Abstract
Nicotinamide adenine dinucleotide (NAD+) is a vital energy intermediate in skeletal muscle. The discovery of dietary-derived NAD+ precursors has led to the rapid development of NAD+ therapeutics designed to manipulate NAD+ content in target tissues. Of those developed, nicotinamide riboside and nicotinamide mononucleotide have been reported to display health benefit in humans under clinical scenarios of NAD+ deficiency. In contrast, relatively little is known regarding the potential benefit of nicotinamide riboside and nicotinamide mononucleotide supplementation in healthy individuals, with questions remaining as to whether NAD+ therapeutics can be used to support training adaptation or improve performance in athletic populations. Examining animal and human nicotinamide riboside supplementation studies, this review discusses current evidence suggesting that NAD+ therapeutics do not alter skeletal muscle metabolism or improve athletic performance in healthy humans. Further, we will highlight potential reasons why nicotinamide riboside supplementation studies do not translate to healthy populations and discuss the futility of testing NAD+ therapeutics outside of the clinical populations where NAD+ deficiency is present.
Key Points
| Nicotinamide adenine dinucleotide is a key energy intermediate in skeletal muscle thought to promote mitochondrial adaptation to endurance exercise. |
| Cellular nicotinamide adenine dinucleotide levels can be altered by the dietary-derived precursors tryptophan, nicotinic acid, nicotinamide, nicotinamide riboside, and nicotinamide mononucleotide. |
| Supplementation of nicotinamide riboside increases mitochondrial biogenesis and functional performance in rodent models of nicotinamide adenine dinucleotide deficiency such as aging, obesity, and mitochondrial myopathy. |
| Nicotinamide riboside alters the NAD+ metabolome in human skeletal muscle but does not increase mitochondrial biogenesis or augment intracellular signaling processes following a single bout of endurance exercise or prolonged endurance training. |
| Current evidence suggests that NAD+-targeted therapeutics do not improve training adaptation or athletic performance in humans. |
| Future work should establish physiological conditions where NAD+ deficiency occurs to identify potential scenarios where NAD+-targeted therapeutics may have a benefit in humans. |
Introduction
Nicotinamide adenine dinucleotide (NAD+) is a ubiquitous cellular coenzyme, first discovered in 1906 [1]. NAD+ is a key metabolic intermediate, capable of harnessing energy from glucose, fatty acids, and amino acids via glycolysis, β-oxidation, and the tricarboxylic acid cycle [2]. In recent years, a role for NAD+ as a signaling moiety that can impact numerous biological processes has emerged, primarily owing to the discovery of cellular NAD+ sensors that can mediate metabolic remodeling in skeletal muscle in response to alterations in NAD+ availability [2]. Accordingly, this area of biology has become a hot topic of investment for biotech companies seeking to explore NAD+ boosters as potential dietary supplements for a variety of metabolic diseases characterized by NAD+ deficiency. The aim of this review is to (i) provide an overview of NAD+ metabolism in skeletal muscle, (ii) discuss how diet and exercise interact to alter NAD+ content, (iii) highlight different oral supplementation approaches to manipulate NAD+ in vivo, and (iv) examine the feasibility of NAD+-targeted therapeutics to improve athletic performance in humans.
The Role of NAD+ in Skeletal Muscle
The term metabolism describes the sum of all the reactions within the body that transduce chemical energy for cellular processes from ingested substrate stores [3, 4]. The most prevalent of these reactions in skeletal muscle is the catabolism of dietary lipid, carbohydrate, or protein stores to generate adenosine triphosphate (ATP), the energy unit of the cell. During endurance exercise, the majority of energy is derived from lipid and carbohydrate stores. This process is tightly regulated, with numerous feedback and feed-forward mechanisms ensuring that the cell can switch between lipid and carbohydrate metabolism when required. The key determinant in the selection of carbohydrate or lipid metabolism as the preferential fuel source is the intensity (how hard) and the duration (how long) of muscle contraction. As a 6-carbon molecule, glucose, the principal dietary carbohydrate, is readily metabolized without the requirement of oxygen, to pyruvate during a ten-step enzyme process termed glycolysis [3, 4]. The formation of pyruvate is key to the progression of metabolism as two reactions stem from this metabolite, first, the generation of lactate, via lactate dehydrogenase, and second, the production of acetyl-CoA via pyruvate carboxylation via the pyruvate dehydrogenase complex. The latter is particularly important as acetyl-CoA, following conversion to citrate forms the principal donor for the tricarboxylic acid cycle, which initiates the cycling of carbon substrate to generate electrons (NAD+ and flavin adenine dinucleotide) for the final stages of oxidative phosphorylation and the synthesis of ATP [3, 4].
During the transition from rest to exercise, the metabolic requirement of mammalian skeletal muscle for ATP synthesis can increase over 100-fold, while the ATP level only decreases marginally [5]. To maintain ATP homeostasis, the pathways of ATP synthesis must be activated rapidly to match the rate of ATP utilization [5]. Glycolysis and oxidative phosphorylation provide most of the ATP used during exercise; in both processes, the interconversion between NAD+ and, its reduced equivalent, NADH, occurs [6]. This redox couple not only participates in reduction–oxidation reactions as co-substrates, but also plays important roles in metabolic regulation in both the cytosol and mitochondria [2, 5]. NADH produced in glycolysis is utilized in reactions catalyzed by lactate dehydrogenase, or transported to the mitochondria for oxidation in the electron transport chain to maintain the redox states (NADH/NAD+) in cytosol and mitochondria [2, 5]. With significantly increased metabolic rates during exercise, it is therefore crucial to maintain the balance of NADH and NAD+ within cytosolic and mitochondrial compartments [2, 5].
Mechanisms of NAD+ Turnover and Homeostasis
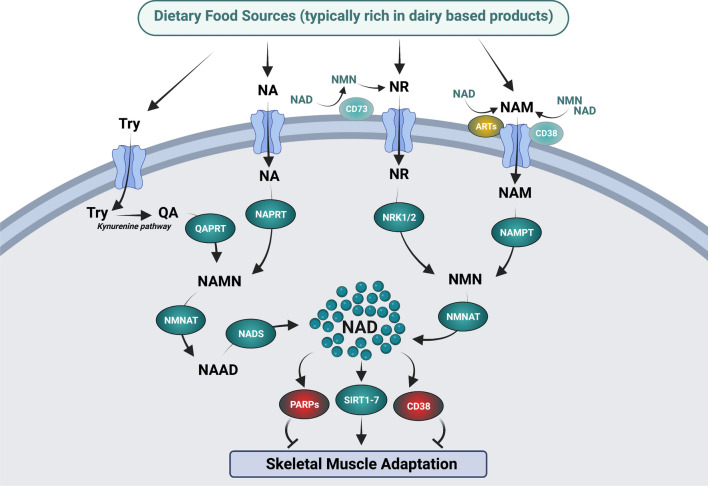
As with any metabolic intermediate, NAD+ content in skeletal muscle is dictated by processes of production and consumption, which maintain the NAD+ pool. The main processes of NAD+ generation in skeletal muscle can be separated into two classes, (i) de novo synthesis and (ii) salvaging pathways. Importantly, NAD+ is sensitive to dietary manipulation, as tryptophan (Try), nicotinic acid (NA), nicotinamide riboside (NR), and nicotinamide (NAM) can all influence NAD+ content (Fig. 1). The de novo synthesis pathway is prevalent in mammalian systems, and functions to convert the amino acid Try into quinolinic acid and then subsequently into nicotinic acid mononucleotide (NAMN). The conversion to NAMN is important as this then serves as a precursor for NAD+ (Fig. 1). Despite Try being the canonical NAD+ precursor, its action is thought to be up to 60 times less efficient than NA, as Try is also used for protein translation and biosynthetic processes [7]. The recommended daily allowance of NAMN derived from NA is 16 mg/day for men and 14 mg/day for women, suggesting that targeting of NAMN from NA is more effective than the substantial amount of Try that would be required (Try 960 mg/day for men) [8]. As such, the use of Try as a NAD+ precursor would not be solely sufficient to support physiological NAD+ requirements in mammals [9].
Fig. 1.
Influence of dietary-derived nicotinamide adenine dinucleotide (NAD+) precursors on the NAD+ metabolome in skeletal muscle. The supplementation of the NAD+ donors tryptophan (Try), nicotinic acid (NA), nicotinamide riboside (NR), and nicotinamide (NAM) activates a regulatory cascade of homeostatic mechanisms that converge to alter cellular levels of NAD+. The de novo synthesis pathway involves the metabolic break down of Try and NA metabolic pathways. Try is converted into quilonic acid via the kynurenine pathway, before undergoing phosphoribosylation from quinolinic acid phosphoribosyltransferase (QAPRT), to produce nicotinic acid mononucleotide (NAMN). Nicotinic acid is also converted into NAMN, as it undergoes phosphoribosylation from NA phosphoribosyltransferase (NAPRT). Following these reactions, NAMN is converted into nicotinic acid adenine dinucleotide (NAAD), via nicotinamide mononucleotide adenylyltransferase (NMNAT) activity. NAAD then undergoes amidation via NAD+ synthetase (NADS) to drive NAD+ synthesis. The NAD+ salvage pathway involves the metabolic break down of NR and NAM. NR and NAM can be sourced through the diet or via the activation of the NAD+ and nicotinamide mononucleotide (NMN) degrading ectoenzymes; CD73 and CD38, respectively. Free NAM can also be sourced via activation of ADP-ribosyltransferases (ARTs). Once NR and NAM cross the plasma membrane, they are converted into NMN. NR undergoes phosphorylation by the NR kinases 1 and 2 (NRK1 and NRK2), while NAM is modified through phosphoribosylation by NAM phosphoribosyltransferase (NAMPT). NMN is then converted into NAD+ via NMNAT. The activation or suppression of these metabolic cascades can influence NAD+ levels, which in turn alters the activity of the NAD+ sensors sirtuins (SIRTs) 1–7, to promote skeletal muscle adaptation. In parallel, the NAD+ consuming proteins PARP1/2 and CD38 compete with SIRTs for NAD+ and are thought to suppress skeletal muscle adaptation. Image created by BioRender.com
In contrast to Try, NA is an established dietary approach to synthesize NAD+ (Fig. 1). Once inside the cell, NA is converted to NAD+ through the Preiss–Handler pathway, in a three-step process involving the phosphoribosylation of NA to NAMN, NAMN adenylation to nicotinic acid adenine dinucleotide, and nicotinic acid adenine dinucleotide amidation to NAD+ by the glutamine-dependent NAD+ synthetase [10]. Whilst NA supplementation was initially viewed as a feasible approach to boost NAD+ levels in vivo, the clinical use of NA has been hindered by observations that NA ingestion results in a pronounced flushing response. This flushing effect appears to be mediated by the binding of NA to the G protein-coupled receptor, GPR109A (HM74A or PUMA-G) [11]. However, in contrast to Try, NA is a potent NAD+ precursor in the liver and kidneys, primarily because nicotinate phosphoribosyltransferase enzymatic activity is exaggerated in these tissues [12]. There is also evidence in vivo that the majority of dietary-derived NA is rapidly metabolized into NAM in the gut and the liver [13], which consequently results in a low circulatory abundance of NAM in plasma [12]. Collectively, these data suggest that Try and NA supplementation would have a limited effect on boosting NAD+ in vivo when provided as a dietary supplement, owing to inefficient synthesis (Try) or excessive clearance via the liver (NA).
The final routes of NAD+ synthesis in mammals are provided through the NAD+ recycling/salvage pathways supported by the NAD+ precursors NAM and NR. NAM is converted into NAD+ initially via the phosphoribosylation action of the enzyme NAM phosphoribosyltransferase (NAMPT), which converts NAM to the intermediate NMN. NMN then undergoes conversion to NAD+, mediated by NMN acetyltransferase (Fig. 1) [10]. NAM appears to have a high turnover rate, given it is a product of NAD+ degradation in a constant cycle of breakdown and recycling, and thus, unsurprisingly, it has a higher concentration in skeletal muscle compared with NAD+ [14]. This suggests that NAMPT is an integral component of NAD+ metabolism. For example, transgenic overexpression of NAMPT leads to an increase in skeletal muscle NAD+ levels [15–17], whilst muscle-specific deletion of NAMPT leads to NAD+ depletion and a muscle dystrophic phenotype [18, 19]. In contrast, NR can be transported into the cell by nucleoside transporters [20], before undergoing phosphorylation by the NR kinases 1 and 2 (NRK1 and NRK2) to generate NMN [20]. Once formed, NMN is then converted to NAD+ via NMN acetyltransferase.
How is NAD+ Metabolism Altered Under Different Physiological Conditions?
NAD+ is thought to have distinct cytosolic, mitochondrial, and nuclear pools within skeletal muscle [21]. However, typically, NAD+ content is reported as whole cell values, offering little distinction between the regional sites of NAD+ abundance. Further, few studies have examined NAD+ turnover in cell systems using stable-isotope tracer approaches, meaning that very little is known about dynamic movement of NAD+ and associated NAD+ metabolites within the cell [21]. Consequently, very little is known about NAD+ cellular levels or localization in skeletal muscle [2].
In resting human muscle, total NAD+ and NADH+ concentrations are estimated to be ~ 1.5–1.9 and ~ 0.08–0.20 mmol/kg dry weight muscle, respectively [2], 10% of which would be assumed to be mitochondrial [22]. Accordingly, the NAD+/NADH ratio in resting skeletal muscle is estimated to be greater in the cytosol compared with mitochondria [2]. During maximal exercise, i.e., above 100% of maximal oxygen uptake (VO2max), NADH has been reported to increase approximately two-fold above resting levels, with no significant change reported in NAD+ levels [23, 24]. In contrast, during submaximal exercise, total muscle NADH concentrations are not thought to change during exercise at 75% VO2max [25] or decrease during exercise at 50% VO2max [26]. Exercise intensity appears to be an important contributor to the differences in measured NAD+(H) and NAD+/NADH ratio during exercise. For example, NADH decreased (and the cytosolic NAD+/NADH ratio was unchanged) from resting values during exercise at 40% VO2max, but both NAD+ and the cytosolic NAD+/NADH ratio were increased above resting values at 75% and 100% VO2max [27].
Do Cellular NAD+ Sensors Drive Skeletal Muscle Mitochondrial Adaptation to Exercise?
Interest in NAD+ donors as skeletal muscle therapeutics has stemmed primarily because of the identification and characterization of sirtuin 1 (SIRT1), a cellular NAD+-activated metabolic sensor and purported regulator of skeletal muscle oxidative capacity. Over the past decade, SIRT1 has been shown to influence the activity of the AMP-activated protein kinase and the transcriptional co-activator, peroxisome proliferator activated receptor-gamma coactivator-1α (PGC-1α) in vitro, representing a metabolic sensing node, translating fluctuations in cellular stresses to mitochondrial remodeling [28].
The first mechanistic link between SIRT1 and mitochondrial adaptation was provided by Nemoto and colleagues, who demonstrated that SIRT1 deacetylates and activates PGC-1α in an NAD+-dependent manner in PC12 neuron-like cells [29]. In parallel, Rodgers and colleagues reported that SIRT1 and PGC-1α protein content were rapidly up-regulated in the liver following fasting and returned to basal conditions upon re-feeding [30], mirroring increased hepatic pyruvate and NAD+ concentrations. Following the observation that PGC-1α was deacetylated following fasting, the authors proposed that fasting increased cellular NAD+ concentrations that initiated an increase in the SIRT1 and PGC-1α association to increase gluconeogenic gene expression [30]. With regard to skeletal muscle, Gerhart-Hines et al. reported that overexpression of SIRT1 led to increased expression of genes involved in mitochondrial respiration and fatty acid utilization in C2C12 myotubes [31]. Further, short hairpin RNA knockdown of SIRT1 reduced the expression of cytochrome-c, isocitrate dehydrogenase-3α, cytochrome-c oxidase subunit IV, medium-chain acyl-CoA dehydrogenase, carnitine palmitoyltransferase-1, pyruvate dehydrogenase kinase-4, and citrate synthase activity, in both C2C12 and mouse primary myotubes [31]. Further, the authors demonstrated that increased mitochondrial metabolic activity in vitro in response to glucose reduction was SIRT1 dependent, as both SIRT1 knockout mouse embryonic fibroblasts and SIRT1 short hairpin RNA knockdown blocked the increase in mitochondrial gene expression and fatty acid utilization observed in wild-type cells [31]. Collectively, these studies established the paradigm that SIRT1 was a key signaling node in vitro, linking alterations in cellular energy status to increased mitochondrial adaptation and β-oxidation, through the targeted deacetylation and activation of PGC-1α.
In contrast to the in vitro studies described above, a role for SIRT1 in the regulation of skeletal muscle mitochondrial biogenesis in vivo is not as clear. For example, Gurd and colleagues reported that a single bout of exhaustive exercise and 7 days of electrical stimulation reduced whole-muscle or nuclear SIRT1 protein content, yet increased whole-muscle and nuclear PGC-1α protein content and SIRT1 activity, respectively [32, 33]. The clearest disconnect between SIRT1 activity and mitochondrial adaptation to endurance exercise has been provided using SIRT1 muscle-specific knockout mice [34], demonstrating that mitochondrial adaptation to acute and chronic exercise is preserved in the absence of SIRT1 activity. Further, muscle-specific SIRT1 overexpression leads to incomplete mitochondrial biogenesis [35] and even a reduction in cytochrome-c oxidase subunit IV and PGC-1α protein content and SIRT1 activity [32]. Thus, when considering these findings together, it appears that SIRT1 is not a critical regulator of skeletal muscle mitochondrial adaptation to exercise, whilst super-physiological increases in SIRT1 can impair mitochondrial function in skeletal muscle.
Nutraceutical Approaches to Target NAD+ Metabolism In Vivo
Nutraceuticals that target NAD+ metabolism have materialized with great interest to the scientific community, primarily involving synthetic precursors of NAD+, i.e., vitamin B3 [36]. Niacin is one of the recognized forms of vitamin B3, a compound constituting both NA and NAM [37, 38]. Supplementation of niacin (i.e., acipimox) resulted in modest but significant increases in state 3 and maximal respiration, increased ATP content, and increased the protein content of mitochondrial electron transport chain complexes 1 and 2 and heat-shock protein 60 [39]. Unfortunately, the authors did not assess NAD+ metabolism or skeletal muscle oxidative capacity, making it difficult to draw conclusions on the direct or indirect mechanism responsible for the mitochondrial adaptation observed from acipimox supplementation.
Regarding NMN action in vivo, a recent clinical trial examined the effect of supplementing 250 mg/day over 10 weeks in pre-diabetic women. Of note, this period of supplementation led to modulation of the skeletal muscle NAD+ metabolome and significant improvements in insulin sensitivity [40]. Less is currently known regarding NMN supplementation and adaptation to exercise. Mills et al. have reported that NMN supplementation at either 100 or 300 mg/kg/day over 12 months increased physical activity in otherwise healthy mice [41], whilst 500 mg/kg/day for 1 month improved exercise capacity in aged mice [42]. Further, a recent human trial of NMN supplementation at 300, 600, and 1200 mg/kg/day over 6 weeks, alongside endurance exercise training, displayed modest effects on whole-body aerobic adaptations. Specifically, there was no change observed to VO2max but an increase in power and oxygen uptake at the ventilatory threshold [43].
NR and Skeletal Muscle Adaptation to Exercise
NR is a naturally occurring form of vitamin B3, a single chemical moiety containing NAM and ribose that is found in milk, meat, poultry, fish, eggs, and green vegetables [44]. As an NAD+ precursor, NR is thought to impact skeletal muscle mitochondrial function through stimulation of the NAD+/SIRT1/PGC-1α signaling cascade [7]. The first evidence for the direct effect of NR on skeletal muscle metabolic function was provided by Canto and colleagues who showed that NR supplementation to C2C12 myotubes increases NAD+ content in the absence of GPR109A mobilization [45]. Feeding NR to mice (400 mg/kg/day) resulted in modest increases in skeletal muscle NAD+ (~ 5%) following 1-week supplementation, in a mechanism independent of changes in NAM phosphoribosyltransferase expression [45]. The authors proposed that the metabolic action of NR supplementation was mediated through SIRT1, as the adaptive response of C2C12 myotubes to NR supplementation was lost when SIRT1 was deleted. Interestingly, NR supplementation protected mice from the deleterious effects of 8 weeks of high-fat feeding, principally through an increase in energy expenditure and a reduction in cholesterol levels [45]. However, the ability of NR to protect against a high-fat diet has recently been challenged by two independent groups [46, 47], who were unable to replicate the findings of Canto et al. [45]. In parallel with metabolic adaptation, Canto et al. reported that endurance capacity also increased by ~ 25% in the NR supplemented mice, coupled with an increase in mitochondrial copy number and increased mitochondrial electron transport chain protein content, concluding that NR supplementation could promote modest increases in skeletal muscle NAD+ content and mitochondrial biogenesis [45]. Recently, Damgaard and colleagues provided contrasting data to Canto et al., reporting that NR supplementation had no impact on mitochondrial respiratory capacity or mitochondrial gene transcription following exercise training in mice, despite increased skeletal muscle NAD+ content [48]. Thus, current evidence suggests that NR supplementation does not promote mitochondrial adaptation to exercise in lean or obese mice. Subsequent research has demonstrated that NR supplementation can improve skeletal muscle NAD+ content and mitochondrial function in mouse models of mitochondrial myopathy [49, 50] and Duchenne muscular dystrophy [51], although again, the validity of this study has recently been challenged [52].
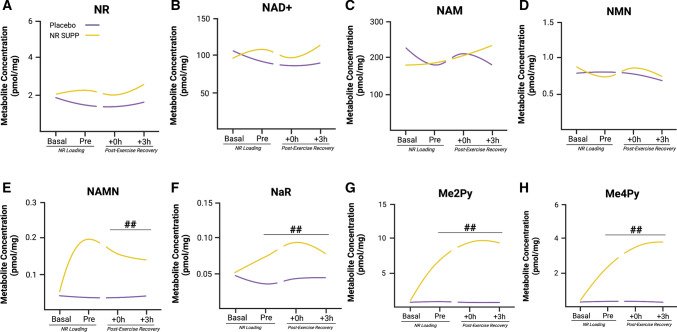
Despite the promise observed in pre-clinical models, the NAD+ boosting effects of NR supplementation have been associated with limited translational progress in humans to date. The most conclusive examination of NR supplementation and skeletal muscle adaptation to training has been provided by Dollerup and colleagues, who demonstrated that NR supplementation (1 g twice daily for 12 weeks) in obese humans with insulin resistance was unable to alter NAD+ and its reduced equivalents in skeletal muscle and had no effect on mitochondrial protein abundance and respiration [53]. These observations align with the work from Elhassan et al., who reported that NR supplementation (1 g daily for 3 weeks) in older male individuals (median age of 75 years) did not increase mitochondrial respiration or citrate synthase activity in skeletal muscle [54]. Interestingly, this study did however demonstrate that NR supplementation could alter the NAD+ metabolome in skeletal muscle, with a liquid chromatography-mass spectrometry analysis reporting an increase in the NAMN derivative nicotinic acid adenine dinucleotide, and the methylated NAM break-down products methylated NAM and N-methyl-2-pyridone-5-carboxamide (Me2PY) [54]. Unfortunately, the absence of a young cohort in this study meant that it was not possible to compare the effect of age on the skeletal muscle NAD+ metabolome, or determine a physiological context to the relevance of the changes in the NAD+ metabolome. Recently, we have investigated the effect of short-term NR loading (1 g daily supplementation for 1 week) on the NAD+ metabolome and related intracellular signaling prior to and following a single bout of exercise in young healthy male volunteers [55]. Consistent with our previous work in older adults [54], NR loading did not alter the metabolite concentrations of NAD+, NR, NAM, and NMN in skeletal muscle (Fig. 2A–D); however, NR loading did increase the concentration of the NAD+ precursor, nicotinic acid riboside, and, methylated NAM break-down products, Me2PY and N-methyl-4-pyridone-5-carboxamide (Me4PY), which was sustained immediately and 3 h post-exercise (Fig. 2E–H). Interestingly, we observed that the NAD+ precursor NAMN was elevated immediately and 3-h post-exercise. Whilst NR loading was able to augment some aspects of the skeletal muscle NAD+ metabolome, we observed no additive effect to exercise in relation to substrate utilization during and in recovery from exercise, nor did we see any amplification in SIRT1 or SIRT3 signaling, or the messenger RNA expression of PGC-1α in the post-exercise recovery period [55].
Fig. 2.
Effect of nicotinamide riboside (NR) loading and acute endurance exercise on the skeletal muscle nicotinamide adenine dinucleotide (NAD+) metabolome. The figure shows the effects of seven days of NR loading (NR SUPP) versus cellulose placebo on NAD+ metabolism in young male volunteers prior to an acute bout of endurance exercise. Specifically, NR loading had no effect on NR, NAD+, nicotinamide (NAM), and nicotinamide mononucleotide (NMN) content in skeletal muscle (A–D). However, deaminated NAD+ precursor, nicotinic acid riboside (NaR), and, methylated breakdown products, N-methyl-2-pyridone-5-carboxamide (Me2Py) and N-methyl-4-pyridone-5-carboxamide (Me4Py), were elevated following NR loading and sustained during the 3-h post-exercise period (E–G; ## denotes p < 0.05 placebo vs NR SUPP). Interestingly, the phosphoribosylated NAD+ precursor, nicotinic acid mononucleotide (NAMN), was only increased post-NR supplementation and exercise (H), whilst exercise alone had no effect on the NAD + metabolome in skeletal muscle (A–G). h hours. Image created by BioRender.com
Why Does NR Not Augment Mitochondrial Adaptation in Human Skeletal Muscle?
Based on the data discussed above, two key questions emerge, (i) why the disparity between cell, animal, and human studies? and (ii), why does NR not alter skeletal muscle metabolism, training adaptation, or athletic performance in humans? Regarding the disparity between rodent and human studies, it appears that NAD+ precursors such as NR are most effective when supplemented in a scenario of NAD+ deficiency (i.e., mitochondrial myopathy). In this context, NR can reverse this NAD+ deficiency and improve metabolic health. However, we are not aware of any studies that have shown a benefit of NR supplementation in young healthy animal models, thus it is not surprising that NR does not have a huge effect in healthy human volunteers in basal conditions, given that there is no compromise in NAD+ metabolism. Similarly, the rapid turnover and replenishment of NAD+ during exercise would also lead to the assumption that NR supplementation would have marginal effects if any during exercise, a statement supported by our recent work [55]. Differences in the impact of NR supplementation between animals and human studies may also be influenced by the scale of dosing between species [56] and the dosing strategy [14], i.e., dose matching, route of administration, and timing [48]. Overall, current evidence would suggest that NR supplementation can only be beneficial in scenarios of rescuing NAD+ deficiency.
The question as to why NR supplementation does not augment training adaptation or athletic performance in humans is also relevant for discussion. The mechanism thought to link NR and skeletal muscle mitochondrial biogenesis is through the SIRT1/PGC-1α signaling cascade, which is proposed to be activated by increased NAD+ availability during and in recovery from exercise. However, there is limited evidence for this pathway to directly mediate skeletal muscle adaptation to endurance exercise in both rodents and humans [28]. For example, we have previously shown that exercise-induced mitochondrial adaptation to endurance exercise training is preserved in muscle-specific SIRT1 knockout mice [34], whilst muscle-specific SIRT1 overexpression in skeletal muscle leads to incomplete mitochondrial biogenesis [35, 57]. In addition, mitochondrial adaptation to endurance exercise training is preserved in skeletal muscle-specific PGC-1α knockout mice [58]. There are also very limited data to support that endurance exercise promotes SIRT1 activation in human skeletal muscle [28, 59]. We recently reported that neither exercise nor exercise in combination with NR loading increased SIRT1 or SIRT3 signaling in human skeletal muscle, despite clear activation of PGC-1α gene expression in the 3-h period post-exercise [55]. Therefore, despite general acceptance for SIRT1 to be an important player in skeletal muscle adaptation to endurance exercise, direct support for this role is lacking and could explain why NR loading is largely ineffective at augmenting adaptation to training as SIRT1 and SIRT3 display substantial redundancy during skeletal muscle adaptation to exercise.
Conclusions and Future Directions
NAD+ therapeutics represent a novel nutritional approach to potentially manipulate skeletal muscle mitochondrial function and metabolic adaptation to endurance exercise. However, despite promising data in cell and rodent studies, the potential of various NAD+ therapeutics has yet to translate in humans unless clear NAD+ deficiency is present [50]. NAD+ metabolism in skeletal muscle, unlike other tissues, appears to be tightly regulated, with limited evidence suggesting that NAD+ deficiency occurs in physiological scenarios of relevance. Thus, based on the present literature, there does not appear to be a scenario currently identified where NR supplementation could be used to benefit skeletal muscle adaptation or athletic performance in vivo. Therefore, if researchers and practitioners are to further explore avenues where NAD+ therapeutics might be of benefit, it seems prudent that the focus should first be placed on determining physiological scenarios where NAD+ deficiency is apparent before examining the ergogenic benefit of NAD+-targeted therapeutics.
Acknowledgements
This supplement is supported by the Gatorade Sports Science Institute (GSSI). The supplement was guest edited by Lawrence L. Spriet, who convened a virtual meeting of the GSSI Expert Panel in October 2021 and received honoraria from the GSSI, a division of PepsiCo, Inc., for his participation in the meeting. Dr. Spriet received no honoraria for guest editing the supplement. Dr. Spriet suggested peer reviewers for each paper, which were sent to the Sports Medicine Editor-in-Chief for approval, prior to any reviewers being approached. Dr. Spriet provided comments on each paper and made an editorial decision based on comments from the peer reviewers and the Editor-in-Chief. Where decisions were uncertain, Dr. Spriet consulted with the Editor-in-Chief. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
Declarations
Funding
This article is based on a presentation by Andrew Philp to the GSSI Expert Panel Virtual Meeting in October 2021. An honorarium for preparation of this article was provided by the GSSI. Andrew Philp also acknowledges funding from the National Health and Medical Research Council and the Rebecca Cooper Medical Research Foundation.
Conflicts of Interest/Competing Interests
Dean Campelj and Andrew Philp have no conflicts of interest that are directly relevant to the content of this review.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
Both DC and AP devised, wrote, and edited the manuscript, and approved the final version of the manuscript prior to submission. Both authors read and approved the final manuscript.
References
- 1.Harden A, Young WJ. The alcoholic ferment of yeast-juice. Part II: the conferment of yeast-juice. Proc R Soc Lond B. 1906;78(526):369–375. doi: 10.1098/rspb.1906.0070. [DOI] [Google Scholar]
- 2.White AT, Schenk S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab. 2012;303(3):E308–E321. doi: 10.1152/ajpendo.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the "crossover" concept. J Appl Physiol (1985) 1994;76(6):2253–2261. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- 4.Brooks GA, Trimmer JK. Glucose kinetics during high-intensity exercise and the crossover concept. J Appl Physiol (1985) 1996;80(3):1073–1075. doi: 10.1152/jappl.1996.80.3.1073. [DOI] [PubMed] [Google Scholar]
- 5.Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990;258(3 Pt 1):C377–C389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- 6.Spriet LL, Howlett RA, Heigenhauser GJ. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med Sci Sports Exerc. 2000;32(4):756–763. doi: 10.1097/00005768-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Canto C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22(1):31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richard DM, Dawes MA, Mathias CW, et al. L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. 2009;2:45–60. doi: 10.4137/IJTR.S2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson LM. Tryptophan's role as a vitamin precursor (Krehl et al., 1945) J Nutr. 1997;127(5 Suppl.):1043S–S1045. [PubMed] [Google Scholar]
- 10.Ruggieri S, Orsomando G, Sorci L, et al. Regulation of NAD biosynthetic enzymes modulates NAD-sensing processes to shape mammalian cell physiology under varying biological cues. Biochim Biophys Acta. 2015;1854(9):1138–1149. doi: 10.1016/j.bbapap.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Gille A, Bodor ET, Ahmed K, et al. Nicotinic acid: pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol. 2008;48:79–106. doi: 10.1146/annurev.pharmtox.48.113006.094746. [DOI] [PubMed] [Google Scholar]
- 12.Hara N, Yamada K, Shibata T, et al. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282(34):24574–24582. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- 13.Collins PB, Chaykin S. The management of nicotinamide and nicotinic acid in the mouse. J Biol Chem. 1972;247(3):778–783. doi: 10.1016/S0021-9258(19)45675-5. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Su X, Quinn WJ, 3rd, et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018;27(5):1067–80.e5. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouwers B, Stephens NA, Costford SR, et al. Elevated nicotinamide phosphoribosyl transferase in skeletal muscle augments exercise performance and mitochondrial respiratory capacity following exercise training. Front Physiol. 2018;9:704. doi: 10.3389/fphys.2018.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costford SR, Brouwers B, Hopf ME, et al. Skeletal muscle overexpression of nicotinamide phosphoribosyl transferase in mice coupled with voluntary exercise augments exercise endurance. Mol Metab. 2018;7:1–11. doi: 10.1016/j.molmet.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frederick DW, Davis JG, Dávila Jr A, et al. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J Biol Chem. 2015;290(3):1546–1558. doi: 10.1074/jbc.M114.579565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frederick DW, Loro E, Liu L, et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 2016;24(2):269–282. doi: 10.1016/j.cmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basse AL, Agerholm M, Farup J, et al. Nampt controls skeletal muscle development by maintaining Ca(2+) homeostasis and mitochondrial integrity. Mol Metab. 2021;53:101271. doi: 10.1016/j.molmet.2021.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikiforov A, Dolle C, Niere M, et al. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286(24):21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oakey LA, Fletcher RS, Elhassan YS, et al. Metabolic tracing reveals novel adaptations to skeletal muscle cell energy production pathways in response to NAD (+) depletion. Wellcome Open Res. 2018;3:147. doi: 10.12688/wellcomeopenres.14898.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabrera ME, Saidel GM, Kalhan SC. Modeling metabolic dynamics: from cellular processes to organ and whole body responses. Prog Biophys Mol Biol. 1998;69(2–3):539–557. doi: 10.1016/S0079-6107(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 23.Henriksson J, Katz A, Sahlin K. Redox state changes in human skeletal muscle after isometric contraction. J Physiol. 1986;380:441–451. doi: 10.1113/jphysiol.1986.sp016296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahlin K. NADH in human skeletal muscle during short-term intense exercise. Pflugers Arch. 1985;403(2):193–196. doi: 10.1007/BF00584099. [DOI] [PubMed] [Google Scholar]
- 25.Sahlin K, Katz A, Broberg S. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am J Physiol. 1990;259(5 Pt 1):C834–C841. doi: 10.1152/ajpcell.1990.259.5.C834. [DOI] [PubMed] [Google Scholar]
- 26.Katz A, Sahlin K. Effect of decreased oxygen availability on NADH and lactate contents in human skeletal muscle during exercise. Acta Physiol Scand. 1987;131(1):119–127. doi: 10.1111/j.1748-1716.1987.tb08213.x. [DOI] [PubMed] [Google Scholar]
- 27.Sahlin K, Katz A, Henriksson J. Redox state and lactate accumulation in human skeletal muscle during dynamic exercise. Biochem J. 1987;245(2):551–556. doi: 10.1042/bj2450551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philp A, Schenk S. Unraveling the complexities of SIRT1-mediated mitochondrial regulation in skeletal muscle. Exerc Sport Sci Rev. 2013;41(3):174–181. doi: 10.1097/JES.0b013e3182956803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1alpha. J Biol Chem. 2005;280(16):16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 31.Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26(7):1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurd BJ, Yoshida Y, Lally J, et al. The deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis. J Physiol. 2009;587(Pt 8):1817–1828. doi: 10.1113/jphysiol.2008.168096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurd BJ, Yoshida Y, McFarlan JT, et al. Nuclear SIRT1 activity, but not protein content, regulates mitochondrial biogenesis in rat and human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):R67–75. doi: 10.1152/ajpregu.00417.2010. [DOI] [PubMed] [Google Scholar]
- 34.Philp A, Chen A, Lan D, et al. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation following endurance exercise. J Biol Chem. 2011;286(35):30561–30570. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White AT, Philp A, Fridolfsson HN, et al. High-fat diet-induced impairment of skeletal muscle insulin sensitivity is not prevented by SIRT1 overexpression. Am J Physiol Endocrinol Metab. 2014;307(9):E764–E772. doi: 10.1152/ajpendo.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 37.Saloranta C, Franssila-Kallunki A, Ekstrand A, et al. Modulation of hepatic glucose production by non-esterified fatty acids in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991;34(6):409–415. doi: 10.1007/BF00403179. [DOI] [PubMed] [Google Scholar]
- 38.Vaag A, Skott P, Damsbo P, et al. Effect of the antilipolytic nicotinic acid analogue acipimox on whole-body and skeletal muscle glucose metabolism in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1991;88(4):1282–1290. doi: 10.1172/JCI115432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Weijer T, Phielix E, Bilet L, et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes. 2015;64(4):1193–1201. doi: 10.2337/db14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshino M, Yoshino J, Kayser BD, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372(6547):1224–1229. doi: 10.1126/science.abe9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills KF, Yoshida S, Stein LR, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24(6):795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das A, Huang GX, Bonkowski MS, et al. Impairment of an endothelial NAD(+)-H(2)S signaling network is a reversible cause of vascular aging. Cell. 2018;173(1):74–89.e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao B, Zhao Y, Wang D, et al. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study. J Int Soc Sports Nutr. 2021;18(1):54. doi: 10.1186/s12970-021-00442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117(4):495–502. doi: 10.1016/S0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 45.Canto C, Houtkooper RH, Pirinen E, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cartwright DM, Oakey LA, Fletcher RS, et al. Nicotinamide riboside has minimal impact on energy metabolism in mouse models of mild obesity. J Endocrinol. 2021;251(1):111–123. doi: 10.1530/JOE-21-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams AS, Koves TR, Pettway YD, et al. Nicotinamide riboside supplementation confers marginal metabolic benefits in obese mice without remodeling the muscle acetyl-proteome. iScience. 2022;25(1):103635. doi: 10.1016/j.isci.2021.103635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Damgaard MV, Nielsen TS, Basse AL, et al. Intravenous nicotinamide riboside elevates mouse skeletal muscle NAD(+) without impacting respiratory capacity or insulin sensitivity. iScience. 2022;25(2):103863. doi: 10.1016/j.isci.2022.103863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerutti R, Pirinen E, Lamperti C, et al. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19(6):1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan NA, Auranen M, Paetau I, et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med. 2014;6(6):721–731. doi: 10.1002/emmm.201403943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryu D, Zhang H, Ropelle ER, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med. 2016;8(361):361ra139. doi: 10.1126/scitranslmed.aaf5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frederick DW, McDougal AV, Semenas M, et al. Complementary NAD(+) replacement strategies fail to functionally protect dystrophin-deficient muscle. Skelet Muscle. 2020;10(1):30. doi: 10.1186/s13395-020-00249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dollerup OL, Chubanava S, Agerholm M, et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J Physiol. 2020;598(4):731–754. doi: 10.1113/JP278752. [DOI] [PubMed] [Google Scholar]
- 54.Elhassan YS, Kluckova K, Fletcher RS, et al. Nicotinamide riboside augments the aged human skeletal muscle NAD(+) metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28(7):1717–28.e6. doi: 10.1016/j.celrep.2019.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stocks B, Ashcroft SP, Joanisse S, et al. Nicotinamide riboside supplementation does not alter whole-body or skeletal muscle metabolic responses to a single bout of endurance exercise. J Physiol. 2021;599(5):1513–1531. doi: 10.1113/JP280825. [DOI] [PubMed] [Google Scholar]
- 56.Blanchard OL, Smoliga JM. Translating dosages from animal models to human clinical trials: revisiting body surface area scaling. FASEB J. 2015;29(5):1629–1634. doi: 10.1096/fj.14-269043. [DOI] [PubMed] [Google Scholar]
- 57.White AT, McCurdy CE, Philp A, et al. Skeletal muscle-specific overexpression of SIRT1 does not enhance whole-body energy expenditure or insulin sensitivity in young mice. Diabetologia. 2013;56(7):1629–1637. doi: 10.1007/s00125-013-2912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rowe GC, El-Khoury R, Patten IS, et al. PGC-1alpha is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS ONE. 2012;7(7):e41817. doi: 10.1371/journal.pone.0041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Islam H, Edgett BA, Gurd BJ. Coordination of mitochondrial biogenesis by PGC-1alpha in human skeletal muscle: a re-evaluation. Metabolism. 2018;79:42–51. doi: 10.1016/j.metabol.2017.11.001. [DOI] [PubMed] [Google Scholar]