Abstract
Although serum amyloid P component (SAP) is known to bind many ligands, its biological function is not yet clear. Recently, it was demonstrated that SAP binds to lipopolysaccharide (LPS). In the present study, SAP was shown to bind to gram-negative bacteria expressing short types of LPS or lipo-oligosaccharide (LOS), such as Salmonella enterica serovar Copenhagen Re and Escherichia coli J5, and also to clinical isolates of Haemophilus influenzae. It was hypothesized that SAP binds to the bacteria via the lipid A part of LPS or LOS, since the htrB mutant of the nontypeable H. influenzae strain NTHi 2019-B29-3, which expresses a nonacetylated lipid A, did not bind SAP. This was in contrast to the parental strain NTHi 2019. The binding of SAP resulted in a clear inhibition of the deposition of complement component C3 on the bacteria. SAP inhibited only the activation of the classical complement pathway; the alternative route remained unaffected. In the classical route, SAP prevented the deposition of the first complement component, Clq, probably by interfering with the binding of Clq to LPS. Since antibody-mediated Clq activation was not inhibited by SAP, SAP seems to inhibit only the LPS-induced classical complement pathway activation. The SAP-induced inhibition of C3 deposition strongly diminished the complement-mediated lysis as well as the phagocytosis of the bacteria. The binding of SAP to gram-negative bacteria, therefore, might influence the pathophysiology of an infection with such bacteria.
Serum amyloid P component (SAP) is a normal constituent of blood and extravascular tissues. It consists of 10 identical, noncovalently linked 25-kDa subunits that are arranged in two parallel cyclic pentagonal structures interacting face to face. Together with C-reactive protein (CRP), SAP belongs to the pentraxin protein family. Pentraxins have remained highly conserved throughout evolution and are found in all vertebrate species. SAP and CRP have a 51% amino acid homology; however, unlike CRP, SAP is not an acute-phase reactant in humans. It is constitutively present in serum at 30 to 50 μg/ml (9). SAP owes its name to its association with all types of amyloid deposits, such as those found in Alzheimer's disease (17). SAP has been said to play a role in the complement cascade, since it can bind to several complement components. For example, it binds to the collagen-like region of Clq and thereby activates the classical pathway (4). SAP has also been reported to bind to C4b-binding protein (C4BP) (11, 24). While some researchers have found that this binding does not influence the function of C4BP (24, 25), others have shown that SAP activates the classical pathway by inhibiting the ability of C4BP to function as a cofactor for factor I in the degradation of C4b (10). Moreover, SAP is known to interact with C3bi (11, 30) and immune complexes, probably via the Fab fragment of immunoglobulin G (IgG) (4, 5). The physiological function of SAP is still unknown; however, it is believed to play a role in the binding and clearance of host- or pathogen-derived cellular debris at sites of inflammation, since it also binds DNA, chromatin, and histones (14).
Lipopolysaccharide (LPS), or endotoxin, is the major component of the outer membranes of gram-negative bacteria. For infections with gram-negative bacteria, LPS is a well-known activator of the humoral and cellular components of the host defense system. Activation of the host defense is essential to fight infection with gram-negative bacteria, although uncontrolled stimulation can also result in the serious, life-threatening symptoms of septic shock (3). LPS consists of three main structural elements: the O-specific polysaccharide chain, the core region, and the lipid A moiety. Based on the presence or absence of the O-specific chain, LPS is characterized as either of the S (smooth) or R (rough) type; the types take their names from the appearance of the bacterial colonies on agar plates. R-type LPS whose inner and outer core elements are not synthesized is called RaLPS to ReLPS, based on the length of the core oligosaccharide (23). The shortest LPS is ReLPS containing the lipid A region and two 2-keto-3-deoxyoctonic acids (13). Lipo-oligosaccharides (LOS) are the major glycolipids expressed on mucosal gram-negative bacteria, including members of the genera Neisseria, Haemophilus, Bordetella, and Branhamella. They are also expressed on some enteric bacteria such as Campylobacter jejuni and Campylobacter coli strains. LOS have lipid A structures similar to those of LPS but lack O-antigen units. Furthermore, the oligosaccharide structures are limited to approximately 10 nonrepeating saccharide units (22).
Recently, SAP was found to bind to smooth and rough types of LPS via the lipid A part (8). The BIAcore technology was used to determine the binding affinity of SAP for LPS from Salmonella enterica serovar Minnesota strain R595 (ReLPS) at 3.9 nM (7). SAP inhibited the binding of fluorescein isothiocyanate (FITC)-labeled ReLPS to human monocytes and the ReLPS-induced priming of the oxidative burst of human neutrophils in the presence of low concentrations of LPS-binding protein (8).
In 1985, Hind et al. showed that SAP bound to Klebsiella rhinoscleromatis and group A Streptococcus pyogenes because of its specificity to methyl-4,6-O-(1-carboxyethylidene)-β-d-galactopyranoside, a particular cyclic pyruvate acetal of galactose present in the cell wall (15). Since no reports that describe SAP binding to gram-negative bacteria via the LPS or LOS part of the cell wall exist, this study was conducted to discover whether SAP can bind to gram-negative bacteria via LPS or LOS and whether SAP binding has an effect on the complement cascade.
MATERIALS AND METHODS
Bacteria.
Escherichia coli O111:B4 and its galactose epimerase-deficient mutant J5 (RcLPS) and Salmonella enterica serovar Copenhagen and its mutant strains Ra, Rc, and Re were obtained from the American Type Culture Collection (Manassas, Va.) strain collection. Staphylococcus aureus 1690 was a clinical isolate. Nontypeable Haemophilus influenzae strain NTHi 2019 and its mutants NTHi 2019-B29-3 (mutant htrB), NTHi 2019-DK1 (mutant rfaD), and NTHi 2019-FK1 (mutant rfaF) were kindly provided by Michael A. Apicella (Department of Microbiology, University of Iowa College of Medicine, Iowa City). Also, clinical isolates of H. influenzae were used.
Cell isolation.
Human neutrophils were isolated from heparinized blood drawn from healthy volunteers as described by Troelstra et al. (26).
Isolation of SAP from serum.
For the isolation of SAP, fresh serum was applied to a column containing DNA cellulose (Sigma, St. Louis, Mo.). The column was then washed with Hanks' balanced salt solution (HBSS; Gibco BRL, Life Technologies, Breda, The Netherlands), and SAP was eluted with an EDTA buffer (140 mM NaCl, 0.01 M Tris-HCl, 10 mM EDTA; pH 8.0). Subsequently, the eluate was applied to a gel filtration column (Hiload 26/60, Superdex 200; Pharmacia, Uppsala, Sweden). Fractions containing SAP were then concentrated in an Amicon filter system (10-kDa cutoff) and dialyzed against phosphate-buffered saline or saline. The purity of the SAP isolate was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequent Coomassie brilliant blue staining, and the SAP concentration was determined by enzyme-linked immunosorbent assay (ELISA), as described previously (8).
SAP, factor D, and antibody depletion of serum.
SAP-depleted (SAP−) serum was obtained by incubating the serum twice for 1 h with DNA cellulose under constant agitation on ice. The serum was then collected and filtered (Spin-X tubes; Costar, Cambridge, Mass.). The depletion of SAP was checked by ELISA. Factor D-depleted (factor D−) serum was obtained by gel filtration (Superdex 200; Pharmacia) of fresh serum at 4°C in HBSS as described by Leijh et al. (20). To obtain serum depleted of both SAP and factor D (SAP−/factor D− serum), factor D− serum was depleted of SAP as described above. For the depletion of IgG antibodies (SAP−/Ab− serum), SAP− serum was applied to a protein A-Sepharose column (Pharmacia) at 4°C. Antibody depletion was checked by opsonization of the bacteria with heat-inactivated SAP− serum and SAP−/Ab− serum for 30 min. After being washed, the bacteria were incubated with phycoerythrin-labeled goat anti-human IgG γ-chain (Tago, Burlingame, Calif.) for detection of bacterium-bound antibodies. All depleted sera still displayed a classical pathway activation, as determined by a CH50 microassay (18). Sera were stored at −70°C.
Purification of anti-C3c MAb.
The anti-C3c monoclonal antibody (MAb) was obtained from the WM1 mouse hybridoma (American Type Culture Collection). Cells were grown in Iscove's modified Dulbecco's medium (Gibco) with 10% fetal calf serum and 10 μg of gentamicin/ml. After the supernatant was collected, the antibodies were purified using GammaBind Plus Sepharose (Pharmacia) according to the manufacturer's instructions. For analysis in the fluorescence-activated cell sorter, the anti-C3c MAb was labeled with FITC as described by Veldkamp et al. (27).
Binding of SAP to bacteria.
All bacteria were grown overnight in Mueller-Hinton broth (Sensititre, Westlake, Ohio) except for the H. influenzae strains, which were grown overnight in Haemophilus test medium (Sensititre). Bacteria (106) were washed with HBSS containing 0.2% human albumin (Central Laboratory for Blood Transfusion, Amsterdam, The Netherlands) and incubated with different concentrations of SAP for 30 min at 37°C. Subsequently, the bacteria were incubated with an anti-human SAP MAb (clone 5; Sigma) for 30 min on ice, followed by FITC-labeled goat anti-mouse Ig (Becton Dickinson, Mountain View, Calif.) for another 30 min on ice. After each incubation, the bacteria were washed twice with HBSS–0.2% albumin. SAP binding was analyzed on a FACScan (Becton Dickinson), using forward and side scatter parameters to gate on bacteria. The results were expressed as the fold increase over background binding.
Complement deposition on bacteria.
Overnight cultures of bacteria were washed with HBSS–0.2% albumin. Bacteria (106) were then incubated in 3% SAP− serum, 3% SAP−/Ab− serum, or 3% SAP−/factor D− serum with increasing concentrations of SAP for 30 min at 37°C. To study just the alternative-pathway activation, bacteria were incubated with complement component factors B (0.5 μg/ml), D (0.005 μg/ml), P (0.27 μg/ml), H (1.2 μg/ml), I (0.09 μg/ml), and C3 (3 μg/ml) in HBSS–0.2% albumin in the presence of increasing concentrations of SAP for 60 min. Factors B, H, I, and C3 were isolated as described by Hammer et al. (12), factor D was isolated as described by Catana and Schifferli (6), and factor P was purchased from Calbiochem-Novabiochem (La Jolla, Calif.). In some experiments using E. coli J5, 3- and 30-μg/ml concentrations of rabbit anti-E. coli J5 antibodies (28) were added together with 3% SAP−/Ab− serum and SAP. In order to detect the deposition of complement component C3 (C3 deposition), bacteria were incubated with an FITC-conjugated anti-C3c MAb for 30 min on ice. For Clq and C4 deposition, anti-Clq or anti-C4 MAbs (Quidel, San Diego, Calif.), respectively, were used, with subsequent incubation with an FITC-labeled goat anti-mouse Ig conjugate. After each incubation, the bacteria were washed twice with HBSS–0.2% albumin. Complement deposition was analyzed on a FACScan. The results were expressed as the mean fluorescence (MFl) of 10,000 bacteria.
Phagocytosis and binding of FITC-labeled bacteria to human neutrophils.
The Re mutant of S. enterica serovar Copenhagen (108 cells/ml) was labeled with FITC (50 μg/ml) in 0.1 M sodium carbonate buffer (pH 9.6) for 1 h at 37°C and washed three times with HBSS. FITC-labeled bacteria (4 × 106) were incubated with human neutrophils (4 × 105) in the presence of 0.3% SAP− serum and different concentrations of SAP for 30 min at 37°C, under continuous agitation. Bacteria, serum, and SAP were all diluted in HBSS–0.2% albumin. The association and phagocytosis of the FITC-labeled bacteria were determined by FACScan analysis, using forward and side scatter parameters to gate on neutrophils. The results were expressed as percent inhibition of phagocytosis compared to that of samples containing no SAP.
Analysis of killing and growth inhibition of bacteria.
To study the effect of SAP on the growth of bacteria, log phase-grown S. enterica serovar Copenhagen (Re) was incubated overnight in HBSS–0.2% albumin–10% Mueller-Hinton broth with different concentrations of SAP− serum in the absence of SAP or in the presence of 20 μg of SAP/ml at 37°C in 96-well flat-bottom plates (Nunc, Nalgene Nunc International, Roskilde, Denmark). The optical density at 665 nm was used to determine growth and killing of the bacteria.
RESULTS
Binding of SAP to bacteria.
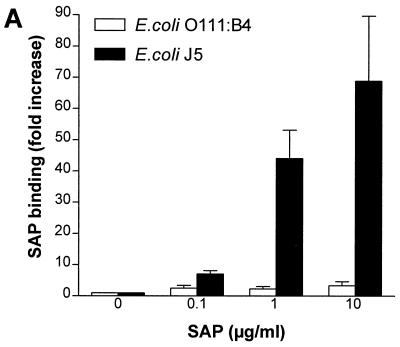
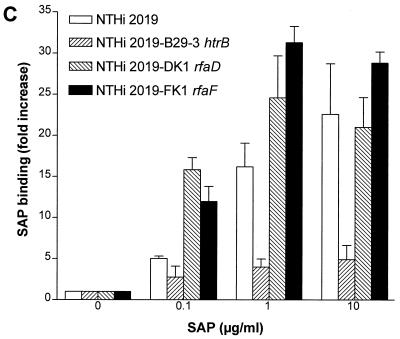
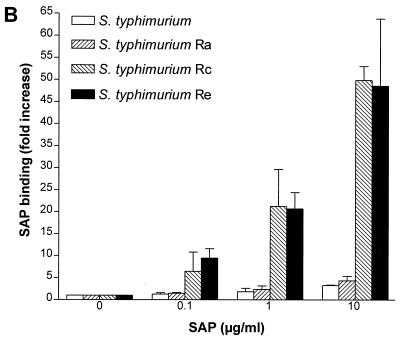
In an earlier report (8), we noted that SAP can bind LPS in solution with LPS-neutralizing effects. In the present study, we first investigated whether SAP could also bind to LPS expressed on the surfaces of gram-negative bacteria. To do this, we incubated purified SAP with E. coli O111:B4 and its Rc mutant J5 and S. enterica serovar Copenhagen and its mutants Ra, Rc, and Re. As shown in Fig. 1A and B, SAP did not bind to either E. coli O111:B4 or serovar Copenhagen but did bind extremely well to E. coli J5. Upon testing the mutants of serovar Copenhagen, we found an increased binding of SAP to the deep rough types of the bacteria. SAP did bind to both smooth strains if they were heat-killed for 10 min at 100°C (data not shown). These results indicate that SAP exerts the highest binding affinity to bacteria expressing short LPS on their outer cell membranes. Since H. influenzae strains also express short LPS or LOS on their outer membranes, we tested five clinical strains of H. influenzae for SAP binding. Two strains bound to SAP (SAP binding [fold increase] ± standard error of the mean [SEM], 16.7 ± 3.0 and 20.5 ± 5.2), while three others showed less or no binding. We also tested the nontypeable H. influenzae strain NTHi 2019. Three mutants of this strain that express short types of LOS exist due to mutations in the htrB, rfaD, and rfaF genes. htrB encodes the acyltransferase for lipid A biosynthesis, while the other two genes encode the ADP-l-glycero-d-manno-heptose-6-epimerase and heptosyltransferase II enzymes, respectively. Figure 1C shows that SAP bound to NTHi 2019 as well as to its rfaD and rfaF mutants, while the htrB mutant hardly bound to any SAP. These results indicate that SAP also binds to naturally occurring, rough-type, gram-negative bacteria. Testing S. aureus strain 1690, a clinical isolate, revealed that SAP did not bind to these gram-positive bacteria (data not shown). These experiments were all performed with isolated SAP in the presence of 1 mM calcium. If the calcium was chelated using EDTA, the binding was abolished. When serovar Copenhagen Re was incubated in normal serum containing about 30 μg of SAP/ml, the association with SAP was equivalent to the addition of 30 μg of SAP/ml in the absence of serum (0.3 and 3% serum, containing approximately 0.1 and 1 μg of SAP/ml gave 5- and 11.6-fold increases in SAP binding, respectively).
FIG. 1.
Binding of SAP to E. coli, S. enterica serovar Copenhagen and nontypeable H. influenzae 2019 strains. All bacteria were grown overnight in Mueller-Hinton broth except for the nontypeable H. influenzae 2019 strains, which were grown in Haemophilus test medium. Bacteria were washed and incubated with increasing concentrations of SAP for 30 min at 37°C. Binding of SAP was detected with an anti-human SAP MAb and goat anti-mouse FITC labeling. (A) Binding of SAP to E. coli O111:B4 and its Rc mutant E. coli J5. (B) Binding of SAP to S. enterica serovar Copenhagen (S. typhimurium) and its Ra, Rc, and Re mutants. (C) Binding of SAP to NTHi 2019 and its htrB, rfaD, and rfaF mutants (NTHi 2019 B29-3, NTHi 2019-DK1, and NTHi 2019-FK1). Data are expressed as the fold increases of SAP binding above the background.
SAP induces inhibition of the complement cascade.
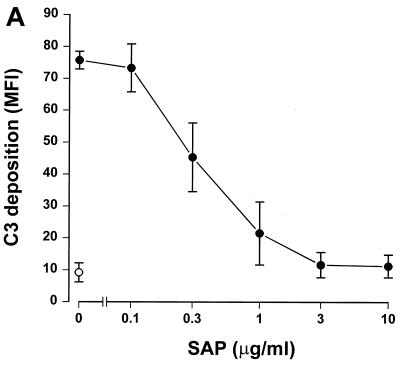
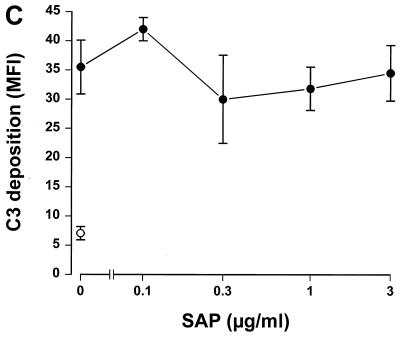
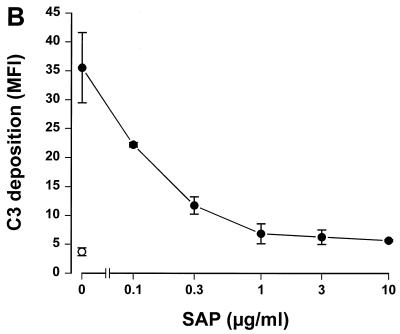
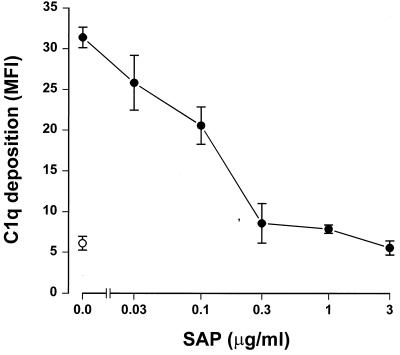
Since SAP binds to gram-negative bacteria, we tested whether this binding had any effect on complement activation. To accomplish this, serovar Copenhagen Re was incubated in 3% SAP− serum in the presence of increasing concentrations of SAP. Figure 2A shows that SAP significantly decreased C3 deposition on serovar Copenhagen Re. When 3% SAP−/factor D− serum was used in order to exclude the alternative pathway, an identical inhibition of C3 deposition was demonstrated (Fig. 2B). This strongly suggests that SAP inhibits the classical pathway of the complement cascade. To investigate the effects of SAP on the alternative pathway, different concentrations of isolated human complement components of the alternative pathway, i.e., factors B, D, P, H, I, and C3, were incubated with serovar Copenhagen Re in the presence of increasing concentrations of SAP for 60 min. Although a clear C3 deposition on the bacteria could be demonstrated, SAP does not inhibit this C3 deposition (Fig. 2C). SAP, therefore, seems to inhibit only the classical pathway of the complement system. When the bacteria were preloaded with SAP, with subsequent washing steps to remove unbound SAP, before the addition of the SAP− serum, the same inhibition of C3 deposition was observed. In order to investigate at what level of the classical pathway activation SAP exerted its effect, the deposition of Clq and C4b on serovar Copenhagen Re was also investigated. As shown in Figure 3, SAP already inhibited the classical pathway at the level of deposition of the first complement component, Clq.
FIG. 2.
SAP inhibits C3 deposition on serovar Copenhagen Re via the classical complement pathway. Serovar Copenhagen Re was grown overnight in Mueller-Hinton broth, washed, and incubated in 3% SAP− serum (A), in 3% SAP−/factor D− serum (B), and in the presence of the alternative complement component factors P, D, B, I, H, and C3 (C) with increasing concentrations of SAP for 30 (A and B) or 60 min (C). C3 deposition was determined via a subsequent incubation with FITC-labeled anti-C3c MAb. Data are MFI for three to six separate experiments ± SEM.
FIG. 3.
SAP inhibits Clq deposition on serovar Copenhagen Re. Serovar Copenhagen Re was grown overnight in Mueller-Hinton broth, washed, and incubated in 3% SAP− serum with increasing concentrations of SAP for 30 min at 37°C. Clq deposition was determined with an anti-human Clq MAb and a subsequent incubation with FITC-labeled goat anti-mouse Ig. Data are MFI for four separate experiments ± SEM.
SAP inhibits the serum-induced killing of serovar Copenhagen Re.
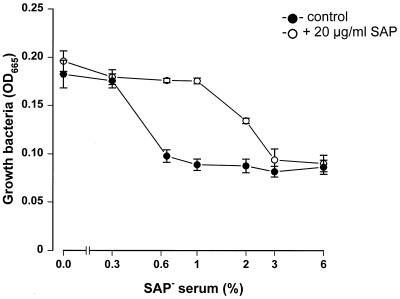
Since SAP inhibits C3 deposition on serovar Copenhagen Re, we were interested in how this would influence the survival of bacteria in the presence of serum. To study this, bacteria were incubated overnight in the presence of increasing concentrations of SAP− serum with and without the reconstitution of SAP. As shown in Fig. 4, bacteria were able to multiply in higher percentages of serum in the presence of 20 μg of SAP/ml than in the absence of SAP. A serum concentration as low as 0.6% was sufficient to lyse or arrest the growth of the bacteria in the absence of SAP, while 3% serum was needed to exert the same effect in the presence of SAP. Thus, the SAP-induced inhibition of C3 deposition on serovar Copenhagen Re corresponds to the finding that more serum is needed to lyse these bacteria.
FIG. 4.
SAP inhibits the lysis of serovar Copenhagen Re by serum. Log phase-grown serovar Copenhagen Re was incubated overnight in HBSS–0.2% albumin–10% Mueller-Hinton broth in different concentrations of SAP− serum in the absence or presence of SAP (20 μg/ml) at 37°C in 96-well flat-bottom plates. The increase in optical density at 665 nm (OD665) was used to determine bacterial growth. Data are means for three separate experiments ± SEM.
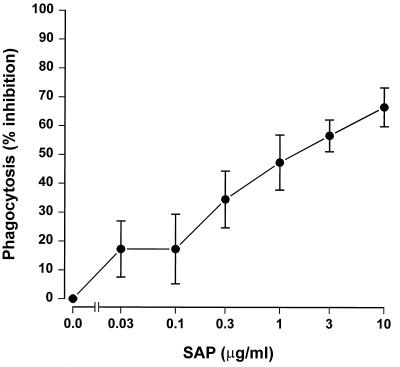
SAP inhibits phagocytosis of serovar Copenhagen Re by human neutrophils.
The inhibition of C3 deposition on bacteria was also expected to influence the subsequent phagocytosis of these bacteria. To investigate this, FITC-labeled serovar Copenhagen Re was incubated for 30 min with human neutrophils in 0.3% SAP− serum in the presence of increasing concentrations of SAP. Figure 5 shows that SAP inhibited the phagocytosis of serovar Copenhagen Re by neutrophils in a dose-dependent fashion. In the absence of SAP− serum, no inhibition or stimulation of the phagocytosis by SAP could be demonstrated. A microscopic and confocal evaluation revealed that approximately 80% of the associated bacteria in this assay were internalized. These results imply that the SAP-induced inhibition of the classical complement pathway also affects the phagocytosis of gram-negative bacteria by human neutrophils.
FIG. 5.
SAP inhibits the phagocytosis of serovar Copenhagen Re by human neutrophils. FITC-labeled serovar Copenhagen Re was incubated with human neutrophils in the presence of 0.3% SAP− serum and different concentrations of SAP for 30 min at 37°C. Association and phagocytosis of the FITC-labeled bacteria were determined by FACScan analysis, using forward and side scatter parameters to gate on neutrophils. Data are mean percentages of inhibition of phagocytosis for four separate experiments ± SEM.
SAP inhibits only the antibody-independent activation of the classical complement pathway.
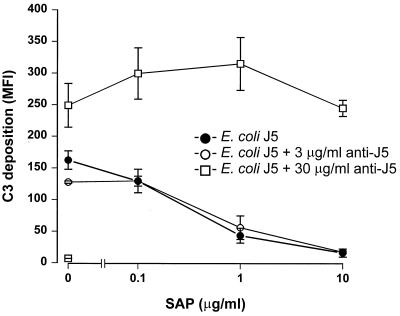
Gram-negative bacteria can, in an antibody-dependent manner and an antibody-independent manner, activate the classical complement pathway in which the antibody-independent route is activated by LPS. In all assays testing the effect of SAP on complement activation, normal human SAP− serum, which most likely contains specific bacterial antibodies, was used. To discriminate between the antibody- and LPS-mediated activation routes, all assays were repeated in the absence of antibodies. Serum was depleted of antibodies by absorption to protein A-Sepharose. Fluorescence-activated cell sorter analysis demonstrated the complete depletion of the specific antibodies: MFI of serovar Copenhagen Re opsonized with no serum, 3% untreated serum, and 3% protein A-treated serum were 5.4, 34.8, and 5.0, respectively. Testing for C3 deposition on serovar Copenhagen Re revealed that there was no difference in the amount of C3 deposited between 3% SAP− and SAP−/Ab− sera. Also the results of the lysis and phagocytosis experiments with serovar Copenhagen Re were the same with both sera. This suggests that the concentration of specific antibodies present in SAP− serum was too low to induce an antibody-mediated activation of the classical complement pathway and/or to enhance phagocytosis in our experiments. This implies that, thus far, we have demonstrated a SAP-induced inhibition of the LPS-dependent classical complement pathway activation. To investigate the effect of SAP on the antibody-dependent classical route, E. coli J5 was incubated in 3% SAP−/Ab− serum in the presence of increasing concentrations of rabbit anti-E. coli J5 antibodies. Figure 6 shows that, in the presence of 3 μg of rabbit anti-E. coli J5 antibodies/ml, C3 deposition on E. coli J5 was not different from that in the absence of antibodies. Only in the presence of 30 μg of rabbit anti-E. coli J5 antibodies/ml could an obvious increase in C3 deposition be seen, indicating that with this concentration of specific antibodies the antibody-dependent classical complement pathway is also activated; 3 μg of specific antibodies/ml was not enough to cause activation. Looking at the effect of SAP on C3 deposition, Fig. 6 shows that only in the presence of 30 μg of rabbit anti-E. coli J5 antibodies/ml was SAP no longer able to prevent activation of the classical complement pathway. These rabbit anti-E. coli J5 antibodies did not affect the binding of SAP to the bacteria (data not shown). These results suggest that SAP can only prevent the classical complement route that is initiated, in an antibody-independent manner, by LPS.
FIG. 6.
SAP inhibits the LPS-mediated, antibody-independent, activation of the classical complement pathway. E. coli J5 was grown overnight in Mueller-Hinton broth, washed, and incubated in 3% SAP−/Ab− serum in the absence of antibodies or in the presence of 3 and 30 μg of rabbit anti-E. coli J5 antibodies/ml with increasing concentrations of SAP for 30 min at 37°C. C3 deposition was determined via a subsequent incubation with FITC-labeled anti-C3c MAb. Data are MFI for three separate experiments ± SEM.
DISCUSSION
SAP is described in the literature as binding many ligands, although no clear biological function is yet known. In recent studies, we described the binding of SAP to different types of smooth and rough LPS via the lipid A part of LPS (7, 8). In the present study, we demonstrate that SAP is also able to bind to certain gram-negative bacteria. SAP showed the highest binding to gram-negative bacteria expressing rough LPS of the Rc or Re type. SAP did not bind to gram-negative bacteria expressing rough LPS of the Ra type or smooth LPS, such as, for example, E. coli O111:B4. However, if the latter bacteria were heat-killed for 10 min at 100°C, they bound to SAP very well. Therefore, it is probably the oligosaccharide chain, by means of sterical hindrance, that prevents SAP from binding to the lipid A part of LPS.
SAP also bound to several naturally occurring H. influenzae strains, including NTHi 2019. It did not, however, bind to the htrB mutant of NTHi 2019. This is a strong indication that SAP indeed binds to the lipid A part of LPS or LOS, since the htrB mutant strain lacks acyltransferase activity (22), resulting in a lipid A deficiency in O-linked fatty acids. It is probably because of this modification in lipid A that the lipid A-binding proteins have lost their ability to bind. Two other mutants of NTHi 2019, the rfaF and rfaD mutant strains, bound to SAP even better than the wild-type strain. This is probably because these mutant strains express shorter LOS on their surfaces due to mutations in the rfaF and rfaD genes. These genes encode the heptosyltransferase II and ADP-l-glycero-d-manno-heptose-6-epimerase enzymes, respectively (22), which means that these mutant strains lack, respectively, two and three of the heptose sugars normally expressed in the core region of NTHi 2019.
A study of C3 deposition on serovar Copenhagen Re, could not demonstrate any difference between untreated serum and antibody-depleted serum, even though the presence of bacterium-specific antibodies could be demonstrated in the untreated serum. This strongly suggests that the concentration of bacterium-specific antibodies was too low to induce an antibody-dependent activation of the complement system. Therefore, in our experiments, only the LPS-mediated activation of the complement system played a role in C3 deposition on the bacteria. Also when C3 deposition on E. coli J5 was studied, it was demonstrated that only higher concentrations of specific anti-E. coli J5 antibodies activated the antibody-dependent complement route. It seems strange that even the phagocytosis of C3-coated serovar Copenhagen Re by human neutrophils was not affected when bacterium-specific antibodies were precleared from the serum, as internalization of bacteria is most efficient through opsonization with both complement and antibodies. This suggests that, also in these experiments, the concentration of bacterium-specific antibodies probably was too low to enhance phagocytosis. Its known from the literature that bacterium-specific antibodies are not obligatory to induce the phagocytosis of nonencapsulated gram-negative bacteria, as LPS can induce the second signal necessary to trigger neutrophils for internalization of bacteria (16).
SAP showed a clear inhibition of C3 deposition on serovar Copenhagen Re. It seems as if only the classical complement pathway is involved in this inhibition, since SAP also inhibits Clq deposition on the bacteria. Moreover, SAP did not have any effect on the alternative complement activation pathway. SAP has been described as binding to Clq and C4BP (4, 10, 11, 24). In both cases, it has been claimed that the binding of SAP activates the classical complement pathway. Since, in the present study, SAP clearly inhibited the classical pathway instead of activating it, the binding of SAP to Clq or C4BP does not seem to be the mechanism of action displayed by SAP. It is more likely that SAP inhibits the classical pathway due to a competition between SAP and Clq for binding to LPS. It has been shown by Betz et al. that LPS expressed on the cell wall of E. coli J5, in the absence of specific antibodies, acts as a nonimmune activator of the classical pathway via direct binding to Clq (1, 2). Since SAP can interact with high affinity especially with rough types of LPS (7, 8), we hypothesize that SAP, via binding to LPS on the bacteria, subsequently prevents the interaction of Clq with LPS. This would explain the inhibition of both C3 and Clq deposition by SAP. This would also predict that the antibody-mediated classical complement pathway activation would not be affected by SAP. Indeed, using E. coli J5, we could demonstrate that the addition of rabbit anti-J5 antibodies to E. coli J5 abolished the SAP-induced inhibition of C3 deposition on the bacteria. However, this could only be demonstrated when the concentration of rabbit anti-J5 antibodies was high enough to induce an antibody-dependent activation of the classical complement pathway. Serum supplemented with a low concentration of rabbit anti-J5 antibodies did not show a higher C3 deposition on E. coli J5 than serum depleted of antibodies, which suggests that, in this situation, only LPS-mediated C3 deposition is active. The fact that SAP, in the presence of these low concentrations of rabbit anti-J5 antibodies, continued to inhibit C3 deposition on E. coli J5, strongly suggests that SAP inhibits C3 deposition only when the classical complement pathway is activated by direct Clq binding to LPS, not in the case of antibody-mediated Clq activation.
It has been shown that SAP can interact with phagocytes via specific receptors (19, 29). Furthermore, the ability of substrate-bound SAP to activate C3b and C3bi receptors of monocytes has been described (29). Since we demonstrated an interaction between SAP and gram-negative bacteria, SAP might play a role as an opsonin, potentiating phagocytosis of C3- or SAP-coated pathogens. However, our experiments clearly demonstrated that SAP inhibited the phagocytosis of complement-coated bacteria by neutrophils. Moreover, in the absence of complement, SAP did not potentiate phagocytosis. Thus, SAP is not opsonic. SAP also inhibited serum-induced lysis, demonstrating that the inhibition of C3 deposition on bacteria was also reflected in subsequent complement-mediated effects.
Since we also showed that SAP binds to naturally occurring gram-negative bacteria, such as H. influenzae, it may influence the pathophysiology of infections by these bacteria. In primary infections, especially in infants and young children, these observed anti-inflammatory properties of SAP can have major implications. SAP may serve as a down-modulator of bacterially driven inflammatory responses while leaving host-driven (antibody-mediated) responses intact, thereby fine tuning and balancing the inflammatory response in infections with gram-negative bacteria. SAP is constitutively expressed in plasma at a level of about 30 to 50 μg/ml. Neonates have levels of about 4 μg/ml in cord sera, but these levels rise rapidly during the first weeks of life to reach the lower level of the adult range (21). In sepsis due to gram-negative bacteria, a two- or threefold increase in the concentration of SAP in plasma can occur (9). In this paper, we showed that these levels of SAP in vivo would be sufficient for a possible role in the pathophysiology of infections with gram-negative bacteria.
REFERENCES
- 1.Betz S J, Isliker H. Antibody-independent interactions between Escherichia coli J5 and human complement components. J Immunol. 1981;127:1748–1754. [PubMed] [Google Scholar]
- 2.Betz S J, Page N, Estrade C, Isliker H. The effect of specific antibody on antibody-independent interactions between E. coli J5 and human complement. J Immunol. 1982;128:707–711. [PubMed] [Google Scholar]
- 3.Bone R C. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 4.Bristow C L, Boackle R J. Evidence for the binding of human serum amyloid P component to Clq and Fab. Mol Immunol. 1986;23:1045–1052. doi: 10.1016/0161-5890(86)90003-9. [DOI] [PubMed] [Google Scholar]
- 5.Brown M R, Anderson B E. Receptor-ligand interactions between serum amyloid P component and model soluble immune complexes. J Immunol. 1993;151:2087–2095. [PubMed] [Google Scholar]
- 6.Catana E, Schifferli J A. Purification of human complement factor D from the peritoneal fluid of patients on chronic ambulatory peritoneal dialysis. J Immunol Methods. 1991;138:265–271. doi: 10.1016/0022-1759(91)90175-f. [DOI] [PubMed] [Google Scholar]
- 7.de Haas C J C, Haas P-J, van Kessel K P M, van Strijp J A G. Affinities of different proteins and peptides for lipopolysaccharide (LPS) as determined by biosensor technology. Biochem Biophys Res Commun. 1998;252:492–496. doi: 10.1006/bbrc.1998.9675. [DOI] [PubMed] [Google Scholar]
- 8.de Haas C J C, van der Tol M E, van Kessel K P M, Verhoef J, van Strijp J A G. A synthetic lipopolysaccharide (LPS)-binding peptide based on amino acids 27–39 of serum amyloid P component inhibits LPS-induced responses in human blood. J Immunol. 1998;161:3607–3615. [PubMed] [Google Scholar]
- 9.Emsley J, White H E, O'Hara B P, Oliva G, Srinivasan N, Tickle I J, Blundell T L, Pepys M B, Wood S P. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 10.Garcia de Frutos P, Dahlback B. Interaction between serum amyloid P component and C4b-binding protein associated with inhibition of factor I-mediated C4b degradation. J Immunol. 1994;152:2430–2437. [PubMed] [Google Scholar]
- 11.Garcia de Frutos P, Hardig Y, Dahlback B. Serum amyloid P component binding to C4b-binding protein. J Biol Chem. 1995;270:26950–26955. doi: 10.1074/jbc.270.45.26950. [DOI] [PubMed] [Google Scholar]
- 12.Hammer C H, Wirtz G H, Renfer L, Gresham H D, Tack B F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981;256:3995–4006. [PubMed] [Google Scholar]
- 13.Helander I M, Lindner B, Brade H, Altmann K, Lindberg A A, Rietschel E T, Zahringer U. Chemical structure of the lipopolysaccharide of Haemophilus influenzae strain I-69 Rd−/b+. Description of a novel deep-rough chemotype. Eur J Biochem. 1988;177:483–492. doi: 10.1111/j.1432-1033.1988.tb14398.x. [DOI] [PubMed] [Google Scholar]
- 14.Hicks P S, Saunero Nava L, Du Clos T W, Mold C. Serum amyloid P component binds to histones and activates the classical complement pathway. J Immunol. 1992;149:3689–3694. [PubMed] [Google Scholar]
- 15.Hind C R, Collins P M, Baltz M L, Pepys M B. Human serum amyloid P component, a circulating lectin with specificity for the cyclic 4,6-pyruvate acetal of galactose. Interactions with various bacteria. Biochem J. 1985;225:107–111. doi: 10.1042/bj2250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz M A. The roles of the Fc and C3 receptors in the phagocytosis and killing of bacteria by human phagocytes. J Reticuloendothel Soc. 1980;28(Suppl.):17s–26s. [PubMed] [Google Scholar]
- 17.Kalaria R N, Galloway P G, Perry G. Widespread serum amyloid P immunoreactivity in cortical amyloid deposits and the neurofibrillary pathology of Alzheimer's disease and other degenerative disorders. Neuropathol Appl Neurobiol. 1991;17:189–201. doi: 10.1111/j.1365-2990.1991.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 18.Klerx J P A M, Beukelman C J, Van Dijk H, Willers J M N. Microassay for colorimetric estimation of complement activity in guinea pig, human and mouse serum. J Immunol Methods. 1983;63:215–220. doi: 10.1016/0022-1759(83)90425-8. [DOI] [PubMed] [Google Scholar]
- 19.Landsmann P, Rosen O, Pontet M, Pras M, Levartowsky D, Shephard E G, Fridkin M. Binding of human serum amyloid P component (hSAP) to human neutrophils. Eur J Biochem. 1994;223:805–811. doi: 10.1111/j.1432-1033.1994.tb19056.x. [DOI] [PubMed] [Google Scholar]
- 20.Leijh P C J, van den Barselaar M T, van Zwet T L, Daha M R, van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Investig. 1979;63:772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepys M B, Dash A C, Markham R E, Thomas H C, Williams B D, Petrie A. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin Exp Immunol. 1978;32:119–124. [PMC free article] [PubMed] [Google Scholar]
- 22.Preston A, Mandrell R E, Gibson B W, Apicella M A. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit Rev Microbiol. 1996;22:139–180. doi: 10.3109/10408419609106458. [DOI] [PubMed] [Google Scholar]
- 23.Raetz C R H. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 24.Schwalbe R A, Dahlback B, Nelsestuen G L. Independent association of serum amyloid P component, protein S, and complement C4b with complement C4b-binding protein and subsequent association of the complex with membranes. J Biol Chem. 1990;265:21749–21757. [PubMed] [Google Scholar]
- 25.Sorensen I J, Nielsen E H, Andersen O, Danielsen B, Svehag S E. Binding of complement proteins Clq and C4bp to serum amyloid P component (SAP) in solid contra liquid phase. Scand J Immunol. 1996;44:401–407. doi: 10.1046/j.1365-3083.1996.d01-326.x. [DOI] [PubMed] [Google Scholar]
- 26.Troelstra A, Giepmans B N G, van Kessel K P M, Lichenstein H S, Verhoef J, van Strijp J A G. Dual effects of soluble CD14 on LPS priming of neutrophils. J Leukoc Biol. 1997;61:173–178. doi: 10.1002/jlb.61.2.173. [DOI] [PubMed] [Google Scholar]
- 27.Veldkamp K E, van Kessel K P M, Verhoef J, van Strijp J A G. Staphylococcal culture supernates stimulate human phagocytes. Inflammation. 1997;21:541–551. doi: 10.1023/a:1027315814817. [DOI] [PubMed] [Google Scholar]
- 28.Vreede R W, Marcelis J H, Verhoef J. Antibodies raised against rough mutants of Escherichia coli and Salmonella strains are opsonic only in the presence of complement. Infect Immun. 1986;52:892–896. doi: 10.1128/iai.52.3.892-896.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright S D, Craigmyle L S, Silverstein S C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983;158:1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying S C, Gewurz A T, Jiang H, Gewurz H. Human serum amyloid P component oligomers bind and activate the classical complement pathway via residues 14–26 and 76–92 of the A chain collagen-like region of Clq. J Immunol. 1993;150:169–176. [PubMed] [Google Scholar]