Abstract
Lumpy skin disease (LSD) is an emerging disease of cattle causing significantly high economic losses. Control of LSD depends on the use of homologous attenuated LSD virus strains isolated originally from South Africa (the Neethling strain). The virus belongs to the genus Capripoxvirus, which includes sheep pox virus and goat pox virus. The present study was conducted to evaluate the safety and efficacy of a new live attenuated LSD vaccine produced by Middle East for Vaccines (MEVAC®) based on the Neethling strain. Tests were performed both in Egypt and Vietnam. Safety was evaluated by inoculation of five cattle with 10 times the recommended dose and observation of the animals for 14 days. Immunogenicity was tested at different periods post-vaccination (PV) in animals receiving the recommended doses of the vaccine using ELISA and virus neutralization test. Five cows were used to determine the protection index (PI) and non-vaccinated control cattle were included. Three calves were challenged by intradermal inoculation of the wild virus (5 × 105 TCID50) 28 days PV. Field or mass vaccination experiments were conducted in Vietnam during national campaigns in the summer of 2021 with 4301 vaccinated animals closely monitored after vaccination. In the field, around 2% (80/4301) of the animals showed hyper-reactivity, and 0.6% (24/4301) showed small skin swellings that disappeared within few hours PV. Abortion was recorded in three animals (0.3% 3/867). Challenged animals were resistant to clinical disease and PI value was 3.5 log10. Meanwhile, antibody levels determined by the ELISA were inconsistent among animals and laboratories during the study period. Overall, the findings point to a new safe and effective LSD vaccine.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11259-022-10037-2.
Keywords: Lumpy skin disease, Vaccine, Neethling strain, Seroconversion, Post-vaccination reaction, Vaccine evaluation
Introduction
Lumpy skin disease (LSD) is a vector-borne transboundary pox disease of cattle characterized by fever, lethargy, eruption of numerous skin nodules, lameness, impaired appetite, decreased milk output, and systemic lymphadenitis (Tuppurainen et al., 2017; Tuppurainen and Oura, 2012). The nodules are usually round, slightly raised, firm, painful and may rupture and become infected. The disease is caused by the lumpy skin disease virus (LSDV), a member of family Poxviridae and the genus Capripoxvirus which is a double-stranded DNA virus (Lefkowitz et al., 2018). Infection is caused by a virus endemic in large parts of the world, including most of the African countries, the Middle East, and Asia, posing a transmission risk to countries which have not yet had LSDV outbreaks (Cornell et al., 2017; Namazi and Khodakaram Tafti, 2021). Outbreaks in Europe have been controlled and eradicated through vaccination (Calistri et al., 2020).
LSD is recognized as one of the greatest threats to dairy and meat farms in Africa, the Middle East and Asia. Because of its transboundary nature and the considerable socioeconomic consequences of skin degradation, reduced milk output, abortions, low weight gain, temporary or permanent infertility, and secondary bacterial infections, the disease has been designated as a notifiable disease by the World Organization for Animal Health (WOAH) (previously named: Office international des epizooties (OIE)) (WOAH, 2022b). Furthermore, in the case of an outbreak, costly control and eradication procedures are needed (Wolff et al., 2020). Besides having a major impact on national output, an outbreak causes a limitation in live animals and animal products global trading (Farra et al., 2022).
Globally, the African countries were the first to encounter LSD outbreaks, but the disease has since extended to the Middle East (though not reported in Morocco, Algeria, Tunisia, Libya), Asia, and more lately, Europe (Molini et al., 2018; Rouby et al., 2021; Tuppurainen and Oura, 2012). The disease was suspected to be spread out of Africa into Israel in August 1989, via a wind-borne route of transmission by the Stomoxys calcitrans fly. This notion was explained by the fact that no new animals were admitted into affected herds, and that LSDV has previously been isolated from stable flies collected while feeding on the infected cattle (Paslaru et al., 2021). In Asia, the first report of LSD was in 2019 in China and the virus moved to Vietnam and nearby countries in 2020 (Roche et al. 2020; Tran et al., 2021). Young calves, lactating cows and underweight animals are the most vulnerable due to compromised immune systems (Namazi and Khodakaram Tafti, 2021). In Egypt, the disease was first identified in 1988 affecting cattle in the northeastern region (Ismailia province) upon livestock importation from Somalia (Ali et al., 1990; House et al., 1990). After 17 years, LSD reemerged in Egypt in 2006 in a major outbreak after the entry of infected cattle imported from Ethiopia, causing serious economic losses to livestock industry (Awadin et al., 2011; Salib and Osman, 2011; Sharawi and Abd El-Rahim, 2011). Those economic losses ranged between 147 and 539 EUR per animal in affected herds in some European countries during 2016 (Casal et al., 2018). Despite widespread vaccination, outbreaks were reported in Egypt in, 2011, 2014, 2017, 2018, and 2020 (Badr et al., 2022; Allam et al., 2020; El-Tholoth and El-Kenawy, 2016; Elhaig et al., 2017; Rouby et al., 2019; Rouby et al., 2021; Shalaby et al., 2016) .
Since sheep pox virus (SPV) and goat pox virus (GPV) belonged to the genus Capripoxvirus, they were cross immunogenic for LSD, but protection of sheep and goat by LSDV against these viruses has not been reported to our knowledge. However, Kenya LSDV strain (long thought to be a sheep and goat pox strain) has been extensively used to protect small ruminants against their respective diseases with inconsistent results (Hamdi et al., 2020). Now that the attenuated sheep or goat pox viruses have been used as heterologous vaccines, with some success, some Neethling-based LSD vaccines showed severe post-vaccination (PV) reactions and vaccination failure. Partial protection of cattle against LSD has been achieved in Egypt by an attenuated Romanian strain of SPV vaccine (Gaber et al., 2022). While it has been reported that vaccination of cattle with LSDV resulted in the appearance of antibodies in 50% of the animals after three weeks, no antibody response was detected with SPV vaccines (Hamdi et al., 2020). In addition, heterologous vaccines sometimes failed due to the appearance of LSD symptoms in some vaccinated animals, raising doubt about their field efficacy (Abutarbush et al., 2015; Brenner et al., 2009). This has been attributed to the nature of some cattle breeds or type of the vaccine used, since heterologous ones are administered at 10-fold higher concentrations than homologous LSD strains (Abdallah et al., 2018; Bamouh et al., 2021). Of the homologous LSD strains, a Neethling strain isolated in South Africa was successfully attenuated and used (Davies, 1991). The vaccine proved innocuous and immunogenic, though some local reactions have been observed in some animals. The vaccine was used in six Balkan countries during 2016–2017 (Bulgaria, Greece, Serbia, Montenegro, Former Yugoslav Republic of Macedonia, and Albania) and the average ratio of its effectiveness was 79.8% (range = 62.5–97%) (Klement et al., 2020). The discrepancies and inconsistency of results obtained from many heterologous and some homologous vaccines against LSD prompted some veterinary vaccines-based corporations to produce a homologous vaccine based on the Neethling strain (MEVAC® live attenuated LSD vaccine). The safety and effectiveness of this product were evaluated in Egypt and Vietnam during an emergency season between 2020 and 2021.
Materials and methods
LSD vaccine
The LSD vaccine used in the current study is a live attenuated LSDV vaccine based on an isolate that was isolated, characterized and attenuated in Egypt. Partial nucleotide sequencing showed that the isolate is genetically related to the reference LSDV Neethling strain (ScienSano report; Supplementary files 1 and 2). The vaccine is manufactured by the Middle East for Vaccines (MEVAC®) by propagation of the virus on Madin-Darby bovine kidney (MDBK) cells or fetal lamb heart cells. Extensive reversion to virulence studies have been conducted during vaccine development and vaccine samples were sent to ScienSano for evaluation according to their method (Agianniotaki et al. 2017). The vaccine is prepared in one dosage form containing103.5 log10 TCID50/dose.
The sterile diluent used for vaccine reconstitution is a sterile phosphate-buffered saline solution. The reconstituted vaccine is kept on ice and should be protected from light until use. The reconstituted vaccine should be used within two hours.
Experimental studies
The experimental studies were conducted using Holstein Friesian cows to determine the safety, efficacy, and potency of the examined vaccine.
Safety
Safety of MEVAC® LSD vaccine was experimentally evaluated in dairy cows that did not have antibodies against LSDV by ELISA or serum neutralization tests (SNT). The cattle were maintained in a large animal unit with a high level of containment (BSL-3) at the Central Laboratory for Evaluation of Veterinary Biologics (CLEVB, Cairo, Egypt). Five vials of the lyophilized vaccine were randomly chosen, reconstituted in sterile diluent, and pooled. Five pregnant cows were inoculated subcutaneously (S/C) with 10 times the recommended dose of the vaccine (10 ml) divided into two injection sites. Two animals were inoculated with the vaccine diluent and used as the control. All animals were clinically inspected daily for any adverse vaccine reactions for two weeks PV. The experiment was conducted in both Egypt and Vietnam before vaccine use.
In Vietnam, a pilot experiment was conducted to examine safety of the vaccine and its effects on the general health status of the animals before the expanded large scale (field) vaccination. In this experiment, 65 animals (25 pregnant heifers, 20 lactating cows, and 20 pregnant dry cows) were inoculated by the in-label dose of the vaccine (1 ml containing 103.5 log10 TCID50). The animals were clinically inspected for any adverse vaccine reactions. Further, main physiological parameters including rumination index, health index, and milk yield were monitored as described previously (Stangaferro et al., 2016; Vanhoudt et al., 2015). Rumination index and health index were automatically monitored by specialized software for cow health management (SCR-Israel). The health index scale ranged between 0 and 100 units and a reading < 86 on at least one day within 2–5 days PV was regarded as a health disorder. Milk yield was monitored by DeLaval DelPro™ farm system (Sweden). Serum samples were also collected from vaccinated animals on days 21, 28, 35 and 42 PV for the antibody testing by using the ID Screen Capripox Double Antigen ELISA kit (ID.Vet, Montepellier, France), to examine vaccine potency and seroconversion percent.
Efficacy
Efficacy of the vaccine was examined by using two methods; first one is the protection index (PI) and the second one is the challenge test. The PI, which is the difference between the virulent virus titer (over log106 TCID50) in vaccinated and non-vaccinated controls (WOAH, 2022b). The experiment was conducted at 28 days PV at the Central Laboratory for Evaluation of Veterinary Biologics (CLEVB, Cairo, Egypt). Six dilutions of the LSDV wild strain locally identified in Egypt (titer < 5 × 106 TCID50) were inoculated intradermally (0.1 ml per inoculum) along the length of the flank in four replicates down the flank of five vaccinated and two non-vaccinated (control) cows. The animals were observed for the development of clinical signs at inoculation sites for 14 days post-inoculation and rectal temperature, general condition, and specific signs of LSD were recorded. However, hypersensitivity reactions that appeared within 24 hours (h) at the sites of inoculation were ignored as they quickly diminished. The virus titer was determined from LSD inoculated flanks of vaccinated and unvaccinated animals two weeks after inoculation. A difference between in vivo titrations of the virus in vaccinated and non-vaccinated animals ≥ log10 2.5 is considered suitable for vaccine release (evidence of protection) (WOAH, 2022b).
The challenge experiment was conducted in Vietnam, as previously described (Wolff et al., 2020). Six calves (6–12 months old) were used. Three of them were vaccinated with the in-label dose and the other three animals were not vaccinated and used as a control. All animals were challenged after 28 days of vaccination with field LSDV (LSD/KN1/2020) isolated in Vietnam by intradermal injection of 5 × 106 TCID50 of the virus (1 ml) at the neck. Animals were observed for clinical signs for two weeks after the challenge test.
Potency
Three small farms in Egypt (23 animals each) received one vaccine dose (1 ml containing 103.5 log10 TCID50). The sera were collected at days 28, 45, 60, 120, and 150 PV to evaluate the antibody response by the IDvet ELISA following the manufacturer’s instructions. The readings were calculated as a percent of the sample optical density (OD) to the positive control OD (S/P %) as follows:
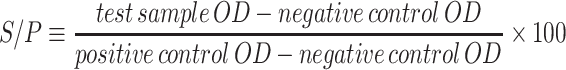 |
Values < 30 were considered negative, and ≥ 30 were positive, according to the manufacturer’s recommendations.
Virus neutralization test (VNT) was also examined for the same sera. The test was performed in 96-microwell plates (Nunc, Thermo Fisher Scientific, USA) and titers were expressed as the logarithm (base 10) of the reciprocal of the last dilution of serum that neutralized 100× TCID50 of the virus in 50% of the wells (Krešić et al., 2020; WOAH, 2022a).
Field study
In a large scale, the vaccine was used to immunize more than 3.5 million cattle during a national campaign in Egypt in the summer of 2021. In Vietnam, more than two million cattle were also vaccinated, with 4301 animals at two farms were closely monitored for protection percent (number of noninfected-vaccinated animals/total vaccinated animals X 100) after one year of vaccination. PV reactions including hyper-reactivity, skin swellings, and abortion were also monitored. In addition, 29 vaccinated animals from each farm were used in an additional experiment, in which serum samples were collected from each animal at days 21, 28, 35 and 42 PV to examine serocoversion by using Idvet ELISA. The sample of each animal was divided into three portions and sent to three different laboratories to assess the consistency of results.
Statistical analysis
In this study, the statistical functions of Microsoft 365 were used in the estimation of central tendency measures, standard deviation, performance of the t-test, correlation coefficient, normal distribution p-values, and design of the tables and graphs (Microsoft Corporation, 2021).
Results
Experimental studies
Safety
In the safety experiment, despite the 10 times doses, MEVAC® LSD vaccine was safe and all vaccinated cows showed normal body temperature (< 39 oC), measured at 24 h PV. Pregnancy was not affected and no adverse reactions, abnormal clinical signs, or local reactions were observed. All five inoculated animals grew stably. When this experiment was repeated in Vietnam, one of the five animals showed shrunken skin at the vaccination site and tears, then spontaneously recovered within few hours. On the other hand, all uninoculated controls did not develop any symptoms during contact and remained seronegative during the observation period.
Genetically, the genome of the vaccine virus was differentiable from the wild-type LSDV and did not contain detectable parts of wild-type LSDV genome (ScienSano report; Supplementary file 1). Further, the vaccine virus was highly akin (≥ 95%) to those of the Neethling vaccine OBP KX76465, cro 216 MG 972,412, SIS lumpyvax KX 764,643, Neethling LW 1959 AF 409,138, Herbivac vaccine KX764644, LSDV 148-GP-RSA-1997 MN6 36,843, isolate 103-GP-1991 MN636839 and isolate LSD 220-2NW-RSA-1993 MN636842 (ScienSano report; Supplementary files 1 and 2).
Of the 65 animals closely observed in Vietnam after vaccination using the recommended dose, right after injection, one cow was found to have hyper-sensitivity reaction signs with some symptoms such as ruffled hair, facial wrinkles (Fig. 1), skin shrinkage at the injection site, and rapid respiration rate. The reacted cow gradually recovered without any complications. Two vaccinated cows showed small swellings in the skin, which gradually disappeared in about five h PV. Abortion didn’t occur in any of the pregnant animals used in this experiment.
Fig. 1.

A cow’s head showing tears, ruffled hair, and wrinkled face skin after lumpy skin disease vaccination
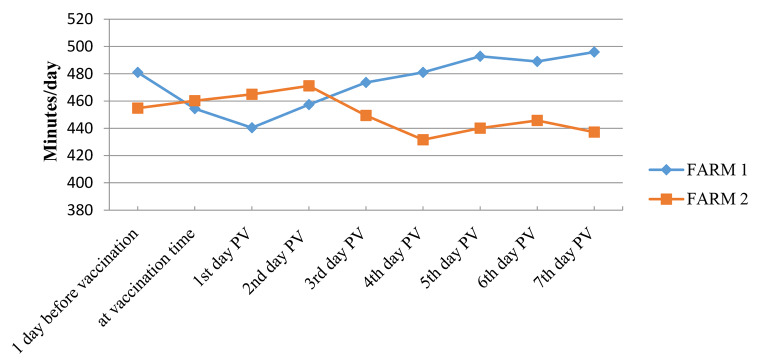
In one farm, rumination index slightly decreased after vaccination then returned to normal (444-449 minutes (min)) by the 3rd day PV. In the other farm, rumination index increased after vaccination to 464 min then gradually decreased after the 3rd day PV to reach 431 min. Statistically, the mean index values for the two farms were not significantly different during the first seven days PV (p = 0.6) as shown in Table 1; Fig. 2.
Table 1.
Mean rumination indices in animals from two farms (n = 65 animals) ± standard deviation (SD)
| Before vaccination | At vaccination | 1 day PV※ | 2 days PV | 3 days PV | 4 days PV | 5 days PV | 6 days PV | 7 days PV | |
|---|---|---|---|---|---|---|---|---|---|
| Average | 467.9 | 457.3 | 452.65 | 464.25 | 461.5 | 456.3 | 466.45 | 467.35 | 466.55 |
| ± SD | 18.53 | 4.10 | 17.32 | 9.69 | 17.11 | 34.93 | 37.26 | 30.62 | 41.51 |
※PV: Post-vaccination.
Fig. 2.
Average rumination index monitored after vaccination in two cattle farms in Vietnam. Farm-1 (n = 30): average rumination index decreased by 1st day post-vaccination (PV), then recovered and tended to increase from 2nd day and remained stable afterwards. Farm-2 (n = 35): average rumination index increased by 2nd day PV then decreased from 3rd to 4th days and was recovered afterwards
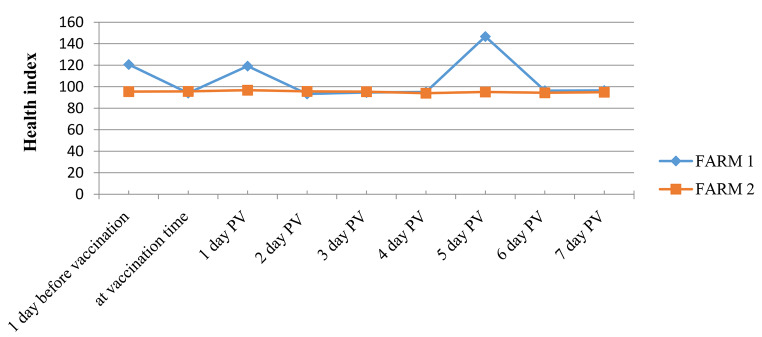
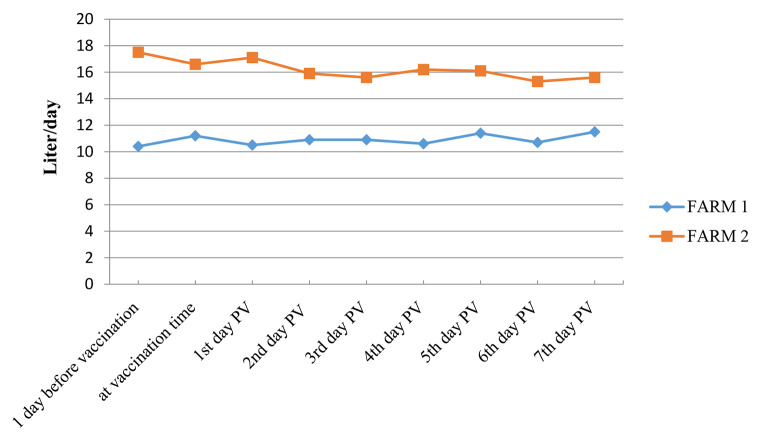
In addition, the physical activity or health index (restlessness, discomfort, lying, and standing) and milk yield showed limited variations during the first seven days PV. Statistically, the mean health index for the two farms was not significantly different during the first seven days PV (p = 0.80), which was also observed for the milk index (p = 0.45) as shown in Tables 2 and 3; Figs. 3 and 4.
Table 2.
Mean health indices in two farms (n = 65 animals) ± standard deviation (SD)
| Before vaccination | At vaccination | 1 day PV※ | 2 days PV | 3 days PV | 4 days PV | 5 days PV | 6 days PV | 7 days PV | |
|---|---|---|---|---|---|---|---|---|---|
| Average | 108.1 | 94.9 | 108.0 | 95.0 | 95.05 | 94.55 | 120.9 | 95.35 | 95.7 |
| ± SD | 17.82 | 1.13 | 15.84 | 2.40 | 0.49 | 0.78 | 36.49 | 1.34 | 1.13 |
※PV: Post-vaccination
Table 3.
Mean milk indices in two farms (n = 65 animals) ± standard deviation (SD)
| Before vaccination | At vaccination | 1 day PV※ | 2 days PV | 3 days PV | 4 days PV | 5 days PV | 6 days PV | 7 days PV | |
|---|---|---|---|---|---|---|---|---|---|
| Average | 13.95 | 13.9 | 13.8 | 13.4 | 13.25 | 13.4 | 13.75 | 12.95 | 13.55 |
| ± SD | 5.02 | 3.82 | 4.67 | 3.54 | 3.32 | 3.96 | 3.32 | 3.32 | 2.90 |
※PV: Post-vaccination
Fig. 3.
Average health index monitored after vaccination in two cattle farms in Vietnam. Farm-1 (n = 30): showed minor fluctuations after vaccination. Farm-2 (n = 35): average health index was stable after vaccination. PV: Post-vaccination
Fig. 4.
Average milk yield monitored after vaccination in two cattle farms in Vietnam. Farm-1 (n = 30): average milk yield tended to increase slightly after vaccination (from 10.4 L/day at 1 day before vaccination to 11.5 L/day at 7th day PV). Farm-2 (n = 35): average milk yield of vaccinated cows decreased slightly after vaccination (from 17.5 L/day at 1 day before vaccination to 15.6 L/day at 7th day PV). PV: Post-vaccination
Efficacy
In the PI experiment conducted in Egypt, the difference between the wild virus titer in vaccinated and non-vaccinated animals was 3.5 log10 ID50, being one log10 higher than the required reference value (> 2.5 log10) (WOAH, 2022b). The control animals’ inoculation sites all developed edematous swellings, but the replicas that received the most diluted inocula showed little to no reaction. Within 24 h, the vaccinated animals developed initial hypersensitivity reactions at the sites of inoculation, which quickly diminished.
In the challenge experiment conducted in Vietnam, the vaccinated animals showed a mild rise in temperature (0.5 − 0.9 ºC), and small swellings at the site of injection (1 cm diameter) that disappeared within few hours. The three control animals showed fever (1-1.5 ºC higher than normal) and severe LSD symptoms (swelling diameter between 3.5 and 5.0 cm at the injection site) after the challenge.
Potency
Baseline antibody levels of unvaccinated animals (Day 0) were equal or less than 0.6 log10 using VNT, while in ELISA values < 30 were considered negative, according to the manufacturer’s recommendations.
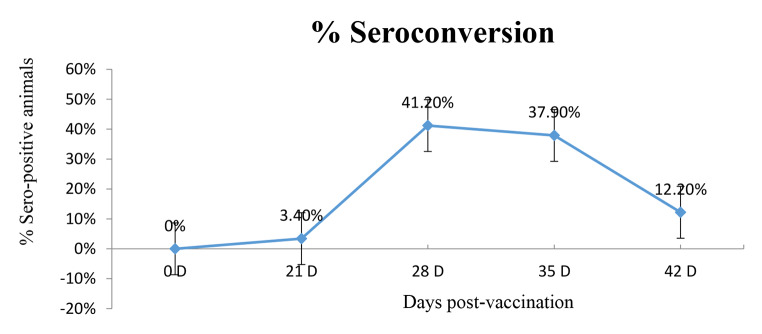
Table 4 demonstrates a comparison between the ELISA and VNT results in three vaccinated farms in Egypt (69 cattle in total). ELISA showed a mean positive percent of 51.7 ± 30.6, reflecting a relatively wide range of variation during the monitoring period, as observed in the other experiment conducted in Vietnam (Fig. 5). The corresponding mean for VNT was 78.38 ± 15.18% (Table 4), denoting a higher sensitivity, with titers ≥ 1.2 log10 regarded as positive. The relationship between the two tests showed a moderate correlation (r = 0.51). In Vietnam, seroconversion was additionally monitored in two farms by ID-VET ELISA performed simultaneously at three different laboratories (Table 5). The readings came out significantly different between the farms at 28 days PV (p = 0.01) and among labs (lab-2, p = 0.05). In Farm-1, all 3 laboratories showed negative or few positive readings (3.4%) throughout the whole monitoring period. In Farm-2, more positive samples were detected by the three laboratories by 28 days PV (range 34-41.2%, average 37.7%). The average positive samples decreased to 25.03% and 18.2% by days 35 and 42 PV respectively (Table 5).
Table 4.
Comparison between the results of virus neutralization test (VNT) and ELISA using t-test; the samples were collected from three Egyptian farms at different intervals post-vaccination (n = 69)
| Days PV † | VNT※ % positive (≥ 1.2 log10) |
ELISA % positive |
|---|---|---|
| 28 | 78.6 | 50 |
| 45 | 77.4 | 30 |
| 60 | 57.1 | 57.1 |
| 120 | 100 | 100 |
| 150 | 78.6 | 21.4 |
| Mean ± SD | 78.38 ± 15.18% | 51.7 ± 30.6 |
†PV: Post-vaccination.
※VNT: Virus neutralization test.
Fig. 5.
Seroconversion percent of vaccinated animals (n = 65) at different intervals post-vaccination using IDvet Capripox ELISA. (D: days post-vaccination)
Table 5.
Comparison of ELISA results performed simultaneously at three different laboratories for samples from two farms in Vietnam using t-test (data from Farm-2 were depicted twice: horizontally for comparison with Farm-1 in terms of ELISA results at different intervals post-vaccination and vertically for comparison of results from each lab for the two farms)
| Seropositive ELISA results % | P-value (t-test) Farm 1 vs. 2 |
||||||
|---|---|---|---|---|---|---|---|
| Days post-vaccination | Farm-1 (n = 29 cows) | Farm-2 (n = 29 cows) | |||||
| Lab-1 | Lab-2 | Lab-3 | Lab-1 | Lab-2 | Lab-3 | ||
| 21 | 0 | 0 | 0 | 0 | 0 | 2.9 | 0.42 |
| 28 | 3.4 | 3.4 | 0 | 34 | 37.9 | 41.2 | 0.01 |
| 35 | 0 | 3.4 | 3.4 | 17.2 | 37.9 | 20 | 0.06 |
| 42 | 0 | 3.4 | 3.4 | 0 | 37.9 | 16.7 | 0.25 |
| Farm-2 (n = 29 cows) | |||||||
| 21 | 0 | 0 | 2.9 | ||||
| 28 | 34 | 37.9 | 41.2 | ||||
| 35 | 17.2 | 37.9 | 20 | ||||
| 42 | 0 | 37.9 | 16.7 | ||||
|
P-value (T-test) Within each Lab |
0.20 | 0.05 | 0.10 | ||||
Field study
In the large-scale field studies, PV clinical signs were similar to those reported in the small-scale experiments. Table 6 presents a detailed stratification of physiological conditions of cattle vaccinated in the two farms (n = 4301) in Vietnam and the recorded PV reactions. Results showed that 1.9% (80/4301) of the animals showed signs of hyper-reaction right after vaccination and 0.6% (24/4301) developed small swellings around the injection site and over the body (Table 6). The hyper-reactivity vanished within 3–5 h, PV. Abortion was recorded between two and nine days PV in three animals only out of 867 pregnant animals (0.3%, 3/867) at 142, 197, and 238 days of gestation.
Table 6.
Stratification of physiological conditions of cattle vaccinated with MEVAC® LSD in two farms in Vietnam: in addition to rate of appearance of post-vaccination reactions
| Vaccination | Cattle (head) | Local hyper- reaction | Skin swellings | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Calves | Young heifers | Pregnant heifers | Milking cows | Dry off cows | Total | No. | % | No. | % | |
| Farm-1 | 471 | 234 | 211 | 529 | 132 | 1577 | 18 | 1.1 | 9 | 0.6 |
| Farm-2 | 1196 | 261 | 656 | 165 | 446 | 2724 | 62 | 2.3 | 15 | 0.6 |
| Total | 1667 | 495 | 867 | 694 | 578 | 4301 | 80 | 1.9 | 24 | 0.6 |
Abortion was recorded in 0.3% (3/867) of the pregnant animals.
Protection percent against natural LSD infection in the two Vietnamese farms-1 and − 2 reached 98 and 100%, respectively after one year of vaccination. However, in one different small farm, with 50 calves born to unvaccinated dams, the vaccine was safe and protection percent was 88%.
Discussion
In this study, the live attenuated MEVAC® LSD vaccine (Neethling strain) was evaluated. In general, the vaccine was well tolerated by all the animals as the impact of vaccination on the general health, ruminal index, and milk yield of vaccinated cattle were almost nonexistent. Data obtained from both experimental and field studies in Egypt and Vietnam showed that the product was safe with no or insignificant PV reactions on the skin (Table 6), health indices (Tables 1 and 2; Figs. 2 and 3) and milk yield (Table 3; Fig. 4). These results suggest a highly satisfactory product, since some other LSD vaccines have shown adverse effects, probably due to higher dose concentration (104 TCID50/mL), or insufficient attenuation leading to localized or generalized skin lesions, with detectable vaccine virus in the nodules, blood, and milk. Those vaccines were rejected for lack of safety requirements stipulated by OIE (Bedeković et al., 2018). Other LSD vaccines showed pronounced multiple swellings at the injection sites in 12% of the animals starting at six days PV and lasting for 2–4 days (Katsoulos et al., 2018) or small-sized lumps (< 0.5 cm) in 9% of the animals between days 8 and 18 PV (Calistri et al., 2019; Tekilegiorgis and Tamir, 2019). In our study, the use of a larger number of inoculated animals in the safety test and batch potency (PI test) relative to OIE requirements (WOAH, 2022b) was to assess any risks associated with the vaccine administration. The fewer number of uninoculated controls used, was based on the expectation of no or negligible signs of disease; meanwhile, large groups of animals were vaccinated in the field using the regular dose.
These PV reactions are in agreement with the observations of Tuppurainen et al. (2021) who stated that available LSD vaccines may show variable results in quality, efficacy, safety, and side effects, probably due to type of seed virus used, level of attenuation, dose volume, and titer. Low-level attenuation and heterogenicity of viruses used in vaccination have been associated with incomplete safety and appearance of clinical disease in vaccinated animals (Tuppurainen et al. 2021). However, it has been reported that vaccines based on the Neethling strain are four times more effective than a sheep pox-based vaccine in preventing LSD (Ben-Gera et al., 2015). The relative vaccine effectiveness of one Neethling strain vaccine was 77% and full protection was achieved one month after vaccination, although evidence of effectiveness after two weeks only was demonstrated (Tekilegiorgis and Tamir, 2019).
In the present study, PV comfort was monitored in Vietnam by evaluating the resting behavior and rumination indices of the animals (Vanhoudt et al., 2015). The mean health, milk, and rumination indices values for the two farms were non-significantly different during the first seven days PV. Cows normally ruminate about 450 to 500 min a day (about 8 h), and the observed differences were non-significant, indicating no effect on the digestive system or well-being of the animals. Additionally, the appearance of skin hypersensitivity reactions or small swellings which were associated with vaccine administration, has been mainly local and transient. Unvaccinated animals in experimental studies never showed these signs. Abortion was recorded in 0.3% (3/867) of the pregnant animals by 2–9 days PV at gestation periods < 4 months, suggesting linkage to vaccine administration.
Immunogenicity studies using MEVAC® LSD vaccine showed a mean positive ELISA percent of 51.7 ± 30.6, while the mean positive titers by VNT (≥ 1.2 log10) was 78.38 + 15.18% (Table 4). This may indicate a higher VNT sensitivity compared to ELISA, though the results were fluctuating and the correlation between the results of the two tests during the observation period was moderate (r = 0.51). Likewise, one study reported VNT antibodies in 50% of the vaccinated cattle (Hamdi et al., 2020), while another study reported a lower value of 34% (Samojlović et al., 2019). ELISA readings were comparable with VNT, being positive in 30% of the sera and the Kappa Index of Inter-rater Reliability (K) between the two tests was 0.8–0.9 (Samojlović et al., 2019). However, the authors included some VNT titers lower than 1.2 log10, and they preferred performing VNT over PI test of OIE, being in vitro, easier, and cheaper (Samojlović et al., 2019). Overall, the observed low serological responses (VNT or ELISA) have been explained by the significant role of cellular immunity in the protection against the disease after vaccination (Norian et al., 2019; Varshovi et al., 2017).
In Vietnam, seroconversion was evaluated by ID-VET ELISA only and, interestingly, the results from the three different laboratories came out significantly different at 28 days PV (p = 0.01), either because of inappropriate timing of sample collection in relation to detectable antibody levels or inconsistencies in the method. For instance, in farm-1, all three laboratories showed negative ELISA readings after vaccination and few animals (3.4%) were positive. In farm-2, considerably higher results were obtained from all three laboratories at days 35 and 42 PV. Earlier, Milovanović et al. (2019) showed that Capripoxvirus-specific antibodies were detected by 46 to 47 weeks after vaccination in only 33.77% of the animals using ELISA, while VNT was positive in 35.06% of them. It appears that lab-2 demonstrated significantly higher values in farm-2 compared to farm-1 (p = 0.05). For this reason, the challenge test was recommended to confirm protection (Gari et al., 2015), since studies to evaluate the cell-mediated immunity against LSD are insufficient or un-straightforward (Abutarbush and Tuppurainen, 2018). Previous researchers reported that the number of animals with antibodies against LSDV decreased with time after vaccination (Samojlović et al., 2019), in agreement with our results (Tables 4 and 5). When the vaccine efficacy/effectiveness was measured by calculating the risk of disease among vaccinated and unvaccinated cattle and the percent reduction in risk of disease among vaccinated and unvaccinated animals was determined, protection by MEVAC® LSD was estimated to be 98 and 100% in two farms in Vietnam and was 88% in a third small farm containing 50 calves born to unvaccinated dams. This lower value may be attributed to the vaccination of calves at one month of age before the full maturation of the immune system. Normally calves born to immunized cows will have a passive immunity that persists for about three to four months (Tuppurainen et al. 2021) and vaccination is administered after this period to avoid neutralization by maternal immunity through colostrum (Agianniotaki et al., 2018).
The obtained results show that MEVAC® LSD vaccine produced minor clinical signs, with around 0.6% of the animals exhibiting minor skin inflammatory reactions (< 2 cm diameter nodules) in the field. Those lesions disappeared within few hours PV in the absence of fever or change of appetite. Abortion was recorded in 0.3% (3/867) of the pregnant animals. Vaccinated calves were resistant to challenge, and PI value was 3.5 log10.
The majority of the statistical methods used in this study are descriptive, which is one of its limitations; however, the controlled experiments performed (i.e., safety, challenge, and PI) support the conclusions drawn from the descriptive statistics. Furthermore, the large number of animals monitored in the field study could add to the evidence. Overall, our results promise a new safe and potent LSD vaccine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by Middle East for Veterinary Vaccines (MEVAC®) company, Egypt. We are grateful to the veterinarians and farmers who helped in the conduction of the study’s experiments and collection of the required data.
Author contributions
All authors contributed to the study conception and design. Abdel-Hamid Bazid, Mohamed Fawzy, Mohamed Abdelmegeid, Randa Y. Thabet, and Asmaa Magouz performed the research; Momtaz Wasfy, Hui Sian Yong, Magdy M. El-Sayed, and Yassien Badr supervised the research; Abdel-Hamid Bazid, Mohamed Fawzy, Mohamed Abdelmegeid,, and Yassien Badr analyzed the data; Abdel-Hamid Bazid wrote the original manuscript; Momtaz Wasfy, Mohamed Nayel, Magdy M. El-Sayed ,and Yassien Badr revised and edited the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Middle East for Veterinary Vaccines (MEVAC®) company, Egypt.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Data supporting the results are available on request from the authors.
Declarations
Conflict of interest
Magdy M. El-Sayed is a member of MEVAC’s board of directors. Momtaz Wasfy and Randa Y. Thabet are employees in Middle East for Vaccines (ME VAC®). Hui Sian Yong is an employer at Kemin Biologisc®; MEVAC® now is a part of Kemin®. However, this association has no effect on the study’s design, data analysis, results’ interpretation, or publication decision.
Ethics statement
This study was approved by the Institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine, University of Sadat City, Menoufia, Egypt (Ethical approval number: VUSC-004-1-22).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary information
Supplementary files 1 and 2 are showing the ScienSano report, containing information on identity and molecular characterization of the virus used to produce the vaccine.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdallah FM, El Damaty HM, Kotb GF. Sporadic cases of lumpy skin disease among cattle in Sharkia province, Egypt: Genetic characterization of lumpy skin disease virus isolates and pathological findings. Vet World. 2018;11(8):1150–1158. doi: 10.14202/vetworld.2018.1150-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutarbush SM, Ababneh MM, Al Zoubi IG, Al Sheyab OM, Al Zoubi MG, Alekish MO, Al Gharabat RJ. Lumpy Skin Disease in Jordan: Disease Emergence, Clinical Signs, Complications and Preliminary-associated Economic Losses. Transbound Emerg Dis. 2015;62(5):549–554. doi: 10.1111/tbed.12177. [DOI] [PubMed] [Google Scholar]
- Abutarbush SM, Tuppurainen ESM. Serological and clinical evaluation of the Yugoslavian RM65 sheep pox strain vaccine use in cattle against lumpy skin disease. Transbound Emerg Dis. 2018;65(6):1657–1663. doi: 10.1111/tbed.12923. [DOI] [PubMed] [Google Scholar]
- Agianniotaki EI, Chaintoutis SC, Haegeman A, Tasioudi KE, De Leeuw I, Katsoulos PD, Sachpatzidis A, De Clercq K, Alexandropoulos T, Polizopoulou ZS, Chondrokouki ED, Dovas CI. Development and validation of a TaqMan probe-based real-time PCR method for the differentiation of wild type lumpy skin disease virus from vaccine virus strains. J Virol Methods. 2017;249:48–57. doi: 10.1016/j.jviromet.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Agianniotaki EI, Babiuk S, Katsoulos P-D, Chaintoutis Serafeim C, Praxitelous A, Quizon K, Boscos C, Polizopoulou ZS, Chondrokouki ED, Dovas CI. Colostrum transfer of neutralizing antibodies against lumpy skin disease virus from vaccinated cows to their calves. Transbound Emerg Dis. 2018;65(6):2043–2048. doi: 10.1111/tbed.12983. [DOI] [PubMed] [Google Scholar]
- Ali AA, Esmat M, Attia H, Selim A, Abdel-Hamid YM. Clinical and pathological studies on lumpy skin disease in Egypt. Vet Rec. 1990;127(22):549–550. [PubMed] [Google Scholar]
- Allam AM, Elbayoumy MK, Abdel-Rahman EH, Hegazi AG, Farag TK. Molecular characterization of the 2018 outbreak of lumpy skin disease in cattle in Upper Egypt. Vet World. 2020;13(7):1262–1268. doi: 10.14202/vetworld.2020.1262-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awadin W, Hussein H, Elseady Y, Babiuk S, Furuoka H. Detection of lumpy skin disease virus antigen and genomic DNA in formalin-fixed paraffin-embedded tissues from an Egyptian outbreak in 2006. Transbound Emerg Dis. 2011;58(5):451–457. doi: 10.1111/j.1865-1682.2011.01238.x. [DOI] [PubMed] [Google Scholar]
- Badr Y, Noreldin AE, Elewa YHA, Ahmed MS, Inoshima Y, Baker NM, Aamer WN, Abas OM, Nayel M, Rahman MM, Elgendy E, Saleh AG, El-Neweshy MS. Cellular infiltration, cytokines, and histopathology of skin lesions associated with different clinical forms and stages of naturally occurring lumpy skin disease in cattle. Comp Immunol Microbiol Infect Dis. 2022;90–91:101894. doi: 10.1016/j.cimid.2022.101894. [DOI] [PubMed] [Google Scholar]
- Bamouh Z, Hamdi J, Fellahi S, Khayi S, Jazouli M, Tadlaoui KO, Fihri OF, Tuppurainen E, Elharrak M. Investigation of post vaccination reactions of two live attenuated vaccines against lumpy skin disease of cattle. Vaccines. 2021;9(6):621. doi: 10.3390/vaccines9060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedeković T, Šimić I, Krešić N, Lojkić I. Detection of lumpy skin disease virus in skin lesions, blood, nasal swabs and milk following preventive vaccination. Transbound Emerg Dis. 2018;65(2):491–496. doi: 10.1111/tbed.12730. [DOI] [PubMed] [Google Scholar]
- Ben-Gera J, Klement E, Khinich E, Stram Y, Shpigel NY. Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep-pox live attenuated vaccines for the prevention of lumpy skin disease - The results of a randomized controlled field study. Vaccine. 2015;33(38):4837–4842. doi: 10.1016/j.vaccine.2015.07.071. [DOI] [PubMed] [Google Scholar]
- Brenner J, Bellaiche M, Gross E, Elad D, Oved Z, Haimovitz M, Wasserman A, Friedgut O, Stram Y, Bumbarov V, Yadin H. Appearance of skin lesions in cattle populations vaccinated against lumpy skin disease: Statutory challenge. Vaccine. 2009;27(10):1500–1503. doi: 10.1016/j.vaccine.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Calistri P, DeClercq K, Gubbins S, Klement E, Stegeman A, Cortiñas Abrahantes J, Antoniou S-E, Broglia A, Gogin A. Lumpy skin disease: III. Data collection and analysis. EFSA J. 2019;17(3):5638. doi: 10.2903/j.efsa.2019.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calistri P, De Clercq K, Gubbins S, Klement E, Stegeman A, Cortiñas Abrahantes J, Marojevic D, Antoniou SE, Broglia A. Lumpy skin disease epidemiological report IV: data collection and analysis. EFSA J. 2020;18(2):e06010. doi: 10.2903/j.efsa.2020.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Allepuz A, Miteva A, Pite L, Tabakovsky B, Terzievski D, Alexandrov T, Beltrán-Alcrudo D. Economic cost of lumpy skin disease outbreaks in three Balkan countries: Albania, Bulgaria and the Former Yugoslav Republic of Macedonia (2016–2017) Transbound Emerg Dis. 2018;65(6):1680–1688. doi: 10.1111/tbed.12926. [DOI] [PubMed] [Google Scholar]
- Cornell T, Awada L, Tizzani P, Sinclair J. Mapping the spread of lumpy skin disease using WAHIS data. In: Éloit M, editor. OIE bulletin. Animal welfare: An asset for livestock production. Paris, France: World Organisation for Animal Health; 2017. [Google Scholar]
- Davies FG. Lumpy skin disease of cattle: A growing problem in Africa and the Near East. World Anim Rev. 1991;68(3):37–42. [Google Scholar]
- El-Tholoth M, El-Kenawy AA. G-Protein-Coupled Chemokine Receptor Gene in Lumpy Skin Disease Virus Isolates from Cattle and Water Buffalo (Bubalus bubalis) in Egypt. Transbound Emerg Dis. 2016;63(6):e288–e295. doi: 10.1111/tbed.12344. [DOI] [PubMed] [Google Scholar]
- Elhaig MM, Selim A, Mahmoud M. Lumpy skin disease in cattle: Frequency of occurrence in a dairy farm and a preliminary assessment of its possible impact on Egyptian buffaloes. Onderstepoort J Vet Res. 2017;84(1):e1–e6. doi: 10.4102/ojvr.v84i1.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farra D, De Nardi M, Lets V, Holopura S, Klymenok O, Stephan R, Boreiko O. Qualitative assessment of the probability of introduction and onward transmission of lumpy skin disease in Ukraine. Microb Risk Anal. 2022;20:100200. doi: 10.1016/j.mran.2021.100200. [DOI] [Google Scholar]
- Gaber A, Rouby S, Elsaied A, El-Sherif A. Assessment of heterologous lumpy skin disease vaccine-induced immunity in pregnant cattle vaccinated at different times of gestation period and their influence on maternally derived antibodies. Vet Immunol Immunopathol. 2022;244:110380. doi: 10.1016/j.vetimm.2021.110380. [DOI] [PubMed] [Google Scholar]
- Gari G, Abie G, Gizaw D, Wubete A, Kidane M, Asgedom H, Bayissa B, Ayelet G, Oura CAL, Roger F, Tuppurainen ESM. Evaluation of the safety, immunogenicity and efficacy of three capripoxvirus vaccine strains against lumpy skin disease virus. Vaccine. 2015;33(28):3256–3261. doi: 10.1016/j.vaccine.2015.01.035. [DOI] [PubMed] [Google Scholar]
- Hamdi J, Boumart Z, Daouam S, El Arkam A, Bamouh Z, Jazouli M, Tadlaoui KO, Fihri OF, Gavrilov B, El Harrak M. Development and Evaluation of an Inactivated Lumpy Skin Disease Vaccine for Cattle. Vet Microbiol. 2020;245:108689. doi: 10.1016/j.vetmic.2020.108689. [DOI] [PubMed] [Google Scholar]
- House JA, Wilson TM, el Nakashly S, Karim IA, Ismail I, el Danaf N, Moussa AM, Ayoub NN. The isolation of lumpy skin disease virus and bovine herpesvirus-4 from cattle in Egypt. J Vet Diagn Invest. 1990;2(2):111–115. doi: 10.1177/104063879000200205. [DOI] [PubMed] [Google Scholar]
- Katsoulos PD, Chaintoutis SC, Dovas CI, Polizopoulou ZS, Brellou GD, Agianniotaki EI, Tasioudi KE, Chondrokouki E, Papadopoulos O, Karatzias H, Boscos C. Investigation on the incidence of adverse reactions, viraemia and haematological changes following field immunization of cattle using a live attenuated vaccine against lumpy skin disease. Transbound Emerg Dis. 2018;65(1):174–185. doi: 10.1111/tbed.12646. [DOI] [PubMed] [Google Scholar]
- Klement E, Broglia A, Antoniou S-E, Tsiamadis V, Plevraki E, Petrović T, Polaček V, Debeljak Z, Miteva A, Alexandrov T, Marojevic D, Pite L, Kondratenko V, Atanasov Z, Gubbins S, Stegeman A, Abrahantes JC. Neethling vaccine proved highly effective in controlling lumpy skin disease epidemics in the Balkans. Prevent Vet Med. 2020;181:104595. doi: 10.1016/j.prevetmed.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Krešić N, Šimić I, Bedeković T, Acinger-Rogić Ž, Lojkić I, Fenwick B. Evaluation of serological tests for detection of antibodies against lumpy skin disease virus. J Clin Microbiol. 2020;58(9):e00348–e00320. doi: 10.1128/JCM.00348-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz EJ, Dempsey DM, Hendrickson RC, Orton RJ, Siddell SG, Smith DB. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV) Nucleic Acids Res. 2018;46(D1):D708–D717. doi: 10.1093/nar/gkx932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microsoft Corporation (2021) Microsoft Excel. Retrived from https://office.microsoft.com/excel
- Milovanović M, Dietze K, Milićević V, Radojičić S, Valčić M, Moritz T, Hoffmann B. Humoral immune response to repeated lumpy skin disease virus vaccination and performance of serological tests. BMC Vet Res. 2019;15(1):80. doi: 10.1186/s12917-019-1831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molini U, Aikukutu G, Khaiseb S, Haindongo NN, Lilungwe AC, Cattoli G, Dundon WG, Lamien CE. Molecular characterization of lumpy skin disease virus in Namibia, 2017. Arch Virol. 2018;163(9):2525–2529. doi: 10.1007/s00705-018-3891-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namazi F, Khodakaram Tafti A. Lumpy skin disease, an emerging transboundary viral disease: A review. Vet Med Sci. 2021;7(3):888–896. doi: 10.1002/vms3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norian R, Afzal Ahangran N, Varshovi HR, Azadmehr A. Comparative efficacy of two heterologous capripox vaccines to control lumpy skin disease in cattle. Bulg J Vet Med. 2019;22(2):171–179. doi: 10.15547/bjvm.2067. [DOI] [Google Scholar]
- Paslaru AI, Verhulst NO, Maurer LM, Brendle A, Pauli N, Vögtlin A, Renzullo S, Ruedin Y, Hoffmann B, Torgerson PR, Mathis A, Veronesi E. Potential mechanical transmission of Lumpy skin disease virus (LSDV) by the stable fly (Stomoxys calcitrans) through regurgitation and defecation. Cur Res Insect Sci. 2021;1:100007. doi: 10.1016/j.cris.2020.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche X, Rozstalnyy A, TagoPacheco D, Pittiglio C, Kamata A, Beltran Alcrudo D, Bisht K, Karki S, Kayamori J, Larfaoui F, Raizman E, VonDobschuetz S, Dhingra MS, Sumption K(2020) Introduction and spread of lumpy skin disease in South, East and Southeast Asia: Qualitative risk assessment and management. In P. FAO animal production and health (Series Ed.), FAO Animal Production and Health Papers. Rome, FAO. FAO animal production and health
- Rouby SR, Bazid A-H, Wasfy M, El-Sayed M. Capripoxviruses: Exploring the genetic relatedness between field and vaccine strains from Egypt. Vet World. 2019;12(12):1924–1930. doi: 10.14202/vetworld.2019.1924-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouby SR, Safwat NM, Hussein KH, Abdel- Ra’ouf AM, Madkour BS, Abdel-Moneim AS, Hosein HI. Lumpy skin disease outbreaks in Egypt during 2017–2018 among sheeppox vaccinated cattle: Epidemiological, pathological, and molecular findings. PLoS ONE. 2021;16(10):e0258755. doi: 10.1371/journal.pone.0258755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salib FA, Osman AH. Incidence of lumpy skin disease among Egyptian cattle in Giza Governorate Egypt. Vet World. 2011;4(4):162–167. [Google Scholar]
- Samojlović M, Polaček V, Gurjanov V, Lupulović D, Lazić G, Petrović T, Lazić S. Detection of antibodies against Lumpy skin disease virus by Virus neutralization test and ELISA methods. Acta Vet. 2019;69(1):47–60. doi: 10.2478/acve-2019-0003. [DOI] [Google Scholar]
- Shalaby MA, El-Deeb A, El-Tholoth M, Hoffmann D, Czerny C-P, Hufert FT, Weidmann M, El Abd A. Recombinase polymerase amplification assay for rapid detection of lumpy skin disease virus. BMC Vet Res. 2016;12(1):244. doi: 10.1186/s12917-016-0875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharawi SS, Abd El-Rahim IH. The utility of polymerase chain reaction for diagnosis of lumpy skin disease in cattle and water buffaloes in Egypt. Rev Sci Tech. 2011;30(3):821–830. doi: 10.20506/rst.30.3.2075. [DOI] [PubMed] [Google Scholar]
- Stangaferro ML, Wijma R, Caixeta LS, Al-Abri MA, Giordano JO. Use of rumination and activity monitoring for the identification of dairy cows with health disorders: Part II. Mastitis. J Dairy Sci. 2016;99(9):7411–7421. doi: 10.3168/jds.2016-10908. [DOI] [PubMed] [Google Scholar]
- Tekilegiorgis T, Tamir D. Review on Alternative Vaccine of LSD. Appro Poult Dairy & Vet Sci. 2019;7(2):618–619. doi: 10.31031/APDV.2019.07.000656. [DOI] [Google Scholar]
- Tran HTT, Truong AD, Dang AK, Ly DV, Nguyen CT, Chu NT, Hoang TV, Nguyen HT, Nguyen VT, Dang HV. Lumpy skin disease outbreaks in vietnam, 2020. Transbound Emerg Dis. 2021;68(3):977–980. doi: 10.1111/tbed.14022. [DOI] [PubMed] [Google Scholar]
- Tuppurainen ES, Alexandrov T, Beltrán-Alcrudo D. Lumpy skin disease field manual–A manual for veterinarians. Rome, Italy: Food and Agriculture Organization of the United Nations (FAO); 2017. [Google Scholar]
- Tuppurainen ES, Dietze K, Wolff J, Bergmann H, Beltran-Alcrudo D, Fahrion A, Lamien CE, Busch F, Sauter-Louis C, Conraths FJ, De Clercq K, Hoffmann B, Knauf S (2021) Review: Vaccines and Vaccination against Lumpy Skin Disease. Vaccines (Basel) 9(10). doi:10.3390/vaccines9101136 [DOI] [PMC free article] [PubMed]
- Tuppurainen ES, Oura CA. Review: lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transbound Emerg Dis. 2012;59(1):40–48. doi: 10.1111/j.1865-1682.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- Vanhoudt A, van Winden S, Fishwick JC, Bell NJ. Monitoring cow comfort and rumen health indices in a cubicle-housed herd with an automatic milking system: a repeated measures approach. Ir Vet J. 2015;68(1):12–12. doi: 10.1186/s13620-015-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshovi HR, Norian R, Azadmehr A, Afzal Ahangaran N. Immune response characteristics of Capri pox virus vaccines following emergency vaccination of cattle against lumpy skin disease virus. Iran J Vet Sci Technol. 2017;9(2):33–40. doi: 10.22067/veterinary.v9i2.65381. [DOI] [Google Scholar]
- WOAH (2022a) Foot and Mouth Disease (infection with foot and mouth disease virus), In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2022: World Organization for Animal health (Previously: Office international des epizooties), Paris, France
- WOAH (2022b) Lumpy Skin Disease, In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2022: World Organization for Animal health (Previously: Office international des epizooties), Paris, France
- Wolff J, Moritz T, Schlottau K, Hoffmann D, Beer M, Hoffmann B. Development of a safe and highly efficient inactivated vaccine candidate against lumpy skin disease virus. Vaccines (Basel) 2020;9(1):4. doi: 10.3390/vaccines9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the results are available on request from the authors.