To the Editor:
The influence of routinely used chronic medications during Coronavirus Disease 2019 (COVID-19) is often controversial. This is the case of the role of proton pump inhibitors (PPI) in influencing susceptibility to severe COVID-19 and risk of fatal outcome 1, 2. Here we investigated whether the measurement of chromogranin A (CgA) plasma levels might influence the clinical outcome and unveil PPI impact on disease outcome.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused a highly contagious disease, which was responsible for more than 6 million deaths worldwide since 2019. A titanic effort has been made to identify the best therapeutic approach to treat COVID-19 patients. Over the past two years, scientists identified several risk factors and biochemical indicators to predict clinical outcome or mortality in acute COVID-19 [3]. Age, sex and pre-existing comorbidities (i.e. arterial hypertension, chronic kidney disease, diabetes mellitus, cardiovascular disease, active neoplasia, chronic obstructive pulmonary disease, etc.) increased morbidity and mortality. Risk factors include the medication commonly used before the infection.
PPI are widely used to treat diseases of the upper digestive apparatus such as gastroesophageal reflux disease or peptic ulcer. They are well tolerated and often used long-term and at high doses. It has been suggested that PPI users have higher risk of acquiring SARS-CoV-2 infection and/or experiencing severe outcomes in COVID-19 disease [1], but no clear evidence has been obtained yet [2]. The frequent co-morbidities (which themselves influence the outcome of COVID-19) among PPI users might act as confounder, hampering the generalizability and reproducibility of results [4].
It is well known that PPI treatment increases CgA secretion, at least in a subpopulation of patients [5]. CgA and its fragments, secreted mainly by endocrine/neuroendocrine cells, regulate vascular homeostasis, cardiac function and immune responses [6]. We have recently demonstrated that CgA levels increased in the plasma of patients with COVID-19 at the time of admission at the Emergency Department, in particular in those who died from the disease. CgA plasma levels emerged as early independent predictors of mortality [6], pointing to a role of the early generation of CgA in the response against SARS-CoV-2.
In the present study, we compared plasma CgA levels in PPI users and non-users and investigated whether CgA differentially predicts mortality depending on PPI status and plasma CgA may predict fatal outcome in patients on PPI treatment. The study was approved by the Hospital Ethics Committee (protocol no. 34/int/2020) and registered on ClinicalTrials.gov (NCT04318366).
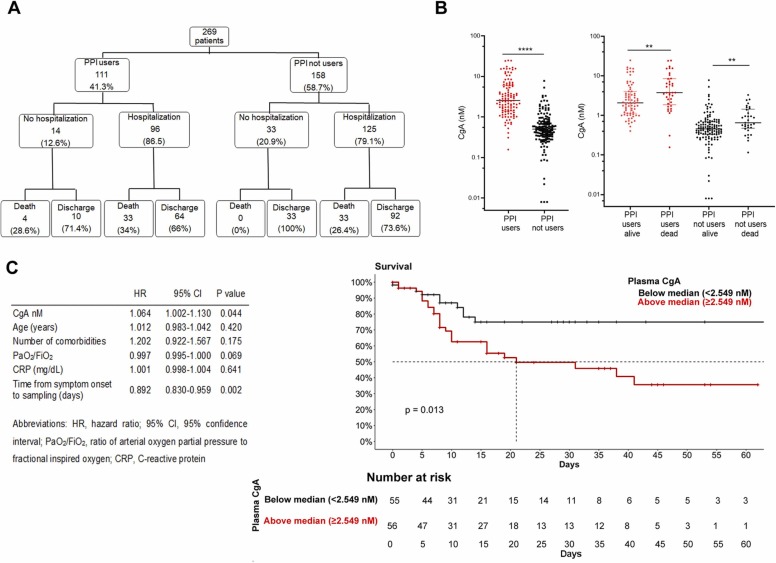
We analyzed plasma level of CgA in 269 patients admitted at the Emergency Department of San Raffaele University Hospital, Milan, from March 18, 2020 to May 5, 2020. Among them, 111 were receiving chronic PPI therapy at admission. Inclusion criteria were: age ≥ 18 years, positive nasopharyngeal swab for SARS-CoV-2 (confirmed by real-time reverse-transcriptase polymerase chain reaction), and clinical or radiological signs or symptoms of COVID-19. Patients were followed up until death or hospital discharge. Patient and disease characteristics were prospectively collected following blood withdrawal, and reported in Table 1 (supporting information). Outcomes of PPI users and non-users are depicted in Fig. 1, panel A.
Fig. 1.
(A) Clinical outcomes of the cohort. The flowchart shows the number of patients and the percentage of the original population. (B) CgA levels in PPI users and non-users (left) and in alive or dead patients (right); Data are in log scale. * ** *= p < 0.0001, * * =p < 0.01. (C) Multivariate Cox regression analysis (left) and Kaplan-Meier survival curves (right) in PPI users (Log rank test, p = 0.013).
No significant difference was found between PPI users and non-users in the hospitalization rate (87.4% vs. 79.1% respectively, p = 0.11) or length of hospital stay (median [interquartile range, IQR] 10.5 [5–28] days vs. 12 [4–22] days, p = 0.96). PPI users more frequently had at least one comorbidity (84.6% in PPI users vs. 46% in non-users, p < 0.0001) and were older (median [IQR] age 70 [61.51–77.4] years in PPI users vs. 60 [49.2–71.2] years in non-users, p < 0.0001). The proportion of PPI users who died was significantly higher than in non-users (Fig. 1 and in Table 1, supporting information).
Plasma CgA levels were measured in blood specimens collected at hospital admission. As expected, we found that CgA levels were higher in PPI users than in non-users (2.5 [1.4–5.9] nM vs. 0.9 [0.4–2.4], respectively), although some patients on PPI therapy showed plasma CgA levels similar to patients not using PPI (Fig. 1, B left panel). In both PPI users and non-users, CgA levels were significantly higher in patients that did not survive (Fig. 1, B right panel).
It is known that CgA plasma levels at the admission correlated with age, degree of hypoxia and plasma creatine phosphokinase [6]. CgA plasma levels correlated with age in both PPI users and not users (R =0.396 and p < 0.0001, R= 0.446 and p value <0.0001, respectively, supporting information, Fig. 1). Elderly is often link to multidrug therapy and PPI are usually prescribed. Multivariate Cox regression analyses performed specifically in PPI users (thus excluding non-users) revealed that CgA plasma levels still predicted mortality when adjusting for age, number of comorbidities, degree of respiratory dysfunction (PaO2/FiO2), systemic inflammation as reflected by C-reactive protein (CRP), levels at admission and time from symptom onset to sampling (Fig. 1, panel C). Kaplan Meier survival analysis confirmed that among PPI users, those with levels of CgA above the median value of 2.49 nM at admission had a higher risk of mortality compared to those with plasma levels of CgA below the median (log rank test, p = 0.013, Fig. 1, panel C). This result demonstrates that, independent of PPI use, CgA plasma levels predicted fatal outcome.
In addition to the frequent pre-infection use of PPI in the general population, COVID-19 patients may specifically require PPIs in certain conditions. Administration of high doses of non-steroidal anti-inflammatory drugs or corticosteroids to control fever and inflammation could damage the gastro-intestinal tract. Moreover, the use of low molecular weight heparin to control thromboembolic complications of COVID-19 might increase the risk of bleeding in pre-existing mucosal lesions. These conditions frequently prompt physicians to add PPIs to therapy for COVID-19. The cellular source of circulating CgA remains to be defined. Secretion of CgA could be increased in endocrine/neuroendocrine cells by stress-induced activation of sympathetic-adrenal-medullary system during the COVID-19 disease. Dispersed neuroendocrine cells that secrete CgA are also physiologically present in the bronchopulmonary tract. Hyperplasia of these cells could be induced in COVID-19-patients due to hypoxia status similarly to chronic lung diseases such as interstitial pneumonia and obstructive lung disease [7]. CgA is also expressed by myocardial and immune cells [6] and could be upregulated and secreted in patients due to the spread inflammatory state contributing to increase of plasma levels. Although the pathogenic role of CgA in COVID-19 have not yet been elucidated, we provide evidence that CgA plasma levels are a key biomarker of the risk of COVID-19 progression and that this effect is not influenced by PPI therapy. Therefore, measuring CgA plasma levels in PPI users (and non-users) at hospital admission might be clinically relevant to predict the risk of adverse outcome.
Declaration of Competing Interest
All authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
Acknowledgement
This work was supported by a COVID-19 program project grant from the IRCCS San Raffaele Hospital and the grant COVID-2020–12371617 from the Italian Ministero della Salute.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.phrs.2022.106601.
Appendix A. Supplementary material
Supplementary material
.
Data availability
Data will be made available on request.
References
- 1.Lee S.W., et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70(1):76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 2.Israelsen S.B., et al. Proton pump inhibitor use is not strongly associated with SARS-CoV-2 related outcomes: a nationwide study and meta-analysis. Clin. Gastroenterol. Hepatol.: Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021;19(9):1845–1854. doi: 10.1016/j.cgh.2021.05.011. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su M., Xu S., Weng J. A bibliometric study of COVID-19 research in Web of Science. Pharmacol. Res. 2021;169 doi: 10.1016/j.phrs.2021.105664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreotti F., et al. Methodological education in response to the quality of COVID-19 publications. Pharmacol. Res. 2021;164 doi: 10.1016/j.phrs.2020.105381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosli H.H., et al. Effect of short-term proton pump inhibitor treatment and its discontinuation on chromogranin A in healthy subjects. J. Clin. Endocrinol. Metab. 2012;97(9):E1731–E1735. doi: 10.1210/jc.2012-1548. [DOI] [PubMed] [Google Scholar]
- 6.De Lorenzo R., et al. Chromogranin A plasma levels predict mortality in COVID-19. PloS One. 2022;17(4) doi: 10.1371/journal.pone.0267235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki Y., et al. A case of multiple lung carcinoid tumors localized in the right lower lobe. Respir. Med. Case Rep. 2022;38 doi: 10.1016/j.rmcr.2022.101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.