Abstract
Th1-type cellular immune responses play a critical role in protection against infection with Leishmania parasites, whereas activation of Th2-type cells results in progressive disease. Cutaneous leishmaniasis caused by Leishmania major is often a self-healing disease; however, persistent nonhealing forms are also known. In the present study, we have described cell-mediated immune responses in nonhealing patients by measuring T-cell proliferation, cytokine production, and phenotypic characterization of these cells. The responses were compared with those of patients with active lesions, patients who had recovered from infection, and healthy controls. Peripheral blood mononuclear cells from patients with active lesions and recovered donors proliferated vigorously and produced Th1-type cytokine when stimulated with L. major antigens, whereas in nonhealing patients the proliferative responses were significantly lower and showed a Th2-type response to Leishmania antigens. Interleukin-10 (IL-10) production was not a feature of L. major stimulation. Flow cytometric analysis revealed that L. major antigen induced proliferation of the CD4-positive population and that these cells were the major source of gamma interferon and IL-4. These results show a distinct dichotomy in the cytokine response to L. major infection.
Leishmania major is the causative agent of zoonotic cutaneous leishmaniasis (CL) and affects millions of people in many parts of the world (30). The disease is prevalent in many areas of Iran. Hyperendemic foci of zoonotic CL in northeastern, southwestern, and central parts of Iran have been reported (9, 10, 31). The most common presentation of CL is one or a few skin lesions, which often heal spontaneously and leave a depressive scar. However, a rare presentation of infection, involving persistent lesions, is also known. These so-called nonhealing forms last for several years and do not respond to conventional chemotherapies. Patients are mostly from areas of hyperendemic infection and include some of the soldiers who were immunized with virulent parasites (leishmanization) during the Iraq war against Iran. Some of these patients experience periods of remission and reactivation at the site of inoculation, and others suffer from chronic lesion for several years.
Extensive studies with experimental models have shown that the outcome of infection is critically dependent on the activation of one of the two subsets of CD4 T cells, Th1 and Th2 (26). Gamma interferon (IFN-γ), secreted by Th1 cells, is the most potent macrophage-activating cytokine leading to host resistance to infection with Leishmania parasites (27, 29), whereas interleukin-4 (IL-4), secreted by Th2 cells, is associated with down-modulation of IFN-γ-mediated macrophage activation (1, 17). However, in human cutaneous leishmaniasis, a clear functional dichotomy in CD4 T cells has not definitely been documented. Concerning the key role of cytokines, we were interested in defining the immune response of nonhealing patients and comparing it with that of individuals who had recovered from infection and patients who were newly infected. Immune response to different species of Leishmania have been studied mostly in subjects with active lesions and/or patients who had recovered (6, 12–15, 24). There are few reports on the immune status of nonhealing patients, particularly those infected with L. major. Treatment of patients might benefit from immunological interventions, if the role of T-cell subsets in disease and resistance is clearly clarified.
MATERIALS AND METHODS
Study groups.
The study groups were as follows. (i) The first consisted of 22 patients newly infected and suffering from active CL. (ii) The second consisted of 17 patients with chronic lesion (nonhealing patients) who had a clinical history for more than 2 years. Five of these patients were soldiers who had undergone leishmanization, and the rest were from areas of hyperendemic infection. All patients had a confirmed diagnosis of leishmaniasis by visualization of Leishmania amastigotes in Giemsa-stained smears and/or culture of scrapings from lesions. Monoclonal antibody XLVI-5B8-B3 (T-1) (kindly provided by F. Modabber TDR/WHO, Geneva, Switzerland) was used to confirm the species of parasite as L. major. (iii) The third group consisted of 17 individuals who had recovered from CL and who had a scar and were leishmanin skin test positive. Donors with past or current CL were from areas of zoonotic endemic infection in Iran. (iv) The final group consisted of 15 healthy Iranian and Danish donors without a history of leishmaniasis.
Isolation of mononuclear cells.
Heparinized peripheral blood was collected and mononuclear cells (PBMC) were isolated by Lymphoprep (Nyegaard, Oslo, Norway) density centrifugation. The cells were frozen and transported in liquid nitrogen. Before use, the cells were rapidly thawed and washed. Their viability was ascertained by trypan blue dye exclusion.
Antigens and mitogen.
Soluble Leishmania antigen (SLA) was prepared by the method described by Scott et al. (28) with some modifications. L. major (MRHO/IR/75/ER) promastigotes at stationary phase were washed three times in phosphate-buffered saline and then resuspended at 109 parasites/ml in 100 mM Tris-HCl–1 mM EDTA (pH 8) supplemented with 50 ng of leupeptin per ml and 1.6 mM phenylmethylsulfonyl fluoride (all from Sigma). The suspension was subjected to ultrasonication and centrifuged at 20,000 × g for 2 h. The supernatant was stored at −70°C until use. The protein concentration was determined with a protein assay kit from Bio-Rad (Brussels, Belgium). Purified protein derivative (PPD) was purchased from Staten Serum Institute (Copenhagen, Denmark). SLA and PPD were used at final concentrations of 70 and 12 μg/ml respectively. Phytohemagglutinin was from Wellcome (Detroit, Mich.) and was used at a concentration of 40 μg/ml.
Lymphocyte proliferation.
PBMC were cultured in RPMI plus 10 mM HEPES, 20 U of penicillin per ml, and 20 μg of streptomycin per ml supplemented with 15% heat-inactivated pooled normal human serum (complete medium). The cells were incubated with SLA, PPD, and phytohemagglutinin at 6.7 × 105 cells/ml in volumes of 170 μl in round-bottom microculture plates (Nunc, Roskilde, Denmark). The cultures were incubated for 7 days at 37°C under 5% CO2 and pulsed with 0.5 μCi of [3H]thymidine (Amersham, Little Chalfont, United Kingdom) per well for the last 18 h of incubation. The stimulation index (SI) was obtained by dividing the cpm of stimulated cultures by the cpm of unstimulated cultures. The culture supernatants were recovered and stored at −20°C for later determination of IFN-γ and IL-10. The cells were harvested onto fiberglass filters and the incorporation of [3H]thymidine into DNA was determined with a matrix counter (Packard-Greve, Denmark). All tests were performed in triplicate. For each set of samples, the mean value was recorded. For the measurement of IL-4 release by antigen-stimulated cultures of PBMC, parallel cultures were carried out for 6 days and then pulsed with 1 μM ionomycin and 50 ng of phorbol myristate acetate (PMA) (both from Sigma Chemical Co., St. Louis, Mo.) for 18 h before the culture supernatants from triplicate wells were harvested.
Cytokine measurements.
IFN-γ and IL-4 in supernatants were measured by a two-site sandwich enzyme-linked immunosorbent assay using specific rabbit polyclonal immunoglobulin G (IgG) to human recombinant IFN-γ and IL-4 and the biotin-avidin system as described elsewhere (15, 16). The assays were calibrated to detect IFN-γ and IL-4 within the range of 120 to 8,500 and 30 to 2,000 pg/ml, respectively. IL-10 was measured with an OptEIA set enzyme-linked immunosorbent assay kit (Pharmingen, San Diego, Calif.) as recommended by the manufacturer.
Intracellular cytokine assay.
Intracellular cytokines were determined by a method described by Kemp et al. (11). Briefly, 106 cells/ml were incubated with antigen for 6 days. Negative control cultures consisted of the medium without antigen. Then 1.5 M monensin, 1 M ionomycin, and 50 ng of PMA per ml were added 6 h prior to the end of the incubation period. The cells were labeled with conjugated antibody directed against the cell surface markers CD3, CD4, CD8 (Dako, Glostrop, Denmark) and were fixed with formaldehyde. During fixation, the samples were vigorously vortexed to avoid cell clumping. Saponin was used to permeabilize the cells. Subsequently, cytokine-specific antibodies against IFN-γ, IL-4, and IL-10 (Pharmingen) were each added at a 1 μg/ml. After adequate washing, the cells were analyzed on FACScan flow cytometer (Becton Dickinson) using Lysis II software. Isotype-matched control antibodies were used to detect nonspecific binding to cells in each step. The cell population was defined by forward- and side-scatter gating. Statistical markers were set using the isotype-matched antibody-negative controls as a reference.
Statistical analysis.
Data were evaluated using SigmaStat 2.0 (Jandel Scientific, San Rafael, Calif.) software. The differences of sample medians were tested by the Wilcoxon-Mann-Whitney test. P < 0.05 was considered significant.
RESULTS
Clinical findings for the study groups.
The major characteristics of the study groups are summarized in Table 1. There were no significant differences in clinical and immunological findings between nonhealing patients who underwent leishmanization and those from areas of hyperendemic infection (data not shown).
TABLE 1.
Major characteristics of study groups
| Characteristic | Study group
|
|||
|---|---|---|---|---|
| Recovered | Active lesions | Non-healing | Healthy controls | |
| No. | 17 | 22 | 17 | 15 |
| Age range (yr) | 14–55 | 11–65 | 11–64 | 20–50 |
| M/F ratioa | 8/9 | 13/9 | 12/5 | 8/7 |
| Mean duration of disease | NDb | 4.1 mo | 6.3 yr | |
| Mean no. of lesions (range) | 3.3c (1–10) | 2.1 (1–7) | 1.6 (1–6) | |
| No. of patients treated with antimonials | 9 | 7 | 13 | |
Male/female ratio.
ND, not determined.
Scar.
Lymphocyte responses to antigens.
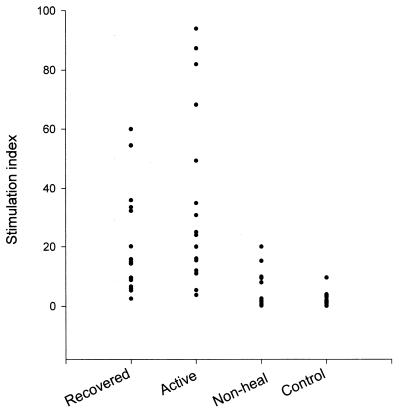
The proliferative responses of all four groups of subjects are shown in Fig. 1. SLA induced a vigorous proliferative response in PBMC from patients with active lesions and patients who had recovered, with median SIs of 25 and 15 (95% confidence intervals, 15.4 to 49.4 and 8.8 to 32.3), respectively. However, PBMC from nonhealing donors and normal controls did not proliferate in response to SLA. A significant difference was observed for SIs for patients with active lesions and patients who had recovered compared with those for nonhealing and normal donors (P < 0.001). The differences between patients with active lesions and those who had recovered, as well as between nonhealing and normal donors, were not significant. No significant differences were found between the groups in response to PPD (data not shown).
FIG. 1.
Proliferative response of PBMC from different study groups to SLA. The responses are shown as SI.
Cytokine production.
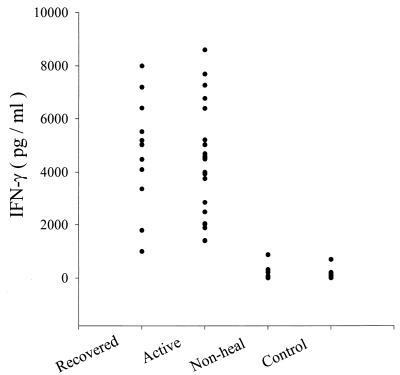
The production of IFN-γ, IL-4, and IL-10 in the supernatants of PBMC cultured with SLA or PPD was determined. Figure 2 shows the concentration of IFN-γ in the culture supernatants of PBMC. No or low IFN-γ production was observed in response to SLA in nonhealing and normal donors. Patients with active lesions and those who had recovered produced significantly larger amounts of IFN-γ than did nonhealing and normal donors (< 0.001). The difference between patients with active lesions and patients who had recovered was not significant. The levels of PPD-induced IFN-γ were comparable in all groups (data not shown). To measure antigen-dependent IL-4 production, cell cultures were provoked with ionomycin and PMA after 6 days of antigenic stimulation. Antigen-induced cytokine production was calculated as the difference in the amount of cytokine between antigen-stimulated and unstimulated cultures. Higher levels of IL-4 were detected in the supernatants of Leishmania-stimulated PBMC cultures from nonhealing patients than in supernatants of cultures from the remaining three groups (P < 0.001) (Table 2). The differences between the last three groups were not significant. Cells from all four groups produced no or little IL-4 when incubated with PPD (data not shown). Table 2 also shows that the IFN-γ/IL-4 ratio was greater in patients with active lesions and those who had recovered than in nonhealing patients. The supernatants from the cultures of PBMC stimulated with SLA were further tested to determine the levels of IL-10. The PBMC from all groups did not produce detectable IL-10 when incubated with either SLA or PPD (data not shown).
FIG. 2.
Concentrations of IFN-γ in culture supernatant of SLA-stimulated cells from different study groups.
TABLE 2.
IL-4 concentrations in supernatants of PBMCs stimulated with SLA and median ratios of IFN-γ to IL-4 concentration after stimulation
| Study group | Median IL-4 level (95% CI) (pg/ml) | IFN-γ/IL-4 ratio |
|---|---|---|
| Recovered | 37.8 (0–91.7) | 133.1 |
| Active lesions | 22.8 (2.4–49.1) | 198.5 |
| Nonhealing | 146.9 (103.1–261.6)a | 0.2 |
| Healthy controls | 47.6 (0–56.6) | 0.0 |
P < 0.001 compared to each of the other three groups.
Flow cytometry.
In a phenotypic analysis of SLA-responding T cells, the CD4/CD8 ratios in PBMC of all four groups after 7 days in culture with or without SLA were determined and are shown in Table 3. The CD4/CD8 ratios in unstimulated cultures from the four groups were almost identical. However, this ratio was significantly different when stimulated cells from the four groups were compared (P < 0.001). Moreover, CD4/CD8 ratios in patients with active lesions and those who had recovered were significantly higher in stimulated cultures than in unstimulated cultures (P < 0.001 and P < 0.001, respectively). In responder mononuclear cells, the forward-scatter signal, reflecting cell size, was increased, particularly in the CD4 cell population. PBMC of nonhealing and control donors failed to show any significant difference in the CD4/CD8 ratio between stimulated and unstimulated cultures (P ≥ 0.3).
TABLE 3.
CD4/CD8 ratios in PBMCs from study groups without and with SLA stimulation
| Study group | CD4/CD8 ratio in:
|
||
|---|---|---|---|
| Unstimulated samples | Stimulated samples | P | |
| Recovered | 2.6 (2, 4.1) | 9.0 (2.4, 44) | <0.001 |
| Active lesions | 3 (2.4, 20.3) | 8.6 (5.7, 12.4) | <0.01 |
| Nonhealing | 1.4 (1.8, 3.8) | 3.7 (2.6, 6) | 0.37 |
| Healthy controls | 0.8 (1.8, 4.4) | 2.3 (1.6, 3.2) | 0.90 |
Analysis of intracellular cytokines.
Intracellular cytokine staining was used to detect the frequency of IFN-γ, IL-4, and IL-10-producing CD4+ and CD8+ T cells. Analysis was carried out for 13 patients with active lesions, 13 who had recovered, 9 nonhealing patients, and 12 healthy controls. No statistically significant differences were observed between unstimulated cultures of PBMC from the four groups. However, incubation with SLA resulted in different intracellular cytokine patterns among different groups. As shown in Table 4, the percentage of IFN-γ-producing cells after stimulation with SLA was significantly higher in patients with active lesions and those who had recovered than in nonhealing and control donors (P < 0.005). In contrast, after SLA stimulation, a higher percentage of cells from the nonhealing group expressed both CD4 and IL-4. The difference was statistically significant (P < 0.001) (Table 4). In all donors tested, the majority of the cells expressing intracellular cytokines after incubation with SLA were CD4+ T cells. There were no significant differences between the percentages of unstimulated and stimulated IFN-γ-producing (Table 5) or IL-4-producing (data not shown) CD8+ cells.
TABLE 4.
Percentage of T cells expressing CD4 and cytokine
| Group | Median % of cellsa expressing CD4 and:
|
|
|---|---|---|
| IFN-γ | IL-4 | |
| Recovered | 3.0 (2.0–11.4) | 10.2 (3.3–14.7) |
| Active lesions | 3.6 (1.8–11.2) | 12.3 (10.0–19.1) |
| Nonhealing | 1.7 (0.2–3.5) | 25.7 (11.4–37.9) |
| Healthy controls | 1.0 (0.7–1.7) | 8.4 (5.3–13.7) |
Numbers in parentheses are 95% CI.
TABLE 5.
Percentages of IFN-γ-producing CD8 T cells with and without SLA stimulation
| Group | Median % of IFN-γ-producing cellsa
|
||
|---|---|---|---|
| Unstimulated | Stimulated | P | |
| Recovered | 0.5 (0.2–0.8) | 0.6 (0.2–1.9) | 0.7 |
| Active lesions | 1.5 (0.6–0.3) | 1.1 (0.6–3.9) | 0.9 |
| Nonhealing | 1.2 (0.3–3.4) | 1.6 (0.5–2.4) | 1 |
| Healthy controls | 0.9 (0.6–4.2) | 1 (0.4–3.0) | 0.5 |
Numbers in parentheses are 95% CI.
DISCUSSION
L. major causes rural cutaneous leishmaniasis, which is endemic in different parts of Iran (9, 10, 31). CL is often a self-healing disease; however, persistent lesion lasting for several years is known (nonhealing form). Immune responses to Leishmania have been studied mostly in subjects with active lesions and/or those who had recovered from infection with Old or New World Leishmania parasites (6, 12–15, 24). There are few reports on the immune status of nonhealing patients, particularly those infected with L. major (25). Since the clinical manifestation of leishmaniasis depends on both the species of the parasite and the immune response of the host, we attempted to define the immunological profile of nonhealing patients and compare it with those of newly infected patients and those of patients who had recovered. PBMC from unexposed Iranian and Danish donors were used as healthy controls.
The proliferative response to Leishmania antigen was compared between the different groups. PBMCs from patients with active lesions and those who had recovered showed strong proliferation in response to SLA; however, the cells from nonhealing patients and healthy controls did not respond to this antigen. This finding is in agreement with the findings of Kemp et al. (13), who found that PBMC from individuals with a history of self-healing CL proliferated vigorously in response to Leishmania antigen, and the findings of Gaafar et al. (6), who reported that patients with severe form of CL showed lower proliferative response to Leishmania antigen. Since there were no significant differences among the groups in response to PPD, the unresponsiveness of cells from nonhealing donors to Leishmania antigen seems to be antigen specific.
PBMC from patients with active lesions and individuals who had recovered produced large amounts of IFN-γ and no or little IL-4 in response to SLA, whereas no or low IFN-γ and high IL-4 production were observed in nonhealing donors. PBMC from normal controls did not produce considerable levels of IFN-γ or IL-4 in response to SLA. These patterns of cytokine production extend the previous observation of IL-4 and no IFN-γ production in nonhealing patients (25) and IFN-γ production in individuals who had recovered (13). Although Gaafar et al. did not find any difference in IL-4 production between patients with mild and severe disease, they found an association between IFN-γ production and severity of the disease, i.e., high IFN-γ production associated with mild disease and low IFN-γ production associated with severe disease (6). However, our results showed activation of Th1-like cells in both patients with active lesions and those who had recovered. Coutinho et al. have reported finding mixed type 1 and type 2 cytokines during the active American CL and type 1 cytokines after therapy (3). The findings of the present study clearly show a functional dichotomy in the cytokine response to L. major promastigote antigens in PBMC from patients with different manifestations of disease. Furthermore, these findings confirm the role of IFN-γ in the healing process and the role of IL-4 in persistence of the lesion in humans, as previously shown in mice (2).
IL-10 is a potent inhibitor of proliferation and cytokine production by Th1 cells (5, 8) and favors the development of a Th2-type immune response (21, 22). IL-10 production by PBMC from patients infected with L. major has not previously been reported. We found no detectable IL-10 production in culture supernatants from the studied groups. This is in contrast to high levels of IL-10 in plasma (7) and high levels of IL-10 production by PBMC from patients with visceral leishmaniasis (8). Furthermore, IL-10 mRNA expression in lesions from patients infected with L. mexicana (20) and L. major (18) have been demonstrated. However, our findings are in agreement with those of Maasho et al. (19), who reported that PBMC from CL patients infected with L. aethiopica did not produce IL-10 in response to L. aethiopica antigens. PBMC do not necessarily reflect the situation at the site of infection (12). Furthermore, there are different types of cells in the lesion that can produce IL-10 (7, 23); therefore, extracted RNA from skin biopsy specimens does not necessarily reflect the mononuclear origin of IL-10.
To identify the source of cytokines and the phenotype of cytokine-producing cells, an intracellular cytokine assay was carried out using flow cytometry. The percentage of IFN-γ-producing cells generated in response to SLA was significantly higher in patients with active lesions and those who had recovered, whereas cells from the nonhealing group expressed more IL-4. The phenotype of T cells activated by SLA was identified by flow cytometry. Increased forward scatter, reflecting cell size, was observed only within the CD4+ T cell population, indicating SLA-induced blastogenesis in this population. In addition, an increased CD4/CD8 ratio after stimulation, which was seen only in responder cells (cells from patients with active lesions and those who had recovered) confirmed this finding. Our results showed that in both patients with active lesions and those who had recovered, Leishmania-reactive cells were CD4+ T cells. These findings are in contrast to those of Coutinho et al. (3) and Da-Cruz et al. (4), who reported the predominance of responding CD8+ T cells in individuals cured from and vaccinated against American CL, respectively. Maasho et al. have also indicated a larger proportional increase of CD8+ cells in response to Leishmania antigen in individuals who had recovered from L. aethiopica infection (28). This disagreement might be due to the different species of parasite. Finally, flow cytometric analysis revealed that in all groups, CD4+ cells were the major source of IFN-γ and IL-4.
The data presented in this study demonstrate the existence of a Th1-like response to L. major antigens in patients with active lesions and those who had recovered and a Th2-like response in nonhealing patients. Production of large amounts of IL-4 and low levels of IFN-γ by PBMC from nonhealing patients and unresponsiveness of these patients to chemotherapies suggest that these patients might benefit from immunological interventions.
ACKNOWLEDGMENTS
We are grateful to Marjan Yousefi, Anne Corfitz, and Gitte Pedersen for technical assistance.
This work was supported by a training grant from UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Disease to S.A., and by the Danish Biotechnology Programme.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan C, Gessner A, Solbuch W, Rollinghoff M. Invasion, control and persistence of Leishmania parasites. Curr Opin Immunol. 1996;8:517–525. doi: 10.1016/s0952-7915(96)80040-9. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho S G, Oliveira M P, Da-Cruz A M, De Luca P M, Mendonca S C F, Berthol A L, Soong L, McMahon-Pratt D. T cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania brasiliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–155. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- 4.Da-Cruz A M, Conceicao-Silva F, Bertho A L, Coutinho S G. Leishmania-reactive CD4 and CD8 T cells associated with cure of human cutaneous leishmaniasis. Infect Immun. 1994;62:2614–2618. doi: 10.1128/iai.62.6.2614-2618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorentino D F, Ziotnik A, Vieira P, Mosmann T R, Howard M, Moore K W, O'Garra A. IL-10 acts on the antigen presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 6.Gaafar A, Kharazmi A, Ismail A, Kemp M, Hey A, Christensen C B V, Dafalla M, El Kadaro A Y, El Hassan A M, Theander T G. Dichotomy of T cell response to leishmania antigens in patients suffering from cutaneous leishmaniasis: absence or scarcity of Th1 activity is associated with severe infections. Clin Exp Immunol. 1995;100:239–245. doi: 10.1111/j.1365-2249.1995.tb03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasim S, El Hassan A M, Khalil E A G, Ismail A, Kadaro A M Y, Kharazmi A, Theander T G. High levels of plasma IL-10 and expression of IL-10 by keratinocytes during visceral leishmaniasis predict subsequent development of post-kala-azar dermal leishmaniasis. Clin Exp Immunol. 1998;111:64–69. doi: 10.1046/j.1365-2249.1998.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghalib H W, Puvezam M R, Skeiky Y M, Siddig M, Hashim F A, El Hassan A M, Russo D M, Reed S G. Interleukin-10 production correlates with pathology in human Leishmania donovani infection. J Clin Investig. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javadian E, Mesghali A. Studies on cutaneous leishmaniasis in Khuzestan, Iran. 1. The leptomonad infection of sandflies. Bull Soc Pathol Exot Fil. 1974;67:513–516. [PubMed] [Google Scholar]
- 10.Javadian E, Nadim A, Tahvildare-Bidrudi G, Assefi N. Epidemiology of cutaneous leishmaniasis in Iran B. Khorassan. V. Report on a focus of zoonotic cutaneous leishmaniasis in Esferayen. Bull Soc Pathol Exot Fil. 1976;69:140–143. [PubMed] [Google Scholar]
- 11.Kemp K, Hviid L, Kharazmi A, Kemp M. IFN-γ production by human T cells and natural killer cells in vitro in response to antigens from two intracellular pathogens Mycobacterium tuberculosis and Leishmania major. Scand J Immunol. 1997;46:495–499. doi: 10.1046/j.1365-3083.1997.d01-154.x. [DOI] [PubMed] [Google Scholar]
- 12.Kemp K, Theander T G, Hviid L, Gafaar A, Kharazmi A, Kemp M. IFN-γ and TNF-α producing cells in humans who are immune to cutaneous leishmaniasis. Scand J Immunol. 1999;49:655–659. doi: 10.1046/j.1365-3083.1999.00554.x. [DOI] [PubMed] [Google Scholar]
- 13.Kemp M, Hey A S, Kurtzhals J A L, Christensen C B V, Gaafar A, Mustafa M D, Kordofani A A Y, Ismail A, Kharazmi A, Theander T G. Dichotomy of the human T cell response to Leishmania antigens. I. Th1-like response to Leishmania major promastigote antigens in individuals recovered from cutaneous leishmaniasis. Clin Exp Immunol. 1994;96:410–415. doi: 10.1111/j.1365-2249.1994.tb06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemp M, Handman E, Kemp K, Ismail A, Mustafa M D, Kordofani A Y, Bendtzen K, Kharazmi A, Theander T G. The Leishmania promastigote surface antigen-2 (PSA-2) is specifically recognized by Th1 cells in humans with naturally acquired immunity to L. major. FEMS Immunol Med Microbiol. 1998;20:209–218. doi: 10.1111/j.1574-695X.1998.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 15.Kemp M, Theander T G, Kharazmi A. The contrasting roles of CD4+ T cells in intracellular infections in human: Leishmania as an example. Immunol Today. 1996;17:13–16. doi: 10.1016/0167-5699(96)80562-7. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzhals J A L, Hey A S, Jardim A, Kemp M, Schaefer K U, Odera E O, Christensen C B V, Githure G I, Olafson R W, Theander T G, Kharazmi A. Dichotomy of the human T cell response to Leishmania antigens. II. Absent or Th2-like response to gp63 and Th1-like response to lipophosphoglycan associated protein in cells from cured visceral leishmaniasis patients. Clin Exp Immunol. 1994;96:416–421. doi: 10.1111/j.1365-2249.1994.tb06044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis J, Himmelrich H, Parra-Lopez C, Tacchini-Cottier F, Launois P. Regulation of protective immunity against Leishmania major in mice. Curr Opin Immunol. 1998;10:459–464. doi: 10.1016/s0952-7915(98)80121-0. [DOI] [PubMed] [Google Scholar]
- 18.Louzir H, Melby P C, Ben Salah A, Marrakchi H, Aoun K, Ben Ismail R, Dellagi K. Immunologic determinants of disease evaluation in localized cutaneous leishmaniasis due to Leishmania major. J Infect Dis. 1998;177:1687–1695. doi: 10.1086/515297. [DOI] [PubMed] [Google Scholar]
- 19.Maasho K, Sanchez F, Schurr E, Hailu A, Akuffo H. Indications of the protective role of natural killer cells in human cutaneous leishmaniasis of endemicity. Infect Immun. 1998;66:2698–2704. doi: 10.1128/iai.66.6.2698-2704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melby P C, Andrade-Narraez F, Darnell B J, Valencia-Pacheco G. In situ expression of interleukin-10 and interleukin-12 in active human cutaneous leishmaniasis. FEMS Immunol Med Microbiol. 1996;15:101–107. doi: 10.1111/j.1574-695X.1996.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore K W, O'Garra A, de-Waal M R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann T R, Moore K W. The role of IL-10 in cross regulation of Th1 and Th2 responses. Immunol Today. 1991;12:49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 23.Nilsen R, Mshana R N. In situ characterization of the cellular immune response in Ethiopian cutaneous leishmaniasis. Scand J Immunol. 1987;26:503–512. doi: 10.1111/j.1365-3083.1987.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 24.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceicao-Silva F, Modlin R. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Investig. 1993;91:1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafati S, Couty-jouve S, Alimohammadian M H, Louis J A. Biochemical analysis and immunogenicity of Leishmania major amastigote fractions in cutaneous leishmaniasis. Clin Exp Immunol. 1997;110:203–211. doi: 10.1111/j.1365-2249.1997.tb08318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiner S L, Locksly R. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 27.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 28.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in murine model I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–227. [PubMed] [Google Scholar]
- 29.Swihart K, Fruth U, Messmer N, Hug K, Behin R, Huang S, Del-Giudice G, Aguet M, Leuis J A. Mice from genetically resistant background lacking IFN-g receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell type-1 CD4 T cell response. J Exp Med. 1995;181:961–971. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Information circulation. Vol. 40. WHO Mediterranian Zoonosis Control Center.; 1996. pp. 11–13. [Google Scholar]
- 31.Yaghoobi-Ershadi M R, Javadian E. Epidemiological study of reservoir hosts in an endemic area of zoonotic cutaneous leishmaniasis. Bull W H O. 1996;74:587–590. [PMC free article] [PubMed] [Google Scholar]