Abstract
The role of polymorphonuclear neutrophils (PMNs) in the development of the specific immune response against Chlamydophila abortus (Chlamydia psittaci serotype 1) infection was studied in a pregnant mouse model involving treatment with RB6-8C5 monoclonal antibody. PMN depletion significantly affected the immune response in the liver, in which the T-lymphocyte and F4/80+ cell populations decreased, particularly the CD8+ T-cell population. A Th1-like response, characterized by high levels of gamma interferon without detectable levels of interleukin 4 (IL-4) in serum, was observed in both depleted and nondepleted mice, although an increased production of IL-10 was detected in the depleted group. Our results suggest that PMNs play a very important role in the recruitment of other leukocyte populations to the inflammatory foci but have little influence in the polarization of the immune specific response toward a Th1-like response.
Chlamydophila abortus (Chlamydia psittaci serotype 1) is a gram-negative obligate intracellular bacterium (11), highly pathogenic for pregnant small ruminants, infecting the placenta and causing abortion during the last third of gestation in ewes and goats (26). The disease is serious because of the economic losses it may cause and the potential zoonotic risk for pregnant women (15). Mouse models have been widely used to study the pathogenesis of chlamydial abortion, since the inoculation of pregnant mice with C. abortus causes late-term abortions similar to those observed in cases of natural or experimentally induced abortion in small ruminants (4, 24).
Polymorphonuclear neutrophils (PMNs) are the predominant cell type recruited into the inflammatory foci of the liver, spleen, and maternal-fetal junctions in the early stages of C. abortus infection (4). It has been reported previously that PMNs play an important role in host defense against Chlamydia trachomatis (1). Furthermore, PMNs are able to destroy chlamydiae in vitro via their phagocytic activity and the production and release of enzymes and reactive oxygen species (22). It has been claimed that PMNs can also destroy parenchymal cells infected by intracellular pathogens such as Listeria monocytogenes (7). Moreover, activated PMNs release several polypeptide mediators of inflammation and cytokines, such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin 8 (IL-8), macrophage inflammatory protein 1α (MIP-1α), and MIP-1β (6, 32), which suggests that, once granulocytes have arrived at the inflammatory site, they can promote further recruitment of neutrophils, as well as the subsequent accumulation and activation of monocytes, macrophages, and lymphocytes. Finally, PMNs can modulate the specific immune response mediated by CD4+ T cells, using their ability to produce IL-12, which encourages a Th1 response, or IL-10, which induces a Th2 response (23). The Th1 immune response is necessary in the resolution of chlamydial infection (19), although the relative importance of the T cells in this response depends on the species of the family Chlamydiaceae. In fact, CD4+ T cells are the essential cell population involved in the resolution of C. trachomatis infection (18, 27), while the CD8+ T cells assume this preponderant role in C. abortus infection (5).
In a previous study, we have shown that PMNs are an important component of the murine host defense against primary infection by C. abortus (3). In mice which had been neutrophil depleted using the RB6-8C5 monoclonal antibody (MAb) treatment (29), abortions were earlier, there was a much higher mortality rate, and the number of chlamydiae isolated from the spleen was greater than that in nondepleted mice. The placenta displayed widespread necrosis, and the liver had numerous chlamydial inclusions in the hepatocytes, while the beginning of focal hepatitis was noticeably delayed. The aim of the present study was to establish the role of PMNs in the development of the specific immune response against C. abortus infection in a pregnant mouse model. For this purpose, female Swiss OF1 mice (8 to 10 weeks old) purchased from Harlan U.K. Ltd. (Blackthorn, United Kingdom) were infected intraperitoneally at day 11 of gestation with 106 inclusion-forming units of C. abortus strain AB7 (10) in 0.2 ml of 0.1 M sterile phosphate-buffered saline (PBS), pH 7.2. A group of these mice were treated at days 0, 3, and 5 postinfection (p.i.) with 0.5 mg of RB6-8C5 MAb (a gift from R. L. Coffman, DNAX Research Institute, Palo Alto, Calif.) as described previously (3). The control infected group received rat immunoglobulin G (IgG) (Sigma, Madrid, Spain) at the same dosage and time. A group of 10 infected mice served as abortion control group for both depleted and nondepleted mice to establish the percentage and time of the abortions. Ten uninfected but depleted pregnant mice served as pregnancy control.
Five to ten mice of each infected group were killed at 3, 5, and 7 days p.i. After subsequent necropsy, samples from liver and placenta were processed for immunohistochemistry. The samples were snap-frozen in 2-methylbutane cooled in liquid nitrogen for the immunophenotypical characterization of leukocytes. Cryosections of 5 μm were immunostained by the avidin-biotin-peroxidase complex technique using MAbs against mouse leukocyte antigens obtained from rats as primary antibody. The following MAbs were purchased from Caltag Laboratories (Burlingame, Calif.): anti-CD4 (clone CT-CD4), anti-CD8 (clone CT-CD8α), antigranulocytes (clone RB6-8C5), and antimacrophages (clone F4/80). As secondary antibody, we used a biotinylated rabbit anti-rat IgG (mouse adsorbed) (Vector Laboratories, Burlingame, Calif.). After incubation with the avidin-biotin-peroxidase complex (Vector Laboratories), peroxidase activity was detected with diaminobenzidine tetrahydrochloride (Sigma) and slides were counterstained with hematoxylin. The incidence and location of CD4+ and CD8+ T cells in the diffuse cellular infiltrate were ascertained by counting the positive cells in 20 areas of 17,000 μm2 of liver and placenta (metrial gland, decidua basalis, and labyrinth) from each mouse. In the case of the focal infiltrate, the positive cells were counted in 20 foci. Since the morphology and staining pattern of F4/80 and RB6-8C5 MAbs meant that it was not possible to individually count the positive cells, their incidence was estimated as scarce, moderate, or abundant. In order to determine the presence of cytokines in response to chlamydial infection and to compare the IgG1 and IgG2a antibody levels, serum samples were collected from mice at the moment of sacrifice. The presence of IFN-γ, IL-4, and IL-10 was analyzed by commercial murine enzyme-linked immunosorbent assay kits (R & D Systems, Inc., Minneapolis, Minn.), as described in the manufacturer's instructions. Antibody levels of IgG1 and IgG2a were measured by an enzyme-linked immunosorbent assay on 96-well plates using purified chlamydial elementary bodies as antigen; rat monoclonal anti-mouse IgG1 or anti-mouse IgG2a (Caltag Laboratories) was used as the secondary antibody, and finally, alkaline phosphatase-conjugated rabbit anti-rat IgG (Caltag Laboratories) was used. Values were obtained as corrected optical densities (OD). The corrected OD was obtained by subtraction of the mean of the OD of sera from uninfected control mice. All the experiments were repeated twice. Differences between depleted and nondepleted mice at the same day p.i. were analyzed by Student's t test.
All the animals in the depleted abortion control group aborted at 3 to 5 days p.i., while 100% of the mice in the nondepleted abortion control group aborted at 7 to 8 days p.i. Immunohistochemical analysis of the placenta of depleted mice showed scarce F4/80+ cells and occasionally CD4+ and CD8+ T cells in the metrial gland (<1 cell per measured area) at day 3 p.i. The immunohistochemical analysis of the placenta of depleted mice at days 5 and 7 p.i. was not possible, since all the mice killed at these days had aborted. In the placenta of nondepleted mice, at day 3 p.i., the metrial gland and decidua basalis showed moderate leukocyte infiltration represented mainly by RB6-8C5+ cells, scarce F4/80+ cells, and occasionally CD4+ and CD8+ T cells. From day 5 p.i. onwards, there was a substantial increase of RB6-8C5+ cells associated with necrosis foci in the metrial gland and decidua basalis, reaching the labyrinth; scarce F4/80+ cells and very few CD4+ and CD8+ T cells (<1 cell per measured area) were observed. These findings support the hypothesis that the traffic of activated macrophages and T cells is abolished in the maternal placenta to allow the survival of fetal trophoblasts (20) and confirm that the inflammatory response against chlamydial infection in maternal placenta basically depends on PMNs. Uninfected depleted control mice had a normal gestation with a mean of 11.8 pups per litter.
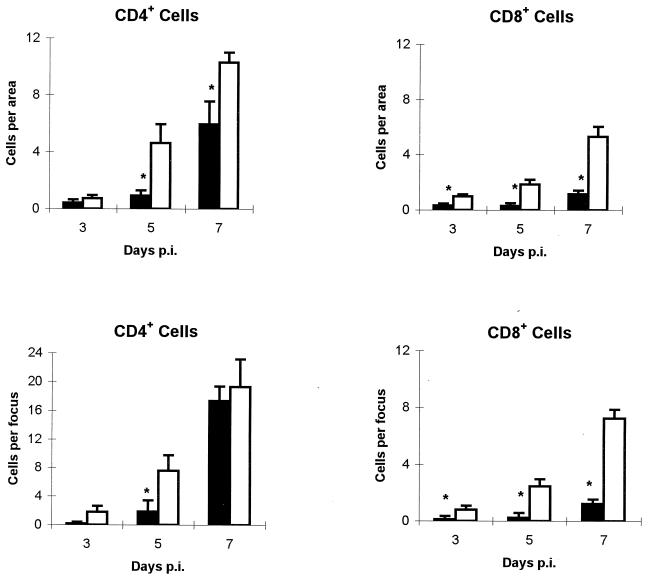
Immunohistochemical analysis of the incidence and location in the liver of lymphocytes (Fig. 1), macrophages, and PMNs showed that, in depleted mice at day 3 p.i., there were scarce and scattered F4/80+ cells and occasional CD4+ and CD8+ T cells. In the liver of nondepleted mice, a diffuse leukocyte infiltration, associated with multiple necrosis foci, was made up of abundant RB6-8C5+ cells, a moderate number of F4/80+ cells, and some CD4+ and CD8+ T cells (P < 0.01 for CD8+ T cells in relation to depleted group). In depleted mice, a slight increase of F4/80+ cells was observed at day 5 p.i., and very few CD4+ and CD8+ T cells were observed, whereas in nondepleted mice the number of CD4+ and CD8+ T cells was significantly higher (P < 0.01 for both CD4+ and CD8+ T cells). In these mice, the RB6-8C5+ cells were found in a moderate number, while F4/80+ cells were abundant. At day 7 p.i. (Fig. 2) in depleted mice, an increased number of CD4+ T cells and a moderate number of F4/80+ cells constituting foci were observed, while CD8+ T cells were very scarce. In nondepleted mice, there was a higher number of both T-lymphocyte subpopulations (P < 0.01 for CD8+ T cells) than in depleted mice; meanwhile, F4/80+ cells were abundant and RB6-8C5+ cells were moderate in number.
FIG. 1.
Effect of the depletion of neutrophils on the recruitment of T cells in the liver of C. abortus-infected mice. The number of CD4+ and CD8+ T cells was counted in both the diffuse and the focal infiltrates in the liver of depleted (black bars) and nondepleted (white bars) mice. For each mouse, the number of positive cells was counted in 20 areas of 17,000 μm2 for the diffuse infiltrate and in 20 foci for the focal infiltrate. Results are a summary of two repeated experiments with 5 to 10 mice per group and experiment. Results are expressed as means ± standard errors of the means. ∗, significant differences (P < 0.01) between depleted and nondepleted mice at the same day p.i.
FIG. 2.
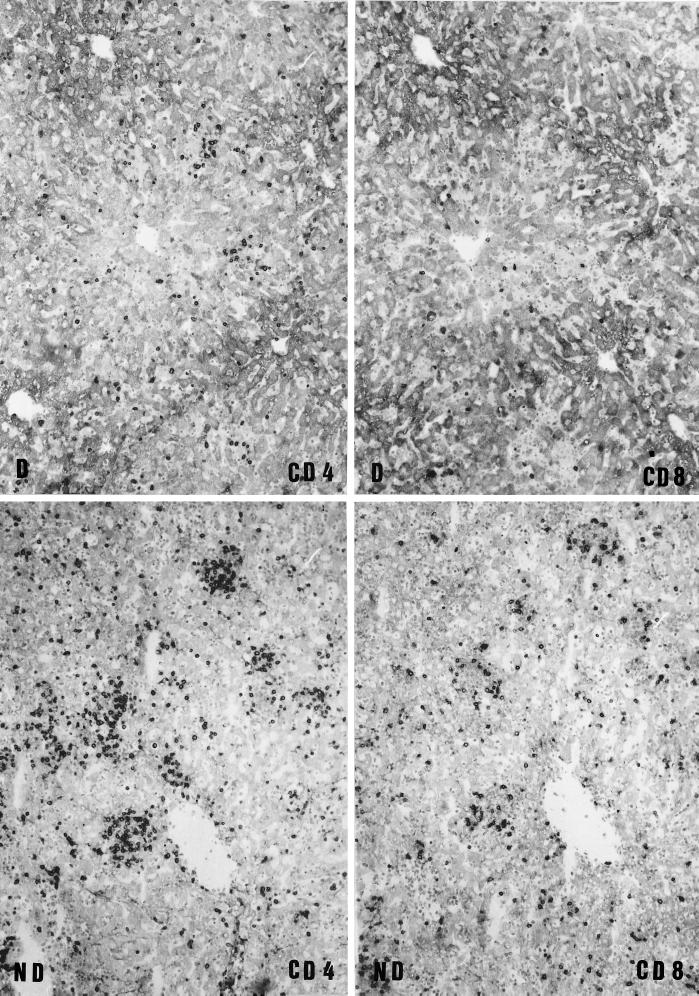
Immunophenotypical characterization of T cells in the liver of mice during C. abortus infection. Adjacent liver sections from depleted (D) and nondepleted (ND) mice at 7 days p.i. were immunostained for CD4+ and CD8+ T cells. Depleted mice show a moderate number of CD4+ T cells and very few CD8+ T cells in the diffuse and focal infiltrates. Nondepleted mice show a large number of CD4+ T cells and a moderate number of CD8+ T cells in the diffuse and focal infiltrates. Magnifications, ×100.
These results suggest that the lack of PMNs in our infection model led to a substantial decrease in the number of macrophages and T cells in the liver, this decrease being especially noticeable in the CD8+ T-cell population. This lack of recruitment of macrophages and lymphocytes in similar mouse models involving RB6-8C5 MAb-induced depletion of PMNs has been described elsewhere in response to tumors (25) and in autoimmune diseases (17). In a similar model in rats, the generation of CD8+ effector T cells was also abolished (28). The mechanism by which PMNs control the influx of other leukocyte populations may be related to the release of cytokines such as IL-8 and MIP-1α that are chemoattractants for PMNs, macrophages, and CD4+ and CD8+ T cells (2, 16). In our study, the recruitment of macrophages and CD4+ T cells was not suppressed but only delayed, probably because of factors that could act as chemoattractants for leukocytes, such as chlamydial endotoxin (14) and the production of proinflammatory cytokines by Chlamydia-infected epithelial cells (20). These factors might partially compensate for the lack of PMNs. Although activated macrophages and CD4+ and CD8+ T cells are involved in a Th1 response, the lack of CD8+ T cells is especially important in C. abortus infection, since this population has been demonstrated as essential in the resolution of infection (5).
It has been reported that RB6-8C5 MAb shows cross-reactivity with the Ly-6C molecule, an antigenic component of some subpopulations of CD8+ T cells (12). To determine the effects of RB6-8C5 MAb treatments on the CD4+ and CD8+ cell populations in our mouse model, groups of five uninfected mice were injected with 0.5 mg of RB6-8C5 or an equivalent amount of rat IgG following the same granulocyte-depletion experimental design described previously (3). Twelve hours later or at day 3, 5, or 7, the mice were killed and the spleen was removed. For the analysis of spleen cell suspensions, cells were incubated for 30 min at 4°C with 25 μl (dilution of 1/60 in PBS) of each of the MAbs labeled with fluorescein isothiocyanate (anti-CD4, clone CT-CD4, or antigranulocytes, clone RB6-8C5) or phycoerythrin (anti-CD8, clone CT-CD8α) (Caltag Laboratories). Samples were washed in PBS and analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). The results (Table 1), which were in accordance with those of Czuprynski et al. (8), indicated that RB6-8C5 MAb treatments significantly reduced (P < 0.01) the percentage of RB6-8C5+ cells in the spleen but had no effect on the percentage of CD4+ T cells and only a moderate effect on the percentage of CD8+ T cells. Furthermore, this effect was observed mainly at 5 days after treatment and not after 12 h when RB6-8C5+ cells were already depleted. This decrease, therefore, was probably due not to a direct effect of the MAb itself but to the effects that PMN depletion had on the T-cell immune response.
TABLE 1.
Effects of RB6-8C5 MAb treatments on RB6-8C5+, CD4+, and CD8+ cell populations in the spleens of uninfected mice as determined by flow cytometry
| Mouse groupa | % positive cellsf
|
||
|---|---|---|---|
| RB6-8C5+ | CD4+ | CD8+ | |
| Non-RB6-8C5-treated miceb | 4.08 ± 1.38 | 21.82 ± 4.43 | 12.06 ± 1.92 |
| RB6-8C5-treated mice | |||
| 12 hb | 0.09 ± 0.01* | 23.15 ± 2.32 | 10.01 ± 2.21 |
| 3 daysc | 0.38 ± 0.08* | 22.61 ± 4.18 | 8.83 ± 2.00 |
| 5 daysd | 0.66 ± 0.27* | 26.23 ± 2.94 | 7.51 ± 2.20 |
| 7 dayse | 0.44 ± 0.18* | 24.47 ± 4.19 | 9.44 ± 1.19 |
All the groups had five mice.
Mice were injected with 0.5 mg of RB6-8C5 MAb or rat IgG. Twelve hours later, the mice were killed and the spleens were removed for flow cytometry analysis.
Mice were treated as described above and killed at day 3.
Mice received a second treatment with 0.5 mg of RB6-8C5 MAb at day 3 and were killed at day 5 after the first treatment.
Mice received a third treatment with 0.5 mg of RB6-8C5 MAb at day 5 and were killed at day 7 after the first treatment.
Asterisks indicate significant differences (P < 0.01) in relation to the nontreated group.
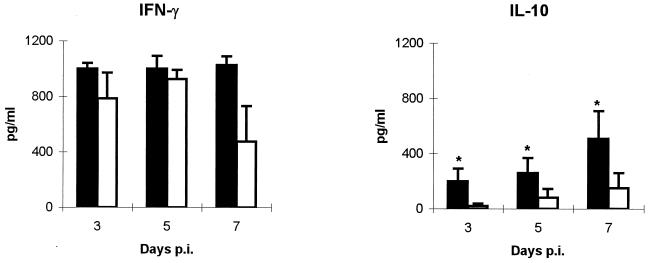
To establish whether the change in the response to C. abortus observed for the liver reflected a generalized alteration of the specific immune response, the levels of IFN-γ, IL-4, and IL-10 in the serum of mice were determined. The levels of IFN-γ were high both in depleted and in nondepleted mice (Fig. 3), although at day 7 p.i. nondepleted mice showed lower levels than their depleted counterparts. The early production of IFN-γ in C. abortus infection seems to be caused by NK cells rather than T cells, as has been reported recently for C. trachomatis infection (30), although in our model of infection the production of this cytokine at day 7 p.i. could be due to the CD4+ T cells that have already been recruited to the infection site. The IL-4 levels in serum were below the detection limit of the technique (2 pg/ml) both in depleted and in nondepleted mice. The levels of IL-10 in depleted mice were always significantly higher (P < 0.05) than in nondepleted groups (Fig. 3). There was no IFN-γ, IL-4, or IL-10 in the serum of uninfected control mice. According to our results, the establishment of a Th1 response is not PMN dependent. The increase in IL-10 levels in the serum of depleted mice could be related to a compensatory mechanism against the early increased TNF-α response caused by the uncontrolled multiplication of chlamydiae in the liver and which coincided with the increased rates of abortion and death observed previously in this model (3). In fact, treatment with TNF-α induces the release of IL-10 in humans (31), and IL-10-deficient mice infected with Toxoplasma gondii died through overproduction of TNF-α and IFN-γ due to the lack of this negative feedback (13). In addition, recent studies in our laboratory showed that CBA/J mice, highly susceptible to C. abortus infection, had high levels of TNF-α in serum after infection and subsequently a peak of IL-10, in contrast with resistant C57BL/6J mice in which no presence of TNF-α or IL-10 was observed (9). This supports the hypothesis that IL-10 could have a regulatory role in C. abortus infection, as it tries to avoid an exacerbated inflammatory cytokine response. Results obtained with cytokine analysis were supported by the study of levels of IgG1 and IgG2a in the serum of mice sacrificed at day 7 p.i., since, although the antibody levels are still low at this day p.i., a clear predominance of OD values of IgG2a in both depleted (0.358 ± 0.092) and nondepleted (0.320 ± 0.070) mice over OD values of IgG1 (0.049 ± 0.013 and 0.046 ± 0.011 for depleted and nondepleted mice, respectively) was observed.
FIG. 3.
Presence of IFN-γ and IL-10 in the serum of C. abortus-infected mice. Levels of IFN-γ and IL-10 in the sera of depleted (black bars) and nondepleted (white bars) mice were measured by enzyme-linked immunosorbent assay. Results are a summary of two repeated experiments with 5 to 10 mice per group and experiment. Results are expressed as means ± standard errors of the means. ∗, significant differences (P < 0.05) between depleted and nondepleted mice at the same day p.i.
In conclusion, our results suggest that PMNs, besides constituting a first defense against chlamydial infection to prevent the uncontrolled multiplication of the pathogen, play a very important role in the recruitment of other leukocyte populations, affecting particularly CD8+ T cells in the liver, although the establishment of the Th1 response is not dependent on PMNs.
Acknowledgments
We thank R. L. Coffman for the generous gift of RB6-8C5 hybridoma cells.
This work was supported by the Comisión Interministerial de Ciencia y Tecnología (CICYT) grant AGF97-0459. R. Montes de Oca was supported by the Universidad Autónoma del Estado de México, México, and the Consejo Nacional de Ciencia y Tecnología (CONACyT). L. Del Río was the recipient of a predoctoral grant from the Ministerio de Educación y Cultura, Spain.
REFERENCES
- 1.Barteneva N, Theodor I, Peterson E M, De la Maza L M. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–4833. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazzoni F, Cassatella M A, Rossi F, Ceska M, Dewald B, Baggiolini M. Phagocytosing neutrophils produce and release high amounts of neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991;173:771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buendía A J, Montes de Oca R, Navarro J A, Sánchez J, Cuello F, Salinas J. Role of polymorphonuclear neutrophils in a murine model of Chlamydia psittaci-induced abortion. Infect Immun. 1999;67:2110–2116. doi: 10.1128/iai.67.5.2110-2116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buendía A J, Sánchez J, Martínez M C, Cámara P, Navarro J A, Rodolakis A, Salinas J. Kinetics of infection and effects on placental cell populations in a murine model of Chlamydia psittaci-induced abortion. Infect Immun. 1998;66:2128–2134. doi: 10.1128/iai.66.5.2128-2134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzoni-Gatel D, Guilloteau L, Bernard F, Bernard S, Chardes T, Rocca A. Protection against Chlamydia psittaci in mice conferred by Lyt-2+ T cells. Immunology. 1992;77:284–288. [PMC free article] [PubMed] [Google Scholar]
- 6.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 7.Conlan J W, North R J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991;174:741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czuprynski C J, Theisen C, Brown J F. Treatment with the antigranulocyte monoclonal antibody RB6-8C5 impairs resistance of mice to gastrointestinal infection with Listeria monocytogenes. Infect Immun. 1996;64:3946–3949. doi: 10.1128/iai.64.9.3946-3949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Río L, Caro M R, Gallego M C, Cuello F, Buendía A J, Salinas J. Immune response to Chlamydia psittaci infection in two inbred mouse lines. In: Naessens J, Oberoi M S, Jand S K, editors. Proc. Fifth Int. Vet. Immunol. Symp. Ludhiana, India: FOIL Printers; 1998. p. 163. [Google Scholar]
- 10.De Sa C, Souriau A, Bernard F, Salinas J, Rodolakis A. An oligomer of the major outer membrane protein of Chlamydia psittaci is recognized by monoclonal antibodies which protect mice from abortion. Infect Immun. 1995;63:4912–4916. doi: 10.1128/iai.63.12.4912-4916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett K D, Bush R M, Andersen A A. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol. 1999;49:415–440. doi: 10.1099/00207713-49-2-415. [DOI] [PubMed] [Google Scholar]
- 12.Fleming T J, Fleming M L, Malek T R. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, Müller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 14.Ingalls R R, Rice P A, Qureshi N, Takayama K, Lin J S, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen D M. Gestational psittacosis in a Montana sheep rancher. Emerg Infect Dis. 1997;3:191–194. doi: 10.3201/eid0302.970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasama T, Strieter R M, Standiford T J, Burdick M D, Kunkel S L. Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1α. J Exp Med. 1993;178:63–72. doi: 10.1084/jem.178.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McColl S R, Staykova M A, Wozniak A, Fordham S, Bruce J, Willenborg D O. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:6421–6426. [PubMed] [Google Scholar]
- 18.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 20.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redline R W, Lu C Y. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988;140:3947–3955. [PubMed] [Google Scholar]
- 22.Register K B, Morgan P A, Wyrick P B. Interaction between Chlamydia spp. and human polymorphonuclear leukocytes in vitro. Infect Immun. 1986;52:664–670. doi: 10.1128/iai.52.3.664-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti Y, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 24.Sánchez J, Buendía A J, Salinas J, Bernabé A, Rodolakis A, Stewart I J. Murine granulated metrial gland cells are susceptible to Chlamydia psittaci infection in vivo. Infect Immun. 1996;64:3897–3900. doi: 10.1128/iai.64.9.3897-3900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoppacciaro A, Melani C, Parenza M, Mastracchio A, Bassi C, Baroni C, Parmiani G, Colombo M P. Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon γ. J Exp Med. 1993;178:151–161. doi: 10.1084/jem.178.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storz J. Intrauterine chlamydial infections and abortions. In: Storz J, editor. Chlamydia and chlamydia induced diseases. Springfield, Ill: Charles C Thomas, Publisher; 1971. pp. 171–201. [Google Scholar]
- 27.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka E, Sendo F. Abrogation of tumor-inhibitory MRC-OX8+ (CD8+) effector T-cell generation in rat by selective depletion of neutrophils in vivo using a monoclonal antibody. Int J Cancer. 1993;54:131–136. doi: 10.1002/ijc.2910540121. [DOI] [PubMed] [Google Scholar]
- 29.Tepper R I, Coffman R L, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;240:516–518. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 30.Tseng C T K, Rank R G. Role of NK cells in early host response to chlamydial genital infection. Infect Immun. 1998;66:5867–5875. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Poll T, Jansen J, Levi M, ten Cate H, ten Cate J W, van Deventer S J H. Regulation of interleukin 10 release by tumor necrosis factor in humans and chimpanzees. J Exp Med. 1995;180:1985–1988. doi: 10.1084/jem.180.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeaman G R, Collins J E, Currie J K, Guyre P M, Wira C R, Fanger M W. IFN-γ is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145–5153. [PubMed] [Google Scholar]