Abstract
The interaction between human neutrophils and wild-type Bordetella pertussis or mutants expressing altered lipopolysaccharide or lacking virulence factors—pertussis toxin, adenylate cyclase toxin, dermonecrotic toxin, filamentous hemagglutinin (FHA), pertactin, or BrkA—was examined. In the absence of antibodies, the wild-type strain and the mutants, with the exception of mutants lacking FHA, attached efficiently to neutrophils. The addition of opsonizing antibodies caused a significant reduction (approximately 50%) in attachment of the wild-type strain and most of the mutants expressing FHA, suggesting that bacterium-mediated attachment is more efficient than Fc-mediated attachment. Phagocytosis was also examined. In the absence of antibodies, about 12% of the wild-type bacteria were phagocytosed. Opsonization caused a statistically significant reduction in phagocytosis (to 3%), possibly a consequence of reduced attachment. Phagocytosis of most of the mutants was similar to that of the wild type, with the exception of the mutants lacking adenylate cyclase toxin. About 70% of the adenylate cyclase toxin mutants were phagocytosed, but only in the presence of opsonizing antibody, suggesting that Fc receptor-mediated signaling may be needed for phagocytosis. These studies indicate that FHA mediates attachment of B. pertussis to neutrophils, but adenylate cyclase toxin blocks phagocytosis.
Bordetella pertussis is the causative agent of whooping cough. During infection the bacteria remain localized to the respiratory tract, causing considerable local damage. However, whooping cough also has aspects of a toxin-mediated disease (30). B. pertussis produces two toxins that are essential for virulence (17, 41). Pertussis toxin catalyzes the transfer of an ADP-ribosyl group to regulatory GTP-binding proteins of mammalian cells, short-circuiting their ability to regulate cellular processes. Exposure to pertussis toxin can inhibit several important cells of the immune system, including neutrophils, macrophages, monocytes, and lymphocytes (10, 38). A second toxin, the adenylate cyclase toxin, catalyzes the conversion of ATP to cyclic AMP in mammalian cells to levels that far exceed what can be achieved by normal cellular mechanisms. Chemotaxis, phagocytosis, superoxide generation, and microbial killing are inhibited in neutrophils and monocytes exposed to adenylate cyclase toxin (9, 38). Adenylate cyclase toxin can also induce apoptosis, or programmed cell death (18). In contrast to pertussis toxin, which is secreted, adenylate cyclase toxin appears to remain on the bacterial surface (23), and it affects only human cells that come into contact with the bacteria.
B. pertussis also produces several adhesins. Several antigenically distinct fimbriae are capable of mediating adhesion (16, 28). The filamentous hemagglutinin (FHA) is a rod-like structure of about 220,000 Da which mediates attachment using an RGD (arginine, glycine, and aspartic acid) integrin receptor motif to bind to mammalian cells and binds to carbohydrate and sulfate groups on lipids and proteins. A family of related outer membrane proteins possessing RGD motifs also promotes adhesion. Pertactin mediates attachment using its RGD motif (26). BrkA also mediates adherence to cells, and in addition it can protect the bacteria from the bactericidal activity of complement (11, 12, 43). Other related proteins include tracheal colonization factor and Vag8 (13, 14). Tracheal colonization factor promotes bacterial growth in the trachea, perhaps by acting as an adhesin (13). Currently, no function has been discovered for Vag8 (14). As a result of the redundancy of adhesins, with the exception of BrkA (39), mutants deficient in the production of a single adhesin are often as virulent as the wild-type strain in animal models of disease (17, 24, 39), and only mutants lacking more than one adhesin have reduced virulence.
Several studies suggest that pertussis vaccines confer better protection from severe disease than from infection (1, 7, 21, 22, 31, 34). A study conducted by Storsaeter et al. (34) showed that 25% of individuals vaccinated with the most efficacious five-component vaccine (pertussis toxin, pertactin, FHA, and fimbriae 2 and 3) had a persistent cough for 21 days or more. Interestingly, in this study (34) and another (7), protection correlated with levels of circulating antibody to pertactin, fimbriae, and to a lesser extent pertussis toxin but did not correlate with levels of antibody to FHA. Since infected people with mild disease are a potential source of infection for susceptible individuals, the ideal vaccine would promote clearance of the organism and would prevent both transmission and severe disease. We have begun to examine the role of bactericidal mechanisms in immunity to pertussis.
Phagocytosis and the subsequent killing of the ingested microorganism compose an immune mechanism that could clear the bacteria from infected individuals. Early reports suggested that B. pertussis is capable of survival and perhaps replication in professional phagocytes (15, 33, 35), but subsequent reports suggest that its intracellular survival is only transient (5, 8, 19, 20, 32). Recently we have shown that only about 1% of B. pertussis cells phagocytosed by neutrophils remain viable, suggesting that phagocytosis could be an important immune defense against B. pertussis (26a). In this study we examined the role of B. pertussis virulence factors and of the presence or absence of opsonizing antibodies in phagocytosis by human neutrophils.
Phagocytosis assay.
Human neutrophils were purified, and 5 × 105 were allowed to adhere to round glass coverslips in 24-well plates for phagocytosis assays as previously described (37). Briefly, green fluorescent protein (GFP)-expressing bacteria (3 × 106) were harvested and incubated with human immune serum or buffer at 37°C for 15 min. Bacterial suspensions were adjusted to 400 μl and were added to adherent neutrophils for 1 h at 37°C in 5% CO2. Where indicated below, bacteria were centrifuged (Marathon 8K; Fisher, Pittsburgh, Pa.) onto the adherent neutrophils at 640 × g for 5 min at room temperature. Phagocytosis was allowed to occur for 1 h at 37°C in 5% CO2. The cells were washed, and adherent extracellular bacteria were stained by adding ethidium bromide (100 μg/ml) for 15 min at room temperature. In this procedure, intracellular GFP-labeled bacteria resist staining with ethidium bromide and remain green, but extracellular ethidium bromide-labeled bacteria appear orange by fluorescence microscopy. Phagocytosis was quantified by fluorescence microscopy.
Role of virulence factors in attachment.
The strains used in this study are described in Table 1. Plasmid CW504, which directs high-level expression of GFP from a constitutive B. pertussis promoter (26a), was introduced into all of the strains by electroporation as described previously (37). Fimbrial expression undergoes phase variation, and individual cells within a population may or may not express fimbriae (27, 28, 39, 42). We have observed temporal variation in fimbrial expression in parental strain BP338, and even different single colonies from the same culture can vary (39, 42). Agglutination using monoclonal antibody BPD5 (against fimbria 2) or BPC10 (against fimbriae 3 and 6) was used to evaluate fimbrial expression as previously described (39, 42). In this assay, more than 10% of the cells in the population must express fimbriae to yield a positive agglutination reaction (39). None of the strains gave a positive agglutination reaction for fimbriae 3 and 6, and as observed previously, expression of fimbria 2 was highly variable (Table 1).
TABLE 1.
Bordetella pertussis strains used in this study
| Strain | Phenotypic designation, description | Reference(s) |
|---|---|---|
| BP338 | Wild type, nalidixic acid-resistant Tohama (29), Fim2−a | 40 |
| BP347 | Bvg, avirulent BP338, Fim2− | 40 |
| BP353 | FhaA, BP338 with transposon insertion in FHA operon, Fim2− | 40 |
| BPM409 | FhaB, BP338 with transposon insertion in the FHA structural gene, Fim2− | 42 |
| BPM3183 | Cyc, BP338 with transposon insertion in adenylate cyclase toxin, Fim2− | 42 |
| BPM2041 | BrkA, BP338 with transposon insertion in BrkA, Fim2+ | 11, 42 |
| BPRA | Ptx, BP338 pertussis toxin deletion, streptomycin resistant, Fim2− | 3 |
| BPM1809 | Dnt, BP338 with transposon insertion in dermonecrotic gene, Fim2− | 42 |
| MLT7 | Lps, BP338 with transposon insertion in LPS operon, Fim2− | 36 |
| BP536 | Wild type, nalidixic acid-resistant, streptomycin-resistant Tohama, Fim2− | 2 |
| bplD | LpsD, BP536 LPS mutant, Fim2+ | 2 |
| bplG | LpsG, BP536 LPS mutant, Fim2+ | 2 |
| bplH | LpsH, BP536 LPS mutant, Fim2+ | 2 |
| bplL | LpsL, BP536 LPS mutant, Fim2+ | 2 |
| BBC8 | Wild type, streptomycin-resistant W28 (29), Fim2+ | 26 |
| BBC9 | Prn, BBC8 pertactin mutant, Fim2+ | 26 |
Fimbrial expression was evaluated using monoclonal antibodies BPD5 (against fimbria 2 [fim2]) and BPC10 (against fimbriae 3 and 6). None of the strains agglutinated with the sera against fimbriae 3 and 6.
The bvg operon encodes a two-component transcriptional activator that is required for expression of the B. pertussis virulence factors (4), including pertussis toxin, adenylate cyclase toxin, dermonecrotic toxin, FHA, pertactin, fimbriae, BrkA, and tracheal colonization factor. An avirulent mutant with an insertion in the bvg operon (BP347) and two mutants deficient in FHA expression, but capable of expression of other bvg-regulated genes, were characterized. BP353 (FhaA) and BPM409 (FhaB) are well-characterized FHA mutants with transposon insertions in genes required for FHA expression. The fhaA gene is required for secretion of FHA and is contained in an operon that also contains genes required for fimbrial secretion (27, 28). Polarity from the fhaA insertion results in a loss of expression of both FHA and fimbriae in mutant BP353. The FhaB mutant has an insertion in the fhaB gene and lacks expression of FHA (42), but a second promoter distal to the fhaB gene (27, 28) could allow this mutant to express fimbriae. However, fimbrial expression was not detected in the parental strain or in any of the FHA mutants (Table 1).
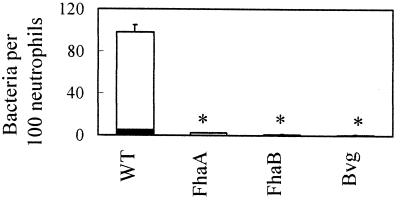
In previously published studies, bacterial suspensions were added to adherent cells and were allowed to settle by gravity. In initial studies, we examined attachment and phagocytosis using these conditions. Approximately 100 wild-type bacteria, or 17% of the total bacterial inoculum (Fig. 1), attached per 100 neutrophils. In contrast, Bvg and FHA mutants bound poorly, with fewer than 5 bacteria attaching per 100 neutrophils, suggesting that FHA is important for attachment to neutrophils. Phagocytosis was also measured. Only 5 wild-type bacteria per 100 neutrophils, or about 1% (Fig. 1), were phagocytosed. No internalized bacteria were observed for the FhaA and Bvg mutants, and only 0.1 bacterium per 100 neutrophils was observed for the FhaB mutant. These numbers seemed quite low, and we speculated that these assay conditions, where bacteria are added to neutrophils and are allowed to settle by gravity, do not promote maximal contact with neutrophils. Further investigation indicated that B. pertussis does not settle significantly within 1 h (26a), limiting its ability to interact with the neutrophils.
FIG. 1.
Effect of FHA on attachment. One hundred consecutive neutrophils were examined for the number of intracellular (green [open bars]) and adherent extracellular (orange [solid bars]) bacteria. Data were analyzed by the Student t test. Each bar represents the mean (± the standard error of the mean) from three to nine independent experiments performed in duplicate. ∗, significantly different from wild type (WT) (P < 0.05).
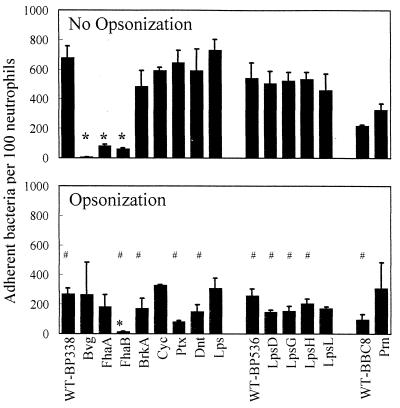
To overcome this problem, we facilitated contact by gently centrifuging the bacteria onto the neutrophils for 5 min at 640 × g. Approximately 700 wild-type bacteria attached to 100 neutrophils, which corresponds to 100% of the bacterial inoculum (Fig. 2, top panel, WT-BP338). Attachment of the strains lacking FHA also was enhanced by centrifugation, but it remained at a significantly lower rate than that of the wild type. Following centrifugation, the rate of attachment of the FhaA mutant increased from 2 to 83 bacteria (P < 0.0001), the rate of attachment of the FhaB mutant increased from 1 to 61 bacteria (P < 0.0004), and the rate of attachment of the avirulent mutant increased from 0.5 to 6 bacteria (P < 0.003). In addition, the rates of attachment of the FhaA (P < 0.003) and the FhaB (P < 0.002) mutants were significantly greater than that of the Bvg avirulent mutant, suggesting that Bvg-regulated adhesins, such as fimbriae, pertactin, BrkA, and tracheal colonization factor, may play a minor role in attachment to neutrophils. Since centrifugation dramatically increased the efficiency of bacterial attachment to neutrophils, subsequent experiments were performed with centrifugation rather than with the previously published procedures.
FIG. 2.
Effects of virulence factors and antibody on attachment. Bacteria were added to adherent neutrophils (no opsonization) or incubated with human immune serum prior to being added to adherent neutrophils (opsonization), centrifugation was performed to promote contact, and phagocytosis was allowed to occur for 1 h. One hundred consecutive neutrophils were examined for the number of adherent extracellular (orange) bacteria. Data were analyzed by the Student t test. Each bar represents the mean (± the standard error of the mean) from 3 to 27 independent experiments performed in duplicate. ∗, significantly different from parental wild type (WT) (P < 0.05); #, significantly different from no opsonization (P < 0.05).
Attachment of mutants lacking the adhesins BrkA (BPM2041) and pertactin (Prn) (BBC9) was examined (Fig. 2, top panel). The rates of attachment of these mutants were comparable to those of their respective parental strains, suggesting that the contribution of these adhesins is masked by the presence of FHA. Mutants expressing altered forms of the lipopolysaccharide (LPS) O chain would be predicted to have significant changes in their surface properties; however, five different LPS mutants attached as efficiently as their parental strains (Fig. 2, top panel).
We also examined attachment of mutants lacking the adenylate cyclase toxin (Cyc) (BPM3183), pertussis toxin (Ptx) (BPRA), or dermonecrotic toxin (Dnt) (BPM1809). None of these mutants was statistically different from the wild-type strain (Fig. 2, top panel). These results show that B. pertussis efficiently attaches to neutrophils via bacterial adhesins, particularly FHA, but other Bvg-regulated adhesins may be involved.
Role of antibody in attachment.
Neutrophils have Fc receptors that allow them to bind the constant region of the immunoglobulin molecules immobilized on a bacterial surface. To investigate the role of opsonization by antibodies in the absence of complement, a serum sample from an individual with occupational exposure to B. pertussis was heat inactivated at 56°C for 30 min. This serum, no. 13 (43), has antibodies to B. pertussis LPS as well as to several surface-localized protein virulence factors. In previous studies, opsonization decreased the rate of attachment of the wild-type strain (26a, 37), and similar results were observed in this study (Fig. 2, bottom panel). Opsonization caused a statistically significant decrease in the attachment rates of all three wild-type strains (BP338, BP536, and BBC8), of mutants lacking FhaB, adenylate cyclase toxin, pertussis toxin, and dermonecrotic toxin, and of four different LPS mutants. Opsonization did not affect attachment of the FhaA, Bvg, LpsL, or pertactin mutants.
When attachment of the opsonized mutants was compared to attachment of the opsonized parental strain, only the FhaB mutant was found to be statistically different and displayed reduced attachment rates (Fig. 2, bottom panel).
Phagocytosis of mutants without opsonization.
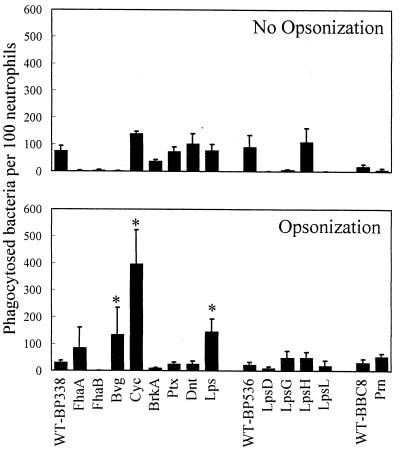
Centrifugation caused a statistically significant increase (P < 0.002) in ingestion of the wild-type strain, from 5 bacteria per 100 neutrophils (Fig. 1) to 75 bacteria per 100 neutrophils (Fig. 3, top panel), which corresponds to 0.8 to 12.5% of the total bacteria added. Phagocytosis of the bacterial mutants was also examined following centrifugation (Fig. 3, top panel). While the mean level of phagocytosis varied, none of the mutants was statistically different from its respective wild-type strain.
FIG. 3.
Effects of virulence factors and antibody on phagocytosis. Nonopsonized or opsonized bacteria were incubated with neutrophils as described in the legend for Fig. 2. One hundred consecutive neutrophils were examined for the number of intracellular (green) bacteria. Data were analyzed by the Student t test. Each bar represents the mean (± the standard error of the mean) from 3 to 27 independent experiments performed in duplicate. ∗, significantly different from wild type (WT) (P < 0.05).
Phagocytosis of mutants with opsonization.
We previously observed that opsonization caused a statistically significant decrease in phagocytosis of the wild-type strain with and without centrifugation (26a, 37), and similar results were observed in this study. The number of wild-type BP338 bacteria that were phagocytosed decreased from 75 (Fig. 3, top panel) to 30 (Fig. 3, bottom panel) following opsonization (P < 0.04).
Phagocytosis of opsonized bacterial mutants was also examined. Previously, we had shown that fluorescein isothiocyanate-labeled bacteria expressed reduced levels of adenylate cyclase toxin and were efficiently phagocytosed when opsonized (37). In this study, phagocytosis of the opsonized adenylate cyclase toxin mutant increased 10-fold compared to that of the wild type (P < 0.9e−7). Similarly, phagocytosis of the Bvg mutant (which fails to express adenylate cyclase toxin in addition to other virulence factors) was fourfold greater than that of the wild-type strain (P < 0.02). In addition, one LPS mutant was phagocytosed more efficiently than the wild-type strain (P < 0.0005). E. coli mutants with alterations in the core region of LPS express reduced levels of functional hemolysin (6), a toxin that is highly related to the adenylate cyclase toxin of B. pertussis. LPS mutant MLT7 produces hemolysis on blood-containing plates, an activity due to functional adenylate cyclase toxin. Western blot analysis was performed as described previously (43), using monoclonal antibodies to adenylate cyclase toxin (3D1, 5D1, 6E1, and 2A12 [25]) at a 1/1,000 dilution. BP338, the wild-type strain, and MLT7, the LPS mutant, expressed similar levels of cell-associated antigenic adenylate cyclase toxin (data not shown), suggesting that the increased phagocytosis of this mutant is due to the LPS defect.
Effect of adenylate cyclase toxin on phagocytosis.
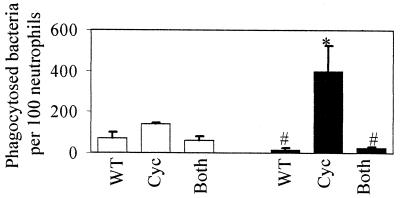
To confirm the role of adenylate cyclase toxin in blocking phagocytosis, the GFP-labeled adenylate cyclase toxin mutant was added directly to neutrophils or mixed with an equal number of unlabeled wild-type bacteria. In the absence of opsonization, neither the GFP-labeled wild-type strain, the GFP-labeled adenylate cyclase toxin mutant, nor the GFP-labeled adenylate cyclase toxin mutant mixed with the unlabeled wild-type strain was efficiently phagocytosed (Fig. 4). Opsonization significantly increased the phagocytosis of the labeled adenylate cyclase toxin mutant but reduced phagocytosis of the wild-type strain as well as of the labeled adenylate cyclase toxin mutant mixed with the unlabeled wild-type strain (Fig. 4). These results suggest that the presence of adenylate cyclase toxin blocks phagocytosis, even when bacteria are not expressing the toxin.
FIG. 4.
Effect of adenylate cyclase toxin on phagocytosis. Phagocytosis studies were performed as described in the legend for Fig. 3, with nonopsonized (open bars) and opsonized (solid bars) bacteria. One hundred consecutive neutrophils were examined for the number of intracellular (green) bacteria. WT, wild-type GFP-labeled BP338; Cyc, GFP-labeled BPM3183; Both, unlabeled BP338 plus GFP-labeled BPM3183. Data were analyzed by the Student t test. Each bar represents the mean (± the standard error of the mean) from three independent experiments. #, significantly different from the Cyc strain (P < 0.05); ∗, significantly different from WT (P < 0.05).
In this study we examined the influence of B. pertussis virulence factors and opsonizing antibodies on phagocytosis by human neutrophils, an immune response that could play a role in eliminating the bacteria from the infected individual. FHA is an important adhesin for B. pertussis (27). Mutants lacking FHA failed to attach to neutrophils, demonstrating a major role for this adhesin in promoting attachment to neutrophils. The lack of efficient attachment of the FHA mutants suggests that pertactin, BrkA, or other putative adhesins, such as tracheal colonization factor, could not compensate for FHA. The role of fimbriae in mediating attachment to neutrophils is uncertain, since neither the FHA mutants nor the parental strain expressed this adhesin. The contribution of fimbriae to adherence would best be examined by constructing mutants that constitutively express this determinant in an FHA-deficient background. While FHA may confer an overall benefit to the pathogen during disease, bacteria expressing FHA stick avidly to neutrophils, and this close association can promote phagocytosis. We have shown that opsonization can reduce the incidence of both attachment and phagocytosis of wild-type strains expressing FHA.
Adenylate cyclase toxin appears to be an important bacterial defense against phagocytosis, as evidenced by the efficient phagocytosis of mutants that fail to express this toxin. However, only opsonized bacteria were efficiently phagocytosed, suggesting that a signal, such as Fc receptor activation, is necessary for neutrophils to recognize B. pertussis as foreign and initiate phagocytosis.
These studies have important implications for vaccine development. While the current vaccines protect against severe diseases, they afford little protection against colonization by the organism (1, 7, 21, 22, 31, 34). Developing a vaccine that prevents bacterial infection as well as serious disease is an important goal. In a previous study, we have shown that B. pertussis is killed following phagocytosis by neutrophils (26a). Identifying the antigens that promote phagocytosis and killing by neutrophils could improve the pertussis vaccine. While antibodies to FHA may confer protection by other means, our studies suggest that antibodies to FHA could antagonize phagocytosis by neutrophils. The role of FHA as a protective immunogen warrants further investigation. In contrast, adenylate cyclase toxin is not included in any of the acellular vaccine formulations, and our studies suggest that neutralizing antibodies to this toxin could be beneficial in preventing infection by B. pertussis. Our studies also suggest that opsonization is needed for efficient phagocytosis, and the identity of opsonizing antibodies remains to be determined.
Acknowledgments
This work was supported in part by grant RO1 AI38415 to A.A.W.
REFERENCES
- 1.Ad Hoc Group for the Study of Pertussis Vaccines. Placebo-controlled trial of two acellular pertussis vaccines in Sweden—protective efficacy and adverse events. Lancet. 1988;i:955–960. [PubMed] [Google Scholar]
- 2.Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 3.Antoine R, Locht C. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect Immun. 1990;58:1518–1526. doi: 10.1128/iai.58.6.1518-1526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arico B, Miller J F, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci USA. 1989;86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banemann A, Gross R. Phase variation affects long-term survival of Bordetella bronchiseptica in professional phagocytes. Infect Immun. 1997;65:3469–3473. doi: 10.1128/iai.65.8.3469-3473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer M E, Welch R A. Pleiotropic effects of a mutation in rfaC on Escherichia coli hemolysin. Infect Immun. 1997;65:2218–2224. doi: 10.1128/iai.65.6.2218-2224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherry J D, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. 1998;16:1901–1906. doi: 10.1016/s0264-410x(98)00226-6. [DOI] [PubMed] [Google Scholar]
- 8.Chhatwal G S, Walker M J, Yan H, Timmis K N, Guzman C A. Temperature dependent expression of an acid phosphatase by Bordetella bronchiseptica: role in intracellular survival. Microb Pathog. 1997;22:257–264. doi: 10.1006/mpat.1996.0118. [DOI] [PubMed] [Google Scholar]
- 9.Confer D L, Eaton J W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 10.Craig F F, Lackie J M, Parton R, Freer J H. Interaction of Bordetella pertussis virulence components with neutrophils: effect on chemiluminescence induced by a chemotactic peptide and intact bacteria. J Gen Microbiol. 1988;134:2201–2211. doi: 10.1099/00221287-134-8-2201. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez R C, Weiss A A. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect Immun. 1994;62:4727–4738. doi: 10.1128/iai.62.11.4727-4738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez R C, Weiss A A. Serum resistance in bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol Lett. 1998;163:57–63. doi: 10.1111/j.1574-6968.1998.tb13026.x. [DOI] [PubMed] [Google Scholar]
- 13.Finn T M, Stevens L A. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 14.Finn T M, Amsbaugh D F. Vag8, a Bordetella pertussis bvg-regulated protein. Infect Immun. 1998;66:3985–3989. doi: 10.1128/iai.66.8.3985-3989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman R L, Nordensson K, Wilson L, Akporiaye E T, Yocum D E. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun. 1992;60:4578–4585. doi: 10.1128/iai.60.11.4578-4585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geuijen C A W, Willems R J L, Bongaerts M, Top J, Gielen H, Mooi F R. Role of the Bordetella pertussis minor fimbrial subunit, FimD, in colonization of the mouse respiratory tract. Infect Immun. 1997;65:4222–4228. doi: 10.1128/iai.65.10.4222-4228.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin M S M, Weiss A A. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect Immun. 1990;58:3445–3447. doi: 10.1128/iai.58.10.3445-3447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gueirard P, Druilhe A, Pretolani M, Guiso N. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect Immun. 1998;66:1718–1725. doi: 10.1128/iai.66.4.1718-1725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzman C A, Rohde M, Timmis K N. Mechanisms involved in uptake of Bordetella bronchiseptica by mouse dendritic cells. Infect Immun. 1994;62:5538–5544. doi: 10.1128/iai.62.12.5538-5544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman C A, Rohde M, Bock M, Timmis K N. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect Immun. 1994;62:5528–5537. doi: 10.1128/iai.62.12.5528-5537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hewlett E L. Pertussis: current concepts of pathogenesis and prevention. Pediatr Infect Dis J. 1997;16(4 suppl.):S78–S84. doi: 10.1097/00006454-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hewlett E L, Halperin S A. Serological correlates of immunity to Bordetella pertussis. Vaccine. 1998;16:1899–1900. doi: 10.1016/s0264-410x(98)00228-x. [DOI] [PubMed] [Google Scholar]
- 23.Hewlett E L, Urban M A, Manclark C R, Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci USA. 1976;73:1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khelef N, Bachelet C-M, Vargaftig B B, Guiso N. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect Immun. 1994;62:2893–2900. doi: 10.1128/iai.62.7.2893-2900.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S-J, Gray M C, Guo L, Sebo P, Hewlett E L. Epitope mapping of monoclonal antibodies against Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1999;67:2090–2095. doi: 10.1128/iai.67.5.2090-2095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leininger E, Roberts M, Kenimer J, Charles I, Fairweather N, Novotny P, Brennan M. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci USA. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Lenz D H, Weingart C L, Weiss A A. Phagocytosed Bordetella pertussis fails to survive in human neutrophils. Infect Immun. 2000;68:956–959. doi: 10.1128/iai.68.2.956-959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locht C, Bertin P, Menozzi F D, Renauld G. The filamentous hemagglutinin, a multifaceted adhesin produced by virulent Bordetella spp. Mol Microbiol. 1993;9:653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 28.Mooi F R, van der Heide H G J, ter Avest A R, Welinder K G, Livey I, van der Zeijst B A M, Gaastra W. Characterization of fimbrial subunits from Bordetella species. Microb Pathog. 1987;2:473–484. doi: 10.1016/0882-4010(87)90054-4. [DOI] [PubMed] [Google Scholar]
- 29.Musser J M, Hewlett E L, Peppler M S, Selander R K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986;166:230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittman M. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev Infect Dis. 1979;1:401–412. doi: 10.1093/clinids/1.3.401. [DOI] [PubMed] [Google Scholar]
- 31.Plotkin S A, Cadoz M. The acellular pertussis vaccine trials: an interpretation. Pediatr Infect Dis J. 1997;16:508–517. doi: 10.1097/00006454-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Schipper H, Krohne G F, Gross R. Epithelial cell invasion and survival of Bordetella bronchiseptica. Infect Immun. 1994;62:3008–3011. doi: 10.1128/iai.62.7.3008-3011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steed L L, Setareh M, Friedman R L. Intracellular survival of virulent Bordetella pertussis in human polymorphonuclear leukocytes. J Leukoc Biol. 1991;50:321–330. doi: 10.1002/jlb.50.4.321. [DOI] [PubMed] [Google Scholar]
- 34.Storsaeter J, Hallander H O, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16:1907–1916. doi: 10.1016/s0264-410x(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 35.Torre D, Ferrario G, Bonetta G, Perversi L, Tambini R, Speranza F. Effects of recombinant human gamma interferon on intracellular survival of Bordetella pertussis in human phagocytic cells. FEMS Immunol Med Microbiol. 1994;9:183–188. doi: 10.1111/j.1574-695X.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 36.Turcotte M L, Martin D, Brodeur B R, Peppler M S. Tn5-induced lipopolysaccharide mutations in Bordetella pertussis that affect outer membrane function. Microbiology. 1997;143:2381–2394. doi: 10.1099/00221287-143-7-2381. [DOI] [PubMed] [Google Scholar]
- 37.Weingart C L, Broitman-Maduro G, Dean G, Newman S, Peppler M, Weiss A A. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 1999;67:4264–4267. doi: 10.1128/iai.67.8.4264-4267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss A. Mucosal immune defenses and the response of Bordetella pertussis. ASM News. 1997;63:22–28. [Google Scholar]
- 39.Weiss A A, Goodwin M S M. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989;57:3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss A A, Hewlett E L, Myers G A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss A A, Hewlett E L, Myers G A, Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984;150:219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- 42.Weiss A A, Melton A R, Walker K E, Andraos-Selim C, Meidl J J. Use of the promoter fusion transposon Tn5 lac to identify mutations in Bordetella pertussis vir-regulated genes. Infect Immun. 1989;57:2674–2682. doi: 10.1128/iai.57.9.2674-2682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss A A, Mobberley P S, Fernandez R C, Mink C M. Characterization of human bactericidal antibodies to Bordetella pertussis. Infect Immun. 1999;67:1424–1431. doi: 10.1128/iai.67.3.1424-1431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]