Abstract
Neoadjuvant chemotherapy has become one of the important means for advanced hypopharyngeal carcinoma. So far, there is no effective index to predict the curative effect. To investigate the value of iodine map of dual-energy computed tomography (CT) in predicting the efficacy of neoadjuvant chemotherapy for hypopharyngeal carcinoma. A total of 54 hypopharyngeal carcinomapatients who underwent two courses of TPF neoadjuvant chemotherapy were recruited in this study. Three cases had a complete response (CR), thirty-six cases had a partial response (PR), eleven cases had stable disease (SD), and four cases had a progressive disease (PD) after the chemotherapy. All patients underwent a dual-source CT scan before chemotherapy and rescanned after chemotherapy. The normalized iodine-related attenuation (NIRA) of the mean of maximum slice and most enhanced region of lesion at arterial and parenchymal phase were measured: NIRAmean-A, NIRAmax-A, NIRAmean-P, and NIRAmax-P, respectively. Correlation analysis was conducted between different metrics of NIRA and the diameter change rate of lesions, and the curative effect was evaluated based on the receiver operating characteristic (ROC) curve. There were a significant correlation between NIRAmean-A, NIRAmax-A, NIRAmean-P, NIRAmax-P and the change rate of lesion’s maximum diameter (ΔD%) (all P < 0.01). The NIRAmax-A, NIRAmean-P, NIRAmax-P had significant differences between CR, PR, SD, PD groups, but NIRAmean-A did not reach a significant difference. All NIRAmean-A, NIRAmax-A, NIRAmean-P, NIRAmax-P had significant differences between effective (CR + PR) and ineffective (SD + PD) groups. The ROC analysis revealed that NIRAmean-P had the largest AUC and prediction efficacy (AUC = 0.809). Dual-energy CT iodine map could predict the efficacy of neoadjuvant chemotherapy and provides imaging evidence to assist in treatment decisions for hypopharyngeal carcinoma patients.
Subject terms: Cancer therapy, Head and neck cancer, Oncology
Introduction
Hypopharyngeal carcinoma is relatively rare and accounts for about 3% of all head and neck cancers (HNCs). But it has a poor prognosis among all the HNCs with a 30–35% 5-year overall survival (OS) rate1. Around 80% of the patients were already at stages III and IV of the disease at the time of admission; only a few cases were detected at an early stage due to its concealed site and infiltrating growth. In recent years, a comprehensive treatment model of neoadjuvant chemotherapy, followed by concurrent radiotherapy and chemotherapy or surgery significantly improved the larynx preservation rate of locally advanced patients and improved the life quality of patients. Thus, predicting the efficacy of chemotherapy is crucial for the optimization of the treatment and avoiding unnecessary system toxicity, cost, and treatment delay. The evaluation of the efficacy of chemotherapy relies mainly on medical imaging. Dual-source dual-energy CT (DECT) could not only obtain single-energy images at different kV but also could generate iodine maps through data processing from different kV images, which might explicate the tissue characteristics of local hypopharyngeal carcinoma quantificationally2. And it was already reported that the enhanced CT iodine map could evaluate the histopathological invasiveness of lung cance3.
This study aimed to explore the predicting value of dual-source DECT in the evaluation of the efficacy of neoadjuvant chemotherapy for hypopharyngeal carcinoma.
Materials and methods
Patients
A total of 54 patients (50 males and 4 females, age range: 42 ~ 81 years old) were recruited in this study in Tianjin First Central Hospital from September 2014 to July 2019. All patients were pathologically confirmed to present hypopharyngeal squamous cell carcinomas, including 48 cases at the piriform fossa (88.9%), 5 cases (9.2%) at the posterior pharyngeal wall, and 1 case (1.9%) at the postcricoid region. All patients were given neoadjuvant chemotherapy with two courses of the modified TPF regimen (Docetaxel + Nedaplatin + Flurouracil). This study was consistent with the principles expressed in the Declaration of Helsinki and approved by the Ethics Committee of Tianjin First Central Hospital. The informed consent was waived for this retrospective study.
Inclusion criteria: (1) admitted to hospital due to hypopharyngeal neoplasms; (2) pathologically confirmed as hypopharyngeal carcinoma; (3) without any systematic treatment at the first diagnosis; (4) had no contraindications to chemotherapy and agreed to receive neoadjuvant chemotherapy; (5) underwent the dual-source DECT plain and enhanced scan before neoadjuvant chemotherapy, and rescanned within one week after the end of the second chemotherapy course.
Exclusion criteria: (1) poor image quality due to artifacts or other factors; (2) neoadjuvant chemotherapy was not performed on time because of complication, contraindication, or adverse reaction of chemotherapy; (3) less than two courses of neoadjuvant chemotherapy due to other reasons.
Scan settings
The SOMATOM Definition Dual-source CT (DSCT; Siemens, Forchheim, Germany) was used to perform a routine plain and dual-energy enhanced scan. Patients lay in a prone position, breathed calmly, and were asked to not cough or swallow during CT scans. The plain scan was performed firstly, followed by an enhanced scan in dual-energy mode (tube voltage, 100 and 140 kV; tube current, Auto mA CARE Dose4D; pitch, 0.9; collimation, 64 × 0.6; reconstruct thickness, 3 mm). A mechanical high-pressure syringe was used to inject the iodine contrast agent (350 mgI/ml) through the elbow vein with a flow of 3 ml/s, dose 1 ~ 1.5 ml/kg and followed by 35 ml physiological saline. The dual-phase enhanced scan was performed at 30 ~ 45 s (arterial phase) and 80 ~ 90 s (parenchymal phase) after injection in energy mode.
Image post-processing
The image data was transmitted to a Siemens workstation (Syngo MMwP 70,971) for post-processing. The fusion image of the anatomy and iodine map was obtained after loading to the Dual-Energy software. The maximum diameter of the tumor before and after chemotherapy was measured three times. The averaged diameter was recorded as DPre and DPost, and the change rate of maximum diameter was denoted as ΔD%.
The measurements of the iodine map were performed on the maximum slice of the lesion. The iodine-related attenuation (IRA) of the mean of the lesion and most enhanced region were measured three times. The averaged IRA of the whole lesion in this slice and the most enhanced region in the arterial phase and parenchymal phase were recorded as IRAmean-A, IRAmax-A, IRAmean-P, and IRAmax-P. Moreover, these metrics were normalized by the IRA value of the common carotid artery on the same side of the lesion and denoted as NIRAmean-A, NIRAmax-A, NIRAmean-P, and NIRAmax-P in percentage, respectively. The ROIs of the most enhanced region were about 8mm2 in general and should include ten voxels at least for the minimum lesions, avoiding the gas, blood vessels, bones, cysts, and necrosis. The ROIs were measured by a 5-year-experience radiologist (R. C.) under the guidance of a radiologist (J. Y.) with over 20 years of experience.
Evaluating metrics
Change rate of lesion’s maximum diameter (ΔD%) = (the tumor’s maximum diameter before chemotherapy (DPre) − the tumor’s maximum diameter after chemotherapy (DPost))/the tumor’s maximum diameter before chemotherapy (DPre) × 100%
Normalized iodine-related attenuation (NIRA) = iodine value of each period/iodine value of common carotid artery in the same period × 100%
According to Response Evaluation Criteria in Solid Tumors (RECIST), (1) complete response (CR): all lesions disappeared without any new lesion; (2) partial response (PR): the sum of the longest diameter of the measurable lesions is reduced by ≥ 30%, and no new lesions appear; (3) stable disease (SD): lesion decline is less than PR or lesion increase is less than that of the progressive disease; (4) progressive disease (PD): the longest diameter of the measurable lesions is increased by ≥ 20%, or new lesion appears. The CR + PR was considered effective while SD + PD was ineffective.
Statistical analysis
Data analyses were conducted using SPSS25.0 software, and the correlation analysis used the Spearman correlation method. Independent-sample Kruskal–Wallis test and independent-sample Mann–Whitney U test was used for enumeration data, and P < 0.05 indicated a statistically significant difference. The receiver operating characteristics curve (ROC) was plotted for curative effect evaluation, and the sensitivity, specificity, area under the curve (AUC), and optimal cutoff value were calculated. A combination of AUC > 0.5 and P < 0.05 in the ROC curve indicated a significant difference.
Results
General information of patients
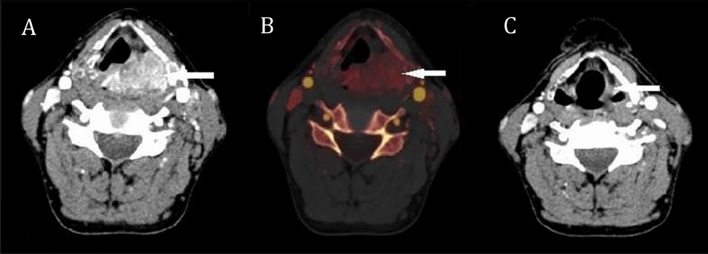
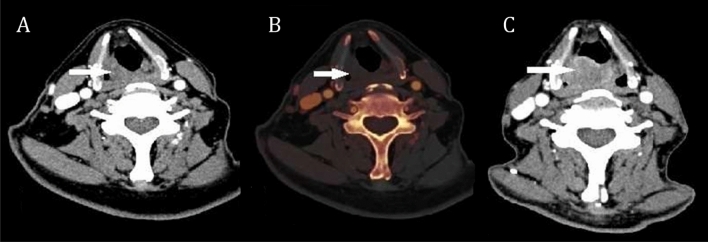
All 54 patients, including 3 cases of CR, 36 cases of PR, 11 cases of SD, and 4 cases of PD, completed neoadjuvant chemotherapy and underwent CT scans as required. These patients were classified into effective and ineffective groups (n = 39 and 15, respectively). The information about the patients and the diameter changes of the lesions were summarized in Table 1. Figure 1 showed a 66-year-old male patient with left piriform fossa carcinoma who had a partial response after chemotherapy. Figure 2 showed a 70-year-old male patient with right piriform fossa progressive carcinoma.
Table 1.
Information of patients and diameter changes of the lesions.
| Effective | Ineffective | Total | |||
|---|---|---|---|---|---|
| CR | PR | SD | PD | ||
| Age (years) | 64.67 ± 15.57 | 60.89 ± 9.16 | 66.27 ± 9.57 | 67.50 ± 5.80 | 62.69 ± 9.52 |
| Male/Female | 3/0 | 34/2 | 10/1 | 3/1 | 50/4 |
| DPre | 2.50 ± 0.94 | 3.11 ± 0.97 | 3.07 ± 1.36 | 2.59 ± 0.48 | 3.03 ± 1.02 |
| DPost | 0.00 ± 0.00 | 1.11 ± 0.63 | 2.49 ± 1.06 | 3.65 ± 0.72 | 1.52 ± 1.13 |
| ΔD% | 1.00 ± 0.00 | 0.63 ± 0.17 | 0.19 ± 0.12 | − 0.42 ± 0.27 | 0.48 ± 0.37 |
CR complete response; PR partial response; SD stable disease; PD progressive disease.
Figure 1.
A representative hypopharyngeal cancer patient showed partial remission after neoadjuvant chemotherapy. (A) Axial CT image of arterial phase before chemotherapy showed an obviously enhanced tumor at left piriform fossa extending to the posterior pharynx wall and postcricoid region. (B) The iodine map of arterial phase before chemotherapy, the red color showed obvious iodine-related attenuation (IRAmean 48.8HU, IRAmax 76.5HU) in the tumor. (C) Followup CT image after chemotherapy, the lesion at the left piriform fossa was significantly smaller.
Figure 2.
A representative patient with progressive hypopharyngeal cancer. (A) Axial CT image of arterial phase before chemotherapy, showed a tumor at the right piriform fossa but no obviouly enhancement. (B) The iodine map of arterial phase before chemotherapy, there was no obvious color in the lesion which implied a low iodine-related attenuation (IRAmean 16.7HU, IRAmax 22.1HU). (C) Followup CT image after chemotherapy, showed that the tumor at right piriform fossa was significantly enlarged.
Normalized iodine-related attenuation (NIRA) and response evaluation (RECIST)
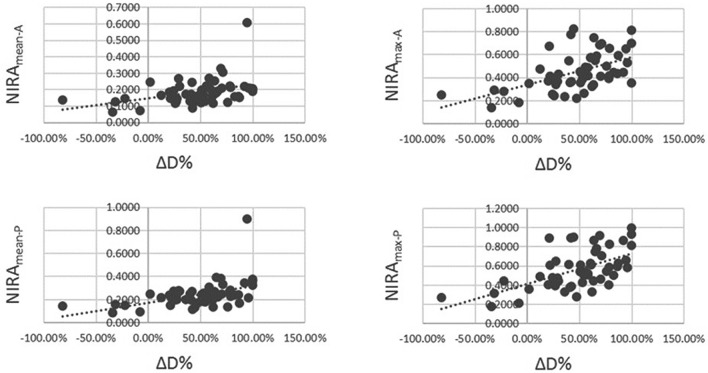
Spearman analysis revealed there was a significant correlation between NIRAmean-A, NIRAmax-A, NIRAmean-P, NIRAmax-P and the change rate of the lesion’s maximum diameter (ΔD%) (all P < 0.01, Fig. 3).
Figure 3.
Scatter plot to illustrate the correlation between normalized iodine-related attenuation (NIRA) and the change rate of lesion’s maximum diameter (ΔD%) (Spearman analysis, all P < 0.01).
Further Kruskal–Wallis test on the NIRAmean-A, NIRAmax-A, NIRAmean-P, and NIRAmax-P between different postchemotherapy response groups showed that NIRAmax-A, NIRAmean-P, NIRAmax-P had significant differences between CR, PR, SD, PD groups (all P < 0.01), but NIRAmean-A did not reach a significant difference (P = 0.05) (Fig. 4 and Table 2).
Figure 4.
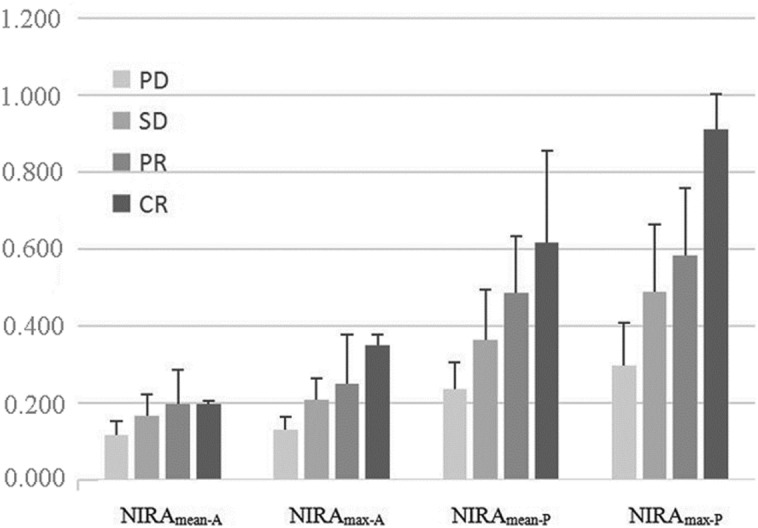
Histogram of the NIRAmean-A, NIRAmax-A, NIRAmean-P, NIRAmax-P between CR, PR, SD, PD groups. NIRAmax-A, NIRAmean-P, NIRAmax-P had significant differences but NIRAmean-A did not reach a significant difference between different postchemotherapy response groups.
Table 2.
Comparison of NIRAs among PD, SD, PR, and CR groups.
| Groups | NIRAmean-A | NIRAmax-A | NIRAmean-P | NIRAmax-P |
|---|---|---|---|---|
| CR | 0.197 ± 0.009 | 0.351 ± 0.027 | 0.618 ± 0.238 | 0.911 ± 0.092 |
| PR | 0.198 ± 0.087 | 0.249 ± 0.129 | 0.485 ± 0.149 | 0.583 ± 0.174 |
| SD | 0.166 ± 0.056 | 0.210 ± 0.054 | 0.364 ± 0.130 | 0.488 ± 0.177 |
| PD | 0.116 ± 0.037 | 0.131 ± 0.033 | 0.237 ± 0.070 | 0.299 ± 0.111 |
| P value | 0.050 | 0.004 | 0.002 | 0.001 |
| Power | 0.403 | 0.473 | 0.857 | 0.890 |
NIRA Normalized iodine-related attenuation; NIRAmean-A the mean NIRA of maximum slice of lesion at arterial phase; NIRAmax-A the NIRA of most enhanced region of lesion at arterial phase; NIRAmean-P the mean NIRA of maximum slice of lesion at parenchymal phase; NIRAmax-P the NIRA of most enhanced region of lesion at parenchymal phase; CR complete response; PR partial response; SD stable disease; PD progressive disease.
To evaluate the efficacy of neoadjuvant chemotherapy, all the cases were divided into an effective group (CR + PR) and an ineffective group (SD + PD). And Mann–Whitney U test revealed that all NIRAmean-A, NIRAmax-A, NIRAmean-P, and NIRAmax-P had significant differences between effective and ineffective groups (Table 3).
Table 3.
Comparison of NIRAs between effective and ineffective groups.
| Groups | NIRAmean-A | NIRAmax-A | NIRAmean-P | NIRAmax-P |
|---|---|---|---|---|
| Ineffective | 0.153 ± 0.055 | 0.189 ± 0.060 | 0.330 ± 0.129 | 0.438 ± 0.180 |
| Effective | 0.198 ± 0.084 | 0.257 ± 0.127 | 0.496 ± 0.157 | 0.609 ± 0.190 |
| P value | 0.022 | 0.030 | < 0.001 | 0.004 |
| Power | 0.450 | 0.490 | 0.895 | 0.755 |
NIRA Normalized iodine-related attenuation; NIRAmean-A the mean NIRA of maximum slice of lesion at arterial phase; NIRAmax-A the NIRA of most enhanced region of lesion at arterial phase; NIRAmean-P the mean NIRA of maximum slice of lesion at parenchymal phase; NIRAmax-P the NIRA of most enhanced region of lesion at parenchymal phase.
ROC analysis and predicting the efficacy of neoadjuvant chemotherapy
The ROC analysis revealed that all NIRAmean-A, NIRAmax-A, NIRAmean-P, and NIRAmax-P could be used to predict the efficacy of neoadjuvant chemotherapy (AUC > 0.5, P < 0.05). The NIRAmean-P had the largest AUC and prediction efficacy (AUC = 0.809) (Fig. 5 and Table 4). For the different parametric of NIRA, the mean NIRA of the maximum slice showed a larger AUC and Youden index than the most enhanced region of the lesion at the same time phase. The NIRA at the parenchymal phase had a larger area under the ROC curve and predicting specificity than the arterial phase, but the NIRAmean-A had a higher sensitivity. The best cutoff values of NIRAmean-A, NIRAmax-A, NIRAmean-P, and NIRAmax-P were 0.148, 0.236, 0.375, and 0.494, respectively (Table 4).
Figure 5.
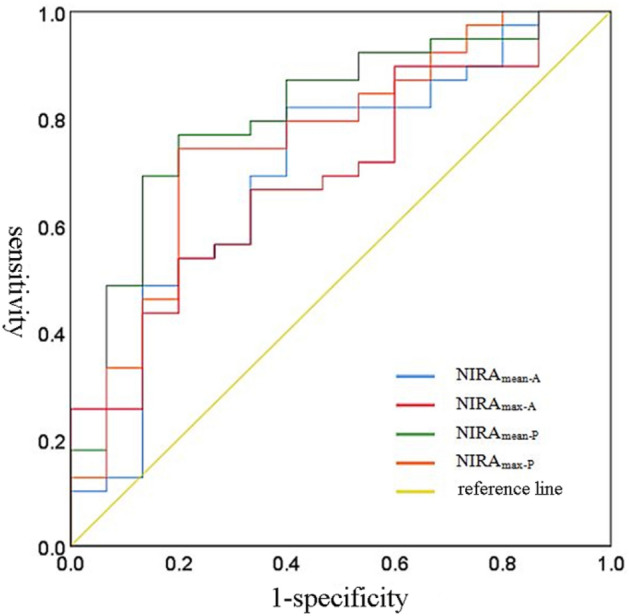
ROC analysis NIRAmean-A, NIRAmax-A, NIRAmean-P, NIRAmax-P to predict the efficacy of neoadjuvant chemotherapy.
Table 4.
The ability of different NIRA parameters in predicting the efficacy of neoadjuvant chemotherapy.
| AUC | P | Cut-off value | Sensitivity | Specificity | Youden index | |
|---|---|---|---|---|---|---|
| NIRAmean-A | 0.703 | 0.022 | 0.148 | 0.821 | 0.600 | 0.421 |
| NIRAmax-A | 0.692 | 0.030 | 0.236 | 0.538 | 0.800 | 0.338 |
| NIRAmean-P | 0.809 | < 0.001 | 0.375 | 0.769 | 0.800 | 0.569 |
| NIRAmax-P | 0.757 | 0.004 | 0.494 | 0.744 | 0.800 | 0.544 |
AUC area under the curve; NIRA Normalized iodine-related attenuation; NIRAmean-A the mean NIRA of maximum slice of lesion at arterial phase; NIRAmax-A the NIRA of most enhanced region of lesion at arterial phase; NIRAmean-P the mean NIRA of maximum slice of lesion at parenchymal phase; NIRAmax-P the NIRA of most enhanced region of lesion at parenchymal phase.
Discussion
Hypopharyngeal cancer is a severe malignancy with a poor prognosis and mortality rate. Neoadjuvant chemotherapy is one of the major methods of comprehensive treatment of head and neck squamous cell carcinoma and has been advocated in recent years with satisfactory results4. The evaluation of the efficacy of chemotherapy as early as possible has an important significance for the treatment and prognosis of patients. Various functional techniques, including positron emission tomography (PET) or magnetic resonance imaging (MRI) diffusion-weighted imaging5,6 or dynamic contrast-enhanced MRI5,7, and CT perfusion8,9 were employed to evaluate the metabolism, hypoxia, cellularity, and perfusion of the tumor. Nonetheless, some drawbacks, such as high cost, long scanning time, and poor resolution of small lesions, limit the clinical application of these methods. As new technology emerged in recent years, dual-source DECT imaging assesses the degree of tumor vascularization10. The applications of DECT in head and neck carcinoma (HNC) has increased, and several studies have shown the benefit of iodine characterization and virtual monoenergetic images for the detection and delineation of HNC11,12, differentiation between metastatic, inflammatory, and benign cervical lymph nodes13–15, or assessment of cartilage invasion16–18. However, the role of DECT-derived quantitative imaging to predict oncological outcomes in hypopharyngeal squamous cell carcinoma has not yet been investigated.
The images obtained in this study were used to evaluate the efficacy based on the morphological changes of RECIST19; however, the internal tumor variation, such as liquefaction necrosis, is unable to make an effective assessment due to these limitations20,21. The RECIST standard assesses the curative effect based on the change in the maximum tumor diameter, which might include tumor and necrotic areas, while the modified RECIST (mRECIST) standard uses “surviving tumors” as the evaluation criteria, excluding the affect of necrotics, recommended by 2010 EASL(European Association for the Study of the Liver) guidelines22. Dual-energy CT can obtain a series of derivative images, including iodine map, virtual non-enhanced image, single energy spectrum image, and nonlinear fusion image. Among these, the iodine-related attenuation measured in the iodine map quantificationally reflects the distribution and uptake of iodine in the lesion to reveal its blood supply condition. Moreover, the iodine-related attenuation is more reliable than the density value if the lesion is enhanced, for example the iodine-related attenuation is not affected by intratumoral hemorrhage. The content of iodine in the tissue can reflect its blood supply, and our results showed it might be a promising biological marker for evaluating the efficacy of chemotherapy. In this study, the patients with hypopharyngeal carcinoma with higher iodine-related attenuation were sensitive to neoadjuvant chemotherapy. Zima et al.23 reported that HNC with high perfusion were sensitive to radiotherapy and chemotherapy, which is consistent with the current conclusion. Thus, it could be postulated that the high perfusion state of tumor tissue can concentrate the local drug than the low perfusion state, thereby promoting the cell killing effect. The hypoperfusion state of tumor tissue might be considered as tumor ischemia and hypoxia, inducing the insensitivity to radiotherapy and chemotherapy.
Neoadjuvant chemotherapy is one of the comprehensive treatment measures for advanced hypopharyngeal cancer. In 1991, the Laryngeal Cancer Group of American Veterans discovered the laryngeal function-sparing effects of chemotherapy and radiotherapy24. In 2003, the US RTOG 91–11 study confirmed that the laryngeal function-sparing rates of concurrent radiotherapy and chemotherapy (CRC), radiotherapy (RT), and neoadjuvant chemotherapy + radiotherapy (IC + RT) were 84%, 66%, and 71%, respectively25. Currently, non-surgical treatments, including CRC, IC + RT, and RT combined with EGFR4 are applied to preserve function in laryngeal and hypopharyngeal cancer patients. However, some patients not sensitive to chemotherapy should need surgery. So that evaluate the effectiveness of the chemotherapy and adjust the treatment plan in time are critical to the survival and prognosis of the patient. Currently, there is no effective method to predict the curative effect before neoadjuvant chemotherapy. This study revealed that Dual-energy CT iodine map could be used to screen out the cases might have favorable effects before chemotherapy and avoid the inappropriate treatment plan for ineffective cases.
The RECIST morphological standard was as the reference to evaluate the tumor curative effect objectively. In order to eliminate the influence of the injection rate and dose of contrast agent among different individuals, a normalized iodine-related attenuation (NIRA) was applied in this study, which is the ratio of the iodine-related attenuation (IRA) in the ROI to the IRA in the carotid artery of the same layer. The present study showed that the NIRAs before chemotherapy was significantly related to the change of tumor diameter, including NIRAmean-A, NIRAmax-A, NIRAmean-P, and NIRAmax-P. For the different parametrics of NIRA, the NIRA at parenchymal phase had a larger area under the ROC curve and predicting specificity than the arterial phase, but the NIRAmean-A had a higher sensitivity. In addition to measuring the mean NIRA of maximum slice of lesion (NIRAmean), we also measured the NIRA of most enhanced region of lesion (NIRAmax) at the arterial and parenchymal phases which might avoid the influence of necrosis within the tumor. But the results showed that NIRAmean had a better predicting effect than NIRAmax, implied that NIRAmean might be better to reflect the global characteristic of tumor. The ROC curve reflects the correlation between the sensitivity and specificity of different NIRA parametrics, thereby rendering it as a comprehensive indicator of the test’s accuracy26. Other researches also confirmed that vascular iodine concentration (IC) reflects the efficacy of chemotherapy drugs within the tissue27–31. Yang et al.32 revealed that patients with higher HU values had a significantly low risk of progression and local recurrence, and DECT could easily identify CR patients and aid in choosing the appropriate treatment regimen for advanced laryngeal and hypopharyngeal squamous cell carcinoma (LHSCC). Bahig et al.33 reported that maximum IC of the primary tumor and the high volume and IC standard deviation of involved lymph nodes predict the local regional recurrence in LHSCC. Therefore, DECT provides valuable information to evaluate the response of hypopharyngeal carcinoma which might be important for the clinical implementation of individualized treatment. In addition, Ge et al.34 compared the radiation dose of DECT and conventional CT scan mode and did not find any significant difference in the mean radiation dose between DECT and the conventional CT scan, indicating that DECT did not cause additional radiation damage and was safe and suitable for efficacy evaluation.
Nevertheless, the present study has several limitations. The evaluation was only for short-term efficacy, and the reliability of the results for the long-term prognosis requires further verification with large sample data. Using dual-source CT iodine-related measurement to predict the effect of chemotherapy is a universal law based on correlation, and its accuracy and feasibility need to be assessed for individual cases. Moreover, the accuracy of iodine-related attenuation measurement is hindered by several factors, such as the inhomogeneity of local structure, the algorithms, and ROI selection. But the progress and improvement of DECT would promote its clinical applications.
Conclusions
In summary, our study showed that DECT iodine-related attenuation might be useful in clinical practice as a tool to stratify patients into appropriate treatment of neoadjuvant chemotherapy.
Acknowledgements
This study was funded by Tianjin Key Medical Discipline Construction Project.
Author contributions
X. W. designed of the study and drafted the article; R. C., H. L. conducted the CT scans and data measurements; M. L., statistical analysis; P. S. collected patients' data; Y. Z. revised the article; L. L. analysis and interpretation of results; J. Y. designed of the study, revised the article critically. All authors reviewed the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xianfeng Wei, Rui Cao.
References
- 1.Garneau JC, Bakst RL, Miles BA. Hypopharyngeal cancer: A state of the art review. Oral Oncol. 2018;86:244–250. doi: 10.1016/j.oraloncology.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Machida H, Tanaka I, Fukui R, et al. Dual-energy spectral CT: Various clinical vascular applications. Radiographics. 2016;36(4):1215–1232. doi: 10.1148/rg.2016150185. [DOI] [PubMed] [Google Scholar]
- 3.Ito R, Iwano S, Shimamoto H, et al. A comparative analysis of dual-phase dual-energy CT and FDG-PET/CT for the prediction of histopathological invasiveness of non-small cell lung cancer. Eur. J. Radiol. 2017;95:186–191. doi: 10.1016/j.ejrad.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 5.Chawla S, Kim S, Dougherty L, et al. Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck. Am. J. Roentgenol. 2013;200(1):35–43. doi: 10.2214/AJR.12.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrecht M, Van Calster B, Vandecaveye V, et al. Integrating pretreatment diffusion weighted MRI into a multivariable prognostic model for head and neck squamous cell carcinoma. Radiother. Oncol. 2014;110(3):429–434. doi: 10.1016/j.radonc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Mortensen LS, Johansen J, Kallehauge J, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother. Oncol. 2012;105(1):14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Hermans R, Meijerink M, Van den Bogaert W, et al. Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003;57(5):1351–1356. doi: 10.1016/S0360-3016(03)00764-8. [DOI] [PubMed] [Google Scholar]
- 9.Razek AA, Tawfik AM, Elsorogy LG, et al. Perfusion CT of head and neck cancer. Eur. J. Radiol. 2014;83(3):537–544. doi: 10.1016/j.ejrad.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Nicola C, Darnell A, Rengo M, et al. Dual-energy CT: Oncologie applications. Am. J. Roentgenol. 2012;199(5 Suppl):S98–S105. doi: 10.2214/AJR.12.9207. [DOI] [PubMed] [Google Scholar]
- 11.Albrecht MH, Scholtz JE, Kraft J, et al. Assessment of an advanced monoenergetic ronstruction technique in dual-energy computed tomography of head and neck cancer. Eur. Radiol. 2015;25(8):2493–2501. doi: 10.1007/s00330-015-3627-1. [DOI] [PubMed] [Google Scholar]
- 12.Toepker M, Czerny C, Ringl H, et al. Can dual energy CT improve the assessment of tumor margins in oral cancer. Oral Oncol. 2014;50(3):221–227. doi: 10.1016/j.oraloncology.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan A, Parker RA, Manjunathan A, et al. Differentiation of benign and malignant neck pathologies: Preliminary experience using spectral computed tomography. J. Comput. Assist. Tomogr. 2013;37:666–672. doi: 10.1097/RCT.0b013e3182976365. [DOI] [PubMed] [Google Scholar]
- 14.Tawfik AM, Razek AA, Kerl JM, et al. Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur. Radiol. 2014;24(3):574–580. doi: 10.1007/s00330-013-3035-3. [DOI] [PubMed] [Google Scholar]
- 15.Liang H, Li A, Li Y, et al. A retrospective study of dual-energy CT for clinical detecting of metastatic cervical lymph nodes in laryngeal and hypopharyngeal squamous cell carcinoma. Acta Otolaryngol. 2015;135(7):722–728. doi: 10.3109/00016489.2015.1015164. [DOI] [PubMed] [Google Scholar]
- 16.Kuno H, Onaya H, Iwata R, et al. Evaluation of cartilage invasion by laryngeal and hypopharyngeal squamous cell carcinoma with dual-energy CT. Radiology. 2012;265(2):488–496. doi: 10.1148/radiol.12111719. [DOI] [PubMed] [Google Scholar]
- 17.Forghani R, Levental M, Gupta R, et al. Different spectral hounsfield unit curve and high-energy virtual monochromatic image characteristics of squamous cell carcinoma compared with nonossified thyroid cartilage. AJNR Am. J. Neuroradiol. 2015;36(6):1194–1200. doi: 10.3174/ajnr.A4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuno H, Sakamaki K, Fujii S, et al. Comparison of MR imaging and dual-energy CT for the evaluation of cartilage invasion by laryngeal and hypopharyngeal squamous cell carcinoma. AJNR Am. J. Neuroradiol. 2018;39(3):524–531. doi: 10.3174/ajnr.A5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J. Natl. Cancer. Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Kim YN, Lee HY, Lee KS, et al. Dual-energy CT in patients treated with anti-angiogenic agents for non-small cell lung cancer: New method of monitoring tumor response? Korean J. Radiol. 2012;13(6):702–710. doi: 10.3348/kjr.2012.13.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HY, Lee KS, Ahn MJ, et al. New CT response criteria in non-small cell lung cancer: Proposal and application in EGFR tyrosine kinase inhibitor therapy. Lung Cancer. 2011;73(1):63–69. doi: 10.1016/j.lungcan.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zima A, Carlos R, Gandhi D, et al. Can pretreatment CT perfusion predict response of advanced squamous cell carcinoma of the upper aerodigestive tract treated with induction chemotherapy? AJR Am. J. Neuroradiol. 2007;28:328–334. [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf GT, Fisher SG, Hong WK, Department of Veterans Affairs Laryngeal Cancer Study Group et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 1991;324(24):1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 25.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N. Engl. J. Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011;48(4):277–287. doi: 10.1007/s13312-011-0055-4. [DOI] [PubMed] [Google Scholar]
- 27.Shimamoto H, Iwano S, Umakoshi H, et al. Evaluation of locoregional invasiveness of small-sized non-small cell lung cancers by enhanced dual-energy computed tomography. Cancer Imaging. 2016;16(1):18. doi: 10.1186/s40644-016-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxa J, Matouskova T, Krakorova G, et al. Dual-phase dual-energy CT in patients treated with erlotinib for advanced non-small cell lung cancer: Possible benefits of iodine quantification in response assessment. Eur. Radiol. 2016;26(8):2828–2836. doi: 10.1007/s00330-015-4092-6. [DOI] [PubMed] [Google Scholar]
- 29.Yanagawa M, Morii E, Hata A, et al. Dualenergy dynamic CT of lung adenocarcinoma: Correlation of iodine uptake with tumor gene expression. Eur. J. Radiol. 2016;85(8):1407–1413. doi: 10.1016/j.ejrad.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Aoki M, Hirose K, Sato M, et al. Prognostic impact of average iodine density assessed by dual-energy spectral imaging for predicting lung tumor recurrence after stereotactic body radiotherapy. J. Radiat. Res. 2016;57(4):381–386. doi: 10.1093/jrr/rrv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Meng X, Ye Z. Iodine quantification to characterize primary lesions, metastatic and non-metastatic lymph nodes in lung cancers by dual energy computed tomography: An initial experience. Eur. J. Radiol. 2016;85(6):1219–1223. doi: 10.1016/j.ejrad.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Luo D, Yi J, et al. Therapy effects of advanced hypopharyngeal and laryngeal squamous cell carcinoma: Evaluated using dual-energy CT quantitative parameters. Sci. Rep. 2018;8(1):9064. doi: 10.1038/s41598-018-27341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahig H, Lapointe A, Bedwani S, et al. Dual-energy computed tomography for prediction of loco-regional recurrence after radiotherapy in larynx and hypopharynx squamous cell carcinoma. Eur. J. Radiol. 2019;110:1–6. doi: 10.1016/j.ejrad.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Ge X, Yu J, Wang Z, et al. Comparative study of dual energy CT iodine imaging and standardized concentrations before and after chemoradiotherapy for esophageal cancer. BMC Cancer. 2018;18(1):1120. doi: 10.1186/s12885-018-5058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.