Abstract
Oral nirmatrelvir plus ritonavir (Paxlovid™) is an effective treatment option for coronavirus disease 2019 (COVID-19), the illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Nirmatrelvir inhibits the main protease of SARS-CoV-2, with ritonavir acting as a pharmacokinetic booster. In the phase II/III EPIC-HR trial, nirmatrelvir plus ritonavir reduced the risk of progression to severe COVID-19 in symptomatic, unvaccinated, non-hospitalized adults with mild-to-moderate COVID-19 at high risk for progression to severe disease. The incidence of COVID-19-related hospitalization or death through day 28 was significantly lower with nirmatrelvir plus ritonavir than with placebo. The efficacy of nirmatrelvir plus ritonavir has also been demonstrated in the real-world setting. Nirmatrelvir plus ritonavir is generally well tolerated, with most adverse events being of mild or moderate severity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40267-022-00971-1.
Plain Language Summary
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the coronavirus disease 2019 (COVID-19) global pandemic. Nirmatrelvir plus ritonavir (Paxlovid™) is an oral antiviral treatment for COVID-19 that reduces the ability of SARS-CoV-2 to multiply in the body. The active substance nirmatrelvir blocks the activity of an enzyme needed by the virus to multiply, while ritonavir slows the breakdown of nirmatrelvir to increase its therapeutic benefit. Nirmatrelvir plus ritonavir reduces the risk of hospitalization or death in patients who are at increased risk for progression to severe COVID-19. The drug is generally well tolerated; most adverse events were mild or moderate in severity. Drug–drug interactions are possible. Nirmatrelvir plus ritonavir is an important treatment option for COVID-19 in patients whose age or underlying health puts them at high risk for becoming severely ill.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40267-022-00971-1.
| Digital Features for this Adis Drug Q&A can be found at 10.6084/m9.figshare.21554598 |
Adis evaluation of nirmatrelvir plus ritonavir in the treatment of COVID-19
| Potent inhibitor of the main protease of SARS-CoV-2, co-packaged with a pharmacokinetic booster |
| Administered orally twice daily for 5 days |
| Reduces the risk of hospitalization or death in patients at high risk of progressing to severe COVID-19 |
| Generally well tolerated |
| May be associated with significant drug–drug interactions |
What is the rationale for developing nirmatrelvir plus ritonavir to treat COVID-19?
Coronavirus disease 2019 (COVID-19), the illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first appeared in late 2019 and was quickly declared a global pandemic [1]. Despite the rapid development and authorisation of numerous COVID-19 vaccines, increasing rates of breakthrough infection due to waning vaccine efficacy and the emergence of new variants of concern have necessitated the use of further measures to control the pandemic, including booster doses and the development of several therapeutic agents [2]. Some treatments are only recommended for use in patients who are at high risk of progression to severe COVID-19 (e.g. older age, immunodeficiencies, comorbidities, lack of vaccination) [3].
Nirmatrelvir plus ritonavir (Paxlovid™) was one of the first orally available antiviral treatments for COVID-19 [4, 5]. Nirmatrelvir is a peptidomimetic inhibitor of the main protease (Mpro) of SARS-CoV-2 [5]. The HIV-1 protease inhibitor ritonavir has no activity against SARS-CoV-2 Mpro, but acts as a pharmacokinetic boosting agent [6, 7]. Nirmatrelvir plus ritonavir is available in several countries, including the USA [7] and those of the EU [6], for the treatment of COVID-19 in patients who are at increased risk for progression to severe disease. Table 1 provides a summary of the prescribing information for nirmatrelvir plus ritonavir in the aforementioned regions.
Table 1.
Summary of the prescribing information of nirmatrelvir plus ritonavir (Paxlovid™) for the treatment of COVID-19 in the USA [7] and the EU [6]
| What is the authorized indication for nirmatrelvir plus ritonavir? | |
| USA | Treatment of mild-to-moderate COVID-19 in adults and paediatric pts (aged ≥ 12 years weighing ≥ 40 kg) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death (EUA) |
| EU | Treatment of COVID-19 in adults who do not require supplemental oxygen and who are at increased risk for progression to severe COVID-19 |
| What is the recommended dosage of nirmatrelvir plus ritonavir? | |
| Nirmatrelvir 300 mg (two 150 mg tablets) with ritonavir 100 mg (one 100 mg tablet) all taken together orally twice daily for 5 days | |
| How should nirmatrelvir plus ritonavir be administered? | |
| Take with or without food; swallow tablets whole (do not chew, break or crush) | |
| Initiate treatment as soon as possible after COVID-19 diagnosis and within 5 days of symptom onset | |
| Complete full 5-day treatment course even if pt requires hospitalization due to severe or critical COVID-19 after starting treatment | |
| What are the contraindications to the use of nirmatrelvir plus ritonavir? | |
| Pts with a history of clinically significant hypersensitivity reactions to nirmatrelvir, ritonavir, or any other components of the product | |
| Pts receiving drugs that are highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious and/or life-threatening reactions | |
| Pts receiving drugs that are potent CYP3A inducers where significantly reduced nirmatrelvir or ritonavir plasma concentrations may be associated with the potential for loss of virological response and possible resistance | |
| How should nirmatrelvir plus ritonavir be used in special populations? | |
| Kidney impairment | Mild: no dosage adjustment required |
| Moderate (eGFR ≥ 30 to < 60 mL/min): reduce dosage of nirmatrelvir to 150 mg twice daily | |
| Severe (eGFR < 30 mL/min): not recommended for use | |
| Hepatic impairment | Mild or moderate (Child-Pugh Class A or B): no dosage adjustment required |
| Severe (Child-Pugh Class C): not recommended for use | |
| Pts receiving other products containing ritonavir or cobicistat | No dosage adjustment required |
| What other special warnings/precautions pertain to the use of nirmatrelvir plus ritonavir? | |
| Hepatotoxicity | May cause hepatic transaminase elevations, clinical hepatitis and jaundice; exercise caution in pts with pre-existing liver disease, liver enzyme abnormalities or hepatitis |
| HIV-1 resistance | May increase risk of HIV-1 developing resistance to HIV protease inhibitors in pts with uncontrolled or undiagnosed HIV-1 infection |
| Allergic reactions/hypersensitivity | May cause hypersensitivity reactions; discontinue immediately if signs/symptoms of clinically significant hypersensitivity or anaphylaxis occur and initiate appropriate medications and/or supportive care (USA) |
Unless otherwise stated, information applies to both the USA and the EU. Consult local prescribing information for further details
COVID-19 coronavirus disease 2019; eGFR estimated glomerular filtration rate, EUA Emergency Use Authorization, pt(s) patient(s), SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
How does nirmatrelvir plus ritonavir work?
Nirmatrelvir is a potent inhibitor of the SARS-CoV-2 Mpro, also known as 3C-like protease or nsp5 protease [7]. SARS-CoV-2 Mpro is responsible for processing two large polyprotein precursors (pp1a and pp1ab) into the smaller functional units required for viral replication [8]. Nirmatrelvir binds directly to the SARS-CoV-2 Mpro active site, inhibiting the activity of SARS-CoV-2 Mpro and thus preventing viral replication [6, 7]. In a biochemical assay, nirmatrelvir inhibited the activity of SARS-CoV-2 Mpro with an inhibition constant of 3.1 nM and a half-maximal inhibitory concentration of 19.2 nM [7]. Ritonavir lacks activity against SARS-CoV-2 Mpro, but increases plasma concentrations of nirmatrelvir by inhibiting the CYP3A-mediated metabolism of nirmatrelvir [6, 7].
Nirmatrelvir demonstrated antiviral activity against SARS-CoV-2 infection of differentiated normal human bronchial epithelial cells, with 50 and 90% maximal effective concentrations of 62 and 181 nM, respectively, after 3 days of drug exposure [6, 7]. The potency of nirmatrelvir against current and emerging SARS-CoV-2 variants is similar to that against wild-type SARS-CoV-2 [9, 10]. In vitro, nirmatrelvir had potent cell culture antiviral activity against all isolates belonging to the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron (B.1.1.529) SARS-CoV-2 variants of concern, and the Lambda (B.1.1.1.37) and Mu (B.1.621) SARS-CoV-2 variants of interest [10, 11]. Nirmatrelvir also demonstrated antiviral activity in a murine model of SARS-CoV-2, reducing lung viral titres and improving disease indicators relative to placebo [7].
The impact of naturally occurring SARS-CoV-2 Mpro polymorphisms (of unknown clinical significance) on nirmatrelvir activity was assessed in a biochemical assay using recombinant Mpro enzyme [6]. G15S, T135I, S144A, H164N, H172Y, Q189K and D248E amino acid substitutions were associated with 3.5- to 233-fold reductions in the activity of nirmatrelvir. Of note, G15S is present in the Lambda variant of SARS-CoV-2, which did not have reduced susceptibility to nirmatrelvir in cell culture. In the phase II/III EPIC-HR trial, a number of treatment-emergent substitutions in SARS-CoV-2 Mpro gene or cleavage regions were detected. However, none of these substitutions occurred in hospitalized patients receiving nirmatrelvir plus ritonavir. The P132H/L/S, A260V and A266V substutitions did not reduce the activity of nirmatrelvir in a biochemical assay. Due to their different mechanisms of action, no cross-resistance is expected between nirmatrelvir and remdesivir or anti-SARS-CoV-2 monoclonal antibodies (mAbs) [6].
What are the pharmacokinetic properties of nirmatrelvir plus ritonavir?
Ritonavir is coadministered with nirmatrelvir and acts as a pharmacokinetic enhancer [6, 7]. In healthy volunteers, ritonavir increased systemic nirmatrelvir exposure and prolonged its half-life [6, 7, 12]. This supported the selection of a regimen of nirmatrelvir 300 mg plus ritonavir 100 mg twice daily for 5 days in subsequent phase II/III trials [12]. The increase in systemic exposure of nirmatrelvir at steady state appears to be less than dose proportional following repeated twice-daily oral administration of nirmatrelvir plus ritonavir (75 mg + 100 mg, 250 mg + 100 mg, and 500 mg + 100 mg) [6]. Steady state is achieved on day 2, with ≈ 2-fold accumulation [6, 7]. Relative to fasting conditions, administration of nirmatrelvir plus ritonavir with a high-fat meal modestly increased nirmatrelvir exposure; therefore, nirmatrelvir plus ritonavir can be administered with or without food (Table 1) [6, 7].
Following a single oral dose of nirmatrelvir 300 mg plus ritonavir 100 mg in healthy volunteers, maximum plasma concentrations of nirmatrelvir and ritonavir were reached in a median of 3.00 and 3.98 h, respectively [6, 7]. Nirmatrelvir is ≈ 69% bound to plasma proteins [6, 7], with a blood-to-plasma ratio of 0.60 [7]. Ritonavir is highly protein-bound (≈ 98–99%) [6, 7], with a blood-to-plasma ratio of 0.14 [7]. The mean volume of distribution is 10.5 L for nirmatrelvir and 112.4 L for ritonavir [7].
Metabolism of nirmatrelvir is by CYP3A4 primarily, but this metabolism is inhibited by the coadministration of ritonavir [6, 7]. Ritonavir is metabolized predominantly by CYP3A4, with a minor contribution from CYP2D6 to form the oxidation metabolite M-2. Nirmatrelvir is excreted via the urine (≈ 50% of a 300 mg dose) and faeces (≈ 35%), mostly as unchanged drug. Ritonavir is eliminated primarily via the hepatobiliary system, with ≈ 86% of a radiolabeled dose excreted via the faeces [6, 7]. The mean oral clearance is 8.99 L/h for nirmatrelvir and 13.92 L/h for ritonavir [7]. The mean half-life of both nirmatrelvir and ritonavir is 6.1 h [6, 7].
Significant and clinically relevant pharmacokinetic drug–drug interactions may occur when nirmatrelvir plus ritonavir is coadministered with various other drugs (e.g. CYP3A substrates, inducers or inhibitors) [6, 7]. Some contraindications apply (Table 1) and dosage adjustments or additional monitoring may be required [6, 7]. Consult local prescribing information for further detailed information.
What is the efficacy of nirmatrelvir plus ritonavir in COVID-19?
Nirmatrelvir plus ritonavir reduces the risk of progression to severe COVID-19 in symptomatic, unvaccinated, non-hospitalized adults with mild-to-moderate COVID-19 at high risk for progression to severe disease, based on the results of a randomized, double-blind, multicentre, placebo-controlled, phase II/III trial (EPIC-HR) [13].
Participants in EPIC-HR were aged ≥ 18 years with confirmed SARS-CoV-2 infection, symptom onset ≤ 5 days prior to randomization, at least one sign or symptom of COVID-19 on the day of randomization, and at least one characteristic or co-existing condition associated with high risk of progression to severe COVID-19 [13]. Exclusion criteria included patients with previous SARS-CoV-2 infection or vaccination. Eligible patients were randomized to receive either nirmatrelvir 300 mg plus ritonavir 100 mg or placebo, administered orally every 12 h for 5 days. Randomization was stratified by geographic region and by receipt or expected receipt of COVID-19 mAbs. At baseline, the median age of patients was 46 years and the median time since first symptom was 3 days (range 0–9 days). Most (71.5%) patients were white and 51.1% of patients were male. The most common risk factors for progression to severe COVID-19 were body mass index (BMI) ≥ 25 kg/m2 (80.5%), current smoking (39.0%) and hypertension (32.9%). The primary endpoint was the proportion of patients with COVID-19-related hospitalization or death from any cause through day 28, assessed in the modified intention-to-treat (mITT) population (i.e. patients whose treatment began within 3 days of symptom onset, who did not receive nor were expected to receive COVID-19 mAbs) [13].
In a planned interim analysis of data from 774 patients in the mITT population, the incidence of COVID-19-related hospitalization or death through day 28 was significantly lower with nirmatrelvir plus ritonavir than with placebo, corresponding to a relative risk reduction (RRR) of 89.1% (Table 2) [13]. Consistent results were seen in the final analysis of data from 1379 patients in the mITT population (Table 2). The efficacy of nirmatrelvir plus ritonavir was also consistent across subgroups based on age (< 65 and ≥ 65 years), sex, BMI (< 25, 25 to > 30 and ≥ 30 kg/m2), diabetes, time since symptom onset (≤ 3 and > 3 days), baseline SARS-CoV-2 serology status (negative and positive) and receipt or expected receipt of COVID-19 mAbs [13].
Table 2.
Efficacy of nirmatrelvir plus ritonavir in the phase II/III EPIC-HR trial [13]
| Endpoint [no. of pts (%)]a | Nirmatrelvir + ritonavir | Placebo | Difference [% (95% CI)] | RRR (%) |
|---|---|---|---|---|
| Interim analysis | (n = 389) | (n = 385) | ||
| COVID-19-related hospitalization or death through day 28b | 3 (0.77)* | 27 (7.01) | − 6.32 (− 9.04, − 3.59) | 89.1 |
| Final analysis | (n = 697) | (n = 682) | ||
| COVID-19-related hospitalization or death through day 28b | 5 (0.72)* | 44 (6.45) | − 5.81 (− 7.78, − 3.84) | 88.9 |
| COVID-19-related hospitalization by day 28 | 5 (0.72) | 44 (6.45) | ||
| Death from any cause by day 28 | 0 | 9 (1.32) |
COVID-19 coronavirus disease 2019, pts patients, RRR relative risk reduction
*p < 0.001 vs placebo
aModified intention-to-treat population
bPrimary endpoint
Among patients whose treatment began within 5 days of symptom onset (n = 2085), eight (0.77%) nirmatrelvir plus ritonavir recipients were hospitalized for COVID-19 or died from any cause through day 28 compared with 66 (6.31%) placebo recipients (difference − 5.62%; 95% CI − 7.21, − 4.03; p < 0.001), corresponding to an 87.8% RRR (key secondary endpoint) [13]. When data from all patients (n = 2224) were analysed, including those who received or were expected to receive mAbs, COVID-19 hospitalization or death from any cause occurred in nine (0.81%) patients in the nirmatrelvir plus ritonavir group and 68 (6.10%) patients in the placebo group (difference − 5.36%; 95% CI − 6.88, − 3.84; p < 0.0001) [13].
At day 5 of treatment, the viral load was significantly (p < 0.001) lower with nirmatrelvir plus ritonavir than with placebo [13]. After adjusting for baseline viral load, serology status and geographic region, the mean difference was − 0.868 log10 copies/mL when treatment was initiated within 3 days of symptom onset and − 0.695 log10 copies/mL when treatment was initiated within 5 days of symptom onset. Similar results were seen when patients who received or were expected to receive mAbs were included in the analysis. Results from subgroup analyses were consistent with those in the overall population, regardless of baseline serology status and viral load [13].
Real-world experience with nirmatrelvir plus ritonavir supports the efficacy results observed during the EPIC-HR trial [14–20]. Reductions in the risk of severe COVID-19 [17], mortality [15–19], hospitalization [14–16, 19, 20] and disease progression (mortality, invasive mechanical ventilation or intensive care unit admission) [15, 18] were reported across studies in Hong Kong [15, 18, 19], Israel [16, 17] and the USA [14, 20] that each included ≥ 1000 patients treated with nirmatrelvir plus ritonavir.
What is the tolerability profile of nirmatrelvir plus ritonavir?
Nirmatrelvir plus ritonavir is generally well tolerated in adult patients with symptomatic SARS-CoV-2 infection, based on data from the EPIC-HR trial [13]. Adverse events (AEs) reported during or after the treatment period occurred in 22.6% of nirmatrelvir plus ritonavir recipients and 23.9% of placebo recipients. The most common (incidence ≥ 2%) AEs with nirmatrelvir plus ritonavir were dysgeusia (5.6% vs 0.3% with placebo), diarrhoea (3.1 vs 1.6%), increased fibrin D-dimer (1.9 vs 2.8%) and increased alanine aminotransferase (1.5 vs 2.4%). Most AEs were of mild or moderate severity. Serious AEs occurred at a lower incidence with nirmatrelvir plus ritonavir than with placebo (1.6 vs 6.6%). AEs led to discontinuation of study medication in 2.1% of nirmatrelvir plus ritonavir recipients and 4.2% of placebo recipients. AEs considered to be related to study medication occurred in 7.8% of nirmatrelvir plus ritonavir recipients and 3.8% of placebo recipients and, in nirmatrelvir plus ritonavir recipients, were most commonly dysgeusia (4.5 vs 0.2%) and diarrhoea (1.3 vs 0.2%) [13].
What is the current clinical role of nirmatrelvir plus ritonavir in COVID-19?
Nirmatrelvir plus ritonavir is an effective treatment option for COVID-19 in patients who are at increased risk for progression to severe disease. Administered orally for 5 days as soon as possible after COVID-19 diagnosis, nirmatrelvir plus ritonavir reduces the risk of hospitalization or death in symptomatic, unvaccinated, non-hospitalized adults with mild-to-moderate disease. The efficacy of nirmatrelvir plus ritonavir has also been demonstrated in the real-world setting. Nirmatrelvir plus ritonavir is generally well tolerated, with most AEs being mild or moderate in severity.
Despite the proven efficacy of nirmatrelvir plus ritonavir, there have been reports of virological rebound (i.e. recurrent COVID-19 symptoms and/or increased viral load) following treatment [21–25]. In a longitudinal cohort study (POSITIVES), virological rebound after treatment with nirmatrelvir plus ritonavir was associated with high viral load and, in some patients, culturable virus [21]. In the EPIC-HR trial, viral load rebound occurred in 2.3% of nirmatrelvir plus ritonavir recipients and 1.7% of placebo recipients, but was not associated with recurrence of severe COVID-19 symptoms [24]. The occurrence of viral load rebound in the placebo group suggests that some patients with COVID-19 may experience the phenomenon as part of the disease’s natural progression [24]. Virological rebound has also been reported after other COVID-19 treatments such as molnupiravir, indicating that the phenomenon is not unique to nirmatrelvir plus ritonavir [25]. Further studies are required to determine the mechanisms, incidence and clinical implications of virological rebound with COVID-19 antivirals, including nirmatrelvir plus ritonavir [21, 22, 25].
A number of treatment options are currently available for patients with non-severe COVID-19 who are at the highest risk of hospitalization, with the choice of treatment depending on factors such as drug availability, route of administration, duration of treatment and time from symptom onset [3]. The most recent update of the living WHO guideline for the treatment of COVID-19 strongly recommends nirmatrelvir plus ritonavir as an option for patients with non-severe COVID-19 at highest risk of hospitalization. The guideline also conditionally recommends against the use of nirmatrelvir plus ritonavir for patients with non-severe COVID-19 at low risk of hospitalization [3]. The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommends the use of nirmatrelvir plus ritonavir in adult, unvaccinated, ambulatory patients with mild-to-moderate COVID-19 at high risk of progression to severe disease within 5 days of symptom onset [26]. Draft guidance from the UK National Institute for Health and Care Excellence (NICE) recommends the use of nirmatrelvir plus ritonavir in the non-hospital setting to treat adults with COVID-19 who do not need supplemental oxygen, but who are at risk of progression to severe disease [27].
To date, no randomized clinical trials have directly compared the efficacy of nirmatrelvir plus ritonavir with other oral antivirals for COVID-19, such as remdesivir and molnupiravir. A recent meta-analysis found that nirmatrelvir plus ritonavir, molnupiravir and fluvoxamine were all effective in reducing the risk of death or hospitalization in patients with COVID-19, with odds ratios of 0.05 (95% CI 0.28–0.72), 0.22 (95% CI 0.10–0.48) and 0.45 (95% CI 0.28–0.72), respectively [28]. However, results of such indirect comparisons should be interpreted with caution. Data evaluating the efficacy and tolerability of nirmatrelvir plus ritonavir relative to other agents in head-to-head clinical trials would be of interest.
High epidemic resurgence of COVID-19 is expected to have a considerable burden on health system capacity [29]. In Hong Kong, both nirmatrelvir plus ritonavir and molnupiravir were associated with significant cost savings [19]. In the outpatient setting, nirmatrelvir plus ritonavir cost $US331,105 to prevent one death, but saved $US5,503 to prevent one death relative to standard care [19]. In Korea, treatment of symptomatic COVID-19 with oral nirmatrelvir plus ritonavir is likely to be cost effective, with incremental cost-effectiveness ratios (ICERs) of $US8878, $US8964 and $US1454 per prevented severe case when targeting all adults, adults with underlying diseases and elderly patients, respectively [29]. Oral molnupiravir is likely to be less cost effective, with ICERs of US$28,492, $US29,575 and $US7915, respectively [29]. According to NICE, nirmatrelvir plus ritonavir is likely a cost-effective use of National Health Service (NHS) resources in the non-hospital setting compared with standard care [27].
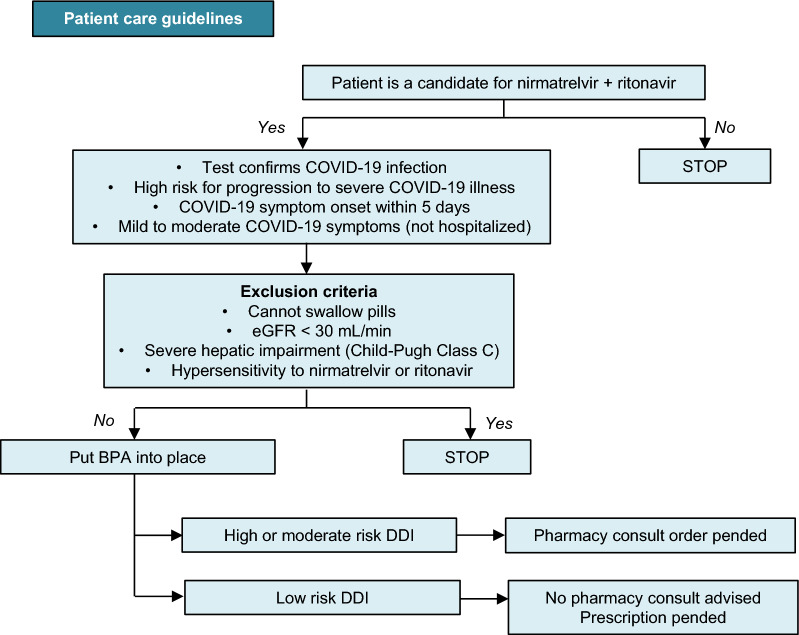
‘Test-and-treat’ approaches to COVID-19 and equitable access to effective oral antivirals are crucial, particularly for high-risk populations [30]. Although affordable generic versions of nirmatrelvir plus ritonavir and molnupiravir are available in ≈ 100 low- and middle-income countries, some middle-income countries (e.g. Argentina, Brazil, Malaysia, Thailand) are excluded from the agreements. Moreover, access to nirmatrelvir plus ritonavir, which must be started within 5 days of symptom onset, will remain limited in low-income countries due to reduced testing availability. Conversely, high-income countries with good access to testing and nirmatrelvir plus ritonavir have very small high-risk populations due to high vaccination rates [30]. Clinical decision support systems have been shown to improve prescribing practice and patient outcomes [31]. For example, in a US academic health system, a best practice advisory model was developed to facilitate equitable access to nirmatrelvir plus ritonavir for patients with COVID-19 who are at high risk of clinical deterioration (Fig. 1) [31].
Fig. 1.
BPA workflow for prescription of nirmatrelvir plus ritonavir to treat COVID-19, as suggested by Millstein et al. [31]. BPA best practice advisory, COVID-19 coronavirus disease 2019, DDI drug–drug interaction, eGFR estimated glomerular filtration rate
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The manuscript was reviewed by: Ye Htut Linn, FAME Pharmaceuticals Industry Co., Ltd., Yangon, Myanmar; A. Singh, Department of Pharmacology, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India. During the peer review process, Pfizer, the marketing authorization holder of nirmatrelvir plus ritonavir, was also offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
H. A. Blair is a salaried employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
References
- 1.Islam T, Hasan M, Rahman MS, et al. Comparative evaluation of authorized drugs for treating Covid-19 patients. Health Sci Rep. 2022;5(4):e671. doi: 10.1002/hsr2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanyaolu A, Okorie C, Marinkovic A, et al. Current advancements and future prospects of COVID-19 vaccines and therapeutics: a narrative review. Ther Adv Vaccines Immunother. 2022 doi: 10.1177/25151355221097559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Therapeutics and COVID-19: living guideline. 2022. https://apps.who.int/iris/bitstream/handle/10665/362843/WHO-2019-nCoV-therapeutics-2022.5-eng.pdf. Accessed 15 Nov 2022. [PubMed]
- 4.Fenton C, Keam SJ. Emerging small molecule antivirals may fit neatly into COVID-19 treatment. Drugs Ther Perspect. 2022;38(3):112–126. doi: 10.1007/s40267-022-00897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamb YN. Nirmatrelvir plus ritonavir: first approval. Drugs. 2022;82(5):585–591. doi: 10.1007/s40265-022-01692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Medicines Agency. Paxlovid 150 mg + 100 mg film-coated tablets: EU summary of product characteristics. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid. Accessed 15 Nov 2022.
- 7.Pfizer Inc. PAXLOVID—nirmatrelvir and ritonavir: US prescribing information. 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bdddfba-bd31-44cb-ba9e-23a4e17a4691. Accessed 15 Nov 2022.
- 8.Duveau DY, Thomas CJ. The remarkable selectivity of nirmatrelvir. ACS Pharmacol Transl Sci. 2022;5(6):445–447. doi: 10.1021/acsptsci.2c00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullrich S, Ekanayake KB, Otting G, et al. Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir. Bioorg Med Chem Lett. 2022 doi: 10.1016/j.bmcl.2022.128629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022 doi: 10.1016/j.antiviral.2022.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rai DK, Yurgelonis I, McMonagle P, et al. Nirmatrelvir, an orally active Mpro inhibitor, is a potent inhibitor of SARS-CoV-2 variants of concern. bioRxiv. 2022. 10.1101/2022.01.17.476644.
- 12.Singh RSP, Toussi SS, Hackman F, et al. Innovative randomized phase I study and dosing regimen selection to accelerate and inform pivotal COVID-19 trial of nirmatrelvir. Clin Pharmacol Ther. 2022;112(1):101–111. doi: 10.1002/cpt.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. medRxiv. 2022. 10.1101/2022.06.14.22276393.
- 15.Wong CKH, Au ICH, Lau KTK, et al. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir against mortality, hospitalization, and in-hospital outcomes among community-dwelling, ambulatory COVID-19 patients during the BA.2.2 wave in Hong Kong: an observational study. medRxiv. 2022. 10.1101/2022.05.26.22275631. [DOI] [PMC free article] [PubMed]
- 16.Arbel R, Sagy YW, Hoshen M, et al. Oral nirmatrelvir and severe Covid-19 outcomes during the Omicron surge. Res Sq. 2022 doi: 10.21203/rs.3.rs-1705061/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong CKH, Au ICH, Lau KTK, et al. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong’s Omicron BA.2 wave: an observational study. medRxiv. 2022. 10.1101/2022.05.19.22275291.
- 19.Wai AK, Chan CY, Cheung AW, et al. Association of molnupiravir and nirmatrelvir-ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health West Pac. 2022 doi: 10.1016/j.lanwpc.2022.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewnard JA, Malden D, Hong V, et al. Effectiveness of nirmatrelvir-ritonavir against hospital admission: a matched cohort study in a large US healthcare system. medRxiv. 2022. 10.1101/2022.10.02.22280623.
- 21.Boucau J, Uddin R, Marino C, et al. Characterization of virologic rebound following nirmatrelvir-ritonavir treatment for COVID-19. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epling BP, Rocco JM, Boswell KL, et al. COVID-19 redux: clinical, virologic, and immunologic evaluation of clinical rebound after nirmatrelvir/ritonavir. medRxiv. 2022. 10.1101/2022.06.16.22276392.
- 23.Ranganath N, O'Horo JC, Challener DW, et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease-2019 in high-risk persons. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares H, Baniecki M, Cardin RD, et al. Viral load rebound in placebo and nirmatrelvir-ritonavir treated COVID-19 patients is not associated with recurrence of severe disease or mutations. Res Sq. 2022 doi: 10.21203/rs.3.rs-1720472/v2. [DOI] [Google Scholar]
- 25.Wang L, Berger NA, Davis PB, et al. COVID-19 rebound after paxlovid and molnupiravir during January-June 2022. medRxiv. 2022. 10.1101/2022.06.21.22276724.
- 26.Bartoletti M, Azap O, Barac A, et al. European society of clinical microbiology and infectious diseases guidelines for coronavirus disease 2019: an update on treatment of patients with mild/moderate disease. Clin Microbiol Infect. 2022 doi: 10.1016/j.cmi.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence. Draft guidance consultation: therapeutics for people with COVID-19. 2022. https://nice.org.uk/guidance/gid-ta10936/documents/129. Accessed 18 Nov 2022.
- 28.Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54(1):516–523. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jo Y, Kim SB, Radnaabaatar M, et al. Model-based cost-effectiveness analysis of oral antivirals against SARS-CoV-2 in Korea. Epidemiol Health. 2022 doi: 10.4178/epih.e2022034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepperrelll T, Ellis L, Wang J, et al. Barriers to worldwide access for Paxlovid, a new treatment for COVID-19. Open Forum Infect Dis. 2022 doi: 10.1093/ofid/ofac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millstein JH, Asch DA, Hamilton K, et al. Decision support and centralized pharmacy consultation for nirmatrelvir-ritonavir prescribing in an academic health system-a model to promote drug access and reduce provider burden. J Gen Intern Med. 2022;38:4028–4031. doi: 10.1007/s11606-022-07752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.