Abstract
Introduction
Pediatric nephrology prenatal consultations for congenital anomalies of the kidney and urinary tract (CAKUT) and criteria for kidney replacement therapy initiation in neonatal end-stage kidney disease (ESKD) are not well described. We evaluated pediatric nephrology approaches to prenatal CAKUT counseling and neonatal dialysis initiation.
Methods
A 35-question Qualtrics survey was distributed via the North American Pediatric Renal Trials and Collaborative Studies email list between January and March 2021. Thirty-nine pediatric nephrology centers completed the survey.
Results
All but one responding center (n = 38) provide prenatal CAKUT consultations and neonatal dialysis, with wide variability in reported multispecialty involvement. Nearly half (47%) of centers utilize written/unwritten criteria for offering neonatal dialysis. The most common contraindications to neonatal dialysis were parental refusal (61%), contraindication to access placement by surgeons (55%), and birth weight (BW) contraindication (55%, with<1,500 g being the most common BW contraindication). Overall, 79% of centers reported caring for<5 neonates with ESKD in the past year, 61% use hemodialysis therapies prior to peritoneal dialysis in neonates requiring dialysis, and 100% transition to peritoneal dialysis by hospital discharge.
Conclusion
Many pediatric nephrology programs provide prenatal CAKUT consultations and neonatal dialysis, but with variability in practice approach. Further multicenter research regarding prenatal consultations and neonatal dialysis outcomes is necessary to further improve care delivery to this population.
Keywords: peritoneal dialysis, chronic peritoneal dialysis, chronic hemodialysis, pediatric ESKD, infants, neonates
Introduction
Congenital anomalies of the kidney and urinary tract (CAKUT) are the most common sonographically identified malformations, occurring in 1 in 500 live births and constituting 20 to 30% of all antenatally diagnosed fetal anomalies.1 In some cases, severe in utero CAKUT is managed with prenatal procedures aimed at enhancing postdelivery survival,2,3 but with minimal to no improvement shown in long-term kidney outcomes following the performance of the prenatal procedures to date. Current advances in maternal-fetal medicine and dialysis-related technology have given rise to an increasing number of decisions regarding the initiation of dialysis in neonates with end-stage kidney disease (ESKD) (defined as the initiation of dialysis<30 days of life) due to severe CAKUT.
In recent years, many pediatric nephrologists have consulted with parents/caregivers and the maternal-fetal medical teams before the birth of neonates identified with severe CAKUT to provide insight about outcomes and recommendations for postnatal patient management. However, little is known about the practices involved in the prenatal evaluation of CAKUT or the extent to which pediatric nephrology programs have standardized their indications and contraindications (both absolute and relative) regarding the initiation of dialysis in the neonate with ESKD. Kidney failure associated with the initiation of chronic dialysis occurs in approximately 0.3 neonates per 100,000 live births in North America and is also associated with a significant risk of mortality.4 Thus, decisions surrounding the initiation of dialysis can be challenging for pediatric nephrologists due to the small number of neonates receiving dialysis in any single center and the medical, surgical, and at times ethical complexity of chronic dialysis in neonates.
To help inform these issues based on input from practicing clinicians, we surveyed pediatric nephrologists across North America to (1) understand practice patterns regarding prenatal consultations for severe fetal CAKUT, (2) evaluate the use of center guidelines (including relative contraindications) for the initiation of dialysis in neonates with ESKD, and (3) understand current practice variation for initiation of dialysis in neonates with ESKD, specifically focusing on dialysis modalities and access used during neonatal dialysis initiation. We hypothesized that a substantial number of pediatric nephrology centers are participating in prenatal consults for severe fetal CAKUT in collaboration with maternal-fetal medicine and neonatology colleagues and are providing neonatal dialysis care when needed, but that there would be significant variation in center-specific practices pertaining to the initiation of dialysis in these patients.
Materials and Methods
A 35-question electronic survey (Qualtrics) was created and distributed to the email listserv of active members of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). NAPRTCS has a pediatric patient registry that has collected information regarding chronic dialysis since 1992 and chronic kidney disease (CKD) since 1994 with 91 total centers from within the United States and Canada (50 of which are considered actively participating centers which have submitted data within the past year, July 2020–June 2021). We requested that only one member from each NAPRTCS site complete the survey and asked that the center representative be either the pediatric dialysis medical director and/or the pediatric nephrologist whose work was most focused on the care of neonates and infants with CKD/ESKD. The survey was anonymous and not offered to trainees/fellows. Pediatric nephrologists were asked to describe the frequency of their participation (reported as always, sometimes, or never) in the prenatal consultations for CAKUT diagnoses, as well as the frequency of participation among various pediatric subspecialties (neonatology, urology, surgery, ethics, and palliative care). They were also queried about contraindications to neonatal dialysis and dialysis modality practice patterns in their respective centers. The survey was open for completion between January 1, 2021 and March 31, 2021. As per the common rule, there was determination that this was not human subjects research given the survey was anonymous and did not include patient-specific data or any identifiers.
Data were reported as median (interquartile range [IQR]) and frequency (percent). All analyses were performed using STATA Version 15.0 (StataCorp, College Station, TX).
Results
We received responses from 39 centers with a median of 7 (IQR 5, 9.5) pediatric nephrology, nontrainee workforce members (Doctor of Medicine/Doctor of Osteopathy, advanced practice registered nurse) per center, giving an overall response rate of 43% among all 91 total NAPRTCS centers, though a 78% response rate among the 50 actively participating centers. Thirty-one of the 39 centers (80%) had five or greater pediatric nephrology workforce members (defined as “large centers”). The centers also reported a median of 8 (IQR 4.5, 13) outpatient chronic hemodialysis (HD) patients and 8 (IQR 4, 13.5) chronic home peritoneal dialysis (PD) patients under the age of 21 years.
Thirty-eight of 39 centers offer chronic dialysis to neonates with ESKD. Most of the centers (71%) reported that only a select number of pediatric nephrology nontrainee workforce members (median 2; IQR 1.5, 3.5) provide these prenatal consultative services. Of the 38 reporting centers who acknowledged participating in pediatric nephrology prenatal consultations, the frequency of pediatric nephrology nontrainee workforce participation in consultations for all prenatal CAKUT diagnoses in a center was reported as “always” by 37% of centers (n = 14) and “sometimes” by 62% of reporting centers (n = 24). The frequency of participation by neonatology and urology in prenatal CAKUT consultations was similar to nephrology participation (neonatology always participates in 46% [n = 16], sometimes participates in 49% [n = 17], and never participates in 6% [n = 2] of prenatal CAKUT diagnoses per center; pediatric urology always participates in 34% [n = 12], sometimes participates in 63% [n = 22], and never participates in 3% [n = 1] of reporting centers, respectively [Fig. 1]). However, compared with the other subspecialties, the lowest reported frequency of participation in prenatal CAKUT consults were among palliative care, pediatric surgery, and ethics services, with “never participation” reported to occur in 31% (n = 10), 34% (n = 11), and 71% (n = 20) of reporting centers, respectively (Fig. 1).
Fig. 1.
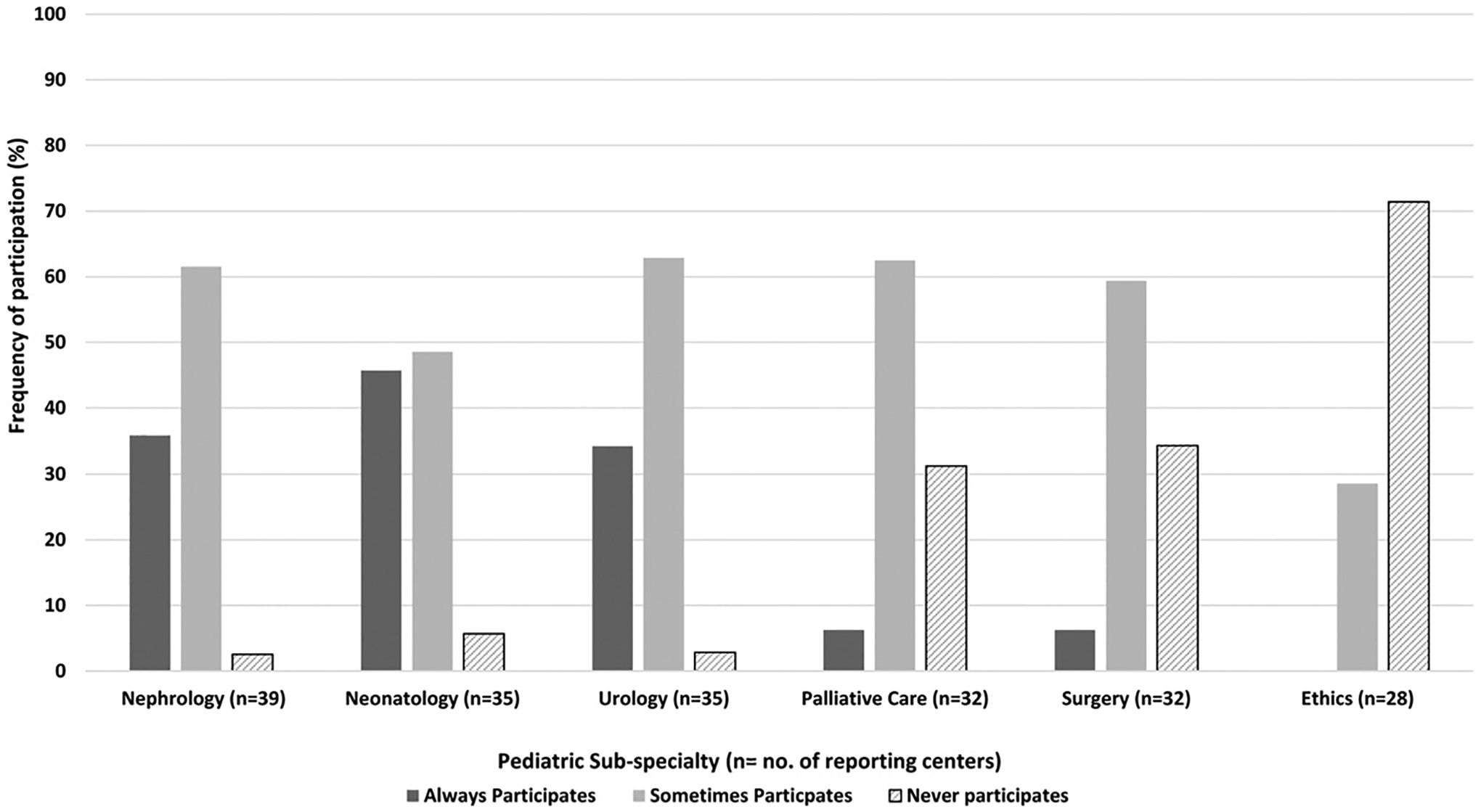
Frequency of pediatric subspecialty participation in prenatal congenital anomalies of the kidney and urinary tract (CAKUT) consultations.
Eighteen of 38 centers (47%) that perform prenatal CAKUT consultations reported the use of written or unwritten guidelines pertaining to the provision of chronic dialysis to neonates with ESKD. Four of these 18 centers (22%) reported having formal written guidelines, whereas the other 14 centers (78%) reported the use of unwritten guidelines (or “consistent approach”) among faculty members regarding the feasibility of offering neonatal dialysis. In centers that reported the use of written or unwritten guidelines, these guidelines are shared with faculty members in neonatology and pediatric surgery in 14 (78%) and 10 (63%) of reporting centers, respectively.
Reported contraindications to providing dialysis to neonates with ESKD among the 38 pediatric nephrology centers which offer neonatal dialysis are listed in Table 1.
Table 1.
Contraindications to dialysis initiation in neonates with ESKD reported among surveyed centers (n = 38 centers)
| Contraindications reported in > 50% of surveyed centers | Contraindications reported in 10–50% of surveyed centers | Contraindications reported in < 10% of surveyed centers | Contraindications reported in zero % of surveyed centers |
|---|---|---|---|
|
|
|
|
Abbreviation: ESKD, end-stage kidney disease.
The most commonly reported contraindications (reported in>50% of centers) include parental/guardian refusal to initiate dialysis (n = 26; 68%), contraindication to dialysis access placement determined by the pediatric surgeon (n = 21; 55%), and birth weight (BW) below a required minimum (n = 21, 55%), with the most common BW-related contraindication being<1,500 g (n = 11, 52%) (Fig. 2).
Fig. 2.

Reported minimum birth weight (g) category for which pediatric nephrology centers would not offer neonatal dialysis (n = 21 total centers).
Additional contraindications reported in 10 to 50% of surveyed centers included severe pulmonary disorder or respiratory disease (n = 17, 47% of centers), presence of a concomitant severe or life-threatening genetic abnormality (n = 13, 34% of centers), refractory hypotension (n = 7, 18% of centers), severe neurologic impairment (n = 7, 18% of centers), and a severe or life-threatening cardiac abnormality (n = 7, 18%). No center reported any of the following as a contraindication to dialysis initiation in a neonate with ESKD: significant colorectal anomaly requiring an ostomy, presence of a specific kidney disease (such as autosomal recessive polycystic kidney disease, or ARPKD; or an inborn error of metabolism), postnatal anuria or oliguria, or social determinants of health that would limit a family’s ability to provide maintenance home dialysis therapy (Table 1).
Queries regarding the center frequency of chronic dialysis in neonates with ESKD revealed that in 30 centers (79%), there had been fewer than five such patients initiated on chronic dialysis over the past year, whereas the eight remaining participating centers (21%) reported that 5 to 9 neonates initiated chronic dialysis over the prior year. Thirty-five centers (92%) reported that neonates with ESKD initiate dialysis in the neonatal intensive care unit setting, while three centers (8%) reported that dialysis initiation takes place in the pediatric intensive care unit. Although 100% of centers reported PD as the standard discharge dialysis modality for neonates with ESKD, 23 centers (61%) reported use of an alternative form of dialysis, such as HD, continuous renal replacement therapy, or prolonged intermittent dialysis using various machines (including the Aquadex FlexFlow ultrafiltration system) as a bridge to PD initiation (Fig. 3).5
Fig. 3.
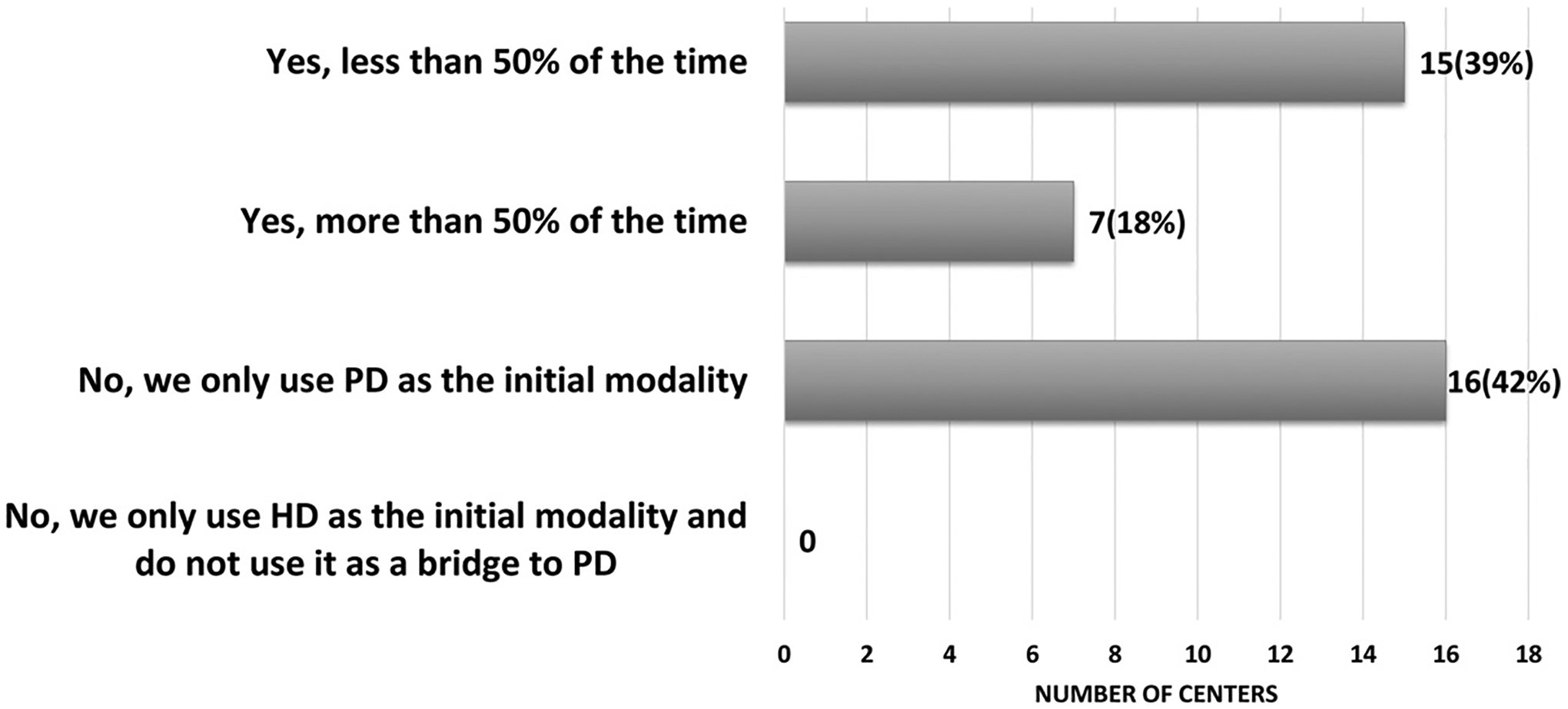
Reported use of hemodialysis/continuous kidney replacement therapy (CKRT)/prolonged intermittent renal replacement therapy (PIRRT)/modified Aquadex* as a bridge prior to the initiation of peritoneal dialysis (PD) in the neonate with end-stage kidney disease (ESKD) (n = 38 total centers).
Although only 23 centers (61%) reported the use of HD therapies in neonates with ESKD, all 38 centers that provide neonatal ESKD care responded to questions regarding HD central venous catheter capabilities for neonatal ESKD management. Nineteen centers (50%) reported that the smallest HD catheter used in their center is a 7-French vascular catheter, followed by 5 centers (13%) reporting the use of 5-French vascular catheters. Three centers (8%) reported using 4-French vascular catheters and two centers (5%) reported 3-French catheters as the smallest available HD dialysis catheter used in their centers.
Thirty-two centers (84%) reported that pediatric surgeons place all chronic PD catheters. Of the 32 centers, 10 centers (31%) indicated that only a limited number of pediatric surgeons within the pediatric surgery group at their center participate in chronic PD catheter placement in neonates with ESKD. Other services reported to place chronic PD catheters included pediatric cardiothoracic surgery (n = 1, 17%), pediatric urology (n = 2, 33%), and other nonspecified subspecialties (n = 3, 50%). Chronic HD central venous catheters were also reported as being placed primarily by pediatric surgery (n = 23, 61%) followed by interventional radiology (n = 13, 34%) and critical care (n = 2, 5%).
Discussion
In this study, we report current pediatric nephrology practice variability in the approach to severe fetal CAKUT prenatal consultation and the provision of dialysis in neonates born with ESKD. Most participating pediatric nephrology centers (97%) reported offering prenatal counseling for severe fetal CAKUT diagnoses as well as neonatal dialysis; however, less than half of those centers who perform prenatal counseling and dialysis for these neonates use written or unwritten center-specific guidelines. This study’s finding of a high frequency of pediatric nephrology prenatal consultations coupled with a lower frequency of a formal guideline or approach to treatment is consistent with our original hypothesis.
Although it can be difficult to precisely predict postnatal kidney function from prenatal studies, prior research has identified some predictive features of neonatal kidney function based upon ultrasound and fetal biochemistry.6–8 There are also established staging systems to assist maternal-fetal medicine clinicians and pediatric nephrologists with prenatal counseling and patient selection for in utero interventions. It is unclear if these studies are being used to assist with pediatric nephrology prenatal counseling and this should be explored in future studies. Uncertainties remain surrounding postnatal survival, kidney function outcomes, and long-term neonatal dialysis outcomes following implementation of maternal-fetal medicine staging systems and interventions, highlighting the need for future pediatric nephrology research.3,9 There is also limited data regarding the association of pediatric nephrology prenatal CAKUT consultations and parental perinatal decision-making (e.g., comfort care vs. dialysis initiation) or short- and long-term outcomes (including both kidney survival and overall survival). We believe this data highlights the need for future multicenter research informed by decision aid support science to provide a consistent, evidence-based patient (parent)-centered approach to prenatal fetal CAKUT consultations with families.10
Nearly all centers reported neonates with ESKD are typically discharged from their hospital on chronic PD, yet over half of the centers offering neonatal dialysis provide some form of HD as a bridge to PD initiation. This practice is in contrast to prior literature describing the neonatal ESKD population as one primarily receiving PD as the only initial dialysis modality. This issue is of critical importance for further study given the rising, more complex nature of the neonatal dialysis population (i.e., infants receiving dialysis with contraindications to PD), who, like all dialysis patients, need long-term preservation of their vasculature.11–13
One of the most common reported contraindications to initiate dialysis for neonatal ESKD was parental/guardian refusal to initiate dialysis. Interestingly, surveys of pediatric nephrologists in 1998 and 2011 reported that 30 to 40% of pediatric nephrologists did not consider parental rights to refuse neonatal dialysis acceptable.14,15 This highlights the ethical challenges that exist regarding dialysis refusal in neonatal ESKD, particularly given that over 70% of pediatric nephrologists in this survey also reported no participation by ethicists in prenatal CAKUT consultations in their centers. Given the increase in medical complexity and comorbidities of neonates being offered dialysis, there has been expanding literature highlighting the ethical challenges and issues that need to be addressed by the medical team with families whenever neonatal dialysis is a consideration.16,17 The ethics surrounding the provision or withdrawal of dialysis have been well described within both the adult and pediatric nephrology literature.15,18–20 Clinical ethicists can be particularly beneficial when medical team members have discordant recommendations or in settings where parents may have difficulty with their own ethical principles surrounding limiting or maximizing support. Studies of pediatric clinical ethics consultation services have shown that they are highly likely to produce intramedical team consensus and reduce medical team-parent dissensus.21–23 Similar to the low frequency of consultations with ethicists in fetal CAKUT prenatal counseling reported in this study, other studies report that despite many hospitals having access to ethics teams, ethical consultations are often underutilized despite a need for ethical guidance and expertise.24 Models of clinical ethics consults in other prenatal counseling of other fetal anomalies exist that can be utilized to support the engagement of ethics when needed in fetal CAKUT consultations.24
The pediatric nephrologists surveyed in this study also reported a lower frequency of participation by pediatric palliative care services for prenatal CAKUT diagnoses, with 30% reporting that palliative care services never participate in prenatal CAKUT consultations at their centers. The American Academy of Pediatrics has previously recommended that pediatric palliative care services should be an integral part of the multidisciplinary care of children with terminal or life-threatening conditions to facilitate discussions about ethical dilemmas, quality of life, and goals of care.25 Published literature provides evidence that pediatric palliative care involvement in prenatal consultations has increased by 40% over the past decade for fetal anomalies and is associated with greater access to comfort care for neonates and interested families.26,27 Though there is currently a paucity of literature regarding the role of palliative care specifically in prenatal CAKUT consultations, the increasing frequency and success with the integration of palliative care in other pediatric specialties, such as reported by pediatric cardiology for pediatric ventricular assist device patients, highlights the potential need for pediatric palliative care consultation for the care of the fetus and neonate with severe CAKUT.28,29 Future research on barriers to access and outcomes of ethics and palliative care consultations for severe fetal CAKUT is needed to support or improve engagement of these teams in the multidisciplinary prenatal CAKUT consultation model.28,29
Ultimately, this study suggests that multidisciplinary prenatal CAKUT consultations are highly variable, whereas emerging data in other pediatric subspecialty models indicates the multidisciplinary consultation approach is increasing and beneficial to clinicians and families. The goals of multidisciplinary prenatal CAKUT consultations should not necessarily be to increase use of comfort care, but instead to help clarify parental understanding and improve parent/guardian access to neonatal dialysis information in efforts to support shared decision making and advance care planning for the neonate with ESKD.30
Strengths of this data are the novel description of common pediatric nephrology practices among North American pediatric nephrology centers. Limitations include the small sample size which reduces the generalizability of this data to all North American pediatric nephrology centers and sample bias given the size of most programs that participated in this survey. Most of the centers (79%) that participated in the survey had 5 or more pediatric nephrology workforce members and by our definition would be considered a “large” pediatric nephrology program.31 While all but one surveyed center reported providing prenatal consultation and neonatal ESKD care, based upon the number of neonates receiving chronic dialysis in the U.S., it is likely therewould be a greater number of pediatric nephrology centers who are not supporting neonatal ESKD care if we were able to survey all North American pediatric nephrology centers. An additional limitation includes the fact that only centers that are engaged in NAPRTCS were included in the survey. However, NAPRTCS is a long-standing, multi-center, observational cohort study that gathers clinical data on pediatric kidney disease and dialysis patients and participants from NAPRTCS sites serve as a rich resource for questions related to pediatric kidney disease. Finally, while we acknowledge that the absence of input from neonatologists or other pediatric specialty providers might be considered a limitation, inclusion of these providers was outside the scope of the current work which was focused on evaluating and informing the scientific community about pediatric nephrologists’ prenatal consult practice patterns and indications and contraindications for neonatal dialysis. In future research, a survey designed to include neonatologists and neonatal intensive care nurse’s perspectives on fetal CAKUT and neonatal dialysis could support a comparison of specialty-based perspectives and practice patterns.
Conclusion
A variety of approaches to care are being used to support neonates with ESKD secondary to CAKUT and their families among pediatric nephrology programs. The pediatric nephrologist has the opportunity to provide an important consultative role by bridging the transition from prenatal care to postnatal life, and integrating medical and ethical concerns regarding dialysis initiation for these complex patients. Additional research is needed to evaluate the impact of the variation in pediatric nephrology prenatal consultation and practice patterns on parental decision-making and pertinent neonatal ESKD outcomes.
Funding
Dr. Warady is supported by that National Institutes of Health, through Grant U01DK66143. Dr. Sanderson is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR002490 and Grant 2015213 from the Doris Duke Charitable Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest
None declared.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
References
- 1.Talati AN, Webster CM, Vora NL. Prenatal genetic considerations of congenital anomalies of the kidney and urinary tract (CAKUT). Prenat Diagn 2019;39(09):679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nassr AA, Shazly SAM, Abdelmagied AM, et al. Effectiveness of vesicoamniotic shunt in fetuses with congenital lower urinary tract obstruction: an updated systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;49(06):696–703 [DOI] [PubMed] [Google Scholar]
- 3.Morris RK, Malin GL, Quinlan-Jones E, et al. ; Percutaneous vesicoamniotic shunting in Lower Urinary Tract Obstructioxxn (PLUTO) Collaborative Group. Percutaneous vesicoamniotic shunting versus conservative management for fetal lower urinary tract obstruction (PLUTO): a randomised trial. Lancet 2013;382(9903):1496–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey WA, Talley LI, Sehring SA, Jaskula JM, Mathias RS. Outcomes of dialysis initiated during the neonatal period for treatment of end-stage renal disease: a North American Pediatric Renal Trials and Collaborative Studies special analysis. Pediatrics 2007;119(02):e468–e473 [DOI] [PubMed] [Google Scholar]
- 5.Askenazi D, Ingram D, White S, et al. Smaller circuits for smaller patients: improving renal support therapy with Aquadex™. Pediatr Nephrol 2016;31(05):853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schürch B, Manegold-Brauer G, Schönberger H, et al. Diagnostic accuracy of an interdisciplinary tertiary center evaluation in children referred for suspected congenital anomalies of the kidney and urinary tract on fetal ultrasound - a retrospective outcome analysis. Pediatr Nephrol 2021;36(12):3885–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein J, Buffin-Meyer B, Boizard F, et al. ; BIOMAN consortium. Amniotic fluid peptides predict postnatal kidney survival in developmental kidney disease. Kidney Int 2021;99(03):737–749 [DOI] [PubMed] [Google Scholar]
- 8.Moscardi PRM, Katsoufis CP, Jahromi M, et al. Prenatal renal parenchymal area as a predictor of early end-stage renal disease in children with vesicoamniotic shunting for lower urinary tract obstruction. J Pediatr Urol 2018;14(04):320.e1–320.e6 [DOI] [PubMed] [Google Scholar]
- 9.Saccone G, D’Alessandro P, Escolino M, et al. Antenatal intervention for congenital fetal lower urinary tract obstruction (LUTO): a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2020;33(15):2664–2670 [DOI] [PubMed] [Google Scholar]
- 10.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4(04):CD001431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanderson KR, Yu Y, Dai H, Willig LK, Warady BA. Outcomes of infants receiving chronic peritoneal dialysis: an analysis of the USRDS registry. Pediatr Nephrol 2019;34(01):155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidal E, van Stralen KJ, Chesnaye NC, et al. ; ESPN/ERA-EDTA Registry. Infants requiring maintenance dialysis: outcomes of hemodialysis and peritoneal dialysis. Am J Kidney Dis 2017;69(05):617–625 [DOI] [PubMed] [Google Scholar]
- 13.Carey WA, Martz KL, Warady BA. Outcome of patients initiating chronic peritoneal dialysis during the first year of life. Pediatrics 2015;136(03):e615–e622 [DOI] [PubMed] [Google Scholar]
- 14.Geary DF. Attitudes of pediatric nephrologists to management of end-stage renal disease in infants. J Pediatr 1998;133(01):154–156 [DOI] [PubMed] [Google Scholar]
- 15.Teh JC, Frieling ML, Sienna JL, Geary DF. Attitudes of caregivers to management of end-stage renal disease in infants. Perit Dial Int 2011;31(04):459–465 [DOI] [PubMed] [Google Scholar]
- 16.Ranchin B, Plaisant F, Demède D, de Guillebon JM, Javouhey E, Bacchetta J. Review: Neonatal dialysis is technically feasible but ethical and global issues need to be addressed. Acta Paediatr 2021;110(03):781–788 [DOI] [PubMed] [Google Scholar]
- 17.Wightman AG, Freeman MA. Update on ethical issues in pediatric dialysis: has pediatric dialysis become morally obligatory? Clin J Am Soc Nephrol 2016;11(08):1456–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lantos JD, Warady BA. The evolving ethics of infant dialysis. Pediatr Nephrol 2013;28(10):1943–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunchman TE. The ethics of infant dialysis. Perit Dial Int 1996;16 (Suppl 1):S505–S508 [PubMed] [Google Scholar]
- 20.Ledermann SE, Scanes ME, Fernando ON, Duffy PG, Madden SJ, Trompeter RS. Long-term outcome of peritoneal dialysis in infants. J Pediatr 2000;136(01):24–29 [DOI] [PubMed] [Google Scholar]
- 21.Streuli JC, Staubli G, Pfändler-Poletti M, Baumann-Hölzle R, Ersch J. Five-year experience of clinical ethics consultations in a pediatric teaching hospital. Eur J Pediatr 2014;173(05):629–636 [DOI] [PubMed] [Google Scholar]
- 22.Loos S, Kemper MJ. Causes of renal oligohydramnios: impact on prenatal counseling and postnatal outcome. Pediatr Nephrol 2018;33(04):541–545 [DOI] [PubMed] [Google Scholar]
- 23.Mehler K, Gottschalk I, Burgmaier K, et al. Prenatal parental decision-making and postnatal outcome in renal oligohydramnios. Pediatr Nephrol 2018;33(04):651–659 [DOI] [PubMed] [Google Scholar]
- 24.Carter B, Brockman M, Garrett J, Knackstedt A, Lantos J. Why are there so few ethics consults in children’s hospitals? HEC Forum 2018;30(02):91–102 [DOI] [PubMed] [Google Scholar]
- 25.Bioethics C Palliative care for children. Pediatrics 2000;106(2 Pt 1):351–357 [PubMed] [Google Scholar]
- 26.Bourdens M, Tadonnet J, Hostalery L, Renesme L, Tosello B. Severe fetal abnormality and outcomes of continued pregnancies: a French multicenter retrospective study. Matern Child Health J 2017;21(10):1901–1910 [DOI] [PubMed] [Google Scholar]
- 27.Kukora S, Gollehon N, Laventhal N. Antenatal palliative care consultation: implications for decision-making and perinatal outcomes in a single-centre experience. Arch Dis Child Fetal Neonatal Ed 2017;102(01):F12–F16 [DOI] [PubMed] [Google Scholar]
- 28.Hope KD, Bhat PN, Dreyer WJ, et al. Pediatric palliative care in the heart failure, ventricular assist device and transplant populations: supporting patients, families and their clinical teams. Children (Basel) 2021;8(06):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knoll C, Kaufman B, Chen S, et al. Palliative care engagement for pediatric ventricular assist device patients: a single-center experience. ASAIO J 2020;66(08):929–932 [DOI] [PubMed] [Google Scholar]
- 30.Craig F, Henderson EM, Patel B, Murtagh FEM, Bluebond-Langner M. Palliative care for children and young people with stage 5 chronic kidney disease. Pediatr Nephrol 2022;37(01):105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akchurin OM, Kogon AJ, Kumar J, et al. Approach to growth hormone therapy in children with chronic kidney disease varies across North America: the Midwest Pediatric Nephrology Consortium report. BMC Nephrol 2017;18(01):181. [DOI] [PMC free article] [PubMed] [Google Scholar]


