Abstract
Background
Fecal microbiota transplantation (FMT) has been well described in the treatment of pediatric diseases; however, the latest updates regarding its use in children are unclear and the concepts involved need to be revisited.
Data sources
We performed advanced searches in the MEDLINE, EMBASE, and Cochrane databases using the keywords “Fecal microbiota transplantation OR Fecal microbiota transfer” in the [Title/Abstract] to identify relevant articles published in English within the last five years. To identify additional studies, reference lists of review articles and included studies were manually searched. Retrieved manuscripts (case reports, reviews, and abstracts) were assessed by the authors.
Results
Among the articles, studies were based on the mechanism (n = 28), sample preparation (n = 9), delivery approaches (n = 23), safety (n = 26), and indications (n = 67), including Clostridium difficile infection (CDI) and recurrent C. difficile infection (rCDI; n = 21), non-alcoholic fatty liver disease (NAFLD; n = 10), irritable bowel syndrome (IBS; n = 5), inflammatory bowel disease (IBD; n = 15), diabetes (n = 5), functional constipation (FC; n = 4), and autism spectrum disorder (ASD; n = 7).
Conclusions
Concepts of FMT in pediatric diseases have been updated with respect to underlying mechanisms, methodology, indications, and safety. Evidence-based clinical trials for the use of FMT in pediatric diseases should be introduced to resolve the challenges of dosage, duration, initiation, and the end point of treatment.
Keywords: Autism spectrum disorder, Children, Clostridium difficile infection, Fecal microbiota transplantation, Functional constipation, Safety
Introduction
Fecal microbiota transplantation (FMT) is a method that transfers stool from a healthy donor to a recipient to restore the intestinal microbiota environment and achieve a therapeutic benefit. FMT was first recorded in the Jin Dynasty in ancient China. A Chinese physician, Hong Ge, elaborated the effect of stool by mouth on patients with food poisoning or severe diarrhea. In the “Compendium of Materia Medica,” written by Shi-Zhen Li in the Ming Dynasty, over 20 FMT methods were documented for treating gastrointestinal diseases such as food poisoning, diarrhea, fever, vomiting, constipation, and abdominal pain [1]. FMT was also applied in veterinary medicine in Europe in the sixteenth century. Additional therapeutic use of human excretions was described in Europe in the eighteenth and nineteenth centuries and in World War II, during which gut bacteria were administered to German soldiers suffering from dysentery in the North African campaign [2]. More scientifically, in 1958, Eismann successfully utilized fecal transplantation via enemas in four patients for the treatment of severe pseudomembranous colitis [3]. Three of the four patients recovered and were discharged from the hospital after several days, while the fourth patient died from non-intestinal-associated diarrhea. Taken together, these results suggested the clinical value of FMT [3].
In early 2011, FMT was proposed to treat gastrointestinal diseases[4]. Physicians called for the use of FMT by colonoscopy, gastroscopy, and gastroduodenal catheterization for Clostridium difficile infection (CDI) instead of surgery in an effort to reduce deaths. Since that time, the number of studies focusing on FMT has increased rapidly. In 2012, Khorouts et al. carried out the first study using standard cryopreserved bacteria [5]. In 2013, Nood et al. reported that FMT was successful in the treatment of a recurrent C. difficile infection (rCDI) in a randomized controlled trial at the University of Amsterdam [6]. At the same time, the Food and Drug Administration (FDA) approved the use of FMT in humans [7]. In the pediatric population, the first use of FMT can be traced to Massachusetts General Hospital in the United States in 2010. Russell et al. reported a two-year-old child with rCDI whose symptoms resolved completely 36 hours after FMT administration and no recurrences or adverse events (AEs) occurred during the six-month follow-up period [8]. Although the enormous potential of FMT is apparent, FMT-related AEs have been identified in the published literature [9–13]. Therefore, the acceptance, use, and safety of FMT are still under investigation.
Mechanism underlying fecal microbiota transplantation
The goal of FMT is to re-establish the intestinal flora by normalizing the amount and activity of immune and inflammatory responses, neurotransmitters and vasoactive substances, and energy metabolism [14]. The diversity of the microbiota prevents the colonization and overgrowth of pathogens when homeostasis is achieved in the gastrointestinal tract. FMT can make the composition of the gut microbiota similar to that of the donor so that the proportion and diversity of beneficial bacteria is balanced [15]. FMT can reduce intestinal permeability and maintain the integrity of the epithelial barrier by increasing the production of short-chain fatty acids, thereby reducing the severity of the disease [14, 16, 17]. Moreover, gut microbiota can activate the humoral immune response and induce the synthesis of immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) through the Toll-like receptor (TLR) pathway, thus protecting the intestinal mucosa [14]. FMT inhibits the secretion of proinflammatory cytokines and promotes T helper 1 (Th1) cell differentiation, T cell activity, leukocyte adhesion, and immune-stimulating factors [7]. FMT also reduces intestinal pH and increases the adhesion of bacteria to H2O2, inhibiting the transport of pathogens [18]. All these findings serve as the presumed mechanism underlying FMT effectiveness [14–18].
Indications
Advanced searches in the MEDLINE, Embase, Cochrane Library, and Cochrane IBD Group Specialized Register databases with the terms “Fecal Microbiota Transplantation OR Fecal Microbiota Transfer” in the [Title/Abstract] field were performed. Based on the research strategy, a bibliometric analysis was performed on the use of FMT in children in the last five years, and 67 articles reported the indications of FMT in children (Fig. 1). The acquired literature included Clostridium difficile infection (CDI) and recurrent Clostridium difficile infection (rCDI; n = 21), non-alcoholic fatty liver disease (NAFLD; n = 10), irritable bowel syndrome (IBS; n = 5), inflammatory bowel disease (IBD; n = 15), diabetes (n = 5), functional constipation (FC; n = 4), and autism spectrum disorder (ASD; n = 7). The references, study design, interventions, and results of the indicated diseases for pediatric FMT were retrieved and are summarized in Table 1.
Fig. 1.
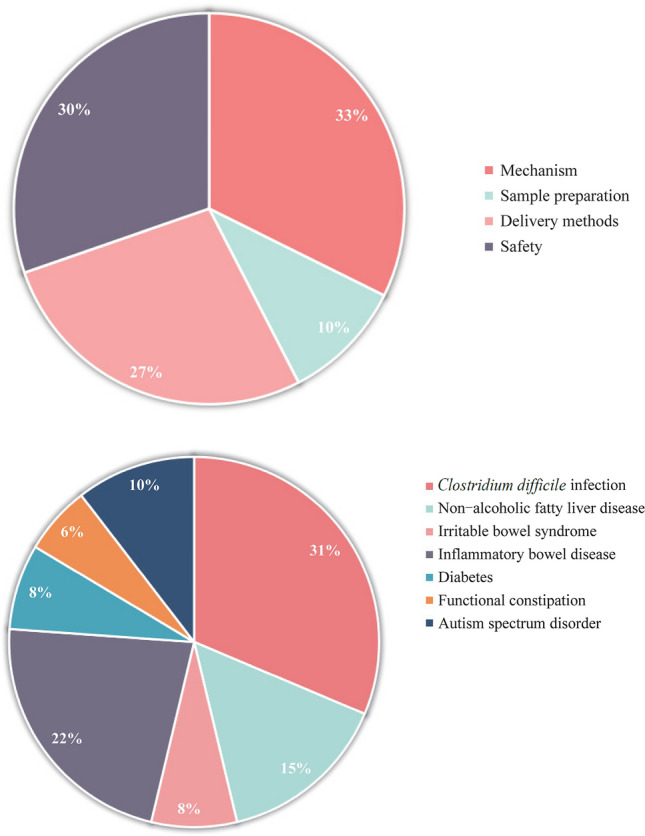
Literature related to fecal microbiota transplantation (FMT) includes indications, mechanism, donor exclusion, sample preparation, and delivery methods
Table 1.
Current potential indications for pediatric FMT
| Diseases | The first author | Year | Identifier (PMID) |
Design | Intervention | Numbers | References |
|---|---|---|---|---|---|---|---|
| CDI | Khoruts A | 2010 | 20048681 | Case report | Colonoscopy | 1 | 9 |
| Hamilton MJ | 2012 | 22290405 | Case control study | Colonoscopy | 43 | 5 | |
| Van Nood E | 2013 | 23323867 | Case control study | Nasoduodenal tube | 32 | 6 | |
| Wang J | 2015 | 25798243 | Case report | Nasal jejunal | 1 | 21 | |
| Kronman MP | 2015 | 25162365 | Case report | Nasogastric tube | 10 | 10 | |
| Walia R | 2014 | 25162365 | Case report | Colonoscopy | 2 | 22 | |
| Kahn SA | 2012 | 23211865 | Case report | Nasogastric tube | 1 | 12 | |
| Russell G | 2010 | 20547640 | Case report | Nasogastric tube | 1 | 8 | |
| Hourigan SK | 2019 | 31660343 | Case report | Colonoscopy | 9 | 23 | |
| IBD | Karolewska-Bochenek K | 2018 | 29151253 | Case report | Nasoduodenal tube | 10 | 24 |
| Kunde S | 2013 | 23542823 | Case report | Enema | 10 | 25 | |
| Goyal A | 2018 | 29361092 | A prospective trial | Endoscopy | 20 | 28 | |
| Cho S | 2019 | 30320666 | A retrospective trial | Colonoscopy | 8 | 29 | |
| Moutinho BD | 2019 | 30632438 | Case report | Colonoscopy | 1 | 30 | |
| Shimizu H | 2016 | 27324973 | Case report | Colonoscopy | 1 | 31 | |
| Hourigan SK | 2015 | 26198180 | Case report | Colonoscopy | 8 | 27 | |
| IBS | Johnsen PH | 2018 | 29100842 | RCT | Colonoscopy | 90 | 33 |
| FC | Tian H | 2016 | 26751143 | A pilot study | Nasojejunal tube | 24 | 35 |
| NAFLD | Philips CA | 2017 | 27816755 | A pilot study | Nasoduodenal tube | 8 | 43 |
| ASD | Kang DW | 2017 | 28122648 | An open-label study | Oral and colonoscopy | 18 | 45 |
| Li N | 2021 | 34737978 | An open-label study | Capsule and colonoscopy | 56 | 46 | |
| Chen Y | 2022 | 35105621 | RCT | Capsule | 318 | 47 | |
| Diabetes | Leiva-Gea I | 2018 | 30224347 | Case-control study | Oral | 43 | 52 |
| Solito A | 2021 | 34229463 | RCT | Oral | 101 | 56 |
FMT fecal microbiota transplantation, CDI Clostridium difficile infection, IBD inflammatory bowel disease, IBS irritable bowel syndrome, FC functional constipation, NAFLD non-alcoholic fatty liver disease, ASD autism spectrum disorder
Established indications
Clostridium difficile infection
Clostridium difficile infection (CDI) and recurrent Clostridium difficile infection (rCDI) are considered the most suitable indications for pediatric FMT [19]. The incidence of rCDIs has been reported to be as high as 90%, some of which are appropriate for traditional therapy [5, 6, 9, 20]. The youngest reported child receiving FMT for CDI was a 13-month-old infant [21]; several other reports involved children over three years of age [10, 12, 22]. These cases were successful, and FMT for CDI has been associated with improved growth in young children [22]. Russell reported the first case of a two-year-old child with rCDI for whom the donor was transported through a nasogastric tube to the child’s small intestine in 2010 [8]. The CDI symptoms resolved completely 36 hours after FMT administration, and there were no recurrences or adverse reactions during six months of follow-up [8]. Suchitra selected nine pairs of donor receptors with an average age of 10 years in 2019 to examine the efficacy of FMT in pediatric CDIs [23]. Three days after FMT treatment, CDI-related symptoms of all recipients were alleviated, and no recurrences occurred. During the follow-up period, one patient had long-term C. difficile-negative diarrhea and intermittent incontinence, which was mild and different in nature compared with CDI symptoms before FMT [23]. Furthermore, a multicenter retrospective cohort study was also conducted on the largest sample size of CDI trials in children [35]. Of the 335 children, 271 (80.9%) were cured by the traditional therapeutic regimen. In the remaining 64 children with rCDIs, 19 (53.1%) were cured after FMT treatment, reaching an overall success rate of 88.6%.
Inflammatory bowel disease
The efficacy and safety of FMT in pediatric IBD has been confirmed in several studies [24–27]. Katarzyna assessed the effectiveness of a two-week FMT course in 10 children (10–17 years of age) with moderate-to-severe IBD by freshly prepared FMT via a nasoduodenal tube and found that a short, intensive course of FMT has a beneficial effect on ulcerative colitis (UC) and Crohn’s disease (CD) [24]. Goyal et al. reported 21 patients with IBD refractory to medical therapy who underwent a single FMT by upper and lower endoscopy with a median age of 12 years; 57% and 28% demonstrated clinical responses one and six months after FMT administration. Two patients with CDI were in full remission at six months [28]. Similarly, in 2019, Cho reported a 75% rate response in eight patients with IBD three months after FMT [29]; however, contrary results have also been reported. In a case report of a 17-year-old male with refractory UC, clinical improvement lasted for only one month before symptoms recurred. A second implementation of FMT also led to no improvements [30]. In another case involving an 11-year-old girl, the first FMT led to exacerbation of UC symptoms, while repeated procedures allowed her to remain in remission with a minimal dose of steroids [31]. There are two points that may contribute to the variation in FMT in childhood IBD. First, the pathogenesis of IBD in children may differ from that in adults. Second, most parents do not allow their children to be research subjects for safety reasons, thus resulting in poor compliance and a high dropout rate in the pediatric population.
Potential indications
Irritable bowel syndrome
In 2019, a systematic review and meta-analysis reported the efficacy of FMT in the IBS through a total of 33 randomized clinical trials (RCTs) involving 4321 patients [32]. The authors pointed out that the clinical symptoms of IBS were alleviated after FMT treatment. Other meta-analyses and cohort studies have shown significant improvement in IBS patients after FMT treatment [33, 34]. FMT has enormous potential in adult IBS. Nevertheless, there is still uncertainty about FMT treatment in pediatric IBS because clinical trials for treating childhood IBS have not been conducted.
Functional constipation
Functional constipation has also been reported as a potential indication for FMT. In 2016, Tian conducted an open-label study of FMT in the treatment of slow-transit constipation (STC) [35]. In this trial, 24 STC patients (20–74 years of age) were enrolled. FMT was performed on three consecutive days through nasal-jejunal tubes, and the patients underwent follow-up for 12 weeks. Clinical improvement was shown in 50% (12/24) of those recruited, and full remission was found in 37.5% (9/24) with no AEs reported [35]. Similar results regarding the use of FMT in childhood constipation have also been reported. In 2016, de Meij TG reported a significant increase in bacterial species in a study using conventional culture techniques in 28 constipated children compared with 14 healthy children [36]. By comparing the fecal flora between eight constipated children and 14 healthy children, the authors observed an increase in the abundance of bifidobacteria in constipated subjects [36]. Together, these results indicated that FMT has enormous potential for treating childhood constipation [37].
Fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) severity is closely related to the dysregulation of intestinal bacteria and changes in metabolic function. Studies have shown differences in the composition of bacteria in the feces of NAFLD and healthy patients [38]. Another study involving non-alcoholic steatohepatitis (NASH) in children mentioned that compared with the control group, the content of bacterial components in NASH children was different. A meta-analysis confirmed that at normal transaminase levels, the laboratory indices of the probiotic experimental treatment group improved significantly compared to the placebo group [39]. Reducing the number of harmful microbiota can also increase the concentration of butyrate in the cecum and the expression of the intestinal tight junction protein (ZO-1). The increased butyrate and tight junction protein, ZO-1, is beneficial because butyrate is the energy source of colonic motility, and ZO-1 can repair the mucosal barrier of the colonic epithelium [40]. A meta-analysis confirmed that probiotics reduced the number of harmful microbiota and increased the level of butyrate and ZO-1 [41]. It has been reported that with the recovery of intestinal flora, the symptoms of portal hypertension and other hepatic symptoms significantly improved [42]. Moreover, the implementation of FMT has not increased the incidence of AEs in any NASH/NAFLD patients [43]. On the basis of existing clinical and experimental data, FMT has therapeutic potential in NASH/NAFLD [43].
Autism spectrum disorder
Gut flora and its metabolites play an important role in the pathophysiology of ASD [44–47]. In 2017, Kang adopted a modified FMT regimen involving 18 participants with ASD (7–16 years of age) [45]. An improvement was observed in 89% of the participants with respect to the symptoms of diarrhea, constipation, dyspepsia, and abdominal pain, but the autism symptoms were not significantly reduced [45]. Subsequently, the author performed a two-year follow-up evaluation, and in 2019, the ASD symptoms of the participants were re-evaluated [45]. Interestingly, a significant improvement in behavior symptoms was observed in all participants compared to the baseline measurements, suggesting the effectiveness of FMT in ASD [48]. Nevertheless, the remission of ASD symptoms of the participants could not be completely attributed to FMT because the improvement of behavioral symptoms occurred two years later. The brain-intestine axis may be a means of communication between the brain and intestinal flora. Several studies have shown how the gut flora may alter brain function [49–51].
Diabetes
To determine the differences in intestinal flora among children with metabolic diseases, Isabel Leiva-Gea published a study in 2018 comparing 15 diabetic and 13 healthy children [52]. Leiva-Gea reported that the intestinal flora in children with type 1 diabetes differed in classification and function from healthy subjects, and there were fundamental differences in non-autoimmune diabetes models. In another study, Vrieze administered FMT to patients with metabolic syndrome, and insulin sensitivity improved significantly after FMT administration, suggesting the feasibility of FMT in metabolic diseases [53–55]. Solito assessed the effects of probiotic supplementation on weight and metabolism in 101 obese and insulin-resistant young children in a cross-over, double-blind, randomized controlled trial [56]. The study demonstrated that eight weeks of intervention was safe, well tolerated, and efficacious in improving insulin sensitivity and supporting weight loss.
Methodology
Donor screening
Stool samples from a healthy donor are the first requirement for FMT. Consent from a parent is required for FMT involving a child. Donor samples must be safe and reliable and cannot introduce iatrogenic diseases. Donor exclusion criteria are shown in Table 2 [19, 33, 53, 57–61]. Based on domestic and international donor screening standards, the main direction and focus of screening FMT donors are the factors that affect the quality and efficacy of the fecal bacteria solution of the donor, such as gastrointestinal tract, infectious diseases, use of drugs, and immunologic conditions. We also do not currently understand how to select an ideal FMT recipient or how other underlying conditions might impact the response [62–64]. Current screening protocols for FMT may be insufficient. Little is known about what qualifies as an effective or safe donor and does not account for the possibility of gut microbiota perturbations. Similarly, in the pediatric population, the donor criteria must fulfill the above-described standards, but specific tests should be added to the criteria for a pediatric donor. Factors that may affect child development need to be taken into account. When considering a donor, it is necessary to exclude child-specific diseases, such as attention-deficit hyperactivity disorder, autism, and other inherited metabolic disorders, because all these diseases have the potential to increase the risk of additional diseases in the FMT recipient.
Table 2.
Disease screening and laboratory test for donor candidates
| Categories |
|---|
| History of diseases |
|
Infectious diseases: AIDS, hepatitis, tuberculosis Gastrointestinal diseases: CDI, IBD, IBS, constipation, gastrointestinal surgery in the past 6 mon Immune system diseases or immunomodulatory treatment Tumors Metabolic syndrome and/or obesity Inherited metabolic diseases: phenylketonuria, lysosomal storage disorders, glycogen storage disease, favism Use of antibiotics within 3 mon |
| Serological screening |
|
Hepatitis series virus antibody: hepatitis A, B, C, I and II virus surface antigen Epstein–Barr virus (IgG and IgM) and cytomegalovirus (IgG and IgM.) AIDS Bacterial test: syphilis reagin test; TPPA/TPHA; tuberculosis Routine blood examination Liver and renal function C-reactive protein, erythrocyte sedimentation rate and anti-streptolysin O test Insulin and blood glucose |
| Stool test |
|
Viral test: viruses associated with diarrhea (RT-PCR): rotavirus, norovirus, astrovirus Parasitic test: Ascaris, Ancylostoma duodenale, Strongyloides stercoralis, Giardia lamblia, Entamoeba histolytica, Trichuris trichiura, Clonorchis sinensis, Blastocystis hominis Bacterial tests: Helicobacter pylori; Salmonella spp., Shigella spp., Vibrio spp., Campylobacter spp., Yersinia enterocolitica and Aeromonas spp. Additional fecal test: fecal white blood cell, Occult blood |
| Additional test |
|
Abdominal ultrasound Abdominal and chest (posteroanterior) radiography COVID-19 tests (only for pandemic period): nasopharyngeal swab, serology for SARS-CoV-2, stool testing for SARS-CoV-2 |
AIDS acquired immune deficiency syndrome, CDI Clostridium difficile infection, IBD inflammatory bowel disease, IBS irritable bowel syndrome, IgG immunoglobulin G, IgM immunoglobulin M, TPPA/TPHA Treponema pallidum particle agglutination assay/Treponema pallidum hemagglutination assay, RT-PCR reverse transcription-polymerase chain reaction, COVID-19 corona virus disease 2019, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
Preparation
Stool that is used for FMT can be fresh or frozen [19, 65–70]. Fresh stool should be disposed of within six hours of donation and stored at room temperature for further treatment. The feces are thoroughly stirred with standard sterile sodium chloride, and the filtrate is drawn into a syringe and injected into the gastrointestinal channel of the recipient. Another type of frozen feces is made by collecting feces from a group of pre-screened donors and storing frozen feces in the feces bank in equal aliquots. Final disposal is in storage at −80 °C. On the day of FMT, the fecal suspension is defrosted in a warm water bath (37°C), then dissolved in normal saline to obtain the expected volume of the suspension. The infusion is performed within six hours after defrosting [7]. Notably, repeated defrosting and freezing should be avoided. Regardless of the methods used to obtain the fecal liquid, the principle of asepsis must be considered in the process of making fecal bacteria liquid for FMT, and the influence of air oxidation on fecal bacteria should be prevented. It is often not possible to determine the amount of fecal bacteria liquid that the patient needs. Compared with the relatively large amount of the infusion, the risk of failure when the infusion amount is < 50 g is more than fourfold higher [20]. The determination of the amount of fecal bacteria solution warrants further experimental determination [60], and there is also no clear consensus on the best method of preservation [67, 68, 71, 72].
Delivery approaches
We consider differences in treatment and the preparation of fecal specimens, and patient acceptance of the different delivery methods. The current delivery approaches for FMT include the following: (1) nasogastric, nasoduodenal, or nasal-jejunal tube; (2) capsule; (3) colonoscopy (stool deposited into the right colon or terminal ileum); (4) oral; and (5) enema [2, 73]. Table 3 shows there are notable differences among delivery methods. Colonoscopy is a good option for the delivery of FMT both in children and adults [72, 74–77]. Compared with other methods, the treatment effects of colonoscopy are better in pediatric patients; however, colonoscopy is invasive, requires sedation, has the standard risks of colonoscopy, and the effectiveness may be limited within the colon (i.e., not the entire intestine) [78]. Capsule technology, which has emerged in recent years, is an effective delivery approach for pediatric FMT and can overcome the psychological problems of “oral feces” [71], but it is very expensive and has high technical requirements that exceed the capabilities of most hospitals. Specifically, an oral capsule is unsuitable in children because an oral capsule has the risks of becoming lodged in the esophagus and aspirated. At the same time, the effectiveness of an oral capsule is tentative because the number of fecal bacteria contained might not meet the requirements for FMT. A nasogastric/nasoduodenal/nasal-jejunal tube is easy to use and has the lowest technical requirements. Retrograde colonic enemas via an anal tube are also a widely used method of delivery. Enemas are easy to perform at home, even in pediatric patients. However, enemas are only effective for colon diseases because the transplanted microbiota may not be distributed throughout the entire intestine. Each FMT method has advantages and disadvantages. Clinicians should, therefore, select the appropriate approach based on the purpose and technical capabilities.
Table 3.
Advantages and disadvantages of the different delivery approaches for FMT
| Approaches | Advantage | Disadvantage |
|---|---|---|
| Nasogastric/nasoduodenal/nasojejunal |
Avoids sedation Low cost |
Discomfort of tube placement Risk of vomiting and aspiration Inability to evaluate mucosa or take biopsies |
| Capsules |
No sedation risk Less invasive Can be administered in office setting |
Expensive Less effective than colonoscopy Capsule burden Risks of vomiting and aspiration |
| Colonoscopy |
Ability to evaluate mucosa and take biopsies Most effective route for treatment of rCDI |
Invasive and requires sedation Standard risks of colonoscopy (discomfort, perforation, bleeding) Expensive |
| Enema |
Low cost Less invasive and avoids sedation Easy to carry out in office or at home |
Donor stool does not reach the entire colon and limited to distal colon Less effective than other routes for rCDI |
FMT fecal microbiota transplantation, rCDI recurrent Clostridium difficile infection
Safety of fecal microbiota transplantation
Although FMT has shown excellent therapeutic effects in pediatric diseases, it is worth noting that many AEs have been reported [34, 79]. The most common AEs included abdominal pain, gastrointestinal flatulence, diarrhea, constipation, fever, nausea, and vomiting [80, 81]. Serious complications, such as sedation-induced aspiration, perforation, bleeding, toxic megacolon with sepsis and peritonitis, fatal aspiration pneumonia, and death under anesthesia, have also been reported [80]. Potential risks for pediatric FMT include infectious diseases, obesity, diabetes, atherosclerosis, cancer, NAFLD, and asthma [80]. In the pediatric population, especially in newborns, specific AEs include belching, abdominal distention, abdominal pain, vomiting, diarrhea, fever, or transient CRP elevation [19, 28, 48, 82]. Kumagai reported that the AEs in clinical course of UC in a child who received FMT was transient fever and abdominal pain [83]. From 2013 to 2018, Zhang focused on AEs in the short and long terms in pediatric FMT patients. Only a few children developed AEs in the short term, while few AEs occurred during the long-term follow-up. Indeed, no fatal AEs associated with FMT have been reported in children [19, 82, 84–87].
Perspectives and future
FMT has become widely practiced over recent years, and interest in FMT has surged among pediatricians and patients. Although the therapeutic effect of FMT in adults is satisfactory, the clinical practice of FMT in pediatrics needs to be improved and supplemented. The gap of FMT in the treatment of pediatric diseases reminds pediatricians that they need to consider many challenges, such as the required dosage, duration, onset, and end point of treatment. Future pediatric guidelines/studies should specify established indications versus potential/scientific indications. Additional larger, controlled, and prospective studies are needed to clarify both the safety and efficacy of FMT in pediatrics.
Author contributions
ZSC:writing–review and editing. GX: formal analysis, writing–original draft. CZH: data curation. CZH and GX contributed equally as the first author.
Funding
This project was funded by the National Natural Science Foundation of China (30700917, 81570465) and Minsheng Foundation of Joint Research Project of Liaoning Province(2021JH2/10300129).
Data availability
Not required.
Declarations
Conflict of interest
No financial benefits have been received from any party related directly or indirectly to the subject of this article.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 2.Vindigni SM, Surawicz CM. Fecal microbiota transplantation. Gastroenterol Clin North Am. 2017;46(1):171–185. doi: 10.1016/j.gtc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859. [PubMed] [Google Scholar]
- 4.Palmer R. Fecal matters. Nat Med. 2011;17(2):150–152. doi: 10.1038/nm0211-150. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 6.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 7.Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, et al. Fecal microbiota transplantation: review and update. J Formos Med Assoc. 2019;118:S23–S31. doi: 10.1016/j.jfma.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Russell G, Kaplan J, Ferraro M, Michelow IC. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics. 2010;126:e239–e242. doi: 10.1542/peds.2009-3363. [DOI] [PubMed] [Google Scholar]
- 9.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- 10.Kronman MP, Nielson HJ, Adler AL, Giefer MJ, Wahbeh G, Singh N, et al. Fecal microbiota transplantation via nasogastric tube for recurrent Clostridium difficile infection in pediatric patients. J Pediatr Gastroenterol Nutr. 2015;60:23–26. doi: 10.1097/MPG.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 11.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 12.Kahn SA, Young S, Rubin DT. Colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection in a child. Am J Gastroenterol. 2012;107:1930–1931. doi: 10.1038/ajg.2012.351. [DOI] [PubMed] [Google Scholar]
- 13.Pierog A, Mencin A, Reilly NR. Fecal microbiota transplantation in children with recurrent Clostridium difficile infection. Pediatr Infect Dis J. 2014;33:1198–1200. doi: 10.1097/INF.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 14.Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, et al. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24:5–14. doi: 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta SK, Girotra M, Garg S, Dutta A, von Rosenvinge EC, Maddox C, et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol. 2014;12:1572–1576. doi: 10.1016/j.cgh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 17.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YX, Chen X, Gan HT. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system: why were TLR3's roles not tested? Gastroenterology. 2014;146:1428. doi: 10.1053/j.gastro.2014.01.069. [DOI] [PubMed] [Google Scholar]
- 19.Gurram B, Sue PK. Fecal microbiota transplantation in children: current concepts. Curr Opin Pediatr. 2019;31:623–629. doi: 10.1097/MOP.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 20.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Xiao Y, Lin K, Song F, Ge T, Zhang T. Pediatric severe pseudomembranous enteritis treated with fecal microbiota transplantation in a 13-month-old infant. Biomed Rep. 2015;3:173–175. doi: 10.3892/br.2014.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walia R, Garg S, Song Y, Girotra M, Cuffari C, Fricke WF, et al. Efficacy of fecal microbiota transplantation in 2 children with recurrent Clostridium difficile infection and its impact on their growth and gut microbiome. J Pediatr Gastroenterol Nutr. 2014;59:565–570. doi: 10.1097/MPG.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 23.Hourigan SK, Ahn M, Gibson KM, Perez-Losada M, Felix G, Weidner M, et al. Fecal transplant in children with Clostridioides difficile gives sustained reduction in antimicrobial resistance and potential pathogen burden. Open Forum Infect Dis. 2019;6:379. doi: 10.1093/ofid/ofz379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karolewska-Bochenek K, Grzesiowski P, Banaszkiewicz A, Gawronska A, Kotowska M, Dziekiewicz M, et al. A two-week fecal microbiota transplantation course in pediatric patients with inflammatory bowel disease. Adv Exp Med Biol. 2018;1047:81–87. doi: 10.1007/5584_2017_123. [DOI] [PubMed] [Google Scholar]
- 25.Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H, Jr, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 26.Caldeira LF, Borba HH, Tonin FS, Wiens A, Fernandez-Llimos F, Pontarolo R. Fecal microbiota transplantation in inflammatory bowel disease patients: a systematic review and meta-analysis. PLoS ONE. 2020;15:e0238910. doi: 10.1371/journal.pone.0238910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hourigan SK, Chen LA, Grigoryan Z, Laroche G, Weidner M, Sears CL, et al. Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42:741–752. doi: 10.1111/apt.13326. [DOI] [PubMed] [Google Scholar]
- 28.Goyal A, Yeh A, Bush BR, Firek BA, Siebold LM, Rogers MB, et al. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:410–421. doi: 10.1093/ibd/izx035. [DOI] [PubMed] [Google Scholar]
- 29.Cho S, Spencer E, Hirten R, Grinspan A, Dubinsky MC. Fecal microbiota transplant for recurrent Clostridium difficile infection in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2019;68:343–347. doi: 10.1097/MPG.0000000000002172. [DOI] [PubMed] [Google Scholar]
- 30.Moutinho BD, Baima JP, Rigo FF, Saad-Hossne R, Rodrigues J, Romeiro FG, et al. Fecal microbiota transplantation in refractory ulcerative colitis - a case report. J Int Med Res. 2019;47:1072–1079. doi: 10.1177/0300060518821790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu H, Arai K, Abe J, Nakabayashi K, Yoshioka T, Hosoi K, et al. Repeated fecal microbiota transplantation in a child with ulcerative colitis. Pediatr Int. 2016;58:781–785. doi: 10.1111/ped.12967. [DOI] [PubMed] [Google Scholar]
- 32.Asha MZ, Khalil SFH. Efficacy and safety of probiotics, prebiotics and synbiotics in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Sultan Qaboos Univ Med J. 2020;20:e13–e24. doi: 10.18295/squmj.2020.20.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnsen PH, Hilpusch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3:17–24. doi: 10.1016/S2468-1253(17)30338-2. [DOI] [PubMed] [Google Scholar]
- 34.Green JE, Davis JA, Berk M, Hair C, Loughman A, Castle D, et al. Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: a systematic review and meta-analysis. Gut Microbes. 2020;12:1–25. doi: 10.1080/19490976.2020.1854640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian H, Ding C, Gong J, Ge X, McFarland LV, Gu L, et al. Treatment of slow transit constipation with fecal microbiota transplantation: a pilot study. J Clin Gastroenterol. 2016;50:865–870. doi: 10.1097/MCG.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 36.de Meij TG, de Groot EF, Eck A, Budding AE, Kneepkens CM, Benninga MA, et al. Characterization of microbiota in children with chronic functional constipation. PLoS ONE. 2016;11:e0164731. doi: 10.1371/journal.pone.0164731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkusa T, Koido S, Nishikawa Y, Sato N. Gut microbiota and chronic constipation: a review and update. Front Med (Lausanne) 2019;6:19. doi: 10.3389/fmed.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clemente MG, Mandato C, Poeta M, Vajro P. Pediatric non-alcoholic fatty liver disease: recent solutions, unresolved issues, and future research directions. World J Gastroenterol. 2016;22:8078–8093. doi: 10.3748/wjg.v22.i36.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig Dis Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 41.Suk KT, Kim DJ. Gut microbiota: novel therapeutic target for nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13:193–204. doi: 10.1080/17474124.2019.1569513. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Lezana T, Raurell I, Bravo M, Torres-Arauz M, Salcedo MT, Santiago A, et al. Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology. 2018;67:1485–1498. doi: 10.1002/hep.29646. [DOI] [PubMed] [Google Scholar]
- 43.Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol. 2017;15:600–602. doi: 10.1016/j.cgh.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Zhou JM. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience. 2016;324:131–139. doi: 10.1016/j.neuroscience.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li N, Chen H, Cheng Y, Xu F, Ruan G, Ying S, et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front Cell Infect Microbiol. 2021;11:759435. doi: 10.3389/fcimb.2021.759435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Xueying Z, Jiaqu C, Qiyi C, Huanlong Q, Ning L, et al. FTACMT study protocol: a multicentre, double-blind, randomised, placebo-controlled trial of faecal microbiota transplantation for autism spectrum disorder. BMJ Open. 2022;12:e051613. doi: 10.1136/bmjopen-2021-051613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, et al. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9:5821. doi: 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9:eaaf6397. doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 50.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O'Donnell TA, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170:185–98e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125:782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leiva-Gea I, Sanchez-Alcoholado L, Martin-Tejedor B, Castellano-Castillo D, Moreno-Indias I, Urda-Cardona A, et al. Gut microbiota differs in composition and functionality between children with type 1 diabetes and MODY2 and healthy control subjects: a case-control study. Diabetes Care. 2018;41:2385–2395. doi: 10.2337/dc18-0253. [DOI] [PubMed] [Google Scholar]
- 53.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 54.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 56.Solito A, Bozzi Cionci N, Calgaro M, Caputo M, Vannini L, Hasballa I, et al. Supplementation with Bifidobacterium breve BR03 and B632 strains improved insulin sensitivity in children and adolescents with obesity in a cross-over, randomized double-blind placebo-controlled trial. Clin Nutr. 2021;40:4585–4594. doi: 10.1016/j.clnu.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015;149:223–237. doi: 10.1053/j.gastro.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woodworth MH, Neish EM, Miller NS, Dhere T, Burd EM, Carpentieri C, et al. Laboratory testing of donors and stool samples for fecal microbiota transplantation for recurrent clostridium difficile infection. J Clin Microbiol. 2017;55:1002–1010. doi: 10.1128/JCM.02327-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr Opin Microbiol. 2013;16:240–245. doi: 10.1016/j.mib.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68:2111–2121. doi: 10.1136/gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cammarota G, Ianiro G, Tilg H, Rajilic-Stojanovic M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khoruts A, Rank KM, Newman KM, Viskocil K, Vaughn BP, Hamilton MJ, et al. Inflammatory bowel disease affects the outcome of fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2016;14:1433–1438. doi: 10.1016/j.cgh.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khanna S, Pardi DS, Kelly CR, Kraft CS, Dhere T, Henn MR, et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J Infect Dis. 2016;214:173–181. doi: 10.1093/infdis/jiv766. [DOI] [PubMed] [Google Scholar]
- 64.Newman KM, Rank KM, Vaughn BP, Khoruts A. Treatment of recurrent Clostridium difficile infection using fecal microbiota transplantation in patients with inflammatory bowel disease. Gut Microbes. 2017;8:303–309. doi: 10.1080/19490976.2017.1279377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kassam Z, Dubois N, Ramakrishna B, Ling K, Qazi T, Smith M, et al. Donor screening for fecal microbiota transplantation. N Engl J Med. 2019;381:2070–2072. doi: 10.1056/NEJMc1913670. [DOI] [PubMed] [Google Scholar]
- 66.Keller JJ, Ooijevaar RE, Hvas CL, Terveer EM, Lieberknecht SC, Hogenauer C, et al. A standardised model for stool banking for faecal microbiota transplantation: a consensus report from a multidisciplinary UEG working group. United European Gastroenterol J. 2021;9:229–247. doi: 10.1177/2050640620967898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164. doi: 10.1001/jama.2018.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sood A, Mahajan R, Singh A, Midha V, Mehta V, Narang V, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis. 2019;13:1311–1317. doi: 10.1093/ecco-jcc/jjz060. [DOI] [PubMed] [Google Scholar]
- 69.Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–1228. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 70.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109e6. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318:1985–1993. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicholson MR, Mitchell PD, Alexander E, Ballal S, Bartlett M, Becker P, et al. Efficacy of fecal microbiota transplantation for Clostridium difficile infection in children. Clin Gastroenterol Hepatol. 2020;18:612–6191. doi: 10.1016/j.cgh.2019.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paramsothy S, Borody TJ, Lin E, Finlayson S, Walsh AJ, Samuel D, et al. Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis. 2015;21:1600–1606. doi: 10.1097/MIB.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 74.Seward E. Recent advances in colonoscopy. F1000Res. 2019;8:1028. doi: 10.12688/f1000research.18503.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nambu R, Hagiwara SI, Kakuta F, Hara T, Shimizu H, Abukawa D, et al. Current role of colonoscopy in infants and young children: a multicenter study. BMC Gastroenterol. 2019;19:149. doi: 10.1186/s12876-019-1060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kellermayer R. Fecal microbiota transplantation: great potential with many challenges. Transl Gastroenterol Hepatol. 2019;4:40. doi: 10.21037/tgh.2019.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhong M, Buch H, Wen Q, Long C, Cui B, Zhang F. Colonic transendoscopic enteral tubing: route for a novel, safe, and convenient delivery of washed microbiota transplantation in children. Gastroenterol Res Pract. 2021;2021:6676962. doi: 10.1155/2021/6676962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim SY, Kim HS, Park HJ. Adverse events related to colonoscopy: global trends and future challenges. World J Gastroenterol. 2019;25:190–204. doi: 10.3748/wjg.v25.i2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park SY, Seo GS. Fecal microbiota transplantation: is it safe? Clin Endosc. 2021;54:157–160. doi: 10.5946/ce.2021.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS ONE. 2016;11:e0161174. doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saha S, Mara K, Pardi DS, Khanna S. Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Gastroenterology. 2021;160:1961–1969.e3. doi: 10.1053/j.gastro.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 82.Zhang XY, Wang YZ, Li XL, Hu H, Liu HF, Li D, et al. Safety of fecal microbiota transplantation in Chinese children: a single-center retrospective study. World J Clin Cases. 2018;6:1121–1127. doi: 10.12998/wjcc.v6.i161.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumagai H, Yokoyama K, Imagawa T, Inoue S, Tulyeu J, Tanaka M, et al. Failure of fecal microbiota transplantation in a three-year-old child with severe refractory ulcerative colitis. Pediatr Gastroenterol Hepatol Nutr. 2016;19:214–220. doi: 10.5223/pghn.2016.19.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baxter M, Ahmad T, Colville A, Sheridan R. Fatal aspiration pneumonia as a complication of fecal microbiota transplant. Clin Infect Dis. 2015;61:136–137. doi: 10.1093/cid/civ247. [DOI] [PubMed] [Google Scholar]
- 86.Hogenauer C, Kump PK, Krause R. Tempered enthusiasm for fecal transplantation? Clin Infect Dis. 2014;59:1348–1349. doi: 10.1093/cid/ciu567. [DOI] [PubMed] [Google Scholar]
- 87.Goldenberg SD, Batra R, Beales I, Digby-Bell JL, Irving PM, Kellingray L, et al. Comparison of different strategies for providing fecal microbiota transplantation to treat patients with recurrent Clostridium difficile infection in two English hospitals: a review. Infect Dis Ther. 2018;7:71–86. doi: 10.1007/s40121-018-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not required.


