Abstract
Recombinant adeno-associated virus (rAAV) vectors are often produced in HEK293 or Spodoptera frugiperda (Sf)-based cell lines. We compared expression profiles of “oversized” (∼5,000 bp) and “standard-sized” (4,600 bp) rAAV5–human α1-antitrypsin (rAAV5-hA1AT) vectors manufactured in HEK293 or Sf cells and investigated molecular mechanisms mediating expression decline. C57BL/6 mice received 6 × 1013 vg/kg of vector, and blood and liver samples were collected through week 57. For all vectors, peak expression (weeks 12–24) declined by 50% to week 57. For Sf- and HEK293-produced oversized vectors, serum hA1AT was initially comparable, but in weeks 12–57, Sf vectors provided significantly higher expression. For HEK293 oversized vectors, liver genomes decreased continuously through week 57 and significantly correlated with A1AT protein. In RNA-sequencing analysis, HEK293 vector-treated mice had significantly higher inflammatory responses in liver at 12 weeks compared with Sf vector- and vehicle-treated mice. Thus, HEK293 vector genome loss led to decreased transgene protein. For Sf-produced vectors, genomes did not decrease from peak expression. Instead, vector genome accessibility significantly decreased from peak to week 57 and correlated with transgene RNA. Vector DNA interactions with active histone marks (H3K27ac/H3K4me3) were significantly reduced from peak to week 57, suggesting that epigenetic regulation impacts transgene expression of Sf-produced vectors.
Keywords: AAV vectors, AAV production, valoctocogene roxaparvovec, oversized AAV vectors
Graphical abstract

AAV vectors used for gene therapy are manufactured in mammalian or insect-derived cell lines. Handyside and colleagues show similar long-term expression between the two manufacturing methods. Decline of expression was mediated by vector DNA loss for mammalian-produced vectors and vector genome interactions with chromatin and chromatin accessibility for insect-produced vectors.
Introduction
Wild-type adeno-associated viruses (AAVs) are small, non-pathogenic, single-stranded DNA parvoviruses with a genome approximately 4.7 kb long.1 Recombinant AAV vectors of multiple serotypes are widely used to deliver therapeutic transgenes, as they efficiently transduce human cells and have relatively low immunogenicity.2,3 However, the limited packaging size of AAV vectors (4.7 kb) poses a challenge for the clinical development of AAV-mediated gene therapy with large gene cassettes. Engineering transgenes by removing unneeded protein domains and shortening the cDNA size can allow use of AAV vectors where size would otherwise be prohibitive. For example, AAV vectors can be used for transgene delivery in Duchenne muscular dystrophy by removing protein domains from the >11-kb dystrophin coding sequence.4,5,6 Gene therapies for hemophilia A using AAV vectors have used similar approaches, as the wild-type factor VIII (FVIII) protein-coding sequence is around 7 kb; in particular, use of B-domain-deleted (BDD) FVIII protein variants originally developed as recombinant protein replacement treatments for hemophilia A enabled successful clinical outcomes.7,8,9,10,11,12 When using BDD FVIII variants, the size of the FVIII protein-coding sequence is reduced to just slightly over the packaging size limit (“oversized”); these vector genomes can be successfully packaged in AAV capsids, but altered gene expression may result compared with standard-sized vector genomes.13 In a study using AAV serotype 8 (AAV8) in mice, an oversized 5.1-kb vector encoding FVIII provided 2- to 3-fold lower plasma FVIII protein expression and levels of vector genomes in hepatocytes than a standard-sized 4.6-kb vector at 3 months post dose.13
AAV vectors for gene therapy are manufactured primarily in either mammalian human embryonic kidney 293 (HEK293; hereafter 293) cells or baculovirus-Spodoptera frugiperda (Sf) insect cell systems.14,15,16,17 Manufacturing in 293 cells was previously the predominant method for producing vectors used in gene therapy;18 however, Sf insect cell systems offer improved scalability for manufacturing the large amounts of vector needed for clinical programs.15 Vector characteristics may differ per production method. Manufacture of AAV8 in Sf cell systems resulted in more empty particles and particles containing short fragments of vector genomes than manufacture of AAV8 capsids carrying the same construct via plasmid transfection in 293 cells.19 Another analysis of controlled production of AAV8 vectors in both 293 and Sf cells found differences in post-translational modification of capsid proteins and methylation of vector genomes per manufacturing method.20 These characteristics could result in differing efficacy of transduction or transgene expression per manufacturing method. In mice, 293-produced AAV1 and AAV8 vectors within the size limit for AAV packaging had higher in vivo potency, defined as expression of the transgene in skeletal muscle and liver, than Sf-produced vectors for up to 8 weeks post dose.20 In similar experiments using AAV5, we found comparable expression levels in mice for mammalian and insect-derived vectors at 5 and 10 weeks post dose.9 Questions remain regarding whether manufacturing methods may contribute to differences in long-term expression of AAV vector; furthermore, it is unknown whether these differences also exist for oversized vectors.
Valoctocogene roxaparvovec (AAV5-hFVIII-SQ) uses an AAV5 vector to deliver a codon-optimized, BDD FVIII coding sequence controlled by a small liver-selective promoter.9 The length of the AAV5-hFVIII-SQ vector genome is just slightly over the AAV packaging limit at approximately 5 kb,9 and AAV5-hFVIII-SQ is manufactured in an Sf cell system.11 A single infusion of 6 × 1013 vector genomes (vg)/kg of AAV5-hFVIII-SQ provided therapeutic levels of FVIII expression and protection from bleeding in adult men with severe hemophilia A for thus far up to 6 years in a phase 1/2 trial and up to 2 years in a phase 3 trial.11,12,21,22,23,24 In both studies, endogenous FVIII expression post gene transfer peaked around week 26 and slowly declined thereafter.11,12,21,22,23,24 Liver biopsies from participants treated with 6 × 1013 vg/kg in the phase 1/2 study that were taken >4 years post dose revealed the presence of 3–4 copies of full-length inverted terminal repeat (ITR)-fused circular episomes per diploid genome, which presumably are capable of giving rise to transgene expression.25 The mechanisms contributing to changes in transgene expression over time are unknown;25,26,27 potential hypotheses include antibody-mediated clearance of transgene protein,28,29 loss of transduced hepatocytes due to cytotoxic T cell responses, cellular stress, or hepatocyte turnover,30 host-mediated genome metabolism,31,32 and epigenetic silencing of vector genomes.33
In this study, we performed head-to-head comparisons in mice of the long-term durability of “oversized” versus “standard-sized” recombinant AAV5 reporter vectors produced in Sf and 293 cell lines over a period of 57 weeks, allowing us to model the expression trajectory observed clinically. We then systematically examined potential molecular mechanisms that may contribute to changes in expression over time and observed differences between 293- and Sf-produced vectors, including in transduction, transcription, protein production, and epigenetic modification.
Results
Production and characterization of oversized and standard-sized vectors manufactured in 293 and Sf systems
First, we performed extensive biophysical characterization of oversized and standard-sized vectors used in this study produced in 293 or Sf cell systems to identify intrinsic differences resulting from different manufacturing processes (Table S1). We used reporter vectors expressing secreted human α1-antitrypsin (hA1AT) (AAV5-HLP-hA1AT) instead of valoctocogene roxaparvovec to minimize sampling variability from serial blood draws using the tail-nick method, which could potentially activate the clotting cascade and lead to consumption of FVIII (data not shown). Oversized (∼5 kb) and standard-sized (4.6 kb) vector constructs were produced using a “triple transfection” process in 293 cells34 or in Sf cells infected with helper and vector baculovirus (Figure 1A).15,34 An alkaline gel electrophoresis analysis evaluating encapsidated DNA size distribution showed that Sf-produced vectors contained more short fragments than did 293-produced vectors (Figure 1B). Similarly, analytical ultracentrifugation determining capsid particle distribution and AAV species heterogeneity found that 293-produced vectors contained a more discrete-sized intermediate population (Figure 1C), indicating relatively more uniform DNA size profile for 293-produced vectors than for Sf-produced vectors after purification. Long-read PacBio (Menlo Park, CA) sequencing confirmed these results (data not shown). Finally, reverse-phase high-performance liquid chromatography found that 293-produced vectors contained more viral protein 1 (VP1) capsid protein content than did Sf-produced vectors (Table 1). Additional characterization found no other differences between vectors produced by either system (data not shown). Overall, 293-produced AAV5 vectors contained more homogeneous encapsidated DNA and relatively higher VP1 content than Sf-produced vectors.
Figure 1.
Characterization of vectors produced in HEK293 and Sf cell systems
(A) Schematic of the vector constructs used in this study. (B) Alkaline gel analysis of encapsidated DNA distribution. (C) AUC analysis of capsid particle distribution. 293, mammalian HEK293 cell system; A1AT, α1-antitrypsin; AUC, area under the curve; HEK, human embryonic kidney; HLP, hybrid liver promoter; ITR, inverted terminal repeat; polyA, polyadenylation sequence; Sf, Spodoptera frugiperda insect cell system.
Table 1.
RP-HPLC analysis of viral capsid protein content
| Samples | % VP1 | % VP2 | % VP3 |
|---|---|---|---|
| Sf-standard | 4.7 | 10.6 | 84.8 |
| Sf-oversized | 4.4 | 11.2 | 84.4 |
| 293-standard | 6.1 | 12.9 | 81.0 |
| 293-oversized | 5.7 | 12.5 | 81.8 |
293, mammalian HEK293 cell system; HEK, human embryonic kidney; RP-HPLC, reverse-phased, high-performance liquid chromatography; Sf, Spodoptera frugiperda insect cell system; VP, viral protein.
Oversized vector achieved lower protein expression levels compared with standard-sized vector
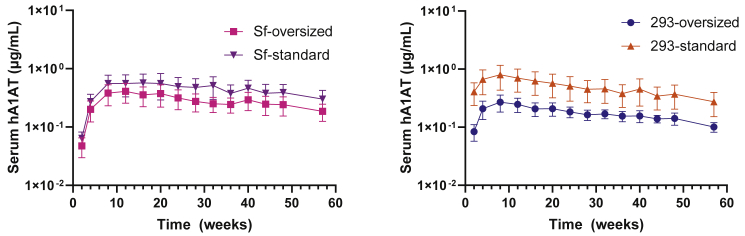
We next sought to assess whether there was a difference in gene transfer efficacy in mice with standard-sized and oversized AAV5-HLP-hA1AT vectors produced in 293 or Sf cell lines. All four vectors were administered to mice intravenously, and levels of serum hA1AT protein were evaluated for up to 57 weeks using serial blood draws. There was no difference in transduction between the standard and oversized vectors in both Sf and 293 cells, suggesting that vector genome size does not impact transgene expression within the cell system (Figure S1). Serum hA1AT protein levels were lower throughout 57 weeks with the oversized vectors as compared with the standard-sized vectors for vector produced in both Sf and 293 cell lines (Figure 2). At week 57, the difference between serum protein produced by oversized and standard-sized vectors was greater for 293-produced vectors (209% higher) than for Sf-produced vectors (65% higher).
Figure 2.
Oversized vectors produced less serum transgene protein compared with standard-sized vectors regardless of production method
Data are mean ± standard deviation (SD), depicted on a log scale. 293, mammalian HEK293 cell system; hA1AT, human α1-antitrypsin; HEK, human embryonic kidney; Sf, Spodoptera frugiperda insect cell system.
Serum protein expression kinetics of 293- and Sf-produced vectors
At early time points, serum hA1AT expression levels were higher for 293-produced vectors than for Sf-produced vectors; the difference in expression level was higher among standard-sized vectors than among oversized vectors (Figure 3A). At week 2, the expression levels for 293-produced vectors were significantly higher, with 6.4-times higher expression for standard-sized vectors and 1.8-times higher expression for oversized vectors. For standard-sized vectors, no significant difference in protein expression levels was seen between Sf- and 293-produced vectors at week 12, but for oversized vectors significantly higher expression levels were reached with Sf-produced vectors as compared with 293 vectors beginning at week 12 and maintained through week 57 (Figure 3B). For all four vectors, hA1AT expression reached peak levels by 12–24 weeks post dose, followed by a decline of 47%–63% through week 57. Together, these data show that even though the early expression kinetics are different, the long-term expression achieved with Sf- and 293-produced AAV5 vectors is similar and that for oversized AAV5 vectors, Sf cell system production results in significantly better long-term expression.
Figure 3.
Transgene protein expression from oversized and standard-sized vectors by production system
(A) Protein expression of HEK293- and Sf-produced vectors at weeks 2 and 12. (B) Comparison of protein expression by vectors produced in Sf and HEK293 cell lines over 57 weeks. ∗∗p < 0.05, ∗∗∗p < 0.001 using Welch’s t test for (A), an unpaired t test at week 57 only for (B) (left), and a repeated-measures mixed-effects model with log transformation and adjusted p values for (B) (right). Data are mean ± SD. Data in (B) are the same as those in Figure 2 but shown on a linear scale. 293, mammalian HEK293 cell system; hA1AT, human α1-antitrypsin; HEK, human embryonic kidney; ns, not significant; SD, standard deviation; Sf, Spodoptera frugiperda insect cell system.
Mechanisms of action mediating the decline of transgene expression
We sought to further understand the mechanism of action mediating the observed decline in transgene protein expression from the peak expression at 12–24 weeks through 57 weeks. As all four vectors had similar overall patterns of decline, we chose to focus specifically on the oversized vectors. Mechanisms mediating the decline in transgene expression could include differences in initial transduction and nuclear transport of vector genome, antibody-mediated clearance of transgene protein, loss of transduced hepatocytes due to cytotoxic T cell responses, cellular stress, or hepatocyte turnover, host-mediated genome metabolism, and epigenetic regulation/silencing of vector genomes. We tested each of these hypotheses using oversized vectors produced by both 293 and Sf cell systems in mice dosed with AAV5-HLP-hA1AT.
We dosed additional mice with 293- or Sf-produced oversized vector and set up take-down cohorts at weeks 1, 3, 12, 24, and 57 to take blood and liver samples. We then compared serum transgene protein expression in the take-down cohorts with that in the cohort from which serial blood samples were taken via tail nick to ensure that the observed transgene expression kinetics were the same. In the take-down cohorts, expression peaked at 12 weeks for mice dosed with 293-produced vector and at 24 weeks for mice dosed with Sf-produced vector (Figure S2).
First, we compared initial transduction of Sf- and 293-produced vector into hepatocytes using immunohistochemistry of VP3 to detect capsid uptake. 293-produced vectors resulted in significantly higher VP3 staining in hepatocytes at weeks 1 and 3; however, there was no difference in the amount of vector genomes present in hepatocyte nuclei from week 3 onward (Figure S3). Together, these data indicate that while 293-produced vectors had higher initial cellular uptake, perhaps due to their higher VP1 content, this did not impact long-term durability.
We next tested the hypothesis that antibody-mediated transgene protein clearance led to decline of expression by measuring anti-hA1AT antibodies using an electrochemiluminescence assay (ECLA). Through 57 weeks, no anti-hA1AT antibodies were detected in mice dosed with either 293- or Sf-produced oversized vector (data not shown), consistent with previous studies showing that hA1AT protein is non-immunogenic in wild-type mice.35,36
We then tested whether loss of episomal vector genomes as a result of hepatocyte proliferation or cell death contributed to the decline in expression. First, we assessed the rate of natural hepatocyte turnover by measuring phosphorylated histone 3 (PHH3) and Ki-67 staining as markers of cell proliferation and division, respectively. In liver samples of mice in the take-down cohort, we observed minimal PHH3 and Ki-67 staining throughout the study, indicating that little hepatocyte turnover occurred (Figure S4). These results align with previous observations of limited cell proliferation in mouse livers beyond 1 week of age.37 We next assessed whether hepatocyte death due to cytotoxic T cell responses or cellular stress was occurring. Similarly, we did not observe significant elevation of cluster of differentiation 4 (CD4)- or CD8-positive T cells (Figure S5), of ER stress as measured with glucose regulated protein 78 (GRP78) staining (Figure S6), or of apoptotic cells as indicated by cleaved caspase 3 (CC3) staining (Figure S7) in any treatment groups. Similarly, no elevation of mean serum alanine aminotransferase (ALT) levels was observed (Figure S8).
We next assessed the frequency of vector DNA integration into the nuclear genome as a potential source of long-term transgene expression that may vary by vector production method. Using target-enrichment sequencing, we found the number of unique integration sites per cell was comparable between oversized Sf- and 293-produced vectors at weeks 12, 24, and 57 (Figure S9). The mean (standard deviation [SD]) unique integration sites per cell (IS/cell) in mice treated with Sf-produced vector were 0.0019 (0.0004), 0.0023 (0.0013), and 0.0022 (0.0006) at weeks 12, 24, and 57, respectively. The mean (SD) unique IS/cell in mice treated with 293-produced vector were 0.0014 (0.0004), 0.0016 (0.0007), and 0.0016 (0.0005) at weeks 12, 24, and 57, respectively. For vectors produced with either method, the similar rate of integration observed during weeks 12 through 57 suggests that integration occurs early and does not increase over time.
Degradation of liver vector genome mediates decline of transgene expression in mice dosed with 293-produced vector
Having ruled out hepatocyte proliferation or loss as the mechanism of decline and confirmed that there were no differences in vector integration, we next determined whether metabolism of vector genomes within hepatocytes was occurring by evaluating levels of vector DNA in the liver over time in each of the take-down cohorts using droplet digital PCR (ddPCR). Overall, genome copy numbers from 293- and Sf-produced oversized vectors declined sharply between weeks 1 and 3 post dose at similar rates, but the pattern of decline diverged after week 3. In the mice dosed with 293-oversized vectors, total copy numbers of genomes in the liver decreased continuously from week 3 through week 57, and liver vector genome levels were significantly correlated with circulating hA1AT protein levels at weeks 12, 24, and 57 (Figure 4A). Similar changes in vector genomes between peak and terminal time points were observed when considering only circular episomes (Figure S10). This suggests that for mice dosed with 293 vector, the decline in protein expression over time is primarily mediated by decline in liver genomes. However, in mice treated with Sf-oversized vectors, copy numbers of vector genomes in the liver were fairly stable after week 3, and liver vector genome levels were not correlated with circulating hA1AT levels (Figure 4B). Therefore, vector genome metabolism in hepatocytes may not be the primary mechanism responsible for the decline of hA1AT protein expression in mice following dosing with Sf-produced oversized vectors.
Figure 4.
Levels of vector DNA in the liver and correlation with serum transgene protein levels
(A) Liver DNA and serum protein in mice dosed with 293-produced oversized vectors. (B) Liver DNA and serum protein in mice dosed with Sf-produced oversized vectors. Bar graphs show mean ± SD. Significance was assessed with a Pearson correlation. 293, mammalian HEK293 cell system; HEK, human embryonic kidney; SD, standard deviation; Sf, Spodoptera frugiperda insect cell system; wk, week.
We next performed RNA sequencing (RNA-seq) of liver tissue and applied gene set enrichment analysis (GSEA) to identify coordinated changes in a gene set within a cellular pathway. 293 vector-treated mice had the highest normalized enrichment score (adjusted p < 0.05) for pathways including tumor necrosis factor α signaling via nuclear factor κB, interferon-α (IFN-α) and IFN-γ responses, transforming growth factor β signaling, and other inflammatory responses at 12 weeks post dose compared with Sf vector-treated mice (Table S2) and vehicle-treated mice (Table S3), respectively. In Sf-treated mice, over-represented pathways compared with vehicle-treated mice included oxidative phosphorylation, G2M checkpoint, and DNA repair, but no significant enrichment of immune pathways was noted (Table S4). Together, these data suggest that a heightened innate immune response to 293-produced vector and potentially immune-related metabolism of vector genomes mediates the decline of transgene expression in mice treated with 293-produced vectors.
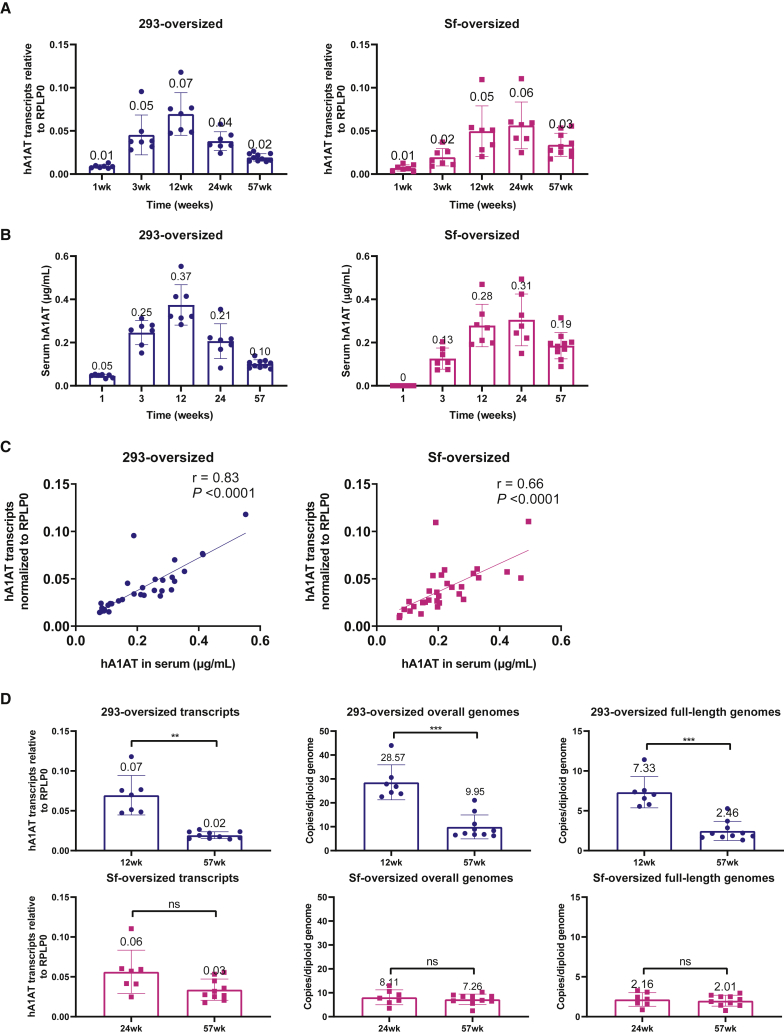
We investigated further by analyzing hA1AT RNA expression levels in liver samples collected from mice in the take-down cohorts and compared them with observed levels of serum protein expression. Across 57 weeks, the RNA transcript expression profile in the liver was similar to hA1AT serum protein expression levels for both 293 and Sf cohorts (Figures 5A and 5B). Indeed, RNA and protein levels were significantly correlated for oversized vectors produced from both manufacturing systems (Figure 5C), indicating that the amount of RNA present in cells contributes directly to the level of transgene protein expression. The ratio of RNA to DNA was significantly higher at weeks 12, 24, and 57 for oversized Sf-produced vectors compared with 293-produced vectors (Figure S11). Of note, the ratio of protein to RNA did not change over time in either cohort (Figure S12); the steady ratio suggests that the level of RNA produced was not saturating for protein production.25
Figure 5.
The patterns of liver RNA and serum protein expression are similar between oversized vectors produced in the two systems
(A) hA1AT RNA production in the liver. (B) Serum hA1AT protein. (C) Correlation between hA1AT RNA and serum hA1AT protein. (D) Decrease in liver vector genome levels (HEK293) or transcriptional activities (Sf) from peak expression (12 weeks for HEK293 and 24 weeks for Sf) to final time point may be mediating the decline of circulating A1AT protein levels. ∗∗∗p <0.001; ∗∗p < 0.01; ns, not significant. Bar graphs show mean ± SD. Squares and circles indicate individual samples. Significance was assessed with a Pearson correlation for (C) and Welch’s t test for (D). 293, mammalian HEK293 cell system; hA1AT, human α1-antitrypsin; HEK, human embryonic kidney; RPLP0, ribosomal protein lateral stalk subunit P0; SD, standard deviation; Sf, Spodoptera frugiperda insect cell system; wk, week.
Altogether, these data suggest that a change in liver genome levels may be mediating the decline of circulating hA1AT protein for 293-produced vector but not for Sf-produced vector (Figure 5D). Other molecular mechanisms must therefore contribute to the observed decline in transgene production from Sf-produced vector. Given that there was no change in vector DNA levels between the peak expression at week 24 and the lowest expression at week 57 but that there was a corresponding decline in transgene RNA and protein, we hypothesized that the decline in protein expression was mediated by a decrease in transcriptional activity of the vector genomes over 57 weeks, perhaps caused by changes in epigenetic modifications of the vector.
Genome accessibility over time for 293- and Sf-produced vectors
To identify changes in the amount of open chromatin available for transcription in vector genomes, we used an assay for transposase-accessible chromatin with high-throughput sequencing (ATAC-seq).38 In ATAC-seq analysis, samples are treated with hyperactive Tn5 transposase to simultaneously fragment and tag genomes in transcriptionally active areas, allowing for evaluation of genome accessibility.38 We performed ATAC-seq on liver samples from mice dosed with 293- or Sf-produced oversized vector in the take-down cohorts when transgene protein expression was at its peak (week 12 for 293 and week 24 for Sf) and at week 57, the final time point. After ATAC-tag counts were corrected for sequencing depths and normalized to the copy number of vector genomes, we found a decrease in genome accessibility from peak to decline in the upstream regions (1–660 bp; spanning the ITR, the promoter, and the beginning of the hA1AT open reading frame) in the Sf-produced vectors but not in the 293-produced vectors (Figure 6A). This decrease in genome accessibility in the promoter region may therefore mediate the decline in RNA and subsequent protein production from Sf-produced vectors. Indeed, genome accessibility of Sf-produced vectors as measured with ATAC-seq tags correlated significantly (r = 0.74; p = 0.0059) with levels of transcriptional efficiency as measured by transgene RNA produced per copies of vector genome DNA (Figure 6B).
Figure 6.
Vector genome accessibility of oversized vector genomes in the livers of mice treated with 293- or Sf-produced vector
(A) ATAC-seq tag counts indicating genome accessibility at the time of peak transgene protein expression and at the final time point. (B) Correlation of ATAC-seq tags with transgene RNA/DNA ratio in mice treated with Sf-produced oversized vector. ∗p < 0.05 using Welch’s t test. Peak samples were from week 12 for 293-produced vectors and week 24 for Sf-produced vectors. Final samples were from week 57. Data in (A) are mean ± SD. Tag counts are ATAC-seq peak regions in the AAV5 promoter and regulatory region (1–660 bp) corrected for sequencing depth and normalized to vector genome copies. 293, mammalian HEK293 cell system; AAV, adeno-associated virus; ATAC-seq, assay for transposase-accessible chromatin with sequencing; HEK, human embryonic kidney; SD, standard deviation; Sf, Spodoptera frugiperda insect cell system.
Epigenetic modifications of vector genomes affecting accessibility
Having observed decreased genome accessibility for Sf-produced vectors that correlated with transgene RNA levels, we further explored epigenetic modifications that could lead to decreased transcriptional efficiency. We assessed histone-vector DNA interactions using chromatin immunoprecipitation sequencing (ChIP-seq) and DNA methylation using bisulfite sequencing in liver samples from mice treated with 293- or Sf-produced vector genomes.
Samples of 293- and Sf-produced vector genomes from the livers of mice in the take-down cohorts at weeks 12, 24, and 57 were used for ChIP-seq to analyze association of active (acetylation of lysine 27 on histone H3 [H3K27ac], tri-methylation of lysine 4 on histone H3 [H3K4me3]) and repressive (tri-methylation of lysine 9 on histone H3 [H3K9me3]) histone marks on vector genomes produced in 293 and Sf cells. ChIP-seq peak calling analysis identified H3K27ac and H3K4me3 binding and enrichment at the AAV promoter sites (Figures 7A and 7B). The read counts spanning the promoter peak regions, normalized for sequencing depth and vector genome copy number, showed no differences in H3K27ac or H3K4me3 binding between the peak and final time points for 293-produced vector. Interestingly, H3K27ac and H3K4me3 binding were significantly reduced from peak to final time points for Sf-produced vector genomes (Figures 7C and 7D). No ChIP-seq H3K9me3 peaks could be called, indicating no binding to the repressive H3K9me3 mark. These data are consistent with the ATAC-seq genome accessibility analysis and suggest that the reduced genome accessibility of Sf-produced vector from the peak to final time points significantly decreases the binding of active histone elements, as indicated by H3K4me3 and H3K27ac, revealing a role for epigenetic regulation in the decline of transgene expression in mice dosed with Sf-produced vector.
Figure 7.
Epigenetic modification of vector genomes by production method
ChIP-seq peak calling analysis showing the occupancy and enrichment of (A) H3K27ac and (B) H3K4me3 binding at the vector promoter sites for HEK293 and Sf-treated mice at peak (week 12/week 24) and final (week 57) time points. Reads in peak counts are ChIP-seq tag counts for (C) H3K4me3 (137–618) and (D) H3K27ac (147–870) corrected for sequencing depth and normalized to vector genome copies. (E) CG sites with significantly different methylation ratios between Sf-produced vectors at week 57 as assessed with bisulfite sequencing. (F) Increasing methylation over time at CG site 765 of the AAV5-HLP-hA1AT oversized vector genome. ∗p < 0.05, ∗∗∗∗p < 0.0001 using two-way ANOVA with Tukey’s multiple comparison. (C) and (D) show peak counts in the yellow shaded areas of (A) and (B) corrected for sequencing depth and normalized to vector genome copies. 293, mammalian HEK293 cell system; ANOVA, analysis of variance; CG, cytosine-guanine; ChIP-seq, chromatin immunoprecipitation sequencing; H3K27ac, acetylation of lysine 27 on histone H3; H3K4me3, tri-methylation of lysine 4 on histone H3; HEK, human embryonic kidney; ITR, inverted terminal repeat; polyA, polyadenylation signal; Sf, Spodoptera frugiperda insect cell system; WT-A1AT, wild-type α1-antitrypsin.
We next treated take-down cohort samples with bisulfite to convert unmethylated cytosines in cytosine-guanine (CG) sites to uracil, therefore allowing the precise identification of CG sites with DNA methylation. In vertebrates, methylation of CG sites is typically associated with silenced expression.39,40 When comparing the number of methylated with total CG sites (both methylated and unmethylated), vectors prepared from either manufacturing system were initially unmethylated (methylation ratio approximately 0.003; data not shown) but became methylated in mouse livers after transduction before week 12. Most CG sites at the ITR, promoter, and A1AT transgene did not show significant differential methylation between 293- and Sf-produced vectors (Figure S13), but sites 579 and 765 in the A1AT transgene had a 1.8- and 2.3-fold higher (p < 0.0001) methylation ratio, respectively, in Sf-produced vector compared with 293-produced vector at 57 weeks (Figure 7E). The extent of methylation for most sites remained similar from 12 weeks to 57 weeks; however, the methylation ratio of site 765 increased over time in both 293- and Sf-produced vectors, suggesting a potential impact on transcriptional gene regulation over time (Figure 7F). Whether the changing methylation over time on Sf-produced vectors in mouse livers resulted in differences in genome accessibility and reduced expression between peak and final time points remains to be determined.
Discussion
Overall, recombinant AAV5 vectors produced in Sf or HEK293 cells showed similar long-term durability of expression in vivo, although mammalian cell-produced vectors expressed and reached peak expression slightly earlier than insect cell-produced vectors, as observed previously in studies of shorter duration.9 Regardless of production system, expression from oversized vectors was lower than for standard-sized vectors. However, long-term expression from the Sf-produced vectors was at least comparable with, if not higher than, that of HEK293-produced oversized AAV5 vectors. For all vectors tested, the long-term expression profile was characterized by peak expression levels at 8–24 weeks followed by decline of approximately 50% beyond 1 year. For standard-sized vectors produced by either system, peak expression was higher and occurred earlier, potentially due to less need for genome repair. For the oversized Sf-produced vector specifically, the expression profile of individual mice shows an initial ramping-up period, a peak at 8–24 weeks, then a decline of about 50% from peak over 57 weeks (Figure S14). These data suggest that individual mice with a higher peak have a more pronounced difference between peak and plateau levels of serum hA1AT protein.
Similar individual expression profiles in plasma FVIII activity over 6 years were observed in humans treated with 6 × 1013 vg/kg of the oversized, Sf-produced, AAV5-mediated gene transfer valoctocogene roxaparvovec in a phase 1/2 trial.11,21,22,24 While all participants had a decline from peak expression, a portion maintained stable expression at lower FVIII activity for up to 6 years.21,24 In the phase 3 trial, transgene expression trajectories of a peak followed by a decline for some participants and steady expression for others was observed over 2 years.12,23 In a phase 1/2 trial of an AAV3-mediated FVIII gene therapy, some participants experienced a decline in transgene levels over 2 years of follow-up while others did not; loss of FVIII expression in two participants was attributed to an anti-AAV capsid cellular immune response.10 Preliminary results from the high-dose cohort of a phase 1/2 trial of giroctocogene fitelparvovec, an AAV2/6-mediated FVIII gene therapy, also showed a steady decline in FVIII levels over 2 years of follow-up.41 In a phase 1/2 trial of an AAV8-mediated FIX gene therapy, transgene expression was not sustained beyond 11 weeks for most participants; the decline was hypothesized to result from enrichment of CpG motifs in the vector genome that acted as pathogen-associated molecular patterns and activated an innate immune response against the vector genome.42 In mice and non-human primates, transgene expression patterns of a peak followed by a decline have been observed after AAV-mediated gene transfer.43,44 The similarities between trial data and our observations in mice suggest that the mechanisms we identified as potentially leading to transgene expression decline in this study may be relevant for clinical outcomes in humans. Further mechanistic study is needed to definitively confirm our findings. For example, experiments in mice removing CG sites from the vector may be informative. However, analysis of serial human liver biopsies would be required to confirm whether levels of methylation correlate with the degree of FVIII expression decline in human participants who received valoctocogene roxaparvovec; such experiments are likely not possible.
Biophysical characterization of Sf- and 293-produced vectors revealed only a few differences that resulted from the production method. We found that vector genomes in Sf-produced vectors were more fragmented and contained less homogeneous DNA than those produced in 293 cells, but differences in long-term durability or rate of vector DNA integration were not observed. These results are similar to previous work showing that AAV2 vectors produced in Sf cells had a higher proportion of truncated and unresolved genomes, as well as greater overall heterogeneity in vector size.19 We also found that 293-produced vectors contained more VP1 capsid protein than did Sf-produced vectors. A higher proportion of VP1 in the capsid may have facilitated the observed higher initial uptake of these vectors, similar to our observations, but the majority of DNA was degraded by week 3 regardless of the production system.26,45 Interestingly, another head-to-head comparison of AAV manufacturing methods found that rAAV5 capsids manufactured in Sf cells had higher VP1 content and transduction efficacy in the brains of mice than those manufactured in 293 cells, but the capsid composition of rAAV9 vectors did not differ by manufacturing method.17
As expected, oversized vectors had lower expression than standard-sized vectors regardless of time point or production method. This is likely due to the fragmented and heterogeneous packaging of vector genomes slightly over the packaging size limit of AAV vectors.13,46 While Sf-produced standard-sized vectors had lower expression than 293-produced oversized vectors, as expected given previous research,20 Sf-produced oversized vectors had comparable, if not better, expression at week 57 than 293-produced oversized vectors. The higher expression at later time points by Sf-produced oversized vectors may be related to the fact that different mechanisms mediate the long-term decline of oversized vector expression depending on the production system.
To identify the mechanisms mediating expression trajectories over time for oversized vectors, we systemically tested multiple hypotheses. As expected, since hA1AT is non-immunogenic in mice,35,36 anti-hA1AT antibodies capable of targeting transgene protein were not detected with vectors produced from either production method. We also found little evidence of hepatocyte turnover due to either natural proliferation or cytotoxic T cell responses, ER stress, or apoptosis. In mice, hepatocyte proliferation is not expected beyond 1 week of age,37,47 and we have previously shown that the weaker promoter used here does not induce ER stress when producing FVIII protein.48 The lack of cell death or cytotoxic responses observed here post AAV5 dosing is reassuring, although previous research suggests that mice might not be the optimal model system for investigating mechanisms contributing to post-gene-therapy ALT rises that are presumed to result from immune responses against transduced hepatocytes.11,12,22,30,49 However, our results are supported by histopathology of liver biopsies taken between 2.6 and 4.1 years post dose from participants in a phase 1/2 trial of AAV5-hFVIII-SQ that showed no fibrosis, chronic inflammation, or ER stress associated with human FVIII protein production.25 We also observed no difference in rates of nuclear genome integration by vector production method.
For 293-produced oversized vectors, we observed a steady decrease in vector genome levels in hepatocytes even after week 3, which was not mirrored in mice dosed with Sf-produced oversized genomes. We previously demonstrated that the processing kinetics of oversized vector genomes packaged in AAV5 are the same as observed for all other AAV vectors, where total vector DNA initially declines rapidly in the first 3 weeks and long-term transgene protein expression is associated with the formation in hepatocytes of stable, circular episomes that contain single or multiple full-length vector genomes.25,26,43,50,51,52,53 In line with those observations, levels of vector genomes decreased sharply in hepatocytes between week 1 and week 3, regardless of production method. During weeks 12 through 57, levels of 293-produced vector genomes continued to decrease steadily while levels of Sf-produced vector genomes did not. This was also observed with other Sf-produced vector genomes with different gene expression cassettes (data not shown). Importantly, levels of hA1AT protein were significantly correlated with hepatocyte vector DNA in 293-dosed mice but not in Sf-dosed mice. These data indicate that the decline in vector genomes is linked to the decline in transgene protein expression only for 293-produced vectors, suggesting differential metabolism of vector genomes.
Our RNA-seq data show that 293 vector-treated mice appear to have significantly higher inflammatory response at 12 weeks post dose compared with Sf vector-treated mice, suggesting that vector genome metabolism may be due to an antiviral immune response. The host immune response is one of the most crucial factors that blocks transgene expression in the long term and remains a challenge for AAV gene therapy. Recombinant AAV vectors produced on different platforms have varying post-translational modifications.20 Moreover, host cell impurities differ between platforms, such as immunogenic N-linked glycans that can activate sensing of AAV vector elements, and pattern recognition receptors such as Toll-like receptors that recognize the foreign particles (pathogen-associated molecular patterns, microbial proteins, and glycolipid structures) and activate intracellular signaling cascades to induce expression of proinflammatory cytokines, chemokines, and IFNs.54,55,56,57 Secreted IFNs and cytokines enhance innate immune responses and induce expression of IFN-stimulated genes that inhibit any step of the viral life cycle, including entry, uncoating, DNA replication, transcription, translation, and assembly.58,59,60 In addition to activating innate immune responses, cellular sensing of viral DNA also primes adaptive immunity and epigenetic silencing of foreign DNA.61,62 Our data show that 293-produced vectors trigger a heightened immune response as compared with Sf-produced vectors and therefore potentially mediate the decline in transgene expression and durability.
For Sf-produced vectors, transgene protein levels were not associated with vector DNA but were associated with vector RNA levels, suggesting that a decrease in transcription occurred over time. However, Sf-produced oversized vectors still provided significantly higher transgene protein expression than 293-produced vectors at week 57. We used high-throughput methods to assess whether vector genome accessibility was changing over time. AAV vectors can stably persist over time as episomes and can thus maintain a chromatin-like structure,43 indicating that AAV minichromosome epigenetic regulation may impact long-term gene expression. Recombination of the ITR aids in concatemer formation and functions as a transcriptional regulatory element.63,64 DNA accessibility is a typical requisite for transcriptional regulation, and the dynamics of genome accessibility changes impact transgene expression. We found that for Sf-produced vectors, the vector genome accessibility for transcriptional regulation decreased between week 24 (peak expression) and week 57 (final time point).
We therefore assessed whether changes in DNA methylation and histone acetylation of vector genomes occurred that could result in changing transgene expression. DNA bisulfite sequencing indicated that Sf-produced vectors had higher ratios of DNA methylation than did 293-produced vectors; increasing methylation over time at certain CG sites may result in declining expression from these vector genomes. Once the methylation ratio increases above a specific threshold, it may trigger a change in expression.65 In addition to regulation of transgene expression by transcription factors and histone occupancy, DNA methylation can change the nucleosome physical properties and accessibility of regulatory elements.66
Previously, histone activation and repression markers have also been identified on AAV vector genomes,67,68 suggesting that the host cell epigenetically regulates vector genomes. Recently, the human silencing hub complex that deposits repressive histone marks was shown to suppress AAV transcript production by epigenetic modification of associated host histones.33 In our study, we found that vector interactions with the active histone marks H3K4me3 and H3K27ac, which are associated with transcriptionally active chromatin, were significantly reduced from the time of peak expression to the final time point for Sf-produced genomes but not 293-produced genomes. H3K4me3 marks stimulate activator-dependent transcription by recruiting proteins, including TATA binding protein (TBP) and TBP-associated factors, and are essential for transcription initiation by RNA polymerase II.69 Thus, the decreasing association of the vector promoter with these active marks over time could lead to less vector transcription.
Changes in epigenetic regulation of vector genomes may underlie clinical observations. In two participants who received 6 × 1013 vg/kg AAV5-hFVIII-SQ who had initially high FVIII activity that declined over several years, liver biopsies revealed at least 3–4 copies of vector genome per diploid genome present in their hepatocytes over 4 years post dose.25 As vector genomes were present as episomes in the cells of these participants, decreasing genome accessibility, perhaps mediated by changes in DNA methylation and loss of active histone marks, may have resulted in the decline of transgene protein production. Furthermore, approximately 50% of these participants’ hepatocytes still contained vector DNA.25 If the transcriptional machinery could be turned back on and vector genomes reopened, the durability and hemostatic benefit of gene transfer could potentially be extended. Application of a histone deacetylase inhibitor significantly improved the expression of an rAAV transgene in cancer cell lines;70 similar methods may work in hepatocytes to modulate epigenetic regulation of vector genomes.
Overall, it appears that for AAV vectors, various molecular mechanisms can affect the trajectory of transgene expression. This is evident in our results, which show that while oversized vectors had similar durability in long-term expression, there were clear differences in mechanisms mediating expression of the same AAV5 construct depending on whether it was produced in Sf or 293 cell lines. While we found that changes in genome accessibility and vector genome copy number mediated changes in expression, recent work in hemophilia A mice showed that a decline in transgene-produced FVIII 8 weeks after AAV8-mediated transduction was due to translational shutdown, as transgene protein levels decreased in spite of persistent mRNA rather than a loss of transduced hepatocytes.71 Some animals had evidence of ER stress linked to hepatic inflammatory cytokine expression; FVIII protein expression was preserved by suppressing CD8+ T and natural-killer cell responses through inhibition of interleukin-15 and its receptor.71 Together, these findings illustrate the many different factors that can govern transgene expression.
Limitations of this study include the small sample size and that liver samples in the analyses comparing the time point with peak expression with the final time point came from different mice. Serial liver biopsies from the same individual or multiple biopsies collected from early, peak, and late time points post dosing, as were taken from participants in the phase 1/2 trial of valoctocogene roxaparvovec,25 would provide additional insight into the mechanisms mediating the decline of transgene expression over time by allowing changes in vector DNA and RNA to be tracked more precisely over time. We also note that these analyses were performed in one group’s labs with AAV5 vectors manufactured per our methods, and the wider generalizability of our results is unknown.
Overall, these results support the use of Sf cells as comparable with 293 cells for the production and commercialization of recombinant AAV5 vectors, particularly for oversized vectors. Furthermore, we found that the decline in transgene protein expression from oversized vectors produced in Sf cells was not due to loss of vector genomes, as for 293-produced vectors, but rather to changes in vector genome accessibility. Additional research is needed to identify mechanisms and potential approaches to maintain and prolong gene expression for human recipients of AAV-mediated gene therapy.
Materials and methods
AAV reporter vector construction
Reporter vectors expressing secreted wild-type hA1AT were used instead of valoctocogene roxaparvovec to give sequence-length flexibility and minimize variability from serial blood sampling using the tail-nick method, which activates the clotting cascade and leads to consumption of FVIII. Additionally, hA1AT protein is non-immunogenic in wild-type mice, enabling long-term studies in immune-competent hosts.35,36 AAV5-HLP-hA1AT consists of a replication-incompetent AAV5 vector containing single-stranded DNA encoding an hA1AT reporter vector controlled by a hybrid liver promoter (HLP) with double-stranded ITRs at its 5′ and 3′ ends. In addition to the hA1AT transgene, both oversized and standard-sized vectors included a stuffer sequence to make up the appropriate sizes (Figure 1A). Both oversized and standard-sized vectors were manufactured using either a “triple transfection” process in 293 cells or in Sf cells infected with helper and vector baculovirus.9,15,34 Manufacturing in 293 cells was performed by SAB Tech (Philadelphia, PA).
Analytical ultracentrifugation
A Beckman Coulter ProteomeLab XL-I AUC (Beckman, Brea, CA) equipped with absorbance and Rayleigh interference optics was used for sample analysis. Samples were loaded into 2-sector sample cells containing Epon centerpieces. Cells were then loaded into an 8-hole rotor. Samples were temperature-equilibrated at 20°C for no less than 2 h. After temperature equilibration, sedimentation velocity centrifugation was performed on samples at 10,000 rpm for 10–12 h and scans were collected at the maximum detection rate of the equipment. Data were analyzed with the c(s) method as implemented in the program Sedfit40 and previously utilized for AAV capsid analysis.72 In brief, Sedfit directly models the data with numerical solutions to the fundamental equation that describes diffusion and sedimentation in a sector-shaped compartment, the Lamm equation: ∂c/∂t = D[(∂2c/∂r2) + 1/r(∂c/∂r)] − sω2[r(∂c/∂r) + 2c], where c is total AAV concentration, t is time, D is diffusion constant, r is radius, s is sedimentation coefficient, and ω is rotor speed.73 The two terms on the right side of the equation describe two competing forces: diffusion and sedimentation. The diffusion force is driven by molecular motion and moves toward a homogeneous solute solution. The sedimentation force is driven by the applied gravitational field and transports solute to the base of the cell.
Alkaline gel analysis
An aliquot of each rAAV sample containing 1 × 1011 vg was combined with 1.5 μL of 10% SDS (Ambion, Austin, TX) and 7.5 μL of 6× alkaline gel loading dye (Alfa Aesar, Haverhill, MA) and brought to 25 μL with 1× alkaline buffer (50 mM NaOH, 1 mM EDTA). The size-separation of samples was performed on an alkaline agarose gel (0.8% of agarose in 1× alkaline buffer) kept on ice for 3.5 h at 60 V. The New England Biolabs (Ipswich, MA) 1-kb ladder was used to determine the approximate molecular weights of DNA in each sample. After three 10-min washes in neutralizing buffer (1 M Tris-HCl, 1 M NaCl; pH 7.40), the gel was stained for 30 min using GelRed (diluted 1.5:10,000 in 0.1× neutralizing buffer; Biotium, Fremont, CA). DNA was visualized on a Chemidoc imaging system using the GelRed read setting.
Study design
Four sets of ten C57BL/6 wild-type mice at 8 weeks of age were treated with a 6 × 1013 vg/kg dose of vector (293-produced standard-sized, 293-produced oversized, Sf-produced standard-sized, and Sf-produced oversized, respectively). Serial blood collections were performed at weeks 2 and 4 and then monthly through week 57. Additional cohorts of mice (n = 7 each) were dosed with vector, and livers were collected for vector genome analysis by ddPCR, immunohistochemistry, ATAC-seq, bisulfite sequencing, and ChIP-seq at weeks 1, 3, 12, 24, and 57. All in vivo mouse experiments were performed in accordance with institutional guidelines under protocols approved by the Institutional Animal Care and Use Committee of the Buck Institute.
Detection of human A1AT protein in serum
Levels of hA1AT protein in mouse serum samples were determined using the Ella microfluidic immunoassay instrument (Protein Simple, San Jose, CA) using 72 × 1 Simple Plex assay cartridges for the detection of human SERPIN A1 (hA1AT). This assay is specific to hA1AT and does not cross-react with endogenous mouse A1AT protein. Samples were diluted 1:25, 1:100, or 1:250 with the provided reagent diluent (Protein Simple). All samples were analyzed in duplicate following the manufacturer’s instructions for the Ella instrument. Average concentrations were calculated from duplicate measurements, and samples with a coefficient of variation of below 20% met acceptance criteria.
Immunohistochemistry
For hepatic expression of the ER stress marker GRP78, slides were deparaffinized and rehydrated in a series of decreasing graded ethanols. Antigen retrieval solution CC1 (Ventana Discovery, Tucson, AZ) was used to retrieve antigen at 95°C for 32 min. Sections were blocked in 2% normal donkey serum (NDS), 0.1% BSA, and 0.3% Triton in 1× Tris-buffered saline (TBS) for 45 min at room temperature. Sections were immunostained using anti-GRP78 antibody (1:1,000; Cell Signaling Technology, Danvers, MA) diluted in Ventana reaction buffer (Ventana Medical Systems, AZ, USA). Slides were incubated overnight at 4°C, then washed in 3× TBS. Anti-GRP78 antibody was detected using donkey anti-rabbit immunoglobulin G (IgG) (H + L) highly cross-adsorbed secondary antibody conjugated with Alexa Fluor 555 (1:1,000, A-21206; Thermo Fisher Scientific, Waltham, MA). Secondary antibodies were diluted in Ventana reaction buffer, and sections were incubated for 1 h at room temperature. Slides were washed in 1× TBS, counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and mounted with Fluoromount G. Slides were imaged on a Zeiss Axio Scan.Z1 using a Plan-Apochromat 20×/0.8 objective equipped with a Hamamatsu (Hamamatsu City, Japan) Orca Flash camera. Image analysis was performed using Visiopharm (Hoersholm, Denmark). Mean signal intensity of GRP78 was measured. Image data were exported, organized, and sorted in Excel spreadsheets (Excel 2013, Microsoft), and statistical analyses were performed using Prism 9 (v9.01, GraphPad).
Immunohistochemistry was also used to detect Ki-67, PHH3, CC3, and CD4 and CD8 protein in hepatocytes. Flash-frozen paraffin-embedded livers were sectioned at 5 μm thickness on Superfrost Plus slides and shipped to Ensigna Biosystems (San Leandro, CA) for immunostaining. Single chromogenic stains were performed to label cells positive for Ki-67 (1:50; BioCare, Pacheco, CA), PHH3 (1:200; Cell Signaling), CC3 (1:250; Cell Signaling), CD4 (1:100; Cell Signaling) and mCD8 (1:400; Cell Signaling). All slides were stained using an automated Ventana autostainer using a heat-induced epitope retrieval at pH 6.2. Slides were blocked for 5 min with peroxidase and 10 min with Background Punisher (BioCare), incubated for 45 min with the primary antibody followed by 30 min with horseradish peroxidase tertiary reagent, and developed using 3,3′-diaminobenzidine. Slides were counterstained using hematoxylin and coverslipped using Permount mounting medium. Slides were shipped back to BioMarin and imaged on a Zeiss Axio Scan.Z1 using a Plan-Apochromat 20×/0.8 objective equipped with a Hamamatsu Orca Flash camera. Image analysis was performed using Visiopharm. Hepatocytes were scored as either positive or negative for the proliferation markers Ki-67 and PHH3 and for the apoptotic marker CC3. Percentages of positive CD4 and CD8 cells were quantified. In addition, CD4- and CD8-positive cell clusters (>10 cells) were manually counted and normalized to tissue area. Image data were exported, organized, and sorted in Excel spreadsheets, and statistical analyses were performed using Prism 9 v9.01.
Distribution of AAV5 capsid protein, VP3, was measured by immunohistochemistry. Slides were deparaffinized and rehydrated in a series of decreasing graded ethanols. Antigen retrieval solution CC1 (Ventana Discovery) was used to retrieve antigen at 95°C for 32 min. Sections were blocked in 2% NDS, 0.1% BSA, and 0.3% Triton X-100 in 1× TBS for 45 min at room temperature. Sections were immunostained with anti-VP3 (1:1,000; Novus) diluted in Ventana reaction buffer. Slides were incubated overnight at 4°C and washed in 3× TBS.
Anti-VP3 antibody was detected using donkey anti-rabbit IgG (H + L) cross-adsorbed secondary antibody conjugated to Alexa Fluor 488 (1:500; Thermo Fisher Scientific). The secondary antibody was diluted in Ventana reaction buffer, and sections were incubated for 1 h at room temperature. Slides were washed in 1× TBS, counterstained with DAPI, and mounted with Fluoromount G. Slides were imaged on a Zeiss Axio Scan.Z1 using a Plan-Apochromat 20×/0.8 objective equipped with a Hamamatsu Orca Flash camera. Image analysis was performed using Visiopharm. Hepatocytes were scored as either positive or negative for VP3 protein. Image data were exported, organized, and sorted in Excel spreadsheets, and statistics were performed using Prism 9 v9.01.
In situ hybridization
Formalin-fixed paraffin-embedded liver sections (5 μm) were collected on Superfrost Plus slides using RNA-free conditions. A singleplex in situ hybridization (ISH) protocol was performed using a Ventana Discovery (Tucson, AZ) Ultra Autostainer, RNAscope (Newark, CA), Universal 2.5 reagent kit, with custom-generated probes to detect vector genome DNA. Slides were imaged on an Axio Scan.Z1 (Zeiss) slide scanner using a Plan-Apochromat 40×/0.8 objective equipped with a Hamamatsu Orca Flash camera. Hepatocyte nuclei staining positive for vector genome DNA were counted, and ISH area per nucleus was quantified using Visiopharm image analysis software. Image data were exported, organized, and sorted in Excel spreadsheets, and statistics were performed using Prism 9 v9.01.
Fluorometric assay of serum ALT activity
The enzyme ALT catalyzes the reversible transfer of an amino group from alanine to α-ketoglutarate, generating glutamate and pyruvate. ALT is found primarily in liver and serum but occurs in other tissues as well. Hepatocellular injury often results in an increase in serum ALT levels; therefore, elevated serum ALT levels can be used as a marker for liver injury.
ALT levels in 1–5 diluted serum samples from selected groups were determined using the Alanine Aminotransferase Activity Assay Kit (ab241035; Abcam, Cambridge, UK) according to the manufacturer’s instructions for fluorometric measurements. Fluorometric detection was performed with a FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA). Raw data were acquired using SoftMax Pro 6.3 (Molecular Devices) and transferred to Excel spreadsheets for analysis and reporting.
RNA-seq methods
The mRNA samples were prepared using Illumina Stranded mRNA kits (Illumina, San Diego, CA, USA) and sequenced on the NovaSeq 6000 v1.5 using single-end 100-bp reads. Reads were aligned to the Mus musculus genome build GRCm38 (mm10).74 Differential expression analysis was performed using the DESeq2 package in R.75 We then performed GSEA with the clusterProfiler package using the results of the differential expression analysis as input.76 Gene sets for GSEA were downloaded from the Molecular Signatures database.77,78
Integration site analysis
Target-enrichment sequencing
Target-enrichment sequencing (TES) was performed by double-capture using two different RNA 120-bp-long bait sets, both designed based on 8× tiling. The first RNA bait set was homologous to the whole vector sequence, and the second bait set covered only vector regions diverging between the vector and the mouse genome, including the ITRs, promoter-transgene junction, and exon-exon junctions within the transgene and the polyA.
TES was first performed on control samples consisting of vector plasmid spiked into untransduced canine genomic DNA simulating a vector load of one vector genome per cell and non-spiked control. Samples were analyzed in duplicates using an input of 1,000 ng of DNA per replicate. DNA was sheared to ∼500 bp length using an ultrasonicator (Covaris, Woburn, MA), and fragment size was verified by TapeStation (Agilent, Santa Clara, CA). Libraries were prepared using the Agilent SureSelectXT2 kit in line with manufacturer’s instructions. Additional hybridization and PCR enrichment steps were performed on the final library. After library concentration and size distribution evaluation, libraries were sequenced by 2 × 250 bp symmetric paired-end on the Illumina MiSeq platform.
Bioinformatic analyses of sequenced TES amplicons
GENE-IS was used for the bioinformatic analyses of TES-derived data.79 Raw sequence data were filtered according to 100% sample barcode identity and trimmed according to sequence quality (Phred 20). Each sample replicate was analyzed individually. Sequencing reads were aligned to the GRCm38 (mm10) mouse genome74 and vector genome for integration site analysis. Furthermore, vector coverages for each replicate were analyzed and illustrated in Integrative Genome Viewer.80 The average vector coverage, normalized by the average coverage on the subgenomic regions, was also used to calculate the vector copy number for the analyzed samples.
ECLA to detect total antibodies to hA1AT in murine serum
Total antibodies against hA1AT were measured in murine serum using a sandwich ECLA method. Uncoated black MSD Multi-Array 96-well plates (Meso Scale Diagnostics, Rockville, MD) were coated with 1 μg/mL hA1AT (Athens Research, Athens, GA) diluted in PBS for approximately 1 h with moderate shaking (400–500 rpm) at room temperature. Plates were then washed with washing buffer (PBS containing 0.1% Tween 20). Plates were blocked with diluent buffer (TBS with 1% casein; Bio-Rad, Hercules, CA) for 1 h at room temperature, with shaking, and followed by another wash. Samples and controls were diluted 1:20 in diluent buffer and loaded into duplicate wells. Negative control was pooled serum from male C57BL/6 mice. Positive controls were rabbit anti-hA1AT polyclonal antibodies (Novus, Centennial, CO) spiked into pooled male mouse serum at 0.5 and 13.3 μg/mL. Incubation was for approximately 1 h with shaking at room temperature. After another wash, antibodies to hA1AT were detected by the addition of 1 μg/mL ruthenylated protein A/G/L diluted in diluent buffer and incubated at room temperature for 30 min with shaking at 400–500 rpm. Using recombinant fusion protein A/G/L, which can bind all IgG subclasses, as well as IgE, IgA, IgD, and IgM, total Ig binding profiles can be reported. After the final wash, 1× MSD Read Buffer T with surfactants (Meso Scale Diagnostics) containing the substrate tripropylamine was added to initiate a chemical reaction with ruthenium. The electrochemiluminescence (ECL) signal (relative light units [RLU]) was detected using the MESO QuickPlex SQ 120 instrument (Meso Scale Diagnostics) using Methodical Mind software version 1.0.36. Raw data were exported to Excel for analysis. Sample results were expressed as signal over noise (S/N) values calculated by dividing the sample ECL units by the average ECL units of all negative controls.
Vector DNA and RNA quantification
DNA and RNA were extracted from liver samples using the AllPrep DNA/RNA Mini Kit (Qiagen, Hilden, Germany) as per the manufacturer’s instructions. Quantities of vector genomes, including circular genomes, and transcripts were measured with drop-phase ddPCR, which quantifies levels of paired target sequences on a single DNA molecule using two different fluorescent tags, as per previously described methods.26
ATAC-seq methods
Flash-frozen mouse liver tissue was manually disassociated, and 100,000 nuclei were tagmented using the enzyme and buffer provided in the Nextera Library Prep Kit (Illumina) as previously described, with some modifications.38,81 Tagmented DNA was then purified using the MinElute PCR purification kit (Qiagen), amplified with ten cycles of PCR, and purified using Agencourt AMPure SPRI beads (Beckman Coulter, Indianapolis, IN). The resulting material was quantified using the KAPA Library Quantification Kit for Illumina platforms (KAPA Biosystems, Wilmington, MA) and sequenced with PE42 sequencing on the NextSeq 500 sequencer (Illumina).
Reads were aligned using the BWA algorithm, and duplicate reads were removed. Reads were aligned to the ∼5,000 bp vector sequence, and peaks were identified using the MACS 2.1.0 algorithm at a cutoff of p value = 10−7. Aligned vector reads (tags) in the 1–660 bp merged region were counted using BEDtools (v2.25.0) followed by the unix wc -l command.82 The resulting reads in peaks (RIP) numbers were corrected for the relative sequencing depth/coverage for each sample and for the determined vector copy number for each sample.
Bisulfite sequencing methods
Sample preparation
Frozen liver tissues were sent to Zymo Research (Irvine, CA) for targeted bisulfite sequencing. Assays were designed to target CpG sites in specified regions of interest for PCR amplification. Amplicon target size was >100 bp and <300 bp; primers were designed to avoid annealing to CpG sites at the region of interest if possible (Table S5). After primer validation, samples were bisulfite converted using the EZ DNA Methylation-Lightning Kit (Zymo Research) as per the manufacturer’s instructions, and PCR amplification of all samples was performed. The resulting amplicons were pooled for harvesting and subsequent barcoding using limited amplification cycles. After barcoding, samples were pooled, purified with DNA Clean & Concentrator-5 (Zymo Research), and prepared for massively parallel sequencing using a MiSeq V2 300bpv2 300 bp reagent kit (Illumina) and paired-end sequencing protocol as per the manufacturer’s guidelines.
Targeted sequence alignments and data analysis
Sequence reads were identified using standard Illumina base-calling software and analyzed in Python. Low-quality nucleotides and adapter sequences were trimmed during analysis quality control. Sequence reads were aligned back to the reference genome using paired-end alignment with the software Bismark, with the “non_directional” parameter applied and default parameters used otherwise.83 Nucleotides in primers were trimmed from amplicons during methylation calling. The methylation level of each sampled cytosine (C) was estimated as the number of reads reporting a C divided by the total number of reads reporting a C or thymine (T).
ChIP-seq methods
Chromatin immunoprecipitation
Frozen tissue was sent to Active Motif Services (Carlsbad, CA) for ChIP-seq. Active Motif prepared chromatin, performed ChIP reactions, generated libraries, sequenced the libraries, and performed basic data analysis. In brief, tissue was submersed in PBS + 1% formaldehyde, cut into small pieces, and incubated at room temperature for 15 min. Fixation was stopped by the addition of 0.125 M glycine (final). The tissue pieces were then treated with a Tissue-Tearor and finally spun down and washed twice in PBS. Chromatin was isolated by adding lysis buffer and then disrupted with a Dounce homogenizer. Lysates were sonicated and the DNA sheared to an average length of 300–500 bp with Active Motif’s EpiShear probe sonicator. Genomic DNA (input) was prepared by treating aliquots of chromatin with RNase, proteinase K, and heat for de-crosslinking, followed by cleanup with SPRI Beads (Beckman Coulter) and quantitation using CLARIOstar (BMG Labtech, Ortenberg, Germany). Extrapolation to the original chromatin volume allowed determination of the total chromatin yield.
An aliquot of chromatin (30 μg) was pre-cleared with protein A agarose beads (Invitrogen, Waltham, MA). Genomic DNA regions of interest were isolated using 4 μg of antibody against H3K4me3 and H3K9me3 (Active Motif). Complexes were washed, eluted from the beads with SDS buffer, and subjected to RNase and proteinase K treatment. Crosslinks were reversed by incubation overnight at 65°C, and ChIP DNA was purified by phenol-chloroform extraction and ethanol precipitation.
Quantitative PCR (qPCR) reactions were carried out in triplicate on specific genomic regions using SYBR Green Supermix (Bio-Rad, Hercules, CA). The resulting signals were normalized for primer efficiency by carrying out qPCR for each primer pair using input DNA.
ChIP sequencing (Illumina)
Illumina sequencing libraries (a custom type derived from the original paired-end library84) were prepared from the ChIP and input DNA on an automated system (Apollo 342; Wafergen Biosystems/Takara, Fremont, CA). After a final PCR amplification step, the resulting DNA libraries were quantified and sequenced on Illumina’s NextSeq 500 (75 nt reads, single end). Reads were aligned separately to the ∼5,000 vector sequence using the Burrows-Wheeler Aligner algorithm (aln mode; default settings).85 Duplicate reads were removed, and only uniquely mapped reads (mapping quality ≥25) were used for further analysis. Peak locations were determined using the MACS algorithm (v2.1.0) with a cutoff of p value of 10−7,86 and peak calling graphs were generated using the Partek Flow software (v10.0; Partek, St. Louis, MO). The read counts were quantified similarly to ATAC-seq, and the RIP numbers were corrected for the relative sequencing depth/coverage for each sample and for the determined vector copy number for each sample.
Statistical methods
Serum hA1AT protein levels from oversized Sf or 293-produced vectors at weeks 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, and 57 were compared using a repeated-measures mixed-effects model with log transformation; p values were adjusted to account for multiple testing using the simulate method in SAS Proc Mixed (SAS, Cary, NC). Genome copy numbers and transcript levels were compared using a Welch’s t test or an unpaired t test. All correlation analyses used simple linear regression with a Pearson correlation. Benjamini-Hochberg adjusted p values were calculated for differential expression and GSEA in RNA-seq experiments, using a p value of <0.05 as a cutoff for inclusion. Data for image analysis of immunohistochemistry were analyzed using one-way ANOVA followed by Sidak’s multiple comparison test. ATAC-seq and histone ChIP-seq tag counts were analyzed using Welch’s unpaired t test. Differential methylation analyses were performed using two-way ANOVA with Tukey’s multiple comparison test.
Acknowledgments
Funding for this study was provided by BioMarin Pharmaceutical. Medical writing support was provided by Kathleen Pieper, PhD, of AlphaBioCom and funded by BioMarin Pharmaceutical. We thank the following employees of BioMarin Pharmaceutical: Micah Robinson for project management support; Oktay Kirak for reviewing the manuscript and providing suggestions; and Katina Ngo for her contributions to the molecular and biochemical analyses. We thank Peter Colosi, formerly of BioMarin Pharmaceutical, for designing the vectors used in this study.
Author contributions
S.F., S. Bunting, and S. Bullens contributed to the study design. B.H., C.-R.S., T.B., and A.M.I. performed the molecular and biochemical analyses. B.Y. and C.K.K. performed immunohistochemical and imaging analyses. L.Z., L.X., and R.M. performed the animal experiments. M.X.D., S.S., R.D.A., O.A.K., N.L.M., and V.S.B. performed vector characterization. All authors critically reviewed the manuscript and contributed to interpretation of the data.
Declaration of interests
B.H., A.M.I., L.Z., B.Y., C.K.K., L.X., C.-R.S., R.M., T.B., N.L.M., M.X.D., E.P., V.S.B., S. Bullens, S. Bunting, and S.F. are employees and stockholders of BioMarin Pharmaceutical Inc. S.S. and R.D.A. are former employees of BioMarin Pharmaceutical Inc. and may hold stock.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.11.001.
Supplemental information
Data availability
Materials and protocols will be distributed to qualified scientific researchers for non-commercial, academic purposes. Part of AAV5-hA1AT vector and the AAV5-hA1AT vector sequence is an ongoing development program and will not be shared.
References
- 1.Srivastava A., Lusby E.W., Berns K.I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J. Virol. 1983;45:555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samulski R.J., Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu. Rev. Virol. 2014;1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- 3.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakim C.H., Wasala N.B., Pan X., Kodippili K., Yue Y., Zhang K., Yao G., Haffner B., Duan S.X., Ramos J., et al. A five-repeat micro-dystrophin gene ameliorated dystrophic phenotype in the severe DBA/2J-mdx model of Duchenne muscular dystrophy. Mol. Ther. Methods Clin. Dev. 2017;6:216–230. doi: 10.1016/j.omtm.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harper S.Q., Hauser M.A., DelloRusso C., Duan D., Crawford R.W., Phelps S.F., Harper H.A., Robinson A.S., Engelhardt J.F., Brooks S.V., Chamberlain J.S. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat. Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 6.Wang B., Li J., Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. USA. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courter S.G., Bedrosian C.L. Clinical evaluation of B-domain deleted recombinant factor VIII in previously untreated patients. Semin. Hematol. 2001;38:52–59. doi: 10.1016/s0037-1963(01)90109-x. [DOI] [PubMed] [Google Scholar]
- 8.McIntosh J., Lenting P.J., Rosales C., Lee D., Rabbanian S., Raj D., Patel N., Tuddenham E.G.D., Christophe O.D., McVey J.H., et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–3344. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunting S., Zhang L., Xie L., Bullens S., Mahimkar R., Fong S., Sandza K., Harmon D., Yates B., Handyside B., et al. Gene therapy with BMN 270 results in therapeutic levels of FVIII in mice and primates and normalization of bleeding in hemophilic mice. Mol. Ther. 2018;26:496–509. doi: 10.1016/j.ymthe.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George L.A., Monahan P.E., Eyster M.E., Sullivan S.K., Ragni M.V., Croteau S.E., Rasko J.E.J., Recht M., Samelson-Jones B.J., MacDougall A., et al. Multiyear factor VIII expression after AAV gene transfer for hemophilia A. N. Engl. J. Med. 2021;385:1961–1973. doi: 10.1056/NEJMoa2104205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 12.Ozelo M.C., Mahlangu J., Pasi K.J., Giermasz A., Leavitt A.D., Laffan M., Symington E., Quon D.V., Wang J.D., Peerlinck K., et al. Valoctocogene roxaparvovec gene therapy for hemophilia A. N. Engl. J. Med. 2022;386:1013–1025. doi: 10.1056/NEJMoa2113708. [DOI] [PubMed] [Google Scholar]
- 13.Kyostio-Moore S., Berthelette P., Piraino S., Sookdeo C., Nambiar B., Jackson R., Burnham B., O'Riordan C.R., Cheng S.H., Armentano D. The impact of minimally oversized adeno-associated viral vectors encoding human factor VIII on vector potency in vivo. Mol. Ther. Methods Clin. Dev. 2016;3:16006. doi: 10.1038/mtm.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chahal P.S., Schulze E., Tran R., Montes J., Kamen A.A. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods. 2014;196:163–173. doi: 10.1016/j.jviromet.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotin R.M., Snyder R.O. Manufacturing clinical grade recombinant adeno-associated virus using invertebrate cell lines. Hum. Gene Ther. 2017;28:350–360. doi: 10.1089/hum.2017.042. [DOI] [PubMed] [Google Scholar]
- 16.Kurasawa J.H., Park A., Sowers C.R., Halpin R.A., Tovchigrechko A., Dobson C.L., Schmelzer A.E., Gao C., Wilson S.D., Ikeda Y. Chemically defined, high-density insect cell-based expression system for scalable AAV vector production. Mol. Ther. Methods Clin. Dev. 2020;19:330–340. doi: 10.1016/j.omtm.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondratov O., Marsic D., Crosson S.M., Mendez-Gomez H.R., Moskalenko O., Mietzsch M., Heilbronn R., Allison J.R., Green K.B., Agbandje-McKenna M., et al. Direct head-to-head evaluation of recombinant adeno-associated viral vectors manufactured in human versus insect cells. Mol. Ther. 2017;25:2661–2675. doi: 10.1016/j.ymthe.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm D., Kern A., Rittner K., Kleinschmidt J.A. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 19.Tran N.T., Lecomte E., Saleun S., Namkung S., Robin C., Weber K., Devine E., Blouin V., Adjali O., Ayuso E., et al. Human and insect cell-produced recombinant adeno-associated viruses show differences in genome heterogeneity. Hum. Gene Ther. 2022;33:371–388. doi: 10.1089/hum.2022.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rumachik N.G., Malaker S.A., Poweleit N., Maynard L.H., Adams C.M., Leib R.D., Cirolia G., Thomas D., Stamnes S., Holt K., et al. Methods matter: standard production platforms for recombinant AAV produce chemically and functionally distinct vectors. Mol. Ther. Methods Clin. Dev. 2020;18:98–118. doi: 10.1016/j.omtm.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasi K.J., Laffan M., Rangarajan S., Robinson T.M., Mitchell N., Lester W., Symington E., Madan B., Yang X., Kim B., et al. Persistence of haemostatic response following gene therapy with valoctocogene roxaparvovec in severe haemophilia A. Haemophilia. 2021;27:947–956. doi: 10.1111/hae.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasi K.J., Rangarajan S., Mitchell N., Lester W., Symington E., Madan B., Laffan M., Russell C.B., Li M., Pierce G.F., et al. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for hemophilia A. N. Engl. J. Med. 2020;382:29–40. doi: 10.1056/NEJMoa1908490. [DOI] [PubMed] [Google Scholar]
- 23.Mahlangu J., Chambost H., Chou S., Dunn A., von Drygalski A., Kaczmarek R., Kenet G., Laffan M., Leavitt A., Madan B., et al. Relationship between transgene-produced FVIII and bleeding rates 2 years after gene transfer with valoctocogene roxaparvovec: results from GENEr8-1. Res. Pract. Thromb. Haemost. 2022;6:e12787. [Google Scholar]
- 24.Laffan M.A., Rangarajan S., Lester W., Symington E., Madan B., Hart D.P., et al. Hemostatic results for up to 6 years following treatment with valoctocogene roxaparvovec, an AAV5-hFVIII-SQ gene therapy for severe hemophilia A. Res. Pract. Thromb. Haemost. 2022:e12787. [Google Scholar]
- 25.Fong S., Yates B., Sihn C.R., Mattis A.N., Mitchell N., Liu S., Russell C.B., Kim B., Lawal A., Rangarajan S., et al. Interindividual variability in transgene mRNA and protein production following adeno-associated virus gene therapy for hemophilia A. Nat. Med. 2022;28:789–797. doi: 10.1038/s41591-022-01751-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sihn C.R., Handyside B., Liu S., Zhang L., Murphy R., Yates B., Xie L., Torres R., Russell C.B., O'Neill C.A., et al. Molecular analysis of AAV5-hFVIII-SQ vector-genome-processing kinetics in transduced mouse and nonhuman primate livers. Mol. Ther. Methods Clin. Dev. 2022;24:142–153. doi: 10.1016/j.omtm.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batty P., Mo A., Hurlbut D., Ishida J., Yates B., Brown C., Harpell L.M., Hough C., Pender A., Rimmer E.K., et al. Long-term follow-up of liver-directed, adeno-associated vector-mediated gene therapy in the canine model of hemophilia A. Blood. 2022 doi: 10.1182/blood.2021014735. [DOI] [PubMed] [Google Scholar]
- 28.Verdera H.C., Kuranda K., Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol. Ther. 2020;28:723–746. doi: 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao O., Dobrzynski E., Wang L., Nayak S., Mingle B., Terhorst C., Herzog R.W. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Rasko J., Ozelo M.C., Hoots K., Blatt P., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 31.Hauck B., Zhao W., High K., Xiao W. Intracellular viral processing, not single-stranded DNA accumulation, is crucial for recombinant adeno-associated virus transduction. J. Virol. 2004;78:13678–13686. doi: 10.1128/JVI.78.24.13678-13686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Xie J., Lu H., Chen L., Hauck B., Samulski R.J., Xiao W. Existence of transient functional double-stranded DNA intermediates during recombinant AAV transduction. Proc. Natl. Acad. Sci. USA. 2007;104:13104–13109. doi: 10.1073/pnas.0702778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das A., Vijayan M., Walton E.M., Stafford V.G., Fiflis D.N., Asokan A. Epigenetic silencing of recombinant adeno-associated virus genomes by NP220 and the HUSH complex. J. Virol. 2022;96:e0203921. doi: 10.1128/jvi.02039-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clément N., Grieger J.C. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther. Methods Clin. Dev. 2016;3:16002. doi: 10.1038/mtm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiuchiolo M.J., Kaminsky S.M., Sondhi D., Hackett N.R., Rosenberg J.B., Frenk E.Z., Hwang Y., Van de Graaf B.G., Hutt J.A., Wang G., et al. Intrapleural administration of an AAVrh.10 vector coding for human alpha1-antitrypsin for the treatment of alpha1-antitrypsin deficiency. Hum. Gene Ther. Clin. Dev. 2013;24:161–173. doi: 10.1089/humc.2013.168. [DOI] [PubMed] [Google Scholar]
- 36.De B., Heguy A., Leopold P.L., Wasif N., Korst R.J., Hackett N.R., Crystal R.G. Intrapleural administration of a serotype 5 adeno-associated virus coding for alpha1-antitrypsin mediates persistent, high lung and serum levels of alpha1-antitrypsin. Mol. Ther. 2004;10:1003–1010. doi: 10.1016/j.ymthe.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L., Yates B., Murphy R., Liu S., Xie L., Handyside B., Sihn C.R., Bouwman T., Galicia N., Tan D., et al. Young mice administered adult doses of AAV5-hFVIII-SQ achieve therapeutic factor VIII expression into adulthood. Mol. Ther. Methods Clin. Dev. 2022;26:519–531. doi: 10.1016/j.omtm.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buenrostro J.D., Giresi P.G., Zaba L.C., Chang H.Y., Greenleaf W.J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinson C., Chatterjee R. CG methylation. Epigenomics. 2012;4:655–663. doi: 10.2217/epi.12.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bock C., Beerman I., Lien W.H., Smith Z.D., Gu H., Boyle P., Gnirke A., Fuchs E., Rossi D.J., Meissner A. DNA methylation dynamics during in vivo differentiation of blood and skin stem cells. Mol. Cell. 2012;47:633–647. doi: 10.1016/j.molcel.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visweshwar N., Harrington T.J., Leavitt A.D., Konkle B.A., Giermasz A., Stine K., Rupon J., Di Russo G., Tseng L.J., de los Angeles Resa M., et al. Updated results of the Alta study, a phase 1/2 study of giroctocogene fitelparvovec (PF-07055480/SB-525) gene therapy in adults with severe hemophilia A. Blood. 2021;138:564–566. [Google Scholar]
- 42.Konkle B.A., Walsh C.E., Escobar M.A., Josephson N.C., Young G., von Drygalski A., McPhee S.W.J., Samulski R.J., Bilic I., de la Rosa M., et al. BAX 335 hemophilia B gene therapy clinical trial results: potential impact of CpG sequences on gene expression. Blood. 2021;137:763–774. doi: 10.1182/blood.2019004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penaud-Budloo M., Le Guiner C., Nowrouzi A., Toromanoff A., Chérel Y., Chenuaud P., Schmidt M., von Kalle C., Rolling F., Moullier P., et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grimm D., Pandey K., Nakai H., Storm T.A., Kay M.A. Liver transduction with recombinant adeno-associated virus is primarily restricted by capsid serotype not vector genotype. J. Virol. 2006;80:426–439. doi: 10.1128/JVI.80.1.426-439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson T.M., Ho M.L., Wahlig B., Gough V., Banta A., Reyes Gamas K., Kang B., Lee E., Chen W., Suh J. An essential N-terminal serine-rich motif in the AAV VP1 and VP2 subunits that may play a role in viral transcription. Virology. 2020;546:127–132. doi: 10.1016/j.virol.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong B., Nakai H., Xiao W. Characterization of genome integrity for oversized recombinant AAV vector. Mol. Ther. 2010;18:87–92. doi: 10.1038/mt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arrojo e Drigo R., Lev-Ram V., Tyagi S., Ramachandra R., Deerinck T., Bushong E., et al. Age mosaicism across multiple scales in adult tissues. Cell Metab. 2019;30:343–351.e343. doi: 10.1016/j.cmet.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fong S., Handyside B., Sihn C.R., Liu S., Zhang L., Xie L., Murphy R., Galicia N., Yates B., Minto W.C., et al. Induction of ER stress by an AAV5 BDD FVIII construct is dependent on the strength of the hepatic-specific promoter. Mol. Ther. Methods Clin. Dev. 2020;18:620–630. doi: 10.1016/j.omtm.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L., Handyside B., Murphy R., Sihn C.R., Xie L., Vitelli C., Harmon D., Sisó S., Liu S., Bullens S., et al. Prednisolone does not regulate factor VIII expression in mice receiving AAV5-hFVIII-SQ: valoctocogene roxaparvovec. Mol. Ther. Methods Clin. Dev. 2020;17:13–20. doi: 10.1016/j.omtm.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan D., Sharma P., Yang J., Yue Y., Dudus L., Zhang Y., Fisher K.J., Engelhardt J.F. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J. Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakai H., Yant S.R., Storm T.A., Fuess S., Meuse L., Kay M.A. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Afione S.A., Conrad C.K., Kearns W.G., Chunduru S., Adams R., Reynolds T.C., Guggino W.B., Cutting G.R., Carter B.J., Flotte T.R. In vivo model of adeno-associated virus vector persistence and rescue. J. Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnepp B.C., Chulay J.D., Ye G.J., Flotte T.R., Trapnell B.C., Johnson P.R. Recombinant adeno-associated virus vector genomes take the form of long-lived, transcriptionally competent episomes in human muscle. Hum. Gene Ther. 2016;27:32–42. doi: 10.1089/hum.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers G.L., Suzuki M., Zolotukhin I., Markusic D.M., Morel L.M., Lee B., Ertl H.C.J., Herzog R.W. Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-mediated gene transfer. J. Innate Immun. 2015;7:302–314. doi: 10.1159/000369273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang X., Yang Y. Innate immune recognition of viruses and viral vectors. Hum. Gene Ther. 2009;20:293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawai T., Sato S., Ishii K.J., Coban C., Hemmi H., Yamamoto M., Terai K., Matsuda M., Inoue J.i., Uematsu S., et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 57.Zhu J., Huang X., Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoggins J.W., Rice C.M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 2011;1:519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unterholzner L., Keating S.E., Baran M., Horan K.A., Jensen S.B., Sharma S., Sirois C.M., Jin T., Latz E., Xiao T.S., et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hotter D., Bosso M., Jønsson K.L., Krapp C., Stürzel C.M., Das A., et al. IFI16 targets the transcription factor Sp1 to suppress HIV-1 transcription and latency reactivation. Cell Host Microbe. 2019;25:858–872.e813. doi: 10.1016/j.chom.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orzalli M.H., Conwell S.E., Berrios C., DeCaprio J.A., Knipe D.M. Nuclear interferon-inducible protein 16 promotes silencing of herpesviral and transfected DNA. Proc. Natl. Acad. Sci. USA. 2013;110:E4492–E4501. doi: 10.1073/pnas.1316194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knipe D.M., Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat. Rev. Microbiol. 2008;6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 63.Haberman R.P., McCown T.J., Samulski R.J. Novel transcriptional regulatory signals in the adeno-associated virus terminal repeat A/D junction element. J. Virol. 2000;74:8732–8739. doi: 10.1128/jvi.74.18.8732-8739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Earley L.F., Conatser L.M., Lue V.M., Dobbins A.L., Li C., Hirsch M.L., Samulski R.J. Adeno-associated virus serotype-specific inverted terminal repeat sequence role in vector transgene expression. Hum. Gene Ther. 2020;31:151–162. doi: 10.1089/hum.2019.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y., Ji Y., Qiu P. Identification of thresholds for dichotomizing DNA methylation data. EURASIP J. Bioinform. Syst. Biol. 2013;2013:8. doi: 10.1186/1687-4153-2013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choy J.S., Wei S., Lee J.Y., Tan S., Chu S., Lee T.H. DNA methylation increases nucleosome compaction and rigidity. J. Am. Chem. Soc. 2010;132:1782–1783. doi: 10.1021/ja910264z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madigan V.J., Yuziuk J.A., Chiarella A.M., Tyson T.O., Meganck R.M., Elmore Z.C., Tse L.V., Hathaway N.A., Asokan A. Ring finger protein 121 is a potent regulator of adeno-associated viral genome transcription. PLoS Pathog. 2019;15:e1007988. doi: 10.1371/journal.ppat.1007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith-Moore S., Neil S.J.D., Fraefel C., Linden R.M., Bollen M., Rowe H.M., Henckaerts E. Adeno-associated virus Rep proteins antagonize phosphatase PP1 to counteract KAP1 repression of the latent viral genome. Proc. Natl. Acad. Sci. USA. 2018;115:E3529–E3538. doi: 10.1073/pnas.1721883115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gates L.A., Foulds C.E., O'Malley B.W. Histone marks in the 'driver's seat': functional roles in steering the transcription cycle. Trends Biochem. Sci. 2017;42:977–989. doi: 10.1016/j.tibs.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okada T., Uchibori R., Iwata-Okada M., Takahashi M., Nomoto T., Nonaka-Sarukawa M., Ito T., Liu Y., Mizukami H., Kume A., et al. A histone deacetylase inhibitor enhances recombinant adeno-associated virus-mediated gene expression in tumor cells. Mol. Ther. 2006;13:738–746. doi: 10.1016/j.ymthe.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Butterfield J.S.S., Yamada K., Bertolini T.B., Syed F., Kumar S.R.P., Li X., Arisa S., Piñeros A.R., Tapia A., Rogers C.A., et al. IL-15 blockade and rapamycin rescue multifactorial loss of factor VIII from AAV-transduced hepatocytes in hemophilia A mice. Mol. Ther. 2022 doi: 10.1016/j.ymthe.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McIntosh N.L., Berguig G.Y., Karim O.A., Cortesio C.L., De Angelis R., Khan A.A., Gold D., Maga J.A., Bhat V.S. Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci. Rep. 2021;11:3012. doi: 10.1038/s41598-021-82599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.The Genome Reference Consortium . 2022. Mouse Reference Genome GRCm38 (mm10)https://www.ncbi.nlm.nih.gov/grc/mouse [Google Scholar]
- 75.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Afzal S., Wilkening S., von Kalle C., Schmidt M., Fronza R. GENE-IS: time-efficient and accurate analysis of viral integration events in large-scale gene therapy data. Mol. Ther. Nucleic Acids. 2017;6:133–139. doi: 10.1016/j.omtn.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thorvaldsdóttir H., Robinson J.T., Mesirov J.P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corces M.R., Trevino A.E., Hamilton E.G., Greenside P.G., Sinnott-Armstrong N.A., Vesuna S., Satpathy A.T., Rubin A.J., Montine K.S., Wu B., et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods. 2017;14:959–962. doi: 10.1038/nmeth.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quinlan A.R., Hall I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krueger F., Andrews S.R. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bentley D.R., Balasubramanian S., Swerdlow H.P., Smith G.P., Milton J., Brown C.G., Hall K.P., Evers D.J., Barnes C.L., Bignell H.R., et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., et al. Model-based analysis of ChIP-seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Materials and protocols will be distributed to qualified scientific researchers for non-commercial, academic purposes. Part of AAV5-hA1AT vector and the AAV5-hA1AT vector sequence is an ongoing development program and will not be shared.