex drug regimens in both the community and acute care setting17,19 and implementing evidence-based tools, especially for older people, e.g. STOPP-START.20
Data from this study highlight the time and travel burden for patients associated with seeing multiple different specialist clinicians. It is important to consider the number and type of healthcare appointments that a patient receives, where they take place and whether any of the advice and information given conflicts. Previous research has highlighted that communication between clinicians and patients, and between clinicians, is often poor and identified a relationship between substandard communication and patient outcomes.21,22 Ensuring continuity of care has been shown to improve both the patient experience and the patient outcome.23,24 When planning the care of patients with multimorbid AF, it is vital that communication between different clinicians and the handover of patient-related knowledge becomes as important as communication with the patient themselves.25 This will positively impact the shared decision-making process and enable the patient to have greater involvement in their healthcare.26–29 It is also important to consider how lifestyle and behaviour change interventions could positively impact specific health conditions or overall health-related quality of life including non-pharmacological treatment options.
Limitations
The survey was administered via a specialist body (EHRA), and therefore, the opinions of those involved in AF management outside of EHRA were not included. As 66% of the responses to this survey were from six European countries, it is important to recognize that the findings of this survey may not be generalizable to Europe as a whole. The low response rate from allied health professionals, with 91% of responses from physicians, which may have a huge impact on the availability of other specialist care. On the other hand, it illustrates the reality that little AF care in Europe at this moment is delivered in multidisciplinary (even nurse-led) settings, which is exactly one of the main findings of this study. Furthermore, as the survey was shared at a local and regional level and submitted anonymously, there was no method for verifying the identity of respondents and therefore the accuracy of the submitted data.
Conclusion
The results of this survey highlighted the current state of clinical practice in the management of multimorbid AF across Europe and the varying levels of specialist services available for managing comorbidities and the lack of a systematic approach for managing multimorbidity. For many respondents, the need for greater collaborative working, education, and a focus on patient self-efficacy was clear. The results demonstrate the need for systematic, integrated management of AF-related comorbidities and these results will inform the next phases of the EHRA-PATHS study.
Contributor Information
Geraldine Lee, Division of Applied Technology for Clinical Care, Florence Nightingale Faculty of Nursing, Midwifery & Palliative Care, King’s College London, James Clerk Maxwell Building, 57 Waterloo Road, London SE1 8WA, UK.
Edward Baker, Division of Applied Technology for Clinical Care, Florence Nightingale Faculty of Nursing, Midwifery & Palliative Care, King’s College London, James Clerk Maxwell Building, 57 Waterloo Road, London SE1 8WA, UK.
Ronan Collins, Age-Related Health Care, Tallaght University Hospital Dublin, Dublin, Ireland; Department of Gerontology, Trinity College Dublin, Dublin, Ireland.
Jose L Merino, Department of Cardiology, La Paz University Hospital, IdiPaz, Universidad Autonoma, Madrid, Spain.
Lien Desteghe, Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium; Heart Centre Hasselt, Jessa Hospital, Hasselt, Belgium; Research Group Cardiovascular Diseases, University of Antwerp, Antwerp, Belgium; Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium.
Hein Heidbuchel, Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium; Research Group Cardiovascular Diseases, University of Antwerp, Antwerp, Belgium; Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the grant agreement No. 945260.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Jani BD, Nicholl BI, McQueenie R, Connelly DT, Hanlon P, Gallacher KIet al. Multimorbidity and comorbidity in atrial fibrillation and effects on survival: findings from UK biobank cohort. Europace 2018;20:f329–f36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker E, Woolley A, Xyrichis A, Norton C, Hopkins P, Lee G. How does the implementation of a patient pathway-based intervention in the acute care of blunt thoracic injury impact on patient outcomes? A systematic review of the literature. Injury 2020;51:1733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts HC, Pickering RM, Onslow E, Clancy M, Powell J, Roberts Aet al. The effectiveness of implementing a care pathway for femoral neck fracture in older people: a prospective controlled before and after study. Age Ageing 2004;33:178–84. [DOI] [PubMed] [Google Scholar]
- 5. Devapriam J, Alexander R, Gumber R, Pither J, Gangadharan S. Impact of care pathway-based approach on outcomes in a specialist intellectual disability inpatient unit. J Intellect Disabil 2014;18:211–20. [DOI] [PubMed] [Google Scholar]
- 6. Kwan J, Hand P, Dennis M, Sandercock P. Effects of introducing an integrated care pathway in an acute stroke unit. Age Ageing 2004;33:362–7. [DOI] [PubMed] [Google Scholar]
- 7. Heidbuchel H, Van Gelder IC, Desteghe L, for EHRA-PATHS Investigators . ESC And EHRA lead a path towards integrated care for multimorbid atrial fibrillation patients: the horizon 2020 EHRA-PATHS project. Eur Heart J 2022;43:1450–2. [DOI] [PubMed] [Google Scholar]
- 8. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. [DOI] [PubMed] [Google Scholar]
- 9. O'Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010;341:c4587. [DOI] [PubMed] [Google Scholar]
- 10. Moseholm E, Fetters M. Conceptual models to guide integration during analysis in convergent mixed methods studies. Methodol Innovations 2017;10:205979911770311. [Google Scholar]
- 11. Roland M, Paddison C. Better management of patients with multimorbidity. BMJ 2013;346:f2510. [DOI] [PubMed] [Google Scholar]
- 12. Valderas JM, Gangannagaripalli J, Nolte E, Boyd CM, Roland M, Sarria-Santamera Aet al. Quality of care assessment for people with multimorbidity. J Intern Med 2019;285:289–300. [DOI] [PubMed] [Google Scholar]
- 13. Irving G, Neves AL, Dambha-Miller H, Oishi A, Tagashira H, Verho Aet al. International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open 2017;7:e017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jutz R. Health inequalities in Eastern Europe. Does the role of the welfare regime differ from Western Europe? Soc Sci Med 2020;267:113357. [DOI] [PubMed] [Google Scholar]
- 15. Mackenbach JP, Valverde JR, Artnik B, Bopp M, Brønnum-Hansen H, Deboosere Pet al. Trends in health inequalities in 27 European countries. Proc Natl Acad Sci U S A 2018;115:6440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Payne RA, Avery AJ, Duerden M, Saunders CL, Simpson CR, Abel GA. Prevalence of polypharmacy in a Scottish primary care population. Eur J Clin Pharmacol 2014;70:575–81. [DOI] [PubMed] [Google Scholar]
- 17. Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ 2015;350:h176. [DOI] [PubMed] [Google Scholar]
- 18. Payne RA, Avery AJ. Polypharmacy: one of the greatest prescribing challenges in general practice. Br J Gen Pract 2011;61:83–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Avery AJ, Rodgers S, Cantrill JA, Armstrong S, Cresswell K, Eden Met al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet 2012;379:1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening tool of older person's prescriptions) and START (screening tool to alert doctors to right treatment). Consensus validation. Int J Clin Pharmacol Ther 2008;46:72–83. [DOI] [PubMed] [Google Scholar]
- 21. Tiwary A, Rimal A, Paudyal B, Sigdel KR, Basnyat B. Poor communication by health care professionals may lead to life-threatening complications: examples from two case reports. Wellcome Open Res 2019;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vermeir P, Vandijck D, Degroote S, Peleman R, Verhaeghe R, Mortier Eet al. Communication in healthcare: a narrative review of the literature and practical recommendations. Int J Clin Pract 2015;69:1257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nyweide DJ, Anthony DL, Bynum JP, Strawderman RL, Weeks WB, Casalino LPet al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med 2013;173:1879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med 2005;3:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sinnott C, Mc Hugh S, Browne J, Bradley C. GPs’ perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open 2013;3:e003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Foster G, Taylor SJ, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev 2007. Art 4:CD005108. [DOI] [PubMed] [Google Scholar]
- 27. Smith SM, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2021;1:CD006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennedy A, Reeves D, Bower P, Lee V, Middleton E, Richardson Get al. The effectiveness and cost effectiveness of a national lay-led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health 2007;61:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham Cet al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346:f2882. [DOI] [PMC free article] [PubMed] [Google Scholar]