Abstract
Mucormycosis is a fungal infection which got worsens with time if not diagnosed and treated. The current COVID-19 pandemic has association with fungal infection specifically with mucormycosis. Already immunocompromised patients are easy target for COVID-19 and mucormycosis as well. COVID-19 infection imparts in weak immune system so chances of infection is comparatively high in COVID-19 patients. Furthermore, diabetes, corticosteroid medicines, and a weakened immune system are the most prevalent risk factors for this infection as we discussed in case studies here. The steroid therapy for COVID-19 patients sometimes have negative impact on the patient health and this state encounters many infections including mucormycosis. There are treatments available but less promising and less effective. So, researchers are focusing on the promising agents against mucormycosis. It is reported that early treatment with liposomal amphotericin B (AmB), manogepix, echinocandins isavuconazole, posacanazole and other promising therapeutic agents have overcome the burden of mucormycosis. Lipid formulations of AmB have become the standard treatment for mucormycosis due to their greater safety and efficacy. In this review article, we have discussed case studies with the infection of mucormycosis in COVID-19 patients. Furthermore, we focused on anti-mucormycosis agents with mechanism of action of various therapeutics, including coverage of new antifungal agents being investigated as part of the urgent global response to control and combat this lethal infection, especially those with established risk factors.
Keywords: Mucormycosis, COVID-19, Amphotericin B (AmB), Azoles, Echinocandins, Statins, GPI biosynthetic Pathways, Antifungal agents
Graphical abstract
Abbreviations
- ARDS
Acute respiratory distress syndrome
- AIDS
Acquired immune deficiency syndrome
- HIV
Human immunodeficiency virus
- RCOM
Rhino-Orbito-Cerebral Mucormycosis
- ICU
Intensive care unit
- EGD
Esophagogastroduodenoscopy
- CT
Computed Tomography
- MRI
Magnetic resonance imaging
- qPCR
quantitative multiplex polymerase chain reaction
- LDM
Lanosterol 14α‐demethylase, AmB, AmB
- GS
β-(1,3)-D-glucan synthase, DKA, Diabetic ketoacidotic
- LAmB
Liposomal Amphotericin-B
- RHPOR
Rhizopus orizae
- GPIs
Glycosylphosphatidylinositols
- Gwt1
GPI-anchored wall protein transfer 1
- MGX
Manogepix
- HMG-CoA
3-hydroxy-3-methylglutaryl-CoA
- ROS
Reactive Oxygen species
- MIC
Minimum inhibitory concentration
- FDA
Food and drug administration
1. Introduction
In the last 20 years or more, an increase in the incidence of fungal infections has been noted, along with a substantial increase in the population of severely immunocompromised people. These infections are primarily caused by viral infections, particularly the human immunodeficiency virus epidemic [1] and currently SARS Co–V, haematological disorders such as various types of leukaemia, organ transplants, and more intensive and aggressive medical practises. Fungal infections can be caused by surgery, catheter use, injections, radiation, chemotherapy, antibiotics, and steroids resulting in an increase in the incidence of infection. Fungus-induced respiratory tract infections cause 4.3 million fatalities each year. The exact incidence of these fungal infections of the respiratory system is unknown because they are largely ignored. Despite therapy, the majority of invasive fungal infections have significant mortality rates of more than 50%. In general, fungal infections of the respiratory system are regarded synonymous with Aspergillus spp.-caused invasive pulmonary infections [2]. Recently the whole world is facing the pandemic condition induced by SARS-CoV-2 which cause a respiratory life-threatening infection. Recently, there are some associated complications which have been reported in COVID-19 patients according to the clinical diagnosis. These associated infections are acute respiratory failure, acute respiratory distress syndrome (ARDS), and cardiac injury. Acute liver injury, pneumonia, acute kidney injury, neurological disorders, secondary infection, and multisystem inflammatory syndrome in children, disseminated intravascular coagulation, septic shock, rhabdomyolysis and chronic fatigue are also associated complications but, the most common complications are ARDS and acute respiratory failure [3]. Being at low immunity level, COVID-19 patients are easy target for the fungal infection as host with weak immune system is best option for opportunistic microbial infections. Therefore, clinicians are worried for them and researchers are also focusing their research on the therapeutic strategies against fungal infections. Viral-bacterial and viral fungal co-infections are among the most prominent medical concerns in the current scenario, resulting in an increased mortality rate [4,5].
A major infected population with COVID-19 suffered from the fungal infection mucormycosis, often known as “black fungus,” as part of the chain of co-infections linked with COVID-19. Black molds are a diverse collection of darkly coloured (dematiaceous) fungus that are extensively spread in the environment and can infect people. Mycetomas, chromoblastomycosis, sinusitis, and superficial, cutaneous, subcutaneous, and systemic phaeohyphomycosis are all part of the infection's clinical spectrum. Apart with COVID-19 problems, these black fungus infections are easily transmitted to immunocompromised people. A case study from the San Francisco Bay area is highlighted here where the annual incidence of infection caused by black molds was one case per million [6]. Black molds were found to be responsible for two out of every 20 invasive mould infections in organ transplant recipients in a recent prospective multicenter investigation [7]. There have been recent reports of nosocomial acquisition of black fungi, including a case of meningitis caused by Bipolaris spicifera following auditory neuroma surgery. These infections are fairly common these days, but the survival rate is very low. However, there are various unique therapeutic tactics or treatments available to tackle this deadly disease. Combination therapy with liposuction is one of these therapies.
Combination therapy using lipid-based AmB and caspofungin, or clinical azoles such as itraconazole or posaconazole, or a combination of all three therapies are among the options. When compared to available alternatives for treating microbial and other fungal infections, the number of therapy solutions for this condition is quite modest. Polyenes and azole-based compounds are currently being used in clinical trials to treat this fungal infection [8].
Despite a lack of clear clinical evidence, treating this fungal infection in highly immunocompromised patients with a combination of antifungal medications is now standard clinical practice all over the world. Synergistic effects and a broader therapy range are advantages of these treatments, but possible antagonism, toxicity, and medication interactions are negatives. The first-line treatment for this life-threatening fungal infection is liposomal AmB. Due to residuals concerns, isavuconazole and new posaconazole formulations have been recommended for clinical use, but only as a second-line treatment after liposomal AmB [9].
2. Black fungus: brief taxonomy
The Zygomycota are a subclass of lower fungi with nonseptate thalli (coenocytic). After isogamic sex organs (gametangia) fuse, a single black, thick-walled, often decorated sexual spore called zygospore is produced. Their wide, aseptate, hyaline, randomly branching hyphal components can be identified in host tissue. There are two classes and 11 orders of Zygomycota. Only two of them have clinically relevant fungi: the Entomophthorales, which have violently ejected spores, and the mucorales, whose spores arise from sporangial plasma cleavage and are passively freed. The Mucorales family is the most clinically significant. Its members are found in food, soil, and the air and are extensively spread. Dolatabadi and Guarro have named and depicted eleven genera containing 22 species of medical relevance. Rhizopus and Absidia are the most common and important mucoralean genera in clinical laboratories [10,11]. Mucormycosis is a rare, invasive, fungal opportunist infection that sometimes responsible for fatal disease caused by a group of molds that belongs to the fungal family, “Mucorales”. This fungus is ubiquitous and naturally found in soil, plants, manures, decaying fruits, and vegetables [12].
3. Epidemiology and pathogenesis of black fungus
About 150 fungal species out of about 1.5 million have been linked to human diseases, and only a dozen or so are commonly encountered in clinical settings. In addition to the scarcity of dangerous fungal species, life-threatening fungal diseases are rare in immunologically healthy human populations when compared to other infectious diseases. The lack of human sickness contrasts with a high prevalence of infection among humans who live in areas where fungi are present. As a result, just a few fungus species are pathogenic, and those that do cause disease are uncommon causes of life-threatening illness [13]. The perception that fungi are less likely to be employed as biological weapons may have been influenced by these epidemiological facts. Depending on whether the organism is obtained from a host or the environment, fungal illnesses can be categorised into two types. Candida spp., Malassessia furfur, and Dermatophytes are examples of pathogenic fungi acquired from the host [14]. These organisms are commonly found in the flora of the host and only cause disease when the host-microbe interaction is disrupted. For example Human candidiasis is linked to a damaged integument, the use of antibacterial drugs, corticosteroid use, and immunological suppression. Fungi obtained from other hosts, such as Candida spp, are generally low pathogenicity organisms that rarely cause disease until the host-microbe interaction is disrupted.
Aspergillus spp, the dimorphic fungus, and Cryptococcus neoformans are examples of pathogenic fungi that can be acquired through the environment. Humans who are exposed to these organisms are frequently infected, although sickness is uncommon unless the host's immunity is compromised [15]. For example, whereas the prevalence of cryptococcosis in the general population is less than one case per 100,000, it was as high as 10% in Acquired immune deficiency syndrome (AIDS) patients prior to the development of effective antiretroviral medication. However, with the introduction of antiretroviral medication, the frequency of cryptococcosis in HIV patients was drastically reduced in areas with access to therapy, demonstrating the disease's reliance on the host population's immunological condition. Initial infection with environmentally acquired fungi is mainly acquired through inhalation, and the result is either asymptomatic or minor disease, while many of these organisms are capable of staying in the host in a dormant form. Even with Coccidioides spp., the vast majority of first infections result in mild or no illness [16]. When fungi from the environment infect the host, the sickness is severe, difficult to treat, and often fatal. None of the fungi obtained in the environment are transmissible from host to host, and disease clusters are mainly the result of unique exposures. For example, pulmonary histoplasmosis outbreaks in apparently immunologically normal people have occurred after tree-cutting operations and cave trips. The lack of communicability of environmentally acquired pathogenic fungus limits their weapon potential for indiscriminate deployment, but it also makes them appealing because they are unlikely to harm non-exposed friendly forces [17].
4. Mode of transmission and associated complications
The fungal spores commonly enter through inhalation and affect the sinus and lungs. They can also penetrate through wounds or an open cut and thus infect the skin. Patient with severe COVID-19, such as those in Intensive care unit (ICU) needs more attention and hygiene because they are particularly vulnerable to bacterial and fungal infection. Apart from black fungus, other fungal infections have already been reported within the past few months all over the globe. The most common fungal infection reported in COVID-19 patients other than black fungus is aspergillosis or invasive candidiasis [18]. All these fungal infections with co-infections are reported more frequently and may cause serious illness and death. Awareness of the possibility of fungal and bacterial co-infection is essential for timely diagnosis, treatment ultimately helpful to prevent serious illness and death caused by these infections [19,20] including COVID-19. According to some health specialists, this infection may be because of steroid medications used while treatment of COVID-19 patients to reduce the inflammation in the lungs for patients but unfortunately resulted into and push up sugar levels in both diabetic and non-diabetic patients in SARS-CoV-2 [21] hence the immunity of the patient has already been compromised. Like, “Diabetes lowers the body immune system, coronavirus exacerbates it, and steroids that used for COVID-19 treatments act like fuel to fire, also drops in the immunity may trigger this black fungus infection very fast. More information about the sudden outbreak of black fungus, symptoms, and other complications with COVID-19 is explained in coming section.
5. Recent complications of black fungus with COVID-19 patients
Coronavirus has been associated with a wide range of opportunistic bacterial and fungal infections [22]. Aspergillosis or invasive candidiasis has been reported as primary fungal pathogens for causing disease in COVID-19 patients [23]. After December 2019 outbreak in China, various modifications in terms of pathophysiology, diagnosis, and complications may be correlated with a wide range of disease forms. In COVID-19, the disease manifesting as rhino, orbital, cerebral mucormycosis. Several such cases have been reported during COVID-19 illness [24]. Patients can include those with iatrogenic immunosuppression, haematological malignancies, diabetes mellitus, acquired immunodeficiency syndrome. In the COVID-19 condition, hypoxic conditions occurred due to endothelial barrier disruption and impaired oxygen diffusion capacity [25]. Profound lymphopenia with a decreased number of T lymphocytes (CD4+T and CD8+T cells) may amend the immune response of COVID-19 patients enhancing the risk of invasive fungal infections. Symptoms of COVID-19 and fungal infections are quite similar.
Some of them are listed here: fever, pain, headache, redness and periocular swelling, drooping of eyelids, limitation of ocular movements, and painful loss of vision [26]. The progression is usually quick, taking only two days on average from the outset. Edema of the eyelids and periocular region, complete ptosis, total ophthalmoplegia proptosis, and relative afferent pupillary defect, unilateral facial or orbital pain, double vision or loss of vision, the sign of exposure keratitis, chemosis, sinusitis, nasal discharge, and neurological signs and symptoms. The clinical profile of coronavirus patients suffering from this life-threatening fungal infection differs depending upon the severity of the disease.
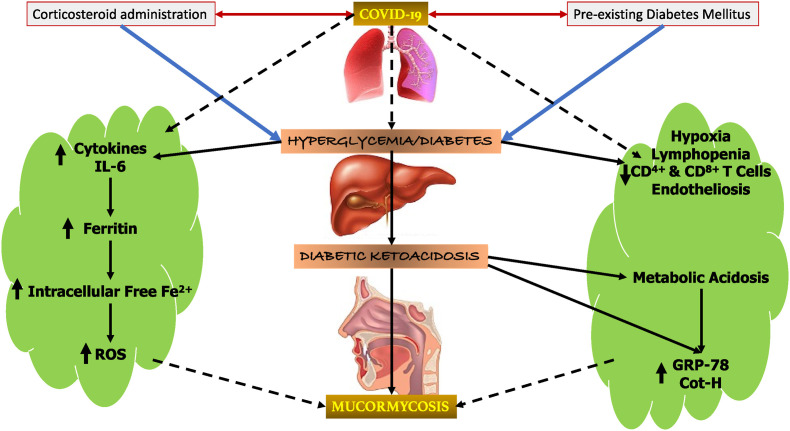
The diagrammatic representation is shown in Fig. 1 to depict the connection of immunological and physiological complications along with improper glucose metabolism which possibly provide a conducive environment for mucormycosis initiation and progression.
Fig. 1.
COVID-19 induced immunological and physiological complications along with improper glucose metabolism may provide a conducive environment for mucormycosis initiation and progression. The aggravation of COVID-19 and hyperglycemic conditions due to steroid administration in a diabetic individual add further fuels the mucormycosis development.
6. Clinical profile and cases of mucormycosis infection in corona patients
In this section, we will discuss the clinical profile of mucormycosis and case studies conducted in different regions of the world. In Rhino-Orbito-Cerebral Mucormycosis (RCOM), fungus mucormycosis affects the nose, eyes, and brain. This fungal infection starts from the nose, where it rapidly spreads and infects the bone cavity, which surrounds the eye and brain. In starting stage, the patient experiences nasal discharge. It can be a bloody, nasal blockage, or pain inside the nose. After that, patient experiences numbness or swelling on face and develop facial pain [27]. As in the second stage, progression reaches the orbit, and the patient experiences headache, orbital pain, periocular edema, eyelid dropping, vision loss, and double vision with pain. The passage is usually rapid, an average of two days from onset. Furthermore, in the last stage, dysfunction in jaw movement occurs. Tooth of upper jaw starts loosening, chilling, burning or numbness occurs in the skin. Black eschar develops near the eyes or nose. Pulmonary mucormycosis generally occurs in patients with immunocompromised conditions. This infection affects the lungs and respiratory system. Fever, breathlessness, cough, chest pain, some experienced cough with blood are known as haemoptysis. As the infection progresses, it worsens, and the patient and pleural effusion was observed as well, in which a fluid build-up occurs between the tissues that line the lungs and chest [28].
A retrospective study was done in the Department of Infectious Disease in Manipal Hospital, Bangalore, India, from August to December 2020.
10 patients with a mean age of 55.8 years were out there with mucormycosis associated with COVID-19. Eight patients had diabetes, complaining about severe eye pain, nasal blockage, and facial pain; out of 10 patients, six received steroids. One patient had received tocilizumab for the treatment of COVID-19. Nine patients were suffering from diabetes, hypertension, and chronic kidney disease. Only one patient was suffering from severe COVID-19 infection while the remaining patients were suffering from mild and moderate disease. They treated all the patients with local debridement of the infected and necrotic tissue and AmB with COVID-19 treatment [29].
Another highlight study was done at Sawai Man Singh Medical Hospital, Jaipur, India, from August to December 2020, and a total l of 23 patients were there. Fifteen were male, and eight were female. During treatment, out of 23 patients, 4 were still COVID-19 positive at that time, and 19 had been recovered. Twenty-one of the patients were diabetic, 12 of them have uncontrolled blood sugar levels, nine patients had controlled diabetes, 14 patients suffering from hypertension, and one patient suffers from renal failure. All 23 patients used steroids for the treatment of COVID-19. Now, all of them suffering from invasive mucormycosis, all of them operated while keeping in mind complete surgical debridement and amphotericin administered intravenously [30].
Another study included 66-year-old male patient who was hospitalised to the University Hospital in Sassari, Italy on March 26, 2020 suffered with COVID infection. For the first ten days, patients were given hydroxychloroquine and lopinavir-ritonavir. Respiratory parameters steadily worsened following admission to the ICU: reduced oxygenation, increased radiological infltrates, and the left lower lobe parenchymal thickening was observed. For prolonged mechanical ventilation, a procedure was conducted at the patient's bedside. Aseptate broad hyphae, sporangia carrying sporangiospores, and aseptate broad hyphae were seen on microscopic examination using lactophenol cotton blue preparation. The mould was identified as Rhizopus spp. based on phenotypic characteristics. The treatment with liposomal AmB, 5 mg/kg intravenously (IV), was started according to the instructions and after consultation with an infectious disease physician. After 40 days in the ICU, the antifungal treatment was changed to isavuconazole, and the liposomal AmB treatment was discontinued. Due to refractory shock and hepatic failure, the patient died on day 62 following ICU admission [31].
A 86-year-old man with a history of arterial hypertension was admitted to a Brazilian emergency room with acute diarrhoea, cough, dyspnea, and fever that started five days before to admission. Due to abrupt respiratory failure and hemodynamic instability, he was admitted to the critical care unit (ICU). In the intensive care unit, the patient was given ceftriaxone, azithromycin, oseltamivir, and hydrocortisone, as well as vasopressors and mechanical ventilation. He was treated with three units of red blood cells and omeprazole. Esophagogastroduodenoscopy (EGD) revealed two huge stomach ulcers with dirty debris and a deep hemorrhagic base without active bleeding in the greater and lesser curvature. A pathology investigation confirmed the presence of mucormycosis [32]. Also, another case study has done on 32 years old lady with uncontrolled diabetes at the Department of Otorhinolaryngology and Head and Neck Surgery, KS Hegde Medical Academy, NITTE University, Mangalore, Karnataka. She was presented with left facial pain and left eye complete ptosis. Her CT scan of the nose and paranasal sinus showed total opacification of the left ethmoid, and maxillary and frontal sinus suggest fungal sinusitis. Doctor asked for immediate endoscopic surgery; a COVID-19 test was done, which came positive; after surgery AmB (25 mg/day) dose had been administered. The patient followed up was done for two months. There was a reduction in facial pain but no improvement in vision [33].
7. Reasons for mucormycosis in COVID-19 patients
There are several reasons reported for the severe black fungal infection. Many research studies have proven that steroids help to reduce mortality in COVID-19 patients with low oxygen saturation levels and reduce the ability to fight against other infections. COVID-19 patients who were already immunocompromised having diabetes, chronic kidney disease, or chronic liver disease were taking steroids regularly for a prolonged duration, and that too not in defined dose. They put a very high risk of mucormycosis because fungus mucormycosis causes illness in people who are immunocompromised and have high sugar levels. Therefore, this surge can be attributed to the improper use of steroids to treat patients with COVID-19 and poor management of diabetes. Steroids increase blood sugar levels, causing the blood to become acidic [34]. This fungus thrives in high blood glucose and acidic environment. This fungus has the potential to cause damage under unsanitary conditions. But steroids are not only villains in this fungal infection; they are one of the reasons behind this infection [35]. According to Dr. Hegde, “The virus is pathogenic, causing blood sugar levels to skyrocket to dangerously high levels. Surprisingly, the fungus is harming a large number of young people.” [36].
The pathophysiology of mucormycosis has also been correlated with mononuclear and polymorphonuclear phagocytes of normal hosts killing. Mucorales by producing oxidative metabolites and defensins, making neutropenic individuals and those with defective phagocytes susceptible to invasive mucormycosis. There is severe lymphopenia in COVID-19, and viral replication exacerbates the inflammatory response and neutrophil and monocyte influx in the bloodstream in advanced infections [29]. As a result of this imbalance in neutrophil and lymphocyte activity, the patient is more susceptible to systemic fungal infections. When we talk about other factors then moisture in the environment and oxygen cylinder can be a significant source of mucormycosis infection because the oxygen cylinder used might be outdated. There are other likely causes for the fungal infection [37]; It may be improper use of oxygen cylinders, unclean masks, or no pure water in the equipment, which has become the norm in most cities that deal with the shortage of hospital beds. Above mentioned reasons are possible key responsible factors to cause mucormycosis in COVID patients. ‘
8. Diagnosis of mucormycosis
Mucormycosis is difficult to diagnose early stage and is associated with a high death rate, particularly in immunocompromised patients [38]. Because underlying co-morbid diseases and clinical presentation are often similar, distinguishing this disorder from invasive aspergillosis is critical, as antifungal treatment may differ. A comprehensive history, physical examination, and imaging are all required to identify probable mucormycosis. A common finding on a cranial CT in diabetes patients is bone destruction. A cranial Magnetic resonance imaging (MRI) is recommended for added sensitivity because the results will reveal any involvement of the brain, sinuses, or orbit. The staging will be indicated on imaging in terms of sinus and brain involvement. A biopsy should be planned and sent for direct microscopy, culture in standard media at 30/37° Celsius. After that, susceptibility testing can be requested for further process [39]. In most rural regions, mucormycosis diagnostic techniques are based on insufficient fundamental microbiology, which has resulted in diagnosis delays. Unlike invasive aspergillosis, mucormycosis diagnosis is not aided by the identification of circulating antigens such as galactomannan and D-1, 3-glucan. As a result, in addition to culture and clinical features, samples from the anatomical site of infection are frequently required for the diagnosis of mucormycosis.
Mucormycosis can now be diagnosed non-invasively with the help of the latest molecular biology tools [40]. Million et al. developed a Mucor/Rhizopus, Lichtheimia, and Rhizomucorxvi 18S rRNA-based quantitative multiplex polymerase chain reaction (qPCR) targeting Mucor/Rhizopus, Lichtheimia, and Rhizomucorxvi. The goal of the PCR assay is to detect Mucorales DNA in the bloodstream (serum) [41]. A study looked at the use of Mucorales-targeting real-time PCR on tissue and respiratory samples in patients with haematological malignancies who had proven or suspected mucormycosis. As a result, the value of reverse halo sign on computed tomography scan combined with serum qPCR targeting Mucorales for the early detection of pulmonary mucormycosis is now required in patients with COVID-19 infections.
9. Antifungal agents against mucormycosis
Researchers are working towards the promising therapeutic invention against mucormycosis. Combinations of echinocandin and lipid polyenes enhanced survival rates in mice with disseminated mucormycosis [42]. AmB is the most widely used drug for the treatment of mucormycosis. Because of the increased risk of nephrotoxicity when using AmB, it is important to pay close attention to kidney function. If the condition is severe, second-line drugs can be considered. Combination therapy of echinocandin and AmB is recommended as a second-line treatment. When echinocandin is coupled with AmB, a polyene skeleton is added, thereby increasing the success rate of treatment. Triazoles, posaconazole, and isoconazole are some other recognized second-line antifungals. Triazoles block 14-demethylation, leading to an increase in the harmful 14-methyl sterol and changing the permeability of fungal membranes. Posaconazole is used for patients who are intolerant to AmB. Isaconazole has a broad-spectrum effect. Therefore, it is the only antifungal drug that can be used to treat invasive mucormycosis [39]. The antifungal treatment should be continued until clinical signs and symptoms are resolved, radiological signals are resolved or stabilized, and underlying immunosuppression is resolved. The ideal dosage for antifungal treatment of mucormycosis is unknown and is mostly determined by the patient's health and laboratory results. Moreover mucormycosis, whether suspected or diagnosed, necessitates consultation with the surgical team. To prevent the spread of the fungal infection, clean margins are essential during surgical debridement. This is used as emergency therapy. Histopathology and microbiological diagnostics can be performed on biopsies taken during surgery. From the above techniques and therapeutic strategies, the fear of such fungal infections can be diminished up to a significant point. Important antifungal drugs for treatment of mucormycosis and their possible mode of actions are presented in Table 1 .
Table 1.
Important antifungal drugs for treatment of Mucormycosis and their possible mode of actions.
| S.No | Antifungal Drug | Target* | Mode of action | References |
|---|---|---|---|---|
| 1 | AmB | Ergosterol in the fungal cytoplasmic membrane | Ion channel formation Ergosterol sequestration Induction of reactive oxygen species |
[43] [44] |
| 2 | Itraconazole | lanosterol 14-α-demethylase | Inhibits the biosynthesis of ergosterol | [45,46] |
| 3 | Posaconazole | lanosterol 14-α-demethylase | Inhibits the biosynthesis of ergosterol | [45,46] |
| 4 | Isavuconazole | lanosterol 14-α-demethylase | Inhibits the biosynthesis of ergosterol | [45] [46] |
| 5 | Echinocandins | β-(1,3)-D-glucan synthase | Inhibition of biosynthesis of β-(1,3)-D-glucan | [47] [48] |
| 6 | Oteseconazole (VT-1161) | lanosterol 14α‐demethylase | Inhibits the synthesis of ergosterol | [49] [50] |
| 7 | Terbinafine | Squalene epoxidase | Inhibits the synthesis of ergosterol | [51] |
| 8 | Rapamycin | FKBP12 |
|
[52] |
| 9 | Tacrolimus (FK506) | FKBP12 | FKBP12-dependant inhibition of PP2B | [53] |
| 6 | Manogepix and prodrug fosmanogepix | Inositol acyltransferases (Gwt1) | Prevents the maturation of GPI-anchored proteins | [54] |
| 7 | Jawsamycin | Catalytic subunit Spt14/Gpi3 of the fungal UDP-glycosyltransferase | Inhibits the biosynthesis of GPI-anchored proteins | [55] |
| 8 | Fluvastatin and other statin | Hydroxymethylglutaryl-CoA (HMGCoA) reductase enzyme | Inhibits the biosynthetic pathway of sterols | [56] |
| 9 | Anti-CotH3 antibodies | 16-mer peptide region of the CotH3 of Mucorales | Bind to glucose-regulated protein 78 (GRP78) on endothelial cells. | [57] |
9.1. Polyenes
Amphotericin B (1, AmB) is most widely used mycosamine polyene macrolide in antifungal therapy. Mechanistically this polyene binds to ergosterol (2) and forms ion channels in the fungal cell membrane this may cause the fungal cell death due to loss of membrane integrity and leakage of vital cytoplasmic components through these channels. According to a study by Burke et al., mycosamine sugar plays a central role to promote direct binding interaction between AmB and hydroxyl group of ergosterol for the formation of ion channels (Fig. 2 ). AmB also binds to the surface of plasma membrane and physically extracts ergosterol (ergosterol sequestration), which leads to depletion of vital constituent of membrane and ultimately dysfunction of cell membrane. AmB may induce reactive oxygen species (ROS) which may cause protein damage, lipid damage and mitochondrial dysfunction (Fig. 3 ) [43,44,58,59]. Various formulations of AmB have allowed its successful use as a fungicide against mucormycosis, and multidrug-resistant. These formulations have demonstrated remarkable and broad-spectrum activity against various fungal species that cause mucormycosis.
Fig. 2.
Structure of Amphotericin B (AmB): Mycosamine sugar of AmB promotes direct binding interaction between AmB and the hydroxyl group of ergosterol for the formation of ion channels which may lead to fungal cell death.
Fig. 3.
Mode of actions of Amphotericin B (AmB): AmB bind to ergosterol, forming ion channels in the cell membrane. The formation of these ion channels leads to increased membrane permeability. Additionally, AmB induces the accumulation of ROS, which have multiple toxic effects on fungal cells.
The clinically suggested dose of AmB deoxycholate is 1–1.5 mg/kg/day [[60], [61], [62]] which was found to be highly toxic for patients suffering from fungal diseases. Interestingly the various lipid formulations of AmB was found considerably less nephrotoxic as compared to AmB. This lipid formulation of an antifungal drug can be safely used at higher doses for a longer duration. As compared to AmB (1 mg/kg/day) high-dose of liposomal AmB (15 mg/kg/day) was found to be significantly effective in vivo in R. oryzae infected murine mice model with diabetic ketoacidosis. In the case of liposomal AmB survival rate was reported nearly double as compared to AmB deoxycholate [63]. Lipid formulations of AmB is a clinically established therapeutic option for the primary treatment of mucormycosis. The efficacy of this Lipid formulation of AmB has been established in preclinical and clinical investigations. Various lipid formulations of AmB, including lipid complex, colloidal dispersion, and liposomal AmB are widely used therapies for the treatment of mucormycosis [64]. Natamycin (3) is another naturally occurring polyene antifungal agent. Walther et al. investigated antifungal activities of natamycin against 101 mucoralean strains belonging to the genus Mucor, the closely related species Cokeromyces recurvatus), Rhizopus, Lichtheimia, Rhizomucor and compared with another five antifungals (AmB, terbinafine, isavuconazole, itraconazole, and posaconazole). Natamycin was found less active as compared to AmB and posaconazole. against various fungal strains [65].
9.2. Azoles
Azoles are widely used in clinical practice as antifungal agents. Mechanistically these therapeutic molecules inhibit the fungal lanosterol 14α‐demethylase (LDM) which catalyse the formation of ergosterol in fungal cells. Lanosterol 14α‐demethylase (ERG11 gene) exhibits variable response against different triazoles. Inhibition of LDM decreases the concentration of ergosterol in the fungal cell membrane, which causes less fluidity, inhibition of growth and death of fungal pathogen [66] (Fig. 4 ). Among antifungal triazoles, fluconazole, itraconazole, and voriconazole were found to exhibit minor or no activity against mucormycosis causing fungi. However, newly reported azoles, such as posaconazole and isavuconazole have exhibited significantly improved in vitro antifungal activity against mucorales and clinical data of these azoles proposing their applications for the treatment of mucormycosis (Fig. 5 ).
Fig. 4.
General mechanism and mode of action of triazole: Ergosterol is a regulator of the fluidity of fungal cell membrane. Inhibition of ergosterol synthesis may cause membrane dysfunction and increase in the permeability of membrane which may lead to the cell lysis and cell death.
Fig. 5.
Structure of important antifungal azoles potentially active against mucormycosis.
9.2.1. Itraconazole
Itraconazole (4) is the marketed antifungal azole based drug, which exhibits excellent in vitro activity against mucormycosis [67]. Various case reports and investigations have confirmed that itraconazole is successful therapy against mucormycosis [[68], [69], [70]]. Itraconazole showed anti-fungal activity against fungal isolates in in vitro investigations but in in vivo studies, Rhizopus and Mucor spp., were not found to be susceptible to itraconazole [71,72]. In contrast, itraconazole exhibited in in vivo activity against a hyper susceptible fungal strain of Absidia with a MIC of 0.03 μg/mL. These findings suggest that itraconazole should not be considered as a first-line treatment against this life-threatening disease, yet its clinical use might be used as adjunctive therapy in certain cases where extremely susceptible fungal pathogens against itraconazole have been cultured.
9.2.2. Posaconazole
Antifungal agents such as posaconazole (5) and ravuconazole (6) are important investigational triazole based drug molecules (Fig. 5). These molecules have demonstrated excellent in vitro activity against the fungal pathogens of mucormycosis [67,73]. Posaconazole was found to be more effective as compared to itraconazole but less effective in comparision to AmB in an in vivo investigation in animal models [74]. There are various expanding case reports for treatment of refractory mucormycosis where posaconazole was found to be effective therapy. Significant results have been observed in the case of a combination of posaconazole with AmB in patients suffering from rhinocerebral mucormycosis [75] in heart and kidney transplant patients who were unresponsive to AmB treatment [76]. Posaconazole has exhibited varied in vitro activity against various species of Mucorales [71]. A study of 131 clinical isolates of Mucorales species against posaconazole confirmed that the median MICs of this azole drug varied between 1.0 and 8.0 μg/mL [77]. In an in vivo investigation, posaconazole was reported most effective against various species of mucorales but it was found inactive against infection caused by Rhizopus spp [70,78,79]. In another study by Lewis et al., it was noticed that more than 4.00 mg/mL serum concentration of posaconazole was required to check the growth of strains of Rhizopus spp with a MIC of 2 μg/mL in immunosuppressed mice murine model of pulmonary mucormycosis [80]. These data raised concerns about the clinical efficacy of this antifungal posaconazole against mucormycosis causing Rhizopus spp at least in the existing standard dose of 0.30 μg/day of extended-release pills.
9.2.3. Isavuconazole
Isavuconazole (7) is a recently discovered broad-spectrum antifungal pharmaceutically active triazole based drug of the prodrug isavuconazonium sulphate. This antifungal molecule was approved in the US and Europe for treating mucormycosis. In Europe, isavuconazole was approved due to the non-feasibility of AmB. This anti-fungal drug can be administered IV and in oral formulations with a dose of 0.20 μg/three times/day for two days and 0.20 μg/day thereafter. Isavuconazole showed various pharmacokinetic and safety benefits over other antifungal azole drugs against mucorales [81,82] such as linear pharmacokinetics, fewer interactions with P450 leading to fewer drug-drug interactions, no liver failure, nephrotoxic cyclodextrin free in the intravenous formulation and dose adjustment in kidney was not required [83,84]. Isavuconazole showed variable in vitro activity against mucorales which was found to be species-dependent and The MIC values of isavuconazole against mucorales were found two to four folds higher as compared to those of posaconazole, that's why isavuconazole should be considered in clinical practice [85,86]. Isavuconazole exhibited comparable efficacy to high-dose of liposomal AmB for the reduction of tissue fungal burden, in the lungs and the brain in the neutropenic mouse model of mucormycosis. Isavuconazole provided survival benefits for 21 days of treatment [87]. Several other investigations have also disclosed that isavuconazole could be an effective treatment for the mucormycosis in severely immunosuppressed patients, including posaconazole failure. Isavuconazonium sulphate (8) (Fig. 5) is a broad-spectrum antifungal drug that was approved by the FDA for the treatment of invasive aspergillosis and invasive mcormycosis. Isavuconazonium sulphate is a prodrug of isavuconazole, which is readily hydrolyzed by the enzymatic action of butylcholinesterase into the active form isavuconazole (BAL-4815) and a non-active cleavage product (BAL-8728) [88].
9.3. Echinocandins
Echinocandins (Fig. 7) such as caspofungin (9), micafungin (10), and anidulafungin (11) are specific and noncompetitive inhibitors of β-(1,3)-D-glucan synthase (GS), which play important role in the synthesis of the essential component of the fungal cell wall. GS complex is located in the fungal cell membrane and is a key constituent in maintaining the integrity and strength of the cell wall. Echinocandins display their antifungal activity through noncompetitive binding to the Fks p subunit of the GS complex leading to inhibiting the synthesis of β-(1,3)-D-glucan [47,89]. This inhibition causes lysis and death of fungal cell due to loss of cell wall integrity and imbalance in the intracellular osmotic pressure (Fig. 6 ). Caspofungin is the first echinocandins drug to be marketed in the US as an antifungal agent. Caspofungin displayed minimal in vitro activity against the fungal pathogens of mucormycosis [90,91]. The combination therapy of caspofungin (1 mg/kg/day) and AmB lipid complex (5 mg/kg/day) responded synergistically for the treatment of disseminated mucormycosis in diabetic ketoacidotic (DKA) mice [92].
Fig. 7.
Structure of various clinically important antifungal echinocandins.
Fig. 6.
Schematic representation of the mechanism of action of echinocandin. Echinocandins inhibit the synthesis of β-(1,3)-D-glucan of the fungal cell wall at the level of the cell membrane. Fks is the catalytic subunit, and Rho is the regulatory subunit of the GS complex.
In another study by Ibrahim et al. Micafungin (1 or 3 mg/kg/day) or anidulafungin (1 or 10 mg/kg/day), monotherapies exhibited no significant improvement for the survival DKA mice as compared to the placebo dose. The combination therapy of lipid-based AmB (LAmB) and micafungin (at 1 mg/kg/day dose) or LAmB and anidulafungin (10 mg/kg/day dose) synergistically improved the survival of DKA mice infected with R. oryzae as compared to the above monotherapies. In combination therapy, the higher dose of caspofungin (3 mg/kg/day) was not found equally synergistic however a paradoxical loss was seen for the efficacy of caspofungin against mucormycosis as compared to low dose combination therapy. Monotherapy with micafungin at 3 mg/kg/day dose exhibited a higher survival rate of DKA mice as compared to the monotherapy with micafungin (1 mg/kg/day dose), but the difference in survival of DKA mice or time duration to death was not significant [93].
9.4. Novel antifungal agents with activity against Mucorales
Oteseconazole (VT-1161) 12 (Fig. 8 ) is a novel metalloenzyme inhibitor which prevent the synthesis of ergosterol through selective inhibition of fungal Cyp51 (lanosterol 14α‐demethylase). VT-1161 was reported as antifungal agent which displayed in vitro activity (0.12–1 μg/mL) against some strains of Mucorales. Gebremariam et al. compared prophylactic or continuous therapy with oteseconazole to that with clinically relevant posaconazole for the treatment of mucormycosis caused by infection of R. arrhizus var. arrhizus. VT-1161 showed lowered tissue fungal burden and improved survival rate of immunosuppressed infected mice in prophylaxis investigations. As compared to the posaconazole drug, VT-1161 resulted in better extending mouse survival time despite its comparable effect in decreasing tissue fungal burden during continuous therapy [94]. Terbinafine (13, Fig. 8) is an allylamine antifungal drug, which is commonly used for the treatment of dermotropic fungal infections. Mechanistically terbinafine inhibits the enzymatic action of fungal squalene epoxidase, which catalyse the biosynthesis of ergosterol in the fungal cell membrane [51]. Terbinafine has exhibited in vitro activity against few species of Mucorales [71,95,96] but showed mild or minimal in vivo efficacy in animal models [97]. Terbinafine has been used as an adjunct therapy in combination with antifungal azoles (posaconazole, and itraconazole), polyenes (AmB) or echinocandins (caspofungin) against the severe drug-resistant or refractory mycosis. All the combinations of this adjunct therapy synergistically reduced the MICs. This synergistic action was probably due to the inhibition of fungal ergosterol. The GM MIC decreased on average, 18-fold for terbinafine and 36-fold for caspofungin. But the MICs of terbinafine and caspofungin were found comparably high [98].
Fig. 8.
Structures of potential new antifungal agents exhibiting activity against mucormycosis.
Ibrexafungerp (14, Fig. 8) is a triterpenoid based antifungal agent which inhibits the biosynthesis of β-(1,3)-D-glucan in the cell wall of the fungal pathogen. Ibrexafungerp has exhibited wide and strong in vitro activity against Aspergillus and Candida spp. ibrexafungerp also has displayed potent activity against azole-resistant isolates, including biofilm-forming Candida spp. and echinocandin-resistant isolates. Unfortunately, Ibrexafungerp exhibited no or weak activity or minimal against Mucorales spp [99]. Recently Colley et al. reported novel triazole, PC1244 (15) which displayed significant antifungal activity against Mucor circinelloides and Rhizomucor pusillus with MIC, 2 μg/mL. PC1244 was found more effective as compared to other azoles such as voriconazole and posaconazole (MIC >8 μg/mL) [100].
Colistin (polymyxin E) (16, Fig. 8) is a positively charged cyclic peptide-based antibiotic. Mechanistically colistin bind to and disrupt the anionic outer cell membrane of Gram -ve bacteria and neutralise the bacterial lipopolysaccharides. This antibiotic has shown activity against Saccharomyces cerevisiae. This provided evidence of the extension of its antimicrobial activity against eukaryotic pathogens. Ben-Ami et al. investigated that colistin shows in vitro activity against Mucorales spp and in vivo study in a murine model of pulmonary mucormycosis [101]. Sofosbuvir (17, Fig. 8) is an FDA approved antiviral drug against Hepatitis C (HCV) which target RNA-dependent RNA polymerase (RdRp) virus. Based on in silico study, Elfiky showed that sofosbuvir can bind to R. oryzae with binding energies comparable to that of HCV NS5b RdRp. It was suggested that RdRp may be a potential target protein against the R. oryzae which cause mucormycosis [102].
The TOR (target of rapamycin) signaling pathway is also an attractive target for antifungal therapy due to its central role in regulating fungal growth. Rapamycin (18) exhibits broad-spectrum antifungal property against various fungal pathogens, including Candida albicans and Cryptococcus neoformans. Rapmycin exerts it therapeutic action through conserved FKBP12-dependent inhibition of a TOR kinase homolog in M. circinelloides. Rapamycin significantly inhibits the growth of various fungal pathogens such as zygomycetes P. blakesleeanus, R. oryzae, and M. circinelloides. Rapamycin showed inhibition up to 80% of growth at 6.26 μg/mL, 12.5 μg/mL, and 100 μg/mL concentrations for P. blakesleeanus, R. oryzae, and M. circinelloides, respectively [52]. Tacrolimus (FK506) (19) also displayed significant inhibition of growth of M. circinelloides R7B (−), M. circinelloides NRRL3631 (+), R. oryzae FGSC9543 and P. blakesleeanus NRRL1555. FK506 exerts its antifungal effect through FKBP12-dependant inhibition of PP2B, but not TOR [103].
Montoir et al. have synthesized a library of azole based antifungal agents fused with pyrrolotriazinone moiety (Fig. 9 ). All the synthesized azoles were screened against various fungal pathogens. Synthesized azoles displayed a broad in vitro activity against fluconazole-susceptible and fluconazole-resistant Candida spp. These azole derivatives were found 10-100-fold more active than voriconazole against two C. Albicans isolates. Compounds 23, 24, 25 and 26 significantly inhibited the growth of Rhizopus orizae (RHPOR1, RHPOR2) and Mucor circinelloides (MURI1).
Fig. 9.
Structure of new antifungal azoles having pyrrolotriazinone scaffold. Compound 26 shows promising antifungal activity against mucormycosis.
Compound 23 showed inhibition against RHPOR1, RHPOR2 and MURI1 with MIC values of 0.19, 0.125 and 0.19 μg/mL respectively. Compound 22 displayed inhibition with MIC values 0.0625, 0.25 and 0.25 μg/mL against the above three strains. Compound 25 displayed its inhibition with the MIC 0.125, 0.25 and 0.75 μg/mL while MIC values of compound 26 were found as 0.0625, 0.094 and 0.19 μg/mL against RHPOR1, RHPOR2 and MURI1 respectively. Compound 26 of reported library exhibited significant in vitro antifungal activity against A. fumigatus, R. oryzae and M. circinelloides. Compound 26 displayed in vivo efficacy against two murine models of lethal systemic infections caused by C. albicans [104].
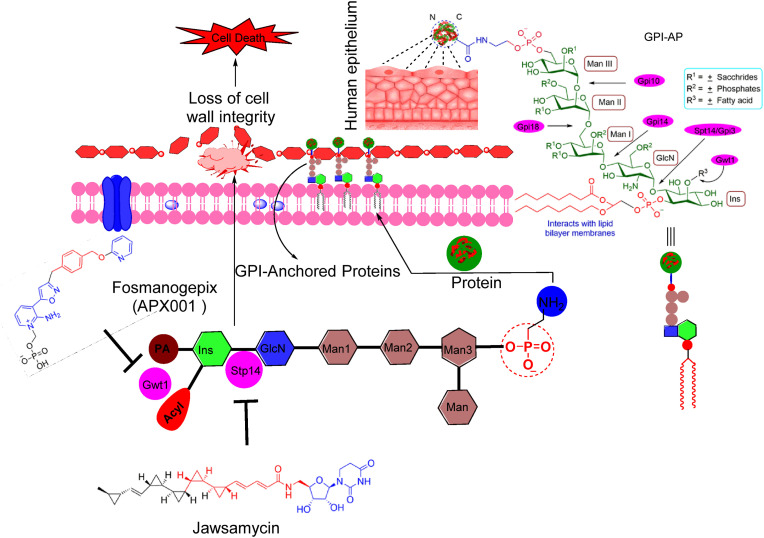
Glycosylphosphatidylinositols (GPIs) are a family of complex glycolipids that are covalently attached to the protein C-terminus through an amide bond. This protein is responsible for adhesion to host epithelium. Recently it was discovered that GPI biosynthetic pathway is a promising target for the treatment of life-threatening fungal infections. GPI biosynthesis is a conserved process, required for anchoring proteins to the fungal cell membrane and essential for the integrity of the cell wall in fungi (yeasts and molds). Inhibition of GPI biosynthesis is damaging to fungal cells as GPI anchor maturation impairment disturbs proteostasis in the endoplasmic reticulum (ER) causing potentially lethal cellular stress. Additionally, lack of GPI-anchored proteins at the cell surface hampers the cell wall integrity ultimately leading to lysis and death of fungal cells. One of the targets of the GPI biosynthetic pathway is the GPI-anchored wall protein transfer 1 (Gwt1), which catalyzes the acylation of inositol during GPI-biosynthesis [105,106]. Inhibition of Gwt1 protein compromises fungal cell wall integrity and produces various fungal growth defects leading to the cell death (Fig. 10 ) [[106], [107], [108]].
Fig. 10.
Various targets and inhibitors of GPI biosynthetic pathways: General structure of GPI-APs. Ethanolamine phosphate (EtNP) is linked to the C-terminus of the protein through amide linkage, this protein is responsible for adhesion of fungal cells to the host epithelium. The lipophilic fatty acids chains attached to the inositol (Ins) interacts with lipid bilayer membranes. Manogepix (MGX) or Fosmanogepix inhibits the conserved fungal Gwt1 enzyme required for acylation of Ins in the GPI biosynthesis pathway. Jawsamycin inhibits the biosynthesis of GPI by targets the catalytic subunit Spt14/Gpi3 of the fungal UDP-glycosyltransferase of GPI.
Manogepix (MGX) (27, Fig. 11 ) is a biologically active form of prodrug fosmanogepix (28) (formerly APX001 and E1210, Fig. 11) which is a broad-spectrum antifungal drug that prevents the maturation of GPI-anchored proteins by inhibiting the enzymatic action of inositol acyltransferases (Gwt1). This inhibition caused pleiotropic effects on the growth of fungal pathogens and virulence [54]. MGX has displayed a broad range of in vitro activity against various fungal pathogens and few rare molds [[109], [110], [111]]. MGX has also displayed variable and moderate in vitro activity against some mucorales strains [111]. Gebremariam et al. have evaluated the activity of fosmanogepix in two mice models. Fosmanogepix is available in both IV and oral formulations, this antifungal prodrug is currently under phase-II clinical investigations as a therapy against life-threatening invasive fungal infections against two strains of R. arrhizus with high Minimum effective concentration (MEC) (4.0 g/mL) and low MEC (0.25 g/mL) values. Treatment of invasive pulmonary infection mice models with 78 mg/kg or 104 mg/kg doses of fosmanogepix prodrug, significantly improved the median survival time and prolonged overall survival of mice as compared to placebo control. Fosmanogepix treatment reduced the fungal burden significantly in both lungs and the brain. Tissue clearance and survival of animals were found comparable to clinically significant doses of FDA approved isavuconazole for the treatment of mucormycosis against 104 mg/kg fosmanogepix dose. These findings suggest sustained development of fosmanogepix as a first-in-class treatment against invasive mucormycosis [112].
Fig. 11.
Inhibitors of inositol acyltransferases (Gwt1) of GPI biosynthesis pathway.
Jawsamycin (29) is an unprecedented and highly complex natural product containing five cyclopropyl rings in the fatty acid tail and dihydro uridine moiety in the head region. Recently Fu et al. reported in vitro antifungal activities against various pathogenic fungi including molds and mucorales, and in vivo in a mouse model of invasive pulmonary mucormycosis due to Rhyzopus delemar infection. Jawsamycin exhibited fungicidal activity against mucorales Rhyzopus delemar (MEC = 0.016 μg/mL), Rhyzopus oryzae (MEC = ≤ 0.008 μg/mL), Absidia corymbifera (MEC = ≤ 0.008 μg/mL), Mucor circinelloides (MEC = 0.016 μg/mL) and Mucor indicus (≤0.008 μg/mL). Chemical derivatization of jawsamycin produced a library of 24 jawsamycin derivatives. This derivatization was achieved by creating variations in tail and head region of this natural product through an efficient synthetic strategy. Unfortunately, none of the tested jawsamycin derivatives displayed improved potency or spectrum compared to the jawsamycin derivatives. Jawsamycin was identified as the first inhibitor of the fungal UDP-GlcNAc phosphatidylinositol transfer complex which exhibited selectivity over the mammalian enzyme and exerts antifungal activities against a wide spectrum of pathogenic fungi in vitro, and an intractable pathogen in a murine disease model [55] (Fig. 12 ).
Fig. 12.
Structure of jawsamycin and various jawsamycin antifungal derivatives.
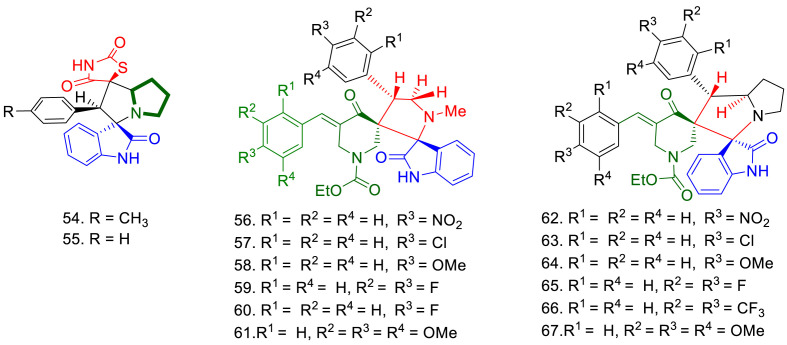
Recently Barakat et al. have reported in vitro antifungal activities of spirooxindole derivatives against Syncephalastrum racemosum RCMB 016001 (rarely lead to complications designated mucormycosis) through agar well diffusion method and compared with AmB and fluconazole. Compound 54 bearing a methyl substituent in phenyl ring, showed better antifungal activity (zone of inhibition = 19 mm) as compared to compound 55 (zone of inhibition = 14 mm). Docking studies of the compounds with lanosterol 14α-demethylase, showed that compound 54 showed four hydrogen bonding interactions with the target protein, while compound 55 only showed one. This might explain the higher antifungal activity of compound 54 (Fig. 13 ).
Fig. 13.
Structures of antifungal spirooxindole, spirooxindole-spiropiperidinonepyrrolidines and spirooxindole-spiropiperidinone-pyrrolizines.
Theses spirooxindoles were found less active as compared to AmB (zone of inhibition = 22 mm) and fluconazole ((zone of inhibition = 22 mm) [113]. Hassaneen et al. have also reported in vitro antifungal activity of spirooxindole-spiropiperidinonepyrrolidines (56–61) and spirooxindole-spiropiperidinone-pyrrolizines (62–67) against S. racemosum. Compounds 56 to 67 exhibited good antifungal activity against S. racemosum (39.46–82.63 mm). Compounds 62, 64 and 68 displayed better activity as compared to the control drug (AmB) while compound 57 and 65 showed antifungal activity comparable to AmB. Compounds 57, 62, 64 and 65 showed MIC 1.95, 0.49, 0.98 and 1.95 μg/mL respectively against S. racemosum. These spiro compounds inhibited the growth of against S. racemosum with the IC50 values of 16.37, 10.64, 11.32 and 11.32 μg/mL respectively. Compound 62 and 64 were found more active than AmB (MIC = 1.95 μg/mL and IC50 14.27 μg/mL) [114].
9.5. Statins
Statins are inhibitors of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, which reduce intracellular levels of mevalonate and farnesyl pyrophosphate (FPP) and ultimately affect the synthesis of ergosterol (Fig. 14). In the mevalonate (MVA) pathway, mevalonate is synthesized from 3-hydroxy-3methylglutaryl-CoA by the action of HMG-CoA reductase which converted to FPP through an enzyme cascade. FPP is then converted to squalene and, subsequently to ergosterol [115] (Fig. 14). Statins prevent the synthesis of mevalonate from HMG-CoA by inhibiting HMG-CoA reductase. Hence statins are able to block ergosterol biosynthesis by inhibition of FPP production in fungal cells. Various in vitro studies have confirmed that statins can decrease the ergosterol content of fungal cells [116]. Statin-induced lowering in ergosterol seems to be dose-dependent [117], and this is believed to be due to the inhibition of HMG-CoA reductase and not related to direct depletion of intracellular ergosterol [118].
Fig. 14.
Mevalonate (MVA) pathway: Statins are competitive inhibitors of HMG-CoA reductase and block the conversion of 3-hydroxy-3-methylglutaryl-CoA to mevalonate and FPP. Synthesis of mevalonate from HMG-CoA is a rate limiting step in the isoprenoid biosynthetic pathway, which is involved in the synthesis of ergosterol in fungal cells.
Various statin drugs, fluvastatin, lovastatin, hydrolyzed lovastatin, rosuvastatin atorvastatin, simvastatin and hydrolyzed simvastatin have been shown to inhibit a wide variety of opportunistic fungal pathogens such as R. oryzae, R. homothallicus, R. stolonifer, R. microspores, R. pusillus, R. miehei, M. racemosus, M. mucedo, M. circinelloides A. corymbifera, A. glauca, M. Africana and S. racemosum. These fungal species are responsible for causing mucormycosis in human beings. Fluvastatin seems to be the most effective antifungal statin with the widest and significant in vitro inhibitory activity against mucormycosis. In R. oryzae (Clinical isolate) fluvastatin exhibited higher inhibitory activity (MIC = 2–3.25 μg/mL) against R. oryzae [119] and R. pusillus (MIC90 = 0.4 μg/mL) [120] as compared to AmB (MIC = 2–4 μg/mL) and (MIC90 = 1.0 μg/mL) respectively. fluvastatin also displayed significant activity against R. microspores, R. miehei, M. racemosus, M. mucedo, M. circinelloides M. circinelloides, A. glauca and S. racemosum. Antifungal activities of various statins against mucormycosis have been summarised in Table 2 .
Table 2.
In vitro antifungal activities of various statins against Mucormycosis with reported MICs ≤128 μg/mL.
| S.No | Statin | Structure | Fungus* | Strain | MIC μg/mL |
References |
|---|---|---|---|---|---|---|
| 1 | Fluvastatin (FLV) | 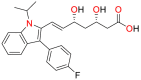 |
R. oryzae | CBS 112.07 | 3.6–11 | [121] |
| CBS 109939 | 2–3.125 | [122] | ||||
| CBS 146.90 | 12.5 | [123] | ||||
| CI | 2–3.125 (<AmB:2–4) | [122] | ||||
| R. microsporus | NRRL 514 | MIC90 = 33–96 | [121] | |||
| R. pusillus | CN(M) 231 | 0.4 (<AmB:1) | [121] | |||
| ETH M4920 | 6.25 | [123] | ||||
| R. miehei | CBS 360.92 | 3.125 | [123] | |||
| CBS 360.92 | MIC90 = 3.6 | [121] | ||||
| M. racemosus | WRL CN(M)304 | 25 | [123] | |||
| M. mucedo | WRL CN(M) 12034 |
6.25 | [123] | |||
| M. circinelloides | MS 12 | 4 | [124] | |||
| A.corymbifera | SZMC 2010 | MIC90 = 3.6 | [121] | |||
| A. glauca | SZMC 11,072 | 6.25 | [123] | |||
| S. racemosum | SZMC 2011 | 11–33 | [121] | |||
| 2. | Atorvastatin (ATO) | 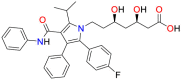 |
R. oryzae | CBS 109939 | 32 | [122] |
| CI | 64–128 | [125] | ||||
| CI | 32 | [119] | ||||
| R. stolonifer | SZMC 11101 | 64 | [126] | |||
| M. circinelloides | MS 12 | 16 | [127] | |||
| S. racemosum | SZMC 2011 | 32 | [126] | |||
| M. africana | NRRL 2978 | 8 | [126] | |||
| 3. | Lovastatin (LOV) |  |
R. oryzae | CBS 109939 | 128 | [120,122] |
| CI | 32–56 | [124] | ||||
| CI | 128 | [119] | ||||
| R. pusillus | CN(M) 231 | MIC90 = 3.6 | [121] | |||
| EI and CI | 1–2 | [128] | ||||
| R. homothallicus | CI | 40–56 | [124] | |||
| R. miehei | EI,CI | 64–128 | [128] | |||
| M. circinelloides | CI | 16–56 | [124] | |||
| S. racemosum | SZMC 2011 | 16 | [126] | |||
| C. bertholletiae | CI | 32–40 | [124] | |||
| 4. | Hydrolyzed lovastatin (HLOV) | 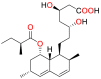 |
R. pusillus | CN(M) 231 | MIC90 = 11 | [121] |
| R. miehei | CBS 360.92 | MIC90 = 11 | [121] | |||
| A. corymbifera | SZMC 2010 | MIC90 = 11 | [121] | |||
| 5. | Rosuvastatin (ROS) | 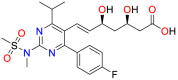 |
R. oryzae | CBS 112.07 | MIC90 = 96 | [121] |
| R. stolonifer | SZMC 11101 | 64 | [126] | |||
| R. pusillus | CN(M) 231 | MIC90 = 11 | [121] | |||
| R. miehei | CBS 360.92 | MIC90 = 11–33 | [121] | |||
| A. corymbifera | SZMC 2010 | MIC90 = 33 | [121] | |||
| S. racemosum | SZMC 2011 | 32 | [126] | |||
| M. africana | NRRL 2978 | 8 | [126] | |||
| M. circinelloides | MS 12 | 32 | [127] | |||
| 6. | Simvastatin (SIM) | 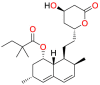 |
R. oryzae | CBS 109939 | 64 | [122] [119] |
| R. pusillus | CN(M) 231 | MIC90 = 11–33 | [121] | |||
| A. corymbifera | SZMC 2010 | MIC90 = 96 | [121] | |||
| S. racemosum | SZMC 2011 | 8 | [126] | |||
| 7. | Hydrolyzed simvastatin (HSIM) |  |
R. pusillus | CN(M) 231 | MIC90 = 1.2–3.6 | [121] |
| A. corymbifera | SZMC 2010 | MIC90 = 11 | [121] | |||
| R. microsporus var. oligosporus | NRRL 514 | MIC90 = 33–96 | [121] |
CI = Clinical isolates EI = Environmental isolates, Genus Rhizopus (R. oryzae, R. homothallicus, R. stolonifer, R. microspores), Genus Rhizomucor (R. pusillus, R. miehei), Genus Mucor (M. racemosus, M. mucedo, M. circinelloides) Genus Absidia (A. corymbifera, A. glauca), Genus Mycotypha (M. Africana) Genus Syncephalastrum (S. racemosum).
10. Conclusions
Mucormycosis is a lethal fungal infection that commonly affects immunocompromised patients with an incompletely understood pathogenesis. This is more common in those who are on hemodialysis, taking high-dose glucocorticoids, have significant burns, or have uncontrolled diabetes mellitus [129]. The overall mortality rate of this malady is more than 40% and reaches 100% in patients suffering from disseminated illness, brain infection or persistent neutropenia. This article provides a comprehensive review of the urgent global efforts, currently underway, towards the discovery and development of therapeutic agents for the treatment of mucormycosis. This review is specifically focused on mechanism of action of various therapeutics, including coverage of new antifungal agents being investigated as part of the urgent global response to control and combat this lethal infection. Various antifungal agents, as indicated in this review can be promising agents to treat this life threatening disease. Although, large-scale clinical investigations are strongly suggested to these issues.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful to their respective institutions for technical and administrative support.
Data availability
No data was used for the research described in the article.
References
- 1.Chowdhary A., Perfect J., de Hoog G.S. Black molds and melanized yeasts pathogenic to humans. Cold Spring Harbor Perspect. Med. 2015;5(8) doi: 10.1101/cshperspect.a019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhary A., Agarwal K., Meis J.F. Filamentous fungi in respiratory infections. What lies beyond Aspergillosis and Mucormycosis? PLoS Pathog. 2016;12(4) doi: 10.1371/journal.ppat.1005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webmd.com . 2021. Complications Coronavirus Can Cause.https://www.webmd.com/lung/coronavirus-complications#1 [Google Scholar]
- 4.Zhu X., et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khurana S., et al. Profile of co-infections & secondary infections in COVID-19 patients at a dedicated COVID-19 facility of a tertiary care Indian hospital: implication on antimicrobial resistance. Indian J. Med. Microbiol. 2021;39(2):147–153. doi: 10.1016/j.ijmmb.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skiada A., Pavleas I., Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J. Fungi. 2020;6(4):265. doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotjanapan P., et al. Epidemiology and clinical characteristics of invasive mould infections: a multicenter, retrospective analysis in five Asian countries. Med. Mycol. 2018;56(2):186–196. doi: 10.1093/mmy/myx029. [DOI] [PubMed] [Google Scholar]
- 8.Pound M.W., et al. Overview of treatment options for invasive fungal infections. Med. Mycol. 2011;49(6):561–580. doi: 10.3109/13693786.2011.560197. [DOI] [PubMed] [Google Scholar]
- 9.Brunet K., Rammaert B. Mucormycosis treatment: recommendations, latest advances, and perspectives. J. Mycolog. Med. 2020;30(3) doi: 10.1016/j.mycmed.2020.101007. [DOI] [PubMed] [Google Scholar]
- 10.Dolatabadi S., et al. Virulence of Rhizopus species compared with two alternative model systems. Mucorales Between Food Infect. 2015:135. [Google Scholar]
- 11.Guarro J., Gené J., Stchigel A.M. Developments in fungal taxonomy. Clin. Microbiol. Rev. 1999;12(3):454–500. doi: 10.1128/cmr.12.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.India T.o. 2021. Explained: Mucormycosis Aka Black Fungus in Covid-19 Patients; Symptoms, Treatment and How to Prevent it.https://timesofindia.indiatimes.com/ [Google Scholar]
- 13.Brown G.D., et al. Hidden killers: human fungal infections. Sci. Transl. Med. 2012;4(165) doi: 10.1126/scitranslmed.3004404. 165rv13-165rv13. [DOI] [PubMed] [Google Scholar]
- 14.Casadevall A., Pirofski L.-A. The weapon potential of human pathogenic fungi. Med. Mycol. 2006;44(8):689–696. doi: 10.1080/13693780600928503. [DOI] [PubMed] [Google Scholar]
- 15.Karkowska-Kuleta J., Rapala-Kozik M., Kozik A. Fungi pathogenic to humans: molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochim. Pol. 2009;56(2) [PubMed] [Google Scholar]
- 16.Denham S.T., Wambaugh M.A., Brown J.C. How environmental fungi cause a range of clinical outcomes in susceptible hosts. J. Mol. Biol. 2019;431(16):2982–3009. doi: 10.1016/j.jmb.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J., et al. Isolated pulmonary mucormycosis in an immunocompetent patient: a case report and systematic review of the literature. BMC Pulm. Med. 2021;21(1):1–8. doi: 10.1186/s12890-021-01504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoenigl M. Invasive fungal disease complicating coronavirus disease 2019: when it Rains, it Spores. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beer K.D., et al. Does pulmonary aspergillosis complicate coronavirus disease 2019? Crit. Care Explor. 2020;2(9) doi: 10.1097/CCE.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone N., Gupta N., Schwartz I. Mucormycosis: time to address this deadly fungal infection. Lancet Microb. 2021;2(8):e343–e344. doi: 10.1016/S2666-5247(21)00148-8. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Molinero A., et al. Association between high-dose steroid therapy, respiratory function, and time to discharge in patients with COVID-19: cohort study. Med. Clínica. 2021;156(1):7–12. doi: 10.1016/j.medcle.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salehi M., et al. Opportunistic fungal infections in the epidemic area of COVID-19: a clinical and diagnostic perspective from Iran. Mycopathologia. 2020;185(4):607–611. doi: 10.1007/s11046-020-00472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firacative C. Memórias do Instituto Oswaldo Cruz; 2020. Invasive Fungal Disease in Humans: Are We Aware of the Real Impact? p. 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 2021;42:264. e5–e264. e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imam S., et al. 2021. Fungus in a Viral Land-Orbital Mucormycosis in Patients with COVID-19 Infection. [Google Scholar]
- 26.Maini A., et al. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int. J. Surg. Case Rep. 2021;82 doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasmin F., et al. COVID-19 associated mucormycosis: a systematic review from diagnostic challenges to management. Diseases. 2021;9(4):65. doi: 10.3390/diseases9040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajoor U., Mahabalshetti A., Dhananjaya M. Clinical profile of mucormycosis: a study from teaching hospital in North Karnataka, India. Int. J. Intg. Med. Sci. 2016;3(3):253–256. [Google Scholar]
- 29.Mishra N., et al. A case series of invasive mucormycosis in patients with COVID-19 infection. Int. J. Otorhinolaryngol. Head Neck Surg. 2021;7(5):867–870. [Google Scholar]
- 30.Sharma S., et al. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021;135(5):442–447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasero D., et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2021;49(5):1055–1060. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.do Monte Junior E.S., et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin. Endosc. 2020;53(6):746. doi: 10.5946/ce.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saldanha M., Reddy R., Vincent M.J. Title of the Article: Paranasal Mucormycosis in COVID-19 Patient. Indian J. Otolaryngol. Head Neck Surg. 2021 doi: 10.1007/s12070-021-02574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamez-Pérez H.E., et al. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J. Diabetes. 2015;6(8):1073–1081. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.India Q. Why does India have so many cases of post-Covid mucormycosis? 2021. https://qz.com/india/2012257/why-india-has-so-many-cases-of-mucormycosis-or-black-fungus
- 36.News B. Mucormycosis: the 'black fungus' maiming Covid patients in India. https://www.bbc.com/news/world-asia-india-57027829 2021
- 37.Mishra A., et al. The rising Havoc of the black, white and Yellow fungi in India. Biotica Res. Today. 2021;3(5):418–420. [Google Scholar]
- 38.Skiada A., et al. Challenges in the diagnosis and treatment of mucormycosis. Med. Mycol. 2018;56(suppl_1):S93–S101. doi: 10.1093/mmy/myx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alekseyev K., Didenko L., Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J. Med. Cases. 2021;12(3):85–89. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dadwal S.S., Kontoyiannis D.P. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev. Mol. Diagn. 2018;18(10):845–854. doi: 10.1080/14737159.2018.1522250. [DOI] [PubMed] [Google Scholar]
- 41.Millon L., et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF) Clin. Microbiol. Infect. 2016;22(9):810. e1–e810. e8. doi: 10.1016/j.cmi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Spellberg B., et al. Combination therapy for mucormycosis: why, what, and how? Clin. Infect. Dis. 2012;54(suppl_1):S73–S78. doi: 10.1093/cid/cir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamiński D.M. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 2014;43(10–11):453–467. doi: 10.1007/s00249-014-0983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson T.M., et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014;10(5):400–406. doi: 10.1038/nchembio.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.K Mazu T., et al. The mechanistic targets of antifungal agents: an overview. Mini Rev. Med. Chem. 2016;16(7):555–578. doi: 10.2174/1389557516666160118112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosam K., Monk B.C., Lackner M. Sterol 14α-demethylase ligand-binding pocket-mediated acquired and intrinsic azole resistance in fungal pathogens. J. Fungi. 2021;7(1):1. doi: 10.3390/jof7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sucher A.J., Chahine E.B., Balcer H.E. Echinocandins: the newest class of antifungals. Ann. Pharmacother. 2009;43(10):1647–1657. doi: 10.1345/aph.1M237. [DOI] [PubMed] [Google Scholar]
- 48.Douglas C., et al. Identification of the FKS1 gene of Candida albicans as the essential target of 1, 3-beta-D-glucan synthase inhibitors. Antimicrob. Agents Chemother. 1997;41(11):2471–2479. doi: 10.1128/aac.41.11.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warrilow A., et al. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob. Agents Chemother. 2014;58(12):7121–7127. doi: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brand S.R., et al. A randomized phase 2 study of VT-1161 for the treatment of acute vulvovaginal Candidiasis. Clin. Infect. Dis. 2021;73(7):e1518–e1524. doi: 10.1093/cid/ciaa1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryder N. Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992;126:2–7. doi: 10.1111/j.1365-2133.1992.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 52.Bastidas R.J., et al. Rapamycin exerts antifungal activity in vitro and in vivo against Mucor circinelloides via FKBP12-dependent inhibition of Tor. Eukaryot. Cell. 2012;11(3):270–281. doi: 10.1128/EC.05284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waldmeier P.C., et al. Cyclophilin D as a drug target. Curr. Med. Chem. 2003;10(16):1485–1506. doi: 10.2174/0929867033457160. [DOI] [PubMed] [Google Scholar]
- 54.Miyazaki M., et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 2011;55(10):4652–4658. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu Y., et al. Jawsamycin exhibits in vivo antifungal properties by inhibiting Spt14/Gpi3-mediated biosynthesis of glycosylphosphatidylinositol. Nat. Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-17221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tavakkoli A., Johnston T.P., Sahebkar A. Antifungal effects of statins. Pharmacol. Therapeut. 2020;208 doi: 10.1016/j.pharmthera.2020.107483. [DOI] [PubMed] [Google Scholar]
- 57.Gebremariam T., et al. Anti-CotH3 antibodies protect mice from mucormycosis by prevention of invasion and augmenting opsonophagocytosis. Sci. Adv. 2019;5(6) doi: 10.1126/sciadv.aaw1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gray K.C., et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. USA. 2012;109(7):2234–2239. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palacios D.S., et al. Synthesis-enabled functional group deletions reveal key underpinnings of amphotericin B ion channel and antifungal activities. Proc. Natl. Acad. Sci. USA. 2011;108(17):6733–6738. doi: 10.1073/pnas.1015023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibrahim A., et al. Clinical Mycology. Zygomycosis. 2003:241–251. [Google Scholar]
- 61.Kwan-Chung K., Young R.C., Orlando M. Pulmonary mucormycosis caused by Cunninghamella elegans in a patient with chronic myelogenous leukemia. Am. J. Clin. Pathol. 1975;64(4):544–548. doi: 10.1093/ajcp/64.4.544. [DOI] [PubMed] [Google Scholar]
- 62.Sugar A. Agents of mucormycosis and related species. Princ. Pract. Infect. Dis. 2005;2:2973–2984. [Google Scholar]
- 63.Spellberg B., Edwards J., Jr., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gamaletsou M.N., et al. Rhino-orbital-cerebral mucormycosis. Curr. Infect. Dis. Rep. 2012;14(4):423–434. doi: 10.1007/s11908-012-0272-6. [DOI] [PubMed] [Google Scholar]
- 65.Wagner L., et al. A revised species concept for opportunistic Mucor species reveals species-specific antifungal susceptibility profiles. Antimicrob. Agents Chemother. 2019;63(8) doi: 10.1128/AAC.00653-19. e00653-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma L.-J., et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009;5(7) doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun Q.N., et al. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob. Agents Chemother. 2002;46(5):1581–1582. doi: 10.1128/AAC.46.5.1581-1582.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quinio D., et al. Zygomycosis caused by Cunninghamella bertholletiae in a kidney transplant recipient. Med. Mycol. 2004;42(2):177–180. doi: 10.1080/13693780310001644644. [DOI] [PubMed] [Google Scholar]
- 69.Eisen D., Robson J. Complete resolution of pulmonary Rhizopus oryzae infection with itraconazole treatment: more evidence of the utility of azoles for zygomycosis. Mycoses. 2004;47(3–4):159–162. doi: 10.1111/j.1439-0507.2004.00959.x. [DOI] [PubMed] [Google Scholar]
- 70.Dannaoui E., et al. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 2003;47(11):3647–3650. doi: 10.1128/AAC.47.11.3647-3650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dannaoui E., et al. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 2003;51(1):45–52. doi: 10.1093/jac/dkg020. [DOI] [PubMed] [Google Scholar]
- 72.Van Cutsem J., et al. Treatment of experimental zygomycosis in Guinea pigs with azoles and with amphotericin B. Chemotherapy. 1989;35(4):267–272. doi: 10.1159/000238681. [DOI] [PubMed] [Google Scholar]
- 73.Pfaller M., et al. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 2002;46(4):1032–1037. doi: 10.1128/AAC.46.4.1032-1037.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Q.N., et al. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. Antimicrob. Agents Chemother. 2002;46(7):2310–2312. doi: 10.1128/AAC.46.7.2310-2312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tobón A.M., et al. Mucormycosis (zygomycosis) in a heart-kidney transplant recipient: recovery after posaconazole therapy. Clin. Infect. Dis. 2003;36(11):1488–1491. doi: 10.1086/375075. [DOI] [PubMed] [Google Scholar]
- 76.Ide L., et al. Zygomycosis in neutropenic patients with past Aspergillus infection: a role for posaconazole? Clin. Microbiol. Infect. 2004;10(9):862–863. doi: 10.1111/j.1469-0691.2004.00945.x. [DOI] [PubMed] [Google Scholar]
- 77.Caramalho R., et al. Etest cannot be recommended for in vitro susceptibility testing of Mucorales. Antimicrob. Agents Chemother. 2015;59(6):3663–3665. doi: 10.1128/AAC.00004-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewis R.E., Albert N.D., Kontoyiannis D.P. Comparative pharmacodynamics of posaconazole in neutropenic murine models of invasive pulmonary aspergillosis and mucormycosis. Antimicrob. Agents Chemother. 2014;58(11):6767–6772. doi: 10.1128/AAC.03569-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bellanger A.-P., et al. Effect of preexposure to triazoles on susceptibility and virulence of Rhizopus oryzae. Antimicrob. Agents Chemother. 2015;59(12):7830–7832. doi: 10.1128/AAC.01583-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dupont B. Pulmonary mucormycosis (zygomycosis) in a lung transplant recipient: recovery after posaconazole therapy. Transplantation. 2005;80(4):544–545. doi: 10.1097/01.tp.0000168343.47569.1c. [DOI] [PubMed] [Google Scholar]
- 81.Sipsas N.V., et al. Therapy of mucormycosis. J. Fungi. 2018;4(3):90. doi: 10.3390/jof4030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arendrup M.C., Jensen R.H., Meletiadis J. In vitro activity of isavuconazole and comparators against clinical isolates of the Mucorales order. Antimicrob. Agents Chemother. 2015;59(12):7735–7742. doi: 10.1128/AAC.01919-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Donnelley M.A., Zhu E.S., Thompson G.R., 3rd Isavuconazole in the treatment of invasive aspergillosis and mucormycosis infections. Infect. Drug Resist. 2016;9:79. doi: 10.2147/IDR.S81416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo G., et al. Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrob. Agents Chemother. 2014;58(4):2450–2453. doi: 10.1128/AAC.02301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peixoto D., et al. Isavuconazole treatment of a patient with disseminated mucormycosis. J. Clin. Microbiol. 2014;52(3):1016–1019. doi: 10.1128/JCM.03176-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ervens J., et al. Successful isavuconazole salvage therapy in a patient with invasive mucormycosis. Infection. 2014;42(2):429–432. doi: 10.1007/s15010-013-0552-6. [DOI] [PubMed] [Google Scholar]
- 87.Graves B., et al. Isavuconazole as salvage therapy for mucormycosis. Med. Mycol. Case Rep. 2016;11:36–39. doi: 10.1016/j.mmcr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flick A.C., et al. Synthetic approaches to new drugs approved during 2018. J. Med. Chem. 2020;63(19):10652–10704. doi: 10.1021/acs.jmedchem.0c00345. [DOI] [PubMed] [Google Scholar]
- 89.Kurtz M., Douglas C. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 1997;35(2):79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 90.Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 1998;36(10):2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Del Poeta M., Schell W.A., Perfect J.R. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 1997;41(8):1835–1836. doi: 10.1128/aac.41.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spellberg B., et al. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob. Agents Chemother. 2005;49(2):830–832. doi: 10.1128/AAC.49.2.830-832.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ibrahim A.S., et al. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob. Agents Chemother. 2008;52(4):1556–1558. doi: 10.1128/AAC.01458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gebremariam T., et al. VT-1161 protects immunosuppressed mice from Rhizopus arrhizus var. arrhizus infection. Antimicrob. Agents Chemother. 2015;59(12):7815–7817. doi: 10.1128/AAC.01437-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vitale R.G., et al. Antifungal susceptibility and phylogeny of opportunistic members of the order mucorales. J. Clin. Microbiol. 2012;50(1):66–75. doi: 10.1128/JCM.06133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chowdhary A., et al. Molecular characterization and in vitro antifungal susceptibility of 80 clinical isolates of mucormycetes in Delhi, India. Mycoses. 2014;57:97–107. doi: 10.1111/myc.12234. [DOI] [PubMed] [Google Scholar]
- 97.Lewis R.E., et al. Efficacy of caspofungin in neutropenic and corticosteroid-immunosuppressed murine models of invasive pulmonary mucormycosis. Antimicrob. Agents Chemother. 2011;55(7):3584–3587. doi: 10.1128/AAC.01812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang S., Li R., Yu J. Drug combinations against Mucor irregularis in vitro. Antimicrob. Agents Chemother. 2013;57(7):3395–3397. doi: 10.1128/AAC.02612-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lamoth F., Alexander B.D. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob. Agents Chemother. 2015;59(7):4308–4311. doi: 10.1128/AAC.00234-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Colley T., et al. In vitro and in vivo efficacy of a novel and long-acting fungicidal azole, PC1244, on Aspergillus fumigatus infection. Antimicrob. Agents Chemother. 2018;62(5) doi: 10.1128/AAC.01941-17. e01941-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ben-Ami R., et al. Antifungal activity of colistin against Mucorales species in vitro and in a murine model of Rhizopus oryzae pulmonary infection. Antimicrob. Agents Chemother. 2010;54(1):484–490. doi: 10.1128/AAC.00956-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elfiky A.A. The antiviral Sofosbuvir against mucormycosis: an in silico perspective. Future Virol. 2019;14(11):739–744. [Google Scholar]
- 103.Harris T.E., Lawrence J.C., Jr. TOR signaling. Sci. STKE. 2003;2003(212) doi: 10.1126/stke.2122003re15. re15-re15. [DOI] [PubMed] [Google Scholar]
- 104.Montoir D., et al. New azole antifungals with a fused triazinone scaffold. Eur. J. Med. Chem. 2020;189 doi: 10.1016/j.ejmech.2020.112082. [DOI] [PubMed] [Google Scholar]
- 105.Tsukahara K., et al. Medicinal genetics approach towards identifying the molecular target of a novel inhibitor of fungal cell wall assembly. Mol. Microbiol. 2003;48(4):1029–1042. doi: 10.1046/j.1365-2958.2003.03481.x. [DOI] [PubMed] [Google Scholar]
- 106.Hata K., et al. Abstr Intersci Conf Antimicrob Agents Chemother, Poster F1-1377. 2011. In vitro and in vivo antifungal activities of E1211, a water-soluble prodrug of E1210. [Google Scholar]
- 107.Hata K., et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob. Agents Chemother. 2011;55(10):4543–4551. doi: 10.1128/AAC.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kapoor M., et al. Evaluation of resistance development to the Gwt1 inhibitor manogepix (APX001A) in Candida species. Antimicrob. Agents Chemother. 2019;64(1) doi: 10.1128/AAC.01387-19. e01387-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rivero-Menendez O., Cuenca-Estrella M., Alastruey-Izquierdo A. In vitro activity of APX001A against rare moulds using EUCAST and CLSI methodologies. J. Antimicrob. Chemother. 2019;74(5):1295–1299. doi: 10.1093/jac/dkz022. [DOI] [PubMed] [Google Scholar]
- 110.Castanheira M., et al. Activities of E1210 and comparator agents tested by CLSI and EUCAST broth microdilution methods against Fusarium and Scedosporium species identified using molecular methods. Antimicrob. Agents Chemother. 2012;56(1):352–357. doi: 10.1128/AAC.05414-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pfaller M.A., et al. In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Aspergillus spp. determined by CLSI and EUCAST broth microdilution methods. Antimicrob. Agents Chemother. 2011;55(11):5155–5158. doi: 10.1128/AAC.00570-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gebremariam T., et al. Fosmanogepix (APX001) is effective in the treatment of pulmonary murine mucormycosis due to Rhizopus arrhizus. Antimicrob. Agents Chemother. 2020;64(6) doi: 10.1128/AAC.00178-20. e00178-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barakat A., et al. New spiro-oxindole constructed with pyrrolidine/thioxothiazolidin-4-one derivatives: regioselective synthesis, x-ray crystal structures, Hirshfeld surface analysis, DFT, docking and antimicrobial studies. J. Mol. Struct. 2018;1152:101–114. [Google Scholar]
- 114.Hassaneen H.M., et al. Facial regioselective synthesis of novel bioactive spiropyrrolidine/pyrrolizine-oxindole derivatives via a three components reaction as potential antimicrobial agents. Molecules. 2017;22(3):357. doi: 10.3390/molecules22030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhattacharya S., Esquivel B.D., White T.C. Overexpression or deletion of ergosterol biosynthesis genes alters doubling time, response to stress agents, and drug susceptibility in Saccharomyces cerevisiae. mBio. 2018;9(4) doi: 10.1128/mBio.01291-18. e01291-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wikhe K., Westermeyer C., Macreadie I. Biological consequences of statins in Candida species and possible implications for human health. Biochem. Soc. Trans. 2007;35(6):1529–1532. doi: 10.1042/BST0351529. [DOI] [PubMed] [Google Scholar]
- 117.Bibikova M., Spiridonova I., Katlinskiĭ A. Lovastatin effect on ergosterol production and growth of Tolypocladium inflatum 106. Antibiotiki i khimioterapiia= Antibiotics and chemoterapy [sic] 2004;49(4):3–6. [PubMed] [Google Scholar]
- 118.Callegari S., et al. Atorvastatin-induced cell toxicity in yeast is linked to disruption of protein isoprenylation. FEMS Yeast Res. 2010;10(2):188–198. doi: 10.1111/j.1567-1364.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 119.Nyilasi I., et al. In vitro interactions between primycin and different statins in their effects against some clinically important fungi. J. Med. Microbiol. 2010;59(2):200–205. doi: 10.1099/jmm.0.013946-0. [DOI] [PubMed] [Google Scholar]
- 120.Nyilasi I., et al. In vitro synergistic interactions of the effects of various statins and azoles against some clinically important fungi. FEMS Microbiol. Lett. 2010;307(2):175–184. doi: 10.1111/j.1574-6968.2010.01972.x. [DOI] [PubMed] [Google Scholar]
- 121.Galgóczy L., et al. Antifungal activity of statins and their interaction with amphotericin B against clinically important Zygomycetes. Acta Biol. Hung. 2010;61(3):356–365. doi: 10.1556/ABiol.61.2010.3.11. [DOI] [PubMed] [Google Scholar]
- 122.Nyilasi I. Effect of different statins on the antifungal activity of polyene antimycotics. Acta Biol. Szeged. 2010;54(1):33–36. [Google Scholar]
- 123.Galgóczy L., et al. Are statins applicable for the prevention and treatment of zygomycosis? Clin. Infect. Dis. 2009;49(3):483–484. doi: 10.1086/600825. [DOI] [PubMed] [Google Scholar]
- 124.Chamilos G., Lewis R.E., Kontoyiannis D.P. Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrob. Agents Chemother. 2006;50(1):96–103. doi: 10.1128/AAC.50.1.96-103.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bellanger A.-P., et al. Statin concentrations below the minimum inhibitory concentration attenuate the virulence of Rhizopus oryzae. J. Infect. Dis. 2016;214(1):114–121. doi: 10.1093/infdis/jiw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galgóczy L., et al. Interactions between statins and Penicillium chrysogenum antifungal protein (PAF) to inhibit the germination of sporangiospores of different sensitive Zygomycetes. FEMS Microbiol. Lett. 2007;270(1):109–115. doi: 10.1111/j.1574-6968.2007.00661.x. [DOI] [PubMed] [Google Scholar]
- 127.Nagy G., et al. CRISPR-Cas9-mediated disruption of the HMG-CoA reductase genes of Mucor circinelloides and subcellular localization of the encoded enzymes. Fungal Genet. Biol. 2019;129:30–39. doi: 10.1016/j.fgb.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 128.Lukács G., et al. Cloning of the Rhizomucor miehei 3-hydroxy-3-methylglutaryl-coenzyme A reductase gene and its heterologous expression in Mucor circinelloides. Antonie Leeuwenhoek. 2009;95(1):55–64. doi: 10.1007/s10482-008-9287-2. [DOI] [PubMed] [Google Scholar]
- 129.Azhar A., et al. Mucormycosis and COVID-19 pandemic: clinical and diagnostic approach. J. Infect. Public Health. 2022;15(4):466–479. doi: 10.1016/j.jiph.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.