Abstract
Objectives
This study aimed to describe the full scope of long-term outcomes and the ongoing pathophysiological alterations among COVID-19 survivors.
Methods
We established a longitudinal cohort of 208 COVID-19 convalescents and followed them at 3.3 (interquartile range [IQR]: 1.3, 4.4, visit 1), 9.2 (IQR: 9.0, 9.6, visit 2), and 18.5 (IQR: 18.2, 19.1, visit 3) months after infection, respectively. Serial changes in multiple physical and psychological outcomes were comprehensively characterized. We, in addition, explored the potential risk factors of SARS-CoV-2 antibody response and sequelae symptoms.
Results
We observed continuous improvement of sequelae symptoms, lung function, chest computed tomography (CT), 6-minute walk test, and the Borg dyspnea scale, whereas sequelae symptoms (at least one) and abnormal chest CT patterns still existed in 45.2% and about 30% of participants at 18.5 months, respectively. Anxiety and depression disorders were alleviated for the convalescents, although depression status was sustained for a longer duration.
Conclusions
Most COVID-19 convalescents had an overall improved physical and psychological health status, whereas sequelae symptoms, residual lesions on lung function, exercise impairment, and mental health disorders were still observed in a small proportion of participants at 18.5 months after infection. Implementing appropriate preventive and management strategies for the ever-growing COVID-19 population is warranted.
Keywords: COVID-19, Longitudinal cohort, Sequelae, Lung function, CT abnormalities, Depression and anxiety
Introduction
SARS-CoV-2, with high covertness and high transmissibility, has caused more than 0.5 billion confirmed cases and 6.3 million deaths globally as of July 13, 2022 [1,2]. Huge burdens for the healthcare system and the whole of society may occur due to the overwhelming COVID-19 pandemic and the rapidly growing population of post-COVID-19 patients worldwide [3]. Emerging evidence has suggested that many COVID-19 survivors suffered from a higher rate of long-term complications and limited day-to-day activities [4], [5], [6] and showed a relatively lower physical and mental health status than the general population [7], [8], [9]. Previous studies primarily focused on sequelae symptoms or respiratory outcomes within 1-year after infection [10], [11], [12], [13], [14], [15], and the majority of studies were limited to the cross-sectional design [10]. Few prospective investigations depicted the overall health outcomes of discharged COVID-19 patients with repeated assessments [9]. Moreover, despite the reported possible persisting myocarditis and inflammation [16,17], less attention is paid to the recovery condition of the myocardial injury. To date, little is known about the natural history of long-term COVID-19. There is still an immediate need for studies to explore the longer health outcomes and the ongoing pathophysiology alterations among COVID-19 survivors.
In this study, we established a longitudinal cohort of COVID-19 convalescents with different disease severity with over 18.5 months of follow-up. We comprehensively characterized the serial changes of multiple indicators, including sequelae symptoms, respiratory outcomes, computed tomography (CT) scans, physical function, a biomarker of myocardial injury, SARS-CoV-2 antibody response, and mental health disorders. We further explored the potential risk factors of the SARS-CoV-2 antibody and sequelae symptoms.
Methods
Study design
In this prospective cohort study, we invited discharged patients with COVID-19 from different hospitals in multiple districts of Wuhan, China. There were 289 convalescents who agreed to participate in this study and attend designated follow-up at Hubei Provincial Hospital of Traditional Chinese Medicine on February 17, 2020. A total of 81 non-consecutive patients were lost to follow-up due to declining participation (64 participants), leaving Wuhan (15 participants), and dying before visit 1 (2 participants). There were 208 participants who were included in the final analysis and participated in three visits. The median duration of three visits was 3.3 (interquartile range [IQR]: 1.3, 4.4, visit 1), 9.2 (IQR: 9.0, 9.6, visit 2), and 18.5 (IQR: 18.2, 19.1, visit 3) months after infection, respectively. The study population and severity classification, admission and discharge criteria, and the statistical analysis were further summarized in the supplementary eText. The flowchart of the study was also shown in supplementary eFigure 1. This study was approved by the Ethics Committee of the School of Public Health, Tongji Medical College, Huazhong University of Science and Technology (approval number: 202001). All participants provided written informed consent.
Procedure
At each visit, the participants underwent a detailed questionnaire interview, physical examination, routine blood test, pulmonary function tests, high-resolution chest CT (HRCT) scan, and a 6-minute walking test (6MWT). Self-reported sequelae symptoms include cough, fatigue or muscle weakness, sleep difficulties, decreased appetite, diarrhea or vomiting, smell or taste disorder, dizziness or headache, sore throat, and chest pain.
Lung function tests were performed before and after 6MWT according to guidelines of the American Thoracic Society [18]. Parameters consisted of forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and forced expiratory flow between 25% and 75% of vital capacity (FEF25-75). All measurements were performed with a spirometer (SP80B, CONTEC Medicine, Qinhuangdao, China) and expressed as predicted percentages of normal values. Measurements assessed after 6MWT were shown in the results.
Participants underwent unenhanced chest CT examinations using a 40-section CT scanner with breath holding at the end of inspiration (uCT 530, United Imaging Healthcare, Shanghai, China). Images were reconstructed at 0.55 mm slice thickness, with 513 mm × 768 mm matrix size. The collimation and rotation time was 22 mm and 0.7 seconds, respectively. Other parameters were set according to the manufacturer's standard routine. CT abnormalities were demonstrated according to the terms of international standards defined by the Fleischner Society glossary and peer-reviewed COVID-19 literature [19], [20], [21]. In our study, two professional radiologists and one experienced pulmonologist constituted an evaluation group, and we invited two groups with the same specialist configuration to read CT reports separately and further assess ground-glass opacity (GGO) scores and reticular pattern (RP) scores. The average score of the two groups was considered the final score for statistical analysis. Details of the GGO scoring system are: 0, no involvement; 1, involvement <25%; 2, 25-50% involvement; 3, 50-75% involvement; 4, involvement >75%. The unilateral lung is divided into three sections: upper (above the level of the carina), middle, and lower (below the level of the right lower pulmonary vein). There are six sections on both sides, including right upper, right middle, right lower, left upper, left middle, and left lower. Each lobe is scored separately, and then the total GGO score of six lobes (0-24) is calculated. Details of the RP scoring systems are: 0, no lines; 1, 1-2 short line/grid shadows; 2, multiple line/grid shadows; 3, <1 lung segment's aggregated line/grid-like shadows in the subpleural area; 4, 1-3 lung segments' large grid shadows; 5, >3 lung segments' diffuse grid shadows and/or deformed lung structure. The right upper lobe contains three segments, the left upper lobe usually contains two segments, the right middle, and the left lingual lung contain two segments and the highest score is 4 points; the lower lobe of both lungs has more than two segments and the highest score is 5 points; thus, the maximum score is 26 points. Each lobe was scored separately, and then we calculated the total RP score of the six lobes (0-26 points). All participants were scored, excluding pre-morbid lung nodules or scars.
A 6MWT was performed on a walk monitoring and analysis system (YK2020A, Wocaring Medical Equipment, Wuhan, China), following the standardized protocol of the American Thoracic Society [22]. Participants walk as far as possible indoors within 6 minutes on a flat and hard surface. Distance and predicted percentage of walking distance were calculated based on Jay's [23] method. In addition, the Borg dyspnea scale was assessed after 6MWT, as participants were asked to grade their level of breath shortness from 0 (no dyspnea at all) to 10 (excessive dyspnea) [24].
For biomarkers of cardiac injury, we measured cardiac troponin T (TnT) using a highly sensitive reagent of TnT (hsTnT) on the Cobas E601 immune analyzer (Roche Diagnostics), following the manufacturer's instruction. The measuring range was 3-10000 ng/l, and the intermediate precision coefficient of variation was <10%. A 99th percentile value of 14 ng/l in the general reference population had been reported previously [25,26]. Myocardial injury is defined as circulating cardiac Tn levels higher than the 99th percentile upper reference limit, regardless of new abnormalities of electrocardiography and echocardiography [27,28].
In addition, we assessed the SARS-CoV-2 neutralizing antibody (NAb) level by a pseudotyped virus-based assay and calculated the half-maximal inhibitory concentration (50% neutralizing titer [NT50]) according to the inhibition rate of each dilution using nonlinear regression. The details have been described in a previous study [29].
To further evaluate participants’ mental health, the validated Chinese versions of the Patient Health Questionnaire-9 (PHQ-9) contained a 9-item depression module ranging from 0 to 27 points and was used to evaluate post-discharge depression status [30]. The depression level was grouped by minimal (0-4), mild (5-9), moderate (10-14), moderately severe (15-19), and severe (20-27) [30]. Meanwhile, the Generalized Anxiety Disorder-7 (GAD-7) included a 7-item anxiety module ranging from 0 to 21 points and was applied to assess post-discharge anxiety conditions [31]. The anxiety severity was leveled as minimal (0-4), mild (5-9), moderate (10-14), and severe (15-21) [31]. Each above item scored from 0 (not at all) to 3 (nearly every day).
Results
The demographics and comorbidities are listed in Table 1 . Among 208 COVID-19 convalescents, there were 146 mild cases and 62 severe cases. The median age of participants was 58.0 years (IQR: 50.0, 64.3), and 100 (48.1%) were men. A total of 68 (32.7%) participants had college or higher education attainment. There were 101 (48.6%) participants who had more than 50,000 RMB per year of household income. The majority of participants were never-smokers (n = 183, 88.0%) and never-drinkers (n = 183, 88.0%). There were 75 (36.1%), 23 (11.1%), and 14 (6.7%) participants who had pre-existing hypertension, diabetes mellitus, and cardiovascular disease (CVD). The median body mass index (BMI) and waist circumferences were 24.3 kg/m2 and 90 cm. There were no significant differences in most variables between mild and severe cases except for BMI, with severe cases having a significantly lower median BMI of 23.4 (IQR: 22.0, 25.5) (P <0.01, Supplementary eTable 1).
Table 1.
Basic characteristics of COVID-19 convalescents.
| Variables | Total population (N = 208) |
|---|---|
| Age, years | 58 (50.0, 64.3) |
| Gender, n (%) | |
| Male | 100 (48.1) |
| Female | 108 (51.9) |
| Education, n (%) | |
| Middle school or lower | 140 (67.3) |
| College or higher | 68 (32.7) |
| Household income, n (%) | |
| <50000 RMB/year | 107 (51.4) |
| ≥50000 RMB/year | 101 (48.6) |
| Cigarette smoking, n (%) | |
| Never-smoker | 183 (88.0) |
| Ever-smoker | 25 (12.0) |
| Alcohol consumption, n (%) | |
| Never-drinker | 183 (88.0) |
| Ever-drinker | 25 (12.0) |
| Comorbidity, n (%) | |
| Hypertension | 75 (36.1) |
| Diabetes | 23 (11.1) |
| Cardiovascular disease | 14 (6.7) |
| Body mass index | 24.3 (22.6, 26.5) |
| Waist circumference | 90 (83, 97) |
| Duration from symptom onset to the last follow-up, months | 18.5 (18.2, 19.1) |
Data were expressed as median (interquartile range) or frequency (percentage).
Table 2 illustrates the longitudinal trend in sequelae symptoms, lung function, chest CT, 6MWT, hs-TnT, NT50 for serum SARS-CoV-2 NAb, and psychological condition of the COVID-19 survivors during the follow-up. The percentage of convalescents with at least one sequelae symptom decreased from 62.0% at 3.3 months to 50.0% and 45.2% at 9.2 months and 18.5 months, respectively. The most commonly reported symptoms in each visit were fatigue or muscle weakness and sleep difficulties (35.1% and 33.2%, respectively, at visit 1), with the frequency gradually declining during the follow-up. A significant decrease was observed for most sequelae symptoms between visit 1 and visit 2, whereas no significant decrease was observed for sequelae between visit 2 and visit 3. The indicators for lung function were slightly increased during the convalescence, although only a significant increase was observed for FEV1/FVC ratio. The FEV1/FVC was 83.0% at visit 1 and increased to 85.7% at visit 3. FEF25-75% was 81.2 (IQR: 71.9, 97.0) % at visit 1, while 91.8 (IQR: 75.6, 110.0) % at visit 3. A total of 95 participants attended chest CT during each visit, and the frequency of GGO decreased from 69 (72.6%) at visit 1 to 28 (29.5%) at visit 3. The CT scores of GGO and RP also experienced a drastic decrease, which was 6 and 7, respectively, at visit 1, while 1 and 3, respectively, at visit 3. The median distance of the 6MWT significantly improved, from 514.9 m (IQR: 480.2, 556.0) at 3.3 months to 535 m (IQR: 509.0, 570.0) at 18.5 months. Similarly, significant improvements were found for the percentage of predicted value and the Borg dyspnea scale after 6MWT between visit 1 and visit 3. Only four participants (4.7%) experienced dyspnea (Borg dyspnea scale ≥1) after 6MWT at 18.5 months, whereas 30 people had a Borg scale ≥ 1 (50.8%) at 3.3 months. The levels of hsTnT experienced a slight decrease over time, from 4.5 ng/l (IQR: 3.0, 7.5) at visit 1 to 4.1 ng/l (IQR: 3.0, 6.6) at visit 3, and myocardial injury frequency (hsTnT ≥14 ng/l) decreased to 3.8% at visit 3 compared with 7.0% at visit 1, although no significant difference was found between each visit. NT50 for serum SARS-CoV-2 NAb experienced a continuous decrease in the unvaccinated group, from 1153.0 (IQR: 473.5, 2095.8) at visit 1 to 281.0 (IQR: 128.2, 499.8) at visit 3. Meanwhile, the trend in the vaccinated group showed a rapid decrease between visit 1 and visit 2, with 765.5 (IQR: 343.5, 1510.5) and 287.5 (IQR: 140.2, 585.0) respectively, and then increased to 910.0 (IQR: 497.0, 1460.8) due to the COVID-19 vaccination after 1 year of pandemic. For mental health disorders, 96 and 97 participants provided information on depression and anxiety disorders during each visit, respectively. Both depression and anxiety scores showed a decreasing trend during the follow-up, with a slight difference among the three visits. The depression score gradually decreased from 5 at 3.3 months to 4 at 9.2 months and then rapidly decreased to 1 at 18.5 months. Meanwhile, the anxiety disorder scores decreased rapidly during the first two visits (median score = 4 and 1 at visit 1 and visit 2, respectively) and remained relatively stable during the last visit (median score = 0). Over 20% of our participants were suffering from at least mild depression or anxiety status at 18.5 months. The overall trends of the above parameters were similar in mild and severe convalescents (Supplementary eTable 2). Compared with the mild convalescents, severe convalescents had a relatively higher frequency of any sequelae symptoms within 18.5 months and higher CT scores of GGO and RP during the early follow-up period, as shown in Supplementary eTable 3. Meanwhile, we compared the variation of lung function, CT, 6MWT, and Borg dyspnea scale during each visit between the mild and the severe convalescents and found that the mild convalescents had a substantially better improvement in Borg dyspnea scale between visit 3 and visit 1 (Δ = -1.000 [-1.000, 0.000], P-value = 0.025) compared with the severe (Supplementary eTable 4).
Table 2.
Physical and psychological health status of convalescents during follow-up.
| Variables | Total population (N = 208) |
||
|---|---|---|---|
| Visit 1 (3.3 months) | Visit 2 (9.2 months) | Visit 3 (18.5 months) | |
| Sequelae symptoms | |||
| Any sequelae symptoms, n (%) | 129 (62.0)a,b | 104 (50.0) | 94 (45.2) |
| Cough, n (%) | 41 (19.7)a,b | 22 (10.6) | 22 (10.6) |
| Fatigue or muscle weakness, n (%) | 73 (35.1)a,b | 45 (21.6) | 37 (17.8) |
| Sleep difficulties, n (%) | 69 (33.2)a,b | 40 (19.2) | 34 (16.3) |
| Decreased appetite, n (%) | 21 (10.1)b | 10 (4.8) | 7 (3.4) |
| Diarrhea or vomiting, n (%) | 21 (10.1) | 9 (4.3) | 10 (4.8) |
| Smell or taste disorder, n (%) | 10 (4.8) | 9 (4.3) | 8 (3.8) |
| Dizziness or headache, n (%) | 9 (4.3) | 7 (3.4) | 8 (3.8) |
| Sore throat, n (%) | 10 (4.8) | 11 (5.3) | 6 (2.9) |
| Chest pain, n (%) | 12 (5.8) | 12 (5.8) | 13 (6.2) |
| Lung function | |||
| FEV1% | 94.3 (82.1, 106.8) | 96.8 (86.1, 104.5) | 96.6 (88.6, 107.6) |
| FVC% | 92.6 (83.5, 108.5) | 93.4 (84.9, 102.5) | 92.9 (83.7, 103.7) |
| FEV1/FVC% | 83.0 (80.2, 85.4)b | 83.3 (81.0, 87.5) | 85.7 (81.1, 89.1) |
| FEF25-75% | 81.2 (71.9, 97.0) | 88.3 (69.8, 103.7) | 91.8 (75.6, 111.0) |
| Chest CT | |||
| CT abnormal of GGO, n (%) | 69 (72.6)a,b | 38 (40.0) | 28 (29.5) |
| CT scores of GGO | 6.0 (3.0, 10.5)a,b | 3.0 (1.0, 5.5)c | 1.0 (0.0, 4.0) |
| CT abnormal of RP, n (%) | 61 (64.2)b | 47 (49.5) | 34 (35.8) |
| CT scores of RP | 7.0 (4.0, 14.0)a,b | 4.0 (2.0, 9.0)c | 3.0 (1.0, 7.0) |
| 6-minute walk test | |||
| Distance, m | 514.9 (480.2, 556.0)a,b | 565.2 (522.2, 610.0)c | 535.0 (509.0, 570.0) |
| Predicted distance% | 92.0 (86.0, 99.0)a,b | 100.0 (94.0, 106.0)c | 96.0 (89.0, 103.0) |
| Borg dyspnea scale ≥1, n (%) | 30 (50.8)a,b | 15 (16.5) c | 4 (4.7) |
| hsTnT (ng/l) | 4.5 (3.0, 7.5) | 4.3 (3.0, 6.8) | 4.1 (3.0, 6.6) |
| hsTnT ≥14 ng/l, n (%) | 10 (7.0) | 5 (3.3) | 6 (3.8) |
| NT50 for serum SARS-CoV-2 neutralizing antibody | 815.0 (396.0, 1804.5)a,b | 293.5 (147.8, 595.0)c | 678.0 (309.5, 1279.5) |
| Unvaccinated group | 1153.0 (473.5, 2095.8)a,b | 346.0 (162.5, 712.2) | 281.0 (128.2, 499.8) |
| Vaccinated group | 765.5 (343.5, 1510.5)a | 287.5 (140.2, 585.0)c | 910.0 (497.0, 1460.8) |
| Mental health disorders | |||
| Depression, score | 5.0 (2.0, 9.0)b | 4.0 (1.0, 8.0)c | 1.0 (0.0, 4.0) |
| Depression score ≥5, n (%) | 53 (55.2)b | 43 (44.8)c | 23 (24) |
| Anxiety disorder, score | 4.0 (0.0, 7.0)b | 1.0 (0.0, 5.0) | 0.0 (0.0, 4.0) |
| Anxiety score ≥5, n (%) | 36 (37.1) | 29 (29.9) | 22 (22.7) |
Data were expressed as median (interquartile range) or frequency (percentage). Kruskal–Wallis test was applied for group comparisons of continuous variables, and χ² test or Fisher Exact tests were performed to analyze the categorical variables.
CT: computed tomography; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; GGO, ground-glass opacity; RP, reticular pattern; hsTnT, highly sensitive troponin T; NT50, the half-maximal inhibitory concentration.
Significant difference between visit 1 and visit 2 groups (P <0.05).
Significant difference between visit 1 and visit 3 groups (P <0.05).
Significant difference between visit 2 and visit 3 groups (P <0.05).
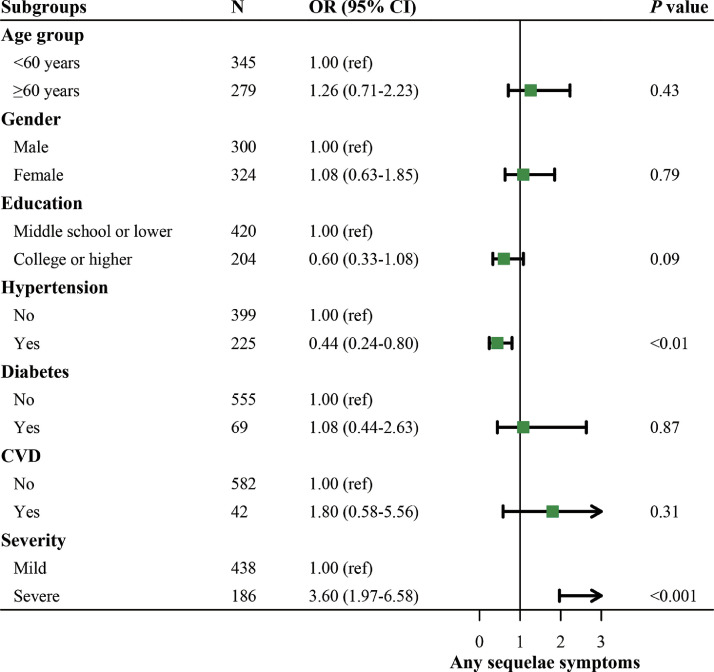
Table 3 provided a summary of the potential risk factors of NT50 for serum SARS-CoV-2 NAb during follow-up. We established a linear mixed model to calculate the effect size of the number of any symptoms, CT scores of GGO and RP, and depression and anxiety scores on NT50 for serum SARS-CoV-2 NAb. The number of any symptoms, CT scores of GGO, and RP were positively associated with a higher level of NT50 for serum SARS-CoV-2 NAb, and the β (SE) were 0.126 (0.045), 0.045 (0.017), and 0.045 (0.015), respectively (all P <0.01). No significant associations were observed for depression and anxiety scores with NT50 (both P >0.05). Figure 1 illustrates the potential risk factors of any sequelae symptoms. Generalized linear mixed models were established to explore the associations of any sequelae symptoms with age group, gender, education, and comorbidities, including hypertension, diabetes mellitus, and CVD, and disease severity, with multivariable adjustment conducted. Compared with participants without hypertension, participants with hypertension had an odds ratio (OR) of 0.44 (95% confidence interval [CI] 0.24-0.80) for any sequelae symptoms. Meanwhile, severe participants had an OR of 3.60 (95% CI 1.97-6.58) compared with mild participants.
Table 3.
Potential risk factors associated with NT50 for serum SARS-CoV-2 neutralizing antibody during follow-up.
| Variables | β (SE) | P-value |
|---|---|---|
| Number of any symptom | 0.126 (0.045) | 0.005 |
| CT scores of ground-glass opacity | 0.045 (0.017) | 0.009 |
| CT scores of reticular pattern | 0.045 (0.015) | 0.004 |
| Depression score | -0.002 (0.015) | 0.901 |
| Anxiety score | 0.019 (0.018) | 0.276 |
Linear mixed model of repeated measures was conducted to estimate fixed effects of any symptom, CT scores, and mental health disorders on log-transformed NT50 of SARS-CoV-2 neutralizing antibody, adjusted for age, gender, and vaccination. Random effects consisted of days of the neutralizing antibody test after symptom onset and days of the first shot of the COVID-19 vaccine since symptom onset of each participant. The number of any symptoms refers to the number of sequelae of a participant.
CT: computed tomography; NT50, the half-maximal inhibitory concentration; SE, standard error of the beta coefficient.
Fig. 1.
The potential risk factors of sequelae symptoms.
Generalized linear mixed models were established to explore the associations of any sequelae symptoms (categorical) with fixed effects. Each patient was included in random effect. For the age group (<60 years/≥60 years), we adjusted gender (male/female) and education (middle school or lower/college or higher). Comorbidities including hypertension (no/yes), diabetes (no/yes), and CVD (no/yes), and disease severity (mild/severe) were excluded due to the potential mediating effects. For gender, we adjusted age group, education, comorbidities, and disease severity. For education, we adjusted age group, gender, and disease severity. Comorbidities were excluded due to the potential mediating effects. For comorbidities (hypertension, diabetes mellitus, and CVD), we adjusted each for age group, gender, education, and comorbidities except itself. Disease severity (mild/severe) was excluded due to the potential mediating effects. For disease severity, we adjusted age group, gender, education, and comorbidities.
CVD, Cardiovascular disease; OR, odds ratio.
In addition, all participants in this study were hospitalized and discharged after recovery (Supplementary eTable 5). The median days of hospitalization were 27 (IQR 17.0, 35.0) for the total population and 25.0 (IQR 15.2, 32.0) and 29.0 (IQR 22.0, 37.0), respectively, for the mild and severe participants. A total of 9 (4.3%) participants were admitted to the intensive care unit (ICU). Only one participant received treatment with invasive mechanical ventilation, whereas no one underwent a tracheostomy or developed renal failure that needed renal function replacement treatment. Compared with mild participants, severe participants had a significantly higher frequency of ICU admission, more intensive treatments, and longer hospital stays. As shown in Supplementary eTable 6, the obvious improvement of CT abnormalities, 6MWT, Borg dyspnea scale, and mental health disorders was observed in both groups (hospitalization <27 days/≥27 days). Substantial improvement of sequelae symptoms was further observed in participants with a stay of ≥27 days. In the vaccinated group, the NT50 levels underwent a decrease and an increase due to the vaccination after visit 2 in both participants with hospitalization less than and more than or equal to 27 days. In Supplementary eTable 7, we observed an obvious recovery trend in any sequelae symptoms, CT abnormalities, 6MWT, Borg dyspnea scale, and mental health disorders in the non-ICU group, while no substantial improvement was observed in the ICU group during three visits.
Discussion
Due to the COVID-19 pandemic, there has been growing concern that survivors might be at higher risk of multiple complications. Large-scale, longitudinal measurements and longer-term data are urgently needed to comprehensively characterize the trend of health consequences of COVID-19 convalescents. In this study, we comprehensively assessed the sequential pathophysiology changes of COVID-19 convalescents based on a considerably long follow-up cohort in Wuhan (over 18.5 months). We observed that most convalescents had substantial improvement in their general health status during the follow-up visits. Meanwhile, some convalescents still have the sequelae symptoms, including fatigue, muscle weakness, sleep difficulties, abnormal CT patterns, and depression and anxiety disorder at 18.5 months after diagnosis. Our finding provides novel evidence for the personalized prevention and intervention of long-term outcomes among COVID-19 convalescents.
Previous studies have reported that fatigue, muscle weakness, and sleep difficulties were the most frequent post-discharge sequelae symptoms and could last at least 12 months [13]. We confirmed this finding and advanced the evidence to 18.5 months after COVID-19 diagnosis. The potential pathogenesis of fatigue and muscle weakness may include viral-induced myositis, long periods of bed rest during convalescence, and the use of systemic corticosteroid therapy [32,33]. We found continuous improvement in chest CT in these participants. In the meantime, the CT appearance of the abnormal radiographic pattern was still detected in around 30% of convalescents at 18.5 months. The CT abnormalities could even be sustained beyond this duration. The improvement with concurrent residual lesions of lung CT was also observed in previous studies with 1 to 2 years of follow-up [9,34,35]. Meanwhile, we observed some fluctuations in the indicators of lung function and the 6MWT. The convalescents experienced fluctuations of FEV1%, FVC%, 6MWT, and predicted distance during the three visits, with an overall recovery trend. The possible mechanisms of this phenomenon include the recovery of early injury and the increase of pulmonary ventilation from 3 to 9 months after symptom onset, and then pulmonary atelectasis and parenchymal fibrosis occurred due to the persistent effect of inflammation in the later stages of the disease course [32,36]. This hypothesis is supported by the CT results in our study, as 35.8% of convalescents still had reticular patterns at 18.5 months. Previous studies of acute respiratory distress syndrome also reported similar trends in lung function and 6MWTs among SARS survivors [37]. Further studies are still warranted to confirm our findings and unravel the underlying mechanisms.
Notably, the results demonstrated that both depression and anxiety disorders were alleviated for the convalescents, although at least mild depression and anxiety status were sustained in over 20% of our participants at 18.5 months. A cohort study suggested that some COVID-19 survivors had psychiatric sequelae (mood, anxiety, or psychotic disorder) and more frequent substance use at 6 months post-COVID-19, although the longitudinal assessment of the outcomes was not available in the study [38]. Moreover, Huang et al. [11] reported that more patients had anxiety or depression at 12 months than at 6 months (26% vs 23%). In addition, another study reported that after 2 years post-symptom onset, there were still 12% of COVID-19 survivors who had anxiety or depression, compared with 5% of participants in matched non-COVID-19 controls [9]. Long-term and multicenter investigations with larger sample sizes and standard assessment methods are warranted to confirm the conclusion.
Long-COVID symptoms [39], pulmonary involvement [40,41], and mental status [42] were associated with antibody immune response, and inflammation mechanisms, including the number of clusters of differentiation 4+ T cells declining and the interleukin-6 level elevation, may partially explain the associations. We included the previously mentioned three indices in multivariable analysis using a linear mixed model and observed that the number of any symptoms, CT scores of GGO, and CT scores of RP were positively associated with higher NT50 for serum SARS-CoV-2 NAb. Higher NAb titer of SARS-CoV-2 indicates a potential persistent inflammation and immune response in our participants for 18.5 months. Previous studies demonstrated that negative psychological experiences, such as less social cohesion, were associated with lower vaccine efficacy [42] and lower Ab titer (β = -0.10, P-value = 0.01) [43]. However, no significant associations were observed for depression and anxiety scores with the NT50 in our participants due to the relatively small sample size. Hence, further psychological and random behavioral intervention studies with a larger sample size are needed to explore the potential mechanism.
Hypertension was associated with lower risks of any sequelae symptoms among our COVID-19 survivors, with adjusted OR = 0.44 (95% CI: 0.24-0.80). Some studies reported that hypertension was associated with higher risks of adverse outcomes in patients with COVID-19 [44,45]. However, Huang et al. [46] reported that hypertension was not an independent risk factor for increasing COVID-19 severity or mortality. Tadic et al. [47] also suggested that hypertension was not an independent predictor of the lethal outcome in patients with COVID-19. A large cohort study included 153,760 COVID-19 individuals, and over 10 million controls suggested that the incident risk of cardiovascular outcome was lower in the hypertension group (hazard ratio [HR] = 1.57, 95% CI: 1.51-1.64) than in the normotensive group (HR = 1.66, 95% CI: 1.61-1.72) [48]. The angiotensin-converting enzyme 2 (ACE2) has been suggested to be a coreceptor for SARS-CoV-2 to enter epithelial cells [49]. Previous studies have indicated that ACE inhibitor (ACEI)/angiotensin Ⅱ receptor blocker (ARB) medication played a protective role in the COVID-19 prognosis [50,51]. A retrospective study involving 1128 adult patients observed that compared with ACEI/ARB non-users, the use of ACEI/ARB among hospitalized patients with COVID-19 and hypertension was associated with a lower risk of all-cause mortality (HR = 0.42, 95% CI: 0.19-0.92) [51]. Meng et al. [50] also reported that patients with COVID-19 with hypertension receiving ACEI/ARB therapy had a lower rate of severe diseases and better clinical outcomes compared with non-ACEI/ARB users. Despite the previously mentioned evidence, the underlying mechanisms for the observed association remain largely unclear. The findings should be interpreted cautiously, and future studies are warranted to confirm our findings and unravel the potential mechanism.
The severe participants had a higher risk for any sequelae symptoms compared with the mild participants, with adjusted OR = 3.60 (95% CI: 1.97-6.58). This is in line with Cao and his colleagues’ studies [9,11,52]. They provided consistent findings that compared with mild patients, severe or critically ill patients had a significantly poor recovery and more post-COVID symptoms at 6, 12, and 24 months after discharge [9,11,52]. A meta-analysis including 18 follow-up studies (N = 8591) also supported this conclusion [6]. Survivors with severe initial illness were more likely to have a higher burden of sequelae symptoms after a year since infection [6].
Compared with the wild-type strain, the current dominant variant Omicron differs in transmissibility, pathogenicity, and immune escape due to the deletions and many mutations in the spike protein [53], [54], [55], [56]. Patients infected with Omicron had milder clinical manifestations and decreased disease severity and mortality compared with those who were infected with the wild-type strain [54]. Meanwhile, a previous study reported that Omicron-infected patients had a largely reduced virus-neutralizing activity [57] and an increased risk of reinfection [58]. A recent study reported that the prevalence of any sequelae symptoms was 4.5% in Omicron cases at 4 weeks infection [53], while 45.2% of our participants (infected with wild-type strain) still experienced any sequelae symptoms at 18.5 months after symptom onset. Therefore, the generalizability of our findings should be tested in other populations infected with new variants.
A longitudinal and comprehensive profile of the disease was observed at 18.5 months of follow-up in our study. Nevertheless, this investigation has several limitations. First, participants in this study were recruited during the early period of the COVID-19 pandemic; thus, the generalizability to other new SARS-CoV-2 variants is limited [53]. Second, more than 70% of our participants were mild patients at the initial stage; further investigations that include participants of various disease severity and larger sample size are needed. Third, not all participants attended three visits, thus, selection bias could be introduced during this process. Although there were 81 non-consecutive patients lost to follow-up, we performed the additional analysis to compare the basic characteristics between included (N = 208) and declined (N = 81) participants (Supplementary eTable 8). No significant difference in basic characteristics was observed for the two groups, which confirmed the robustness of our finding. The selection bias could be minimal. Fourth, we did not assess the transfer factor for carbon monoxide (TLCO) in the pulmonary function test. We intended to conduct a comprehensive study including multiple physical and mental health indices and only assessed the key indicators for each category. The TLCO should be tested in future studies to distinguish diffusion deficit.
In summary, this longitudinal study suggested that most COVID-19 convalescents had an improved physical and psychological health status in general, whereas post-discharge sequelae symptoms, residual lesions on lung function and exercise impairment, and mental health disorders were still observed in a small proportion of our participants for 18.5 months. Clinicians and policymakers should be aware of the risk of physical and mental complications in the ever-growing COVID-19 convalescents. The development of targeted strategies for the early prevention of post-discharge sequelae symptoms, CT abnormalities, and mental health problems is warranted.
Acknowledgments
Declaration of competing interest
No potential conflict of interest was reported by the author(s).
Funding
This work was supported by the Emergency Key Program of Guangzhou Laboratory (EKPG21-30), the National Science Foundation of China (82204113 and 72061137006), and the Fellowship of China Postdoctoral Science Foundation (2020T130034ZX).
Ethical approval
This study was approved by the Ethics Committee of the School of Public Health, Tongji Medical College, Huazhong University of Science and Technology (approval number: 202001). All participants provided written informed consent.
Acknowledgments
We thank all the study participants and project staff from Tongji Medical College, Huazhong University of Science and Technology, and Hubei Provincial Hospital of Traditional Chinese Medicine for the work they have done.
Author contributions
Yi Guo, Hao Wang, Mingzhong Xiao, and Xin Guan: conceptualization, data collection, analysis, interpretation, and writing; Yanshou Lei, Tingyue Diao, Pinpin Long, Rui Zeng, Xuefeng Lai: data collection and interpretation, review; Hao Cai, Yutong You, Yuying Wen, Wenhui Li, Xi Wang, Yufei Wang, Qinlin Chen, Yuchan Yang, Yutong Qiu, Jishuai Chen, Huidan Zeng: data collection and investigation; Wei Ni, Youyun Zhao, Kani Ouyang, Jingzhi Wang: conceptualization, methodology, and investigation; Qi Wang, Li Liu, Lulu Song, Youjie Wang, Huan Guo: resources, supervision, and review; Xiaodong Li, Tangchun Wu and Yu Yuan: study design and conceptualization, methodology, data interpretation, writing, and review; All authors have read and approved the final manuscript. Yi Guo, Hao Wang, Mingzhong Xiao, and Xin Guan are joint first authors; Xiaodong Li, Tangchun Wu and Yu Yuan are joint senior authors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.12.008.
Appendix. Supplementary materials
References
- 1.World Health Organization. COVID-19 weekly epidemiological update, 2022, https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—13-july-2022; [accessed 13 July 2022].
- 2.Hao X, Cheng S, Wu D, Wu T, Lin X, Wang C. Reconstruction of the full transmission dynamics of COVID-19 in Wuhan. Nature. 2020;584:420–424. doi: 10.1038/s41586-020-2554-8. [DOI] [PubMed] [Google Scholar]
- 3.Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323:1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayoubkhani D. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 1 April 2021; https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021 [accessed on 1 April 2021].
- 5.Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11:269. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, et al. Post-COVID syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, Li X, Gu X, Zhang H, Ren L, Guo L, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863–876. doi: 10.1016/S2213-2600(22)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021;92:55–70. doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeßle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort study. Clin Infect Dis. 2022;74:1191–1198. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynberg E, van Willigen HDG, Dijkstra M, Boyd A, Kootstra NA, van den Aardweg JG, et al. Evolution of COVID-19 symptoms during the first 12 months after illness onset. Clin Infect Dis. 2022;75:e482–e490. doi: 10.1093/cid/ciab759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Wang F, Shen Y, Zhang X, Cen Y, Wang B, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Thoracic Society Standardization of spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 20.Pan F, Yang L, Liang B, Ye T, Li L, Li L, et al. Chest CT patterns from diagnosis to 1 year of follow-up in patients with COVID-19. Radiology. 2022;302:709–719. doi: 10.1148/radiol.2021211199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 23.Jay SJ. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 2000;161:1396. doi: 10.1164/ajrccm.161.4.16147a. [DOI] [PubMed] [Google Scholar]
- 24.Just N, Bautin N, Danel-Brunaud V, Debroucker V, Matran R, Perez T. The Borg dyspnoea score: a relevant clinical marker of inspiratory muscle weakness in amyotrophic lateral sclerosis. Eur Respir J. 2010;35:353–360. doi: 10.1183/09031936.00184908. [DOI] [PubMed] [Google Scholar]
- 25.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 26.Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta. 2011;412:748–754. doi: 10.1016/j.cca.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Yuan Y, Xiao M, Chen L, Zhao Y, Haiwei Z, et al. Dynamics of the SARS-CoV-2 antibody response up to 10 months after infection. Cell Mol Immunol. 2021;18:1832–1834. doi: 10.1038/s41423-021-00708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 32.Hui DS, Wong KT, Ko FW, Tam LS, Chan DP, Woo J, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peiris JSM, Chu CM, Cheng VCC, Chan KS, Hung IFN, Poon LLM, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/s0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barini M, Percivale I, Danna P, Longo V, Costantini P, Paladini A, et al. 18 months computed tomography follow-up after COVID-19 interstitial pneumonia. J Public Health Res. 2022;11 doi: 10.4081/jphr.2022.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Ding C, Yu L, Guo W, Feng X, Yu L, et al. One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med. 2021;19:191. doi: 10.1186/s12916-021-02056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholls JM, Poon LLM, Lee KC, Ng WF, Lai ST, Leung CY, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/s0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 38.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJLP. 6-month neurological and psychiatric outcomes in 236,379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Abellán J, Padilla S, Fernández-González M, García JA, Agulló V, Andreo M, et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. J Clin Immunol. 2021;41:1490–1501. doi: 10.1007/s10875-021-01083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Başaran S, Şimşek-Yavuz S, Meşe S, Çağatay A, Medetalibeyoğlu A, Öncül O, et al. The effect of tocilizumab, anakinra and prednisolone on antibody response to SARS-CoV-2 in patients with COVID-19: a prospective cohort study with multivariate analysis of factors affecting the antibody response. Int J Infect Dis. 2021;105:756–762. doi: 10.1016/j.ijid.2021.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He S, Zhou C, Lu D, Yang H, Xu H, Wu G, et al. Relationship between chest CT manifestations and immune response in COVID-19 patients. Int J Infect Dis. 2020;98:125–129. doi: 10.1016/j.ijid.2020.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madison AA, Shrout MR, Renna ME, Kiecolt-Glaser JK. Psychological and behavioral predictors of vaccine efficacy: considerations for COVID-19. Perspect Psychol Sci. 2021;16:191–203. doi: 10.1177/1745691621989243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephen G, Siobhán H, Muldoon OT, Whittaker AC. Social cohesion and loneliness are associated with the antibody response to COVID-19 vaccination. Brain Behav Immun. 2022;103:179–185. doi: 10.1016/j.bbi.2022.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang S, Wang J, Liu F, Liu J, Cao G, Yang C, et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020;43:824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tadic M, Cuspidi C, Mancia G, Dell'Oro R, Grassi G. COVID-19, hypertension and cardiovascular diseases: should we change the therapy? Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with Delta versus Omicron variants of SARS-CoV-2. Lancet. 2022;399:2263–2264. doi: 10.1016/S0140-6736(22)00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA. 2022;327:583–584. doi: 10.1001/jama.2021.24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torjesen I. COVID-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. 2021;375:n2943. doi: 10.1136/bmj.n2943. [DOI] [PubMed] [Google Scholar]
- 57.Carreño JM, Alshammary H, Tcheou J, Singh G, Raskin AJ, Kawabata H, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–688. doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]
- 58.Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;376:eabn4947. doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.