Abstract
Objective:
To examine the influence of sexual arousal on vaginal mucosal inflammatory cytokine and antibody production in healthy women with and without histories of childhood and/or adult sexual violence.
Methods:
Ninety-one premenopausal healthy women (ages 18–42) attended a single laboratory session in which they provided vaginal fluid samples before and after viewing one neutral and one erotic film. While viewing the films, participants’ vaginal sexual arousal was recorded using vaginal photoplethysmography.
Results:
Of the 91 participants, 41 (45%) reported no history of sexual violence, 17 (19%) reported a history of childhood sexual abuse (CSA) only, 19 (21%) reported a history of adult sexual assault (ASA) only, and 10 (11%) reported a history of both CSA and ASA, with 4 participants choosing not to provide information on their sexual violence history. For women with a history of ASA but not CSA, there was a significant increase in vaginal IL-1β following arousal, while for women with a history of CSA (with or without ASA), there was a significant decrease. Women without CSA histories had a significant increase in vaginal IgA following sexual arousal, while women with CSA histories had a decrease.
Conclusion:
Sexual arousal possibly plays a role in modifying vaginal immune responses in young, healthy women. Moreover, these effects may vary depending upon sexual assault histories, such that relative to women without assault histories, women with a history of early life sexual trauma showed significantly altered vaginal immune responses following sexual arousal. If replicated, these findings may help explain the increased risk for sexually transmitted infections observed among women with sexual assault histories.
Keywords: Cytokine, Antibody, Vaginal, Sexual arousal, Sexual violence, Childhood sexual abuse, Women
1. Introduction
Sexual trauma, including childhood sexual abuse (CSA), has been extensively linked to mental, physical, and sexual health concerns (Irish et al., 2010; Mercer et al., 2013; Pulverman et al., 2018; Zinzow et al., 2012). Much work suggests that female survivors of sexual trauma exhibit differences in reproductive pathophysiology, including higher rates of sexually transmitted infections (STIs; Williams et al., 2010), cervical dysplasia (Sadler et al., 2011), candidiasis (Turchik et al., 2010), and bacterial vaginosis (BV; Cammack et al., 2011).These disparities in reproductive functioning between women with and without histories of sexual trauma have historically been attributed to mental health and behavioral factors. For instance, psychopathology and heightened stress response are emphasized as mechanisms by which sexual trauma exposure leads to adverse outcomes, including STIs (Latack et al., 2015). Acute stress is known to downregulate cell-mediated immunity (Elenkov, 2004), which can compromise mucosal defenses against STI pathogens (Scheidell et al., 2020) and alter vaginal flora in ways that increase susceptibility to candidiasis and BV (Meyer et al., 2006; Nansel et al., 2006).
It has additionally been suggested that high rates of STIs among survivors of sexual abuse are mediated by trauma-precipitated increases in behaviors that increase STI exposure, including earlier age of partnered sexual debut, condom non-use, transactional sex, and higher numbers of concurrent sexual partners (Sadler et al., 2011; Stahlman et al., 2014; Strauss et al., 2011). Sexual trauma survivors may be more likely to use alcohol or drugs before sex (Cooper, 2010; Walsh et al., 2014), which may also confer particular risk for infection given that intoxication modulates immune responses (Goral et al., 2008). Behavioral risk factors are not restricted to partnered sexual activity, as exposure to intimate partner violence has also been linked to increased vaginal douching (Weisman et al., 2007), which can cause irritation and increase vulnerability to infection.
As a strength, these studies highlight that not all trauma survivors are affected in the same way. Some authors have nonetheless critiqued this literature’s reliance on clinical samples (e.g., those with a diagnosis, or those presenting to outpatient settings for any reason), which may exaggerate the sequelae of sexual trauma (Bigras et al., 2021; Irish et al., 2010). In the broader population, psychosocial and behavioral factors add to our understanding of reproductive pathology but do not fully account for the disproportionate vulnerabilities that affect survivors of sexual violence. For instance, the nationally representative NESARC survey reported that mental health diagnoses only partially mediated the link between CSA and STIs among adult women (Sweet et al., 2013). Similarly, in a large cohort of girls followed longitudinally from middle school to early adulthood in the Add Health study, CSA remained a significant predictor of STIs even when depression and risky sexual activity were included in the models (Fix et al., 2019; Upchurch & Kusunoki, 2004). Thus, behavior cannot be the sole mechanism for the association between sexual trauma and increased STI risk.
One possibility is that sexual trauma – particularly early in life – may influence how the immune system responds to stressors in the future (Meyer et al., 2006; Tsuyuki et al., 2019). Individuals with different life experiences may come to exhibit different immune activity in response to stressors, regardless of the overt psychological or behavioral repercussions of those experiences. Support for this hypothesis comes from a meta-analysis by Baumeister and colleagues (Baumeister et al., 2016), which linked childhood trauma in general and CSA in particular with elevated inflammatory cytokines (specifically, interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α)) later in adulthood.
Additionally, some work suggests that women’s current sexual activity is related to immune functioning. In one set of studies using saliva samples taken at four points across the menstrual cycle, women who reported sexual activity with a partner exhibited decreasing levels of the mucosal antibody immunoglobulin A (IgA) as well as reduced pro-inflammatory cytokines (including IL-6 and interferon γ (IFN-γ) from menses to ovulation, while women who were sexually abstinent showed little to no change (Lorenz et al., 2015; Lorenz et al., 2017). These findings suggest that sexual activity modulates immune parameters in ways that may be conducive to greater fertility. Conversely, women engaging in regular partnered sexual activity who also had high baseline levels of the inflammatory marker C-reactive protein showed less physiological arousal in response to a sexual video in the laboratory (Clephane et al., 2021). It is thus plausible that other aspects of women’s sexuality, including histories of unwanted sexual activity, relate to immune components of reproductive tract physiology in response to sexual stimulation.
To better understand how sexual trauma relates to reproductive pathophysiology, we explored immune markers within the vaginal mucosa of women with histories of CSA, adult sexual assault (ASA), both, and neither. Specifically, our study investigated how acute sexual response (as indexed by the degree of change in vaginal blood flow in response to a sexual film) influenced vaginal inflammation and antibody production among a sample of women with and without histories of sexual trauma. We assessed four cytokines – interleukin 1β (IL-1β), IL-6, TNF-α, and IFN-γ – as well as the mucosal antibody IgA. Each of these markers has been shown to be detectable in vaginal fluid (Dezzutti et al., 2011) and contribute to a variety of obstetric conditions, including susceptibility to STIs (Archary et al., 2015). In line with sexual trauma affecting how the immune system responds to subsequent sexual scenarios, we hypothesized that vaginal inflammation would be similar among study participants at baseline but different following sexual arousal induction. Survivors of sexual trauma (i.e., those with CSA, ASA, or both) were expected to exhibit increased inflammation in response to the sexual film, while women with no such history were expected to show decreases or no change in immune markers. Such findings would support the hypothesis that trauma exposure affects reciprocal relationships between women’s sexual and immune functioning, potentially contributing to reproductive health disparities more generally.
2. Methods
Data for the current analyses come from a study conducted in two sites, in the Midwestern US (n = 51) and the Southeastern US (n = 40), using similar methods and materials summarized below. Procedures were approved by the Institutional Review Boards at Indiana University and the University of North Carolina at Charlotte, and all participants provided written informed consent. Additional information about the project design, analysis plan, and copies of de-identified data used in these analyses, can be found at the study registration page at https://bit.ly/3d9JqcZ.
2.1. Participants
Participants were recruited via paper flyers and online community boards, social media advertisements, and online course-credit pools from two universities. Eligibility screens were conducted via phone and online survey. To be included, participants needed to report being sexually active with a partner within the last month, some history of visual sexual stimuli use, and ability to become aroused while viewing heterosexual sexual stimuli. Participants were excluded if they endorsed chronic physical health conditions, recreational drug use within the past month, use of medications known to impact either sexual response or immune function (e.g., psychotropics, antibiotics), or evidence of current urinary tract infection (UTI) on the day of testing (see Procedure).
The present analyses were conducted using data from healthy, premenopausal females (n = 91) who met the inclusion and exclusion criteria. Of note, some participants (n = 22) were using hormonal contraceptives at the time of their participation. The number of participants included across analyses varied as a function of data completeness (with some participants missing data on individual variables, see below). Linear multiple regression estimates in G*Power (Franz et al., 2007) indicated that 40 participants would be sufficient to capture medium effect sizes (f2 = 0.02 – 0.15) with 80% power.
2.2. Procedure
Participants were asked to schedule their study appointment during the self-reported luteal phase of their menstrual cycle. All sessions were conducted in the afternoon and participants were asked to abstain from vigorous physical activity (including sexual activity) for 24 h before their study session to reduce transient changes in vaginal inflammation (e.g., due to recent exposure to a partner). Participants were given time to ask any questions prior to starting and thoroughly read the informed consent statement in private. Once participants agreed to participate, they completed a urine sample, which was tested for human chorionic gonadotropin (a marker of pregnancy) and UTI using commercially available immunoassay dipstick tests (Wondfo One Step test strips). As pregnancy and/or UTI would alter our measures of interest (i.e., immune activation), if participants tested positive for either test, they were given instruction to follow up with a medical provider and discontinued from testing that day. Participants with indication of a UTI (n = 8) were invited to reschedule after receiving care from their primary care physician.
The researcher measured participants’ height and weight before giving them privacy to complete the vaginal fluid sample and the first film scale (see below). After removing the tampon, they were then asked to fit the vaginal photoplethysmograph (detailed below). Then, participants viewed both a nature video and a sexual video, completed the second film survey, provided a second vaginal fluid sample, and completed the study survey battery.
2.3. Measures
2.3.1. Audio-visual stimuli
Segments from National Geographic and abbywinters.com (a women-oriented erotic film site) were selected and edited to be the neutral and erotic videos, respectively. The nature video (3 min) was always presented first and included scenes of people hiking, kayaking, and talking about nature. The erotic video (7 min) included scenes of a heterosexual couple engaging in foreplay, oral sex, and vaginal intercourse. This order of stimuli presentation is well-validated for inducing sexual arousal (Velten et al., 2018) while capturing a baseline measurement of vaginal blood flow. Before and after the audio-visual stimuli, participants completed a validated film scale that assessed their subjective response to the stimuli, including positive and negative emotions (Heiman & Rowland, 1983). While subjective response data are presented in detail elsewhere (Clephane et al., 2022; Jones et al., 2021), we here note that there were no significant differences in subjective response between women with and without histories of sexual trauma.
2.3.2. Genital sexual arousal measurement
Vaginal photoplethysmography (VPP) was used to measure vaginal pulse amplitude (VPA), which is an index of vaginal blood flow. The VPP has an orange-red spectrum light source encased in a 4.75 cm × 1.25 cm plastic tube (BIOPAC Systems Inc., Goleta, California). The VPA signal was recorded at 200 Hz and band-pass filtered (0.5 – 30 Hz) for the entirety of both nature and erotic audiovisual stimuli. Data were collected with BioPac transducers and amplifiers, then processed with AcqKnowledge version 4.5 (BIOPAC Systems Inc.). Trained research assistants reviewed and cleaned data to remove movement artifacts according to standard protocols (Clephane & Lorenz, 2021).
Percent change in VPA from nature to erotic videos was the primary metric of magnitude of arousal and was computed for each participant individually. Greater percent change values correspond with larger increase in blood flow, which was interpreted to indicate greater sexual arousal. Across participants, the average percent change in VPA was 179.6% (SD = 202.6; range: −100% to 1083%; see Table 1 for averages by group).
Table 1.
Demographics by group.
| No sexual violence history |
History of childhood sexual abuse |
History of adult sexual assault |
Combined childhood and adult sexual violence history |
Total 1 |
Differences between groups 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | F | P | |
|
| ||||||||||||
| Age | 24.8 | 12.7 | 22.9 | 4.63 | 25.1 | 7.27 | 22.1 | 2.33 | 27.5 | 9.57 | 1.07 | 0.37 |
| Sexual orientation 2 | 21.3 | 26. 4 | 25.9 | 19.9 | 23.1 | 20.5 | 39.4 | 26.9 | 24.7 | 24.4 | 1.54 | 0.21 |
| Body mass index | 25 | 5.34 | 29 | 10.4 | 26.6 | 7.29 | 23.2 | 1.88 | 25.9 | 6.9 | 2.01 | 0.12 |
| n | % | n | % | n | % | n | % | n | % | x2 | P | |
| Race/Ethnicity | 17.99 | 0.12 | ||||||||||
| White | 15 | 37 | 6 | 35 | 11 | 58 | 9 | 64 | 41 | 45 | ||
| Black | 10 | 24 | 5 | 29 | 0 | 0 | 0 | 0 | 15 | 16 | ||
| Asian | 5 | 12 | 1 | 6 | 3 | 16 | 0 | 0 | 9 | 10 | ||
| Latinx | 3 | 7 | 1 | 6 | 2 | 11 | 1 | 7 | 7 | 8 | ||
| Multiracial | 3 | 7 | 3 | 18 | 1 | 5 | 0 | 0 | 7 | 8 | ||
| Data missing | 5 | 12 | 1 | 6 | 2 | 11 | 4 | 29 | 12 | 13 | ||
| Relationship status | 1.43 | 0.96 | ||||||||||
| Single | 4 | 10 | 1 | 6 | 2 | 11 | 1 | 7 | 8 | 9 | ||
| In a relationship | 36 | 88 | 16 | 94 | 17 | 89 | 9 | 64 | 78 | 86 | ||
| Data missing | 1 | 2 | 0 | 0 | 0 | 0 | 4 | 29 | 5 | 5 | ||
Notes:
Four participants were missing data on the sexual violence history, but are included in the total values
Sexual orientation was rated on a scale from 0 (exclusively other-sex oriented) to 100 (exclusively same-sex oriented)
There were no significant differences across groups in any demographic tested.
2.3.3. Vaginal fluid measurement
To maximize participant comfort, we used a self-sampling intravaginal tampon collection protocol that has been validated for measurement of vaginal cytokines and immunoglobulins (Scherpenisse et al., 2013), immunoactive proteins (Fortenberry et al., 2014; Garcia et al., 2021) and a variety of other diagnostic markers (Apalata et al., 2020; Sangtani et al., 2019). Before and after the sexual film, participants were instructed to put on a pair of gloves and insert a small, unbleached rayon tampon. The tampons remained inserted for a minimum of 10 min (pre-film average time = 14.88 min, SD = 5.97; post-film average time = 15.2 min, SD = 6.57). After this absorption period, participants removed the tampons and placed them in polypropylene tubes with 20 mL sterile phosphate buffered saline. These tubes were refrigerated until the end of the experimental session, then moved to a −80 °C freezer where they were stored until assay. When preparing for assay, tubes were thawed and weighed. Fluid from tampons was extracted in a sterile environment into new sterile tubes, which were vortexed to homogenize the sample. Eluted vaginal fluid was then aliquoted and either run same-day or refrozen at −80 °C; no aliquot was subjected to more than one freeze–thaw cycle.
2.3.4. Cytokine and immunoglobulin assay
Cytokines (IL-1β, IL-6, TNF-α, IFN-γ) were measured via high-sensitivity multiplex electrochemiluminescence assay with validated VPLEX kits from MesoScale Discovery (MSD, Rockville Maryland), according to manufacturer’s instructions and recommendations for testing vaginal fluid (Fichorova et al., 2008; Taylor et al., 2013). Relative to other common assays used to quantify cytokines in mucosal samples (e. g., Luminex bead array), electrochemiluminescence assays have been shown to have superior signal-to-noise ratios, with higher sensitivity and wider dynamic range (Günther et al., 2020), allowing us to characterize even very low analyte concentrations in diluted samples. To further maximize assay sensitivity, we used overnight incubations at 4 °C for primary incubation steps. Sensitivity of assays as reported by manufacturer were as follows: IL-1β, 0.05 pg/mL; IL-6, 0.06 pg/mL; TNFα, 0.04 pg/mL; IFN-γ, 0.37 pg/mL. Observed sensitivity was good, with lower limit of detections ranging from 0.11 pg/mL – 0.54 pg/mL. Similarly, inter- and intra-assay coefficients of variance (CVs) were good (intra-assay: 7.0%–12.41%; inter-assay: 9.75%–13.63%).
Secretory immunoglobublin A (IgA) was measured via high-sensitivity enzyme-linked immunosorbent assay (ELISA) with validated kits from Invitrogen (ThermoFisher Scientific, Waltham, MA) according to manufacturer’s instructions and recommendations for testing mucosal samples (de Silva et al., 2017; Wu et al., 2000). As above, we used overnight incubations to improve assay sensitivity. Manufacturer-reported sensitivity of the assay was 1.6 ng/mL; observed sensitivity was good (lower limit of detection = 3.64 ng/ml) and assay CVs were good (inter-assay: 1.54–2.19%; intra-assay: 3.86–16.82%).
2.3.5. Data preparation and analytic plan
Sexual trauma history was coded from three items in the self-report survey, which assessed if participants had ever been forced or coerced into oral, vaginal, or anal sex, genital touching, or other unwanted sexual experiences. For each question, if the participant indicated any life history, they were then asked to indicate the age(s) at which this happened. From these data, we coded presence or absence of CSA and ASA. A history of CSA was coded if there was at least one instance of unwanted sex prior to age 16, while ASA was coded if there was at least one instance after age 16. When participants indicated a range of ages rather than discrete instances, these were coded as representing the minimum and maximum ages indicated by that range (e.g., “repeated rapes from 12 to 19” would be coded as representing both CSA and ASA).
Vaginal cytokine and IgA concentrations were adjusted for individual differences in sample volumes using dilution factor calculations recommended by Short et al. (2018). We applied a conservative cutoff for outliers by winsorizing the upper and lower 5% of all data using the winsor normalization function of the psych package in R (Revelle, 2017); this systematically addressed both the very high values and undetectably low values (impacting a total of 35 out of 704 data points). As is typical with immune parameters in healthy volunteers, both cytokine and IgA values were right-skewed; to address this non-normality, we log-transformed these values. Finally, approximately 9% of vaginal fluid samples were discarded due to trace amounts of blood (e.g., due to menstrual spotting) or handling errors (e.g., not refrigerated immediately after collection) and 24% of vaginal arousal data was missing due to excessive movement artifacts or equipment malfunction during the experimental session. As these data were considered missing at random, we estimated missing data using multiple imputation with the mice package (van Buuren & Groothuis-Oudshoorn, 2011; van Buuren et al., 2022). The mice package uses chained equation algorithms to make successively more informed predictions of missing data using available data (van Buuren & Groothuis-Oudshoorn, 2011). Data were imputed from the nearest available non-missing data within a data type (e.g., values missing from individual cytokines were imputed from the three other cytokines at the same timepoint), specifying 20 imputations as recommended by van Buuren (van Buuren, 2018). For cytokines and IgA, we conducted separate repeated measures linear mixed-effect models with subject-level intercepts to account for individual differences at baseline and time-nested variance. Fixed effects included the effects of time (pre- vs post-arousal), sexual violence history, degree of change in vaginal blood flow, and their interactions. Mixed effects models were conducted using the lme4 and lmertest packages (Bates et al., 2014; Kuznetsova et al., 2017).
3. Results
3.1. Demographics and baseline characteristics
Participants had an average age of 27.50 (SD = 9.57), and the majority (86%) reported being in a relationship. Slightly less than half (45%) of the sample reported their race/ethnicity as white, with 16% Black, 10% Asian, 8% Latinx, and 8% multiracial participants. Full demographics by group are presented in Table 1. In terms of sexual violence history, 41 participants (45% of total sample) reported no history of sexual violence, 17 (19%) reported a history of CSA only, 19 (21%) reported a history of ASA only, and 10 (11%) reported a history of both CSA and ASA, with 4 participants choosing not to provide information on their sexual violence history. Participants who did not provide sexual violence information were dropped from analyses that included these measures; however, they were retained for analysis of vaginal arousal effects on immune parameters. There were no differences between women with different violence histories across demographics (see Table 1).
There were no differences between women with different sexual violence histories in terms of vaginal fluid volumes (F(3, 157) = 0.55, p = 0.65) or percent change in vaginal blood flow during sexual arousal (F (3, 65) = 0.66, p = 0.59; see Table 2 for values by group). In other words, there was no evidence of a significant effect of sexual trauma history on either degree of genital arousal or lubrication response. All vaginal cytokines were significantly correlated (Fig. 1).
Table 2.
Average group-wise differences in vaginal immune parameters.
| No sexual violence history |
History of childhood sexual abuse |
History of adult sexual assault |
Combined childhood and adult sexual violence history |
Total 1 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | |
|
| ||||||||||
| Percent change vaginal blood flow during arousal | 192.67 | 248.91 | 136.82 | 229.15 | 224.05 | 251.15 | 112.64 | 112.85 | 178.28 | 229.95 |
| Vaginal fluid weight (g) | ||||||||||
| Pre-arousal | 0.69 | 0.52 | 0.59 | 0.56 | 0.70 | 0.59 | 0.71 | 0.58 | 0.67 | 0.54 |
| Post-arousal | 0.59 | 0.43 | 0.67 | 0.53 | 0.50 | 0.50 | 0.76 | 0.48 | 0.61 | 0.47 |
| Median 2 | MAD | Median | MAD | Median | MAD | Median | MAD | Median | MAD | |
| Interleukin 1β (pg/mL) | ||||||||||
| Pre-arousal | 390.57 | 568.24 | 171.98 | 235.38 | 405.65 | 596.81 | 120.49 | 168.48 | 346.75 | 509.47 |
| Post-arousal | 243.05 | 318.74 | 93.63 | 130.57 | 308.26 | 452.13 | 36.51 | 40.81 | 153.58 | 219.92 |
| Interleukin-6 (pg/mL) | ||||||||||
| Pre-arousal | 9.26 | 12.01 | 11.93 | 13.57 | 12.33 | 13.16 | 11.83 | 13.56 | 11.78 | 15.29 |
| Post-arousal | 18.03 | 22.19 | 8.03 | 8.86 | 58.23 | 80.11 | 15.28 | 17.72 | 18.17 | 23.95 |
| Tumor necrosis factor α (pg/mL) | ||||||||||
| Pre-arousal | 4.95 | 5.02 | 49.37 | 67.60 | 12.23 | 15.17 | 20.59 | 22.76 | 6.06 | 7.29 |
| Post-arousal | 6.47 | 7.90 | 22.15 | 24.13 | 29.04 | 34.71 | 5.50 | 3.86 | 12.91 | 16.20 |
| Interferon γ (pg/mL) | ||||||||||
| Pre-arousal | 46.06 | 51.41 | 27.44 | 23.91 | 56.20 | 68.74 | 65.56 | 91.94 | 43.78 | 52.86 |
| Post-arousal | 22.25 | 23.47 | 15.69 | 18.01 | 54.21 | 51.18 | 15.96 | 11.85 | 30.33 | 34.01 |
| Immunoglobulin A (mg/L) | ||||||||||
| Pre-arousal | 18.87 | 11.81 | 31.75 | 22.62 | 42.07 | 44.26 | 27.06 | 23.43 | 22.95 | 18.17 |
| Post-arousal | 34.15 | 27.54 | 31.00 | 23.04 | 69.26 | 81.18 | 16.25 | 7.87 | 34.35 | 32.71 |
Four participants were missing data on the sexual violence history, but are included in the total values.
As raw cytokine and immunoglobulin values were highly skewed, we report on the median and median absolute devation (MAD). Statistical models used the natural log of cytokine and immunoglobulin concentrations.
Fig. 1.
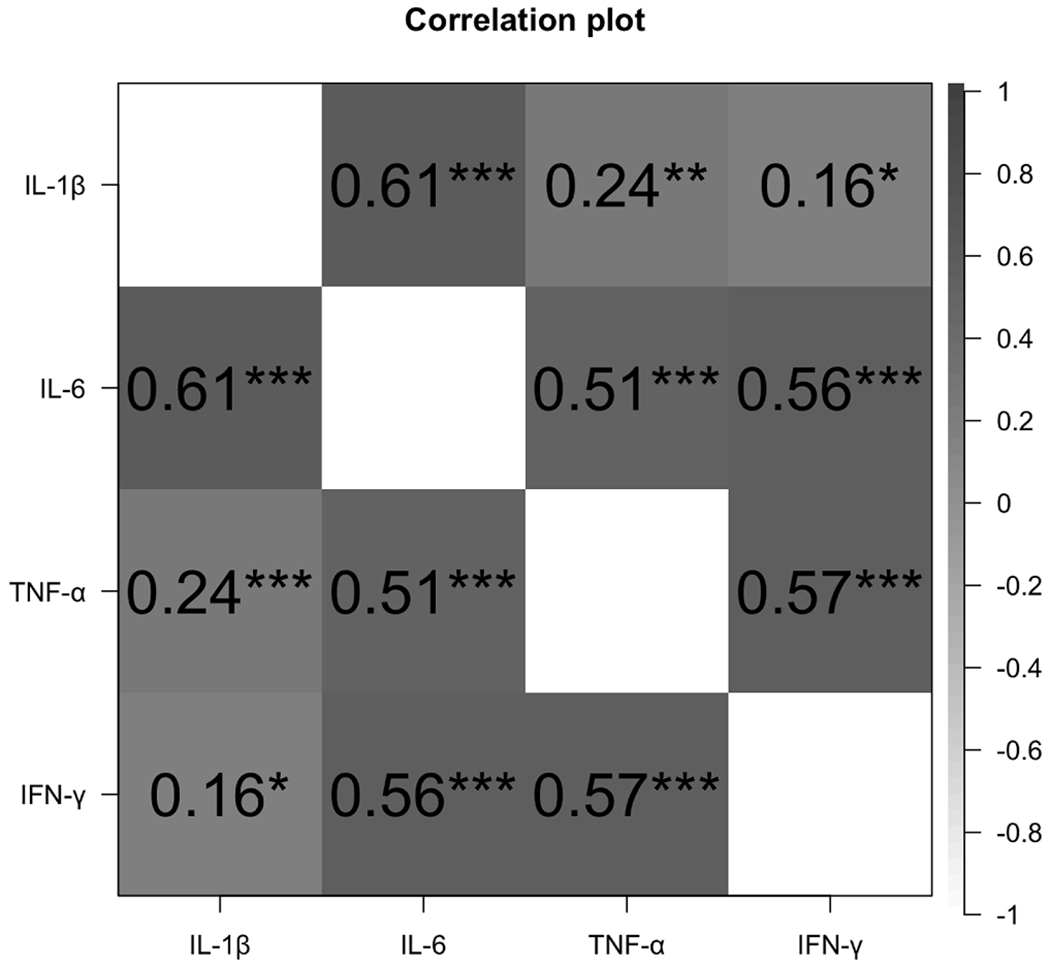
Correlations between vaginal cytokine concentrations. All values are adjusted for weight/dilution, and compared to similar timepoints (pre- or post-arousal) within individuals. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.2. Vaginal immune response to sexual arousal
3.2.1. Effects of vaginal arousal on change in vaginal immune parameters
Across all study participants, for IL-6, TNF, and IFN-γ, there was a significant interaction between degree of vaginal arousal and time (IL-6: F(1, 86.92) = 13.55, p < 0.001; TNF: F(1, 88.76) = 9.03, p = 0.003; IFN: F(1, 88.96) = 9.16, p = 0.003). For interpretation and presentation of these interactions, we created a median split of the degree of vaginal arousal and categorized participants into “high” and “low” categories (Fig. 2a–c). Higher vaginal blood flow during sexual stimulation was associated with decreases in vaginal cytokines from pre- to post-arousal, while lower vaginal blood flow was associated with either no change or moderate increases in vaginal cytokines. Degree of vaginal blood flow did not predict change in IL-1β (F(1, 85.92) = 2.01, p = 0.160).
Fig. 2.
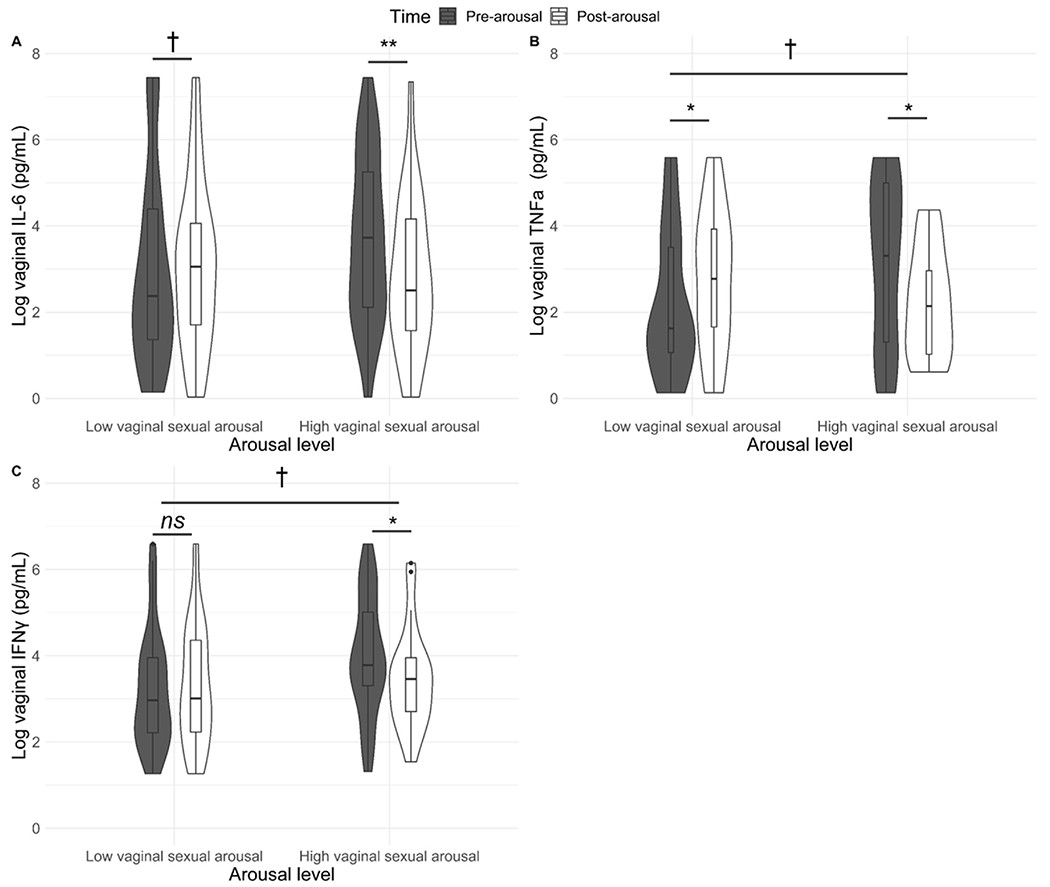
a–c. Changes in vaginal cytokines following sexual stimulation as a function of degree of vaginal sexual arousal. All values are adjusted for weight/dilution. For presentation we designated high and low arousal by median split, but analyses all used the continuous variable of percent change in vaginal pulse amplitude. Higher vaginal blood flow during sexual stimulation was associated with significant decreases in vaginal cytokines from pre- to post-arousal, while lower vaginal blood flow was associated with either no change or moderate increases in vaginal cytokines. There were marginally significant differences at the pre-arousal timepoint in vaginal TNF and IFN between women who went on to have either high or low vaginal arousal. † p < 0.1, * p < 0.05; ** p < 0.01; *** p < 0.001, ns = not significant.
For IgA, there was a significant interaction between degree of vaginal arousal and time (F(1, 87.46) = 4.26, p = 0.042) across all study participants. As seen in Fig. 3, there was a significant decrease in vaginal IgA among women who had lower vaginal arousal, while women with higher levels of vaginal arousal showed a (non-significant) increase in IgA following sexual stimulation.
Fig. 3.
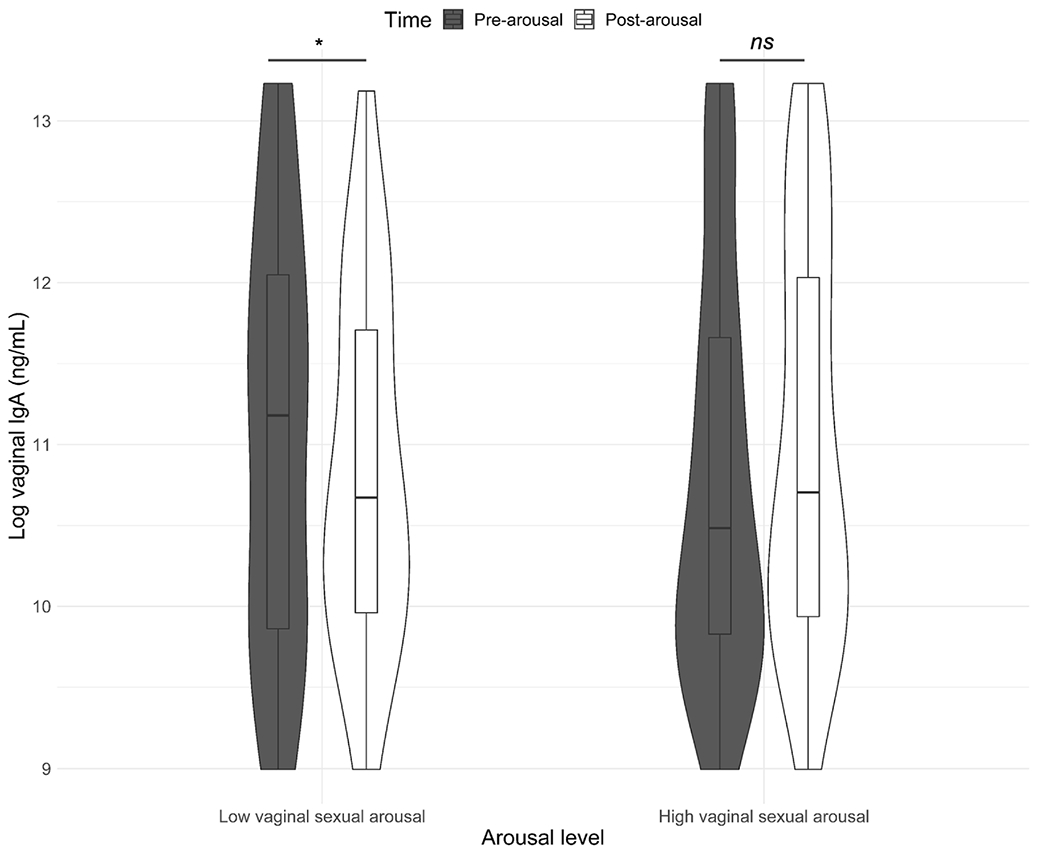
Changes in vaginal immunoglobulin A (IgA) following sexual stimulation as a function of degree of vaginal sexual arousal. All values are adjusted for weight/dilution. Note that for presentation we designated high and low arousal as defined by median split, but analyses all used the continuous variable of percent change in vaginal pulse amplitude. There was a decrease in vaginal IgA among women who had lower vaginal arousal, while women with higher levels of vaginal arousal showed a non-significant increase in IgA following sexual stimulation. † p < 0.1, * p < 0.05; ** p < 0.01; *** p < 0.001, ns = not significant.
3.3. Effects of sexual violence history on change in vaginal immune parameters
For IL-1β, there was a significant interaction between CSA history and time (F(1, 85.58) = 4.94, p = 0.029) and between ASA history and time (F(1, 85.34) = 5.34, p = 0.023). As seen in Fig. 4a, for women with no history of sexual violence, there was a small (non-significant) decrease in vaginal IL-1β from pre- to post-arousal. For women with a history of ASA but not CSA, there was a significant increase in vaginal IL-1β following arousal, while for women with a history of CSA (with or without ASA histories), there was a significant decrease.
Fig. 4.
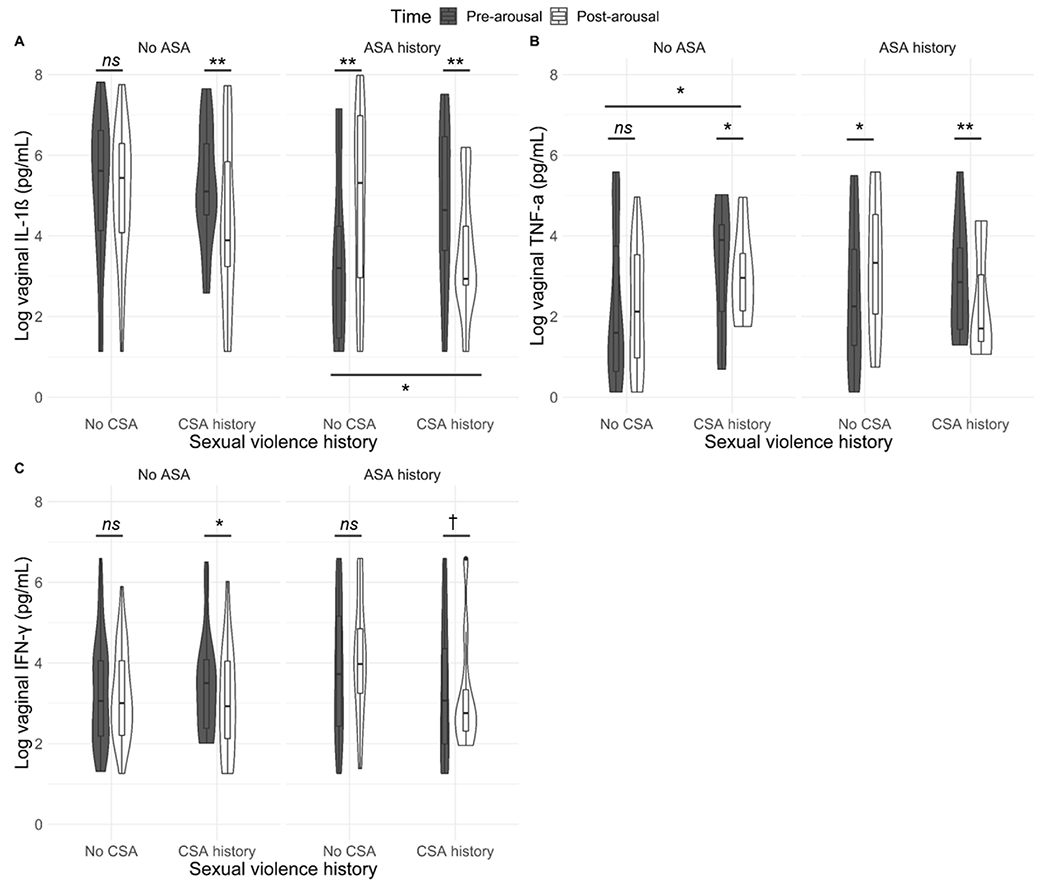
a–c. Changes in vaginal cytokines following sexual stimulation as a function of sexual violence history. All values are adjusted for weight/dilution. CSA = Childhood sexual abuse; ASA = Adult sexual assault. For women with a history of ASA but not CSA, there was a significant increase in vaginal IL-1β following arousal, while for women with a history of CSA (with or without ASA histories), there was a significant decrease. Women with a CSA history had a significant decrease in vaginal TNF-α and IFN-γ following sexual arousal, while women without CSA histories had either no change or an increase. Among women with ASA histories, there were significant differences at the pre-arousal timepoint in vaginal IL1β between women with vs without CSA histories. Among women without ASA histories, there were significant differences in the pre-arousal timepoint in vaginal TNF- α between women with vs. without CSA histories. † p < 0.1, * p < 0.05; ** p < 0.01; *** p < 0.001, ns = not significant.
For TNF-α and IFN, there was a marginally significant interaction between CSA history, ASA history, and time (TNF-α: F(1, 86.93) = 3.58, p = 0.062; IFN-γ: F(1, 87.54) = 3.62, p = 0.060). As seen in Fig. 4b–c, women with a CSA history had a significant decrease in vaginal TNF-α and IFN-γ following sexual arousal, while women without CSA histories had either no change or an increase.
Sexual violence history did not predict changes in IL-6 (CSA: F(1, 86.39) = 1.36, p = 0.247; ASA: F(1, 85.93) = 0.54, p = 0.463).
Finally, for IgA, there was a significant interaction between CSA history and time (F(1, 86.45) = 4.44, p = 0.038). As seen in Fig. 5, women without CSA histories showed a significant increase in vaginal IgA following sexual arousal, while women with CSA histories showed a decrease.
Fig. 5.
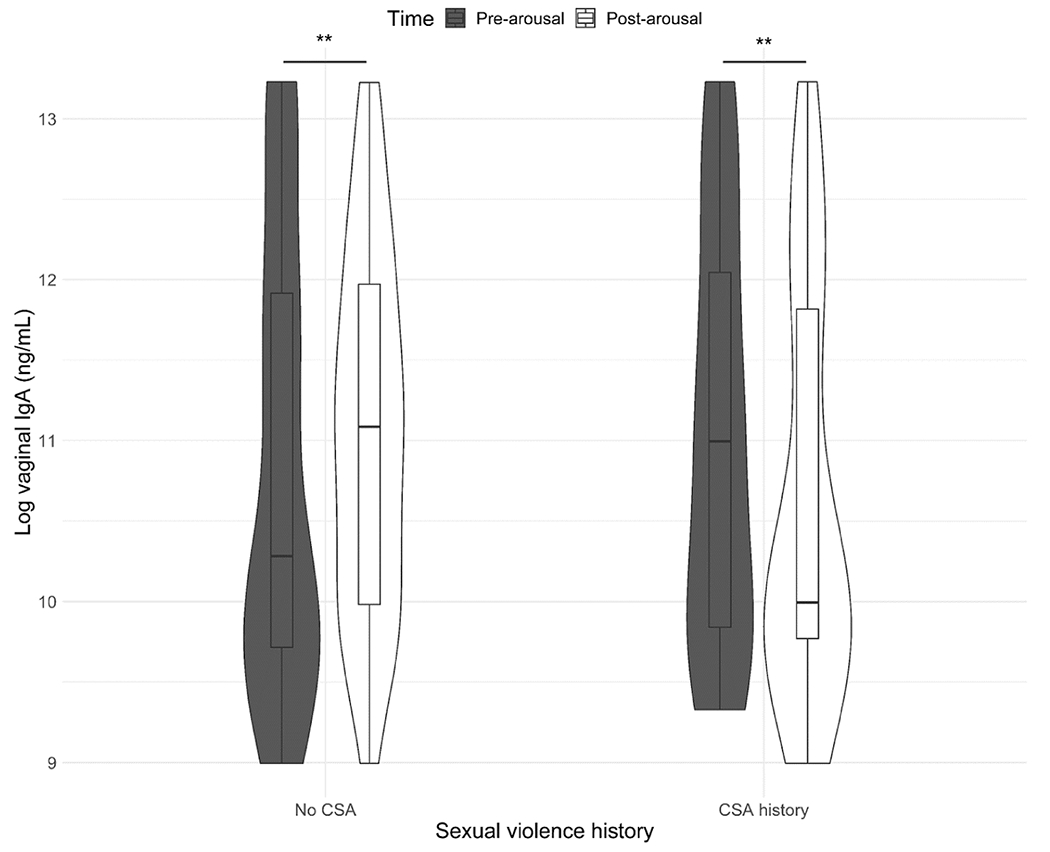
Changes in vaginal immunoglobulin A (IgA) following sexual stimulation as a function of sexual violence history. All values are adjusted for weight/dilution. CSA = Childhood sexual abuse. Women without CSA histories showed a significant increase in vaginal IgA following sexual arousal, while women with CSA histories had a significant decrease. † p < 0.1, * p < 0.05; ** p < 0.01; *** p < 0.001, ns = not significant.
4. Discussion
This study examined the influence of sexual arousal on vaginal cytokine production among women with varying sexual trauma histories. Though results varied, there were two through-lines: sexual arousal was associated with changes in vaginal cytokine and IgA concentrations, and those changes were shaped by sexual trauma history. Considering across women with and without sexual trauma histories, higher levels of arousal were associated with lower vaginal cytokine concentration and (marginally) higher vaginal IgA concentration post-arousal, while lower levels of arousal were associated with increased cytokine concentration post-arousal and decreased IgA levels. This may have been a function of the higher baseline cytokine levels observed in women who went on to have higher vaginal arousal – essentially, becoming aroused brought down the higher levels of cytokines to similar levels as the women who started with lower baselines. When considering the effect of sexual trauma histories, those with CSA histories tended to have different immune profiles post-arousal than those who had ASA histories without CSA history. CSA was associated with decreased IgA following arousal as well as lower cytokine levels – which was especially apparent if there was also an ASA history. However, those with an ASA history without CSA showed increased vaginal cytokines following sexual arousal.
These results paint a telling picture of sexual arousal’s potential influence on women’s mucosal immune function. Sexual arousal may induce a coordinated response that reduces inflammation and maintains antibody-mediated response even as lubrication volume increases. If so, this would reduce the potential that inflammation in the reproductive tract would interfere with conception (Lorzadeh et al., 2020), while still providing protection against STIs. In other words, sexual arousal may stimulate a highly adaptive process that balances trade-offs between creating a hostile environment to pathogens, while avoiding the same hostility to sperm or embryo before it has the chance to establish itself in the uterus. While subtle, this effect would be strongly selected for throughout human evolution, as arousal would promote the conditions necessary for fertility at precisely the moment those conditions would be most relevant – namely, following intercourse – while minimizing the costs of maintaining those conditions. Possibly, these effects are mediated by sympathetic activation during sexual arousal, as activation of the sympathetic nervous system is associated with increased heart rate, faster breathing rate, and heightened vaginal arousal during sexual stimulation (Lorenz et al., 2012).
However, the difference in inflammation profile after arousal for women who have a sexual trauma history indicates that, for these women, facilitative arousal-induced immune processes may be disrupted. Childhood trauma and early life stress have been linked to a number of physiological and psychological disturbances in adulthood, such as autoimmune conditions and depression (Baumeister et al., 2016; Cattaneo et al., 2015; Chase et al., 2019; Danese et al., 2017; Geiger et al., 2019). Early trauma often increases stress and may chronically activate the hypothalamic–pituitaryadrenal (HPA) axis, which can in turn dysregulate immune activation (Danese et al., 2017). Childhood is an important time for the development of the adaptive immune system, particularly the mucosal immune system (Brugman et al., 2015; Zhao & Elson, 2018), thus significant adversity during this time may negatively impact the development of these systems. In addition to the direct effects of stress and HPA activation, the adaptive immune system may be influenced by external biological factors. For instance, a meta-analysis of childhood abuse histories and cytokine profiles in adulthood found that CSA was associated with a different cytokine profile than emotional or physical abuse (Baumeister et al., 2016). Specifically, those who had a CSA history tended to have increased levels of TNF-α and IL-6 (Baumeister et al., 2016). These results parallel our findings, which also highlight greater levels of TNF-α pre-arousal in those who had a CSA history. It is possible that during CSA, exposure to another person’s biological materials (such as semen) paired with stress from abuse teaches the adaptive immune system a different pattern of association and activation. Further investigations into the mechanisms that biologically differentiate childhood sexual abuse from other types of abuse are warranted.
While general cytokine profiles are interesting in and of themselves, this study broadens the picture of cytokine changes found in vaginal fluid pre- and post-arousal. As the different groups of participants started at varying baselines, the influence vaginal arousal had on cytokine recruitment is noteworthy. For women who had a CSA history, pro-inflammatory cytokines were dampened post-arousal. However, for those with an ASA history but without a CSA history, IL-1β was significantly increased, while other cytokine profiles showed little change from pre- to post-arousal. These findings may reflect the different roles that sexual trauma in early vs. later life played in defining immune response to sexual stimulation. Early life exposure to sexual stimulation (albeit in an abusive situation) appears to potentiate some aspects of a pro-conceptive facilitative response (lowering inflammation) but reduces protection against STIs (decreasing antibodies). Stress calibrates reproductive strategies across the lifespan, emphasizing early reproduction in girls exposed to early life adversity even at the expense of long-term health (Del Giudice et al., 2011); tentatively, our findings may reflect this tendency for CSA survivors to develop more so-called “fast” life history trajectories (Chisholm et al., 1993). If so, this would explain why sexual trauma experienced in adulthood had such different effects on arousal-induced changes in vaginal immunity.
It is important to note that our study was designed to recruit a general, healthy population of women; and yet, one half of the sample reported some form of sexual trauma. In order to participate in our study, participants had to report that they were not currently distressed by any sexual trauma history. Further, subjective negative responses to the film such as self-reported disgust and fear were low and not significantly different between women with and without trauma experiences (Clephane et al., 2022; Jones et al., 2021). While participants did not report distress in response to the film, it is possible that women with trauma histories processed sexual stimuli differently than those without trauma histories at an automatic or pre-conscious level (Rellini et al., 2011). For example, it is possible that women with sexual trauma histories attended to different aspects of the sexual stimulus (Latack, Moyer, Simon, & Davila, 2017; Velten, Milani, Margraf, & Brotto, 2021), subtly changing their response at a level that did not reach the surface of conscious reporting in our sample. Taken together, these factors highlight two important things: firstly, that sexual trauma is pervasive and impacts many people. Secondly, there is something about experiencing sexual trauma, rather than the current (conscious) experience of distress, driving these effects. Further research examining the pervasiveness and scale of these discrepancies among distressed and nondistressed samples is warranted.
4.1. Limitations
Participants were brought into a controlled laboratory environment and the arousal induction was conducted via a sexual video and (optional) use of a vibrator, thus the generalizability to partnered sex is unclear. However, evidence of cytokine changes in a controlled laboratory setting is promising for the potential magnitude of changes exhibited in vivo. Along the same lines, participants were only given 7 min to become aroused. Though this duration has been found to be enough time for women to become sufficiently aroused to orgasm through self-stimulation (Kinsey et al., 1998), it may not be representative of arousal in participants’ solitary or partnered activities. It is possible that an even greater change could be detected the longer participants are aroused, but this requires further investigation. Additionally, these participants generally reported most of their lifetime sexual activity was with partners with penises. Women who are sexually active with men may have different immune profiles in response to sexual activity than women who have sex with women (Brown et al., 2008). Our sample was young, healthy, and relatively homogenous in terms of race and ethnicity; generalizability to a wider diversity of women is unknown. Finally, our immune measures were enumerative (i.e., concentrations of immune markers) and thus do not necessarily reflect immune function; future work may examine effects of arousal on microbiocidal activity or other metrics of immune function. This last point is particularly important for translation of these findings: we cannot conclude, based solely on these preliminary data, that the effects of sexual trauma history on vaginal immune response is sufficient to significantly alter STI susceptibility, fertility, or other meaningful clinical endpoints. Until these findings have been replicated and extended to such endpoints, caution is needed in their interpretation.
5. Future directions
In summary, these results shed light on potential immune differences in response to sexual stimuli which may be influenced by adverse childhood experiences. While these results are noteworthy, more work examining this phenomenon is warranted. Specifically, there seems to be an important distinction in the body’s immune response to sexual stimuli based on CSA and ASA histories. The mechanism behind this differentiation is unclear and warrants investigation. Future research may also benefit from assessing the influence of automatic or pre-conscious processing of sexual stimuli in directing immune response. Perhaps experiencing CSA creates an underlying distress signal that – while not consciously experienced – could trigger automatic physiological reactions in response to sexual stimuli. Additionally, replication in settings with greater ecological validity – such as in the context of partnered sex – is needed. Finally, this line of research would benefit from assessing functional immune response, and investigating such functional responses as potential mediators of the disparities in STI rates that have been documented among trauma survivors.
6. Conclusions
Our study points to a possible role for sexual arousal in modifying vaginal immune responses in young, healthy women. Moreover, relative to women without sexual assault histories, women with a history of early life sexual trauma showed significantly altered vaginal immune responses following sexual arousal. Our findings warrant replication in a broader sample with greater diversity and in response to a wider range of sexual activity, including partnered sex. If replicated, these findings would point to a novel mechanism by which CSA is associated with higher STI risk – and highlight the need to develop behavioral interventions that promote positive sexual well-being as a means of promoting broader reproductive health.
Acknowledgements
This work was supported by the National Institute of Child Health and Human Development [grant number T32 HD049336] and by internal funds from the University of North Carolina at Charlotte and the University of Nebraska-Lincoln Department of Psychology; the Nebraska Tobacco Settlement Biomedical Research Development Fund; and the UNL Office of Research and Economic Development. KC and TL are additionally supported by funds from the National Institute of General Medical Sciences of the National Institutes of Health [P20GM130461] and the Rural Drug Addiction Research Center at the University of Nebraska-Lincoln. CW is supported by the National Science Foundation (NSF) Graduate Research Fellowship Program [grant number 1342962]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Science Foundation, the University of Nebraska-Lincoln, or the University of North Carolina at Charlotte.
Abbreviations:
- CSA
childhood sexual abuse
- ASA
adult sexual assault
- STI
sexually transmitted infection
- IgA
immunoglobulin A
- VPA
vaginal pulse amplitude
- UTI
urinary tract infection.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Apalata T, Longo-Mbenza B, Prasad S, 2020. The role of T helper 17 (Th17) and regulatory T cells (Treg) in the pathogenesis of vulvovaginal candidiasis among HIV-infected women. Int. J. Microbiol 2020, 1–10. [Google Scholar]
- Archary D, Liebenberg LJ, Werner L, Tulsi S, Majola N, Naicker N, Dlamini S, Hope TJ, Samsunder N, Abdool Karim SS, Morris L, Passmore JA, Garrett NJ, Tachedjian G, 2015. Randomized cross-sectional study to compare HIV-1 specific antibody and cytokine concentrations in female genital secretions obtained by menstrual cup and cervicovaginal lavage. PLoS ONE 10 (7), e0131906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S. (2014). Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823 [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V, 2016. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry 21 (5), 642–649. 10.1038/mp.2015.67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigras N, Vaillancourt-Morel M-P, Nolin M-C, Bergeron S, 2021. Associations between childhood sexual abuse and sexual well-being in adulthood: a systematic literature review. J. Child Sex. Abuse 30 (3), 332–352. 10.1080/10538712.2020.1825148. [DOI] [PubMed] [Google Scholar]
- Brown SG, Morrison LA, Calibuso MJ, Christiansen TM, 2008. The menstrual cycle and sexual behavior: relationship to eating, exercise, sleep, and health patterns. Women Health 48 (4), 429–444. 10.1080/03630240802575179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman S, Perdijk O, van Neerven RJJ, Savelkoul HFJ, 2015. Mucosal immune development in early life: setting the stage. Arch. Immunol. Therap. Exp 63 (4), 251–268. 10.1007/s00005-015-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack AL, Buss C, Entringer S, Hogue CJ, Hobel CJ, Wadhwa PD, 2011. The association between early life adversity and bacterial vaginosis during pregnancy. Am. J. Obstet. Gynecol 204 (5), 431.e1–431.e8. 10.1016/j.ajog.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A Macchi F Plazzotta G Veronica B Bocchio-Chiavetto L Riva MA Pariante CM Inflammation and neuronal plasticity: A link between childhood trauma and depression pathogenesis. Frontiers in Cellular Neuroscience Front. Cell. Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase KA, Melbourne JK, Rosen C, McCarthy-Jones S, Jones N, Feiner BM, Sharma RP, 2019. Traumagenics: at the intersect of childhood trauma, immunity and psychosis. Psychiatry Res. 273, 369–377. 10.1016/j.psychres.2018.12.097.. [DOI] [PubMed] [Google Scholar]
- Chisholm JS, Ellison PT, Evans J, Lee PC, Lieberman LS, Pavlik Z, Ryan AS, Salter EM, Stini WA, Worthman CM, 1993. Death, hope, and sex: Life-history theory and the development of reproductive strategies [and comments and reply]. Curr. Anthropol 34 (1), 1–24. [Google Scholar]
- Clephane K Lorenz T Vaginal Pulse Amplitude Data Cleaning Guide 2021. 10.17605/OSF.IO/T67QN.. [DOI]
- Clephane K, O’Loughlin JI, Bodnar TS, Wilson MC, Stariha JT, Craig AN, Weinberg J, Brotto LA, Lorenz TK, 2022. Lack of evidence for a relationship between salivary CRP and women’s sexual desire: an investigation across clinical and healthy samples. J. Sex.Med 19 (5), 745–760. 10.1016/j.jsxm.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clephane K, Wilson MC, Craig AN, Heiman JR, Lorenz TK, 2021. Inflammation predicts sexual arousability in healthy women. Comprehen. Psychoneuroendocrinol 8, 100086 10.1016/j.cpnec.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, 2010. Toward a person × situation model of sexual risk-taking behaviors: Illuminating the conditional effects of traits across sexual situations and relationship contexts. J. Pers. Soc. Psychol 98 (2), 319–341. 10.1037/a0017785.. [DOI] [PubMed] [Google Scholar]
- Danese A J Lewis S, & J Lewis S Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology 42 1 2017. 99–114. 10.1038/npp.2016.198.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva TI, Gould V, Mohammed NI, Cope A, Meijer A, Zutt I, Reimerink J, Kampmann B, Hoschler K, Zambon M, Tregoning JS, 2017. Comparison of mucosal lining fluid sampling methods and influenza-specific IgA detection assays for use in human studies of influenza immunity. J. Immunol. Methods 449, 1–6. 10.1016/j.jim.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA, 2011. The adaptive calibration model of stress responsivity. Neurosci. Biobehav. Rev 35 (7), 1562–1592. 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezzutti CS, Hendrix CW, Marrazzo JM, Pan Z, Wang L, Louissaint N, Kalyoussef S, Torres NM, Hladik F, Parikh U, Mellors J, Hillier SL, Herold BC, Kelly KA, 2011. Performance of swabs, lavage, and diluents to quantify biomarkers of female genital tract soluble mucosal mediators. PLoS ONE 6 (8), e23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, 2004. Glucocorticoids and the Th1/Th2 Balance. Ann. N. Y. Acad. Sci 1024 (1), 138–146. 10.1196/annals.1321.010.. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti CS, Donoval B, Doncel GF, Donaghay M, Grivel J-C, Guzman E, Hayes M, Herold B, Hillier S, Lackman-Smith C, Landay A, Margolis L, Mayer KH, Pasicznyk J-M, Pallansch-Cokonis M, Poli G, Reichelderfer P, Roberts P, Rodriguez I, Saidi H, Sassi RR, Shattock R, Cummins JE Jr., 2008. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal. Chem 80 (12), 4741–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix RL, Assini-Meytin LC, Le PD, 2019. Gender and race informed pathways from childhood sexual abuse to sexually transmitted infections: a moderated mediation analysis using nationally representative data. J. Adolesc. Health 65 (2), 267–273. 10.1016/j.jadohealth.2019.02.015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenberry JD, Rogers M, Fordyce K, King S, Aronoff DM, Van Anders SM, 2014. HIV inhibition and variation in anti-microbial peptides associated with intercourse. Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- Franz F, Edgar E, Albert-Georg L, Axel B, 2007. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39 (2), 175–191. [DOI] [PubMed] [Google Scholar]
- Garcia B, Arthur A, Patel B, Chang J, Chen D, & Lane F. (2021). A Non-Invasive Determination of LOXL1 and Fibulin-5 Levels in the Vaginal Secretions of Women with and Without Pelvic Organ Prolapse. Journal of Medical Research and Surgery, 2 (2), 10.52916/jmrs214042. 10.52916/jmrs214042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger ML, Boeck C, Koenig AM, Schury K, Waller C, Kolassa S, Karabatsiakis A, Kolassa I-T, 2019. Investigating the effects of childhood maltreatment on pro-inflammatory signaling: the influence of cortisol and DHEA on cytokine secretion ex vivo. Mental Health Prevent. 13, 176–186. 10.1016/j.mhp.2018.04.002. [DOI] [Google Scholar]
- Goral J, Karavitis J, Kovacs EJ, 2008. Exposure-dependent effects of ethanol on the innate immune system. Alcohol 42 (4), 237–247. 10.1016/j.alcohol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther A, Becker M, Göpfert J, Joos T, Schneiderhan-Marra N, 2020. Comparison of bead-based fluorescence versus planar electrochemiluminescence multiplex immunoassays for measuring cytokines in human plasma. Front. Immunol 11, 2486. 10.3389/fimmu.2020.572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman JR, Rowland DL, 1983. Affective and physiological sexual response patterns: the effects of instructions on sexually functional and dysfunctional men. J. Psychosom. Res 27 (2), 105–116. [DOI] [PubMed] [Google Scholar]
- Irish L, Kobayashi I, Delahanty DL, 2010. Long-term physical health consequences of childhood sexual abuse: a meta-analytic review. J. Pediatr. Psychol 35 (5), 450–461. 10.1093/jpepsy/jspl18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H, Lorenz T, & Sartin-Tarm A. (2021). Mechanisms of Arousal, Inflammation and Disgust (MAID). 10.17605/OSF.10/4DC9S. [DOI]
- Kinsey AC, Pomeroy WB, Martin CE, Gebhard PH, 1998. Sexual Behavior in the Human Female. Indiana University Press. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RH, 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw 82, 1–26. [Google Scholar]
- Latack JA, Moyer A, Simon VA, Davila J, 2017. Attentional bias for sexual threat among sexual victimization survivors: a meta-analytic review. Trauma Violence Abuse 18 (2), 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latack JA, Rodriguez-Seijas C, Stohl M, Blanco C, Hasin DS, Eaton NR, 2015. Transdiagnostic psychopathology mediates the relationship between childhood sexual abuse and HIV/AIDS and other sexually transmitted infections in adulthood. Compr. Psychiatry 62, 71–79. 10.1016/j.comppsych.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Lorenz TA, Harte CB, Hamilton LD, Meston CM, 2012. Evidence for a curvilinear relationship between sympathetic nervous system activation and women’s physiological sexual arousal. Psychophysiology 49 (1), 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TK, Demas GE, Heiman JR, 2017. Partnered sexual activity moderates menstrual cycle–related changes in inflammation markers in healthy women: An exploratory observational study. Fertil. Steril 107 (3), 763–773.e3. 10.1016/j.fertnstert.2016.11.010.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TK, Worthman CM, Vitzthum VJ, 2015. Links among inflammation, sexual activity and ovulationEvolutionary trade-offs and clinical implications. Evolution, Medicine, and Public Health 2015 (1), 304–324. 10.1093/emph/eov029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorzadeh N, Kazemirad N, Kazemirad Y, 2020. Human immunodeficiency: extragonadal comorbidities of infertility in women. Immun. Inflamm. Dis 8 (3), 447–457. 10.1002/iid3.327.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer CH, Tanton C, Prah P, Erens B, Sonnenberg P, Clifton S, Macdowall W, Lewis R, Field N, Datta J, Copas AJ, Phelps A, Wellings K, Johnson AM, 2013. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 382 (9907), 1781–1794. 10.1016/S0140-6736(3)62035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H, Goettlicher S, Mendling W, 2006. Stress as a cause of chronic recurrent vulvovaginal candidosis and the effectiveness of the conventional antimycotic therapy. Mycoses 49 (3), 202–209. 10.1111/j.l439-0507.2006.01235.x. [DOI] [PubMed] [Google Scholar]
- Nansel TR, Riggs MA, Yu K-F, Andrews WW, Schwebke JR, Klebanoff MA, 2006. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am. J. Obstet. Gynecol 194 (2), 381–386. 10.10l6/j.ajog.2005.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulverman CS, Meston CM, Hixon JG, 2018. Automated artifact-detection procedure for vaginal photoplethysmography. J. Sex Marital Ther 44 (6), 566–590. 10.1080/0092623X.2018.1436627.. [DOI] [PubMed] [Google Scholar]
- Rellini AH, Ing AD, Meston CM, 2011. Implicit and explicit cognitive sexual processes in survivors of childhood sexual abuse. J. Sex. Med 8 (11), 3098–3107. 10.llll/j.1743-6109.2011.02356.x. [DOI] [PubMed] [Google Scholar]
- Revelle WR (2017). psych: Procedures for personality and psychological research. [Google Scholar]
- Sadler AG, Mengeling MA, Syrop CH, Torner JC, Booth BM, 2011. Lifetime sexual assault and cervical cytologic abnormalities among military women. J. Women’s Health 20 (11), 1693–1701. 10.1089/jwh.2010.2399. [DOI] [PubMed] [Google Scholar]
- Sangtani A, Bakkum-Gamez J, Kipp B, Kerr S, Abyzov A, Voss J, Wang C, 2019. Combining methylation markers, genomic instability, and next generation sequencing as a panel for endometrial cancer detection via intravaginal tampon collection. Gynecol. Oncol 154 (1), e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidell JD, Thorpe LE, Adimora AA, Caniglia EC, Lejuez CW, Troxel AB, Khan MR, 2020. Perceived stress, sexually transmitted infection, and pelvic inflammatory disease: examination of differences in associations among black and white women. Sex. Transm. Dis 47 (9), 617–624. 10.1097/OLQ.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherpenisse M, Mollers M, Schepp RM, Meijer CJ, de Melker HE, Berbers GA, van der Klis FR, 2013. Detection of systemic and mucosal HPV-specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination. Human Vaccines & Immunotherap. 9 (2), 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short CS, Quinlan R, Bennett P, Shattock RJ, Taylor GP, 2018. Optimising the collection of female genital tract fluid for cytokine analysis in pregnant women. J. Immunol. Methods 458, 15–20. 10.1016/j.jim.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlman S, Javanbakht M, Cochran S, Hamilton AB, Shoptaw S, Gorbach PM, 2014. Self-reported STIs and sexual risk behaviors in the U.S. military: how gender influences risk. Sex. Transm. Dis 41 (6), 359–364. 10.1097/OLQ.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss JL, Marx CE, Weitlauf JC, Stechuchak KM, Straits-Tröster K, Worjoloh AW, Sherrod CB, Olsen MK, Butterfield MI, Calhoun PS, 2011. Is military sexual trauma associated with trading sex among women veterans seeking outpatient mental health care? J. Trauma Dissoc 12 (3), 290–304. 10.1080/15299732.2011.551509. [DOI] [PubMed] [Google Scholar]
- Sweet T, Polansky M, Welles SL, 2013. Mediation of HIV/STI risk by mental health disorders among persons living in the united states reporting childhood sexual abuse. JAIDS J. Acq. Immune Deficiency Syndromes 62 (1), 81–89. 10.1097/QAI.0b013e318273b0c7. [DOI] [PubMed] [Google Scholar]
- Taylor BD, Holzman CB, Fichorova RN, Tian Y, Jones NM, Fu W, Senagore PK, 2013. Inflammation biomarkers in vaginal fluid and preterm delivery. Hum. Reprod 28 (4), 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyuki K, Al-Alusi NA, Campbell JC, Murry, DeMarjion, Cimino AN, Servin AE, Stockman JK, Seedat S, 2019. Adverse childhood experiences (ACEs) are associated with forced and very early sexual initiation among Black women accessing publicly funded STD clinics in Baltimore, MD. PLOS ONE 14 (5), e0216279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchik JA, Garske JP, Probst DR, Irvin CR, 2010. Personality, sexuality, and substance use as predictors of sexual risk taking in college students. J. Sex Res 47 (5), 411–419. [DOI] [PubMed] [Google Scholar]
- Upchurch DM, Kusunoki Y, 2004. Associations between forced sex, sexual and protective practices, and sexually transmitted diseases among a national sample of adolescent girls. Women’s Health Issues 14 (3), 75–84. 10.1016/j.whi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- van Buuren S. (2018). Flexible imputation of missing data. (Second Ed. ed.)New York, NY. CRC press. [Google Scholar]
- van Buuren S, & Groothuis-Oudshoorn K. (2011, 12/12). mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software, 45(3), 1–67. 10.18637/iss.v045.i03.. [DOI] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K, Robitzsch A, Vink G, Doove L, & Jolani S. (2022). Package ‘mice’. https://cran.r-project.org/package=mice. [Google Scholar]
- Velten J, Chivers ML, Brotto LA, 2018. Does repeated testing impact concordance between genital and self-reported sexual arousal in women? Arch. Sex. Behav 47 (3), 651–660. [DOI] [PubMed] [Google Scholar]
- Velten J, Milani S, Margraf J, Brotto LA, 2021. Visual attention to sexual stimuli in women with clinical, subclinical, and normal sexual functioning: An eye-tracking study. J. Sex. Med 18 (1), 144–155. [DOI] [PubMed] [Google Scholar]
- Walsh K, Latzman NE, Latzman RD, 2014. Pathway from child sexual and physical abuse to risky sex among emerging adults: the role of trauma-related intrusions and alcohol problems. J. Adolesc. Health 54 (4), 442–448. 10.1016/j.jadohea1th.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman CS, Grimley DM, Annang L, Hillemeier MM, Chase GA, Dyer A-M, 2007. Vaginal douching and intimate partner violence: is there an association? Women’s Health Issues 17 (5), 310–315. 10.1016/j.whi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Williams C, Larsen U, McCloskey LA, 2010. The impact of childhood sexual abuse and intimate partner violence on sexually transmitted infections. Violence Vict. 25 (6), 787–798. 10.1891/0886-6708.25.6.787.. [DOI] [PubMed] [Google Scholar]
- Wu H-Y, Abdu S, Stinson D, Russell MW, 2000. Generation of female genital tract antibody responses by local or central (common) mucosal immunization. Infect. Immun 68 (10), 5539–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Elson CO, 2018. Adaptive immune education by gut microbiota antigens. Immunology 154 (1), 28–37. 10.llll/imm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzow HM, Resnick HS, Barr SC, Danielson CK, Kilpatrick DG, 2012. Receipt of post-rape medical care in a national sample of female victims. Am. J. Prev. Med 43 (2), 183–187. 10.1016/j.amepre.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


