Abstract
Background
The number of cardiologically relevant genetic findings will continue to increase. This is due to the use of high-throughput sequencing techniques and the critical role of incidental findings in cardiac disease genes. Telemedicine can be a useful diagnostic tool to monitor the heart rhythm of patients with inborn cardiac diseases.
Methods
Patients were screened once they had been referred to our outpatient department for rare cardiac diseases between January 2020 and May 2022. Those patients who underwent genetic testing and were consequently diagnosed with a genetic disorder were included in this study. Their medical records were evaluated regarding implanted cardiac electronic devices and findings in the telemedical monitoring.
Results
304 patients were seen in our outpatient department for rare cardiac diseases in the mentioned period. In 100 cases, genetic testing was performed. 10 patients (10%) with an identified inborn cardiac disease were monitored via telemedicine until the end of May 2022. 4 patients were monitored by implantable loop recorders (ILR), 4 patients were monitored by Implantable Cardioverter Defibrillators (ICD), and 2 patients received both devices. Clinical relevant arrhythmias making medical intervention necessary were identified in 4 cases. In two cases, data interpretation was hampered by sinus tachycardia caused by physical exercise.
Discussion
Telemonitoring of the heart rhythm by medical devices is beneficial for patients with monogenic heart diseases. Especially, when the indication for an ICD is not clear, implantation of a telemonitored ILR can be a suitable choice. However, rhythm analysis can be challenging in young patients who are physically active.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00335-022-09972-x.
Background
During the last years, molecular diagnostics experienced a boost by the growing use of next-generation sequencing. Exome sequencing enables the complete analysis of the coding regions of the human DNA. The use of this technique has become a popular method to identify a growing number of disease genes. Eleven different genes have shown a definitive disease-causing association with non-syndromic dilated cardiomyopathy (DCM) (Hershberger and Jordan 1993), whilst variants in nine genes, partly overlapping with the aforementioned, were proven to cause non-syndromic hypertrophic cardiomyopathy (Cirino and Ho 1993). The importance of cardiac disease genes causing cardiomyopathies and/or primary arrhythmias is strengthened by their role as so called “actionable genes”. Even in asymptomatic individuals, pathogenic variants in these genes should be reported as incidental findings which can occur using high-throughput technologies (Green et al. 2013). This list of genes was recently extended by TTN (Miller et al. 2021) since certain truncating variants in this gene are associated with an increased risk for the development of a DCM phenotype (Haggerty et al. 2019). Hence, the number of patients with rare and complex cardiac diseases in need of a close follow-up by experienced cardiologists will be further increasing. This in turn requires a personalized risk assessment as well as individual prophylactic and therapeutic interventions in order to reduce the number of sudden cardiac deaths in the young caused by preventable cardiac arrhythmias.
Telemedicine facilitates outpatient monitoring and heart rhythm analysis. It can be combined with cardiac implantable devices such as the Implantable Cardioverter Defibrillators (ICD) that can terminate a life-threatening arrhythmia. Another used device in telemedicine are implantable loop recorders (ILR) that solely record the heart rhythm without any possibility of intervention (Jamal et al. 2021). It was shown that telemonitoring reduces hospitalization in patients with heart failure (Winkler et al. 2021). Therefore, its use is recommended in those patients by the guidelines of the European Society of Cardiology (Theresa AM et al. 2022). Especially during the COVID-19 pandemic, telemedicine played a significant role in patient care (Monaghesh E, Hajizadeh A 2020). The use of telemedicine is not restricted to the distant monitoring of biosignals. It was also shown that patients referred for cardiogenetic visits benefited from the use of telemedicine for genetic counselling (Liang LW et al. 2022).
In this study, we provide an overview of our experiences with telemonitoring of patients with inherited cardiac diseases in our tertiary centre. The aim is to point out the advantages and limitations of telemonitoring in these rare diseases.
Methods
Study design
Patients were screened once they had been referred to our outpatient department for rare cardiac diseases (“Zentrum für seltene Erkrankungen”, Department of Internal Medicine I, Klinikum rechts der Isar, Technical University Munich, Germany) between January 2020 and May 2022. Those patients who underwent genetic testing and were consequently diagnosed with a genetic disorder were included in this study. Their medical records were evaluated regarding implanted cardiac electronic devices and findings in the telemedical monitoring. The study was performed according to the declaration of Helsinki. Written consent was obtained from all patients.
Telemedical surveillance
Telemedically monitored devices were screened on a daily basis by our telemedicine centre for one week. In cases of automatically detected arrhythmias, all alerts were manually reviewed by medical experts. If an arrhythmia was confirmed, the respective patient was informed by telephone or by video-visit and, if needed, invited to our outpatient department or the cardiological ward, respectively, for further evaluation.
Results
Most patients were affected by dilated cardiomyopathy
304 patients were seen in our outpatient department for rare cardiac diseases in the mentioned period of time. Genetic testing was performed in 91 of these cases because of a suspected monogenic disease. In nine additional cases, genetic testing had already been performed.
The average age at presentation of these combined 100 patients was 47 years (minimum: 16 years, maximum: 80 years) with a 50:50 sex ratio. The most common referral diagnosis of patients was “dilated cardiomyopathy” (DCM) followed by “hypertrophic cardiomyopathy” (HCM). Four patients were clinically unaffected relatives that were predictively tested for a known familial variant (Fig. 1, overview of all patients: Supplementary Table).
Fig. 1.

Referral diagnosis of patients that received genetic testing in our outpatient department or had already been diagnosed with a genetic disease. ACM arrhythmogenic cardiomyopathy, BrS Brugada syndrome, CTD connective tissue disorder, DCM dilated cardiomyopathy, HCM hypertrophic cardiomyopathy
A genetic variant that was at least classified as a variant of an unknown significance (VUS) or (likely) pathogenic according to the recommendations published by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG) (Richards S et al. 2015) was identified in a total of 31 patients. Two (likely) benign variants were detected in a mother of a deceased index case (patient ID 66, for case description see Supplementary case reports). In 59 out of the 91 cases that received genetic testing in our centre (57.1%), neither a disease-causing variant nor a VUS could be detected (Table 1).
Table 1.
Number of identified variants in our patients
| Identified variants | Number of patients (genetic testing in our centre) | Number of patients (external genetic testing) |
|---|---|---|
| Pathogenic | 13 | 5 |
| Likely pathogenic | 9 | 3 |
| VUS | 7 | 1 |
| Likely pathogenic + VUS | 1 | none |
| Likely pathogenic + likely benign + benign | 1 | none |
| Likely benign + benign | 1 | none |
| No variant detected | 59 | none |
| ∑ | 91 | 9 |
VUS variant of unknown significance
Telemedical monitoring identified clinically relevant arrhythmias in four out of ten cases
Ten patients (10%) with an inborn cardiac disease of known genetic origin were monitored telemedically until the end of May 2022 (Table 2, clinical description of all cases provided as supplementary case reports). In a further case, inclusion was planned (patient ID: 83). Implanted and monitored devices were as follows: four patients were monitored by an ILR, four patients were monitored by an Implantable Cardioverter Defibrillator (ICD), and two patients received both devices. In these cases, an ILR was used combined with a subcutaneous ICD (sICD) since rhythm disturbances with a cycle length out of the therapy zone such as slow ventricular tachycardias (slow VT) cannot be monitored with the sICD-algorithm. On average, patients were monitored for a period of 11.5 months [2–31 months]. Clinically relevant arrhythmias with the necessity of medical intervention were identified in four cases (patient IDs 28, 63, 92, 100).
Table 2.
Telemonitored patients with identified genetic variants
| Patient ID | Diagnosis | Genetic variant | Implanted device | Time period of telemonitoring | Detected arrhythmia |
|---|---|---|---|---|---|
| 2 | BrS | c.934G > T, p.(Glu312*), heterozygous in SCN5A (NM_198056.3) | sICD | 21 months | none |
| 15 | HCM | c.3130C > T, p.(Gln1044*), heterozygous in MYBPC3 (NM_000256.3) | ILR | 16 months | sinus tachycardia due to physical exercising |
| 28a | ACM | c.(1510 + 1_1511-1)_(1688 + 1_1689-1)del, heterozygous in PKP2 (NM_004572.3) | sICD/ILR | 2 months | sinus bradycardia, nsVTs |
| 29a | ACM | c.(1510 + 1_1511-1)_(1688 + 1_1689-1)del, heterozygous in PKP2 (NM_004572.3) | sICD | 6 months | none |
| 43 | BrS | c.2804A > T, p.(Glu935Val), heterozygous in MYH7 (NM_000257.4), incidental finding | ICD | 11 months | none |
| 63b | DCM | c.21304C > T, p.(Arg7102*), heterozygous in TTN (NM_003319.4), c.1673 A > G, p.(His558Arg), heterozygous and c.1715 C > A, p.(Ala572Asp), heterozygous in SCN5A (NM_198056.3) | ILR/sICD | 13 months | nsVT |
| 65b | DCM | c.21304C > T, p.(Arg7102*), heterozygous in TTN (NM_003319.4) | ILR | 4 months | none |
| 92 | Survived SCD | c.433 T > C, p.(Phe145Leu), heterozygous in NKX2-5 (NM_004387.3) | ICD | 3 months | several nsVTs |
| 97 | ACM | c.4789G > T, p.(Glu1597*), heterozygous in DSP (NM_004415.4) | ILR | 8 months | sinus tachycardia due to physical exercising |
| 100 | FA | 800 ± 100 and 900 ± 100 GAA repeats in FXN (NM_000144.5) | ILR | 31 months | sinus tachycardia, SVES, recurrent AF |
ACM arrhythmogenic cardiomyopathy, AF atrial fibrillation, BrS Brugada Syndrome, FA Friedreich Ataxia, HCM hypertrophic cardiomyopathy, ILR implantable loop recorder, sICD subcutaneous implantable cardioverter-defibrillator, nsVT non-sustained ventricular tachycardia, SVES supraventricular extrasystole, PVC premature ventricular contraction, SCD sudden cardiac death
aMother and son, bFather and son
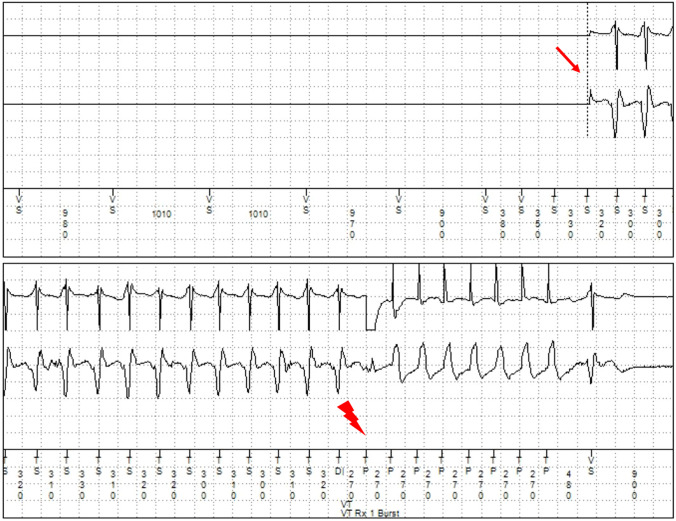
In patient ID 28, a 20-year-old male diagnosed with arrhythmogenic cardiomyopathy (ACM), sICD monitoring identified an episode of VT with a heart rate between 249 and 251/min. An ILR was implanted subsequently for detection of arrhythmias beneath the monitor zone. The patient contracted SARS-CoV-2 and a total of four VTs (cycle length 245–265 ms) could be monitored at that time (Fig. 2). He was admitted to a hospital until recovery and betablocker therapy was intensified. Indication for ablation therapy will be evaluated if intensification of pharmaceutical therapy is insufficient to reduce the arrhythmic burden.
Fig. 2.
Episode of a ventricular tachycardia (VT) in the telemonitored implantable loop recorder. The arrow marks the beginning of the VT. Right before the beginning, four ventricular extrasystoles occurred as bigeminus (asterisks)
The brother of patient ID 65 died of sudden cardiac death (SCD) at the age of 20 years. The patient himself showed signs of DCM in cardiac MRI. Genetic workup identified two proarrhythmic polymorphisms in SCN5A as well as the heterozygous nonsense variant in TTN (NM_003319.4) in the patient’s and the deceased brother’s DNA. A telemedically monitored ILR was implanted before genetic testing. Two months later, a sICD was implanted because of a detected nsVT.
The 40-year-old female patient ID 92 was affected by recurrent idiopathic ventricular fibrillation. An ICD was implanted subsequently and shocked repeatedly because of polymorphic VTs and VF. Previously performed genetic analysis had identified the heterozygous polymorphism c.433 T > C, p.(Phe145Leu) in NKX2-5 (NM_004387.3) which is associated with an elevated odds ratio for VTs and SCD next to structural heart defects (Sveinbjornsson G et al. 2018). Since recurrent VTs were detected in the monitoring of the ICD (Fig. 3), a therapy of mexiletine was started.
Fig. 3.
Telemedically detected ventricular tachycardia (VT). The detected VT was terminated by the ICD using six beats of antitachycardia pacing. Arrow = start VT, Lightning = therapy with antitachycardiac pacing
Patient ID 100 was diagnosed with Friedreich’s ataxia caused by biallelic GAA repeats in FXN. The patient was affected by recurrent atrial fibrillation (AF) and flutter, respectively, and received several ablations and cardioversion therapies. An ILR was implanted at the age of 22 years. From the age of 28 years, the patient noted episodes of tachycardia which could be correlated with episodes of AF as well as sinus tachycardia in the telemonitoring. Implantation of a Cardiac Resynchronization Therapy (CRT) pacemaker and ablation of the atrioventricular node was discussed with the patient.
Sinus tachycardia induced by physical exercising is monitored telemedically
In two further cases, data interpretation was hampered by sinus tachycardia caused by physical exercising (patient IDs 15, 97). Patient ID 15 is a 39-year-old male affected by a HCM caused by a pathogenic heterozygous nonsense variant in the MYBPC3 gene (NM_000256.3) Since the HCM Risk-SCD (O’Mahony C et al. 2014) was below the threshold of 5%, there was no clear indication for ICD implantation according to the guideline of the European Society of Cardiology (ESC) (Authors TF, M. et al. 2014). An ILR was implanted instead. Sinus tachycardia caused by regular physical exercising was repeatedly recorded.
Patient ID 97’s older sister was severely affected by left ventricular ACM caused by a heterozygous nonsense variant in DSP with multiple episodes of electrical storm, resuscitation, and performed VT ablations (Westphal DS et al. 2022). Patient ID 97 is her 25-year-old brother who also inherited the variant in DSP. He is asymptomatic; however, cardiac MRI revealed left ventricular LGE-positive areas. Based on the positive family history, an ILR was implanted. The patient was recommended to reduce physical exercise because of his extensive training. Nevertheless, telemedical monitoring was overlaid by episodes of sinus tachycardia caused by continuous training (Fig. 4).
Fig. 4.
Monitored sinus tachycardia with 180 beats per minute (bpm). The implanted implantable loop recorder detected several episodes of tachycardia. Analysis revealed episodes of sinus tachycardia is correlated with physical exercising
Discussion
From a total of 100 patients with suspected genetic diseases, 10 patients (10%) with an inborn cardiac disease of known origin were monitored via telemedicine in our centre. Although the time period of monitoring was relatively short with an average of 11.5 months [minimum: 2 months, maximum: 31 months], clinically relevant arrhythmia could be detected in 40% of the cases (4/10 cases). In patient ID 28, nsVTs during a SARS-CoV-2 infection led to admission to hospital. Because the patient did not intend to visit the hospital despite his infectious disease, these nsVTs would not have been noticed until the next control of the implanted devices in best case. Patient ID 63 received a sICD after detection of an nsVT in the telemonitoring of the ILR, whilst monitored nsVTs in patient ID 98 led to a prompt change in medication and the start of mexiletine. AF could be differentiated from benign sinus tachycardia which was repeatedly noted by patient ID 100, diagnosed with Friedreich’s ataxia. These cases illustrate the relevance of the monitored arrhythmias and the direct consequence on the further therapy in patients with diagnosed inborn arrhythmias or cardiomyopathies.
There are recommendations regarding implantation of an ICD for different genetic diseases (O’Mahony C et al. 2014; Towbin JA et al. 2019; Wilde AAM, Amin AS, Postema PG 2022). Despite recommendations, indication is not always clear. In ACM, for example, detection of nsVTs is a major criterion that can be decisive for the recommendation of an ICD implantation (Towbin JA et al. 2019). These nsVTs can escape Holter ECGs that only monitors a very limited period of time. In these cases, implantation of a telemonitored ILR may prevent losing precious time which could be vital for the patient. Although all described patients in this study were affected by known genetic diseases, telemonitoring in patients with an unsolved disease origin can be useful too. For example, implantation of an ILR can be a helpful tool if an ICD is not indicated and genetic testing identified a VUS in a proarrhythmic gene. The identification of a VUS can have challenging impacts, not only on the clinical management but also on the psychological outcome of the affected patients (Mighton C, Shickh S, Uleryk E, Pechlivanoglou P, Bombard Y 2021). Although an ILR is not able to intervene, the knowledge of a monitored heart rhythm can give the feeling of security to a certain degree (Leppert F et al. 2021).
However, there are limitations that can hamper telemonitoring. In two of the reports (patient IDs 15 and 97), analysis of the telemonitoring was disturbed by recurrent sinus tachycardia that were caused by physical exercising. Especially in patient 95, physical exercise is performed despite the recommended restriction due to the genetic predisposition for ACM. These episodes did not only make the analysis a difficult task but also led to full storages of the ILR. In principle, recordings of such benign arrhythmias can be avoided by changing the programming. However, less slow ventricular arrhythmias are not recorded if this is done. In addition, the monitoring of excessive sporting activity is also useful in individual cases for advising patients. These problems mostly arise in young patients who regular participate in sport. Considering that the average age of the patients in our centre for rare cardiac diseases is 47 years [16–80 years] which is lower than the age of usual adult cardiological patients and that the acceptance for telemonitoring is decreasing with age (Siggemann BG, C., Mensing, M., Classen, T., Hornberg, C. & Terschuren, C. 2013), this problem might play an essential role in using telemedicine in cardiogenetic patients. Apart from that, there are also general ethical issues that should be considered when implementing telemedicine such as data privacy and the obligation to continue the patient care despite the distance (Chaet D et al. 2017).
Conclusion
Cardiac arrhythmias and technical problems can be diagnosed in a short time followed by appropriate therapy in patients with a monogenic heart disease. Indication for ICD therapy can be substantiated by documented arrhythmias. Although sporting activity and sinus tachycardia bedevil telemonitoring, it can be useful for advising patients. Remote monitoring should be recommended not only for patients with severe heart failure—as in the ESC Guidelines—but also for patients with rare diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the patients for participating in this study.
Author Contribution
DSW, EM, and FH—Drafting the manuscript. SSW, TJ, BB, and SK—Genetic analysis. DSW—Genetic counselling. DF, AS, TV, EM, and FH—Telemonitoring. All authors—Critical reviewing the manuscript and final approval.
Funding
Britt Beckmann received funding by the “Dr. Rolf Schwiete Stiftung”.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Consent for publication
Written informed consent to publish the data was obtained from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eimo Martens and Franziska Hahn have contributed equally to this work.
References
- Authors TF, m. , et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(2733–2779):2014. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- Chaet D, et al. Ethical practice in Telehealth and Telemedicine. J Gen Intern Med. 2017;32:1136–1140. doi: 10.1007/s11606-017-4082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino, A. L. & Ho, C. in GeneReviews((R)) (eds M. P. Adam et al.) (1993).
- Green RC, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15:565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty CM, et al. Genomics-first evaluation of heart disease associated with titin-truncating variants. Circulation. 2019;140:42–54. doi: 10.1161/CIRCULATIONAHA.119.039573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger RE, Jordan E, et al. Dilated cardiomyopathy overview. In: Adam MP, et al., editors. GeneReviews((R)) Seattle: University of Washington; 1993. [Google Scholar]
- Jamal NE, Abi-Saleh B, Isma'eel H. Advances in telemedicine for the management of the elderly cardiac patient. J Geriatr Cardiol. 2021;18:759–767. doi: 10.11909/j.issn.1671-5411.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert F, et al. The INFluence of Remote monitoring on Anxiety/depRession, quality of lifE, and Device acceptance in ICD patients: a prospective, randomized, controlled, single-center trial. Clin Res Cardiol. 2021;110:789–800. doi: 10.1007/s00392-020-01667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang LW, et al. The use of telemedicine in cardiogenetics clinical practice during the COVID-19 pandemic. Mol Genet Genomic Med. 2022;10:e1946. doi: 10.1002/mgg3.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mighton C, Shickh S, Uleryk E, Pechlivanoglou P, Bombard Y. Clinical and psychological outcomes of receiving a variant of uncertain significance from multigene panel testing or genomic sequencing: a systematic review and meta-analysis. Genet Med. 2021;23:22–33. doi: 10.1038/s41436-020-00957-2. [DOI] [PubMed] [Google Scholar]
- Miller DT, et al. ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2021;23:1381–1390. doi: 10.1038/s41436-021-01172-3. [DOI] [PubMed] [Google Scholar]
- Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20:1193. doi: 10.1186/s12889-020-09301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony C, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD) Eur Heart J. 2014;35:2010–2020. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggemann BG, C., Mensing, M., Classen, T., Hornberg, C. & Terschuren, C. Specific health status has an impact on the willingness to use telemonitoring: data from a 2009 health survey in north rhine-westphalia, Germany. Telemed J E Health. 2013;19:692–698. doi: 10.1089/tmj.2012.0214. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsson G, et al. Variants in NKX2-5 and FLNC cause dilated cardiomyopathy and sudden cardiac death. Circ Genom Precis Med. 2018;11:e002151. doi: 10.1161/CIRCGEN.117.002151. [DOI] [PubMed] [Google Scholar]
- Theresa AM et al. (2022) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail24, 4–131 [DOI] [PubMed]
- Towbin JA, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Westphal DS, et al. Myocarditis or inherited disease? - The multifaceted presentation of arrhythmogenic cardiomyopathy. Gene. 2022;827:146470. doi: 10.1016/j.gene.2022.146470. [DOI] [PubMed] [Google Scholar]
- Wilde AAM, Amin AS, Postema PG. Diagnosis, management and therapeutic strategies for congenital long QT syndrome. Heart. 2022;108:332–338. doi: 10.1136/heartjnl-2020-318259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler S, et al. Is 24/7 remote patient management in heart failure necessary? Results of the telemedical emergency service used in the TIM-HF and in the TIM-HF2 trials. ESC Heart Fail. 2021;8:3613–3620. doi: 10.1002/ehf2.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.