Abstract
Olaparib, niraparib, rucaparib, and talazoparib are poly (ADP-ribose) polymerase (PARP) inhibitors approved for the treatment of ovarian, breast, pancreatic, and/or prostate cancer. Poly (ADP-ribose) polymerase inhibitors are potent inhibitors of the PARP enzymes with comparable half-maximal inhibitory concentrations in the nanomolar range. Olaparib and rucaparib are orally dosed twice a day, extensively metabolized by cytochrome P450 enzymes, and inhibitors of several enzymes and drug transporters with a high risk for drug–drug interactions. Niraparib and talazoparib are orally dosed once a day with a lower risk for niraparib and a minimal risk for talazoparib to cause drug–drug interactions. All four PARP inhibitors show moderate-to-high interindividual variability in plasma exposure. Higher exposure is associated with an increase in toxicity, mostly hematological toxicity. For talazoparib, exposure–efficacy relationships have been described, but for olaparib, niraparib, and rucaparib this relationship remains inconclusive. Further studies are required to investigate exposure–response relationships to improve dosing of PARP inhibitors, in which therapeutic drug monitoring could play an important role. In this review, we give an overview of the pharmacokinetic properties of the four PARP inhibitors, including considerations for patients with renal dysfunction or hepatic impairment, the effect of food, and drug–drug interactions. Furthermore, we focus on the pharmacodynamics and summarize the available exposure–efficacy and exposure–toxicity relationships.
Key Points
| The approved poly (ADP-ribose) polymerase inhibitors olaparib, niraparib, rucaparib, and talazoparib show moderate-to-high interindividual variability in plasma exposure. |
| Olaparib and rucaparib have a high potential for drug–drug interactions, while this risk is lower for niraparib and minimal for talazoparib. |
| Exposure has been associated with toxicity for all poly (ADP-ribose) polymerase inhibitors, mainly with hematological toxicity. |
| Exposure–efficacy relationships have been described for talazoparib, but remain inconclusive for olaparib, niraparib, and rucaparib. |
Introduction
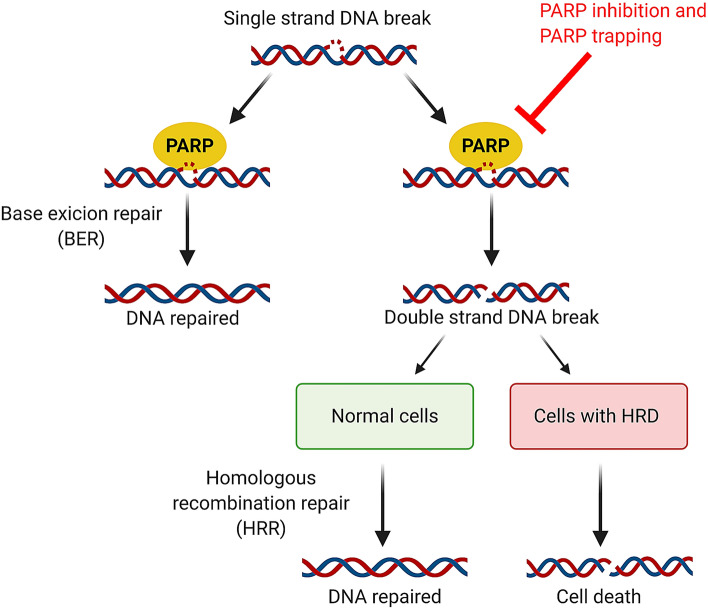
A relatively new class of targeted anticancer agents are the poly (ADP-ribose) polymerase (PARP) inhibitors. Poly (ADP-ribose) polymerase inhibitors primarily inhibit the catalytic activity of PARP-1 and PARP-2 enzymes, which are involved in base excision repair of DNA single-strand breaks. Poly (ADP-ribose) polymerase inhibition leads to accumulation of single-strand breaks, ultimately resulting in double-strand breaks (DSBs) [1]. In addition to catalytic inhibition, PARP inhibitors trap the PARP enzyme-DNA complex on single-strand breaks resulting in DSBs [2]. Poly (ADP-ribose) polymerase trapping is considered the major mechanism of anti-tumor activity [3]. While PARP inhibition is not effective in healthy cells, as they alternatively can utilize the functional homologous recombination repair mechanism for repair of DSBs, it is particularly effective in cells harboring homologous recombination deficiencies (HRD), such as pathogenic breast cancer (BRCA)-1 or BRCA-2 mutations [2]. This concept is called synthetic lethality: simultaneous loss of function of two or more key molecules results in cell death, while a deficiency in only one is not lethal (Fig. 1) [1].
Fig. 1.
Mechanism of action of poly (ADP) ribose polymerase (PARP) inhibitors. Single-strand breaks in DNA are repaired through base excision repair mediated by PARP enzymes. Inhibition of PARP or trapping of PARP on the DNA by PARP inhibitors, result in double-strand breaks in DNA. In normal cells harboring the homologous recombination repair mechanism, double-strand breaks are repaired and the cell survives. In cells with an homologous recombination deficiency (HRD), including breast cancer (BRCA) 1 and 2 mutations, this repair mechanism is absent leading to accumulation of double-strand breaks and cell death
The introduction of PARP inhibitors has accomplished many breakthroughs in the treatment of ovarian, breast, pancreatic, and prostate cancer. It improved progression-free survival (PFS) and quality of life, but there are still challenges to overcome. Drug resistance and adverse effects are common and can limit long-term treatment. Poly (ADP-ribose) polymerase inhibitors are orally administered, given in a fixed dose, and are substrates for different metabolizing enzymes and drug transporters [4–7]. Consequently, large variability in pharmacokinetic exposure between patients is not exceptional. Low exposure may lead to suboptimal efficacy, while high exposure can cause toxicities. This gives the opportunity for precision dosing, for example, by therapeutic drug monitoring [8–11]. Indications of PARP inhibitors are rapidly expanding from monotherapy in patients with BRCA mutations, to patients with other HRD and no HRD, to combination therapy with DNA-damaging agents, radiation, targeted therapies, and immunotherapy [1, 12]. In this review, we aim to summarize the available pharmacokinetic and pharmacodynamic data for the approved PARP inhibitors olaparib, niraparib, rucaparib, and talazoparib.
Methods
A comprehensive literature search was performed using PubMed and EMBASE. The term ‘pharmacokinetics’ was combined with the different PARP inhibitors and relevant studies were selected. The snowballing method was used to find additional relevant studies. The Committee for Medicinal Products for Human Use Assessment Reports from the European Medicines Agency (EMA) and the US Food and Drug Administration Clinical Pharmacology and Biopharmaceutics review of niraparib, olaparib, rucaparib, and talazoparib were consulted as well.
Pharmacokinetics and Pharmacodynamics of PARP Inhibitors
Table 1 gives an overview of the EMA-approved PARP inhibitors and indications. Information on the preclinical pharmacology of PARP inhibitors is shown in Table 2. The clinical pharmacokinetics at steady state is summarized in Table 3. Tables 4 and 5 describes the impact of renal and hepatic impairment, respectively, and other potential factors influencing the pharmacokinetics of PARP inhibitors are discussed as well. The results of food-effect studies are shown in Table 6 and drug–drug interaction (DDI) studies are summarized in Table 7. The data and the implications of the data presented in the tables are further discussed for each compound.
Table 1.
Overview of the approved PARP inhibitors and indications by the EMA
| PARP inhibitor | Registered trade name | Company | Year of approval | Indication | Mutation | Setting | Approved EMA indication | Approved dose |
|---|---|---|---|---|---|---|---|---|
| Olaparib (AZD-2281) | Lynparza | AstraZeneca | 2014 | Ovarian | g/sBRCAm | Maintenance treatment | Platinum-sensitive, relapsed, high-grade serous EO, FT, PP cancer in complete or partial response to platinum-based chemotherapy | 400 mg BID (capsules) |
| 2018 | Ovarian | – | Maintenance treatment | Platinum-sensitive, relapsed, high-grade serous EO, FT, PP cancer in complete or partial response to platinum-based chemotherapy | 300 mg BID (tablets) | |||
| 2019 | Breast | gBRCAm and HER2- | Treatment | Locally advanced or metastatic breast cancer previously treated with an anthracycline and taxane in the (neo)adjuvant or metastatic setting unless not suitable for these treatments. Patients with HR-positive breast cancer should have progressed on or after prior endocrine therapy, or be considered unsuitable for endocrine therapy | 300 mg BID (tablets) | |||
| 2019 | Ovarian | g/sBRCAm | Maintenance treatment | Advanced high-grade EO, FT, PP cancer in complete or partial response following completion of first-line platinum-based chemotherapy | 300 mg BID (tablets) | |||
| 2020 | Pancreas | gBRCAm | Maintenance treatment | Metastatic pancreatic adenocarcinoma without progression after a minimum of 16 weeks of platinum treatment within a first-line chemotherapy regimen | 300 mg BID (tablets) | |||
| 2020 | Ovarian | HRD+ | Maintenance treatment | Advanced high-grade EO, FT, PP cancer in complete or partial response following completion of first-line platinum-based chemotherapy | 300 mg BID (tablets) | |||
| 2020 | Prostate | g/sBRCAm | Treatment | Metastatic castration-resistant prostate cancer who have progressed following prior therapy that included a new hormonal agent | 300 mg BID (tablets) | |||
| 2020 | Ovarian | HRD+ | Maintenance treatment icm bevacizumab | Advanced high-grade EO, FT, PP cancer in complete or partial response following completion of first-line platinum based chemotherapy with bevacizumab | 300 mg BID (tablets) | |||
| Rucaparib (AG014699) | Rubraca | Clovis Oncology | 2018 | Ovarian | g/sBRCAm | Treatment | Platinum-sensitive, relapsed, or progressive high-grade serous EO, FT, PP cancer previously treated with two or more prior lines of platinum-based chemotherapy and unable to tolerate further platinum-based chemotherapy | 600 mg BID |
| 2018 | Ovarian | – | Maintenance treatment | Platinum-sensitive, relapsed, high-grade serous EO, FT, PP cancer in complete or partial response to platinum-based chemotherapy | 600 mg BID | |||
| Niraparib (MK-4827) | Zejula | Tesaro/GSK | 2017 | Ovarian | – | Maintenance treatment | Platinum-sensitive, relapsed, high-grade serous EO, FT, PP cancer in complete or partial response to platinum-based chemotherapy | 300 mg QD |
| 2020 | Ovarian | – | Maintenance treatment | Advanced high-grade EO, FT, PP cancer in complete or partial response following completion of first-line platinum-based chemotherapy | 300 mg QD | |||
| Talazoparib (BMN 673) | Talzenna | Pfizer | 2019 | Breast | gBRCAm and HER2- | Treatment | Locally advanced or metastatic breast cancer previously treated with an anthracycline and/or a taxane in the (neo)adjuvant, locally advanced, or metastatic setting unless patients were not suitable for these treatments. Patients with HR-positive breast cancer should have progressed on or after prior endocrine therapy, or be considered unsuitable for endocrine therapy | 1 mg QD |
BID twice a day, BRCA Breast Cancer, EO epithelial ovarian, FT fallopian tube, g germline, HR hormone receptor, HRD homologous recombination deficiency, HRR homologous recombination repair, HER2 human epidermal growth factor receptor 2, m mutation, PARP poly (ADP-ribose) polymerase, PP primary peritoneal, QD once a day, s somatic
Table 2.
Preclinical pharmacology: the in vitro interaction of PARP inhibitors with their target, enzymes, and transporters and average unbound steady-state concentrations in patients at clinical doses
| PARP inhibitor | Average unbound steady-state Cmin in patients at clinical doses (nM)a | Average unbound steady-state Cmax in patients at clinical doses (nM)b | Target | Target IC50 (nM) | Catalytic inhibitionc (IC50, nM) | Cytotoxictyd (EC50, nM) |
PARP trapping potencye | Metabolized by | Inductor of | Inhibitor of | Down regulator of | Substrate to | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Olaparib Capsules Tablets |
460 672 |
2825 3779 |
PARP1 PARP2 PARP3 TNKS1 |
5 1 4 1500 |
6 | 259 | 1 |
CYP1A1a CYP2A6a CYP3A4 |
CYP2B6 CYP3A4 |
CYP3A4 MATE1 MATE2K OATP1B1 OAT3 OCT1 OCT2 P-gp UGT1A1 |
– | P-gp | [22, 23, 25, 26] |
| Niraparib | 364 | 937 |
PARP1 PARP2 PARP3 |
3.8 2.1 1300 |
60 | 650 | 2 |
CES CYP1A2f CYP2D6f CYP3A4/5f UGT |
CYP1A2g |
BCRP DAT MAO-B MATE1 MATE2-k NET SERT |
– |
BCRP P-gp |
[51] |
| Rucaparib | 1702 | 2010 |
PARP1 PARP2 PARP3 |
0.5 0.8 28 |
21 | 609 | 1 |
CYP1A2 CYP2D6 CYP3A4 |
CYP1A2 |
BCRP CYP1A2 CYP2C9 CYP2C19 CYP2D6 CYP3A4 MATE1 MATE2-k Non-selective sigma channel OCT1 OCT2 P-gp Several kinasesh Sodium channel 2 UGT1A1 |
CYP3A4 CYP2B6 |
BCRP P-gp |
[75, 80, 88, 92] |
| Talazoparib | 2.5 | 14.4 |
PARP1 PARP2 PARP3 TNKS1 TNKS2 |
0.6 0.3 22.0 13.5 4.7 |
4 | 5 | 100 | – | – | – | – |
BCRP P-gp |
[102] |
CES carboxylesterase, Cmax maximum plasma concentration, Cmin minimum plasma concentration, CYP cytochrome P450, BCRP breast cancer resistance protein, DAT dopamine transporter, EC50 half-maximal effective concentration, IC50 half-maximal inhibitory concentration, MAO-B monoamine oxidase inhibitor B, MATE multidrug and toxin extrusion protein, NET norepinephrine transporter, OAT organic anion transporter, OATP organic anion transporting polypeptide, OCT organic cation transporter, PARP poly (ADP-ribose) polymerase, P-gp P-glycoprotein, SERT serotonin transporter, TNKS tankyrase, UGT uridine 5'-diphospho-glucuronosyltransferase (UDP)-glucuronosyltransferase
aCalculated based on protein binding and weighed average Cmin levels at steady state reported in Table 3
bCalculated based on protein binding and weighed average Cmax levels at steady state reported in Table 3
cMeasured as PAR level inhibitions in DT40 cells [114, 115]
dMeasured in the human Capan-1 cell line, which is BRCA2 deficient [3, 100]
eRelative to olaparib
fMinimal contribution
gWeak inhibitor
hCDK16/cyclin Y (PCTAIRE), PASK, CDK17/cyclin Y (PCTK2), PIM3, DYRK1/DYRK1A, DYRK1B, CDK18/cyclin Y (PCTK3), MYO3b, MYO3A, PIM1, CDK6/cyclin D1
Table 3.
Pharmacokinetic parameters at steady state
| PARP inhibitor | N | Dose (mg) |
Tmax (h) Mean (range) |
Cmin (ng/mL) | Cmax (ng/mL) | AUC0–tau (ng/mL*h) a | t1/2 (h) | References |
|---|---|---|---|---|---|---|---|---|
| Olaparib capsules | 6 | 400 BID | 2.0 (1.5–3.0) | 1290 (76%) | 7650 (27%) | 44,900 (39%) | NR | [15] |
| 17 | 400 BID | 1.25 (1.0–8.0)b | 1040 (230–8490) | 6360 (3880–13,300) | 41,500 (18,700–147,000) | 11.9 ± 4.82b | [16] | |
| 10 | 400 BID | 1.25 (1.0–8.0)b | 1860 (530–6670) | 5700 (2380–10,9000) | 43,100 (18,100–98,600) | 11.9 ± 4.82b | [16] | |
| 5c | 400 BID | 2.1 (1.5–4.0) | NR | 5900 (19.7%) | 33,300 (22.3%) d | 10.7 (3.8–18.9)e | [33] | |
| 6 | 400 BID | 2.0 (1.5–3.0) | NR | 7900 (26%) | 44,000 (38%) | NR | [25] | |
| 4 | 400 BID | 2.0 (2.0–3.0)f | 1600 (46.1%) | 9100 (27.2%) | 58,100 (29.4%) | NR | [128] | |
| Olaparib tablets | 17 | 300 BID | NR | 1840 (340–3830) (67%) | 9370 (2280–14,700) (47%) | 58,400 (23,100–96,000) (47%) | NR | [16, 25] |
| 15g | 300 BID | 1.50 (0.97–3.00) | 800 (118%) | 8270 (35.0%) | 44,000 (48.4%) | 6.52 ± 1.35h | [129] | |
| 6c | 300 BID | 3.00 (1.50–3.93) | 1290 (157.6%) | 8430 (35.05%) | 52,340 (68.17%) | 9.43 (6.45–14.7)i | [130] | |
| 29 | 300 BID | NR | 2000 (89.8%)j | 9500 (41.5%)j | 62,100 (51.6%)k | NR | [38] | |
| Niraparib | 10 | 300 QD | 3.5 (2.0–4.2) | 687 ± 303 (44%)l | 1399 ± 608 (43%)l | 21,407 ± 9168 (43%)l | 36.2 ±14.6 | [46] |
| 12g | 300 QD | 3.05 (2.9–6.1) | NR | 2070 (29.3%)l | 27,852 (28.6%)l | 36.45 ± 17.21 | [59] | |
| 4c | 300 QD | 3.7 ±1.6 | 592.3 ± 138.2 | 1167 ± 194.9 | 19,540 ± 3117 | NR | [131] | |
| Rucaparib | 7 | 600 BID | 4 (2.53–10) | NR | 2420 (45%) | 21,400 (61%)m | NR | [71] |
| 196 | 600 BID | NR | 2026 ± 1147 (57%) | NR | NR | NR | [72] | |
| 16 | 600 BID | 2.5 (0.5–3.1) | NR | 2650 (57%) | 25,800 (57%) | NR | [92] | |
| 18 | 600 BID | 1.92 (0–5.98) | NR | 1940 (54%) | 16,900 (54%)n | 12.6 (54%)n | [91] | |
| 375 | 600 BID | NR | 1754 ± 805 (46%) | 2169 ± 890 (41%) | 47,507 ± 20,436 (43%) | NR | [88] | |
| Talazoparib | 6 | 1.0 QD | 1.02 (0.75–2.00) | 3.720 ± 1.590 (43%) | 21.000 ± 7.990 (38%) | 202 ± 54 (27%) | 50.0 ± 16.6 (33%) | [99] |
| 27 | 1.0 QD | 2.00 (0.97–6.00) | 4.950 (56%) | 16.400 (32%) | 208 (37%) | NR | [132] | |
| 6c | 1.0 QD | 1.03 (0.7–1.9) | 3.650 (49%) | 32.840 (14%) | 244.7 (21%) | 50.73 ± 10.1 (20%)o | [133] |
AUC area under the plasma concentration–time curve, AUC0–tau AUC from time zero to the end of the dosing interval (tau), BID twice a day, Cmax maximum plasma concentration, Cmin minimum plasma concentration, CV% percentage coefficient of variation, N number of subjects, NR not reported, SD standard deviation, t½ elimination half-life, Tmax time to maximum plasma concentration, QD once a day
Variability is reported as ± SD, (CV%), (range)
aFor olaparib and rucaparib, AUC from 0 to 12 hours, for niraparib and talazoparib AUC from 0 to 24 hours
bBased on 6 patients receiving a single dose of 400 mg
cJapanese patients
dAUC from 0 to 10 hours
eBased on 6 patients receiving a single dose of 400 mg
fMedian (range)
gChinese patients
hBased on 16 patients receiving a single dose of 300 mg
iBased on 7 patients receiving a single dose of 300 mg
jBased on 27 patients
kBased on 26 patients
lCalculated based the molecular weight of 320.4 g/mol
mBased on 4 patients
nBased on 12 patients
oBased on 6 patients receiving a single dose of 1.0 mg
Table 4.
Impact of renal impairment on the pharmacokinetics of PARP inhibitors
| PARP inhibitor | Method | Renal impairment | PK parameter | GLS mean ratio | 90% CI | Effect on PK parameter | Advice | References |
|---|---|---|---|---|---|---|---|---|
| Olaparib tablets | Clinical studya,b | Mild | Cmax | 1.15 | 1.04–1.27 | ↑ 15% | No dose adjustments required | [28, 29] |
| AUC0–∞ | 1.24 | 1.06–1.47 | ↑ 24% | |||||
| Moderate | Cmax | 1.26 | 1.06–1.48 | ↑ 26% | Decrease the dose to 200 mg BID | |||
| AUC0–∞ | 1.44 | 1.10–1.89 | ↑ 44% | |||||
| PBPK modela | Mild | Cmax | 1.04 | 1.03–1.04 | ↑ 4% | No dose adjustments required | [29, 32] | |
| AUC | 1.40 | 1.39–1.40 | ↑ 40% | |||||
| Moderate | Cmax | 1.09 | 1.07–1.10 | ↑ 9% | Decrease the dose to 200 mg BID | |||
| AUC | 1.89 | 1.89–1.90 | ↑ 89% | |||||
| Severe | Cmax | 1.11 | 1.10–1.12 | ↑ 11% | Olaparib is not recommended | |||
| AUC | 2.21 | 2.19–2.22 | ↑ 121% | |||||
| Olaparib capsules | PBPK modela | Mild | Cmax | 1.21 | 1.19–1.24 | ↑ 21% | No dose adjustments required | [30, 32] |
| AUC | 1.48 | 1.44–1.52 | ↑ 48% | |||||
| Moderate | Cmax | 1.28 | 1.26–1.31 | ↑ 28% | Decrease the dose to 300 mg BID | |||
| AUC | 1.95 | 1.92–1.98 | ↑ 95% | |||||
| Severe | Cmax | 1.31 | 1.28–1.33 | ↑ 31% | Olaparib is not recommended | |||
| AUC | 1.27 | 2.25–2.29 | ↑ 27% | |||||
| Niraparib | PopPK modelc | Mild | Exposured | NR | NR | ↔ | No dose adjustments required | [51, 54, 134] |
| Moderate | Exposured | NR | NR | ↔ | No dose adjustments required | |||
| Rucaparib | PopPK modelc | Mild | AUCss | NR | NR | ↑ 15% | No dose adjustments required | [84, 88] |
| Moderate | AUCss | NR | NR | ↑ 32% | No dose adjustments required | |||
| Talazoparib | Clinical studyc,e | Mild | Cmax | 1.11 | 0.74–1.66 | ↔ | No dose adjustments required | [107] |
| AUC | 1.12 | 0.80–1.57 | ↔ | |||||
| Moderate | Cmax | 1.32 | 0.89–1.94 | ↔ | Decrease the dose to 0.75 mg QD | |||
| AUC | 1.43 | 1.03–1.98 | ↑ 43% | |||||
| Severe | Cmax | 1.89 | 1.27–2.83 | ↑ 89% | Decrease the dose to 0.5 mg QD | |||
| AUC | 2.63 | 1.88–3.69 | ↑ 163% | |||||
| PopPK modelc | Mild | CL/F | NR | NR | ↓ 15% | No dose adjustments required | [106] | |
| Moderate | CL/F | NR | NR | ↓ 38% | Decrease the dose to 0.75 mg QD |
AUC area under the plasma concentration–time curve, AUC0–∞ AUC from zero to infinity, AUCss AUC at steady state, AUC0–24 AUC from 0 to 24 hours, BID twice a day, CI confidence interval, CL/F apparent oral clearance, Cmax maximum plasma concentration, GLS mean geometric least-squares mean, NR not reported, PARP poly (ADP-ribose) polymerase, PBPK physiologically based pharmacokinetic, PK pharmacokinetic, PopPK population pharmacokinetic, QD once a day, ↑ indicates increase, ↓ indicates decrease, ↔ indicates no change
aClassification for renal impairment based on the Committee for Medicinal Products for Human Use guidance (CHMP/EWP/225/02 [135]). Normal renal function: creatinine clearance >80 mL/min; mild renal impairment: creatinine clearance 51–80 mL/min; moderate renal impairment: creatinine clearance 31–50 mL/min; severe renal impairment: creatinine clearance ≤30mL/min
bCreatinine clearance calculated according to the Cockcroft–Gault equation
Classification for renal impairment based on the Committee for Medicinal Products for Human Use guidance (EMA/CHMP/83874/2014 [136]). Normal renal function: creatinine clearance ≥90 mL/min; mild renal impairment: creatinine clearance 60–89 mL/min; moderate renal impairment: creatinine clearance 30–59 mL/ min; severe renal impairment: creatinine clearance ≤30 mL/min
dNot specified
eEstimated glomerular filtration rate, calculated using the Modification of Diet in Renal Disease formula
Table 5.
Impact of hepatic impairment on the pharmacokinetics of PARP inhibitors
| PARP inhibitor | Method | Renal impairment | PK parameter | GLS mean ratio | 90% CI | Effect on PK parameter | Advice | References |
|---|---|---|---|---|---|---|---|---|
| Olaparib tablets | Clinical studya | Mild | Cmax | 1.13 | 0.82–1.56 | ↔ | No dose adjustment required | [31] |
| AUC0–∞ | 1.15 | 0.72–1.93 | ↔ | |||||
| Moderate | Cmax | 0.87 | 0.63–1.22 | ↔ | No dose adjustment required | |||
| AUC0–∞ | 1.08 | 0.66–1.74 | ↔ | |||||
| PBPK modela | Mild | Cmax | 1.06 | 1.05–1.07 | ↑ 6% | No dose adjustment required | [32] | |
| AUC | 1.26 | 1.26–1.28 | ↑ 26% | |||||
| Moderate | Cmax | 0.78 | 0.77–0.80 | ↓ 22% | No dose adjustment required | |||
| AUC | 1.26 | 1.15–1.32 | ↑ 26% | |||||
| Severe | Cmax | 0.59 | 0.58–0.59 | ↓ 41% | Olaparib is not recommended | |||
| AUC | 1.06 | 1.03–1.08 | ↑ 6% | |||||
| Olaparib capsules | PBPK modela | Mild | Cmax | 1.16 | 1.15–1.16 | ↑ 16% | No dose adjustment required | [32] |
| AUC | 0.95 | 0.94–0.97 | ↓ 5% | |||||
| Moderate | Cmax | 1.27 | 1.26–1.28 | ↑ 27% | No dose adjustment required | |||
| AUC | 1.54 | 1.52–1.56 | ↑ 54% | |||||
| Severe | Cmax | 1.04 | 1.03–1.06 | ↑ 4% | Olaparib is not recommended | |||
| AUC | 2.20 | 2.13–2.28 | ↑ 120% | |||||
| Niraparib | PopPK modelb | Mild | Exposurec | NR | NR | ↔ | No dose adjustment required | [51, 54] |
| Clinical studyb | Moderate | Cmax | 0.93 | 0.64–1.36 | ↔ | Decrease the dose to 200 mg QD | [57] | |
| AUC0–∞ | 1.56 | 1.06–2.30 | ↑ 56% | |||||
| Rucaparib | PopPK modelb | Mild | Cmin | NR | NR | ↔ | No dose adjustment required | [75, 84, 88] |
| AUCss | NR | NR | ↔ | |||||
| Moderate | Cmin | NR | NR | ↔ | No dose adjustment required | |||
| AUCss | NR | NR | ↑ 32% | |||||
| Clinical studyb | Moderate | Cmax | 0.91 | 0.61–1.36 | ↔ | No dose adjustment required | [87] | |
| AUC0–∞ | 1.45 | 0.67–3.13 | ↔ | |||||
| Talazoparib | Clinical study/PopPKb | Mild | Exposurec and CL/F | NR | NR | ↔ | No dose adjustment required | [108] |
| Moderate | Exposurec and CL/F | NR | NR | ↔ | No dose adjustment required | |||
| Severe | Exposurec and CL/F | NR | NR | ↔ | No dose adjustment required | |||
| PopPK modelb | Mild | CL/F | NR | NR | ↔ | No dose adjustment required | [106] |
AUC area under the plasma concentration–time curve, AUC0–∞ AUC from zero to infinity, AUCss AUC at steady state, CI confidence interval, CL/F apparent oral clearance, Cmax maximum plasma concentration, GLS mean geometric least-squares mean, NR not reported, PARP poly (ADP-ribose) polymerase, PBPK physiologically based pharmacokinetic, PopPK population pharmacokinetic, PK pharmacokinetic, QD once a day, ↑ indicates increase, ↓ indicates decrease, ↔ indicates no change
aClassification for hepatic impairment based on the Committee for Medicinal Products for Human Use guidance (CHMP/EWP/2339/02) [137]. Mild hepatic impairment: Child-Pugh class A; moderate hepatic impairment: Child-Pugh class B; severe hepatic impairment: Child-Pugh class C
bClassification for hepatic impairment defined by National Cancer Institute Organ Dysfunction Working Group Criteria criteria [138]
cNot specified
Table 6.
Effect of food on the pharmacokinetics of PARP inhibitors after a single dose
| PARP inhibitor | Condition | N | Dose (mg) | t½ (h) | Tmax (h) | Cmax (ng/mL) | AUC0–last (ng × h/mL) |
AUC0–∞ (ng × h/mL) |
Result meal vs fasted state | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Olaparib capsules | High-fat meal | 31 | 400 | 12.20 ± 4.53a | 4.03 (2.00–8.03)a | 6,070 (45.1%)a | 64,620 (63.3%)a | 65,440 (64.2%)a |
↓ t½ 34% ↑ Tmax 134% ↔Cmax ↑AUC0–last 22% ↑AUC0–∞ 19% |
[139] |
| Standard meal | 31 | 400 | 15.42 ± 5.92a | 4.00 (1.00–8.00)b | 6,970 (45.9%)b | 67,710 (86.4%)c | 70,190 (80.5%)a |
↓ t½ 16% ↑ Tmax 133% ↑Cmax 10% ↑AUC0–last 20% ↑AUC0–∞ 21% |
||
| Fasted | 31 | 400 | 18.39 ± 6.99b | 1.72 (0.92–4.05)d | 6,350 (40.9%)d | 58,400 (75.6%)d | 61,060 (78.1%)b | |||
| Olaparib tablets | High-fat meal | 54 | 300 | 11.1 ± 4.09e | 4.00 (1.00–12.0) | 5,480 (40.5%) | 46,000 (56.6%)e | 45,400 (57.1%)e |
↔ t½ ↑ Tmax 167% ↓Cmax 21% ↑AUC0–last 8% ↑AUC0–∞ 8% |
[36] |
| Fasted | 55 | 300 | 12.2 ± 5.31f | 1.50 (0.50–5.85) | 7,000 (35.0%) | 43,600 (54.3%)f | 43,000 (55.2%)g | |||
| Niraparib | High-fat meal | 15 | 300 | 47.9 ± 17.5h | 8.0 ± 4.9i | 582.1 (39%) | 27,186.4 (52%) | 31,194 (54%)h |
↔ t½ ↑ Tmax 128% ↓Cmax 27% ↔AUC0–last ↔AUC0–∞ |
[61] |
| Fasted | 16 | 300 | 50.5 ± 17.9 | 3.5 ± 1.2i | 803.7 (50%) | 28,638.1 (63%) | 29,016.1 (63%)j | |||
| Rucaparib | High-fat meal | 26 | 600 | 16.8 ± 9.5k | 7.83 (1.5–24.45) | 959 (73%) | 13,900l (74%) | NR |
↔ t½ ↑ Tmax 95% ↑Cmax 20% ↑AUC0–24h 38% |
[91] |
| Fasted | 26 | 600 | 18.7 ± 9.9m | 4.02 (0.53–24.83) | 819 (84%) | 10,000l (76%) | NR | |||
| Talazoparib | High-fat meal | 18 | 0.5 | 113.6 ± 38.3 | 4.00 (0.75–5.00) | 0.996 (22%) | 58.215 (19%) | 61.065 (19%) |
↔ t½ ↑ Tmax 300% ↓Cmax 46% ↔AUC0–last ↔AUC0–∞ |
[102] |
| Fasted | 18 | 0.5 g | 116.7 ± 31.9 | 1.00 (0.50–1.52) | 1.849 (41%) | 59.694 (19%) | 62.551 (18%) |
AUC area under the plasma concentration–time curve, AUC0–24h AUC from zero to 24 hours, AUC0–last AUC from zero to the last measurable timepoint, AUC0–∞ AUC from zero to infinity, Cmax maximum plasma concentration, CV% percentage coefficient of variation, N number of subjects, NR not reported, PARP poly (ADP-ribose) polymerase, SD standard deviation, t½ elimination half-life, Tmax time to maximum plasma concentration, ↑ indicates increase, ↓ indicates decrease, ↔ indicates no change
Data are presented as mean (CV%) for Cmax, AUC0–last and AUC0–∞, as median (range) for tmax and mean ± SD for t1/2
aBased on 27 patients
bBased on 29 patients
cBased on 28 patients
dBased on 30 patients
eBased on 51 patients
fBased on 54 patients
gBased on 52 patients
hBased on 14 patients
itmax is presented as mean ± SD
jBased on 15 patients
kBased on 11 patients
lAUC0–24h,m based on 19 patients
Table 7.
Overview of drug–drug interaction studies
| PARP inhibitor | Method | Compound | Enzymatic target | Effect on PARP inhibitor | PARP inhibitor effect on compound | Conclusion | References |
|---|---|---|---|---|---|---|---|
| Olaparib capsules | PBPK model | Itraconazole | Strong CYP3A4 inhibitor |
↑ Cmax 33% ↑ AUC 152% |
– | Strong CYP3A4 enzyme inhibitors should be avoided during olaparib treatment. If coadministration cannot be avoided, the olaparib dose should be reduced from 400 mg to 150 mg BID. | [32] |
| Fluconazole | Moderate CYP3A4 inhibitor |
↑ Cmax 17% ↑ AUC 98% |
– | Moderate CYP3A4 enzyme inhibitors should be avoided during olaparib treatment. If coadministration cannot be avoided, the olaparib dose should be reduced from 400 mg to 200 mg BID. | |||
| Fluvoxamine | Weak CYP3A4 inhibitor |
↔ Cmax ↔ AUC |
– | Co-administration of olaparib with weak CYP3A4 inhibitors is permitted with olaparib treatment. | |||
| Rifampin | Strong CYP3A4 inducer |
↓ Cmax 45% ↓ AUC 71% |
– | Strong CYP3A4 enzyme inducers should be avoided during olaparib treatment | |||
| Efavirenz | Moderate CYP3A4 inducer |
↓ Cmax 34% ↓ AUC 53% |
– | Strong CYP3A4 enzyme inducers should be avoided during olaparib treatment | |||
| Dexamethasone | Weak CYP3A4 inducer |
↔ Cmax ↔ AUC |
Co-administration of olaparib with weak CYP3A4 inducers is permitted with olaparib treatment. | ||||
| Midazolam | CYP3A4 substrate | – |
↑ Cmax 11% ↑ AUC 45% |
Caution should be exercised when CYP3A4 substrates with a narrow therapeutic window are combined with olaparib | |||
| Simvastatin | CYP3A4 substrate | – |
↑ Cmax 27% ↑ AUC 47% |
Caution should be exercised when CYP3A4 substrates with a narrow therapeutic window are combined with olaparib | |||
| Digoxin | P-gp substrate | – |
↔ Cmax ↔ AUC |
Clinically relevant interactions with P-gp substrates cannot be excluded. Appropriate clinical monitoring is advised | |||
| Raltegravir | UGT1A1 substrate | – |
↔ Cmax ↔ AUC |
No clinical relevant interaction | |||
| Olaparib tablets | In vivo | Itraconazole | Strong CYP3A4 inhibitor |
↑ Cmax 42% ↑ AUC0–last 166% ↑ AUC0–∞ 170% ↔ t½ |
- | Strong CYP3A4 enzyme inhibitors should be avoided during olaparib treatment | [37] |
| Rifampin | Strong CYP3A4 inducer |
↓ Cmax 71% ↓ AUC0–last 88% ↓ AUC0–∞ 87% ↔ t½ |
– | Strong CYP3A4 enzyme inducers should be avoided during olaparib treatment | |||
| PBPK model | Itraconazole | Strong CYP3A4 inhibitor |
↑ Cmax 20% ↑ AUC 255% |
– | Strong CYP3A4 enzyme inhibitors should be avoided during olaparib treatment. If coadministration cannot be avoided, the olaparib dose should be reduced from 300 mg to 100 mg BID | [32] | |
| Fluconazole | Moderate CYP3A4 inhibitor |
↑ Cmax 14% ↑ AUC 121% |
– | Moderate CYP3A4 enzyme inhibitors should be avoided during olaparib treatment. If coadministration cannot be avoided, the olaparib dose should be reduced from 300 mg to 150 mg BID | |||
| Fluvoxamine | Weak CYP3A4 inhibitor |
↔ Cmax ↔ AUC |
– | Co-administration of olaparib with weak CYP3A4 inhibitors is permitted with olaparib treatment | |||
| Rifampin | Strong CYP3A4 inducer |
↓ Cmax 44% ↓ AUC 75% |
– | Strong CYP3A4 enzyme inducers should be avoided during olaparib treatment | |||
| Efavirenz | Moderate CYP3A4 inducer |
↓ Cmax 31% ↓ AUC 60% |
– | Strong CYP3A4 enzyme inducers should be avoided during olaparib treatment | |||
| Dexamethasone | Weak CYP3A4 inducer |
↔ Cmax ↔ AUC |
– | Co-administration of olaparib with weak CYP3A4 inducers is permitted with olaparib treatment | |||
| Midazolam | CYP3A4 supstrate | – |
↑ Cmax 18% ↑ AUC 61% |
Caution should be exercised when CYP3A4 substrates with a narrow therapeutic window are combined with olaparib | |||
| Simvastatin | CYP3A4 substrate | – |
↑ Cmax 33% ↑ AUC 54% |
Caution should be exercised when CYP3A4 substrates with a narrow therapeutic window are combined with olaparib | |||
| Digoxin | P-gp substrate | – |
↔ Cmax ↔ AUC |
Clinically relevant interactions with P-gp substrates cannot be excluded. Appropriate clinical monitoring is advised | |||
| Raltegravir | UGT1A1 substrate | – |
↔ Cmax ↔ AUC |
No clinical relevant interaction | |||
| In vivo | Tamoxifen | CYP3A4 inducer |
↓ Cmax 20% ↓ AUC0–tau 27% |
↑ Cmax 13% ↑ AUC0–tau 16% N-DMT ↔ Cmax ↓ AUC0–tau 8% Endoxifen ↔ Cmax ↔ AUC0–tau |
Co-administration of olaparib with tamoxifen shows no clinical relevant interaction | [38] | |
| Anastrazole | CYP3A4 substrate |
↔ Cmax ↔ AUC0–tau |
↓ Cmax 10% ↓ AUC0–tau 14% |
Co-administration of olaparib with anastrazole shows no clinical relevant interaction | |||
| Letrozole | CYP3A4 substrate |
↔ Cmax ↑ AUC0–tau 15% |
↓ Cmax 6% ↓ AUC0–tau 5% |
Co-administration of olaparib with letrozole shows no clinical relevant interaction | |||
| PBPK model | Grapefruit juice (bergamottin) | CYP3A4 inhibitor |
↑ Cmax 1.04-fold ↑ AUC 1.11-fold |
No clinically relevant interaction | [140] | ||
|
St. John’s wort (hyperforin) |
CYP3A4/5 inducer |
↓ Cmax 0.84-fold ↓AUC 0.54-fold |
No clinically relevant interaction | ||||
|
Turmeric (curcumin) |
CYP3A4/5 inhibitor |
↑ Cmax 1.22-fold ↑AUC 1.40-fold |
No clinically relevant interaction | ||||
| Rucaparib | In vivo | Caffeine | CYP1A2 substrate |
↔ Cmax ↑ AUC0–72h 126% |
Dose adjustments should be considered for CYP1A2 substrates with a narrow therapeutic window (e.g., tizanide, theophylline) when concomitantly used with rucaparib | [83, 92] | |
| S-warfarin | CYP2C9 substrate |
↔ Cmax ↑ AUC0–96h 49% |
Dose adjustments should be considered for CYP2C9 substrates with a narrow therapeutic window (e.g., warfarin, phenytoin) when concomitantly used with rucaparib | ||||
| Omeprazole | CYP2C19 substrate |
↔ Cmax ↑ AUC0–72h 55% |
The risk for a relevant effect on the exposure of PPIs is small. No dose adjustments are required for CYP2C19 substrates | ||||
| Midazolam | CYP3A4 substrate |
↔ Cmax ↑ AUC0–72h 39% |
Dose adjustments should be considered for CYP3A4 substrates with a narrow therapeutic window (e.g., cyclosporine, tacrolimus) when concomitantly used with rucaparib | ||||
| Digoxin | P-gp substrate |
↔ Cmax ↑ AUC0–72h 20% |
No clinically relevant interaction | ||||
| Rosuvastatin | BCRP substrate |
↑ Cmax 29% ↑ AUC0–last 34% |
Dose adjustments are not necessary when concomitantly used with rucaparib | ||||
| Ethinylestradiol | CYP3A and CYP2C9 substrate |
↔ Cmax ↑ AUC0–last 43% |
Dose adjustments are not necessary when concomitantly used with rucaparib | ||||
| Levonorgestrel | CYP3A substrate |
↔ Cmax ↑ AUC0–last 56% |
Dose adjustments are not necessary when concomitantly used with rucaparib | ||||
| PopPK model | PPI | ↑ F1 15% | – | Rucaparib exposure is not affected by the concomitant use of proton pump inhibitors. No dose adjustments are required | [75] | ||
| NR |
P-gp inhibitor CYP1A2 inhibitor CYP2D6 inhibitor |
↔ Exposure | – | Concomitant use of strong P-gp, CYP1A2, or CYP2D6 inhibitors has no impact rucaparib pharmacokinetics | |||
| Talazoparib | PopPK model | P-gp inhibitors | ↑ F1 45% | – | A dose reduction from 1 to 0.75 mg is required in patients taking potent P-gp inhibitors | [106] | |
| Acid-reducing agents (PPI, H2-receptor antagonists) | ↔ Exposure | – | No dose adjustments are required | ||||
| In vivo | Itraconazole | P-gp inhibitor |
↑ Cmax 40% ↑ AUC 0-last 51% ↑ AUC 0-inf 56% |
– | Coadministration of talazoparib with potent P-gp inhibitors should be avoided. If coadministration cannot be avoided, the talazoparib dose should be reduced from 1 to 0.75 mg QD | [109] | |
| Rifampicin | P-gp inducer |
↑ Cmax 37% ↔AUC 0-last ↔ AUC 0-inf |
– | The effect on rucaparib exposure is limited. No dose adjustments are required |
AUC area under the plasma concentration–time curve, AUC0–last AUC from zero to the last measurable time point, AUC0–∞ AUC from zero to infinity, AUC0–tau AUC from time zero to the end of the dosing interval (tau), BID twice a day, Cmax maximum plasma concentration, CV% percentage coefficient of variation, CYP cytochrome P450, F1 relative bioavailability, H2 histamine 2, N number of subjects, NR not reported, PARP , PBPK pharmacologically based pharmacokinetic, P-gp P-glycoprotein, PopPK population pharmacokinetic, PPI proton pump inhibitor, QD once day, SD standard deviation, t½ elimination half-life, Tmax time to maximum plasma concentration, UGT uridine 5′-diphospho-glucuronosyltransferase (UDP)-glucuronosyltransferase, ↑ indicates increase, ↓ indicates decrease, ↔ indicates no change
Olaparib
Olaparib was the first approved PARP inhibitor by the EMA in 2014 (Table 1). In study 19, maintenance treatment of olaparib capsules in patients with platinum-sensitive, relapsed, high-grade epithelial ovarian cancer, in response to platinum-based chemotherapy, improved median PFS in the overall population compared with placebo (8.4 vs 4.8 months; hazard ratio [HR] 0.35 (95% confidence interval [CI] 0.25–0.49), p < 0.0001) [13]. The greatest benefit was found in germline (g) or somatic (s) BRCA1/2 mutated patients [11.2 vs 4.3 months; HR 0.18 (95% CI 0.10–0.31), p < 0.0001] with a lower benefit for patients with wild-type BRCA (BRCA variants of unknown significance and no known or reported BRCA mutation) [7.4. vs 5.5 months; HR 0.54 (95% CI 0.34–0.85), p < 0.0075] [14]. The approved dose of 400 mg twice a day was the maximum tolerated dose (MTD) [15]. The high administration burden of the 50-mg capsules has led to the development of an alternative solid dispersion tablet formulation (100 and 150 mg). Because capsules and tablets are not bioequivalent, Study 24 was performed resulting in an optimal tablet dose of 300 mg BID [16]. The tablet formulation was approved in 2018 based on the SOLO2 trial with prolonged PFS in patients using olaparib compared with placebo [19.1 vs 5.5 months; HR 0.30 (95% CI 0.22–0.41), p < 0.0001] [17]. Approval was granted regardless of BRCA status, as overall survival in study 19 was prolonged irrespective of BRCA status [HR 0.73 (95% CI 0.55‒0.95), p = 0.02138] [18]. Indications expanded to breast, pancreas, and prostate cancer. The tablet formulation will mainly be discussed in this review, as capsules are being phased out of the marked.
Preclinical Pharmacology
The in vitro interaction of olaparib with enzymes and transporters is shown in Table 2. Olaparib inhibits the organic cation transporter (OCT) 2, multidrug and toxin extrusion protein (MATE) 1 and MATE2K involved in the tubular secretion of creatinine. Inhibition by olaparib has been associated with increased creatinine levels without affecting renal function. Therefore, the creatinine-derived estimated glomerular filtration rate can underestimate the renal function and an alternative marker such as cystatin C should be used to assess renal function [19, 20]. Furthermore, olaparib penetrates the brain in vivo, but is rapidly cleared from the brain, probably owing to P-glycoprotein (P-gp) efflux transporters [10].
Olaparib is mainly metabolized by cytochrome P450 (CYP) 3A4/5 with three major metabolites formed (M12, M15, and M18). Their potency to inhibit growth of BRCA1 mutant cells and PARP-1 is 30-fold, 30-fold, and four-fold lower, respectively, than olaparib itself [21]. In addition to being a substrate to CYP3A, olaparib inhibits and induces CYP3A. The net effect on CYP3A is weak inhibition, possibly increasing exposure to CYP3A substrates, which could be important for drugs with a narrow therapeutic window [22]. In vivo, olaparib exerts single-agent activity in BRCA1-deficeint and BRCA2-deficient cells, but is less effective in ovarian and/or breast cancer wild-type models [10, 23].
Clinical Pharmacokinetics
Steady-state pharmacokinetic parameters of olaparib capsules and tablets are summarized in Table 3. Formulations of capsules and tablets are not bioequivalent [16]. The 300-mg tablet formulation with improved bioavailability has a 13% higher mean relative exposure (area under the curve [AUC]) at steady state than the 400-mg capsule formulation [24]. Absolute bioavailability has not been investigated, but is probably low, as olaparib is classified as a Biopharmaceutical Classification System (BCS) class IV compound (low solubility, low permeability) [23]. Mean protein binding (albumin and alpha-1 acid glycoprotein) is high (89%), which decreases to 82% at concentrations of >10,000 ng/mL in vitro [5, 25]. Olaparib has an apparent volume of distribution of 167 L (capsules) and 158 L (tablets) [23, 26]. Olaparib is metabolized by CYP enzymes with three major metabolites (M12, M15, and M18) accounting for 9–14% of plasma radioactivity [23]. Considering preclinical data (Sect. 3.1.1), the clinical activity of these metabolites is negligible [21]. Olaparib is hepatically and renally cleared, with 44% (15% unchanged) of the radioactive dose recovered in urine and 42% (6% unchanged) in feces [25, 27].
Pharmacokinetics in Special Populations
Patients with Renal Impairment
The impact of renal impairment on the pharmacokinetics of olaparib is shown in Table 4. Area under the curve and maximum concentration (Cmax) are significantly increased in patients with renal impairment. Although no increase in adverse events were observed, higher exposure might eventually result in increased toxicity, mainly hematological toxicities [28]. Dose adjustments are required in patients with moderate renal impairment and olaparib is not recommended in patients with severe renal impairment [28–30]. Dose adjustments during olaparib treatment should be considered carefully, as the creatinine-derived estimated glomerular filtration rate can underestimate renal function with the risk of underdosing [20].
Patients with Hepatic Impairment
The impact of hepatic impairment on olaparib exposure is shown in Table 5. Olaparib exposure was not significantly altered in patients with mild or moderate hepatic impairment and therefore no dose adjustments are required [31]. Physiologically based pharmacokinetic simulations estimated an negligible increase in AUC for patients with severe hepatic impairment [32]. Until a dedicated clinical study is performed, olaparib is not recommended in patients with severe hepatic impairment [31].
Other Factors Influencing the Pharmacokinetics of Olaparib
Olaparib exposure was 50% higher in patients with advanced solid tumors [15, 33] compared with patients having a non-advanced disease state (patients with breast cancer scheduled for elective surgery). This can partly be explained by the fed versus fasted state, in these studies, but also the disease state might influence the pharmacokinetics [34]. The impact of body weight, age, sex, race, serum creatinine, creatinine clearance, line of treatment, Eastern Cooperative Oncology Group performance status, and tumor type on the pharmacokinetics of olaparib was evaluated in two population pharmacokinetic models. Only Eastern Cooperative Oncology Group performance status had a significant effect on olaparib clearance without a clear biological explanation [24, 35]
Food Effect
The results of the two food-effect studies are described in Table 6. A small significant increase in olaparib exposure was observed when olaparib tablets were administered with a high-fat meal. The inter-patient variability was not affected and no important differences between adverse events were observed under fed/fasted conditions. The current advice is that olaparib can be administered with or without food [36].
Drug–Drug Interactions
Table 7 gives an overview of the performed DDI studies. Olaparib is metabolized by CYP3A4, and exposure is significantly changed when combined with strong CYP3A4 inhibitors or inducers [37]. It is advised to reduce the olaparib tablet dose to 100 and 150 mg BID when co-administered with strong and moderate CYP3A4 inhibitors, respectively, if avoidance is not possible. Moderate and strong CP3A4 inducers should be avoided. Furthermore, clinically relevant interactions between olaparib and CYP3A4 substrates with a narrow therapeutic index (e.g., cyclosporine, tacrolimus) occur [32]. However, this was not observed for the CYP3A4 substrates anastrazole and letrozole [38]. Inhibition is probably weak, as olaparib is an inhibitor and inducer of CYP3A4 with a net effect of weak inhibition (Sect. 3.1.1) [22]. Additionally, interactions with olaparib as a perpetrator could occur with substrates to OCT1, OCT2, OATP1B1, OAT3, MATE1, and MATE2K (Table 2) [39].
Clinical Pharmacodynamics
Exposure Efficacy
Inhibition of PARP in peripheral blood mononuclear cells is highly variable [34]. Maximum PARP inhibition (> 90% from baseline) is reached at doses of ≥ 60 mg BID (capsules) and tumor responses are observed at doses ≥ 100 mg BID [15, 40].
Dose–efficacy relationships were demonstrated; the objective response rate (ORR) was 41% versus 22% with a median PFS of 5.7 months versus 3.8 months in patients with BRCA-mutated breast cancer receiving 400 mg BID and 100 mg BID, respectively [41]. A similar result was observed in patients with BRCA-mutated ovarian cancer (ORR: 33% vs 13%, median PFS: 5.8 months vs 1.9 months, for 400 mg BID and 100 mg BID, respectively) [42].
Exposure–efficacy relationships are not very clear. In patients with prostate cancer (PROfound study, n = 74), Cox proportional hazard modeling showed no significant correlation between exposure and PFS [AUC: HR 0.98 (95% CI 0.97–1.00), Cmax: HR 0.89 (95% CI 0.75–1.02), minimum concentration: HR 0.77 (95% CI 0.56–1.06)]. However, patient numbers were small [43]. Results from an exposure-PFS Cox proportional hazard model using data from patients with solid tumors (n = 410) indicate that 300 mg BID (steady state Cmax 7.67 µg/mL) is superior to 200 mg BID (Cmax,ss 6.99 µg/mL) [HR 0.96 (95% CI 0.94–0.99)], but the difference is small [44]. In summary, the olaparib dose is related to efficacy, but looking at exposure within the registered doses, no clear exposure–efficacy relationship has been demonstrated.
Exposure Toxicity
Hematological toxicities were more frequently reported with the 300-mg tablet formulation compared with the 400-mg capsule formulation [24]. As exposure of the 300-mg tablet formulation is 13% higher, an exposure–toxicity relationship is apparent.
An exposure–toxicity analysis with data from multiple clinical trials showed an exposure–toxicity relationship between the probability of grade 1–4 anemia and steady-state minimum concentrations (p = 0.001) and predicted Cmax (p = 0.013) of the 400-mg capsule formulation [25]. In addition, an exposure–safety (categorical adverse events and hemoglobin) model has been developed using data from multiple clinical trials (n = 757). The probability of safety events and hemoglobin decrease were comparable in all exposure groups [300-mg BID capsules (Cmax 7.67 µg/mL), 400-mg BID capsules (Cmax 6.99 µg/mL), 200-mg BID tablets (Cmax 6.18 µg/mL)], suggesting a minimal effect of olaparib exposure on safety [44].
In a retrospective study (n = 27), olaparib exposure was significantly associated with early adverse events in patients with BRCA1/2-mutated ovarian cancer. A trough concentration of 2500 ng/mL was identified as a threshold that can help to guide dose adjustments [11].
Niraparib
In 2017, niraparib has been approved by the EMA for the maintenance treatment of platinum-sensitive, recurrent, high-grade epithelial ovarian cancer regardless of BRCA status (Table 1). In the phase III NOVA trial, niraparib maintenance treatment resulted in a prolonged median PFS in the gBRCA-mutated cohort [21.0 vs 5.5 months; HR 0.27 (95% CI 0.173–0.410), p < 0.001], the cohort with an HRD deficiency [12.9 vs 3.8 months; HR 0.38 (95% CI 0.243–0.586), p < 0.001], and the non-gBRCA-mutated cohort [9.3 vs 3.9 months; HR 0.45 (95% CI 0.338–0.607), p < 0.001] [45]. The approved dose of 300 mg once a day (QD) was the MTD with fatigue, pneumonitis, and thrombocytopenia as dose-limiting toxicities [46]. Niraparib was additionally approved in 2020 as maintenance treatment following first-line platinum therapy based on the PRIMA trial with prolonged PFS in the overall niraparib population [13.8 vs 8.2 months; HR 0.62 (95% CI 0.50–0.76), p < 0.001] [47].
Preclinical Pharmacology
In Table 2, the in vitro interaction of niraparib with enzymes and transporters is summarized. Niraparib has the potential to cause off-target effects on the cardiovascular and central nervous systems, as it inhibits the neuronal dopamine, norepinephrine, and serotonin transporters. Except for the inhibition of MATE-1 and MATE-2 and being a substrate to P-gp and breast cancer resistance protein (BCRP), niraparib is no substrate to, or inhibitor of other important enzymes or transporters [48].
In vivo, niraparib treatment resulted in tumor regression in a BRCA-1 mutant mouse xenograft model [49], as well as BRCA wild-type models [10]. Although niraparib is substrate of P-gp and BCRP, it is able to permeate the blood–brain barrier with sustainable brain exposure in mice. The high permeability might overcome the transporter-mediated efflux of niraparib [10, 49]. Concentrations in tumor tissue (subcutaneous breast and ovarian cancer xenograft models) three times higher than in plasma have been reported. However, niraparib also has the unfavorable property of distributing into the bone marrow where platelets are generated [10, 49].
Clinical Pharmacokinetics
Table 3 shows the steady-state pharmacokinetic parameters of niraparib. Niraparib is classified as a BCS class II compound (low solubility, high permeability) with a high bioavailability and protein binding (73% and 83%, respectively). It has a high volume of distribution of 1220 L and preferably distributes into red blood cells with a blood-to-plasma ratio of 1.6 [48, 50–52]. The intra-individual variability in exposure is 36.9%, which has been determined in a population pharmacokinetic (PopPK) model [48, 51]. Metabolism mainly takes place by carboxylesterases with M1 as the main metabolite. M1 undergoes glucuronidation by uridine 5′-diphospho-glucuronosyltransferase to form M10. The M1 and M10 metabolites are inactive. Niraparib and its metabolites are eliminated by hepatic and renal routes, with 32% and 40% of total administered dose being recovered in feces and urine, respectively [51, 53].
Pharmacokinetics in Special Populations
Patients with Renal Impairment
The effect of renal impairment on the pharmacokinetics of niraparib was investigated in a PopPK model (Table 4). As no differences were observed in exposure between patients with a normal, mild, and moderate renal function, no dose adjustments are required [51, 54]. The effect of severe renal impairment has not been assessed. Niraparib itself can mildly affect the estimated glomerular filtration rate. This is probably not an effect of inhibition of the tubular creatinine secretion, like olaparib [20, 39, 55], but of hemodynamic impairment due to dopamine and norepinephrine transporter inhibition. As the effect is mild and reversible in most cases, this is not an indication of treatment discontinuation [56].
Patients with Hepatic Impairment
Niraparib exposure was significantly increased in patients with moderate hepatic impairment (Table 5). Therefore, a starting dose of 200 mg is recommended [57]. In a PopPK model, exposure in patients with mild hepatic impairment (n = 27) was not different from exposure in patients with a normal hepatic function (n = 351), thus no dose adjustments are advised in this group [51, 54]. The effect of severe impaired hepatic function on niraparib pharmacokinetics has not been established.
Other Factors Influencing the Pharmacokinetics of Niraparib
In a PopPK model, the impact of age, sex, ethnicity, and body weight on niraparib pharmacokinetics was evaluated. These variables could not explain the moderate-to-high interindividual variability (e.g., 52.5% for oral clearance) [51, 54]. However, clinical studies demonstrated low bodyweight (< 77 kg) to be correlated with a higher exposure (Cmax and AUC). These patients might benefit from a lower starting dose of 200 mg/day, which is currently advised [58, 59]. No effect of age was demonstrated in the PopPK model, which was confirmed in an efficacy and safety analysis. Patients aged > 70 years (n = 61) had comparable PFS benefits and incidence of adverse events, compared to patients aged < 70 years (n = 311) [60].
Food Effect
The results of the food-effect study are shown in Table 6. A high-fat meal delays the time to Cmax and decreases the Cmax of niraparib significantly, but the extent of absorption was not altered. The efficacy and safety profile of niraparib was not affected, therefore niraparib can be taken with or without food [61].
Drug–Drug Interactions
No in vivo DDI studies are performed. The risk of DDIs with CYP enzyme inhibitors or inducers is minimal, as the major route of metabolism is mediated by carboxylesterases. Additionally, gastric-reducing agents are unlikely to alter exposure because niraparib solubility is independent of a pH below its pKa of 9.95 [51]. Co-administration of niraparib with substrates to MATE-1 or MATE-2 (e.g., metformin) could potentially result in increased plasma concentrations of the co-administered drug [48].
Clinical Pharmacodynamics
Exposure Efficacy
In patients, efficacious PARP inhibition (> 90% inhibition of PARP in tumor tissue) was reached at doses of 80 mg/day and above and durable responses measured by Response Evaluation Criteria in Solid Tumors (RECIST) were observed at doses of 60 mg/day [46, 62]. Dose–efficacy relationships were investigated using data from two clinical trials. In the retrospective analysis of the NOVA safety population (n = 553), PFS was similar in patients using 100, 200, and 300 mg/day in gBRCA-mutated and non-gBRCA-mutated patients. However, dose modifications (80%) and interruptions (73%) were common [58]. This is in line with the results of the QUADRA study (n = 463). Clinical benefit rate (ORR), disease control rate, and clinical benefit rate at 24 weeks (CBR24) was similar between patients receiving a mean niraparib dose of ≤ 200 mg/day (8%, 58%, and 19%, respectively) and patients receiving > 200 mg/day (7%, 39%, and 15%, respectively) [63].
A pharmacokinetic model was developed using phase I and III data (NOVA trial, n = 512) to investigate exposure–efficacy relationships. A trend towards increased PFS with increased exposure (AUC) was observed in the non-gBRCA group [11.5 vs 7.5 months; HR 0.70 (95% CI 0.49–0.99)], while this relationship was absent in the gBRCA group [> 15.7 vs 15.9 months; HR 0.91 (95% CI 0.54–1.52)] [48, 64]. More research should be conducted to investigate a possible exposure–efficacy relationship, as these data are inconclusive.
Exposure Toxicity
In the phase I dose-escalation trial, hematological toxicities were more often observed at higher doses and seemed dose proportional [46]. The incidence of nausea, thrombocytopenia, and fatigue was 74%, 61%, and 59%, respectively, in patients using the recommended dose of 300 mg/day in the phase III NOVA trial (n = 367) [45]. The incidence was significantly lower in patients initiating niraparib at 200 mg/day (16%, 14%, and 24% respectively) in a real-world cohort (n = 153) [65]. Furthermore, 66.5% of the patients in the phase III NOVA trial needed a dose reduction and 68.9% had dose interruptions. Dose reductions reduced the incidence of grade 3 and 4 thrombocytopenia, anemia, and neutropenia [45, 66].
A PopPK model was developed to investigate exposure–response relationships using data from the NOVA trial. Exposure (AUC, Cmax, minimum concentration) was significantly associated with any grade of thrombocytopenia and other hematologic and non-hematologic treatment-emergent adverse events [67].
In addition, patients with a low bodyweight (< 77 kg) or low platelet counts (< 150.000/mL) at baseline had a higher risk of grade > 3 thrombocytopenia (35% vs 12%) [58]. Bodyweight was correlated with higher exposure (Cmax and AUC) [59] and it is recommended to start with a dose of 200 mg/day for patients with a bodyweight < 77 kg and/or baseline platelets of < 150.000/mL [58, 59]. This individualized dosing strategy was further investigated in the PRIMA trial (n = 733) [47, 68] and NORA trial (n = 177) [69], with safety being significantly improved while efficacy not being affected. This was confirmed in two real-life cohorts [62, 67]. In summary, data clearly show a relationship between the dose and exposure of niraparib and toxicity.
Rucaparib
In the ARIEL2 study and study 10, rucaparib treatment of patients with g/sBRCA-mutated platinum-sensitive, relapsed, high-grade ovarian cancer resulted in an ORR, complete response, and partial response of 53.8%, 8.5%, and 45.3%, respectively, leading to the accelerated first approval of rucaparib in 2016 (Table 1) [71–73]. The recommended dose of 600 mg BID was selected based on toxicity and clinical activity with no MTD [71]. Additional approval was granted for the maintenance treatment of platinum-sensitive, relapsed, high-grade ovarian cancer regardless of BRCA status with a prolonged median PFS in the BRCA group [16.6 vs 5.4 months; HR 0.23 (95% CI 0.16–0.34), p < 0.0001], HRD group [13.6 vs 5.4 months; HR 0.32 (95% CI 0.24–0.42), p < 0.0001], and total group [10.8 vs 5.4 months; HR 0.36, (95% CI 0.30–0.45), p< 0 .0001] [74].
Preclinical Pharmacology
Table 2 shows the in vitro interaction of rucaparib with enzymes and transporters. Rucaparib inhibits many enzymes and transporters, causing a high risk for DDIs in patients (Sect. 3.3.6). Inhibition of the renal transporters OCT2, MATE-1, and MATE-2K have been related to an increase in creatinine levels without affecting renal function [19, 20]. Furthermore, the antagonistic activity towards the non-selective sigma receptor and several kinases [75] are likely to cause off-target side effects (e.g., increase in cholesterol), but are unlikely to exert anti-tumor activity [76].
P-glycoprotein and BCRP are restricting oral availability and brain accumulation in mice, causing tumor resistance and limiting the use against brain metastasis [77]. Despite limited brain penetration in glioblastoma xenografts [78], antitumor activity was still observed in an intracranial BRCA1-mutated model [79].
Clinical Pharmacokinetics
Steady-state pharmacokinetic parameters of rucaparib are shown in Table 3. Rucaparib is a BCS class IV compound (low solubility and low permeability). Bioavailability is low (36%) with a concentration-independent protein binding of 70.2% in vitro [80]. Rucaparib has a mean volume of distribution of 211 L [81] and preferentially distributes into red blood cells with an average blood-to-plasma ratio of 1.83 [80]. Rucaparib is extensively metabolized by CYP enzymes (Table 2), undergoing phase I and phase II reactions with M324 as the major metabolite. M324 is 30 times less potent compared with rucaparib and mainly eliminated by the kidneys. In a mass balance study, the mean recovery of the administered dose was 17.4% and 71.9% for urine and feces, respectively (7.6% and 63.9% unchanged) [82, 83].
Pharmacokinetics in Special Populations
Patients with Renal Impairment
The effect of renal impairment on the pharmacokinetics of rucaparib is summarized in Table 4. Although exposure of rucaparib was slightly higher in patients with mild and moderate renal impairment, no dose adjustments are required because the side effects and efficacy were not affected [84]. In patients with severe renal impairment or in patients undergoing dialysis, rucaparib is not recommended [7, 75, 85]. However, rucaparib therapy was safe in a single patient with dialysis-dependent renal failure using trough concentrations for dose optimization [86]. Therefore, therapeutic drug monitoring might be useful in patients with severe renal impairment or patients undergoing dialysis.
Patients with Hepatic Impairment
The effect of hepatic impairment on rucaparib exposure is shown in Table 5. No dose adjustments are required in patients with mild or moderate hepatic impairment, but the advice is to monitor patients for adverse events [75, 84, 87, 88]. Until the effect of severe hepatic impairment is investigated, rucaparib is not recommended in patients with severe hepatic impairment [7].
Other Factors Influencing Pharmacokinetic Parameters
Bodyweight [75, 89], body mass index, race, alpha-1 acid glycoprotein, and age have no significant effect on pharmacokinetic parameters of rucaparib [75]. Efficacy and safety were similar in age subgroups, indicating no effect of age on rucaparib pharmacokinetics [90]. Steady-state exposure (AUC) at 600 mg BID was not different between CYP2D6 phenotypes (poor metabolizers, n = 9; intermediate metabolizers, n = 71; normal metabolizers, n = 76; ultra-rapid metabolizers, n = 4) or CYP1A2 phenotypes (normal metabolizers, n = 28, hyper-inducers, n = 136). Therefore, no dose adjustments are needed [84].
Food Effect
The results of the food-effect study are summarized in Table 6. A high-fat meal delays the time to Cmax and increases the AUC and Cmax significantly. This was confirmed in a PopPK model with an increase in bioavailability from 32.7 to 51.7% when rucaparib was taken with a high-fat meal [84]. Food might increase intestinal solubility, as rucaparib is poorly water soluble. The increase in exposure is clinically insignificant because pharmacokinetic variability is not reduced and efficacy and safety are acceptable [91]. Therefore, rucaparib can be taken with or without food.
Drug–Drug Interactions
The results of DDI studies are summarized in Table 7. Rucaparib is extensively metabolized by CYP enzymes; however, CYP1A2 or CYP2D6 inhibitors did not impact rucaparib exposure. As rucaparib is metabolized by CYP3A4, the effect of strong CYP3A4 inhibitors and inducers should be explored [75]. Concomitant use of proton pump inhibitors showed no meaningful effect on rucaparib pharmacokinetics [85].
In addition, dose adjustments should be considered for CYP1A2, CYP2C9, and CYP3A4 substrates with a narrow therapeutic window when administered with rucaparib [92]. Rucaparib had a marginal effect on digoxin exposure, but the effects could be underestimated, as digoxin is not the most selective P-gp probe [75, 93, 94]. Rucaparib weakly increased exposure to oral contraceptives and rosuvastatin. As hormone levels vary widely between individuals, it is unlikely that efficacy is affected and toxicity increased. Although no dose adjustments are recommended for rosuvastatin, attention should be used in case of genetic polymorphisms in genes for BCRP and when extrapolating to other BCRP substates [95]. Furthermore, there is a high potential for DDIs when rucaparib is co-administered with substrates of MATE-1, MATE2-l, OCT1, and OCT2 (e.g., metformin) (Table 2) [75].
Clinical Pharmacodynamics
Exposure Efficacy
Mean PARP inhibition in peripheral blood lymphocytes in patients was > 90% and not dose dependent between doses of 92 mg QD and 600 mg BID [96]. A PopPK model was developed using data from Study 10 and ARIEL2 to explore exposure–efficacy relationships in patients with BRCA-mutated ovarian cancer (n = 121). The AUC averaged by the actual dose received over time was correlated with an investigator radiologist review-assessed RECIST response in the subgroup of platinum-sensitive recurrent disease (n = 75, p = 0.017). Other efficacy endpoints were not correlated. Sample size was small, thus no definite conclusion can be drawn [89, 97].
Exposure Toxicity
In patients taking the recommended dose of 600 mg BID in the phase I/II ARIEL2 study and study 10, adverse events were common and frequently led to dose reductions (69%) and treatment interruptions (64%) [71]. In addition, dose-limiting toxicities were reported in patients receiving doses above 480 mg BID, while doses below were well tolerated [96].
In an exposure–safety analysis with data from Study 10 and ARIEL2 in patients with BRCA-mutated ovarian cancer (n = 393), Cmax,ss was associated with grade ≥2 creatinine (p < 0.001), grade ≥3 alanine aminotransferase (p = 0.033), grade ≥3 aspartate aminotransferase (p = 0.027), fatigue (p = 0.029), platelet decrease (p = 0.04), and a maximum hemoglobin change from baseline (p < 0.001) [89, 97]. The rise in creatinine levels is likely a result of inhibition of renal transporters without an impacting renal function [55]. These results indicate a relationship between exposure and toxicity.
Talazoparib
Talazoparib approval was granted in 2019 by the EMA for the treatment of gBRCA-mutated, human epidermal growth factor receptor-2 negative metastatic breast cancer (Table 1). In the phase III EMBRACA trial, talazoparib treatment resulted in a significantly longer median PFS [8.6 vs 5.6 months; HR 0.54 (95% CI 0.41–0.71), p < 0.001] and a higher ORR (62.6% vs 27.2%; OR 5.0, p < 0.001) compared with standard therapy [98]. The approved dose of 1.0 mg QD was also the MTD [99].
Preclinical Pharmacology
In Table 2, the in vitro interaction of talazoparib with enzymes and transporters is summarized. Talazoparib is the most potent catalytic PARP inhibitor with the highest trapping potency [100–102]. It inhibits tankyrase 1 and tankyrase 2 (PARP5a and b) causing an anti-cancer and anti-fibrotic effect, but also the induction of bone loss with increased osteoclasts [103]. Talazoparib has no effect on enzymes and transporters, but is a substrate to P-gp and BCRP. This is confirmed in vivo, with 1.9 times and 15 times higher plasma and brain concentrations, respectively, in P-gp and BCRP knockout mice [102].
Clinical Pharmacokinetics
Pharmacokinetic parameters of talazoparib at steady state are described in Table 3. Talazoparib is a BCS class II or IV compound (low solubility, moderate permeability) with an estimated bioavailability of at least 55% based on a mass balance study and protein binding of 74% (in vitro) [104, 105]. The apparent volume of distribution is 420 L [102, 104] with no preferable distribution into red blood cells [105]. Metabolism of talazoparib is minimal and the major route of elimination is renal excretion. Mean recovery of the total administered dose is 68.7% (54.6% unchanged) in urine and 19.7% (13.6% unchanged) in feces [104, 105].
Pharmacokinetics in Special Populations
Patients with Renal Impairment
The effect of renal impairment on the pharmacokinetics of talazoparib is summarized in Table 4. Dose adjustments are recommended for patients with moderate or severe renal impairment, as clearance is decreased [106] and exposure significantly increased [107].
Patients with Hepatic Impairment
The effect of hepatic impairment on talazoparib exposure is shown in Table 5. No effect of mild, moderate, or severe hepatic impairment was observed on talazoparib pharmacokinetics. Therefore, no dose adjustments are required [106, 108].
Other Factors Influencing Pharmacokinetic Parameters
The effect of several covariates on the pharmacokinetics of talazoparib was explored by a PopPK model. Age, sex, and body weight had no clinical relevant effect on talazoparib exposure. Talazoparib clearance was 24.7% higher and exposure approximately 20% lower in Asian patients compared with non-Asian patients. P-glycoprotein and BCRP polymorphisms are ethnicity dependent with a higher frequency of single nucleotide polymorphisms in Asian individuals compared with white individuals. This might contribute to the lower exposure in Asian individuals, but no dose adjustments are recommended, as 1 mg QD is the MTD [106].
Food Effect
The effect of food on talazoparib pharmacokinetics is shown in Table 6. A high-fat meal delays the time to Cmax and decreases the Cmax significantly, but does not influence the extent of absorption [102]. These findings are consistent with a PopPK analysis where the absorption rate is decreased (Ka) without any change in the extent of absorption (F1) [106]. In conclusion, talazoparib can be taken with or without food.
Drug–Drug Interactions
Table 7 summarizes the results of DDI studies. Concomitant use of potent P-gp inhibitors increases bioavailability and exposure of talazoparib significantly. Therefore, a reduced dose of 0.75 mg is advised when talazoparib is co-administered with potent P-gp inhibitors. Gastric-reducing agents had no effect on talazoparib exposure, which was expected based on the pH-independent solubility [106, 109]. As talazoparib is a substrate to BCRP, the effect of BCRP inhibitors cannot be excluded and should be further investigated.
Clinical Pharmacodynamics
Exposure Efficacy
Talazoparib shows a dose-dependent and exposure-dependent PARP activity in peripheral blood mononuclear cells with sustained PARP inhibition at and above doses of 0.6 mg/day [99]. Exposure–efficacy relationships were demonstrated in the EMBRACA and ABRAZO trials. In the EMBRACA trial (n = 281), the time-varying average talazoparib concentration (Cavg,t, [to account for dose modifications]) was significantly associated with longer PFS [110]. Dose reductions resulted in a trend towards a marginally less favorable PFS outcome compared with patients without dose reductions. However, dose reductions itself could lead to a shorter PFS, but it could also be a marker of worse prognosis and therefore a shorter PFS [111]. An exposure–efficacy analysis using data from the phase II ABROZO trial (n = 81) found a trend towards a higher ORR with higher exposure, but no relationship with PFS. However, patient numbers were small [112]. These data suggest an exposure–efficacy relationship is apparent.
Exposure Toxicity
An exposure–safety analysis was performed with pooled data from the EMBRACA (n = 285) and ABRAZO trials (n = 82). Patients above the median exposure (Cavg,t) experienced more events of anemia and thrombocytopenia. In the final Cox proportional hazard model, a higher Cavg,t was associated with a higher risk for anemia and thrombocytopenia and there was a trend towards a higher log-transformed Cavg,t and risk for neutropenia [111, 113]. These results indicate an exposure–toxicity relationship.
Discussion
We provided an overview of the (pre)-clinical pharmacology, clinical pharmacokinetics, and clinical pharmacodynamics of the four approved PARP inhibitors. This review reveals that PARP inhibitors have overlapping characteristics and unique properties as well. While all four PARP inhibitors are potent inhibitors of PARP enzymes with comparable half-maximal inhibitory concentration values, they differ in their PARP-trapping potency. Talazoparib has the most rigid structure with two chiral centers, which likely accounts for its potent trapping ability (50–100 times higher) compared with olaparib, niraparib, and rucaparib [103]. Cytotoxicity of PARP inhibitors as a single agent is correlated with PARP trapping and not with catalytic inhibition of PARP [114, 115]. Talazoparib shows the greatest PARP trapping potency, which is also correlated with increased toxicity in normal cells. Therefore, the MTD of talazoparib is much lower than other PARP inhibitors [116, 117]. Furthermore, the approved dose of talazoparib is in the range of the half-maximal inhibitory concentration for PARP inhibition, and therefore the only PARP inhibitor showing a dose-dependent and exposure-dependent inhibition of PARP in peripheral blood mononuclear cells. Unbound steady-state concentrations of olaparib, niraparib, and rucaparib exceed the half-maximal inhibitory concentration for PARP inhibition, which probably means that maximal PARP inhibition is reached at doses far below the recommended dose in patients.
Interestingly, olaparib and niraparib have similar catalytic activities and cytotoxicity against BRCA mutant cells and xenograft models [114], but niraparib is more efficacious in BRCA wild-type models [10]. This is also observed in patients with BRCA wild-type ovarian cancer, with a 5.4-month improvement in PFS for niraparib [45] compared with 1.9 months for olaparib. [14]. BRCA wild-type cells might require higher concentrations of the PARP inhibitor than BRCA-mutated cells, which explains the greater efficacy of niraparib; niraparib concentrations in wild-type tumors in mice were ten times higher compared with olaparib at therapeutic and comparable doses [118]. Initially, PARP inhibitor treatment was restricted to BRCA-mutated patients. As BRCA wild-type patients with HRD-positive tumors and no mutations in homologous recombination repair genes also benefit from PARP inhibitor treatment (but to a lesser extent), indications are expanding. It becomes more clear that biomarkers beyond BRCA, like other deficiencies in homologous recombination repair, play a role in the susceptibility to PARP inhibitors [119, 120].
Olaparib and rucaparib are substrates and inhibitors of several enzymes and transporters. Both PARP inhibitors increase creatinine without affecting renal function and have a high risk for DDIs. Niraparib has less effect on enzymes and transporters and the contribution of talazoparib is minimal. This can be an advantage for patients with comorbidities using multiple drugs. All four PARP inhibitors are substrates of the P-gp efflux transporter causing interactions and limiting the brain penetration. However, niraparib is classified as a BSC II compound with a high permeability that might (partly) overcome the P-gp-mediated efflux that could justify its use in the case of brain metastasis.
Poly (ADP-ribose) polymerase inhibitors differ in the way they are metabolized and excreted. While olaparib and rucaparib are metabolized by CYP enzymes, metabolism of niraparib is mainly mediated by carboxylesterase enzymes and metabolism of talazoparib is minimal. Poly (ADP-ribose) polymerase inhibitors are hepatically and renally cleared with no preferred route for olaparib and niraparib and the liver being the main route of excretion for rucaparib and the kidneys for talazoparib. Dose adjustments are necessary in patients with renal dysfunction for olaparib and talazoparib and for niraparib in patients with hepatic impairment.
Exposure is dose proportional for all PARP inhibitors, except for olaparib capsules because of limited solubility. The improved tablet formulation increased bioavailability and decreased the high administration burden. Niraparib and talazoparib have convenient long half-lives allowing QD dosing while olaparib and rucaparib have shorter half-lives and are dosed BID. All PARP inhibitors are classified as BCS class II or IV compounds with low solubility [23, 48, 75, 121]. This might contribute to the moderate-to-high interindividual variability in exposure; however, exposure is not drastically affected by intake with food.
While a PARP exposure–efficacy relationship is present for talazoparib, this relationship remains inconclusive for olaparib, niraparib, and rucaparib. Average unbound steady-state concentrations of rucaparib at the recommended dose of 600 mg BID are much higher than the required exposure for durable anti-tumor response in preclinical models [75] and exceed the half-maximal effective concentration for cytotoxicity. Based on these data and because dose findings of targeted anti-cancer agents are still mostly based on toxicity, rather than efficacy, the optimal dose of rucaparib might be lower than the current recommended dose. However, further clinical studies should investigate and confirm efficacy at lower dose levels.
Although PARP inhibitors have the same mechanism of action, they differ in their toxicity profile. Rucaparib has the most reported adverse drug reaction, which could be expected based on its many off-target effects (Table 2) [122]. Hypercholesterolemia is specific for rucaparib mediated through off-target kinase inhibition [76]. Hypertransaminasemia has been reported for rucaparib and niraparib and less for olaparib [123]. Niraparib is the only PARP inhibitor causing hypertension, due to off-target inhibition of neuronal dopamine, norepinephrine, and serotonin transporters, increasing neurotransmitters with inotropic effects on the heart. These neurotransmitters are involved in the psychiatric and nervous system disorders as well, which explains the association with niraparib. Gastrointestinal adverse events are very common and a class effect of PARP inhibitors. Furthermore, hematological toxicities, such as anemia, thrombocytopenia, and neutropenia are frequently reported and an on-target class effect. PARP-1 trapping is not only related to cytotoxicity in cancer cells with HRD, but also drives bone marrow toxicity [124]. Additionally, inhibition of PARP-2 is directly related to anemia, due to impaired differentiation of erythroid progenitors and a shortened lifespan of erythrocytes [125]. Awareness of the delayed adverse events of myelodysplastic syndrome and acute myeloid leukemia is important, as these adverse events can be lethal and occur after several months [126]. Niraparib has the highest number of reported hematological toxicities followed by rucaparib and olaparib, related to the volume of distribution [123]. Dose reductions and treatment interruptions occurred frequently with niraparib, but efficacy was not affected [58]. Therefore, the recommended dose of 300 mg/day is possibly higher than necessary for sufficient efficacy, especially for gBRCA-mutated patients, and lower doses of niraparib might be justified. While BRCA status is predictive for efficacy, it is not related to toxicity [127]. Higher exposure is associated with an increase in efficacy for talazoparib and with an increase in hematological toxicities for all PARP inhibitors and thereby might be a rationale for therapeutic drug monitoring.
Conclusions
Poly (ADP-ribose) polymerase inhibitors are valuable anticancer agents with rapidly expanding indications. The understanding of the overlapping and unique pharmacokinetic and pharmacodynamic properties of PARP inhibitors can guide the choice of the PARP inhibitor, support treatment optimization, and improve clinical outcomes.
Declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
Gabe Sonke is the steering committee member of the SOLO-1 and SOLO-2 trials and reports research support paid to the institution from AstraZeneca, Merck, Novartis, and Roche.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author Contributions
MB performed the literature search and wrote the first draft of the manuscript. All authors critically reviewed the manuscript and approved the final manuscript.
References
- 1.Ohmoto A, Yachida S. Current status of poly(ADP-ribose) polymerase inhibitors and future directions. Onco Targets Ther. 2017 doi: 10.2147/OTT.S139336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook SA, Tinker AV. PARP inhibitors and the evolving landscape of ovarian cancer management: a review. BioDrugs. 2019 doi: 10.1007/s40259-019-00347-4. [DOI] [PubMed] [Google Scholar]
- 3.Shen Y, Aoyagi-Scharber M, Wang B. Trapping poly(ADP-ribose) polymerase. J Pharmacol Exp Ther. 2015 doi: 10.1124/jpet.114.222448. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration (FDA). Talzenna (talazoparib): US prescribing information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211651s000lbl.pdf. Accessed 10 Aug 2022.
- 5.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Summary of product characteristics: olaparib. 2019. https://www.ema.europa.eu/en/documents/product-information/lynparza-epar-product-information_en.pdf. Accessed 10 Aug 2022.
- 6.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Summary of product characteristics: niraparib. 2017. https://www.ema.europa.eu/en/documents/product-information/zejula-epar-product-information_en.pdf. Accessed 10 Aug 2022.
- 7.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Summary of product characteristics: rucaparib. 2021. https://www.ema.europa.eu/en/documents/product-information/rubraca-epar-product-information_en.pdf. Accessed 10 Aug 2022.
- 8.Widmer N, Bardin C, Chatelut E, Paci A, Beijnen J, Levêque D, et al. Review of therapeutic drug monitoring of anticancer drugs part two: targeted therapies. Eur J Cancer. 2014 doi: 10.1016/j.ejca.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Bruin MAC, de Vries N, Lucas L, Rosing H, Huitema ADR, Beijnen JH. Development and validation of an integrated LC-MS/MS assay for therapeutic drug monitoring of five PARP-inhibitors. J Chromatogr B. 2020 doi: 10.1016/j.jchromb.2019.121925. [DOI] [PubMed] [Google Scholar]
- 10.Sun K, Mikule K, Wang Z, Poon G, Vaidyanathan A, Smith G, et al. A comparative pharmacokinetic study of PARP inhibitors demonstrates favorable properties for niraparib efficacy in preclinical tumor models. Oncotarget. 2018 doi: 10.18632/oncotarget.26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velev M, Puszkiel A, Blanchet B, de Percin S, Delanoy N, Medioni J, et al. Association between olaparib exposure and early toxicity in brca-mutated ovarian cancer patients: results from a retrospective multicenter study. Pharmaceuticals. 2021 doi: 10.3390/ph14080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geenen JJJ, Linn SC, Beijnen JH, Schellens JHM. PARP inhibitors in the treatment of triple-negative breast cancer. Clin Pharmacokinet. 2018 doi: 10.1007/s40262-017-0587-4. [DOI] [PubMed] [Google Scholar]
- 13.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 14.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014 doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 15.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009 doi: 10.1056/nejmoa0900212. [DOI] [PubMed] [Google Scholar]
- 16.Mateo J, Moreno V, Gupta A, Kaye SB, Dean E, Middleton MR, et al. An adaptive study to determine the optimal dose of the tablet formulation of the PARP inhibitor olaparib. Target Oncol. 2016 doi: 10.1007/s11523-016-0435-8. [DOI] [PubMed] [Google Scholar]
- 17.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 18.Friedlander M, Matulonis U, Gourley C, du Bois A, Vergote I, Rustin G, et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br J Cancer. 2018 doi: 10.1038/s41416-018-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu X, Bleasby K, Chan GH, Nunes I, Evers R. The complexities of interpreting reversible elevated serum creatinine levels in drug development: does a correlation with inhibition of renal transporters exist? Drug Metab Dispos. 2016 doi: 10.1124/dmd.115.067694. [DOI] [PubMed] [Google Scholar]
- 20.Bruin MAC, Korse CM, van Wijnen B, de Jong VMT, Linn SC, van Triest B, et al. A real or apparent decrease in glomerular filtration rate in patients using olaparib? Eur J Clin Pharmacol. 2020 doi: 10.1007/s00228-020-03070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Public assessment report on extension of marketing authorisation grouped with a variation olaparib. 2018. https://www.ema.europa.eu/en/documents/variation-report/lynparza-h-c-3726-x-0016-g-epar-assessment-report-extension_en.pdf. Accessed 10 Aug 2022.
- 22.McCormick A, Swaisland H, Reddy VP, Learoyd M, Scarfe G. In vitro evaluation of the inhibition and induction potential of olaparib, a potent poly(ADP-ribose) polymerase inhibitor, on cytochrome P450. Xenobiotica. 2018 doi: 10.1080/00498254.2017.1346332. [DOI] [PubMed] [Google Scholar]
- 23.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Public assessment report olaparib. 2014. https://www.ema.europa.eu/en/documents/assessment-report/lynparza-epar-public-assessment-report_en.pdf. Accessed 10 Aug 2022.
- 24.Zhou D, Li J, Bui K, Learoyd M, Berges A, Milenkova T, et al. Bridging olaparib capsule and tablet formulations using population pharmacokinetic meta-analysis in oncology patients. Clin Pharmacokinet. 2019 doi: 10.1007/s40262-018-0714-x. [DOI] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration (FDA). Center for drug evaluation and research-clinical pharmacology and biopharmaceutics review(s), Olaparib. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206162Orig1s000ClinPharmR.pdf. Accessed 10 Aug 2022.
- 26.US Food and Drug Administration (FDA). Center for drug evaluation and research-multi-discipline review, Olaparib. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208558Orig1s000MultidisciplineR.pdf. Accessed 10 Aug 2022.
- 27.Ang JE, Clarkson-Jones JA, Swaisland H, Brunetto AT, Lal R, Farnsworth AP, et al. A mass balance study to investigate the metabolism, excretion and pharmacokinetics of [14C]-olaparib (AZD2281) in patients with advanced solid tumours refractory to standard treatments [conference abstract] Eur J Cancer Suppl. 2010 doi: 10.1016/s1359-6349(10)72112-1. [DOI] [Google Scholar]
- 28.Rolfo C, de Vos-Geelen J, Isambert N, Molife LR, Schellens JHM, De Grève J, et al. Pharmacokinetics and safety of olaparib in patients with advanced solid tumours and renal impairment. Clin Pharmacokinet. 2019 doi: 10.1007/s40262-019-00754-4. [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration (FDA). Lynparza (Olaparib) tablets: US prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf. Accessed 10 Aug 2022.
- 30.US Food and Drug Administration (FDA). Lynparza (Olaparib) capsules: US prescribing information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206162s011lbl.pdf. Accessed 10 Aug 2022.
- 31.Rolfo C, Isambert N, Italiano A, Molife LR, Schellens JHM, Blay J, et al. Pharmacokinetics and safety of olaparib in patients with advanced solid tumours and mild or moderate hepatic impairment. Br J Clin Pharmacol. 2020 doi: 10.1111/bcp.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilla Reddy V, Bui K, Scarfe G, Zhou D, Learoyd M. Physiologically based pharmacokinetic modeling for olaparib dosing recommendations: bridging formulations, drug interactions, and patient populations. Clin Pharmacol Ther. 2019 doi: 10.1002/cpt.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto N, Nokihara H, Yamada Y, Goto Y, Tanioka M, Shibata T, et al. A phase I, dose-finding and pharmacokinetic study of olaparib (AZD2281) in Japanese patients with advanced solid tumors. Cancer Sci. 2012 doi: 10.1111/j.1349-7006.2011.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bundred N, Gardovskis J, Jaskiewicz J, Eglitis J, Paramonov V, Mccormack P, et al. Evaluation of the pharmacodynamics and pharmacokinetics of the PARP inhibitor olaparib: a phase I multicentre trial in patients scheduled for elective breast cancer surgery. Invest New Drugs. 2013 doi: 10.1007/s10637-012-9922-7. [DOI] [PubMed] [Google Scholar]
- 35.Peer CJ, Lee JM, Roth J, Rodgers L, Nguyen J, Annunziata CM, et al. Population pharmacokinetic analyses of the effect of carboplatin pretreatment on olaparib in recurrent or refractory women’s cancers. Cancer Chemother Pharmacol. 2017 doi: 10.1007/s00280-017-3346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plummer R, Swaisland H, Leunen K, van Herpen CML, Jerusalem G, De Grève J, et al. Olaparib tablet formulation: effect of food on the pharmacokinetics after oral dosing in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2015 doi: 10.1007/s00280-015-2836-2. [DOI] [PubMed] [Google Scholar]
- 37.Dirix L, Swaisland H, Verheul HMW, Rottey S, Leunen K, Jerusalem G, et al. Effect of itraconazole and rifampin on the pharmacokinetics of olaparib in patients with advanced solid tumors: results of two phase I open-label studies. Clin Ther. 2016 doi: 10.1016/j.clinthera.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Plummer R, Verheul HM, De Vos FYFL, Leunen K, Molife LR, Rolfo C, et al. Pharmacokinetic effects and safety of olaparib administered with endocrine therapy: a phase I study in patients with advanced solid tumours. Adv Ther. 2018 doi: 10.1007/s12325-018-0804-z. [DOI] [PubMed] [Google Scholar]
- 39.McCormick A, Swaisland H. In vitro assessment of the roles of drug transporters in the disposition and drug–drug interaction potential of olaparib. Xenobiotica. 2017 doi: 10.1080/00498254.2016.1241449. [DOI] [PubMed] [Google Scholar]
- 40.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 41.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010 doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 42.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010 doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 43.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Public assessment report on extension of marketing authorisation grouped with a variation, olaparib. 2018. https://www.ema.europa.eu/en/documents/variation-report/lynparza-h-c-3726-x-0016-g-epar-assessment-report-extension_en.pdf. Accessed 10 Aug 2022.
- 44.Zhou D, Li J, Learoyd M, Bui K, Berges A, Milenkova T, et al. Efficacy and safety exposure-response analyses of olaparib capsule and tablet formulations in oncology patients. Clin Pharmacol Ther. 2019 doi: 10.1002/cpt.1338. [DOI] [PubMed] [Google Scholar]
- 45.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016 doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 46.Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 47.González-Martín A, Pothuri B, Vergote I, DePont CR, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019 doi: 10.1056/nejmoa1910962. [DOI] [PubMed] [Google Scholar]
- 48.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Public assessment report niraparib. 2017. https://www.ema.europa.eu/en/documents/assessment-report/zejula-epar-public-assessment-report_en.pdf. Accessed 10 Aug 2022.
- 49.Jones P, Altamura S, Boueres J, Ferrigno F, Fonsi M, Giomini C, et al. Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J Med Chem. 2009 doi: 10.1021/jm901188v. [DOI] [PubMed] [Google Scholar]
- 50.van Andel L, Rosing H, Zhang Z, Hughes L, Kansra V, Sanghvi M, et al. Determination of the absolute oral bioavailability of niraparib by simultaneous administration of a 14C-microtracer and therapeutic dose in cancer patients. Cancer Chemother Pharmacol. 2018 doi: 10.1007/s00280-017-3455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.US Food and Drug Administration (FDA). Center for drug evaluation and research-multi-discipline review, niraparib. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208447orig1s000multidiscipliner.pdf. Accessed 10 Aug 2022.
- 52.Zhang Z, Milton A. Volume of distribution: a relevant, possibly overlooked pharmacokinetic parameter in drug development. Curr Trends Pharmacol Clin Trials. 2019. https://academicstrive.com/CTPCT/CTPCT190014.pdf. Accessed 10 Aug 2022.
- 53.van Andel L, Zhang Z, Lu S, Kansra V, Agarwal S, Hughes L, et al. Human mass balance study and metabolite profiling of 14C-niraparib, a novel poly(ADP-Ribose) polymerase (PARP)-1 and PARP-2 inhibitor, in patients with advanced cancer. Invest New Drugs. 2017 doi: 10.1007/s10637-017-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z-Y, Wang X, Wang J, Pentikis HSS, Kansra V, Oza AM, et al. Modeling and impact of organ function on the population pharmacokinetics (PK) of niraparib, a selective poly (ADP-ribose) polymerase (PARP)-1 and -2 inhibitor [conference abstract] Ann Oncol. 2017 doi: 10.1093/annonc/mdx372.035. [DOI] [Google Scholar]
- 55.Zibetti Dal Molin G, Westin SN, Msaouel P, Gomes LM, Dickens A, Coleman RL. Discrepancy in calculated and measured glomerular filtration rates in patients treated with PARP inhibitors. Int J Gynecol Cancer. 2020 doi: 10.1136/ijgc-2019-000714. [DOI] [PubMed] [Google Scholar]
- 56.Lazareth H, Delanoy N, Cohen R, Boissier E, Ayari H, Combe P, et al. Nephrotoxicity associated with niraparib. Am J Kidney Dis. 2020 doi: 10.1053/j.ajkd.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Akce M, El-Khoueiry A, Piha-Paul SA, Bacque E, Pan P, Zhang ZY, et al. Pharmacokinetics and safety of niraparib in patients with moderate hepatic impairment. Cancer Chemother Pharmacol. 2021 doi: 10.1007/S00280-021-04329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berek JS, Matulonis UA, Peen U, Ghatage P, Mahner S, Redondo A, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018 doi: 10.1093/annonc/mdy181. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Zheng H, Gao Y, Lou G, Yin R, Ji D, et al. Phase I pharmacokinetic study of niraparib in Chinese patients with epithelial ovarian cancer. Oncologist. 2020 doi: 10.1634/theoncologist.2019-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fabbro M, Moore KN, Dørum A, Tinker AV, Mahner S, Bover I, et al. Efficacy and safety of niraparib as maintenance treatment in older patients (≥ 70 years) with recurrent ovarian cancer: results from the ENGOT-OV16/NOVA trial. Gynecol Oncol. 2019 doi: 10.1016/j.ygyno.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Moore K, Zhang ZY, Agarwal S, Burris H, Patel MR, Kansra V. The effect of food on the pharmacokinetics of niraparib, a poly(ADP-ribose) polymerase (PARP) inhibitor, in patients with recurrent ovarian cancer. Cancer Chemother Pharmacol. 2018 doi: 10.1007/s00280-017-3512-5. [DOI] [PubMed] [Google Scholar]
- 62.Jones P, Wilcoxen K, Rowley M, Toniatti C. Niraparib: a poly(ADP-ribose) polymerase (PARP) inhibitor for the treatment of tumors with defective Homologous recombination. J Med Chem. 2015 doi: 10.1021/jm5018237. [DOI] [PubMed] [Google Scholar]
- 63.Matulonis UA, Monk BJ, Secord AA, Geller MA, Miller DS, Cloven NG, et al. Baseline platelet count and body weight as predictors of early dose modification in the quadra trial of niraparib monotherapy for the treatment of heavily pretreated (≥4th line), advanced, recurrent high-grade serous ovarian cancer. Gynecol Oncol. 2019 doi: 10.1016/j.ygyno.2019.04.017. [DOI] [Google Scholar]
- 64.Wang J, Zhang Z-Y, Mirza MR, Gilbert L, Fabbro M, Tinker AV, et al. The exposure-response relationship of niraparib in patients with gBRCAmut and non-gBRCAmut: results from the ENGOT-OV16/NOVA Trial [conference abstract] Ann Oncol. 2017 doi: 10.1093/annonc/mdx372.003. [DOI] [Google Scholar]
- 65.Gallagher JR, Heap KJ, Carroll S, Travers K, Harrow B, Westin SN. Real-world adverse events with niraparib 200 mg/day maintenance therapy in ovarian cancer: a retrospective study. Future Oncol. 2019 doi: 10.2217/fon-2019-0471. [DOI] [PubMed] [Google Scholar]
- 66.Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018 doi: 10.1016/j.ygyno.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Sunthankar K, Amrite A, Taylor A, Jamsen K, Zhang Z. Exposure-response analysis of the PARP inhibitor niraparib to help inform dose optimization for patients with ovarian cancer [conference poster presentation]. American Society of Health-System Pharmacists Midyear Clinical Meeting; 2018.
- 68.Mirza MR, Gonzalez Martin A, Graybill W, O’Malley DM, Gaba L, Yap OWS, et al. Evaluation of an individualized starting-dose of niraparib in the PRIMA/ENGOT-OV26/GOG-3012 study. J Clin Oncol. 2020 doi: 10.1200/jco.2020.38.15_suppl.6050. [DOI] [Google Scholar]
- 69.Wu XH, Zhu JQ, Yin RT, Yang JX, Liu JH, Wang J, et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2021 doi: 10.1016/J.ANNONC.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Zhu J. Real-world hematological adverse events in Chinese patients with advanced ovarian cancer treated with an individualized starting dose of niraparib. Ann Transl Med. 2021 doi: 10.21037/ATM-21-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kristeleit R, Shapiro GI, Burris HA, Oza AM, LoRusso P, Patel MR, et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2796. [DOI] [PubMed] [Google Scholar]
- 72.Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 73.Oza AM, Tinker AV, Oaknin A, Shapira-Frommer R, McNeish IA, Swisher EM, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol. 2017 doi: 10.1016/j.ygyno.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 74.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017 doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Public assessement report rucaparib. 2018. https://www.ema.europa.eu/en/documents/assessment-report/rubraca-epar-public-assessment-report_en.pdf. Accessed 10 Aug 2022.
- 76.Antolin AA, Ameratunga M, Banerji U, Clarke PA, Workman P, Al-Lazikani B. The kinase polypharmacology landscape of clinical PARP inhibitors. Sci Rep. 2020 doi: 10.1038/s41598-020-59074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Durmus S, Sparidans RW, Van Esch A, Wagenaar E, Beijnen JH, Schinkel AH. Breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1) restrict oral availability and brain accumulation of the PARP inhibitor rucaparib (AG-014699) Pharm Res. 2015 doi: 10.1016/j.jpba.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 78.Parrish KE, Cen L, Murray J, Calligaris D, Kizilbash S, Mittapalli RK, et al. Efficacy of PARP inhibitor rucaparib in orthotopic glioblastoma xenografts is limited by ineffective drug penetration into the central nervous system. Mol Cancer Ther. 2015 doi: 10.1158/1535-7163.MCT-15-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen M, Robillard L, Harding TC, Xiao JJ, Simmons AD, Kristeleit H, et al. Intracranial evaluation of the in vivo pharmacokinetics, brain distribution, and efficacy of rucaparib in BRCA -mutant, triple-negative breast cancer [conference abstract] Cancer Res. 2019;79(13):3888. doi: 10.1158/1538-7445.SABCS18-3888. [DOI] [Google Scholar]
- 80.Liao M, Jaw-Tsai S, Beltman J, Simmons AD, Harding TC, Xiao JJ. Evaluation of in vitro absorption, distribution, metabolism, and excretion and assessment of drug-drug interaction of rucaparib, an orally potent poly(ADP-ribose) polymerase inhibitor. Xenobiotica. 2020 doi: 10.1080/00498254.2020.1737759. [DOI] [PubMed] [Google Scholar]
- 81.Wilson RH, Evans TJ, Middleton MR, Molife LR, Spicer J, Dieras V, et al. A phase i study of intravenous and oral rucaparib in combination with chemotherapy in patients with advanced solid tumours. Br J Cancer. 2017 doi: 10.1038/bjc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liao M, Watkins S, Nash E, Isaacson J, Etter J, Beltman J, et al. Evaluation of absorption, distribution, metabolism, and excretion of [14C]-rucaparib, a poly(ADP-ribose) polymerase inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2020 doi: 10.1007/s10637-019-00815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Summary of product characteristics: rucaparib. 2019. https://www.ema.europa.eu/en/documents/product-information/rubraca-epar-product-information_en.pdf. Accessed 10 Aug 2022.
- 84.Jim J. Xiao, Michelle Green MG. Population pharmacokinetics (PK) of rucaparib (CO-338) in patients with advanced ovarian cancer (AOC) or other solid tumors [conference poster presentation]. Clin Pharmacol Ther. 2017;101:S92. http://clovisoncology.com/files/Xiao_POP_PK_2017_ASCPT_FINAL.pdf. Accessed 10 Aug 2022.
- 85.US Food and Drug Administration (FDA). Rubraca (Rucaparib): US prescribing information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209115s003lbl.pdf. Accessed 10 Aug 2022.
- 86.Harold JA, Free SC, Bradley WH. Pharmacokinetics and clinical response to single agent rucaparib in a dialysis dependent patient with BRCA associated breast and recurrent ovarian cancer. Gynecol Oncol Reports. 2018 doi: 10.1016/j.gore.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grechko N, Skarbova V, Tomaszewska-Kiecana M, Ramlau R, Centkowski P, Drew Y, et al. Pharmacokinetics and safety of rucaparib in patients with advanced solid tumors and hepatic impairment. Cancer Chemother Pharmacol. 2021 doi: 10.1007/S00280-021-04278-2/TABLES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.US Food and Drug Administration (FDA). Center for drug evaluation and research-multi-discipline review, rucaparib. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/209115Orig1s000MultiDisciplineR.pdf. Accessed 10 Aug 2022.
- 89.Konecny GE, Oza AM, Tinker AV, Oaknin A, Shapira-Frommer R, Ray-Coquard I, et al. Population exposure-efficacy and exposure-safety analyses for rucaparib in patients with recurrent ovarian carcinoma from Study 10 and ARIEL2. Gynecol Oncol. 2021 doi: 10.1136/ijgc-2019-000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colombo N, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. The effect of age on efficacy, safety and patient-centered outcomes with rucaparib: a post hoc exploratory analysis of ARIEL3, a phase 3, randomized, maintenance study in patients with recurrent ovarian carcinoma. Gynecol Oncol. 2020 doi: 10.1016/J.YGYNO.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shapiro GI, Kristeleit RS, Burris HA, LoRusso P, Patel MR, Drew Y, et al. Pharmacokinetic study of rucaparib in patients with advanced solid tumors. Clin Pharmacol Drug Dev. 2019 doi: 10.1002/cpdd.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao JJ, Nowak D, Ramlau R, Tomaszewska-Kiecana M, Wysocki PJ, Isaacson J, et al. Evaluation of drug–drug interactions of rucaparib and CYP1A2, CYP2C9, CYP2C19, CYP3A, and P-gp substrates in patients with an advanced solid tumor. Clin Transl Sci. 2019 doi: 10.1111/cts.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chu X, Galetin A, Zamek-Gliszczynski MJ, Zhang L, Tweedie DJ. Dabigatran etexilate and digoxin: comparison as clinical probe substrates for evaluation of P-gp inhibition. Clin Pharmacol Ther. 2018 doi: 10.1002/cpt.1213. [DOI] [PubMed] [Google Scholar]
- 94.Nader AM, Foster DR. Suitability of digoxin as a P-glycoprotein probe: Implications of other transporters on sensitivity and specificity. J Clin Pharmacol. 2014 doi: 10.1002/JCPH.200. [DOI] [PubMed] [Google Scholar]
- 95.Liao M, Jeziorski KG, Tomaszewska-Kiecana M, Láng I, Jasiówka M, Skarbová V, et al. A phase 1, open-label, drug–drug interaction study of rucaparib with rosuvastatin and oral contraceptives in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2021 doi: 10.1007/S00280-021-04338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Drew Y, Ledermann J, Hall G, Rea D, Glasspool R, Highley M, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br J Cancer. 2016 doi: 10.1038/bjc.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Konecny GE, Oza AM, Tinker AV, Oaknin A, Shapira-Frommer R, Ray-Coquard I, et al. Population exposure-safety and exposure-efficacy analyses for rucaparib in patients with recurrent ovarian carcinoma from Study 10 and ARIEL2 [conference poster presentation]. SGO 2020 Annual Meeting on Women’s Cancer. https://clovisoncology.com/media/1183/sgo2020_gkonecny_poster.pdf. Accessed 10 Aug 2022.
- 98.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018 doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017 doi: 10.1158/2159-8290.CD-16-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang B, Chu D, Feng Y, Shen Y, Aoyagi-Scharber M, Post LE. Discovery and characterization of (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-2,7,8,9-tetrahydro-3H-pyrido[4,3,2-de]phthalazin-3-one (BMN 673, talazoparib), a novel, highly potent, and orally efficacious poly(ADP-ribose) polymer. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.5b01498. [DOI] [PubMed] [Google Scholar]
- 102.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Public assessment report talazoparib. 2019. https://www.ema.europa.eu/en/documents/assessment-report/talzenna-epar-public-assessment-report_en.pdf. Accessed 10 Aug 2022.
- 103.Mukai T, Fujita S, Morita Y. Tankyrase (PARP5) inhibition induces bone loss through accumulation of its substrate SH3BP2. Cells. 2019 doi: 10.3390/cells8020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.US Food and Drug Administration (FDA). Center for drug evaluation and research-multi-discipline review, talazoparib. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211651Orig1s000MultidisciplineR.pdf. Accessed 10 Aug 2022.
- 105.Yu Y, Chung CH, Plotka A, Quinn K, Shi H, Pápai Z, et al. A phase 1 mass balance study of 14 C-labeled talazoparib in patients with advanced solid tumors. J Clin Pharmacol. 2019 doi: 10.1002/jcph.1415. [DOI] [PubMed] [Google Scholar]
- 106.Yu Y, Durairaj C, Shi H, Wang DD. Population pharmacokinetics of talazoparib in patients with advanced cancer. J Clin Pharmacol. 2020 doi: 10.1002/jcph.1520. [DOI] [PubMed] [Google Scholar]
- 107.Durairaj C, Chakrabarti J, Ferrario C, Hirte HW, Babu S, Piha-Paul SA, et al. The effect of renal impairment on the pharmacokinetics and safety of talazoparib in patients with advanced solid tumors. Clin Pharmacokinet. 2021 doi: 10.1007/S40262-020-00983-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo C, Yu Y, Chakrabarti J, Piha-Paul SA, Moroose R, Plotka A, et al. Evaluation of pharmacokinetics and safety of talazoparib in patients with advanced cancer and varying degrees of hepatic impairment. Br J Clin Pharmacol. 2022 doi: 10.1111/BCP.15294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elmeliegy M, Láng I, Smolyarchuk EA, Chung CH, Plotka A, Shi H, et al. Evaluation of the effect of P-glycoprotein inhibition and induction on talazoparib disposition in patients with advanced solid tumours. Br J Clin Pharmacol. 2020 doi: 10.1111/bcp.14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu Y, Elmeliegy M, Litton JK, Tudor IC, Czibere A, Zheng J, et al. Talazoparib exposure-efficacy analysis in patients with advanced breast cancer and germline BRCA1/2 mutations in the EMBRACA trial. J Clin Pharmacol. 2020 doi: 10.1002/jcph.1623. [DOI] [PubMed] [Google Scholar]
- 111.Hurvitz SA, Gonçalves A, Rugo HS, Lee K, Fehrenbacher L, Mina LA, et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: detailed safety analyses from the phase III EMBRACA trial. Oncologist. 2020 doi: 10.1634/theoncologist.2019-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Telli ML, Turner NC, Mailliez A, Ettl J, Grischke EM, Mina LA, et al. ABRAZO: Exposure-efficacy and -safety analyses of breast cancer patients with germline BRCA1/2 mutations receiving talazoparib in a phase 2 open-label trial [conference poster presentation] Am Assoc Cancer Res (AACR). 2017 doi: 10.1158/1538-7445.sabcs17-p1-14-03. [DOI] [Google Scholar]
- 113.Elmeliegy M, Yu Y, Litton JK, Czibere A, Wilson GG, Tudor IC, et al. Exposure-safety analyses of talazoparib in patients with advanced breast cancer and germline BRCA1/2 mutations in the EMBRACA and ABRAZO trials. J Clin Pharmacol. 2020 doi: 10.1002/jcph.1626. [DOI] [PubMed] [Google Scholar]
- 114.Murai J, Huang SYN, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murai J, Huang S-YN, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014 doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pommier Y, O’Connor MJ, De Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016 doi: 10.1126/scitranslmed.aaf9246. [DOI] [PubMed] [Google Scholar]
- 117.Curtin NJ, Sharma R. PARP inhibitors for cancer therapy. Cancer Drug Discov. Dev. 2015 doi: 10.1007/978-3-319-14151-0. [DOI] [Google Scholar]
- 118.Morosi L, Matteo C, Ceruti T, Giordano S, Ponzo M, Frapolli R, et al. Quantitative determination of niraparib and olaparib tumor distribution by mass spectrometry imaging. Int J Biol Sci. 2020 doi: 10.7150/ijbs.41395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hodgson DR, Dougherty BA, Lai Z, Fielding A, Grinsted L, Spencer S, et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer. 2018 doi: 10.1038/s41416-018-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lheureux S, Lai Z, Dougherty BA, Runswick S, Hodgson DR, Timms KM, et al. Long-term responders on olaparib maintenance in high-grade serous ovarian cancer: clinical and molecular characterization. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2615. [DOI] [PubMed] [Google Scholar]
- 121.US Food and Drug Administration (FDA). Center for drug evaluation and research: product quality review, Talazoparib. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211651Orig1s000ChemR.pdf. Accessed 10 Aug 2022.
- 122.Sandhu D, Antolin AA, Cox AR, Jones AM. Identification of different side effects between PARP inhibitors and their polypharmacological multi-target rationale. Br J Clin Pharmacol. 2022 doi: 10.1111/BCP.15015. [DOI] [PubMed] [Google Scholar]
- 123.LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL, LaFargue CJ, Dal Molin GZ, et al. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019 doi: 10.1016/S1470-2045(18)30786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hopkins TA, Ainsworth WB, Ellis PA, Donawho CK, DiGiammarino EL, Panchal SC, et al. PARP1 trapping by PARP inhibitors drives cytotoxicity in both cancer cells and healthy bone marrow. Mol Cancer Res. 2019 doi: 10.1158/1541-7786.MCR-18-0138. [DOI] [PubMed] [Google Scholar]
- 125.Farrés J, Llacuna L, Martin-Caballero J, Martínez C, Lozano JJ, Ampurdanés C, et al. PARP-2 sustains erythropoiesis in mice by limiting replicative stress in erythroid progenitors. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morice PM, Leary A, Dolladille C, Chrétien B, Poulain L, González-Martín A, et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021 doi: 10.1016/S2352-3026(20)30360-4. [DOI] [PubMed] [Google Scholar]
- 127.Kotsopoulos J, Willows K, Trat S, Kim RH, Volenik A, Sun P, et al. BRCA mutation status is not associated with increased hematologic toxicity among patients undergoing platinum-based chemotherapy for ovarian cancer. Int J Gynecol Cancer. 2018 doi: 10.1097/IGC.0000000000001144. [DOI] [PubMed] [Google Scholar]
- 128.Dean E, Middleton MR, Pwint T, Swaisland H, Carmichael J, Goodege-Kunwar P, et al. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer. 2012 doi: 10.1038/bjc.2011.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuan P, Shentu J, Xu J, Burke W, Hsu K, Learoyd M, et al. Pharmacokinetics and safety of olaparib tablets as monotherapy and in combination with paclitaxel: results of a phase I study in Chinese patients with advanced solid tumours. Cancer Chemother Pharmacol. 2019 doi: 10.1007/s00280-019-03799-1. [DOI] [PubMed] [Google Scholar]
- 130.Yonemori K, Tamura K, Kodaira M, Fujikawa K, Sagawa T, Esaki T, et al. Safety and tolerability of the olaparib tablet formulation in Japanese patients with advanced solid tumours. Cancer Chemother Pharmacol. 2016 doi: 10.1007/s00280-016-3106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yonemori K, Shimizu T, Kondo S, Iwasa S, Koyama T, Kitano S, et al. The safety, tolerability and pharmacokinetics of niraparib in Japanese patients with solid tumours: results of a phase I dose-escalation study. Jpn J Clin Oncol. 2021 doi: 10.1093/JJCO/HYAB013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hoffman J, Chakrabarti J, Plotka A, Milillo Naraine A, Kanamori D, Moroose R, et al. Talazoparib has no clinically relevant effect on QTc interval in patients with advanced solid tumors. Anticancer Drugs. 2019 doi: 10.1097/CAD.0000000000000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Naito Y, Kuboki Y, Ikeda M, Harano K, Matsubara N, Toyoizumi S, et al. Safety, pharmacokinetics, and preliminary efficacy of the PARP inhibitor talazoparib in Japanese patients with advanced solid tumors: phase 1 study. Invest New Drugs. 2021 doi: 10.1007/s10637-021-01120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.US Food and Drug Administration (FDA). Zejula (Niraparib): US prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208447lbl.pdf. Accessed 10 Aug 2022.
- 135.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Note for guidance on the evaluation of the pharmacokinetics of medicinal products in patients with impaired renal function. 2004. https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidance-evaluation-pharmacokinetics-medical-products-patients-impaired-renal-function_en.pdf. Accessed 10 Aug 2022.
- 136.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency (EMA). Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with decreased renal function. 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-decreased-renal-function_en.pdf. Accessed 10 Aug 2022.
- 137.(EMA) C for MP for HU (CHMP) EMA. Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function. 2005. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-impaired-hepatic-function_en.pdf. Accessed 10 Aug 2022.
- 138.Guideline National Cancer Institute Organ Dysfunction Working Group Criteria (NCI- ODWG). https://ctep.cancer.gov/protocoldevelopment/docs/hepatic_dysfunction_v3.doc. Accessed 10 Aug 2022.
- 139.Rolfo C, Swaisland H, Leunen K, Rutten A, Soetekouw P, Slater S, et al. Effect of food on the pharmacokinetics of olaparib after oral dosing of the capsule formulation in patients with advanced solid tumors. Adv Ther. 2015 doi: 10.1007/s12325-015-0214-4. [DOI] [PubMed] [Google Scholar]
- 140.Pilla Reddy V, Jo H, Neuhoff S. Food constituent– and herb–drug interactions in oncology: influence of quantitative modelling on drug labelling. Br J Clin Pharmacol. 2021 doi: 10.1111/BCP.14822. [DOI] [PubMed] [Google Scholar]