Abstract
Identifying brain alterations associated with suicidal thoughts and behaviors (STBs) in young people is critical to understanding their development and improving early intervention and prevention. The ENIGMA Suicidal Thoughts and Behaviours (ENIGMA-STB) consortium analyzed neuroimaging data harmonized across sites to examine brain morphology associated with STBs in youth. We performed analyses in three separate stages, in samples ranging from most to least homogeneous in terms of suicide assessment instrument and mental disorder. First, in a sample of 577 young people with mood disorders, in which STBs were assessed with the Columbia Suicide Severity Rating Scale (C-SSRS). Second, in a sample of young people with mood disorders, in which STB were assessed using different instruments, MRI metrics were compared among healthy controls without STBs (HC; N = 519), clinical controls with a mood disorder but without STBs (CC; N = 246) and young people with current suicidal ideation (N = 223). In separate analyses, MRI metrics were compared among HCs (N = 253), CCs (N = 217), and suicide attempters (N = 64). Third, in a larger transdiagnostic sample with various assessment instruments (HC = 606; CC = 419; Ideation = 289; HC = 253; CC = 432; Attempt=91). In the homogeneous C-SSRS sample, surface area of the frontal pole was lower in young people with mood disorders and a history of actual suicide attempts (N = 163) than those without a lifetime suicide attempt (N = 323; FDR-p = 0.035, Cohen’s d = 0.34). No associations with suicidal ideation were found. When examining more heterogeneous samples, we did not observe significant associations. Lower frontal pole surface area may represent a vulnerability for a (non-interrupted and non-aborted) suicide attempt; however, more research is needed to understand the nature of its relationship to suicide risk.
Subject terms: Depression, Neuroscience, Bipolar disorder
Introduction
Suicide is the second leading cause of death for young people aged between 15 and 29 [1]. Suicidal thoughts and behaviors (STBs) typically emerge during adolescence [2]. It has been estimated that between 11 and 29% of adolescents report suicidal ideation (suicidal thoughts), and 2–10% of adolescents attempted suicide in the past year [3]. Unfortunately, the number of suicide attempts among children and adolescents has continued to increase sharply despite national and international prevention efforts [4].
To improve targeting of prevention and intervention efforts and thereby reduce the number of deaths by suicide in this age group, we must increase our understanding of the mechanisms underlying both suicidal thoughts and suicidal behaviors (including suicide attempts) in young people. Neuroimaging, including Magnetic Resonance Imaging (MRI), is a useful tool with which to identify biological risk markers for STBs in vivo and non-invasively. Many neuroimaging studies have been published examining the neural substrates of STBs in the past 20 years, but few have focused on STBs in youth (for a review, see [5]). Although several of these studies support lower regional brain volumes, particularly in ventral and dorsal prefrontal and also in temporal regions [6–9] in suicide attempters with mood disorders, negative findings have also been reported [10, 11]. Structural brain alterations related to suicidal ideation in young people have inconsistently been reported in the striatum and temporal lobes [12–14].
In addition to the small number of studies focusing on youth, neuroimaging studies investigating associations between structural brain measures and STBs have also been limited by small sample sizes [5]. There are multiple limitations associated with small sample sizes. First, small sample sizes decrease power, increase the probability of false-negative effects, and inflate the effect size estimate when an actual effect is observed [15]. Second, there may be small yet clinically significant associations between STBs and brain structure. To reliably identify these effects, larger samples are needed. Another significant limitation of previous work is that clinical controls (CC) are often not included, making it difficult to understand if alterations are specific to STBs or reflect mental health disorders in general [5].
To address these limitations, the suicide project within the ENIGMA Major Depressive Disorder (ENIGMA-MDD) consortium pooled data from 18 different studies worldwide to examine associations between brain morphology and suicide attempt in major depressive disorder (MDD) patients [16, 17]. Findings showed a lower volume of the thalamus and pallidum and a smaller surface area of the inferior parietal lobe in adults with MDD and a history of suicide attempts (N = 679) compared to individuals with MDD without a history of suicide attempt (N = 5484). However, these studies did not examine structural MRI correlates of suicidal ideation. In addition, studies within ENIGMA-MDD are limited to individuals with MDD, while STBs are transdiagnostic phenomena, and the extent of neurobiological mechanisms underlying STBs that are common to or may differ across psychiatric disorders is unknown. Finally, these previous studies did not examine structural brain alterations in children and adolescents.
Therefore, we established the transdiagnostic ENIGMA Suicidal Thoughts and Behaviours (ENIGMA-STB) consortium, which allows investigation of neural correlates of STBs across a range of psychiatric conditions, leveraging many samples worldwide. This large dataset enables assessment of structural brain alterations that are common across groups (e.g., groups with a variety of psychiatric conditions including mood disorders, anxiety disorders, post-traumatic stress disorder, addiction, and obsessive-compulsive disorder), and also alterations that are specific to subgroups, such as males or females. For this ENIGMA-STB study, we focused specifically on young persons, as there is limited information concerning the mechanisms underlying STB in this group.
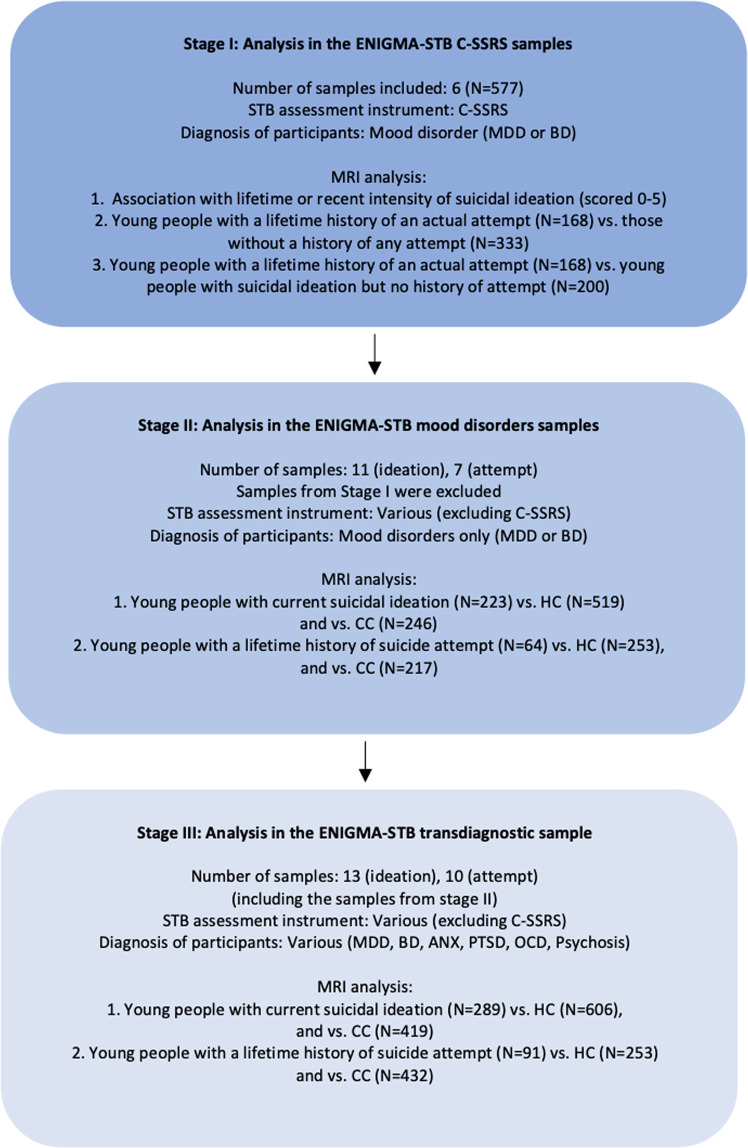
As we expected the effect sizes would be small due to clinical heterogeneity and use of different instruments to assess STBs, we started with the most homogeneous sample in terms of assessment instruments and type of psychiatric disorder (i.e., mood disorders). These six homogeneous samples were enriched for STBs and conducted a more in-depth assessment of STBs (e.g., not only the presence but also the intensity of suicidal ideation) by use of the Columbia Suicide Severity Rating Scale. Here we aimed to investigate differences in structural MRI measures between young persons with a lifetime history of suicide attempt compared to those without and examine associations with the intensity of suicidal ideation. We next evaluated associations between MRI metrics and suicidal thoughts and behavior in a larger sample with mood disorders but more heterogeneity in assessment instruments. In this sample we aimed to identify structural brain alterations in young persons with (1) a lifetime history of a suicide attempt; and (2) current (in the past week, 2 weeks, or month) suicidal ideation (but no history of attempt), compared to healthy controls (HC) and CC. Finally, we examined these associations in the largest sample including youth ENIGMA STB samples with heterogeneity in both diagnosis and assessment instruments (see Fig. 1). Based on previous findings in adolescents, we predicted that STBs would be associated with structural alterations in the prefrontal cortex (PFC) [6, 8, 9], temporal cortex [12], and caudate [14].
Fig. 1. Overview of the three stages of the analysis.
The color indicates the homogeneity of the samples (dark blue=most homogeneous in terms of STB assessment instruments and type of psychiatric disorders, light blue=most heterogeneous in terms of STB assessment instruments and type of psychiatric disorders). C-SSRS Columbia Suicide Severity Rating Scale; HC healthy controls; CC clinical controls.
Patients and methods
Samples
This mega-analysis included data from 21 international studies from ten countries to examine the association between STBs and brain structure in young people ages 8–25 years. The inclusion/exclusion criteria for the different studies are presented in Table S1. All sites obtained ethics approval from their local institutional review boards and ethics committees. All participants who were 18 years old and over provided written informed consent, and those aged under age 18 years provided written informed assent in addition to written informed consent from a parent/guardian at the local institution.
Image processing and harmonization
Structural T1-weighted brain MRI scans were acquired at each site. Information regarding the acquisition parameters, software versions, and scanner characteristics for the different sites is presented in Table S2. The T1-weighted images were analyzed locally using harmonized analysis and quality control protocols for FreeSurfer [18] (http://surfer.nmr.mgh.harvard.edu/), developed by the ENIGMA consortium (http://enigma.ini.usc.edu/protocols/imaging-protocols/). The ENIGMA FreeSurfer protocol provides tools for quality control of the segmented cortical and subcortical phenotypes. Each site visually inspected the segmentation and excluded regions that were not appropriately segmented. To reduce the number of statistical tests and avoid issues related to left-right flipping that may have occurred at the various sites, we combined regional measures across both hemispheres by taking the mean of the left and right hemisphere regions. We examined the volume of eight subcortical regions and cortical thickness and surface area of 34 regions, defined by the Desikan-Killiany atlas [19]. In addition, two global measures were calculated: mean cortical thickness and total surface area across both hemispheres, creating a total of 78 brain measures.
Before the statistical analysis, neuroimaging measures were harmonized across sites using the ComBat algorithm in R [20, 21], with age, sex, and psychiatric diagnosis as covariates. ComBat uses an empirical Bayes approach to adjust for variability between scanners while still preserving biological variability related to age, sex, and diagnosis. All brain measures included in the statistical analyses were ComBat-corrected. After correction, within-site outliers (measures greater than three standard deviations away from the mean of that region) were excluded from the analysis.
Statistical analysis
As we anticipated small effect sizes due to heterogeneity in diagnosis and instruments used to assess STBs, we performed the analyses in three separate stages, moving from homogeneous samples to more heterogeneous samples (please see Fig. 1 and the description of analyses per stage below). All reported p-values were corrected for multiple comparisons (for the 78 brain measures) using the Benjamini Hochberg correction in R to ensure an FDR < 0.05. 95% confidence intervals are reported for all analyses in supplemental tables.
Stage I: Analysis in the ENIGMA-STB Columbia Suicide Severity Rating Scale (C-SSRS) sample
We first examined associations between brain structure and STBs in a subsample of six cohorts, all of which used an instrument designed specifically to assess suicidal ideation and suicide attempt, the C-SSRS (see Table 1). The C-SSRS is a reliable and well-validated interview, specifically developed to assess intensity and severity of suicidal thoughts, and suicidal behavior [22]. These cohorts included participants with MDD or bipolar disorder (BD) diagnoses (N = 577, age range 11–25) (HC samples were excluded from analyses due to no or limited C-SSRS data). Multiple linear regression analyses were conducted in R, and age, sex, and age-by-sex interactions were included as covariates in all analyses. Intracranial volume (ICV) was included as an additional covariate in analyses of subcortical volume and cortical surface area. Because we had estimated and controlled for the contribution of site and scanner using ComBat prior to conducting the analysis (see above), these measures were not included as covariates. In the regression models, the structural brain measures were included as dependent variables. For suicidal ideation analyses, the continuous C-SSRS measure of recent and lifetime intensity of suicidal ideation were included as predictors. This variable was coded 0–5 (0: no ideation; 1: passive ideation; 2: non-specific active ideation; 3: active ideation with a method, but no plan or intent; 4: active ideation with intent, but no plan; 5: active ideation with a plan and intent). We then examined differences in brain morphology between young people with a lifetime history of any attempt (actual, aborted or interrupted attempts) and young people with no lifetime history of attempt.
Table 1.
Descriptive statistics for studies included in the C-SSRS sample.
| Site | Main diagnosis in sample | Age (years) | % Female | Total N |
|---|---|---|---|---|
| Melbourne (YODA) | MDD | 19.0 range 15–25 | 57.6 | 139 |
| MR-IMPACT | MDD | 15.0 range 11–17 | 76.1 | 113 |
| Stanford TAD | MDD | 16.5 range 14–18 | 76.2 | 42 |
| Stanford TIGER | MDD | 16.0 range 13–18 | 67.6 | 34 |
| UCSF | MDD | 16.0 range 13–18 | 63.4 | 71 |
| Yale School of Medicine | MDD + BD | 19.0 range: 13–25 | 64.0 | 178 |
| Total | MDD + BD | 17.0 range 11–25 | 65.9 | 577 |
Presented here are age (median, minimum-maximum) and sex for the six sites in the C-SSRS sample.
MDD major depressive disorder, BD bipolar disorder.
We also examined differences in brain structure between individuals with a lifetime history of an actual suicide attempt (but not interrupted or aborted attempts) and those without any lifetime attempt. We examined actual attempts and did not include interrupted or aborted attempts, as previous work suggests that actual suicide attempt may represent a more clinically severe and reliable phenotype than interrupted and aborted attempts [23, 24]. Finally, we compared brain morphology between individuals with a lifetime history of suicidal ideation (but no lifetime history of an actual attempt) and those with a lifetime history of an actual attempt. In secondary analyses, we examined the difference in brain structure between individuals with a lifetime history of any attempt (aborted, interrupted or actual attempt) compared to those without any attempt (see Supplemental Note 3 for a description and findings). Effect size estimates were calculated using the Cohen’s d metric for group comparisons and the standardized beta for associations with the continuous recent or lifetime intensity of suicidal ideation measure.
Stage II: Analysis in the ENIGMA-STB mood disorders samples
We subsequently examined associations between STBs and brain structure in a combined sample of cohorts that assessed STBs using various instruments other than the C-SSRS. For demographic characteristics of these cohorts, please see Tables 2A and 3A. This larger sample (which did not include the six cohorts from Stage I) included HC and individuals with a current or lifetime diagnosis of MDD or BD. Various instruments were used to assess current suicidal ideation and lifetime history of suicide attempts across cohorts. An overview of these instruments is presented in Table S3, and the approach used to harmonize these measures across cohorts is described in Supplemental Note 1. In short, history of lifetime suicidal attempt (yes/no) was determined using diagnostic interviews [e.g., 25, 26]. Current suicidal ideation (in the past week, 2 weeks or month; yes/no) was determined using a diagnostic interview, or items from depression severity rating scales [e.g., [27, 28]. Because only five sites had information on both suicidal ideation and suicide attempt, and previous work in adults has documented differences between the neural correlates of ideation and attempt [29], we conducted separate analyses for suicidal ideation and suicide attempt to optimize the sample size for each analysis. To examine suicidal attempts, we compared three groups: 1) HC, without a current or lifetime psychiatric diagnosis or lifetime history of suicide attempt (“healthy controls”); 2) “clinical controls”, with a current or lifetime mood disorder, but no lifetime history of suicide attempt and 3) “clinical attempters”; young people with a current or lifetime mood disorder and lifetime history of suicide attempt. To examine current suicidal ideation, we created three groups: 1) HC without a current or lifetime psychiatric diagnosis or lifetime history of suicide attempt or current suicidal ideation; 2) CC with a current or lifetime mood disorder but no current suicidal ideation or lifetime history of suicide attempt; and 3) young people with a current or lifetime mood disorder and current suicidal ideation, but no lifetime history of suicide attempt.
Table 2.
Descriptive statistics for studies included in the ideation analysis.
| Site | Age HC (years) | Age CC (years) | Age ideation | % female HC | % female CC | % female ideation | Total N HC | Total N CC | Total N Ideation |
|---|---|---|---|---|---|---|---|---|---|
| A. Mood disorders only sample | |||||||||
| Boystown (USA) | 17.0 (14–18) | 17.0 (13–19) | 17.0 (14–18) | 22.2 | 60.0 | 54.4 | 9 | 10 | 11 |
| DEP-ARREST-CLIN – MOODS (France) | 21.0 (20–25) | - | 22.0 (18–25) | 54.5 | - | 70.0 | 11 | 0 | 10 |
| EPISCA (Netherlands) | 14.0 (13–19) | 15.5 (13–16) | 16.0 (13–20) | 86.2 | 83.3 | 88.9 | 29 | 6 | 18 |
| FOR2107-Marburg | 23.0 (18–25) | 24.0 (18–25) | 23.0 (18–25) | 67.2 | 84.4 | 49.1 | 125 | 32 | 57 |
| FOR2107-Münster (Germany) | 23.0 (18–25) | 22.0 (18–25) | 23.0 (19–25) | 71.7 | 77.3 | 50.0 | 106 | 22 | 26 |
| Houston BD (USA) | 14.0 (8–25) | 14.0 (8–25) | 14.0 (10–24) | 54.3 | 31.6 | 66.7 | 81 | 57 | 9 |
| MDD Cohort (China) | 23.0 (22–24) | 22.0 (18–25) | 21.0 (16–25) | 80.0 | 44.4 | 66.7 | 5 | 9 | 12 |
| Muenster Neuroimaging Cohort (Germany) | 22.0 (17–25) | 23.0 (16–25) | 22.0 (17–25) | 47.5 | 57.9 | 50.0 | 80 | 19 | 40 |
| Sydney Brain and Mind Centre (Australia) | 23.0 (18–25) | 19.5 (12–25) | 17.0 (15–22) | 60.0 | 61.4 | 77.8 | 25 | 44 | 9 |
| University of Minnesota (USA) | 16.0 (12–19) | 16.0 (13–20) | 16.0 (12–19) | 54.5 | 74.3 | 84.0 | 22 | 35 | 25 |
| University of Texas- Austin -Bipolar Seed Program (USA) | 21.0 (18–25) | 21.0 (18–25) | 20.5 (19–25) | 69.2 | 75.0 | 83.3 | 26 | 12 | 6 |
| Total | 22.0 (8–25) | 19.0 (8–25) | 20.0 (10–25) | 62.4 | 61.0 | 61.4 | 519 | 246 | 223 |
| B. Transdiagnostic sample | |||||||||
| Boystown (USA) | 17.0 (14–18) | 16.0 (12–19) | 16.0 (12–18) | 22.2 | 30.7 | 63.0 | 9 | 114 | 27 |
| DEP-ARREST-CLIN – MOODS (France) | 21.0 (20–25) | 22.0 (18–25) | 54.5 | 70.0 | 11 | 0 | 10 | ||
| EPISCA (Netherlands) | 14.0 (13–19) | 16.0 (13–20) | 16.0 (12–20) | 86.2 | 83.3 | 87.5 | 29 | 12 | 24 |
| FOR2107-Marburg (Germany) | 23.0 (18–25) | 24.0 (18–25) | 23.0 (18–25) | 67.2 | 84.4 | 49.1 | 125 | 32 | 57 |
| FOR2107-Münster (Germany) | 23.0 (18–25) | 22.0 (18–25) | 23.0 (19–25) | 71.7 | 77.3 | 50.0 | 106 | 22 | 26 |
| Houston BD (USA) | 14.0 (8–25) | 14.0 (8–25) | 14.0 (10–24) | 54.3 | 31.6 | 66.7 | 81 | 57 | 9 |
| MDD Cohort (China) | 23.0 (22–24) | 22.0 (18–25) | 21.0 (16–25) | 80.0 | 44.4 | 66.7 | 5 | 9 | 12 |
| Muenster Neuroimaging Cohort (Germany) | 22.0 (17–25) | 23.0 (16–25) | 22.0 (17–25) | 47.5 | 57.9 | 50.0 | 80 | 19 | 40 |
| SOCAT (Turkey) | 23.0 (17–25) | 23.0 (19–25) | 100.0 | 95.0 | 37 | 0 | 20 | ||
| Sydney Brain and Mind Centre (Australia) | 23.0 (18–25) | 19.0 (12–25) | 20.0 (14–25) | 60.0 | 61.4 | 85.7 | 25 | 83 | 21 |
| University of Minnesota (USA) | 16.0 (12–19) | 16.0 (13–20) | 16.0 (12–19) | 54.5 | 74.3 | 84.0 | 22 | 35 | 25 |
| University of Texas- Austin -Bipolar Seed Program (USA) | 21.0 (18–25) | 21.0 (18–25) | 20.5 (19–25) | 69.2 | 75.0 | 83.3 | 26 | 12 | 6 |
| UWashington/Harvard (USA) | 11.0 (8–16) | 10.5 (8–16) | 14.5 (8–16) | 50.0 | 50.0 | 58.3 | 50 | 24 | 12 |
| Total | 21.0 (8–25) | 17.0 (8–25) | 20.0 (8–25) | 63.7 | 52.5 | 65.7 | 606 | 419 | 289 |
Presented here are age (median, minimum, maximum) and sex for the three groups (HC healthy controls, CC clinical controls, Ideation: group with current suicidal ideation) for the different sites included in the analysis on suicidal ideation in the stage II mood disorder only sample (A) and stage III transdiagnostic sample (B).
Table 3.
Descriptive statistics for sites included in the attempt analysis.
| Site | Age HC (years) | Age CC (years) | Age Attempt | % female HC | % female CC | % female Attempt | Total N HC | Total N CC | Total N Attempt |
|---|---|---|---|---|---|---|---|---|---|
| A. Mood disorders only sample | |||||||||
| DEP-ARREST-CLIN - MOODS (France) | - | 22.0 (18–25) | 19.0 (18–23) | - | 70.0 | 55.6 | 0 | 10 | 9 |
| Houston BD (USA) | 14.0 (8–25) | 15.0 (8–25) | 17.0 (11–24) | 48.5 | 34.7 | 69.2 | 97 | 75 | 13 |
| Sydney Bipolar Kids and Siblings | 21.0 (13–25) | 22.5 (16–25) | 22.0 (18–25) | 50.0 | 62.5 | 66.7 | 64 | 16 | 9 |
| Sydney Brain and Mind Centre (Australia) | - | 19.5 (15–25) | 20.5 (15–24) | - | 66.7 | 100.0 | 0 | 48 | 8 |
| University of Minnesota (USA) | 16.0 (14–20) | 16.0 (12–19) | 17.5 (12–19) | 66.7 | 78.0 | 62.5 | 12 | 41 | 8 |
| University of Texas- Austin - Bipolar Seed Program (USA) | 21.0 (18–25) | 21.0 (18–25) | 21.0 (19–25) | 69.2 | 77.8 | 66.7 | 26 | 18 | 9 |
| UWashington/Harvard (USA) | 11.5 (8–16) | 15.0 (12–16) | 14.5 (9–17) | 53.7 | 55.6 | 75.0 | 54 | 9 | 8 |
| Total | 16.0 (8–25) | 17.0 (8–25) | 19.0 (9–25) | 53.0 | 58.1 | 70.3 | 253 | 217 | 64 |
| B. Transdiagnostic sample | |||||||||
| DEP-ARREST-CLIN - MOODS (France) | 22.0 (18–25) | 19.0 (18–23) | 70.0 | 55.6 | 0 | 10 | 9 | ||
| Fondazione Santa Lucia - Schizophrenia sample (Italy) | 23.0 (16–25) | 24.0 (20–25) | 5.3 | 57.1 | 0 | 19 | 7 | ||
| Houston BD (USA) | 14.0 (8–25) | 15.0 (8–25) | 17.0 (11–24) | 48.5 | 34.7 | 69.2 | 97 | 75 | 13 |
| PAFIP1 (Spain) | 22.0 (17–25) | 22.0 (19–24) | 25.5 | 14.3 | 0 | 51 | 7 | ||
| PAFIP2 (Spain) | 21.0 (17–25) | 22.5 (17–25) | 24.1 | 50.0 | 0 | 58 | 10 | ||
| Sydney Bipolar Kids and Siblings (Australia) | 21.0 (13–25) | 22.0 (16–25) | 22.0 (18–25) | 50.0 | 60.9 | 66.7 | 64 | 23 | 9 |
| Sydney Brain and Mind Centre (Australia) | 19.0 (13–25) | 19.0 (15–24) | 68.3 | 100 | 0 | 101 | 10 | ||
| University of Minnesota (USA) | 16.0 (14–20) | 16.0 (12–19) | 17.5 (12–19) | 66.7 | 78.0 | 62.5 | 12 | 41 | 8 |
| University of Texas- Austin - Bipolar Seed Program (USA) | 21.0 (18–25) | 21.0 (18–25) | 21.0 (19–25) | 69.2 | 77.8 | 66.7 | 26 | 18 | 9 |
| UWashington/Harvard (USA) | 11.5 (8–16) | 12.5 (8–16) | 13.0 (9–17) | 53.7 | 52.8 | 77.8 | 54 | 36 | 9 |
| Total | 16.0 (8–25) | 19.5 (8–25) | 20.0 (9–25) | 53.0 | 48.4 | 63.7 | 253 | 432 | 91 |
Presented here are age (median, minimum-maximum) and sex for the three groups (HC healthy controls, CC clinical controls, attempt: group with lifetime suicide attempt) for the different sites included in the analysis on lifetime history of suicide attempts in the stage II mood disorder only sample (A) and stage III transdiagnostic sample (B).
Similar to the analyses in the C-SSRS sample, group differences in subcortical volume, cortical thickness, and cortical surface area were compared using multiple linear regression models in R. Because we were specifically interested in differences between individuals with current suicidal ideation or past suicide attempt(s) versus HC or CC, we included a group predictor variable to compare the suicide attempt group to either CC or to HC (in two-group comparisons). In analyses of current suicidal ideation, a group predictor was included to compare the ideation group to either CC or HC. Covariates in the models included age, sex, and age-by-sex interaction. In addition, we corrected for ICV when analyzing subcortical volumes and cortical surface area measures. We calculated effect size estimates using Cohen’s d metric.
Stage III: Analysis in the ENIGMA-STB transdiagnostic sample
To further investigate the effect of heterogeneity related to type of diagnosis and to provide additional power to potentially detect any differences not identified in the analyses restricted to mood disorders in stage II, we examined the correlates of current suicidal ideation and lifetime history of suicide attempt in a transdiagnostic sample from multiple international cohorts (N cohorts for the ideation analysis = 13; N cohorts for the attempt analysis = 10). For demographic characteristics of these cohorts, please see Tables 2B and 3B. This transdiagnostic sample included the cohorts included in stage II, with additional cohorts of individuals with mental disorders other than MDD or BD. The analyses performed were similar to the analyses performed in stage II. Given the large sample size of this sample, we were able to conduct additional analyses, and examine subgroups. We conducted the above-mentioned analyses in this larger transdiagnostic ENIGMA-STB sample separately for males and females, including age and ICV as covariates. Data on lifetime psychiatric diagnosis were available in a subsample of participants (N = 371 in the ideation analysis and N = 380 in the attempt analysis), as some sites only assessed current disorders. Therefore, in secondary analyses, diagnosis type was included as an additional covariate (see Supplemental Note 2).
Results
Stage I: Associations with suicidal ideation and attempts in the ENIGMA-STB C-SSRS sample
There were no significant associations between lifetime or recent intensity of ideation and cortical thickness, cortical surface area, and subcortical volume measures (N = 438 and 510 respectively; Tables S4 and S5). Surface area of the frontal pole was lower in young people with a lifetime history of an actual suicide attempt (N = 163) compared to those with no lifetime attempt history (N = 323; FDR p value = 0.035; Cohen’s d:−0.342; lower bound CI: −0.531; upper bound CI: −0.152; Table S6 and Fig. 2). Finally, there were no significant differences between those with lifetime ideation (but no history of a prior actual suicide attempt) (N = 200) and those with a lifetime history of an actual attempt (N = 168; Table S7).
Fig. 2. Boxplot showing the mean surface area of the frontal pole in young people without a lifetime history of any suicide attempt (in red), and those with a lifetime history of an actual suicide attempt (in blue).
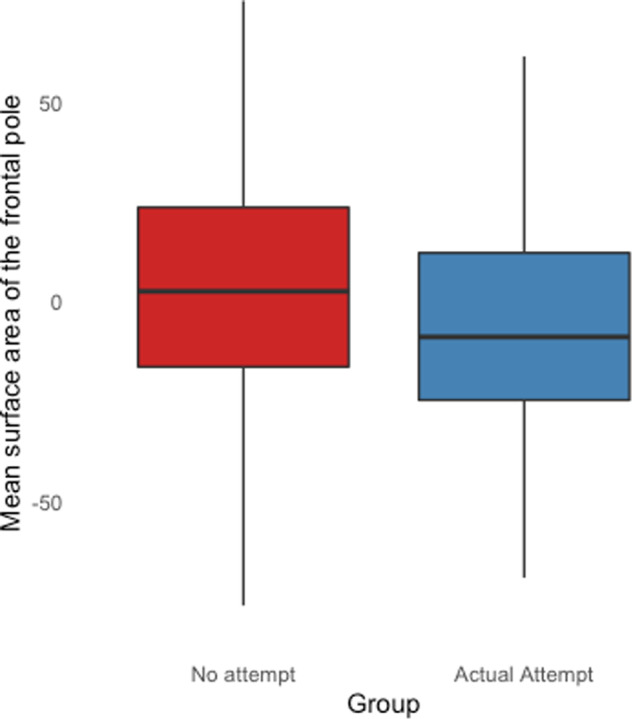
Lifetime history of an actual suicide attempt was assessed using the C-SSRS.
Stage II: Associations with current suicidal ideation and history of attempt in the ENIGMA-STB mood disorders samples
Current suicidal ideation
In the ENIGMA-STB sample of participants with mood disorders (eleven cohorts; excluding the six C-SSRS samples) in which STBs were assessed using various instruments, no brain structure measure differed significantly between young people with current suicidal ideation (N = 223) and HC (N = 519; Table S8) or CC (N = 246; Table S9) groups.
Lifetime history of suicide attempt
In this sample from seven cohorts (the six C-SSRS samples were excluded), MRI measures also did not differ significantly between the suicide attempt group (N = 64) and HC (N = 253; Table S10) or CC (N = 217; Table S11) groups.
Stage III: Associations with current suicidal ideation and history of attempt in the ENIGMA-STB transdiagnostic sample
Current suicidal ideation
In the transdiagnostic ENIGMA-STB sample (not restricted to MDD or BD diagnosis; 13 cohorts) no brain structure measure differed significantly between young people with current suicidal ideation (N = 289) and HC (N = 606; Table S12) or CC (N = 419; Table S13) groups. No differences were observed when additionally adjusting for primary diagnosis type (Table S14), nor when conducting separate analyses in males and females (N HC = 145, N CC = 109, N ideation = 77 in males; N HC = 343, N CC = 181, N ideation = 146 in females; Tables S15, S16, S17 and S18).
Lifetime history of suicide attempt
In the larger transdiagnostic ENIGMA-STB sample (not restricted to MDD or BD diagnosis; ten cohorts), MRI measures also did not differ significantly between the suicide attempt group (N = 91) and HC (N = 253; Table S19) or CC (N = 432; Table S20) groups. No differences were observed when correcting for primary diagnosis type (Table S21) or conducting separate sex-stratified analyses (N CC = 82, N attempt=11 in males; N HC = 134, N CC = 195, N attempt = 53 in females; Tables S22, S23 and S24).
Discussion
In this study we examined the associations between STBs and structural MRI measures in young people in samples from the ENIGMA-STB consortium. In a homogeneous combined sample (six sites, N = 577) assessed with the same well-validated and widely-established instrument specifically developed to assess STBs (C-SSRS) and including only young people with MDD or BD (age range 11–25 years), we found a significantly smaller surface area in the frontal pole in those with a lifetime history of an actual suicide attempt compared to those without any history of attempt.
The frontal pole is the rostral-most aspect of the prefrontal cortex and plays an essential role in higher-order functions involved in emotion and other behavioral regulation, notably, decision-making and cognitive inhibition, as well as social cognition processes (e.g., self-referential processes) implicated in STBs [30–33]. As cortical surface area is highly heritable [34] and is less affected by environmental factors during development and in later life, than is the cortical thickness [35], alterations in frontal pole surface area may represent a pre-existing vulnerability for suicidal behavior in adolescents. Longitudinal studies are needed to elucidate whether structural alterations, in particular cortical surface area, in this region precede the onset of STBs in youth. In a previous longitudinal study, structural alterations in the frontal pole (amongst other frontal regions) were associated with a family history of BD, which is also associated with increased risk of developing STBs [36]. In another longitudinal study of a sample of 46 young people with mood disorders, decreases in rostral prefrontal volume were found to be associated with future suicide attempts, although thickness and surface area were not studied separately [9]). Together with the findings of this study, results suggest that decreases in rostral PFC surface area warrant further study as potential predictors of and targets for the prevention of suicide.
In more heterogeneous samples in terms of diagnosis type or instruments to assess STBs, we did not observe any significant group differences related to ideation or attempt, which may be (partially) due to clinical heterogeneity, and the less specific and consistent definition used for suicide attempts in these samples. In contrast to the C-SSRS, the measures used to assess STBs in these more heterogeneous combined samples, do not distinguish between interrupted or aborted suicide attempts, and actual suicide attempts, therefore the attempt group may have included less severe phenotypes, decreasing our ability to discriminate those who do and do not attempt suicide based on the three MRI metrics examined here. This is supported by a supplementary analysis in the C-SSRS sample (stage I) comparing a group with interrupted, aborted or actual attempt (i.e., not restricted to actual attempt) to those without a history of attempt, which showed a reduction in effect size for the association with surface area of the frontal pole, compared to the analysis including only actual attempters (Cohen’s d = −0.295 versus −0.342 respectively; see Supplemental Note 3).
In line with our hypothesis that interrupted or aborted attempts may represent a less severe phenotype, Rogers and colleagues [24] reported less severe clinical symptoms in individuals with a history of an aborted attempt, compared to individuals with an actual attempt. However, a second study [37] did not find differences in symptom severity between individuals with an interrupted or aborted suicide attempt compared to individuals with an actual suicide attempt. A potential alternative explanation for our findings may be that interrupted or aborted attempts are qualitatively different and therefore may have revealed different associations with brain morphology. While there is limited research on this topic, previous work suggests that aborted or interrupted attempts and actual attempts do not differ in terms of lethality or intentionality [38, 39]. Another factor that may play a role in explaining our findings are differences in the reliability of assessing interrupted, aborted or actual suicide attempts. Mundt et al. [23] showed that inter-rater reliability for interrupted or aborted attempt (kappa = 0.48 and 0.89, respectively), may be lower than for actual attempt (kappa = 1.0). Thus variability across ENIGMA-STB cohorts in how interrupted or aborted attempts were coded, may have increased noise and reduced our ability to identify associations between brain structure and suicide attempt, when interrupted or aborted attempts are included in the definition of attempt.
While we observed lower frontal pole surface area to be associated with actual suicide attempts, a recent study that examined the association between STBs and brain structure in over 6000 younger children aged 9–10 years in the Adolescent Brain Cognitive Development (ABCD) study did not reveal significant structural alterations in association with STBs [40]. This may be related to the fact that the ABCD study is a general population sample with only a few children diagnosed with mood disorders, and STBs were less common and severe. Given the important role of puberty-related developmental processes in STBs, the ABCD sample may have been too young to detect brain alterations [41]. In addition, the study did not distinguish between actual attempts, and interrupted or aborted attempts, which may have also decreased the ability to identify significant alterations associated with suicide attempt. A prior ENIGMA-MDD study did find significant differences in brain structure in adults with MDD and a history of suicide attempts, including in the thalamus, pallidum and inferior parietal lobe [17]. However, the previous ENIGMA-MDD suicide study focused on adults and included only people with MDD and HC, whereas here, we included a transdiagnostic sample of young people. We hypothesize that the structural alterations associated with STBs may be stronger in adults, than in children, given the potential for prolonged exposure to stress and reduced neural plasticity in adults [42]. In future work in the ENIGMA-STB consortium, we will be able to test this new hypothesis.
This study shows the importance of using well-validated and detailed phenotyping of STBs, such as the C-SSRS, when pooling data. Therefore, a strength of this study includes the large sample sizes that allowed the examination of more detailed and homogeneous phenotypes. An additional strength of the study is the use of harmonized protocols for image processing and quality control. We should also note a few limitations of this study. First, different instruments were used to assess STBs across cohorts for analyses in the larger ENIGMA-STB samples (stage II and III), although we used a detailed process to harmonize measures across studies. Moreover, when multiple instruments were used to assess suicidal ideation or attempts within one cohort, we defined STBs in that sample using instruments that showed strong correlations with the instruments used by other cohorts to assess STBs [43]. Future multi-site collaborations would be improved by prospective harmonization in data collection and/or measurement. A second limitation was the cross-sectional study design. Although the findings are consistent with a prior report on future suicide attempts [9], we cannot determine whether brain structure increases the risk for STBs or whether prior attempts affect brain structure. Finally, while including participants from many international studies, the samples mainly included Caucasian participants from high-income countries.
In conclusion, by harmonizing neuroimaging data from research groups worldwide, we found that a deficit in the surface area of the frontal pole was related to actual (non-interrupted and non-aborted) suicide attempts in young people with mood disorders, which we interpret may represent a preexisting vulnerability to suicide attempts. Future studies which aim to pool data across studies, require well-validated measures and detailed phenotyping of STBs. Future studies focusing on the frontal pole may elucidate the structural and functional neurobiological mechanisms through which this region contributes to the development of STBs in young people.
Supplementary information
Acknowledgements
This work was supported by the MQ Brighter Futures Award MQBFC/2 (LS, LC, LV, MRD, LvV, ALvH, HB) and the U.S. National Institute of Mental Health under Award Number R01MH117601 (LS, LvV, NJ). LvV received funding through the National Suicide Prevention Research Fund, managed by Suicide Prevention Australia. LS is supported by an NHMRC Career Development Fellowship (1140764). ALvH is funded through the Social Safety and Resilience program of Leiden University. SA, NB, FP, and GS acknowledge that data collected in IRCCS Santa Lucia Foundation, Rome, Italy was funded by a study funded by the Italian Ministry of Health grant RC17-18-19-20-21/A. ZB, KC, B K-D acknowledge data collected at the University of Minnesota was funded by the National Institute of Mental Health (K23MH090421), the National Alliance for Research on Schizophrenia and Depression, the University of Minnesota Graduate School, the Minnesota Medical Foundation, and the Biotechnology Research Center (P41 RR008079 to the Center for Magnetic Resonance Research), University of Minnesota, and the Deborah E. Powell Center for Women’s Health Seed Grant, University of Minnesota. HB acknowledges data collected at the Yale School of Medicine, New Haven, CT, USA, was funded by: MQ Brighter Futures, R61MH111929RC1MH088366, R01MH070902, R01MH069747, American Foundation for Suicide Prevention, International Bipolar Foundation, Brain and Behavior Research Foundation, For the Love of Travis Foundation and Women’s Health Research at Yale. LC is supported by Interdisziplinäres Zentrum für Klinische Forschung, UKJ. BCD was funded by a CJ Martin Fellowship (NHMRC app 1161356). BCD research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06. CGD and BJH acknowledge that data collected in Melbourne, Australia, was supported by Australian National Health and Medical Research Council of Australia (NHMRC) Project Grants 1064643 (principal investigator, BJH) and 1024570 (principal investigator, CGD). BJH and CGD were supported by NHMRC Career Development Fellowships (1124472 and 1061757, respectively). UD and TH acknowledge data collected at the FOR2107-Münster was funded by the German Research Foundation (DFG, grant FOR2107-DA1151/5-1 and DA1151/5-2 to UD, and DFG grants HA7070/2-2, HA7070/3, HA7070/4 to TH). AJ and TK acknowledges data collected at the FOR2107-Marburg was funded by the German Research Foundation (DFG, grant FOR2107-JA 1890/7-1 and JA 1890/7-2 to AJ, and DFG, grant FOR2107-KI588/14-1 and FOR2107-KI588/14-2 to TK). KD acknowledges data collected for the Münster Neuroimaging Cohort was funded by the Medical Faculty Münster, Innovative Medizinische Forschung (Grant IMF KO 1218 06 to KD). JMF, PBM, BJO, and GR acknowledge that the “Kids and Sibs” Study was supported by the Australian National Medical and Health Research Council (Program Grant 1037196 and Investigator Grant 1177991 to PBM, Project Grant 1066177 to JMF), the Lansdowne Foundation, Good Talk and the Keith Pettigrew Family Bequest (PM). JMF gratefully acknowledges the Janette Mary O’Neil Research Fellowship. IHG is supported in part by R37MH101495. Support for TAD comes from the National Institute of Mental Health (K01MH106805). TH acknowledges support for TIGER includes the Klingenstein Third Generation Foundation, the National Institute of Mental Health (K01MH117442), the Stanford Maternal Child Health Research Institute, and the Stanford Center for Cognitive and Neurobiological Imaging. TCH receives partial support from the Ray and Dagmar Dolby Family Fund. KAM, ABM, MAS acknowledge data collected at Harvard University was funded by the National Institute of Mental Health (R01-MH103291). IN is supported by grants of the Deutsche Forschungsgemeinschaft (DFG grants NE2254/1-2, NE2254/3-1, NE2254/4-1).This study was supported by the National Center for Complementary and Integrative Health (NCCIH) R21AT009173 and R61AT009864 to TTY; by the National Center for Advancing Translational Sciences (CTSI), National Institutes of Health, through UCSF-CTSI UL1TR001872 to TTY; by the American Foundation for Suicide Prevention (AFSP) SRG-1-141-18 to TTY; by UCSF Research Evaluation and Allocation Committee (REAC) and J. Jacobson Fund to TTY; by the National Institute of Mental Health (NIMH) R01MH085734 and the Brain and Behavior Research Foundation (formerly NARSAD) to TTY. YC acknowledges the Medical Leader Foundation of Yunnan Province (L2019011) and Famous Doctors Project of Yunnan Province Plan (YNWR-MY-2018-041). DTG, BCF and RAA wish to thank all PAFIP patients and family members who participated in the study as well as PAFIP´s research team and Instituto de Investigación Marqués de Valdecilla. Work by the PAFIP group has been funded by Instituto de Salud Carlos III through the projects PI14/00639, PI14/00918 and PI17/01056 (Co-funded by European Regional Development Fund/European Social Fund “Investing in your future”) and Fundación Instituto de Investigación Marqués de Valdecilla (NCT0235832 and NCT02534363). MER received support from the Australian National Health and Medical Research Council (NHMRC) Centre for Research Excellence on Suicide Prevention (CRESP) [GNT1042580]. ETCL is supported by grants from NIAAA (K01AA027573, R21AA027884) and the American Foundation for Suicide Prevention. All authors thank the participants for volunteering their time and supporting our research.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Competing interests
IBH was an inaugural Commissioner on Australia’s National Mental Health Commission Sydney. The BMC operates an early-intervention youth service at Camperdown under contract to headspace. He is the Chief Scientific Advisor to, and a 5% equity shareholder in, InnoWell Pty Ltd. deliver the $30M Australian Government-funded Project Synergy (2017–20; a 3-year program forInnoWell was formed by the University of Sydney (45% equity) and PwC (Australia; 45% equity) to the transformation of mental health services) and to lead the transformation of mental health services internationally through the use of innovative technologies. ETCL has previously received salary support for the effort in kind from a Janssen-sponsored study at the University of Texas at Austin. JCS has received research grants from Compass, Alkermes, and Allergan; and has served as a consultant for Pfizer, Sunovion, Sanofi, Johnson & Johnson, Livanova, and Boehringer Ingelheim. NJ and PMT received partial grant support from Biogen, Inc. for research unrelated to this manuscript. All other authors have no financial relationships with commercial interests to report.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Laura S. van Velzen, Email: laura.vanvelzen@unimelb.edu.au
ENIGMA Suicidal Thoughts and Behaviours Consortium:
Laura S. van Velzen, Nic J. A. van der Wee, Steven J. van der Werff, and Anne-Laura van Harmelen
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01734-0.
References
- 1.World Health Organization. Suicide in the world—Global Health Estimates. 2019.
- 2.Hawton K, Saunders KEA, O’Connor RC. Self-harm and suicide in adolescents. Lancet. 2012;379:2373–82. doi: 10.1016/S0140-6736(12)60322-5. [DOI] [PubMed] [Google Scholar]
- 3.Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133–54. doi: 10.1093/epirev/mxn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Sumner SA, Simon TR, Crosby AE, Annor FB, Gaylor E, et al. Trends in the incidence and lethality of suicidal acts in the United States, 2006 to 2015. JAMA Psychiatry. 2020;77:684–93. doi: 10.1001/jamapsychiatry.2020.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmaal L, van Harmelen A-L, Chatzi V, Lippard ETC, Toenders YJ, Averill LA, et al. Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry. 2020;25:408–27. doi: 10.1038/s41380-019-0587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, Liu J, et al. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am J Psychiatry. 2017;174:667–75. doi: 10.1176/appi.ajp.2016.15050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan LA, Ramos L, Segreti A, Brent DA, Phillips ML. Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br J Psychiatry. 2015;206:339–40. doi: 10.1192/bjp.bp.114.151316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan S, Lippard ETC, Sankar A, Wallace A, Johnston JAY, Wang F, et al. Gray and white matter differences in adolescents and young adults with prior suicide attempts across bipolar and major depressive disorders. J Affect Disord. 2019;245:1089–97. doi: 10.1016/j.jad.2018.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippard ETC, Cox Lippard ET, Johnston JAY, Spencer L, Quatrano S, Fan S, et al. Preliminary examination of gray and white matter structure and longitudinal structural changes in frontal systems associated with future suicide attempts in adolescents and young adults with mood disorders. J Affect Disord. 2019;245:1139–48. doi: 10.1016/j.jad.2018.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J, Chen X, Chen J, Ai M, Gan Y, Wang W, et al. Resting-state functional MRI of abnormal baseline brain activity in young depressed patients with and without suicidal behavior. J Affect Disord. 2016;205:252–63. doi: 10.1016/j.jad.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Gifuni AJ, Chakravarty MM, Lepage M, Ho TC, Geoffroy M-C, Lacourse E, et al. Brain cortical and subcortical morphology in adolescents with depression and a history of suicide attempt. J Psychiatry Neurosci. 2021;46:E347–E357.. doi: 10.1503/jpn.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan R, Siddarth P, Levitt J, Gurbani S, Shields WD, Sankar R. Suicidality and brain volumes in pediatric epilepsy. Epilepsy Behav. 2010;18:286–90. doi: 10.1016/j.yebeh.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Ho TC, Teresi GI, Ojha A, Walker JC, Kirshenbaum JS, Singh MK, et al. Smaller caudate gray matter volume is associated with greater implicit suicidal ideation in depressed adolescents. J Affect Disord. 2021;278:650–7. doi: 10.1016/j.jad.2020.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho TC, Cichocki AC, Gifuni AJ, Catalina Camacho M, Ordaz SJ, Singh MK, et al. Reduced dorsal striatal gray matter volume predicts implicit suicidal ideation in adolescents. Soc Cogn Affect Neurosci. 2018;13:1215–24. doi: 10.1093/scan/nsy089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 16.Renteria ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, et al. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry. 2017;7:e1116–e1116. doi: 10.1038/tp.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos AI, Thompson PM, Veltman DJ, Pozzi E, van Veltzen LS, Jahanshad N, et al. Brain correlates of suicide attempt in 18,925 participants across 18 international cohorts. Biol Psychiatry. 2021;90:243–52. doi: 10.1016/j.biopsych.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 19.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Fortin J-P, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–20. doi: 10.1016/j.neuroimage.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radua J, Vieta E, Shinohara R, Kochunov P, Quidé Y, Green MJ, et al. Increased power by harmonizing structural MRI site differences with the ComBat batch adjustment method in ENIGMA. Neuroimage. 2020;218:116956. doi: 10.1016/j.neuroimage.2020.116956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, et al. Columbia-suicide severity rating scale (C-SSRS). New York, NY: Columbia University Medical Center; 2008.
- 23.Mundt JC, Greist JH, Gelenberg AJ, Katzelnick DJ, Jefferson JW, Modell JG. Feasibility and validation of a computer-automated Columbia-Suicide Severity Rating Scale using interactive voice response technology. J Psychiatr Res. 2010;44:1224–8. doi: 10.1016/j.jpsychires.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Rogers ML, Hom MA, Dougherty SP, Gallyer AJ, Joiner TE. Comparing suicide risk factors among individuals with a history of aborted, interrupted, and actual suicide attempts. Arch Suicide Res. 2020;24:57–74. doi: 10.1080/13811118.2018.1522283. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 26.First MB. Structured clinical interview for DSM-IV Axis I disorders SCID-I: clinician version, scoresheet. American Psychiatric Press; 1997.
- 27.Beck AT, Steer RA, Brown GK, et al. Beck depression inventory-II. San Antonio. 1996;78:490–8. [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner G, Li M, Sacchet MD, Richard-Devantoy S, Turecki G, Bär K-J, et al. Functional network alterations differently associated with suicidal ideas and acts in depressed patients: an indirect support to the transition model. Transl Psychiatry. 2021;11:100. doi: 10.1038/s41398-021-01232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bludau S, Eickhoff SB, Mohlberg H, Caspers S, Laird AR, Fox PT, et al. Cytoarchitecture, probability maps and functions of the human frontal pole. NeuroImage. 2014;93:260–75. doi: 10.1016/j.neuroimage.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermann A, Neudert MK, Schäfer A, Zehtner RI, Fricke S, Seinsche RJ, et al. Lasting effects of cognitive emotion regulation: neural correlates of reinterpretation and distancing. Soc Cogn Affect Neurosci. 2021;16:268–79. doi: 10.1093/scan/nsaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bramson B, Folloni D, Verhagen L, Hartogsveld B, Mars RB, Toni I, et al. Human lateral frontal pole contributes to control over emotional approach–avoidance actions. J Neurosci. 2020;40:2925–34. doi: 10.1523/JNEUROSCI.2048-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gifuni AJ, Perret LC, Lacourse E, Geoffroy M-C, Mbekou V, Jollant F, et al. Decision-making and cognitive control in adolescent suicidal behaviors: a qualitative systematic review of the literature. Eur Child Adolesc Psychiatry. 2020. 10.1007/s00787-020-01550-3. [DOI] [PubMed]
- 34.Patel S, Patel R, Park MTM, Masellis M, Knight J, Chakravarty MM. Heritability estimates of cortical anatomy: the influence and reliability of different estimation strategies. Neuroimage. 2018;178:78–91. doi: 10.1016/j.neuroimage.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, et al. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci. 2014;34:8488–98. doi: 10.1523/JNEUROSCI.0391-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts G, Lenroot R, Overs B, Fullerton J, Leung V, Ridgway K, et al. Accelerated cortical thinning and volume reduction over time in young people at high genetic risk for bipolar disorder. Psychol Med. 2022;52:1344–55. [DOI] [PubMed]
- 37.Burke TA, Hamilton JL, Ammerman BA, Stange JP, Alloy LB. Suicide risk characteristics among aborted, interrupted, and actual suicide attempters. Psychiatry Res. 2016;242:357–64. doi: 10.1016/j.psychres.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steer RA, Beck AT, Garrison B, Lester D. Eventual suicide in interrupted and uninterrupted attempters: a challenge to the cry-for-help hypothesis. Suicide Life-Threat Behav. 1988;18:119–28. doi: 10.1111/j.1943-278X.1988.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 39.Barber ME, Marzuk PM, Leon AC, Portera L. Aborted suicide attempts: a new classification of suicidal behavior. Am J Psychiatry. 1998;155:385–9. doi: 10.1176/ajp.155.3.385. [DOI] [PubMed] [Google Scholar]
- 40.Vidal-Ribas P, Janiri D, Doucet GE, Pornpattananangkul N, Nielson DM, Frangou S, et al. Multimodal Neuroimaging of Suicidal Thoughts and Behaviors in a U.S. Population-Based Sample of School-Age Children. Am J Psychiatry. 2021;178:321–32. [DOI] [PMC free article] [PubMed]
- 41.Ho TC, Gifuni AJ, Gotlib IH. Psychobiological risk factors for suicidal thoughts and behaviors in adolescence: a consideration of the role of puberty. Mol Psychiatry. 2021. 10.1038/s41380-021-01171-5. [DOI] [PMC free article] [PubMed]
- 42.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campos AI, van Velzen LS, Veltman DJ, Pozzi E, Ambrogi S, Ballard ED, et al. Concurrent validity and reliability of suicide risk assessment instruments: A meta analysis of 20 instruments across 27 international cohorts. medRxiv 2021. 10.1101/2021.09.15.21263562. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.