Abstract
Direct putaminal infusion of adeno-associated virus vector (serotype 2) (AAV2) containing the human glial cell line-derived neurotrophic factor (GDNF) transgene was studied in a phase I clinical trial of participants with advanced Parkinson’s disease (PD). Convection-enhanced delivery of AAV2-GDNF with a surrogate imaging tracer (gadoteridol) was used to track infusate distribution during real-time intraoperative magnetic resonance imaging (iMRI). Pre-, intra-, and serial postoperative (up to 5 years after infusion) MRI were analyzed in 13 participants with PD treated with bilateral putaminal co-infusions (52 infusions in total) of AAV2-GDNF and gadoteridol (infusion volume, 450 mL per putamen). Real-time iMRI confirmed infusion cannula placement, anatomic quantification of volumetric perfusion within the putamen, and direct visualization of off-target leakage or cannula reflux (which permitted corresponding infusion rate/cannula adjustments). Serial post-treatment MRI assessment (n = 13) demonstrated no evidence of cerebral parenchyma toxicity in the corresponding regions of AAV2-GDNF and gadoteridol co-infusion or surrounding regions over long-term follow-up. Direct confirmation of key intraoperative safety and efficacy parameters underscores the safety and tissue targeting value of real-time imaging with co-infused gadoteridol and putative therapeutic agents (i.e., AAV2-GDNF). This delivery-imaging platform enhances safety, permits delivery personalization, improves therapeutic distribution, and facilitates assessment of efficacy and dosing effect.
Keywords: Parkinson's disease, gene therapy, GDNF, MRI-guided, AAV, gadoteridol, safety, convection-enhanced delivery, putamen
Graphical abstract
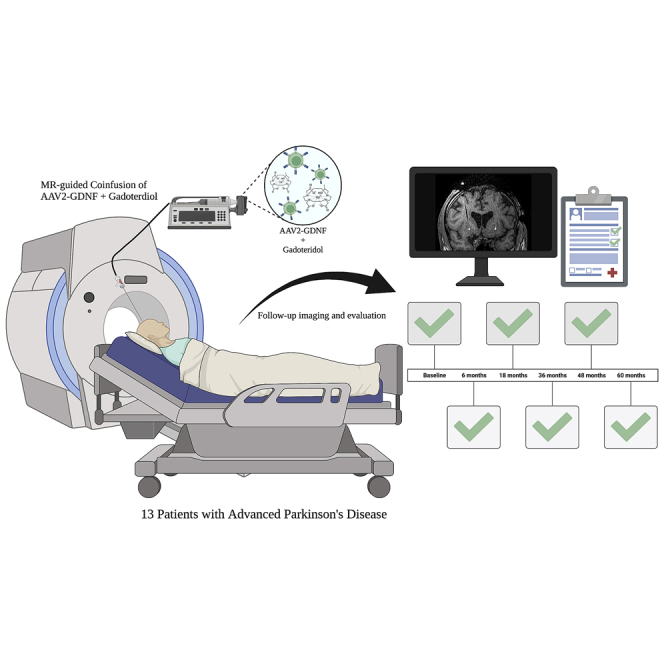
The study examined participants with advanced Parkinson’s disease, serially scanned up to 5 years after co-infusions of AAV-GDNF (glial cell line-derived neurotrophic factor) and gadoteridol, via convection-enhanced delivery with real-time intraoperative MRI. The results indicate no evidence of parenchymal toxicity evaluated in each MRI study at each time point.
Introduction
Direct convection-enhanced delivery (CED) of putative therapeutic agents1,2 has been used to distribute therapeutic infusate to the brain parenchyma, bypassing the blood-brain barrier, in a reliable, targeted, and homogeneous manner.1,2,3,4,5,6,7 Direct intracranial convective delivery of viral vector-based gene therapies (and other therapeutic agents) can cover clinically relevant brain volumes by utilizing a delivery platform3 that includes real-time magnetic resonance imaging (MRI) to directly visualize infusate distribution5 and allow mitigation of potential infusate reflux or off-target distribution.6 Co-infusion of gadoteridol with gene therapy-based therapeutic agents (including adeno-associated virus [AAV] vectors) has been used to confirm volumetric distribution at the perfused site.8,9
Although the short-term preclinical and clinical safety and feasibility of brain parenchymal co-infusions using gadoteridol and putative therapeutic agents has been documented in certain neurological diseases,7,10,11,12,13,14,15,16,17,18 the long-term safety (years) and feasibility of this MRI-based infusion paradigm has not been defined for gene therapy approaches. To determine the long-term safety and feasibility of this imaging-based delivery approach, we analyzed pre-, intra-, and serial postoperative MRI (up to 5 years after treatment) from consecutive participants with Parkinson’s disease (PD) who underwent bilateral putaminal CED of AAV (serotype 2) (AAV2) containing the human glial cell line-derived neurotrophic factor (GDNF) transgene co-infused with gadoteridol in a phase I, first-in-human clinical trial.19
Results
Participants
Thirteen adult participants with advanced PD (Hoehn and Yahr stages III–IV) were enrolled and received AAV2-GDNF. Participants included 10 men and 3 women. Mean age at study entrance was 65.1 ± 6.4 years (range, 51–75 years). Mean PD duration at study entrance was 12.9 ± 5.4 years (range, 6–27 years). Post-dosing follow-up, including brain MRI, ranged from 36–60 months. All participants underwent successful infusion of 450 mL per putamen bilaterally (52 total cannula tracks and infusions). MRI was performed pre-operatively (screening) and intraoperatively in all participants. Serial follow-up MRI was performed in participants up to 5 years after infusion (Table 1). Sixty-five MRI studies were obtained and analyzed. The PD-related clinical findings in these participants have been described previously in detail.19 Briefly, there was PD symptom/sign stability, as defined by the participants’ Unified Parkinson’s Disease Rating Scale scores, over the study period. 18F-fluoro dopa positron emission tomography revealed increased uptake at 18 months from baseline studies in 92% of participants. All CED infusions were well tolerated, and no participants experienced clinical evidence of toxicity throughout the follow-up period (up to 5 years).
Table 1.
The time points when each participant received MRI scans for analysis
| Participant # | Day 0 | 6 months | 18 months | 36 months | 48 months | 60 months |
|---|---|---|---|---|---|---|
| 1 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 2 | ✔ | ✔ | ✔ | ✔ | ✔ | X |
| 3 | ✔ | ✔ | ✔ | X | X | ✔ |
| 4 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 5 | ✔ | ✔ | ✔ | ✔ | X | X |
| 6 | ✔ | ✔ | X | ✔ | ✔ | ✔ |
| 7 | ✔ | ✔ | ✔ | ✔ | X | ✔ |
| 8 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 9 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 10 | ✔ | ✔ | ✔ | ✔ | ✔ | X |
| 11 | ✔ | ✔ | ✔ | ✔ | X | X |
| 12 | ✔ | ✔ | ✔ | ✔ | ✔ | X |
| 13 | ✔ | ✔ | ✔ | ✔ | X | X |
The adverse event (AE) profile was consistent with similar studies evaluating participants with advanced PD undergoing a surgical procedure.20 Many AEs were frequently related to general anesthesia or the cranial procedure (postoperative headaches, delirium, nausea/vomiting), and/or the underlying PD (increased falls, dyskinesias, hallucinations), as noted during the postoperative period and as reported previously.19 2 events were deemed to be associated with the surgical procedure. This included scalp wound dehiscence and radiation exposure from a PET (positron emission tomography) scan that did not yield usable data. Scalp wound dehiscence was considered medically significant, but not life threatening. One death occurred 3.5 years after gene therapy, resulting from complications of an elective spinal surgery, and was determined not to be attributable to the study drug or surgical procedure. No serious AEs were attributed to the AAV2-GDNF/gadoteridol co-infusions.
MRI assessment
Pre-infusion assessment
Pre-infusion MRI permitted development of optimal targeting and trajectory planning in all cases. The neurosurgical team determined optimal cannula trajectories that avoided critical structures (e.g., eloquent cortex, sulci, vessels, ventricles) but allowed prescribed achievement of putaminal targeting.
Intraoperative assessment
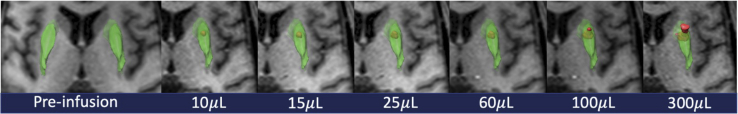
Intraoperative MRI (iMRI) confirmed accurate infusion cannula placement to target in all cases. All participants underwent successful real-time MRI-guided delivery of the 450 mL of infusate to each putamen. During convective perfusion of the anterior and posterior putamen, the co-infused gadoteridol was clearly defined on T1-weighted sequences, allowing precise determination of anatomic distribution of AAV2-GDNF and quantitative volumetric analysis. Progressive filling of the targeted regions of the anterior and posterior putamen was distinctly visualized and provided defined information regarding infusate flow (Figure 1; Videos S1 and S2).
Figure 1.
Intraoperative T1-weighted axial MRI during putaminal convection-enhanced delivery (CED)
Serial images depict the static putaminal volume (green) and serial enlargement of an anterior putaminal infusion volume (red). From left to right, the infusion volume delivered increases and is displayed under the respective image. Putaminal volume representation and contrast co-infusion volume of distribution are depicted using the BrainLab iPlan software package.
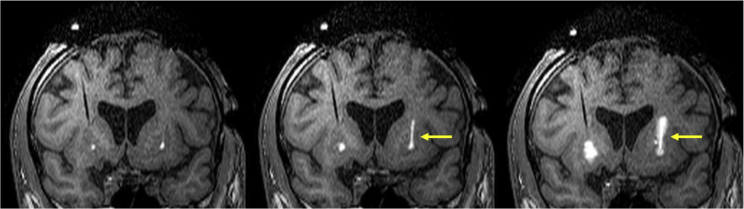
Specifically, real-time imaging revealed infusate reflux along the cannula track and infusion extending beyond the boundaries of the putamina in 98% infusions (51 of 52) (Figure 2). Real-time MRI also demonstrated preferential infusate flow along the perivascular spaces of the lenticulostriate vessels in 35% infusions (18 of 52).
Figure 2.
Intraoperative T1-weighted coronal MRI obtained during putaminal CED co-infusions
Bilateral inserted cannulae are providing convective delivery in the anterior putamen bilaterally. Note the contrast reflux unilaterally along the cannula (yellow arrows) but not on the contralateral side. No significant perivascular leakage is noted in this imaging series.
The mean volume of distribution within the parenchyma was 2.63 ± 1.09 cm3 (mean ± SD; range, 0.82–4.36 cm3). The volume of distribution to infusion volume ratio (Vd:Vi) was 2.93 ± 1.21. The putaminal coverage of infused fluid (AAV2-GDNF) was 0.995 cm3 ± 0.376 cm3 (mean ± SD; range, 0.315–1.881 cm3), approximately 26% of the putaminal volume. Measurements were documented and reported previously.19
Postoperative imaging assessment
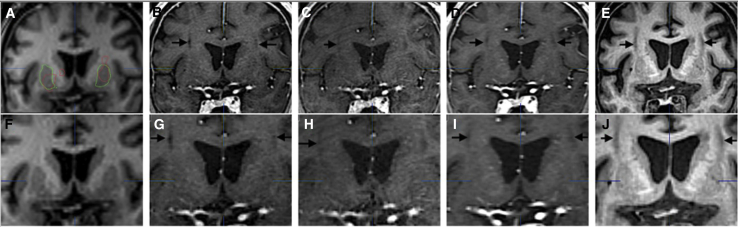
No evidence of parenchymal toxicity was identified in the perfusion region or elsewhere throughout the brain on MRI over the study period in all participants (Figure 3).
Figure 3.
A single participant’s representative set of T1-weighted coronal MRI images over time
(A–E) Pre-operation day 0 (A), 6-month scans (B), 18-month scans (C), 36-month scans (D), 60-month scans (E). (F–J) Higher magnification of the images in the top row. Note visible cannula tracks (black arrows) in postoperative images.
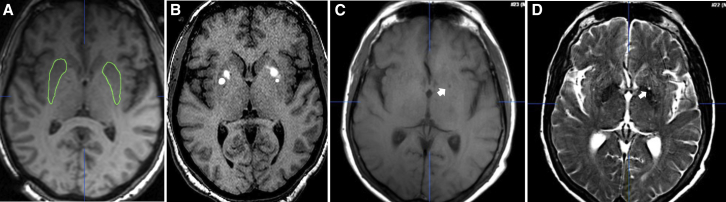
One participant (Figure 4) in the lowest-dose cohort displayed a new unilateral T1 hypointensity on the 6-month follow-up scan. It was of undetermined significance, according to the neuroradiologists evaluating the scans, and was without a clinical correlate on serial neurologic examinations.
Figure 4.
Serial axial MRI images from the participant with new postoperative unilateral putaminal lesion
(A and B) Pre-operative (A) and intraoperative (B) axial T1-weighted images. (C and D) 60-month (C) axial T1-weighted and (D) axial T2-weighted images showing persistence of the new finding (white arrow) initially noted by both neuroradiologists 6 months after the operation but not in pre-surgical and surgical images. Note the absence of significant abnormalities on the contralateral side.
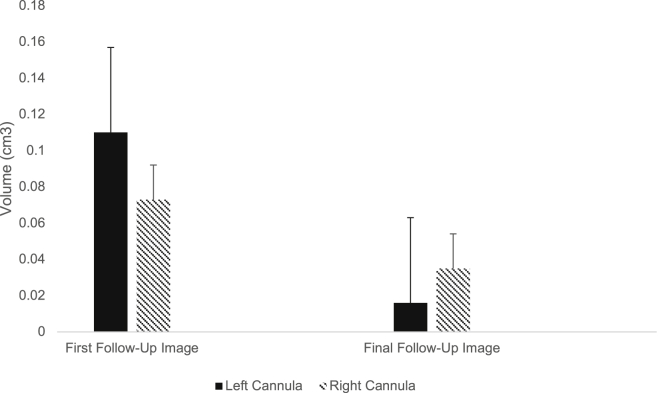
Forty-six cannula tracks (85% of infusion cannulae placed; 23 in each hemisphere) were identified on postoperative MRI T1-weighted sequence as continuous areas of hypointensity coursing through the cortical, sub-cortical, and putamen. Imaging evidence of the cannula tracks was similarly associated with 25 anterior (96.2% of infusion cannulae placed anteriorly) and 21 posterior (80.8% of infusion cannulae placed posteriorly) cannulae (p = 0.08; chi-square test). Six cannula tracks were not found on the first follow-up images obtained at 6 moths and therefore were not included in calculations. Overall, the mean volume of cannula tracks decreased significantly over study follow-up from 0.094 ± 0.133 cm3 (range, 0.009–0.634 cm3) to 0.025 ± 0.057 cm3 (range, 0.005–0.052 cm3) (p = 0.004, t test). When analyzing volumetric changes between right and left cannula tracks, we found no significant difference between hemisphere-related visualized cannula tracks. Four cannula tracks were no longer visible in the last follow-up images (36 months, 48 months, and 60 months) (Figure 5).
Figure 5.
Changes in postoperative MRI-visualized cannula track volumes over time
Shown are bar graph representations of quantitative volumetric data (defined via the BrainLab iPlan software package) from left (n = 23) and right (n = 23) cannula track volumes determined from the first and last available postoperative scans (typically using coronal or sagittal views). Error bars represent the standard error of mean (SEM). Note the statistically significant reductions in cannula track volumes over time (up to 54 months between the first and last scans). Minus bars are not displayed.
Discussion
Direct central nervous system gene therapy delivery
Based on new molecular biology insights and improved direct delivery strategies, the number of gene therapy trials for treating central nervous system (CNS) disorders21 has dramatically increased over the past decade. In 2020, 68 clinical trials in the United States National Library of Medicine database used a gene therapy approach to treat inadequately treated or untreatable neurologic disorders. Based on the technology developed by our group,3 including the current study, nearly half (44%) of the trials used direct intraparenchymal CED of AAV vectors (90% of direct-infusion gene therapy trials used AAVs, 67% employed AAV2) with indication-specific therapeutic transgenes. A key advantage of direct CNS delivery is the ability to bypass the blood-brain barrier and perfuse targeted regions of the brain or diseased neuronal circuitry.
Most direct gene therapy trials are now using co-infusion of an AAV vector and gadoteridol to non-invasively monitor infusate distribution in real time with serial MRI. Such an approach ensures targeting accuracy, improves distribution in targeted brain regions (via minimization of reflux, tailored cannula advancement strategies, and rate adjustment), allows quantification of treatment distribution, and enhances safety (by reducing off-target infusion and avoidance of cerebral vasculature).7,10,11,12,13,14,15,16,17,18 Preclinical data indicate that this delivery paradigm accurately correlates T1-weighted gadoteridol distribution with the extent of AAV2-induced transgene expression of infusion site neurons.8,9 Preclinical and other gene therapy studies in participants with PD indicate the importance of these measures to optimize dosing of a gene therapy product. The clinical efficacy of this PD gene therapy delivery paradigm is supported by early-phase, uncontrolled studies demonstrating greater improvement of PD clinical assessments with a higher volume of distribution; however, additional controlled studies are needed.22,23 To assess feasibility, the impact of real-time MRI, and long-term safety in gene therapy, we analyzed the imaging and clinical findings in consecutive participants with advanced PD infused with AAV-GDNF co-infused with gadoteridol.
Materials and methods
Participants
Consecutive adult participants with advanced PD were enrolled at the NIH Clinical Center in an institutional review board-approved, single-center, phase I, open-label clinical trial (ClinicalTrials.gov: NCT01621581).19 All participants gave informed consent. Participants underwent bilateral putaminal co-infusion of AAV2-GDNF and gadoteridol (anterior and posterior infusion in each putamen) using real-time MRI.
Infusion equipment
The ClearPoint Neuro (Solana Beach, CA) navigation platform (i.e., MRI-compatible headframe, SmartFrame cranial guidance platforms, and ClearPoint software package) and SmartFlow cannulae were utilized for infusions. Cannulae were connected to MRI-safe MedFusion 3500 syringe pumps to control infusion rates. Cannulae were advanced to the pre-planned putaminal target depths, and their positions were confirmed using real-time iMRI.
Infusion
Real-time convective co-infusion of AAV2-GDNF and gadolinium-based contrast agent (1 mM gadoteridol [ProHance], Bracco Diagnostics; Milan, Italy) was monitored using T1-weighted MRI sequences (see Imaging) during infusion until the planned infusion volume was delivered. All study participants received co-infusions of varying AAV2-GDNF vector concentrations (per protocol; dose 1, 9 × 1010 vg [n = 6]; dose 2, 3 × 1011 vg [n = 6]; dose 3, 9 × 1011 vg [n = 1]).19 Study participants received bilateral infusions in anterior and posterior putaminal sites for a total of 450 mL per putamen (300 mL to the anterior putamen and 150 mL to the posterior putamen). Dynamic CED infusion rates were used to maximize the distribution of the vector within the target. During parenchymal placement of the SmartFlow cannulae, the initial rate was 0.5 mL/min to prevent tissue from lodging within the distal cannula tip. When the correct initial position of the cannula tip was confirmed via MRI, infusion flow rates were raised to 1.0 mL/min for 10 min, then 2.0 mL/min for 10 min, then 3.0 mL/min for 10 min, and finally 5.0 mL/min for 40 min for anterior putaminal targets. Posterior putaminal infusion rates were the same except that the 5.0-mL/min rate was maintained for 10 min.
Imaging and assessment
All study participants underwent screening, intraoperative, and follow-up brain MRI scans with and/or without intravenous contrast enhancement. Pre-operative MRI was used to plan transfrontal cannula trajectories targeting each putaminal infusion site. Intraoperative MRI confirmed the pre-operative brain anatomy, target registration, and cannula trajectories. Intraoperative T1-weighted imaging provided real-time visual monitoring to assess the distribution of the co-infused gadoteridol at the individual infusion sites. Serial postoperative MRI provided radiographic evidence of safety by direct visualization of the perfused putaminal regions and the surrounding brain parenchyma up to 5 years after infusion (Table 1).
MRI DICOM datasets for each study participant were qualitatively reviewed by two independent neuroradiologists, including a neuroradiologist from the original study site (NIH) and a blinded neuroradiologist at a different institution (The Ohio State University). Quantitative volumetric assessment of the putaminal volumes of infusion (based on gadoteridol distribution on T1-weighted sequences on iMRI) was determined using the iPlan autosegmentation software package (v.3.0) (BrainLab, Feldkirchen, Germany) using the Windows 10 operating system. A similar quantification technique was used to measure the extent of visible cannula tracks on serial postoperative imaging.
Statistical analysis
Statistical significance was determined at a p-value of 0.5 or less. Specific statistical tests are defined in the text.
Current study
Parenchymal imaging findings
The neuroradiologists reviewing the MRI data did not detect evidence of significant, persistent localized trauma or an inflammatory response (acute or delayed) to the therapeutic intervention during the 5-year interval after infusions. This finding is consistent with the well-established CNS safety profile of intravenously administered gadolinium-based contrast agents as well as preclinical and early clinical safety data regarding direct co-infusion of gadoteridol and viral vectors in the nervous system.4,11,16
Brain parenchymal enhancement after intravenous infusion of gadolinium-based contrast agents (noted on T1-weighted images) is indicative of blood-brain barrier breakdown and associated pathology. Repeated intravenous administration of gadolinium-based contrast agents can result in gadolinium retention within the brain parenchyma,24 including the putamen and cerebellum.25 Evidence of gadolinium retention on MRI has not been reported in the literature to translate into neurologic toxicity.24 We are unaware of peer-reviewed publications describing clinically significant AEs or MRI abnormalities ascribed to intraparenchymally delivered gadolinium-based contrast agents within the brain. However, a phase 2 clinical study of AAV2-AADC/gadoteridol co-infusion was placed on clinical hold after reports of “MRI abnormalities observed in trial participants” by the study sponsor (Voyager Therapeutics).26
The results described in this manuscript are consistent with non-human primate feasibility and safety studies that used real-time MRI monitoring of gene therapy co-infusions using gadoteridol and the putative therapeutic agents, ultimately leading to AAV2-AADC and AAV2-GDNF clinical trials.4,11,16 Limited mean volumetric putaminal coverage (26%) with AAV2-GDNF/gadoteridol in this phase I trial, as reported previously,19 has led to a current phase Ib trial, where we achieve a 63% mean putaminal coverage using a higher total AAV2-GDNF dose and up to 1,800-μL infusion volume with continued safety and tolerability.27 Recent results obtained by our group with administration of AAV2-AADC/gadoteridol gene therapy into the midbrain of children suffering from AADC deficiency have not shown any “MRI abnormalities”18 similar to those described by Voyager. The AADC deficiency study imaging results, therefore, suggest that the Voyager “MRI abnormalities” associated with AAV2-AADC/gadoteridol-treated PD participants may be a rather unique finding and not the norm. Such MRI-defined brain parenchymal changes after AAV2-AADC/gadoteridol gene therapy for PD may have been related to specific vector manufacturing differences between therapeutic agents in recent Voyager trials and trials for AADC-deficient children and treatments of initial PD clinical cohorts.22,23
In clinical trials, short-term MRI analysis of viral vector infusions (without gadoteridol co-infusion) has been performed. Post-infusion MRI was carried out in participants undergoing direct intraparenchymal delivery of AAVrh.10h-CLN2 for Batten’s disease. T2 hyperintensities were found in participants 48 h after infusion and persisted up to 12 months after infusion without clinical sequelae. The T2 signal was attributed to edema and inflammation at the cannula tip infusate delivery site.13 The MRI findings were consistent with similar results noted in non-human primates infused with AAVrh.10hARSA, featuring T cell, B cell, microglia, and macrophage infiltration without clinical consequences.14 Clinical and non-clinical results in this program may be related to a high local concentration of the vector at the cannula tip (because of the absence of convection) or possibly a neuroinflammatory reaction to the transgene and/or AAV serotype used.
Cannula tracks
MRI evidence of a cannula track following the trajectory to the infusion site was seen in 85% of infusions in the current study. The persistent cannula tracks did not produce clinical sequelae. This finding coincides with extensive clinical experience with cannula/electrode passage effects into brain parenchyma for monitoring or treatment.28 Insertion of any device, including microdialysis catheters, intracranial pressure monitors, stimulation electrodes, biopsy needles, and ventricular drain catheters, can evoke a local inflammatory response. Device-related mechanical disruption of the extracellular matrix and blood-brain barrier initiates this process and can initiate cellular sheath and gliosis around the foreign body. These features can be detected on MRI as “tracks” and were found to be without clinical impact in this study.
The study’s delivery platform utilized a cannula with an outer diameter of 1,650 or 2,100 μm (16G and 14G size, respectively), which is substantially smaller than frequently used ventricular catheters and deep brain stimulator leads. The cannulae are stereotactically inserted into the brain’s cortical surface and then through the subcortical white matter to the deep brain target site. As with any foreign body placement in the brain parenchyma, the magnitude of brain parenchymal pathologic reactions depends on the device’s cross-sectional diameter and displacement volume. Typically, microglia, astrocytes, and other inflammatory cells can be found within and immediately surrounding the device’s passage track without stimulating a brain-wide neuroinflammatory response.
Conclusion
Co-infusion of AAV-GDNF and gadoteridol was well tolerated in participants with advanced PD up to 5 years after treatment. The majority of MRI findings in this study were anticipated and not associated with clinical or functional sequelae and demonstrated interval improvement over the course of the study. This delivery-imaging platform enhances safety and permits anatomic customization of delivery, which improves therapeutic distribution and facilitates assessment of efficacy/dosing effects.
Acknowledgments
We thank Dr. Amber Van Laar and Dr. Adrian P. Kells of AskBio for assistance with editing the manuscript. This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health by NIH-RAID X01NS065758-01 (to K.S.B.).
Author contributions
M.T.R., M.S.F., J.D.H., and K.S.B. conceived the idea and experimental design. M.T.R., A.S.A., D.J.E., M.S.F., and K.S.B. refined the experimental design. G.C.S. gathered clinical information. G.C.S., C.L., M.H., and J.D.H. performed clinical follow-up. A.A. performed MRI radiology analysis. M.T.R., A.S.A., and V.M. performed coverage analysis. A.S.A. and V.M. performed statistical analysis. M.T.R., A.S.A., V.M., M.S.F., and K.S.B. wrote the paper. M.T.R., A.S.A., D.J.E., G.C.S., C.L., V.M., A.A., R.R.L., M.S.F., M.H., J.D.H., and K.S.B. performed editing. M.T.R., A.S.A., V.M., M.S.F., and K.S.B. performed final editing.
Declaration of interests
M.S.F. is an employee of Asklepios BioPharmaceutical, Inc. (AskBio). C.L. reports honoraria for editorial work from Elsevier, Inc. The work submitted here was conducted in the course of employment for the National Institute of Neurological Disorders and Stroke, an agency of the US Government. K.S.B. is a consultant to AskBio, Aviado Bio, and Scribe Tx and a patent holder on relevant technologies utilized in this study. K.S.B. was a co-founder of Voyager Therapeutics, has not been associated with the company for over 3 years, and reports no current financial interests in that company.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.08.003.
References
- 1.Bobo R.H., Laske D.W., Akbasak A., Morrison P.F., Dedrick R.L., Oldfield E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison P.F., Laske D.W., Bobo H., Oldfield E.H., Dedrick R.L. High-flow microinfusion: tissue penetration and pharmacodynamics. Am. J. Phys. 1994;266:R292–R305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 3.Fiandaca M.S., Forsayeth J.R., Dickinson P.J., Bankiewicz K.S. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5:123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krauze M.T., Saito R., Noble C., Tamas M., Bringas J., Park J.W., Berger M.S., Bankiewicz K. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J. Neurosurg. 2005;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krauze M.T., McKnight T.R., Yamashita Y., Bringas J., Noble C.O., Saito R., Geletneky K., Forsayeth J., Berger M.S., Jackson P., et al. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain. Res. Brain. Res. Protoc. 2005;16:20–26. doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Varenika V., Dickinson P., Bringas J., LeCouteur R., Higgins R., Park J., Fiandaca M., Berger M., Sampson J., Bankiewicz K. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. J. Neurosurg. 2008;109:874–880. doi: 10.3171/jns/2008/109/11/0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonser R.R., Sarntinoranont M., Morrison P.F., Oldfield E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015;122:697–706. doi: 10.3171/2014.10.Jns14229. [DOI] [PubMed] [Google Scholar]
- 8.Su X., Kells A.P., Salegio E.A., Salegio E.A., Richardson R.M., Hadaczek P., Beyer J., Bringas J., Pivirotto P., Forsayeth J., Bankiewicz K.S. Real-time MR imaging with Gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Mol. Ther. 2010;18:1490–1495. doi: 10.1038/mt.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bankiewicz K.S., Sudhakar V., Samaranch L., San Sebastian W., Bringas J., Forsayeth J. AAV viral vector delivery to the brain by shape-conforming MR-guided infusions. J. Control Release. 2016;240:434–442. doi: 10.1016/j.jconrel.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Lonser R.R., Walbridge S., Garmestani K., Butman J.A., Walters H.A., Vortmeyer A.O., Morrison P.F., Brechbiel M.W., Oldfield E.H. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J. Neurosurg. 2002;97:905–913. doi: 10.3171/jns.2002.97.4.0905. [DOI] [PubMed] [Google Scholar]
- 11.Lonser R.R., Schiffman R., Robison R.A., Butman J.A., Quezado Z., Walker M.L., Morrison P.F., Walbridge S., Murray G.J., Park D.M., et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68:254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 12.Murad G.J.A., Walbridge S., Morrison P.F., Garmestani K., Degen J.W., Brechbiel M.W., Oldfield E.H., Lonser R.R. Real-time, image-guided, convection-enhanced delivery of interleukin 13 bound to pseudomonas exotoxin. Clin. Cancer. Res. 2006;12:3145–3151. doi: 10.1158/1078-0432.Ccr-05-2583. [DOI] [PubMed] [Google Scholar]
- 13.Lonser R.R., Warren K.E., Butman J.A., Quezado Z., Robison R.A., Walbridge S., Schiffman R., Merrill M., Walker M.L., Park D.M., et al. Real-time image-guided direct convective perfusion of intrinsic brainstem lesions. Technical note. J. Neurosurg. 2007;107:190–197. doi: 10.3171/jns-07/07/0190. [DOI] [PubMed] [Google Scholar]
- 14.Murad G.J.A., Walbridge S., Morrison P.F., Szerlip N., Butman J.A., Oldfield E.H., Lonser R.R. Image-guided convection-enhanced delivery of gemcitabine to the brainstem. J. Neurosurg. 2007;106:351–356. doi: 10.3171/jns.2007.106.2.351. [DOI] [PubMed] [Google Scholar]
- 15.Szerlip N.J., Walbridge S., Yang L., Morrison P.F., Degen J.W., Jarrell S.T., Kouri J., Kerr P.B., Kotin R., Oldfield E.H., Lonser R.R. Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. J. Neurosurg. 2007;107:560–567. doi: 10.3171/jns-07/09/0560. [DOI] [PubMed] [Google Scholar]
- 16.Richardson R.M., Kells A.P., Rosenbluth K.H., Salegio E.A., Fiandaca M.S., Larson P.S., Starr P.A., Martin A.J., Lonser R.R., Federoff H.J., et al. Interventional MRI-guided putaminal delivery of AAV2-GDNF for a planned clinical trial in Parkinson's disease. Mol. Ther. 2011;19:1048–1057. doi: 10.1038/mt.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonser R.R. Imaging of convective drug delivery in the nervous system. Neurosurg. Clin. N. Am. 2017;28:615–622. doi: 10.1016/j.nec.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson T.S., Gupta N., San Sebastian W., Imamura-Ching J., Viehoever A., Grijalvo-Perez A., Fay A.J., Seth N., Lundy S.M., Seo Y., et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat. Commun. 2021;12:4251. doi: 10.1038/s41467-021-24524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heiss J.D., Lungu C., Hammoud D.A., Herscovitch P., Ehrlich D.J., Argersinger D.P., Sinharay S., Scott G., Wu T., Federoff H.J., et al. Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson's disease. Mov. Disord. 2019;34:1073–1078. doi: 10.1002/mds.27724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepper P.V., Goldstein M.K. Postoperative complications in Parkinson's disease. J. Am. Geriatr. Soc. 1999;47:967–972. doi: 10.1111/j.1532-5415.1999.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 21.Lonser R.R., Akhter A.S., Zabek M., Elder J.B., Bankiewicz K.S. Direct convective delivery of adeno-associated virus gene therapy for treatment of neurological disorders. J. Neurosurg. 2020;134:1751–1763. doi: 10.3171/2020.4.Jns20701. [DOI] [PubMed] [Google Scholar]
- 22.Christine C.W., Bankiewicz K.S., Van Laar A.D., Richardson R.M., Ravina B., Kells A.P., Boot B., Martin A.J., Nutt J., Thompson M.E., Larson P.S. Magnetic resonance imaging-guided phase 1 trial of putaminal AADC gene therapy for Parkinson's disease. Ann. Neurol. 2019;85:704–714. doi: 10.1002/ana.25450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson R.M., Bankiewicz K.S., Christine C.W., Van Laar A.D., Gross R.E., Lonser R., Factor S.A., Kostyk S.K., Kells A.P., Ravina B., Larson P.S. Data-driven evolution of neurosurgical gene therapy delivery in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2020;91:1210–1218. doi: 10.1136/jnnp-2020-322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanescu A.L., Shaw D.W., Murata N., Murata K., Rutledge J.C., Maloney E., Maravilla K.R. Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: pathological confirmation. Pediatr. Radiol. 2020;50:388–396. doi: 10.1007/s00247-019-04535-w. [DOI] [PubMed] [Google Scholar]
- 25.Chehabeddine L., Al Saleh T., Baalbaki M., Saleh E., Khoury S.J., Hannoun S. Cumulative administrations of gadolinium-based contrast agents: risks of accumulation and toxicity of linear vs macrocyclic agents. Crit. Rev. Toxicol. 2019;49:262–279. doi: 10.1080/10408444.2019.1592109. [DOI] [PubMed] [Google Scholar]
- 26.Voyager Therapeutics Inc Press release: voyager therapeutics provides update on NBIb-1817 (VY-AADC) gene therapy program. 2021. https://ir.voyagertherapeutics.com/news-releases/news-release-details/voyager-therapeutics-provides-update-nbib-1817-vy-aadc-gene-0
- 27.Van Laar A.D., Christine C.W., Merola A., Phielipp N.M., Elder J.B., Larson P.S., Stoicea N., Sebastian W.S., Fiandaca M.S., Kells A.P. Abstract. Safety and preliminary clinical findings of neurosurgical AAV2-GDNF delivery for Parkinson's disease. Mol. Ther. 2022;30:374–375. [Google Scholar]
- 28.Kozai T.D.Y., Jaquins-Gerstl A.S., Vazquez A.L., Michael A.C., Cui X.T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015;6:48–67. doi: 10.1021/cn500256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.