Abstract
Background
The long COVID-19 syndrome has been recently described and some reports have suggested that acute pericarditis represents important manifestation of long COVID-19 syndrome. The aim of this study was to identify the prevalence and clinical characteristics of patients with long COVID-19, presenting with acute pericarditis.
Methods
We retrospectively included 180 patients (median age 47 years, 62% female) previously diagnosed with COVID-19, exhibiting persistence or new-onset symptoms ≥12 weeks from a negative naso-pharyngeal SARS CoV2 swamp test. The original diagnosis of COVID-19 infection was determined by a positive swab. All patients had undergone a thorough physical examination. Patients with suspected heart involvement were referred to a complete cardiovascular evaluation. Echocardiography was performed based on clinical need and diagnosis of acute pericarditis was achieved according to current guidelines.
Results
Among the study population, shortness of breath/fatigue was reported in 52%, chest pain/discomfort in 34% and heart palpitations/arrhythmias in 37%. Diagnosis of acute pericarditis was made in 39 patients (22%). Mild-to-moderate pericardial effusion was reported in 12, while thickened and bright pericardial layers with small effusions (< 5 mm) with or without comet tails arising from the pericardium (pericardial B-lines) in 27. Heart palpitations/arrhythmias (OR:3.748, p = 0.0030), and autoimmune disease and allergic disorders (OR:4.147, p = 0.0073) were independently related to the diagnosis of acute pericarditis, with a borderline contribution of less likelihood of hospitalization during COVID-19 (OR: 0.100, p = 0.0512).
Conclusion
Our findings suggest a high prevalence of acute pericarditis in patients with long COVID-19 syndrome. Autoimmune and allergic disorders, and palpitations/arrhythmias were frequently associated with pericardial disease.
Keywords: COVID-19, SARS-CoV2, Acute pericarditis
Abbreviations: COVID-19:, Coronavirus Disease-2019; Sars-CoV2:, Coronavirus-2; OD:, Odds ratio; TTE:, Transthoracic echocardiogram; NSAIDs:, Nonsteroidal anti inflammatory drugs; CMR:, Cardiovascular magnetic resonance
1. Introduction
Coronavirus Disease-2019 (COVID-19) is a multi-organ disease whose severity ranges from asymptomatic to very serious illness. A large number of patients, who have had COVID-19, experience persistent symptoms or new onset symptoms, after the initial recovery with a negative test, that are not explained by alternative diagnoses. A wide range of symptoms may be present varying from shortness of breath to chest pain. This very debilitating condition has been termed long COVID-19 syndrome, also known as post-acute COVID syndrome, post-acute sequelae or chronic COVID syndrome [[1], [2], [3], [4]]. The origin of symptoms and number of patients who experience long COVID-19 is unknown and varies according to the definition used and the population studied and it is unclear what cardiovascular disorders may be contributing to these symptoms [5,6]. To further characterize patients with long COVID-19 syndrome, we evaluated the clinical, instrumental and biochemical features of patients who developed persistent or new onset symptoms after the initial recovery from COVID-19 with a negative naso-pharyngeal swab test for acute respiratory syndrome-coronavirus-2 (SARS-CoV2) nucleic acid.
2. Methods
In this retrospective study, we collected data from ambulatory centers specifically devoted to diagnosis and treatment of the long COVID-19 syndrome. We retrospectively included 180 consecutive patients (median age 47 years, 62% female) previously diagnosed with COVID-19, who exhibited persistent symptoms or new onset symptoms after at least 12 weeks from a negative swamp for SARS-CoV2 nucleic acid. All patients included in this study signed an individual informed consent form prior to data collection. The original diagnosis of COVID-19 was confirmed by a positive naso-pharyngeal swab test for SARS-CoV2 nucleic acid. Clinical history of COVID-19 was carefully obtained. In particular, fever was defined as intra-auricular temperature > 38 °C, tachycardia as heart rate ≥ 90 beats/min, and hypoxemia as peripheral finger oxygen saturation ≤ 94%. All patients had undergone a thorough physical examination, and blood samples were sent for a series of hematological and biochemical investigations including, white blood cells, platelets, C reactive protein, erythrocyte sedimentation rate, D-dimer, fibrinogen and high-sensitivity cardiac troponin when needed. Patients with suspected heart involvement were referred to a complete cardiovascular evaluation, including an electrocardiogram and possibly a transthoracic echocardiogram (TTE). Echocardiography was performed based on clinical need, according to the published recommendations on the optimization of echocardiographic examinations during COVID-19 pandemic[7]. Other imaging and functional tests were performed, as clinically indicated.
The diagnosis of pericarditis was made by the presence of at least two of the following criteria: 1) typical chest pain, 2) pericardial rubs, 3) electrocardiographic changes, 4) pericardial effusion[8]. Patients were divided into two groups according to the diagnosis of acute pericarditis: patients who met the criteria, and patients who did not.
2.1. Statistical analysis
Categorical variables were presented as the proportion of valid cases and continuous variables were expressed as mean ± standard deviation. Differences were assessed by Student's t-test for continuous variables or contingency tables for categorical variables. Comparisons between continuous variables were analyzed by the standard parametric methods. Non-parametrical variables were expressed by the median value and interquartile range. The Mann and Whitney test was used for comparison. Significance was set at p < 0.05. Logistic regression was used to explore the determinants of acute pericarditis. All variables showing p value <0.1 at univariable analysis were tested in multivariable models using an “Enter” procedure. Data were analyzed using the Statistical Package for the Social Sciences version 26.0 for Windows statistical software program (SPSS, Chicago, Illinois).
3. Results
The age of the study participants ranged between 14 and 82 years. Among the study population, 40 (22%) had at least one comorbidity and 6 (3%) presented a history of cardiovascular disease.
All patients were clinically symptomatic and diagnosis was confirmed by a positive naso-pharyngeal SARS-CoV2 swamp test. Most patients recovered at home, while the minority required hospitalization. The median time interval between initial recovery with a negative test and the time of assessment at the ambulatory center was 131 days. The most common symptoms were fatigue/ shortness of breath (52%), followed by heart palpitations (37%) and chest pain (34%). Chest pain was mostly described as sharp and postural. Clinical examination was unremarkable in all, with normal heart sounds and non-raised jugular venous pressure. Friction rub was reported only in one patient. There was no clear relationship between cardiovascular manifestations and pre-existing cardiovascular disease.
TTE showed normal myocardial structure and function in all patients with the exception of 10; 8 (5%) of whom exhibited a dysfunctional right ventricle, one was diagnosed as post COVID-19 dilated cardiomyopathy and one had regional left ventricular dysfunction due to a previous myocardial infarction. Thirty-nine (22%) patients fulfilled the criteria for acute pericarditis according to ESC guidelines. Twenty-eight presented two classical criteria; 10 with three criteria and one with four criteria for pericarditis. Mild-to-moderate pericardial effusion (5–12 mm) was reported in 12 (31%) (Fig. 1 ). In 27 (69%) patients, pericardial layers were thickened and bright with small or negligible effusions (< 5 mm) with or without comet tails arising from the pericardium (pericardial B-lines) (Fig. 2 ). In 15 (39%) patients, the electrocardiogram showed concave ST segment elevation in most leads or focal T wave inversion in several leads.
Fig. 1.
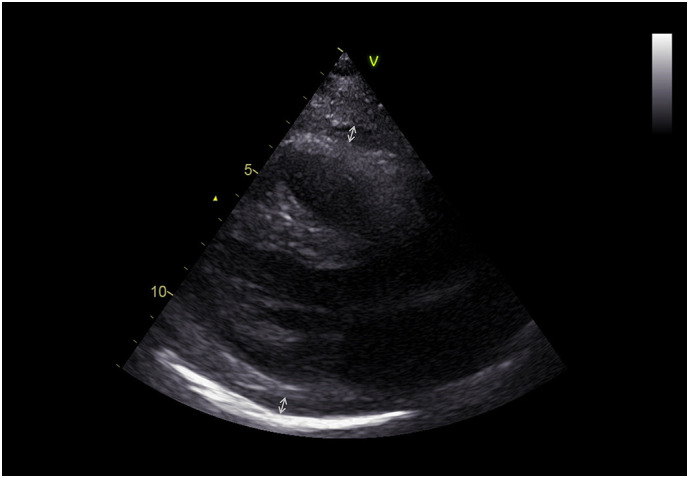
Echocardiographic parasternal long axis view showing a thickened pericardium with an effusion of 10 mm between the layers.
Fig. 2.
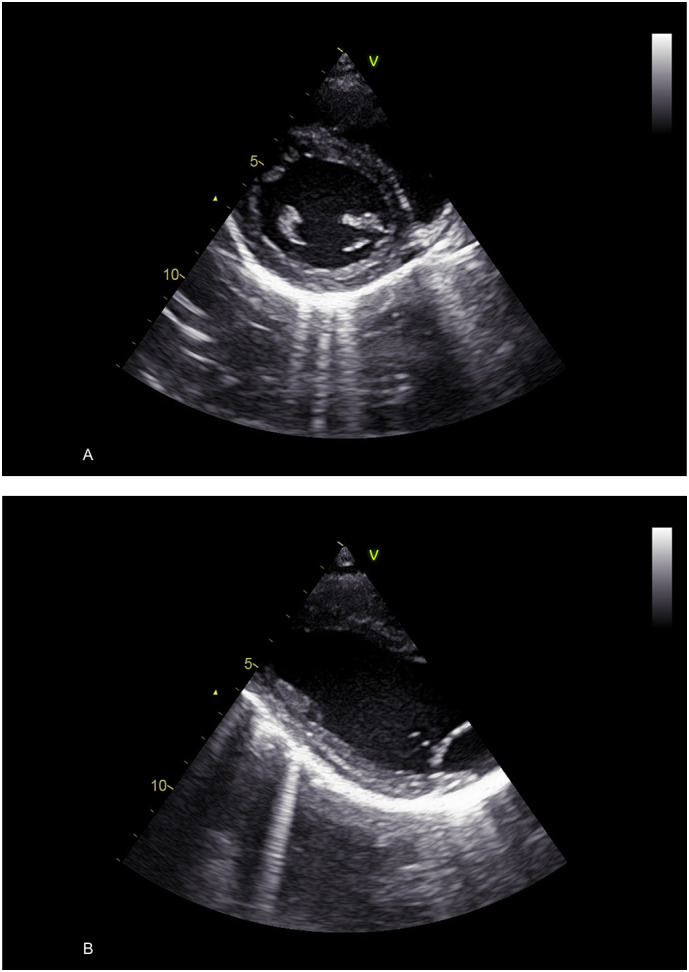
Increased echogenity of the pericardium in the left basal segments with thickened layers and numerous comet tails (pericardial B-lines). A. Short-axis view. B. Long-axis view.
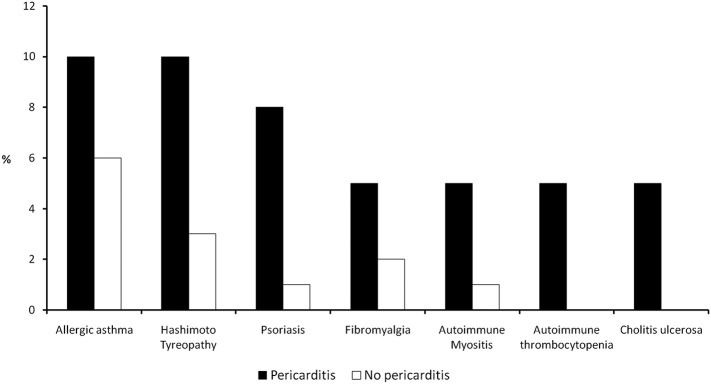
Table 1 shows the characteristics of the study patients. Compared to patients without diagnosis of pericarditis, those with pericarditis were slightly younger and were more often women. Patients with acute pericarditis also had more frequent history of autoimmune disease and allergic disorders (Fig. 3 ). Raised inflammatory markers were more frequent in patients diagnosed with pericarditis; raised erythrocyte sedimentation rate in 26% and high C-reactive protein in 21%. Markers of myocyte injury (high-sensitivity troponin) were normal. Heart palpitations and arrhythmias were more frequent in patients with pericarditis. The most common arrhythmia was sinus tachycardia (69%), but there were patients showing repetitive supraventricular and ventricular premature beats.
Table 1.
Baseline clinical features, clinical complains and laboratoryfindingsof our study cohort.
| Whole population (n = 180) | Acute pericarditis (n = 39) | No acute pericarditis (n = 141) | P value | |
|---|---|---|---|---|
| Baseline features | ||||
| Age (years) | 48 ± 16 | 45 ± 14 | 48 ± 16 | 0.320 |
| Sex (female) | 112 (62) | 31 (80) | 81 (57) | 0.0120 |
| Hypertension, (%) | 30 (17) | 4 (10) | 26 (18) | 0.201 |
| Diabetes | 4 (2) | 1 (3) | 3 (2) | 0.870 |
| Dyslipidemia | 11 (6) | 3 (7) | 8 (6) | 0.641 |
| Coronary artery disease | 4 (2) | 1 (3) | 3 (2) | 0.870 |
| Impaired renal function | 4 (2) | 0(0) | 4 (3) | 0.288 |
| Asthma/Chronic obstructive pulmonary disease | 10 (6) | 3 (8) | 7 (5) | 0.510 |
| Autoimmune and allergic disorders | 29 (16) | 15 (38) | 14 (10) | <0.0001 |
| Duration of positivity at SARS-CoV2 test (days) | 21 [14–41] | 19 [14–41] | 22 [14–32] | 0.6997 |
| Duration of symptoms (days) | 131 [94–255] | 166 [116–255] | 122[94–183] | 0.0106 |
| Prior COVID-19 vaccination (%) | 68 (38) | 22 (56) | 46 (33) | 0.00670 |
| Prior COVID-19 hospitalization | 23 (13) | 1 (3) | 22 (16) | 0.0310 |
| Emergency department visits | 34 (19) | 5 (13) | 29 (21) | 0.274 |
| Oxygen desaturation during COVID-19 | 37 (21) | 3 (8) | 34 (24) | 0.0247 |
| Temperature > 38 °C during COVID-19 | 62 (34) | 8 (21) | 54 (38) | 0.0386 |
| Clinical complaints | ||||
| Brain fog/lack of concentration (%) | 18 (10) | 5 (13) | 13 (9) | 0.507 |
| Chest pain/discomfort | 61 (34) | 35 (90) | 26 (18) | <0.0001 |
| Cough | 19 (11) | 4 (10) | 15 (11) | 0.945 |
| Headache | 7 (4) | 1 (3) | 6 (4) | 0.629 |
| Heart palpitations/arrhythmias | 66 (37) | 26 (67) | 40 (28) | <0.0001 |
| Shortness of breath/fatigue | 93 (52) | 13 (33) | 80 (57) | 0.00964 |
| Other symptoms | 41 (23) | 10 (26) | 31 (22) | 0.573 |
| Laboratory analyses | ||||
| Abnormal C reactive protein (%) | 18 (10) | 8 (21) | 9 (6) | 0.00101 |
| Abnormal erythrocyte sedimentation rate | 16 (9) | 10 (26) | 6 (4) | <0.0001 |
| Abnormal D dimer | 17 (9) | 4 (10) | 13 (9) | 0.845 |
Fig. 3.
Histograms describing prevalence of autoimmune and allergic disorders in long COVID-19 patients with or without pericarditis.
Among patients diagnosed with acute pericarditis, 25 were initially treated with nonsteroidal anti inflammatory drugs (NSAIDs), 8 with corticosteroids and 26 with colchicine. Seven non-responders to NSAIDs were thereafter shifted to corticosteroids. Thirty-three patients recoverd from symptoms between one and four weeks of therapy, while 6 had persistent symptoms and were considered non-responders to therapy. Patients in whom symptoms persisted long after the optimization of treatment were especially those in whom too much time elapsed between the onset of symptoms and the diagnosis of pericardial disease. Recurrence of acute pericarditis (after a minimum symptom-free interval of one month) was observed in 10. Relapsing pericarditis was attributed to idiopathic pericarditis in 6, to COVID-19 vaccination-related pericarditis in 5 and to pericarditis due to COVID-19 recurrence in one.
At logistic regression analysis, relations of variables with acute pericarditis, diagnosed according to the ESC criteria, are displayed in Table 2 . Abnormal C-reactive protein, abnormal erythrocyte sedimentation rate, autoimmune and allergic disorders, heart palpitations/arrhythmias, prior COVID-19 hospitalization, previous vaccination and female gender were found to be univariate predictors of acute pericarditis. Heart palpitations/arrhythmias, autoimmune disease and allergic disorders were related to the diagnosis of acute pericarditis on multivariate analysis. Previous COVID-19 hospitalization was less often independently associated with pericarditis.
Table 2.
Univariate and multivariate analysis of selected variables for the prediction of acute pericarditis.
| Univariate analysis [OR and CI (95%)] | Unadjusted P value | Multivariate analysis [OR and CI (95%)] | Adjusted P value | |
|---|---|---|---|---|
| Abnormal C reactive protein | 3.785 [1.352–10.598] | 0.011 | 2.697 [0.674–10.795] | 0.161 |
| Abnormal D dimer | 1.125 [0.345–3.667] | 0.845 | ||
| Abnormal erythrocyte sedimentation rate | 7.759 [2.612–23.047] | <0.001 | 2.569 [0.637–10.359] | 0.185 |
| Age | 0.988 [0.966–1.011] | 0.319 | ||
| Autoimmune/allergic disorders | 5.670 [2.426–13.252] | <0.001 | 4.147 [1.466–11.728] | 0.007 |
| Heart palpitations/arrhythmias | 5.050 [2.362–10.796] | < 0.001 | 3.748 [1.565–8.976] | 0.003 |
| Persistence of symptoms | 2.557 [1.200–5.446] | 0.015 | 1.579 [0.652–3.822] | 0.311 |
| Prior COVID-19 vaccination | 2.673 [1.295–5.514] | 0.008 | 1.624 [0.695–3.795] | 0.262 |
| Prior COVID-19 hospitalization | 0.142 [0.019–1.091] | 0.061 | 0.100 [0.010–1.012] | 0.051 |
| Sex | 0.348 [0.150–0.812] | 0.015 | 0.7773 [0.282–2.119] | 0.617 |
CI, confidence interval; OR, odds ratio.
4. Discussion
The results of this study showed that acute pericarditis was an important manifestation of long COVID-19 syndrome. There was evidence for an association of acute pericarditis presenting with palpitations/arrhythmias, and autoimmune and allergic disorders. Objective assessment of pericardial disease in long COVID-19 patients was mostly characterized at TTE by thickened and bright pericardial layers with small or negligible effusions.
COVID-19 disease is caused by SARS-CoV2 infection, which leads to a wide spectrum of clinical manifestations, ranging from complete lack of symptoms to severe illness, including respiratory failure and multi-organ dysfunction. The lungs are the organs most affected by COVID-19 and this may lead to aggressive bilateral pneumonia with a high fatality rate. While many patients with COVID-19 fully recover, a significant percentage experience long-term health consequences [[9], [10], [11]].
Little is known about cardiovascular manifestations/complications occurring after clinical and virology recovery from SARS-CoV2 infection and there are few studies focusing on a comprehensive evaluation of long COVID-19 patients that aim at establishing the extent of cardiovascular disturbance contribution to the development of symptoms after recovery [[12], [13], [14], [15]].
It is well known that pericarditis is among the most frequent cardiac complications after viral infections [[16], [17], [18]]. Case reports have recently documented pericarditis with or without pericardial effusion as delayed complications of COVID-19, but the real prevalence of pericarditis in long COVID-19 patients is still unknown [19,20]. In our study, diagnosis of delayed cases of acute pericarditis was based on the presence of at least two of the following criteria: typical sharp and positional chest pain, presence of pericardial effusion of any degree, and electrocardiographic changes. Most study patients diagnosed with acute pericarditis showed thickened and bright pericardial layers with small or negligible effusion. Pericarditis was diagnosed in patients with thickened pericardium albeit no signs of effusion, only in the presence of typical chest pain and electrocardiographic alterations. The association with typical chest pain, electrocardiographic changes and eventually the response to anti-inflammatory medications has also been helpful in substantiating the final diagnosis.
De novo palpitations/arrhythmias were common in the study population. The occurrence of either supraventricular or ventricular arrhythmias have previously been documented in post COVID-19 patients and were found to be often associated with right ventricular dysfunction [21]. Other studies have suggested a possible relation between autonomic nervous system disturbances and post COVID-19 sequelae [22]. Our results show that acute pericarditis was often accompanied by concomitant heart rhythm disturbances. It is reasonable to believe that pericarditis could extend to the epicardial layer of the myocardium, with myocardial damage from inflammatory cascade and subsequent fibrosis, remodeling, and arrhythmias [23].
Although echocardiography is considered the standard cardiac imaging technique, inflammation of the pericardial layers may not always be easily detected by TTE.
Cardiovascular magnetic resonance (CMR) can provide comprehensive information on pericardial disease, including assessment of pericardial thickness and small or negligible effusions. In patients recovered from COVID-19, CMR studies performed more than two months after the infection have shown that as many as 78% had cardiac involvement with abnormal findings, while signs of ongoing myocardial inflammation were present in 60% [24]. Twenty percent had pericardial effusion >10 mm versus 7% in case-matched controls. Despite such high prevalence, cardiac involvement was not related to the severity of COVID-19 and persisted well beyond the acute phase [5]. Recently, cardiovascular complications have been also reported from COVID-19 vaccines and this may suggest common pathways shared by the untoward effects of anti-SARS CoV2 vaccination and the manifestations of the long COVID-19 syndrome [25].
The pathogenesis of long COVID-19 syndrome remains not well defined but seems to be different from acute COVID-19. Several mechanisms have been proposed to explain the occurrence of the long COVID-19, including ongoing inflammation activated by the virus and host of factors comprising allergic conditions, autoimmune reactions, and vascular injury caused by hypercoagulability and thrombosis [26]. It has been recognized that inadequate or excessive immune response driven by T and B cell-mediated mechanisms may be implicated in the occurrence of pericarditis and myocarditis after viral infections [27,28]. Our finding of more frequent occurrence of pericarditis in patients presenting an history of autoimmune and allergic disorders suggests that an association might be present between the two conditions and that the immune system continues to over-react after the coronavirus infection and is unable to reset itself to idle.
Limitations.
An important limitation in this study is that the prevalence of pericarditis in long COVID-19 may vary according to the setting. Studied patients were those referred to ambulatory centers mostly dealing with cardiovascular diseases and this might have led to an overestimation of the real prevalence of pericardial disease among patients with long COVID-19 syndrome [29]. Cardiovascular symptoms, such as chest pain and palpitations commonly occur in long COVID-19 syndrome. In our study cohort, chest pain was reported in just above one third of patients while acute pericarditis was diagnosed in 22%, which suggests that pericarditis could be an underdiagnosed disease, and therefore not optimally managed. There was a discrepancy between the relatively high occurrence of pericarditis in our long COVID-19 patients and that reported in a large study performed in post COVID-19 unvaccinated patients, where the incidence was very low [30]. Possible explanations for the latter finding is that post COVID-19 patients are intrinsically different from those with long COVID-19 syndrome. Moreover, patients with pericarditis frequently show thickened pericardial layers with small or negligible effusions and diagnosis of pericarditis may be difficult unless an echocardiographic examination especially focused on the pericardium is performed.
5. Conclusion
To our knowledge this is the first study describing acute pericarditis associated with long COVID-19 in consecutive patients who have had symptomatic COVID-19 and a negative SARS-CoV2 nucleic acid test. Female gender, the presence of chest pain, history of autoimmune and allergic disorders, and palpitations/arrhythmias were risk factors for the development of acute pericarditis.
Authors' contribution
FLD, MH contributed to study conception and design; FLD, UB, NRP contributed to data collection; FLD, GB and IB performed data analysis; FLD, MH drafted the manuscript, FLD, MH, GB critically revised the work. All the authors read and approved the final version of the paper.
References
- 1.Carfì A., Bernabei R., Landi F., Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. (PMID: 32644129; PMCID: PMC7349096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs L.G., Gourna Paleoudis E., Lesky-Di Bari D., Nyirenda T., Friedman T., Gupta A., Rasouli L., Zetkulic M., Balani B., Ogedegbe C., Bawa H., Berrol L., Qureshi N., Aschner J.L. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243882. (PMID: 33306721; PMCID: PMC7732078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munblit D., Bobkova P., Spiridonova E., Shikhaleva A., Gamirova A., Blyuss O., Nekliudov N., Bugaeva P., Andreeva M., Dunn Galvin A., Comberiati P., Apfelbacher C., Genuneit J., Avdeev S., Kapustina V., Guekht A., Fomin V., Svistunov A.A., Timashev P., Subbot V.S., Royuk V.V., Drake T.M., Hanson S.W., Merson L., Carson G., Horby P., Sigfrid L., Scott J.T., Semple M.G., Warner J.O., Vos T., Olliaro P., Glybochko P., Butnaru D., Sechenov StopCOVID Research Team Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy. 2021;51(9):1107–1120. doi: 10.1111/cea.13997. (Epub 2021 Aug 12. PMID: 34351016; PMCID: PMC8444748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boscolo-Rizzo P., Guida F., Polesel J., Marcuzzo A.V., Capriotti V., D’Alessandro A., Zanelli E., Marzolino R., Lazzarin C., Antonucci P., Sacchet E., Tofanelli M., Borsetto D., Gardenal N., Pengo M., Tirelli G. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19) Int Forum Allergy Rhinol. 2021 doi: 10.1002/alr.22832. (Epub ahead of print. PMID: 34109765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixit N.M., Churchill A., Nsair A., Hsu J.J. Post-acute COVID-19 syndrome and the cardiovascular system: what is known? Am Heart J Plus. 2021;5 doi: 10.1016/j.ahjo.2021.100025. (Epub 2021 Jun 24. PMID: 34192289; PMCID: PMC8223036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang Y., Chen T., Mui D., Ferrari V., Jagasia D., Scherrer-Crosbie M., Chen Y., Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106(15):1132–1141. doi: 10.1136/heartjnl-2020-317056. (Epub 2020 Apr 30. PMID: 32354800; PMCID: PMC7211105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameli M., Pastore M.C., Soliman Aboumarie H., Mandoli G.E., D’Ascenzi F., Cameli P., Bigio E., Franchi F., Mondillo S., Valente S. Usefulness of echocardiography to detect cardiac involvement in COVID-19 patients. Echocardiography. 2020;37(8):1278–1286. doi: 10.1111/echo.14779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler Y., Charron P., Imazio M., Badano L., Barón-Esquivias G., Bogaert J., Brucato A., Gueret P., Klingel K., Lionis C., Maisch B., Mayosi B., Pavie A., Ristic A.D., Sabaté Tenas M., Seferovic P., Swedberg K., Tomkowski W., ESC Scientific Document Group 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. (Epub 2015 Aug 29. PMID: 26320112; PMCID: PMC7539677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalbandian A., Sehgal K., Gupta A. Post-acute COVID-19 syndrome. Nat. Med. 2021 doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korompoki E., Gavriatopoulou M., Hicklen R.S., Ntanasis-Stathopoulos I., Kastritis E., Fotiou D., Stamatelopoulos K., Terpos E., Kotanidou A., Hagberg C.A., Dimopoulos M.A., Kontoyiannis D.P. Epidemiology and organ specific sequelae of post-acute COVID19: A narrative review. J Infect. 2021;83(1):1–16. doi: 10.1016/j.jinf.2021.05.004. (Epub 2021 May 14. PMID: 33992686; PMCID: PMC8118709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. (Epub 2021 Jan 8. PMID: 33428867; PMCID: PMC7833295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhakal B.P., Sweitzer N.K., Indik J.H., Acharya D., William P. SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ. 2020;29(7):973–987. doi: 10.1016/j.hlc.2020.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carubbi F., Alunno A., Leone S., Di Gregorio N., Mancini B., Viscido A., Del Pinto R., Cicogna S., Grassi D., Ferri C. Pericarditis after SARS-CoV-2 infection: another pebble in the mosaic of long COVID? Viruses. 2021;13(10):1997. doi: 10.3390/v13101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021;23(12) doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixit N.M., Churchill A., Nsair A., Hsu J.J. Post-acute COVID-19 syndrome and the cardiovascular system: what is known? Am Heart J Plus. 2021;5 doi: 10.1016/j.ahjo.2021.100025. (Epub 2021 Jun 24. PMID: 34192289; PMCID: PMC8223036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiabrando J.G., Bonaventura A., Vecchié A., Wohlford G.F., Mauro A.G., Jordan J.H., Grizzard J.D., Montecucco F., Berrocal D.H., Brucato A., Imazio M., Abbate A. Management of Acute and Recurrent Pericarditis: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(1):76–92. doi: 10.1016/j.jacc.2019.11.021. (PMID: 31918837) [DOI] [PubMed] [Google Scholar]
- 17.Furqan M.M., Verma B.R., Cremer P.C., Imazio M., Klein A.L. Pericardial diseases in COVID19: a contemporary review. Curr. Cardiol. Rep. 2021;23(7):90. doi: 10.1007/s11886-021-01519-x. (PMID: 34081219; PMCID: PMC8173318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Arocutipa C., Saucedo-Chinchay J., Imazio M. Pericarditis in patients with COVID-19: a systematic review. J. Cardiovasc. Med. (Hagerstown) 2021;22(9):693–700. doi: 10.2459/JCM.0000000000001202. (PMID: 33927144) [DOI] [PubMed] [Google Scholar]
- 19.Soewono K.Y., Raney K.C., 3rd, Sidhu M.S. Pericarditis with pericardial effusion as a delayed complication of COVID-19. Proc (Bayl Univ Med Cent). 2021;34(5):629–630. doi: 10.1080/08998280.2021.1918975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khasnavis S., Habib M., Kaawar F., Lee S., Capo A., Atoot A. New perspectives on long COVID syndrome: the development of unusually delayed and recurring pericarditis after a primary SARS-CoV-2 infection. Cureus. 2022;14(6) doi: 10.7759/cureus.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingul C.B., Grimsmo J., Mecinaj A., Trebinjac D., Berger Nossen M., Andrup S., Grenne B., Dalen H., Einvik G., Stavem K., Follestad T., Josefsen T., Omland T., Jensen T. Cardiac dysfunction and arrhythmias 3 months after hospitalization for COVID-19. J Am Heart Assoc. 2022;11(3):e023473. doi: 10.1161/JAHA.121.023473. (Epub 2022 Jan 20. PMID: 35048715; PMCID: PMC9238505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisaccia G., Ricci F., Recce V., Serio A., Iannetti G., Chahal A.A., Ståhlberg M., Khanji M.Y., Fedorowski A., Gallina S. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8(11):156. doi: 10.3390/jcdd8110156. (PMID: 34821709; PMCID: PMC8621226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visco V., Vitale C., Rispoli A., Izzo C., Virtuoso N., Ferruzzi G.J., Santopietro M., Melfi A., Rusciano M.R., Maglio A., Di Pietro P., Carrizzo A., Galasso G., Vatrella A., Vecchione C., Ciccarelli M. Post-COVID-19 syndrome: involvement and interactions between respiratory, cardiovascular and nervous systems. J. Clin. Med. 2022;11(3):524. doi: 10.3390/jcm11030524. (PMID: 35159974; PMCID: PMC8836767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., Shchendrygina A., Escher F., Vasa-Nicotera M., Zeiher A.M., Vehreschild M., Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dini F.L., Franzoni F., Scarfò G., Pugliese N.R., Imazio M. Acute pericarditis in patients receiving coronavirus disease 2019 vaccines: a case series from the community. J. Cardiovasc. Med. (Hagerstown) 2022;23(8):551–558. doi: 10.2459/JCM.0000000000001342. (PMID: 35904995) [DOI] [PubMed] [Google Scholar]
- 26.Silva Andrade B., Siqueira S., de Assis Soares W.R., de Souza Rangel F., Santos N.O., Dos Santos Freitas A., Ribeiro da Silveira P., Tiwari S., Alzahrani K.J., Góes-Neto A., Azevedo V., Ghosh P., Barh D. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700. doi: 10.3390/v13040700. (PMID: 33919537; PMCID: PMC8072585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D., Pia L., Risson E., Saffern M., Salomé B., Esai Selvan M., Spindler M.P., Tan J., van der Heide V., Gregory J.K., Alexandropoulos K., Bhardwaj N., Brown B.D., Greenbaum B., Gümüş Z.H., Homann D., Horowitz A., Kamphorst A.O., Curotto de Lafaille M.A., Mehandru S., Merad M., Samstein R.M. Sinai immunology review project. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. (Epub 2020 May 6. PMID: 32505227; PMCID: PMC7200337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokolowska M., Lukasik Z.M., Agache I., Akdis C.A., Akdis D., Akdis M., Barcik W., Brough H.A., Eiwegger T., Eljaszewicz A., Eyerich S., Feleszko W., Gomez-Casado C., Hoffmann-Sommergruber K., Janda J., Jiménez-Saiz R., Jutel M., Knol E.F., Kortekaas Krohn I., Kothari A., Makowska J., Moniuszko M., Morita H., O’Mahony L., Nadeau K., Ozdemir C., Pali-Schöll I., Palomares O., Papaleo F., Prunicki M., Schmidt-Weber C.B., Sediva A., Schwarze J., Shamji M.H., Tramper-Stranders G.A., van de Veen W., Untersmayr E. Immunology of COVID-19: mechanisms, clinical outcome, diagnostics, and perspectives-a report of the European academy of allergy and clinical immunology (EAACI) Allergy. 2020;75(10):2445–2476. doi: 10.1111/all.14462. (PMID: 32584441; PMCID: PMC7361752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raman B., Bluemke D.A., Lüscher T.F., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022;43(11):1157–1172. doi: 10.1093/eurheartj/ehac031. (PMID: 35176758; PMCID: PMC8903393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuvali O., Tshori S., Derazne E., Hannuna R.R., Afek A., Haberman D., Sella G., George J. The incidence of myocarditis and pericarditis in post COVID-19 unvaccinated patients-a large population-based study. J. Clin. Med. 2022;11(8):2219. doi: 10.3390/jcm11082219. (PMID: 35456309; PMCID: PMC9025013) [DOI] [PMC free article] [PubMed] [Google Scholar]