Abstract
Introduction
Venous thromboembolism (VTE) is a leading cause of morbidity and mortality globally. The direct oral anticoagulants, including rivaroxaban, are relatively novel therapeutic options in the treatment and prevention of VTE. There is a conflicting and inconclusive evidence surrounding the pharmacokinetics (PK) of rivaroxaban in patients with VTE who are obese.
Objectives
We conducted a systematic review to provide an overview, and to synthesize the available evidence in the current literature pertaining to rivaroxaban PK in obese subjects who are healthy or diseased.
Methods
The PubMed, Embase, ScienceDirect, Rayyan, and Cochrane Library databases were systematically searched from 1 May 2021 through 28 February 2022. Studies investigating rivaroxaban PK in adult obese subjects were included in the review. Pertinent data, including anthropometric parameters, rivaroxaban dosage regimen, PK parameters, PK model, and outcome measures were extracted. Reference values of rivaroxaban PK parameters in the general population were used for comparison purposes. The review protocol was registered in the PROSPERO database (CRD42020177770).
Results
In the 11 studies included in this systematic review, over 7140 healthy or diseased subjects received rivaroxaban therapy, with varying clinical indications in the diseased population. The reported PK parameters of rivaroxaban in obese subjects compared with reference values in the general population were variable. The reported values of the volume of distribution (Vd) among obese subjects (73.4–82.8 L) fell within the range of values reported/calculated for the general population (59.4–104 L), assuming complete bioavailability. However, some of the reported values of clearance (CL) in obese subjects (7.86–16.8 L.h−1) do not fall within the range of values reported/calculated for the general population (5.57–11.3 L.h−1). The reported maximum plasma concentrations in obese subjects versus the general population following a 10 mg dose were 149 vs. 143–180 µg.L−1, and following a 20 mg dose were 214–305 vs. 299–360 µg.L−1, respectively. The area under the plasma concentration versus time curves (AUC) over different intervals in obese subjects versus the general population following a 10 mg dose were 1155 (AUC from time zero to infinity [AUC∞]) vs. 1029 (AUC∞) µg.h.L−1; and 1204–2800 (AUC from time zero to 24 h [AUC24]) vs. 3200 (AUC24) µg.h.L−1, respectively, following a 20 mg dose. The reported values of half-life and time to reach the maximum plasma concentration in obese subjects versus the general population were not consistent across studies.
Conclusion
Variable changes and inconsistencies in different rivaroxaban PK parameters were reported in obese subjects. Further well-designed studies are warranted to better characterize the PK and clinical outcomes of rivaroxaban in subjects with obesity.
Key Points
| Obesity has a variable effect on different rivaroxaban pharmacokinetic (PK) parameters and needs be used with caution in this population, as changes in the anticoagulation activity may occur upon long-term therapy. |
| Well-designed, prospective studies addressing the quality issues identified here in the Crowe Critical Appraisal Tool (CCAT) and Critical Appraisal of Clinical Pharmacokinetic Studies (CACPK) assessments need to be conducted to investigate rivaroxaban PK in the obese population. |
| This will assist to establish solid and definitive evidence to be used in clinical practice guidelines for effective and safe dosage regimen recommendations of rivaroxaban in this population. |
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is associated with high rates of morbidity and mortality, and poses a considerable burden on healthcare systems globally [1–3]. It is a leading cause of hospital deaths [4, 5], with 10 million hospital-associated incidences of VTE reported every year [2, 6]. The huge cost and burden that VTE causes globally cannot be underestimated. For example, the cost of VTE treatment in the US was estimated to be approximately US$10 billion (US$9.4–US$28.4 for a single VTE episode) [7].
The treatment of patients at risk of VTE with appropriate anticoagulants is the optimal way to decrease DVT-related morbidity and mortality [4]. In 2010, direct oral anticoagulants (DOACs) were introduced as therapeutic alternatives to vitamin K antagonists, and were indicated for the prevention and treatment of several thromboembolic conditions [8, 9]. The choice of DOACs is largely due to their superior safety profile and other advantages, including limited interactions with food and other drugs, and predictable pharmacokinetics (PK) and pharmacodynamics (PD), which allow for fixed dosing and therefore less monitoring and follow-up was required [10–15]. Clinical studies suggest that these agents were associated with lower mortality and less severe intracranial haemorrhage when compared with warfarin [16–18]. Accordingly, the prescription of DOACs has surpassed other long-standing anticoagulants over the last decade [19]. DOACs are indicated for the prevention/treatment of DVT and PE [20], as well as for lowering the risk of stroke and embolism in non-valvular atrial fibrillation (NVAF) [21, 22]. Due to similar efficacy and lower bleeding risks, the current guidelines recommend the use of DOACs over warfarin in patients with NVAF for stroke and thromboembolism prevention [23].
Rivaroxaban is one of the DOACs and it directly inhibits factor Xa, which is a key factor in the coagulation pathway. The use of rivaroxaban, as an alternative anticoagulant, is rapidly increasing in clinical practice and has rapidly become one of the drugs of choice for anticoagulation therapy in secondary care [24]. Rivaroxaban is administered orally, in fixed doses, with few documented drug and food interactions, and possesses predictable PK and PD profiles [25]. Although the PK of rivaroxaban is well-established in healthy and diseased individuals with normal body weight, prospective multiple-dosing studies to establish the PK profile and thus its effective dosing regimen in extreme weight patients are still lacking [26, 27]. During rivaroxaban drug development, several studies were conducted to investigate the PD profile of rivaroxaban [28, 29]; however, only few studies were published with a focus on its PK profile [30, 31]. The PD studies found that rivaroxaban was an effective anticoagulant among obese/morbidly obese patients, with no signs of stroke or systemic embolism reported [32–35]. However, studies investigating the effect of obesity on rivaroxaban PK and its potential adverse effects in long-term therapy among the obese population are still limited [35]. Some case reports have documented that obese patients developed stroke or PE during their long-term dabigatran anticoagulation therapy [36, 37]. This has raised a serious concern about the efficacy of DOACs, including rivaroxaban, as they may fail to achieve the desired anticoagulant activity in obese patients. Moreover, the fact that some old anticoagulants such as heparin are administered in a weight-adjusted manner for patients has increased the uncertainty about whether this is also the case for DOACs when treating obese patients [35, 38].
The PK of rivaroxaban following administration of a single 10 mg oral dose in healthy individuals has been described [39]. A maximum plasma rivaroxaban concentration (Cmax,) was reached after 4 h (tmax) [39]. An increase in the Cmax and area under the plasma concentration versus time curve (AUC) values was proportional to rivaroxaban doses of ≤ 10 mg (but not with higher doses [39]). While the absorption of rivaroxaban was not affected by the fed and fasted states at ≤ 10 mg doses, the bioavailability at higher doses decreased to 66% under fasting conditions [40] and increased to ≥ 80% under fed states [40]. Rivaroxaban has high (95%) and reversible plasma protein binding, mainly with albumin [41, 42]. Furthermore, PK modelling studies showed that the volume of distribution (Vd) of rivaroxaban indicates moderate affinity to peripheral tissues [31]. Around 50% of the rivaroxaban dose undergoes hepatic metabolism [11], 66% is excreted renally, mainly by active secretion via P-glycoprotein and breast cancer-resistance protein [43, 44], and 28% is excreted through faeces [11, 45]. The systemic clearance (CL) of rivaroxaban is 10 L.h−1 (0.14 L.h−1.kg−1) [11], while its elimination half-life (t½) is around 8.3 h [46].
Rivaroxaban PK parameters may change in certain populations [47–49]. In obese individuals, PK parameters of some medications could be affected, and thus under- or overdosing would be expected due to changes in the distribution, metabolism, and excretion processes [50]. However, changes in plasma concentrations are < 25% upon using a 10 mg tablet of rivaroxaban in subjects with extreme weights (< 50 kg or > 120 kg), as reported in the Canadian product monograph of rivaroxaban (XareltoTM), and thus no dosage adjustment is recommended [41]. In a meta-analysis that evaluated the impact of body weight on the efficacy and safety of DOACs and warfarin, the results suggested that DOACs showed a similar efficacy to warfarin in obese patients [51]. In 2017, a study meta-analysed 11 randomized controlled trials (RCTs), aiming to investigate the impact of body weight on outcomes in patients who were receiving fixed doses of DOACs [52]. The study demonstrated that dose adjustment of DOACs, according to patients’ body weights, was unlikely to improve the safety or efficacy of these agents [52].
Although the American College of Chest Physicians (CHEST) guidelines recommended that DOACs can be used effectively and safely in patients with extreme body weights for VTE prevention and treatment, these guidelines suggested that larger studies are necessary to confirm the safety and efficacy profiles of DOACs in such a population [20, 53, 54]. Furthermore, the International Society of Thrombosis and Haemostasis (ISTH) guidance does not support the use of DOACs in patients with a body mass index (BMI) of > 40 kg/m2 or weight of > 120 kg due to the weak evidence driven by the lack of adequate clinical data for these specific populations [47]. Such controversy among RCTs and guidelines can explain, in part, the physicians’ hesitancy in prescribing rivaroxaban for overweight/obese patients for the treatment and prevention of VTE and for stroke and thromboembolism prevention in NVAF [35]. Therefore, there is a conflicting and inconclusive evidence surrounding the PK of rivaroxaban in patients with VTE who are obese.
Despite the fact that obesity is one of the risk factors for VTE onset and recurrence in both men and women [55–57], which is directly associated with mortality [58, 59], and even though obese patients have a higher risk of VTE incidence and a large proportion of obese patients who have VTE or AF need to use DOACs for anticoagulation, there is a lack of comprehensive and definitive evidence about rivaroxaban PK in the obese population [60]. Previously published systematic reviews were investigating the efficacy and safety of rivaroxaban, without extracting the relevant PK parameter values; thus, the analysis/comparison of these parameters and their impact were not sufficiently examined. Conducting new research and generating updated knowledge is crucial to building new evidence and to widening the clinical use of rivaroxaban in special populations, particularly patients with obesity [42, 61].
Therefore, the objective of this study was to provide a critical overview of rivaroxaban PK in obese subjects who are healthy or diseased compared with the general population, synthesize the available evidence, and identify the gaps in the literature.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for conducting and reporting this systematic review [62, 63]. The review protocol was registered in the PROSPERO database (CRD42020177770).
Data Sources and Search Strategy
Five electronic bibliographic databases or applications (PubMed, Embase, ScienceDirect, Cochrane Library, and Rayyan web application) were systematically searched by the authors from 1 May 2021 through 28 February 2022 [64, 65]. The following keywords were used to search the databases: ‘rivaroxaban’ or ‘BAY 59-7939’ or ‘anticoagulant’ AND ‘pharmacokinetics’ or ‘half-life’ or ‘concentration’ or ‘volume of distribution’ or ‘bioavailability’ or ‘clearance’ AND ‘obesity’ or ‘obese’ or ‘overweight’ or ‘extreme weight’ or ‘BMI’ or ‘body mass index’ (Table 1). The keywords were customized according to each bibliographic database and using specific indexing terms as appropriate. Limits and filters such as language and human studies were applied according to the functionalities and features of each database. Furthermore, the reference lists of included studies were manually reviewed, and the grey literature was searched to identify any potentially missed studies from the aforementioned electronic search.
Table 1.
Detailed keyword search in each database
| Database | Keywords used for the search |
|---|---|
| PubMed |
(Rivaroxaban) OR (BAY 59-7939) OR (anticoagulant) AND (Pharmacokinetics) OR (half-life) OR (concentration) OR (volume of distribution) OR (bioavailability) OR (clearance) AND (Obesity) OR (obese) OR (overweight) OR (extreme weight) OR (BMI) OR (body mass index) |
| Embase |
‘rivaroxaban’/exp OR rivaroxaban OR (bay AND ’59 7939’) OR anticoagulant AND ‘pharmacokinetics’/exp OR pharmacokinetics OR ‘half life’ OR ‘concentration’/exp OR concentration OR ‘volume of distribution’ OR ((‘volume’/exp OR volume) AND (‘distribution’/exp OR distribution)) OR bioavailability OR clearance AND ‘obesity’/exp OR obesity OR obese OR ’overweight’/exp OR overweight OR ‘extreme weight’ OR (extreme AND (‘weight’/exp OR weight)) OR bmi OR (body AND mass AND index) |
| ScienceDirect |
((Rivaroxaban) OR (BAY 59-7939)) AND ((Pharmacokinetics) OR (half-lfe) OR (concentration) OR (volume of distribution)) AND ((Obesity) OR (obese) OR (body mass index)) |
| Cochrane Library |
Rivaroxaban OR BAY 59 7939 OR anticoagulant AND Pharmacokinetics OR half-life OR concentration OR volume of distribution OR bioavailability OR clearance AND Obesity OR obese OR overweight OR extreme weight OR BMI OR body mass index |
The search results from the targeted databases were combined, duplicates were removed, and the remainder of the identified studies were screened based on their titles and abstracts. The prespecified eligibility criteria were used to assess the identified studies from the databases. Any doubt about a study during title/abstract screening was resolved by conducting a full-text reading by the authors. Articles that were potentially eligible underwent full-text screening. This was achieved by two independent reviewers (MA and OR) and discrepancies were resolved through consensus and adjudication by a third reviewer (AA) whenever necessary. Finally, studies that fulfilled the eligibility criteria were included in the systematic review.
Eligibility Criteria and Study Selection
This review included studies that investigated rivaroxaban PK in obese subjects who are healthy or diseased. Studies were included in the review if they fulfilled the following criteria: investigating rivaroxaban PK in obese (BMI ≥ 30 kg/m2 or body weight > 120 kg) and/or overweight (BMI 25–29.9 kg/m2) subjects (including those studies that included mixed obese/overweight and normal weight subjects, referred to here in this systematic review as the general population), being an original investigation, and involving subjects aged 18 years or older. Studies were excluded if they involved non-human subjects (e.g., animal studies) or were case studies, commentaries, reviews, expert opinions or topic discussions, guidelines, editorials, and conference abstracts, or were written in non-English language.
Methodological Quality Assessment
Two reviewers (MA and AA or OR) independently conducted the risk of bias assessment, and any differences were resolved through consensus by the research team. Two different appraisal tools were used to assess the methodological quality of the selected studies: the Crowe Critical Appraisal Tool (CCAT) and the Critical Appraisal of Clinical Pharmacokinetic Studies (CACPK) tool [66–68]. The CCAT tool was chosen to be used as it was designed to assess the quality of varying types of research designs. It consists of two main parts: the CCAT checklist, which was filled for each included study, and the CCAT user guide that was used along with the checklist to ensure the validity and reliability of the scoring [66, 67]. The CCAT is a score-based instrument that provides a composite quantitative score of a study’s quality. It consists of eight categories: preliminaries, introduction, design, sampling, data collection, ethical matters, results, and discussion. Each category contains multiple items that are scored as present, absent, or not applicable, while the overall category is scored on a scale of 0–5 (0, no evidence; 5, high evidence). Consequently, the total maximum obtainable quality score is 40, which can be converted to a percentage score. The CACPK tool is designed specifically to assess the quality of clinical PK studies. It consists of 21 questions that assess the background (2 questions), study design and experimental methods (15 questions), applied statistics (1 question), and results (3 questions). In addition to the tool, two appendices were used to guide in answering some specific questions within the ‘study design and experimental methods’ section of the CACPK tool. Each question has a rating scale of ‘yes’, ‘no’, ‘I do not know’, and ‘not applicable’ [68].
Data Extraction and Synthesis
The data extraction was performed by one reviewer (MA) and independently verified by two other reviewers (OR or AA). A final reconciled version of the extracted data was achieved. From each included study, relevant data were extracted, including first author’s name, publication year, country of the study, study design, study groups (if any), sample size, general demographics (e.g., baseline age and sex distribution of the study population), clinical conditions, anthropometric parameters (body weight and/or BMI), rivaroxaban dosage regimen, PK model and parameters, and outcomes of the study.
The included studies were observed in terms of their characteristics. Due to heterogeneity in terms of study design, PK model, population studied, and variability in the type of PK parameters determined, meta-analyses of the PK parameters was not feasible. Consequently, we applied a narrative approach to data synthesis. We compared between different studies in terms of PK parameters reported and other important key variables.
Results
Literature Search Findings
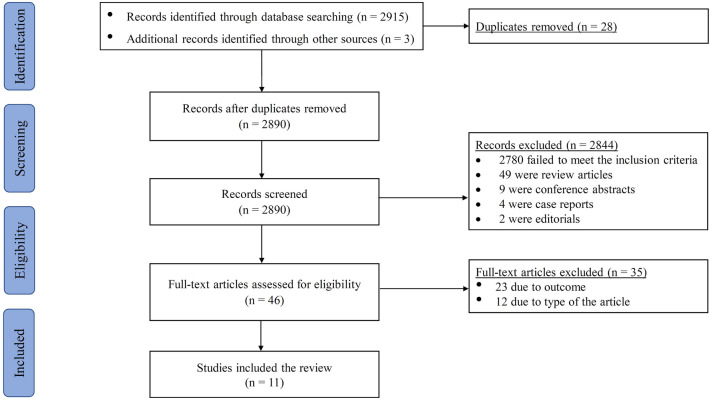
Using the systematic search strategy described earlier, 2918 articles were identified, of which 28 articles were duplicates and were thus excluded. Of the remaining 2890 studies, 2,844 were excluded based on title and abstract screening. Uncertainty of the eligibility of any study was resolved by further reading and confirmation by a second reviewer. A full-text reading of the remaining 46 potentially eligible studies was performed, of which 35 studies were excluded for different reasons, including study type and study outcomes. Finally, 11 studies were included in the review [30, 31, 69–77]. The detailed PRISMA flow diagram that summarizes the search results and the study selection process is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram for literature search and study selection process. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Characteristics and Key Pharmacokinetic-Related Findings of the Included Studies
The characteristics and key PK-related findings of the included studies are presented in Tables 2 and 3. The 11 studies included in this review were published between 2007 and 2022. Four of the studies were retrospective cohort studies [69, 72, 75, 77], one was an RCT [31], two were observational prospective studies [74, 76], and the remaining four were PK modelling studies [30, 70, 71, 73], one of which was pooled from seven RCTs [70]. Two studies were multinational and multicentre [70, 71], while the remaining nine studies were conducted in the UK (three studies), Canada (two studies), Germany, France, Italy, and the Netherlands (one each) [30, 31, 69, 72–77]. In the 11 studies included, over 7140 healthy and diseased subjects received rivaroxaban therapy (sample size ranged from 12 to 4918). The two multinational and multicentre clinical trials contributed to the majority of the subjects (5,927) [70, 71]. Except for one RCT and one PK modelling study, which both lacked any clear indication of categorization of subjects based on body weight or BMI [70, 71], 351 of the 1488 subjects in the remaining studies (approximately 23.6%) were obese (detailed categorization can be found in Table 2).
Table 2.
Overview of the general study characteristics in the included studies
| Study reference | Authors and year of publication | Country | Sample size | Study design | Population | Clinical condition | Groups | CCAT quality scores (%) | CACPK number of ‘yes’ responsesa |
|---|---|---|---|---|---|---|---|---|---|
| [69] | Piran et al. (2018) | Canada | 38, of whom 21 subjects receiving rivaroxaban had weight > 120 kg | Retrospective cohort study that included subjects treated between June 2017 and February 2018 |
Mean age (±SD): 64 (±11) years Males: 79% |
AF: 22 subjects (58%) VTE: 14 subjects (37%) AF + VTE: 1 subject (3%) Others: 1 subject (3%) |
Three groups: Taking apixaban: 7 (18%) Taking dabigatran: 10 (26%) Taking rivaroxaban: 21 (55%) |
85 | 10 |
| [30] | Barsam et al. (2017) | UK | 101, of whom 41 subjects had BMI ≥ 30 kg/m2 |
PK model development study that was based on real-world subjects |
Mean age (range): 52 (20–86) years Males: 58% White 74%, Afro-Caribbean 21%, other 5% |
Acute VTE first event (58%) Acute recurrent event (26%) Prevention of VTE (12%) Elective orthopaedic VTE prophylaxis (4%) |
One group: all were taking prophylactic rivaroxaban or treatment doses for the prevention or treatment of VTE |
95 | 16 |
| [31] | Kubitza et al. (2007) | Germany | 48, of whom 12 subjects receiving rivaroxaban had weight > 120 kg | Single-centre, randomized, single-blind, placebo-controlled, parallel-group study |
Mean age (range): 34.75 (20–54) years Males: 33.3% Caucasian: healthy |
Subjects are all healthy |
Three groups based on weight: (a) ≤50 kg (b) 70–80 kg (c) > 120 kg In each group, 12 healthy subjects were receiving rivaroxaban, and 4 healthy subjects were receiving placebo |
90 | 15 |
| [70] | Willmann et al. (2018) | Data from >27 countries, multinational and multicentre | 4918, data for subgroups based on weight or BMI categories were not available | Integrated population PK model, pooled data from seven randomized controlled trials |
Mean age (±SD): 60.53 (±11.82) years Males: 60.7% |
Prevention of VTE in subjects undergoing elective hip or knee replacement surgery Treatment of acute symptomatic VTE Prevention of cardiovascular events in subjects with ACS Stroke prevention in subjects with AF |
Seven global clinical trials, all subjects were receiving rivaroxaban therapy for different indications | 98 | 17 |
| [71] | Mueck et al. (2008) | Multinational subjects | 1009, data for subgroups based on weight or BMI categories were not available | PK modelling study; randomized, double-blind, double-dummy, active comparator -controlled | Median age (range): 65 (26–87) years in the hip study, and 67 (39–92) years in the knee study | Prevention of VTE in subjects undergoing elective total hip or knee replacement |
Two groups: Hip study (n = 517) Knee study (n = 492) |
83 | 16 |
| [72] | Abdulrehman et al. (2021) | Canada, in a single center | 43, all had weight of > 120 kg | Retrospective study that reviewed patient records between 2014 and 2019 |
Mean age: 54.2 years Males: 60.5% |
Treatment or secondary prevention of VTE | One group: all were receiving rivaroxaban treatment. Peak rivaroxaban plasma levels were measured 2–4 h after rivaroxaban ingestion | 85 | 13 |
| [73] | Speed et al. (2020) | UK | 913, of whom 86 were > 120 kg and 74 had BMI > 40 kg/m2 | PK model generation/development |
Mean age (±SD): 67.03 (±15) years Males: 57.2% |
AF: 110 subjects (68.9%) VTE: 47 subjects (29.4%) Other: 3 subjects (1.9%) |
One group: all were receiving fixed doses of rivaroxaban, according to their indication | 93 | 17 |
| [74] | Ballerie et al. (2021) | France | 146, of whom 77 patients were receiving rivaroxaban and had BMI ≥ 30 kg/m2 | Observational prospective |
Median age (min–max): 61 (19–86) years Males: 52% |
All patients were treated for VTE |
Two groups: Taking rivaroxaban: 77 (53%) Taking apixaban: 69 (47%) |
80 | 14 |
| [75] | Kok et al. (2022) | The Netherlands | 41, of whom 12 patients were taking rivaroxaban and had a BMI mean of 42.6 ± 5.9 kg/m2 | Retrospective, cross-sectional, longitudinal study |
Mean age (SD): 57.9 (8.4) years Males: 33.3% |
AF: 8 subjects (66.7%) VTE: 4 subjects (33.3%) |
Two groups: Taking rivaroxaban: 12 (29%) Taking apixaban: 29 (71%) |
90 | 13 |
| [76] | Martin et al. (2021) | UK |
100, all were ≥ 120 kg Peak rivaroxaban concentrations obtained from 58 subjects Trough rivaroxaban concentrations obtained from 21 subjects |
Prospective, observational study |
Median age (range): 58 (23–78) years Males: 69% |
AF: 58 subjects (58%) VTE: 42 subjects (42%) |
Two groups: Taking rivaroxaban Taking apixaban The number of patients in each group was not reported |
78 | 12 |
| [77] | Russo et al. (2021) | Italy | 58, with a BMI of ≥ 40 kg/m2, of whom 9 subjects were receiving rivaroxaban | Retrospective observational design |
Mean age (±SD): 70.93 (±98.73) years Male: 60% |
All patients were treated for AF |
Four groups: Taking rivaroxaban: 9 (15.5%) Taking apixaban: 24 (41.4%) Taking dabigatran: 12 (20.7%) Taking edoxaban: 13 (22.4%) |
95 | 15 |
SD standard deviation, AF atrial fibrillation, VTE venous thromboembolism, ACS acute coronary syndromes, CCAT Crowe Critical Appraisal Tool, CACPK Critical Appraisal of Clinical Pharmacokinetic Studies, BMI body mass index, PK pharmacokinetics, min minimum, max maximum
aThe number of questions in the CACPK tool with ‘yes’ responses
Table 3.
Overview of the pharmacokinetic models, parameters and outcome measures in the included studies
| Study reference | Weight/BMI | Rivaroxaban dosing regimen | PK model | PK parameter | Outcomes |
|---|---|---|---|---|---|
| [69] |
Median weight: 132.5 kg BMI (IQR): 41 (37.6–47.6) kg/m2 |
Every patient received their medication as prescribed for their condition, which was generally ≥ 15 mg/day | Not reported | Median peak plasma concentration after > 15 mg rivaroxaban: 249 µg.L−1 | No obese subjects receiving rivaroxaban therapy had Cmax below the median trough; however, 28% of the obese subjects receiving rivaroxaban had a Cmax that was below the 5th percentile peak concentration |
| [30] |
Mean weight (±SD) for the whole sample: 88.0 (±23.4) kg BMI, kg/m2 (%) 16–18.49 (1) 18.5–24.9 (27) 25–29.9 (32) 30–34.9 (21) 35–39.9 (14) ≥40 (6) |
15 mg twice daily (57%) 20 mg once daily (38%) 10 mg once daily (4%) 15 mg once daily (1%) |
One-compartment model |
Value (%SD): CL: 8.59 (7%) L.h−1 Vd: 104 (13%) L Ka: 1.32 (24%) h−1 |
The findings suggest that rivaroxaban behaves differently from other traditional anticoagulants, in the extremes of weight population and that the dose does not need to be increased with weight The results suggest that the most important covariate impacting rivaroxaban PK is creatinine clearance, and weight has only a little effect The study showed that weight did not feature as a significant covariant in the study |
| [31] |
Mean BMI (±SD) kg/m2: 28.5 (±10.4) for the placebo group 19.3 (±1.1) for the intervention group who are ≤50 kg 24.3 (±2.3) for the intervention group who are 70–80 kg 43.5 (±4.2) for the intervention group who are > 120 kg |
10 mg/day or placebo in a 3:1 ratio, therefore 12 subjects in each weight group received rivaroxaban and 4 received placebo | Non-compartmental analysis |
Values expressed as means (coefficient of variation), except the tmax, which was expressed as median (range) after a 10 mg dose of rivaroxaban: For subjects > 120 kg: AUC∞: 1155 (15.60%) µg.h.L−1 AUC∞, norm: 15,230 (18.50%) g.h.L−1 Cmax: 149 (20.40%) µg.L−1 Cmax, norm: 1964 (23.10%) g.L−1 tmax: 4.00 (2.00-4.00) h t½: 7.30 (25.40%) h Vz/F: 0.69 (35.80%) L.kg−1 For subjects 70–80 kg: AUC∞: 1029 (20.10%) µg.h.L−1 AUC0, norm: 7611 (19.20%) g.h.L−1 Cmax: 143 (26.50%) µg.L−1 Cmax, norm: 1061 (25.90%) g.L−1 tmax: 3.50 (1.00–4.00) h t½: 7.20 (42.10%) h Vz/F: 1.36 (37.40%) L.kg−1 |
Rivaroxaban was well tolerated in subjects with extremes of body weight, as in subjects with normal body weight, and its PK and PD were not influenced by body weight to an extent considered likely to be clinically relevant |
| [70] |
Mean weight (±SD) for the whole sample: 82.48 (±16.87) kg BMI, kg/m2: <18.5, 18.5 to <25, 25 to <30, 30 to <40, ≥40 |
Ranged from 2.5 mg once daily to 30 mg twice daily | One-compartment model |
Value (% SE): Ka: 0.821 h−1 CL/F: 6.58 (2.33) L.h−1 V/F: 62.5 (2.04) L Population medians varied for AUC, Cmax, and Ctrough by 7.5%, 7.6%, and 44%, respectively, among the BMI categories compared with the reference groups |
The influence of body weight on rivaroxaban PK was minor, demonstrating that fixed doses of rivaroxaban can be prescribed in adult subjects without adjustment for body weight The study showed that the BMI had a minor influence on exposure of rivaroxaban |
| [71] |
Median weight (range): 76 (45–125) kg in the hip study 86 (50–173) kg in the knee study |
2.5, 5, 10, 20 or 30 mg every 12 ± 1 h (5, 10, 20, 40 or 60 mg total daily dose) | One-compartment model |
Values expressed as geometric means [except tmax, which was expressed as median (range)]: After a 10 mg dose of rivaroxaban: AUC12: 974 (63.0) µg.h.L−1 AUC12, norm: 7817 (57.4) g.h.L−1 Cmax: 180 (74.1) µg.L−1 Cmax, norm: 1423 (62.1) g.L−1 tmax: 1 (1–12) h CL/F: 9.80 (59.5) L.h−1 After a 20 mg dose of rivaroxaban: AUC12: 1764 (53.4) µg.h.L−1 AUC12, norm: 7267 (61.2) g.h.L−1 Cmax: 299 (45.6) µg.L−1 Cmax, norm: 1234 (49.8) g.L−1 tmax: 2 (1–4) h CL/F: 11.3 (53.4) L.h−1 |
Rivaroxaban has predictable PK and PD in subjects undergoing major orthopaedic surgery Body weight affected the volume of distribution of rivaroxaban in hip and knee studies; however, the effects of covariates on the PK of rivaroxaban were generally small |
| [72] |
Mean weight (±SD): 140.9 (±16.9) kg, with range 120–181 kg |
20 mg daily | Not reported |
Cmax (IQR): 222 (186–313) µg.L−1 |
There was no significant correlation between body weight and peak plasma concentration of rivaroxaban The peak rivaroxaban concentrations are largely unaffected in subjects weighing ≥120 kg |
| [73] |
Weight, kg (%): < 50 (3.3) 50–100 (73.2) 100–120 (14.1) > 120 (9.4) BMI, kg/m2 (%): < 18.5 (2.1) 18.5–25 (25.3) 25–30 (30.9) 30–35 (20.3) 35–40 (13.4) > 40 (8.1) |
Ranged from 10 to 30 mg once daily | One-compartment model |
After a 20 mg dose of rivaroxaban in patients with weight 125 kg:a AUC24: 2800 µg.h.L−1 Cmax: 305 µg.L−1 After a 20 mg dose of rivaroxaban in patients with normal weight:a AUC24: 3200 µg.h.L−1 Cmax: 360 µg.L−1 |
Creatinine clearance was the single best predictor of rivaroxaban exposure Weight alone was not the most significant factor influencing rivaroxaban PK To conclusively answer the question of whether rivaroxaban is as well tolerated and effective as warfarin at extremes of bodyweight, a large randomized controlled trial would be required; however, conducting a prospective analysis of patients weighing <50 kg or > 120 kg, or with a BMI > 40 kg/m2, would be challenging |
| [74] |
Median weight (range): 100 (73–178) kg BMI, kg/m2 [n (%)] 30–34.9 [79 (54)] 35–39.9 [38 (26)] ≥40 [29 (20)] |
20 mg once daily | Not reported | The median (min–max) of rivaroxaban concentration reported at a median time of 7 h after dose intake: 108 (20–453) µg.L−1 | DOAC use in obese patients treated for VTE at fixed doses led to concentrations similar to those of the general population in a high proportion of patients, without a noticed BMI effect |
| [75] |
Mean weight (±SD): 134.9 (±16.7) kg for the apixaban group 133.2 (±21.9) kg for the rivaroxaban group Mean BMI (±SD): 44.5 (±5.1) kg/m2 for the apixaban group 42.3 (±5.9) kg/m2 for the rivaroxaban group |
20 mg once daily | Not reported |
For subjects with weight from 120 to 130 kg:a Peak range of anti-Xa level: 200–380 µg.L−1 For subjects with weight > 130 to 150 kg:a Peak range of anti-Xa level: 320–350 µg.L−1 |
Plasma anti-Xa level measurements were predominantly within the therapeutic range in morbidly obese patients. In patients using rivaroxaban, no statistically significant relation between plasma anti-Xa levels and body weight was found |
| [76] |
BMI, kg/m2 [n (%)] 30–34.99 [3 (3.2)] 35–39.99 [12 (12.7)] ≥40 [54 (57.5)] ≥50 [16 (17)] ≥60 [9 (9.6)] |
20 mg once daily | Not reported |
In the AF cohort, values expressed as mean (5th–95th): Cmax: 214 (61–672) Ctrough: 59 (14–148) In the VTE cohort, values expressed as mean (5th–95th): Cmax: 220 (99–474) Ctrough: 98 (20–299) |
Subjects with high body weight or morbid obesity receiving factor Xa inhibitors for AF or VTE were unlikely to be underexposed to anticoagulant therapy when administered in standard doses |
| [77] |
Mean BMI (±SD) for the whole sample: 44.43 (±3.54) kg/m2 |
20 mg once daily | One-compartment model |
Values expressed as median (IQR): AUC24: 1204 (861–1390) µg.h.L−1 CL/F: 16.8 (14.4–23.3) L.h−1 Vd: 73.4 (63.5–97.9) L |
Subjects with extreme obesity receiving DOAC therapy for AF had a DOAC plasma concentration in the expected range. The inappropriate DOAC underdosing seems to be the only independent factor associated with the drugs’ plasma concentration out of the expected range |
BMI body mass index, PK pharmacokinetics, PD pharmacodynamics, IQR interquartile range, Cmax maximum plasma concentration, SD standard deviation, CL clearance, Vd volume of distribution, Ka absorption rate constant, AUC∞ area under the plasma concentration-time curve from time zero to infinity, tmax time needed to reach Cmax, t½ elimination half-life, Vz/F apparent volume of distribution during the terminal phase, SE standard error, CL/F apparent clearance, V/F apparent volume of distribution, Ctrough trough concentration, AUCx area under the plasma concentration time curve from time zero to x h, min minimum, max maximum, DOAC direct oral anticoagulant
aValues were visually extracted from study graphs
Most subjects, except in one study that included healthy subjects [31], were taking rivaroxaban for one or more of the following clinical indications: treatment or prevention of VTE, prevention of VTE in patients undergoing elective hip or knee replacement, and prevention of stroke and systemic embolism in patients with NVAF. Rivaroxaban dose across the studies ranged between 2.5 mg once daily and 30 mg twice daily.
The characteristics of the studies (author[s], year of publication, country of the study, sample size, study design, general population demographics, clinical condition of the subjects, rivaroxaban dosage regimen, PK parameters that were reported in the studies, and the outcomes of the studies) are presented in Tables 2 and 3. A one-compartment model or non-compartmental analysis was reported in some studies. Seven PK parameters were extracted from the included studies: Cmax, tmax, AUC, absorption rate constant (ka), Vd, CL, and t½. Overall, the systematic review results showed that rivaroxaban PK parameters changed slightly or considerably in the extreme body weight groups.
Drug exposure (AUC and Cmax) in all included studies except one [31] was measured during steady state. The mean Cmax of rivaroxaban in obese subjects was reported in one study to be 149 µg.L−1 following a 10 mg dose [31], and in one other study was reported to be 305 µg.L−1 following a 20 mg dose [73]. These reported values fall within the range of reported Cmax values in the general population: 143–180 µg.L−1 following a 10 mg dose [31, 71, 78] and 299–360 µg.L−1 following a 20 mg dose [71, 73, 78]. However, in other studies, the reported Cmax values of 214, 215, and 222 µg.L−1 following a 20 mg rivaroxaban dose was slightly lower than anticipated [69, 72, 76]. The AUC of rivaroxaban following a 10 mg dose was reported to be 1155 µg.h.L−1 (AUC from time zero to infinity [AUC∞]) in obese subjects [31] compared with 1029 µg.h.L−1 (AUC∞) in the general population [31]. Similarly, the AUC of rivaroxaban following a 20 mg dose was found to be 1204–2800 µg.h.L−1 (AUC from time zero to 24 h [AUC24] in obese subjects [73, 77] compared with 3200 µg.h.L−1−1 (AUC24) in the general population [73].
The reported/calculated CL value in obese subjects was 7.86–16.8 L.h−1 [31, 77] compared with 5.57–11.3 L.h−1 in the general population [30, 73, 79, 80], i.e., some of the values in obese subjects did not fall within the CL value range reported in the general population. The reported/calculated value of Vd was 73.4–82.8 L in the obese population [31, 77] and 59.4–104 L [30, 31, 70, 73] in the general population, indicating a wide range in the reported/calculated values in the general population; therefore, the effect of obesity on Vd is yet to be investigated. A comparison among the PK parameters in both populations is shown in Table 4.
Table 4.
Comparison between the values of selected pharmacokinetic parameters in the included studiesa
| Parameter (units) | Obese | General population | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kubitza et al. (2007) [31] | Piran et al. (2018) [69] | Speed et al. (2020) [73] | Abdulrehman et al. (2021) [72] | Ballerie et al. (2021) [74] | Kok et al. (2022) [75] | Martin et al. (2021) [76] | Russo et al. (2021) [77] | Speed et al. (2020) [73] | Barsam et al. (2017) [30] | Willmann et al. (2018) [70] | Mueck et al. (2008) [71] | Kubitza et al. (2005)b [78] | Kubitza et al. (2007) [31] |
|
| AUC after a 10 mg dose (µg.h.L−1) | 1155 [AUC∞] (16%) | – | – | – | – | – | – | – | – | – | – | 974 [AUC12] (63.0) |
864 [AUC12] (18.6) |
1029 [AUC∞] (20%) |
| AUC after a 20 mg dose (µg.h.L−1) | – | – | 2800d [AUC24] in a 125 kg subject | – | – | – | – | 1204e [AUC24] | 3200d [AUC24] | – | – | 1764 [AUC12] (53) | 1903 [AUC12] (25) | – |
| Cmax after a 10 mg dose (µg.L−1) | 149 (20%) | – | – | – | – | – | – | – | – | – | – | 180 (74) | 158 (19) | 143 (27%) |
| Cmax after a 20 mg dose (µg.L−1) | – | 215f |
305d in a 125 kg subject |
222 | – |
200–380d in 120–130 kg subjects 320–350a in 130–150 kg subjects |
214 (in AF) 220 (in VTE) |
– | 360d | – | – | 299 (46) | 318.1 (19) | – |
| C7h after a 20 mg dose (µg.L−1) | – | – | – | – | 108e | – | – | – | – | – | – | – | – | – |
| CL (L.h−1) | 7.86g | – | – | – | – | – | – |
16.8e expressed as CL/F |
5.57 (5.34–5.82) expressed as CL/F | 8.59 (7%) | 6.58 (2.33) expressed as CL/F |
9.80 (59.5) after a 10 mg dose 11.3 (53.4) after a 20 mg dose expressed as CL/F |
– | – |
| Vd (L)c |
82.8h expressed as Vz/F or 0.69 (36%) L.kg−1 |
– | – | – | – | – | – | 73.4e |
59.4 (55–64) expressed as Vd/F |
104 (13%)i |
62.5 (2) expressed as V/F |
– | – | 95.2h expressed as Vz/F or 1.36 (37%) L.kg−1 |
AUC area under the plasma concentration-time curve, AUC∞ AUC from time zero to infinity, AUCx AUC from time zero to time x h, Cmax maximum plasma concentration, CL clearance, Vd apparent volume of distribution, PK pharmacokinetic, AF atrial fibrillation, VTE venous thromboembolism, CL/F apparent clearance, Vz/F apparent volume of distribution during the terminal phase
aValues are expressed as geometric means (percentage coefficient of variation) unless otherwise stated
bThis study was not included in this systematic review but was used in Table 4 to extract further PK parameters from the general population for comparison purposes
cAssuming bioavailability of 100% for the whole range of rivaroxaban doses under fed conditions[40]
dAUC and Cmax values were visually extracted from the study graphs
eMedian (range)
fMedian Cmax after an unspecified dose of rivaroxaban (≥ 15 mg)
gCL was calculated using other available PK parameters
hVd (L.kg−1) × weight of the corresponding category
i Percentage standard error
The studies had variable methodological quality characteristics. The CCAT total scores for the studies ranged from 31 to 39 out of 40, with an average of 35.4 out of 40. Average percentage scores of the studies were 88.4% (range 78–98%), with individual study CCAT scores of 78% [76], 80% [74], 83% [71], 85% [69, 72], 90% [31, 75], 93% [73], 95% [30, 77], and 98% [70]. For example, one included study lacked the explanatory diagrams, secondary questions among its objective, and did not account for interindividual variability or potential bias [69]. Another study lacked important criteria in its title, and its sample size was questionable for PK model development purposes [30]. The reporting of study design/intervention description, as well as information about ethical considerations, were lacking in some studies [71, 72, 76]. The CACPK tool rating trend was in agreement with the scoring trend of CCAT in a number of the included 11 studies. The three most highly rated studies using the CACPK tool have scored > 90% in the CCAT tool; similarly, the three least rated by CACPK have scored ≤ 85% in the CCAT tool. A number of questions within the ‘study design and experimental methods’ domain in the CACPK tool were not applicable in 6 of the 11 included studies. This is expected as the CACPK tool is not designed specifically for retrospective studies. More details of all the score categories are shown in Tables 5 and 6.
Table 5.
Quality assessment of the 11 included studies using CCAT
| Study no. | Reference | Preliminary | Introduction | Design | Sampling | Data collection | Ethical matters | Results | Discussion | Total | Corresponding total percentage |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [69] | 5 | 4 | 4 | 4 | 4 | 5 | 4 | 4 | 34 | 85 |
| 2 | [30] | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 | 38 | 95 |
| 3 | [31] | 5 | 5 | 4 | 5 | 5 | 3 | 5 | 4 | 36 | 90 |
| 4 | [70] | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 39 | 98 |
| 5 | [71] | 5 | 5 | 5 | 4 | 4 | 2 | 4 | 4 | 33 | 83 |
| 6 | [72] | 3 | 4 | 4 | 5 | 5 | 5 | 4 | 4 | 34 | 85 |
| 7 | [73] | 5 | 5 | 5 | 4 | 5 | 5 | 4 | 4 | 37 | 93 |
| 8 | [74] | 4 | 5 | 4 | 4 | 4 | 4 | 3 | 4 | 32 | 80 |
| 9 | [75] | 5 | 5 | 4 | 5 | 5 | 5 | 4 | 4 | 36 | 90 |
| 10 | [76] | 5 | 4 | 3 | 3 | 5 | 2 | 5 | 4 | 31 | 78 |
| 11 | [77] | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 38 | 95 |
The CCAT tool is based on a rating score on an overall scale of 0–5 (0, no evidence; 5, high evidence) in each of eight categories
CCAT Crowe Critical Appraisal Tool
Table 6.
Quality assessment of the 11 included studies using CACPK
| Study no. | Reference | Background (2 questions) | Study design and experimental methods (15 questions) | Applied statistics (1 question) | Results (3 questions) | Total number of ‘yes’ responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | NA | Yes | No | NA | Yes | No | NA | Yes | No | NA | |||
| 1 | [69] | 2 | – | – | 4 | 2 | 9 | 1 | – | – | 3 | – | – | 10 |
| 2 | [30] | 2 | – | – | 11 | 4 | – | 1 | – | – | 2 | 1 | – | 16 |
| 3 | [31] | 2 | – | – | 9 | 4 | 2 | 1 | – | – | 3 | – | – | 15 |
| 4 | [70] | 2 | – | – | 11 | 4 | – | 1 | – | – | 3 | – | – | 17 |
| 5 | [71] | 2 | – | – | 11 | 4 | – | 1 | – | – | 2 | 1 | – | 16 |
| 6 | [72] | 2 | – | – | 7 | 5 | 3 | 1 | – | – | 3 | – | – | 13 |
| 7 | [73] | 2 | – | – | 11 | 4 | – | 1 | – | – | 3 | – | – | 17 |
| 8 | [74] | 2 | – | – | 10 | 5 | – | 1 | – | – | 1 | 2 | – | 14 |
| 9 | [75] | 2 | – | – | 8 | 5 | 2 | 1 | – | – | 2 | 1 | – | 13 |
| 10 | [76] | 2 | – | – | 7 | 5 | 3 | 1 | – | – | 2 | 1 | – | 12 |
| 11 | [77] | 2 | – | – | 10 | 3 | 2 | 1 | – | – | 2 | 1 | – | 15 |
The CACPK tool is based on a rating scale of ‘yes’, ‘no’, ‘I do not know’, and ‘not applicable’ in each of the 21 questions in four categories. As there were no ‘I do not know’ answers, this was not included as an option in this table
CACPK Critical Appraisal of Clinical Pharmacokinetic Studies, NA not applicable
Discussion
Several studies have reported that obesity is a significant risk factor for VTE [58, 81, 82]. Since a large proportion of patients who receive anticoagulants, including rivaroxaban, are obese, having definitive evidence about the PK of rivaroxaban in this population will be of great importance [55, 56]. Previously published reviews have provided an overview of the effectiveness and safety of DOACs in general and rivaroxaban in particular among the obese population [83–85]. These reviews discussed the pharmacology of DOACs in obese patients in general, including aspects related to its PK. Therefore, the strength of the current systematic review is that it focuses solely on the PK of rivaroxaban, where all available PK parameters (AUC, Cmax, tmax, Vd, CL, t½) among obese subjects, compared with the general population, would be pooled and extensively analysed in order to assess the effect of obesity on the parameters. To our knowledge, this is the first systematic review that has been conducted to analyse the changes in different PK parameters in obese subjects compared with the general population, which in some instances included a mix of different BMI/body weight categories.
The findings reported here provide evidence that obesity has a variable impact on some of the rivaroxaban PK parameters (particularly Cmax, AUC, CL and Vd). Obesity was found to lower the Cmax of rivaroxaban following a 20 mg dose [69, 72, 76], lower the AUC24 following a 20 mg dose [73, 77], increase the CL (the values were comparatively higher and have not fallen within the CL range reported for general populations) [31, 77], and had an inconclusive effect on the Vd [31, 77]. In general, the data extracted in this systematic review are consistent with previously published rivaroxaban PK studies in the general population. The comparisons in this discussion are held between obese subjects (data exclusively extracted from the included studies in this systematic review [30, 31, 69–77]) and the general population (data extracted from the included studies in this systematic review as well as other published studies) [11, 30, 31, 61, 70, 71, 73, 78]. The current findings regarding the Cmax of rivaroxaban in obese subjects are comparable with previous studies. For example, three studies [31, 71, 78] reported the Cmax of rivaroxaban after a 10 mg dose in the general population to be between 143 and 180 µg.L−1, a range that covers a value in one of the included studies for obese subjects (149 µg.L−1) [31]. However, the Cmax following a 20 mg dose was found to be within 214–305 µg.L−1 in four of the included studies for obese subjects [69, 72, 73, 76], three of which are considerably lower than the values reported in the general population (299–360 µg.L−1) [71, 73, 78], implying a possible impact of body weight on Cmax after a 20 mg dose. The lower than expected rivaroxaban levels reported in the studies by Martin et al. and Piran et al. [69, 76] might have been underestimated if data were sparse and the compartmental modelling was inappropriate [70, 73]. Therefore, a specific comparison of ka and oral CL values after 10 and 20 mg doses of rivaroxaban in obese subjects versus the general population is warranted.
In one of the included studies, the AUC value following a 10 mg rivaroxaban dose was 1155 µg.h.L−1 [31], which is higher than the reported values in a range of 864–1029 µg.h.L−1 in the general population [31, 71, 78]. This comes in agreement with guideline recommendations that endorse that rivaroxaban could produce the desired exposure in obese subjects and thus could be prescribed without dose adjustment [41, 86]. A CL value of 7.86 L.h−1 and a Vd value of 73.4–82.8 L in the obese population [31, 77] were interestingly falling in the middle of the range of values reported in the general population (CL ranged from 5.57 to 11.3 L.h−1 and Vd ranged from 59.4 to 104 L) [30, 70, 71, 73], and thus the same trend was also calculated for t½ (using the formula: ). As the CL and Vd values in the obese population were extracted from two studies, their corresponding values in the general population were extracted from five studies. Such comparison is inconclusive to show a clear impact of obesity on CL and Vd, as the values reported in the general population were about onefold different in separate studies. In addition, the CL values reported in two studies of obese subjects had a 100% difference (Table 4).
High body weight is known to significantly increase the Vd of many medications [87, 88]. Rivaroxaban moderate lipophilicity could partly explain the change in Vd that was observed in obese subjects in some studies [30, 31]. A population PK model for rivaroxaban showed a positive correlation between Vd and body weight [89]. Some factors may affect the PK processes in obesity. For example, a change in albumin binding with an acidic drug (i.e., rivaroxaban) due to displacement by fatty acids, and thus changes in Vd, CL and t½, are expected in the obese population. Moreover, the potential decrease in hepatic activity of cytochrome P450 (CYP) 3A4 in obesity may also partially explain any change in the PK parameters of rivaroxaban in the obese population [90, 91].
Virtual subpopulations were simulated in the models developed in some of the included studies. For example, subpopulations with different weight and BMI categories, including the obese, were defined and the effects of these covariates on PK parameters such as the apparent CL and Vd were simulated [70]. The covariate analysis revealed a minor effect of weight or BMI on rivaroxaban exposure. On the other hand, another modelling study has demonstrated a greater effect of some covariates on PK parameters such as Vd of rivaroxaban, emphasizing that the estimates of PK parameters may not reflect real-world populations and suggesting that larger datasets from real-world patients are warranted [30]. While appraising the included studies for methodological quality and data extraction, some methodological issues were identified [92]. A comparatively short-term follow-up of patients was noted, as two of the included studies were single-dosed and a third study was for about 10 days, all with only one time-point blood sampling. However, longer duration and multiple time points of follow-up and blood sampling may represent a better and clearer picture of rivaroxaban PK profile in the obese population. Furthermore, in two of the included studies, the PK of rivaroxaban were examined retrospectively, which made it difficult to ensure that the data were measured consistently, without confounding factors that could affect the results. Conducting this systematic review was challenging, as the data generated from the included studies have considerable heterogeneity in study designs, clinical indications, rivaroxaban dosage regimens, and the PK parameters that were evaluated and reported. Due to the heterogeneity of data in the included studies, the narrative approach was particularly selected for data synthesis, and thus the meta-analysis approach was deemed inappropriate. To accurately perform data synthesis utilizing the narrative approach, two authors (MA and OR) systematically summarized the key points of each study’s methodology and results to reliably synthesize the findings/conclusions of this systematic review.
One of the limitations of the selected studies is the lack of any study that reported the three related parameters (Vd, CL and t½) in its results; thus, the comparison of these PK parameters between obese subjects and the general population was based on values extracted from different studies or calculated using PK values from the same study. In other words, the missing link between the observed changes in Vd and CL (and the calculated t½) might be due to extracting the PK values from different studies. Another limitation is that the included studies were not specifically designed to investigate the impact of body weight on rivaroxaban PK parameters (except five studies that included only obese/morbidly obese subjects to investigate the PK parameters, however this was a retrospective design study [72, 74–77]). Furthermore, the obese/morbidly obese subjects accounted for an unknown or small proportion of the whole sample size (as low as 18%) among the included studies, potentially skewing the results obtained from this systematic review [30, 31, 69–73]. Finally, some of the included studies have not analysed their findings based on BMI or weight categories, except in three studies that have partially covered the PK parameters in obese subjects [31, 72, 73]. These limitations could clearly highlight the difficulty of analysing the available data and coming up with the current outcomes regarding rivaroxaban PK in obese subjects.
Despite that the included studies contributed to the understanding of rivaroxaban’s PK parameter changes in the obese population, these studies did not distinguish between large and obese subjects upon recruitment and thus have not examined the difference in PK parameters. There is a noteworthy difference in body fat composition between these two groups, which may significantly affect the PK parameters for lipophilic medications such as rivaroxaban, and consequently the interpretation of the findings and outcomes of the review.
As previously mentioned, the current guidelines suggest that rivaroxaban (and other DOACs) should not be used in patients with a BMI of > 40 kg/m2 or a weight of > 120 kg, because limited clinical data exist in this population. Despite the fact that many studies have been conducted investigating the PK/PD with associated clinical outcomes after rivaroxaban administration, and have suggested that decreased drug exposure, reduced peak concentration and shorter half-life (contributing to concerns about underdosing in this population) occur with increasing weight among the obese population, the resultant evidence is still weak and these studies could not confirm the safety/effectiveness of fixed rivaroxaban dosing in this population. Moreover, and according to the guidelines, clinicians should apply their clinical judgement in such a population. Our study aimed to gather/analyse the available evidence in literature, and it was found that the reviewed evidence is insufficient to conclude whether the differences in the reported PK parameter values were clinically relevant or not (and thus whether or not dose adjustment is needed).
Conclusion
In light of this systematic review, it was found that obesity has a variable effect on different rivaroxaban PK parameters. Rivaroxaban should be used with caution in this population, as changes in anticoagulation activity may occur upon long-term therapy. In order to achieve conclusive evidence, it is recommended that well-designed prospective studies be conducted addressing the quality issues identified here in the CCAT and CACPK assessments, to investigate rivaroxaban PK in the obese population. This will assist in establishing solid and definitive evidence to be used in clinical practice guidelines for effective and safe dosage regimen recommendations in this population.
Declarations
Funding
No external funds were used in preparing this manuscript. Open Access funding provided by the Qatar National Library.
Conflicts of interest/competing interests
Majdoleen Alalawneh, Ahmed Awaisu, and Ousama Rachid have no relevant financial or non-financial interests to disclose.
Ethics approval
Ethics approval was not required for this systematic review.
Patient consent
Not applicable.
Consent for publication
Not applicable.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
All authors contributed to the idea of this systematic review. The literature search and data analysis were performed by MA, AA, and OR. The first draft of the manuscript was written by MA, and all authors commented on previous versions of the manuscript. All authors read, critically revised, and approved the final manuscript.
Contributor Information
Majdoleen Alalawneh, Email: malawneh@qu.edu.qa.
Ahmed Awaisu, Email: aawaisu@qu.edu.qa.
Ousama Rachid, Email: orachid@qu.edu.qa.
References
- 1.Cogo A, Bernardi E, Prandoni P, Girolami B, Noventa F, Simioni P, et al. Acquired risk factors for deep-vein thrombosis in symptomatic outpatients. Arch Intern Med. 1994;154(2):164–168. doi: 10.1001/archinte.1994.00420020066008. [DOI] [PubMed] [Google Scholar]
- 2.Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34(11):2363–2371. doi: 10.1161/ATVBAHA.114.304488. [DOI] [PubMed] [Google Scholar]
- 3.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4):S495–S501. doi: 10.1016/j.amepre.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340–1347. doi: 10.1161/CIRCRESAHA.115.306841. [DOI] [PubMed] [Google Scholar]
- 5.ISCfWT D. Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. 2014;12(10):1580-90. [DOI] [PubMed]
- 6.Jha AK, Larizgoitia I, Audera-Lopez C, Prasopa-Plaizier N, Waters H, Bates DW. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Qual Saf. 2013;22(10):809–815. doi: 10.1136/bmjqs-2012-001748. [DOI] [PubMed] [Google Scholar]
- 7.Saunders R, Ozols AA. Cost burden of venous thromboembolism and its prophylaxis in the United States. Value Health. 2016;19(3):A244. doi: 10.1016/j.jval.2016.03.1041. [DOI] [Google Scholar]
- 8.Chen A, Stecker E, Warden AB. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559. doi: 10.1161/JAHA.120.017559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack CV. Introduction to direct oral anticoagulants and rationale for specific reversal agents. Am J Emerg Med. 2016;34(11):1–2. doi: 10.1016/j.ajem.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 10.Bratsos S. Pharmacokinetic properties of rivaroxaban in healthy human subjects. Cureus. 2019;11(8):e5484. [DOI] [PMC free article] [PubMed]
- 11.Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53(1):1–16. doi: 10.1007/s40262-013-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose DK, Bar B. Direct oral anticoagulant agents: pharmacologic profile, indications, coagulation monitoring, and reversal agents. J Stroke Cerebrovasc Dis. 2018;27(8):2049–2058. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128(12):1300–5.e2. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summers RL, Sterling SA. Emergent bleeding in patients receiving direct oral anticoagulants. Air Med J. 2016;35(3):148–155. doi: 10.1016/j.amj.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Drug usage statistics in the USA/rivaroxaban. Available at: https://clincalc.com/DrugStats/Drugs/Rivaroxaban Accessed 23 Apr 2020.
- 16.Kurogi R, Nishimura K, Nakai M, Kada A, Kamitani S, Nakagawara J, et al. Comparing intracerebral hemorrhages associated with direct oral anticoagulants or warfarin. Neurology. 2018;90(13):e1143–e1149. doi: 10.1212/WNL.0000000000005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 18.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 19.Ho KH, van Hove M, Leng G. Trends in anticoagulant prescribing: a review of local policies in English primary care. BMC Health Serv Res. 2020;20(1):1–8. doi: 10.1186/s12913-020-5058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Makam RCP, Hoaglin DC, McManus DD, Wang V, Gore JM, Spencer FA, et al. Efficacy and safety of direct oral anticoagulants approved for cardiovascular indications: Systematic review and meta-analysis. PLoS ONE. 2018;13(5):e0197583. doi: 10.1371/journal.pone.0197583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milling TJ, Jr, Frontera JA. Exploring indications for the use of direct oral anticoagulants and the associated risks of major bleeding. Am J Manag Care. 2017;23(4 Suppl):S67. [PMC free article] [PubMed] [Google Scholar]
- 23.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Ferro CJ, Solkhon F, Jalal Z, Al-Hamid AM, Jones AM. Relevance of physicochemical properties and functional pharmacology data to predict the clinical safety profile of direct oral anticoagulants. Pharmacol Res Perspect. 2020;8(3):e00603. doi: 10.1002/prp2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967. doi: 10.2147/TCRM.S84210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Güler E, Güler GB, Demir GG, Hatipoğlu S. A review of the fixed dose use of new oral anticoagulants in obese patients: Is it really enough? Anatol J Cardiol. 2016;15(12):1020. doi: 10.5152/AnatolJCardiol.2015.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahlmann A, Gehrisch S, Beyer-Westendorf J. Pharmacokinetics of rivaroxaban after bariatric surgery: a case report. J Thromb Thrombol. 2013;36(4):533–535. doi: 10.1007/s11239-013-0891-2. [DOI] [PubMed] [Google Scholar]
- 28.Lucijanic M, Jurin I, Jurin H, Lucijanic T, Starcevic B, Skelin M, et al. Patients with higher body mass index treated with direct/novel oral anticoagulants (DOAC/NOAC) for atrial fibrillation experience worse clinical outcomes. Int J Cardiol. 2020;301:90–95. doi: 10.1016/j.ijcard.2019.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Pathak R, Karmacharya P, Giri S, Poudel DR, Aryal MR, Bhatt VR, et al. Meta-analysis on efficacy and safety of new oral anticoagulants for venous thromboembolism prophylaxis in overweight and obese postarthroplasty patients. Blood Coag Fibrinol. 2015;26(6):635–642. doi: 10.1097/MBC.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 30.Barsam SJ, Patel JP, Roberts LN, Kavarthapu V, Patel RK, Green B, et al. The impact of body weight on rivaroxaban pharmacokinetics. Res Pract Thromb Haemost. 2017;1(2):180–187. doi: 10.1002/rth2.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59–7939) in healthy subjects. J Clin Pharmacol. 2007;47(2):218–226. doi: 10.1177/0091270006296058. [DOI] [PubMed] [Google Scholar]
- 32.Peterson ED, Ashton V, Chen Y-W, Wu B, Spyropoulos AC. Comparative effectiveness, safety, and costs of rivaroxaban and warfarin among morbidly obese patients with atrial fibrillation. Am Heart J. 2019;212:113–119. doi: 10.1016/j.ahj.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Balla SR, Cyr DD, Lokhnygina Y, Becker RC, Berkowitz SD, Breithardt G, et al. Relation of risk of stroke in patients with atrial fibrillation to body mass index (from patients treated with rivaroxaban and warfarin in the rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation trial) Am J Cardiol. 2017;119(12):1989–1996. doi: 10.1016/j.amjcard.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Bates D, Edwards J, Shrum J, Chan C, Manga S, MacKay E. Rivaroxaban for a patient with class III obesity: case report with literature review. Can J Hosp Pharm. 2018;71(1):36. [PMC free article] [PubMed] [Google Scholar]
- 35.Uprichard J. Management of rivaroxaban in relation to bodyweight and body mass index. Ther Adv Cardiovasc Dis. 2016;10(5):294–303. doi: 10.1177/1753944716643645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breuer L, Ringwald J, Schwab S, Köhrmann M. Ischemic stroke in an obese patient receiving dabigatran. N Engl J Med. 2013;368(25):2440–2442. doi: 10.1056/NEJMc1215900. [DOI] [PubMed] [Google Scholar]
- 37.Rafferty JA, Prom R, Kujawski SZ. Acute pulmonary emboli in a patient on long-term dabigatran therapy. Ann Pharmacother. 2013;47(4):20. doi: 10.1345/aph.1R752. [DOI] [PubMed] [Google Scholar]
- 38.Wilson SJ-A, Wilbur K, Burton E, Anderson DR. Effect of patient weight on the anticoagulant response to adjusted therapeutic dosage of low-molecular-weight heparin for the treatment of venous thromboembolism. Pathophysiology of Haemostasis and Thrombosis. 2001;31(1):42-8. [DOI] [PubMed]
- 39.Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59–7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78(4):412–421. doi: 10.1016/j.clpt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51(7):549–561. doi: 10.5414/CP201812. [DOI] [PubMed] [Google Scholar]
- 41.Bayer Inc MBE, Canada. Xarelto, Product monograph. https://www.bayer.com/sites/default/files/2020-11/xarelto-pm-en.pdf (Date of revision 10 June 2022). Accessed 22 Sept 2022.
- 42.Perzborn E, Roehrig S, Straub A, Kubitza D, Mueck W, Laux V. Rivaroxaban: a new oral factor Xa inhibitor. Arterioscler Thromb Vasc Biol. 2010;30(3):376–381. doi: 10.1161/ATVBAHA.110.202978. [DOI] [PubMed] [Google Scholar]
- 43.Hanigan S, Das J, Pogue K, Barnes GD, Dorsch MP. The real world use of combined P-glycoprotein and moderate CYP3A4 inhibitors with rivaroxaban or apixaban increases bleeding. J Thromb Thrombolysis. 2020;49(4):636–643. doi: 10.1007/s11239-020-02037-3. [DOI] [PubMed] [Google Scholar]
- 44.Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos. 2009;37(5):1056–1064. doi: 10.1124/dmd.108.025569. [DOI] [PubMed] [Google Scholar]
- 45.Lang D, Freudenberger C, Weinz C. In vitro metabolism of rivaroxaban, an oral, direct factor Xa inhibitor, in liver microsomes and hepatocytes of rats, dogs, and humans. Drug Metab Dispos. 2009;37(5):1046–1055. doi: 10.1124/dmd.108.025551. [DOI] [PubMed] [Google Scholar]
- 46.Kubitza D, Becka M, Mueck W, Halabi A, Maatouk H, Klause N, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2010;70(5):703–712. doi: 10.1111/j.1365-2125.2010.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin K, Beyer-Westendorf J, Davidson B, Huisman M, Sandset P, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308. doi: 10.1111/jth.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore KT, Kröll D. Influences of obesity and bariatric surgery on the clinical and pharmacologic profile of rivaroxaban. Am J Med. 2017;130(9):1024–1032. doi: 10.1016/j.amjmed.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Domienik-Karłowicz J, Pruszczyk P. The use of anticoagulants in morbidly obese patients. Cardiol J. 2016;23(1):12–16. doi: 10.5603/CJ.a2015.0054. [DOI] [PubMed] [Google Scholar]
- 50.Kubiak E. differences in pharmacokinetics in obesity. Do obese persons require changes in therapeutic schemes? Adv Clin Exp Med. 2006;15(4):669–676. [Google Scholar]
- 51.Di Minno MND, Lupoli R, Di Minno A, Ambrosino P, Scalera A, Dentali F. Effect of body weight on efficacy and safety of direct oral anticoagulants in the treatment of patients with acute venous thromboembolism: a meta-analysis of randomized controlled trials. Ann Med. 2015;47(1):61–68. doi: 10.3109/07853890.2014.982064. [DOI] [PubMed] [Google Scholar]
- 52.Boonyawat K, Caron F, Li A, Chai-Adisaksopha C, Lim W, Iorio A, et al. Association of body weight with efficacy and safety outcomes in phase III randomized controlled trials of direct oral anticoagulants: a systematic review and meta-analysis. J Thromb Haemost. 2017;15(7):1322–1333. doi: 10.1111/jth.13701. [DOI] [PubMed] [Google Scholar]
- 53.Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing G-J, et al. Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel. Chest. 2021;160(6):e545–e608. doi: 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
- 54.Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing G-J, et al. Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. CHEST. [DOI] [PubMed]
- 55.Eichinger S, Hron G, Bialonczyk C, Hirschl M, Minar E, Wagner O, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. 2008;168(15):1678–1683. doi: 10.1001/archinte.168.15.1678. [DOI] [PubMed] [Google Scholar]
- 56.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118(9):978–980. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Printen K, Miller E, Mason E, Barnes R. Venous thromboembolism in the morbidly obese. Surgery, gynecology & obstetrics. 1978;147(1):63–64. [PubMed] [Google Scholar]
- 58.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 59.Coutinho T, Goel K, de Sá DC, Kragelund C, Kanaya AM, Zeller M, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011;57(19):1877–1886. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 60.Moll S, Crona DJ, Martin K. Direct oral anticoagulants in extremely obese patients: OK to use? Research and Practice in Thrombosis and Haemostasis. 2019;3(2):152. doi: 10.1002/rth2.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mueck W, Schwers S, Stampfuss J. Rivaroxaban and other novel oral anticoagulants: pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring. Thromb J. 2013;11(1):10. doi: 10.1186/1477-9560-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151(4):W-65-W-94. [DOI] [PubMed]
- 63.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 64.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison H, Griffin SJ, Kuhn I, Usher-Smith JA. Software tools to support title and abstract screening for systematic reviews in healthcare: an evaluation. BMC Med Res Methodol. 2020;20(1):1–12. doi: 10.1186/s12874-020-0897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crowe M. Crowe critical appraisal tool (CCAT) user guide. Conchra House: Scotland; 2013.
- 67.Crowe M, Sheppard L. A general critical appraisal tool: an evaluation of construct validity. Int J Nurs Stud. 2011;48(12):1505–1516. doi: 10.1016/j.ijnurstu.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Soliman ABE, Pawluk SA, Wilby KJ, Rachid O. The use of a modified Delphi technique to develop a critical appraisal tool for clinical pharmacokinetic studies. Int J Clin Pharm. 2022;44(4):894–903. 10.1007/s11096-022-01390-y. [DOI] [PMC free article] [PubMed]
- 69.Piran S, Traquair H, Chan N, Bhagirath V, Schulman S. Peak plasma concentration of direct oral anticoagulants in obese patients weighing over 120 kilograms: A retrospective study. Research and Practice in Thrombosis and Haemostasis. 2018;2(4):684–688. doi: 10.1002/rth2.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willmann S, Zhang L, Frede M, Kubitza D, Mueck W, Schmidt S, et al. Integrated population pharmacokinetic analysis of rivaroxaban across multiple patient populations. CPT: Pharmacometrics and Systems Pharmacology. 2018;7(5):309-20. [DOI] [PMC free article] [PubMed]
- 71.Mueck W, Eriksson BI, Bauer KA, Borris L, Dahl OE, Fisher WD, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47(3):203–216. doi: 10.2165/00003088-200847030-00006. [DOI] [PubMed] [Google Scholar]
- 72.Abdulrehman J, Selby R, Joundi RA, Yeo E. Peak plasma rivaroxaban levels in patients weighing 120 kg or greater. Thromb Res. 2021;201:15–17. doi: 10.1016/j.thromres.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 73.Speed V, Green B, Roberts LN, Woolcombe S, Bartoli-Abdou J, Barsam S, et al. Fixed dose rivaroxaban can be used in extremes of bodyweight: A population pharmacokinetic analysis. J Thromb Haemost. 2020;18(9):2296–2307. doi: 10.1111/jth.14948. [DOI] [PubMed] [Google Scholar]
- 74.Ballerie A, Van RN, Lacut K, Galinat H, Rousseau C, Pontis A, et al. Apixaban and rivaroxaban in obese patients treated for venous thromboembolism: Drug levels and clinical outcomes. Thromb Res. 2021;208:39–44. doi: 10.1016/j.thromres.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 75.Kok T, de Boer H, Witteman B, Hovens M, van Luin M, Monajemi H. Anti-Xa levels in morbidly obese patients using apixaban or rivaroxaban, before and after bariatric surgery. Obes Surg. 2022;32(3):607–614. doi: 10.1007/s11695-021-05814-y. [DOI] [PubMed] [Google Scholar]
- 76.Martin AC, Thomas W, Mahir Z, Crowley MP, Dowling T, Breen K, et al. Direct oral anticoagulant concentrations in obese and high body weight patients: a cohort study. Thromb Haemost. 2021;121(2):224–233. doi: 10.1055/s-0040-1715834. [DOI] [PubMed] [Google Scholar]
- 77.Russo V, Cattaneo D, Giannetti L, Bottino R, Laezza N, Atripaldi U, et al. Pharmacokinetics of direct oral anticoagulants in patients with atrial fibrillation and extreme obesity. Clin Ther. 2021;43(9):e255–e263. doi: 10.1016/j.clinthera.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59–7939 - An oral, direct Factor Xa inhibitor - After multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61(12):873–880. doi: 10.1007/s00228-005-0043-5. [DOI] [PubMed] [Google Scholar]
- 79.Willmann S, Coboeken K, Zhang Y, Mayer H, Ince I, Mesic E, et al. Population pharmacokinetic analysis of rivaroxaban in children and comparison to prospective physiologically-based pharmacokinetic predictions. CPT. 2021;10(10):1195–1207. doi: 10.1002/psp4.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban–an oral, direct factor xa inhibitor–in healthy subjects. Int J Clin Pharmacol Ther. 2007;45(6):335–344. doi: 10.5414/CPP45335. [DOI] [PubMed] [Google Scholar]
- 81.Lentz SR. Thrombosis in the setting of obesity or inflammatory bowel disease. Hematology 2014, the American Society of Hematology Education Program Book. 2016;2016(1):180-7. [DOI] [PMC free article] [PubMed]
- 82.World Health Organization. Obesity and overweight. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 15 Apr 2020.
- 83.Kido K, Lee JC, Hellwig T, Gulseth MP. Use of direct oral anticoagulants in morbidly obese patients. Pharmacotherapy. 2020;40(1):72–83. doi: 10.1002/phar.2353. [DOI] [PubMed] [Google Scholar]
- 84.Ashton V, Mudarris L, Moore KT. The pharmacology, efficacy, and safety of rivaroxaban in obese patient populations. Am J Cardiovasc Drugs. 2021;21(3):283–297. doi: 10.1007/s40256-020-00434-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elshafei MN, Mohamed MF, El-Bardissy A, Ahmed MB, Abdallah I, Elewa H, et al. Comparative effectiveness and safety of direct oral anticoagulants compared to warfarin in morbidly obese patients with acute venous thromboembolism: systematic review and a meta-analysis. J Thromb Thrombolysis. 2021;51(2):388–396. doi: 10.1007/s11239-020-02179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Charlene Kalani EAH, Alexander T, Udeani G, Surani S. Efficacy and Safety of Direct Oral Anticoagulants (DOACs) in Morbidly Obese Patients. In: Part of special issue: CHEST 2018 Annual Meeting Abstracts. 2018;154(4):108.
- 87.Cheymol G. Clinical pharmacokinetics of drugs in obesity. Clin Pharmacokinet. 1993;25(2):103–114. doi: 10.2165/00003088-199325020-00003. [DOI] [PubMed] [Google Scholar]
- 88.Cheymol G. Effects of obesity on pharmacokinetics. Clin Pharmacokinet. 2000;39(3):215–231. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 89.Mueck WAWAL, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50(10):675–686. doi: 10.2165/11595320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 90.Brill MJ, Diepstraten J, van Rongen A, Van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 91.Smit C, De Hoogd S, Brüggemann RJ, Knibbe CA. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol. 2018;14(3):275–285. doi: 10.1080/17425255.2018.1440287. [DOI] [PubMed] [Google Scholar]
- 92.Soliman ABE, Pawluk SA, Wilby KJ, Rachid O. Creation of an inventory of quality markers used to evaluate pharmacokinetic literature: a systematic review. J Clin Pharm Ther. 2022;47(2):178–183. doi: 10.1111/jcpt.13543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.