Abstract
This article provides an encompassing review of the current pipeline of putative and developed treatments for tuberculosis, including multidrug-resistant strains. The review has organized each compound according to its site of activity. To provide context, mention of drugs within current recommended treatment regimens is made, thereafter followed by discussion on recently developed and upcoming molecules at established and novel targets. The review is designed to provide a clinically applicable understanding of the compounds that are deemed most currently relevant, including those already under clinical study and those that have shown promising pre-clinical results. An extensive review of the efficacy and safety data for key contemporary drugs already incorporated into treatment regimens, such as bedaquiline, pretomanid, and linezolid, is provided. The three levels of the bacterial cell wall (mycolic acid, arabinogalactan, and peptidoglycan layers) are highlighted and important compounds designed to target each layer are delineated. Amongst others, the highly optimistic and potent anti-mycobacterial activity of agents such as BTZ-043, PBTZ 169, and OPC-167832 are emphasized. The evolving spectrum of oxazolidinones, such as sutezolid, delpazolid, and TBI-223, all aiming to exceed the efficacy achieved with linezolid yet offer a safer alternative to the potential toxicity, are reviewed. New and exciting prospective agents with novel mechanisms of impact against TB, including 3-aminomethyl benzoxaboroles and telacebec, are underscored. We describe new diaryloquinolines in development, striving to build on the immense success of bedaquiline. Finally, we discuss some of these compounds that have shown encouraging additive or synergistic benefit when used in combination, providing some promise for the future in treating this ancient scourge.
Key Points
| The mycobacterial cell wall is composed of three layers (the outer mycolic acid, the middle branched arabinogalactan, and the inner peptidoglycan) that offer unique target sites for tuberculosis (TB) therapies that are being exploited in drug development. |
| The discovery and development of bedaquiline was revolutionary in TB therapy and has provided the impetus for exciting further investigation into additional diarylquinolines. |
| Pretomanid possesses potent bactericidal activity and sterilizing capability for non-replicating bacilli that is additive to that achieved with bedaquiline and linezolid. |
| Repeated clinical studies suggest the extensive activity of linezolid against TB, and recent data indicate its toxicity may be mitigated with therapeutic drug (trough) monitoring. |
| Benzothiazinones and their derivatives offer some of the most potent antimycobacterial activity seen in vitro, providing great optimism for further clinical study. |
| Telacebec (Q203) and GSK656 are new and exciting therapies designed to target novel regions of the mycobacterial cell |
Introduction
It has been 140 years since Robert Koch discovered the bacillus identified as Mycobacterium tuberculosis (MTB), yet despite major international efforts, tuberculosis (TB) remains a major health problem. There is archaeological evidence of TB in the Neolithic period, and it has reached epidemic proportions in recent centuries [1, 2]. It was also recognized and named by the ancients: schachepheth in the Old Testament and phthisis by the ancient Greeks [1]. John Bunyan described it as the Captain of all these men of death in 1680, and it was termed the White Plague in eighteenth century England [3]. Unlike the most common causes of death now, cardiovascular disease, cancer and respiratory diseases, which generally kill the elderly, TB struck people down during the prime of their lives. Apart from the COVID-19 pandemic, TB is the most common cause of death from an infectious disease worldwide and the tenth most common cause of death overall [4]. The COVID-19 pandemic has disrupted TB care globally resulting in a reduction in new case notifications in 2020, the provision of fewer treatment regimens for rifampin-resistant disease, and fewer preventative treatments, yet more than 1.5 million died from TB in 2020, an increase from 2019 [4].
Challenges with current therapy against TB include prolonged duration of therapy and intolerance due to side effects. Additionally, particularly in settings of high TB prevalence, access to healthcare and cost remain major issues. Drug resistance, which is becoming more prevalent, complicates therapy and reduces the likelihood of successful treatment since it requires longer and more complex medication regimens than drug susceptible (DS)-TB. Additionally, it is often accompanied by more major adverse effects, is considerably more expensive to treat, and success rates are lower than with regimens for DS disease [4].
After decades without newly developed antimycobacterials, or repurpose of alternative antibacterials for use in TB, novel agents were approved for use in 2012 [5] and 2014 [6]. By utilizing genome sequencing, the identification of novel sites of action has prompted the further pursuit of similar agents that may be even more potent and less toxic than traditional therapies (Table 1). Time will tell with clinical studies ongoing, but it is reasonable to state that the pipeline of anti-TB medication is promising. Helpful updates on the status of medication trials are available and updated regularly [7].
Table 1.
Classification and status of anti-tubercular compounds in development
| Drug | Class | Site of action | Development phase | References |
|---|---|---|---|---|
| Bedaquiline | Diarylquinoline | Mycobacterial ATPase | Marketed | [18, 146, 147, 149] |
| Sudapyridine (WX-081) | Diarylquinoline | Mycobacterial ATPase | 2 | [167] |
| TBAJ-876 | Diarylquinoline | Mycobacterial ATPase | 1 | [18, 19] |
| TBAJ-587 | Diarylquinoline | Mycobacterial ATPase | 1 | [160, 165] |
| Telacebec (Q203) | Imidazopyridine | Cytochrome BC1 complex | 2 | [67, 169, 170] |
| Delamanid | Nitroimidazole | Mycolic acid synthesis/nitric acid | Marketed | [38, 41, 42] |
| Pretomanid | Nitroimidazole | Mycolic acid synthesis/nitric acid | 3 | [16, 41, 53] |
| BVL-GSK098 | Amido-piperidine | MymA, ethionamide enzyme activator | 1 | [34] |
| SQ109 | Ethylenediamine | Mycolic acid transport MmpL3 | 2 | [59, 60] |
| BTZ043 | Benzothiazinone | DprE1 inhibitor, arabinoglycan synthesis | 2 | [78, 79] |
| PBTZ169 | Benzothiazinone | DprE1 inhibitor, arabinoglycan synthesis | 1 | [76, 78] |
| OPC-167832 | Carbostyril | DprE1 inhibitor, arabinoglycan synthesis | 2 | [73, 78] |
| TBA-7371 | 1,4-azaindole | DprE1 inhibitor, arabinoglycan synthesis | 2 | [76, 87] |
| Sanfetrinem | Carbapenem | Peptidoglycan synthesis | 2 | [97] |
| Moxifloxacin | Fluoroquinolone | DNA gyrase | Marketed | [182] |
| Fodrepodacin (SPR720) | Aminobenzimidazole | DNA gyrase B | 2 | [184] |
| VXC-486 | Aminobenzimidazole | DNA gyrase B | 2 | [187] |
| Rifapentine | Rifamycin | RNA Polymerase | Marketed | [12, 138] |
| Linezolid | Oxazolidinone | Protein Synthesis | Marketed | [16, 99] |
| Sutezolid | Oxazolidinone | Protein Synthesis | 2 | [119–121] |
| Tedizolid | Oxazolidinone | Protein Synthesis | No current TB trials | [109, 111–113] |
| Delpazolid | Oxazolidinone | Protein Synthesis | 2 | [125] |
| TBI-223 | Oxazolidinone | Protein Synthesis | 1 | [40] |
| OTB-658 | Oxazolidinone | Protein Synthesis | Pre-clinical | [130, 131] |
| GSK656 | Oxaborole | leucyl t-RNA, protein synthesis | 2 | [134, 135, 137] |
| Clofazimine | Riminophenazine | Reactive Oxygen Species | Marketed | [99, 176] |
| Pyrifazimine (TBI-166) | Riminophenazine | Reactive Oxygen Species | 1 | [66, 175, 176] |
| GSK2556286 | Pyrimidine-2,4-dione | Cholesterol Metabolism | 1 | [22, 188] |
| FNDR-20081 | Quinoline | Uncertain | Pre-clinical | - |
The purpose of this review is to provide a clinically relevant perspective on several new and repurposed compounds that are deemed to show promising early results and to describe the available clinical data for these drugs. Undoubtedly, some of these compounds will not be marketed. To provide a framework for organization, we have structured the review based on sites of activity of compounds. This includes providing sufficient detail to, for example, emphasize the unique targets within the various levels of the cell wall (Fig. 1) [8]. In preparation for this review, the PubMed database was searched using treatment of TB, DR-TB, rifampin-resistant (RR)-TB, MDR-TB, and extensively drug-resistant (XDR)-TB, and for each drug individually. As well, the bibliographies of the selected articles were reviewed for relevant articles. Moreover, the clinicaltrials.gov and www.newtbdrugs.org sites were reviewed (Fig. 2).
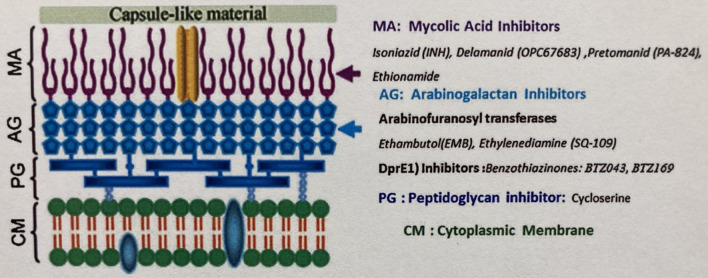
Fig. 1.
The mycobacterial cell wall with target sites of current therapies and those in development.
Reproduced with permission from Elsevier Masson from: Shanib Bhat Z, Ahmad Rather M, Maqbool M, Lah HU, Khalid Yousuf S, Ahmad Z. Cell wall: A versatile fountain of drug targets in Mycobacterium tuberculosis. Biomed Pharmacother. 2017;95:1520–34 [8]
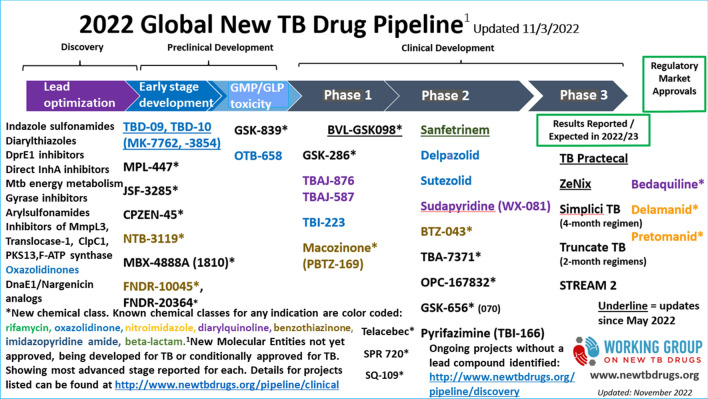
Fig. 2.
2022 global new tuberculosis (TB) drug pipeline, updated 3 November 2022.
Reproduced with permission from the: Stop TB Partnership Working Group on New TB Drugs [7]. Figure is available at: www.newtbdrugs.org/pipeline/clinical
Current Key Drug Targets
Current standard of care of DS-TB remains a combination of isoniazid, rifampin, pyrazinamide, and ethambutol [9, 10]. Both isoniazid and ethambutol are active against mycobacterial cell wall production, inhibiting outer mycolic acid production and the middle arabinogalactan level, respectively. Pyrazinamide is a prodrug and enzymatically converted to a highly bactericidal and acidic substrate, pyrazinoic acid, that is believed to interrupt the production of coenzyme A through aspartate decarboxylase [11]. Rifampin remains the cornerstone of DS-TB treatment due to its combined bactericidal and sterilizing effects, allowing for treatment shortening from at least 18 months to 9. It mediates its concentration-dependent effect through inhibition of the DNA-dependent RNA polymerase, which seems to interfere with the growing RNA strand [12].
For multidrug-resistant TB (MDR-TB), current guidelines advocate for multidrug treatment generally consisting of bedaquiline, linezolid, moxifloxacin or levofloxacin, clofazimine, and cycloserine or terizidone [13–15]; however, growing data on the potency of agents such as bedaquiline, oxazolidinones, and pretomanid have provided justification for shortened treatment regimens, including the BPaL and BPaL(M) regimens [16, 17]. If the isolate is susceptible, pyrazinamide is also recommended for the first 6 months [9].
Recent research has aimed to develop antimycobacterial drugs that exploit novel sites to counter growing antimycobacterial resistance. This research has identified some of the most potent chemicals by in vitro testing to date, including diarylquinolines such as bedaquiline and its derivatives, which target adenosine triphosphate (ATP) synthesis and uncoupling of the electron transport chain [18, 19].
Mycobacterial Cell Wall
It is prudent to briefly deconstruct the mycobacterial cell wall as this structure offers multiple potential sites for drug targeting. The cell wall consists of an extensive lipid-carbohydrate network that serves as an impermeable defense against hydrophilic compounds and has long been recognized as an important antimicrobial target [8]. It possesses an inner phospholipid bilayer cell membrane. Beyond this lies (i) the crosslinked peptidoglycan, (ii) an intermediate, branched arabinogalactan polysaccharide, (iii) and the outer waxy long-chain mycolic acids [20].
The inner peptidoglycan component of the cell wall possesses some unique properties from Gram-positive and Gram-negative bacteria, and specific to TB is the oxidation of N-acetylmuramic acid to N-glycolylmuramic acid, which may increase pathogenicity as well as stability from host intracellular lysozyme [8]. Drugs previously recognized to interfere with mycobacterial peptidoglycan synthesis include cycloserine and carbapenems [8].
The middle arabinogalactan layer is composed of branches of arabinose and galactose and is generated through multiple steps including the use of decaprenylphosphoryl-d-arabinose (DPA). This is a recognized target for nitro-benzothiazinones (BTZs) and dinitrobenzamides, which inhibit decaprenyl-phosphoribose 2′ epimerase (DprE1) enzymatic activity in the generation of DPA [20]. Ethambutol is another agent that targets this middle layer by inhibiting the arabinosyl transferase enzyme [21].
The outer cell wall layer contains mycolic acids: long-chain fatty acids consisting of an α-alkyl and a β-hydroxy chain. It also contains lipoglycans, phospholipids, glycopeptidolipids, and trehalose mycolates [22]. The mycolic acid-containing outer layer is covalently linked to the arabinogalactan layer, which, in turn, is covalently linked to the peptidoglycan layer forming the mycolyl-arabinogalactan-peptidoglycan complex [8, 23]. Generation of the outer mycolic acid layer utilizes many enzymatic steps already targeted by multiple antimycobacterials. Isoniazid, ethionamide, delamanid, and pretomanid all target this component of the cell wall. Finally, a protein and polysaccharide capsule surrounds the cell wall [8, 23].
Outer Mycolic Acid Layer
Isoniazid
Isoniazid (INH) was introduced 70 years ago and remains a first-line drug for the treatment of active and latent TB infection (LTBI) [24]. It inactivates enoyl-ACP reductase [inhA] and beta-ketoacyl ACP synthase [kasA], important enzymes involved in the elongation of long chain fatty acids [25–27]. INH is a prodrug activated by the catalase-peroxidase [katG] enzyme, generating an isoniazid-nicotinamide adenine dinucleotide (NAD) adduct that impairs inhA activity, interfering with its ability to generate NAD (+) and disrupting mycolic acid chain elongation [24]. Mutation of the katG gene is the most common cause of INH resistance, followed by mutations of the inhA gene [24]. Other mycobacterial gene mutations that can cause resistance are less common and act by altering drug metabolism or by facilitating drug efflux [24].
For the years 2003–2017, the global prevalence of INH resistance was 7.4% among new TB cases and 11.4% among previously treated patients; 8.5% of all cases worldwide are INH monoresistant [28, 29]. Among isoniazid monoresistant cases, 78.6% had katG mutations and 14.6% had both katG and inhA mutations [30].
Ethionamide
Ethionamide is a structural analogue of INH and also targets inhA [31]. Similar to INH, ethionamide is a prodrug but is activated by another enzyme, the mycobacterial monooxygenase, ethA, so it maintains its activity in the presence of katG mutations but not inhA mutations [32]. It is considered a second-line drug because of adverse effects. Prothionamide is another thioamide, similar to ethionamide, but may be better tolerated in MDR-TB treatment regimens [33].
BVL-GSK098
BVL-GSK098, an amido-piperidine, boosts bactericidal activity of ethionamide and prothionamide, restoring sensitivity in ethionamide-resistant MTB strains. It is believed to stimulate an additional enzymatic activator for the prodrug ethionamide, called MymA [34]. It is currently in phase I development and a phase IIa trial is planned [35].
Thiacetazone
Thiacetzone is a prodrug that is activated by ethA and once activated inhibits mycolic acid cyclopropane synthesis. It is an inexpensive drug but unfortunately may cause hepatotoxicity and severe cutaneous reactions, particularly in patients coinfected with HIV [36].
Thiadiazoles-Based Chemical Inhibitors
An open collaborative model for TB lead optimization (ORCHID) consortium is developing thiadiazole-based chemical inhibitors that directly inhibit inhA, circumventing the need for cellular activation [37]. GSK693 is a thiadiazole that demonstrated good activity inside and outside macrophages and is not cytotoxic to hepatocytes nor does it inhibit the human ‘Ether-a-go-go’-related gene, KCNH2 (hERG) [22]. Currently, no trials with this drug are registered with clinicaltrials.gov (accessed 6 June 2022).
Delamanid (Previously OPC-67683)
Delamanid is one of two recently approved novel anti-mycobacterial agents for the treatment of TB. While global experience with it appears significantly less than with the other recently approved agent bedaquiline, there are numerous ongoing studies of its use [7].
Delamanid is a first-of-its-kind bicyclic nitroimidazooxazole derivative and is a prodrug that requires metabolic activation by the mycobacterial f420 enzyme to have anti-TB activity [35]. The catalytic enzyme responsible for this activation is Rv3547 [38]. It is not active against routinely encountered bacteria [39]. Similar to INH, its primary mechanism of action is through inhibition of mycolic acid synthesis providing it bactericidal activity; however, it may also exert activity through generation of reactive oxygen intermediates, impairing respiration [38]. It is proposed to inhibit mycolic acid biosynthesis at a site distinct from other agents like isoniazid and ethionamide, specifically by inhibiting methoxy-mycolic acid and keto-mycolic acid, but not α-mycolic acid biosynthesis [40].
Delamanid has activity against both rifampin-susceptible and rifampin-resistant TB and is significantly potent in vitro, even more so than pretomanid [40–42]. The usual dose of 100 mg twice daily generates sufficient concentrations above the epidemiological cut-off values in most individuals [42]. It exerts activity against dormant, non-replicating bacilli, as well as those harboured within macrophages [40, 43].
Delamanid may manifest a low minimum inhibitory concentration (MIC) against some slow-growing nontuberculous mycobacteria, but it frequently has elevated MICs to the most commonly encountered organisms, including M. avium and M. intracellulare, suggesting it is unlikely to offer clinical utility, and it also has no activity against rapidly growing mycobacteria species [39]. Resistance to delamanid is rare so far, but when encountered is frequently due to mutations in the nitroreductase that activates it [44]. Cross-resistance to other nitroimidazoles, such as pretomanid, may occur [41, 42]. Bioavailability is increased with fatty food consumption [45, 46]. Uniquely, delamanid is metabolised by circulating albumin and does not have any notable impact on cytochrome P450 (CYP) enzymes, limiting the potential for significant drug-drug interactions [39, 43, 47]. The half-life is approximately 30 h and most excretion is through the feces, with minimal urinary excretion. Overall, delamanid appears well tolerated, with the main reported adverse events (AEs) including mild gastrointestinal symptoms or QTc prolongation [39, 48].
The sentinel sponsored phase II study that prompted application for European Medicines Agency (EMA) conditional approval of delamanid demonstrated expedited sputum-culture conversion at 2 months in comparison to placebo in patients with MDR-TB or XDR-TB, when combined with an optimal background regimen (45.4% vs. 29.6%, p = 0.008) [6, 48]. Long-term observational follow-up of these patients identified that those who received at least 6 months of delamanid (vs. none or 2 months) had more favorable outcomes (74.5% vs. 55%, relative risk (RR) 1.35; confidence interval (CI) 1.17–1.56; p < 0.001) and lower mortality (1% vs. 8.3%, p < 0.001) [49]. Additionally, some post-marketing studies have also shown promising culture conversion results [50, 51].
Nonetheless, delamanid trials have had conflicting results. In contrast to the above studies, when added to an optimized background regimen for MDR-TB, delamanid did not shorten the time to culture conversion versus placebo (51 days vs. 57 days; hazard ratio (HR) 1.17; 95% CI 0.91–1.51, p = 0.2157) [52]. Due to these discrepant results and limited data available about efficacy (including mortality) when added to background regimens, the medication was categorized as a Group C medication (for addition when other preferred medications cannot be utilized) by the World Health Organization (WHO), and was unable to be included in the most recent recommendations by the official ATS/CDC/ERS/IDSA clinical practice guideline [14].
Pretomanid
Pretomanid is a pro-drug nitroimidazooxazine molecule that also inhibits mycolic acid synthesis in replicating bacilli. However, it is also active against non-replicating bacilli, releasing reactive oxygen species including nitric oxide under anaerobic conditions [53]. These multiple functions provide it with potent bactericidal and sterilizing activity in mouse studies [54]. Mutations mediating cross-resistance between pretomanid and delamanid are already recognized, and are most commonly located in genes required for activation of the nitroimidazoles [55], glucose-6-phosphate dehydrogenase (FGD1) or the deazaflavin cofactor f420 [56].
Activity is present against both DS- and MDR-TB (including XDR-TB) and yet it has no significant activity against Gram-positive and Gram-negative bacteria [8]. The individual contribution of pretomanid to highly effective combination treatments has been demonstrated in mouse models. Specifically, compared to the combination of bedaquiline, moxifloxacin, and pyrazinamide, adding pretomanid (BPaMZ) resulted in a greater reduction in lung colony forming unit (CFU) counts and reduced the risk of selecting bedaquiline-resistant isolates and relapse [57, 58]. Furthermore, pretomanid also affords additional sterilizing activity when added to a regimen of bedaquiline and linezolid (BPaL) [57].
The Nix-TB trial was the premier introduction to the clinical use of pretomanid in combination with bedaquiline and linezolid in XDR-TB or those with MDR-TB intolerant or not responding to standard therapy [16]. After 6 months of this combination, at least 90% of patients were found to have a favorable outcome (absence of failure, relapse, retreatment) in both the intention-to-treat and per-protocol analyses up to 6 months beyond treatment completion. This 109-person phase III study led the way for expedited approval for pretomanid by the US Food and Drug Administration (FDA) in 2019 solely as part of a three-drug regimen for complicated MDR-TB and XDR-TB [16]. Notably, a Grade 3 or higher AE was reported by 57% of the patients on the regimen, although this was likely impacted by linezolid. This included 26.6% with nervous system disorders, 18.3% with musculoskeletal disorders, 12.8% with hematologic disorders, and scattered incidence of various metabolic disorders (acidosis, elevated lipase/amylase, electrolyte abnormalities). Additionally, one of the main drawbacks of the study was the absence of a comparator group.
The SimpliciTB phase II/III trial builds off the recognized high bactericidal activity of the combination of bedaquiline, pretomanid, moxifloxacin, and pyrazinamide (BPaMZ), which benefits from the synergistic activity between pyrazinamide and bedaquiline [58]. This study is ongoing and compares the efficacy and safety of the above combination for 4 months compared to standard 6-month treatment for DS-TB and this regimen for 6 months for DR-TB.
TB-PRACTECAL is another completed phase II/III trial evaluating shortened regimens of bedaquiline, pretomanid, moxifloxacin, linezolid, and clofazimine, with publication pending [17].
Ethylenediamines
SQ109 is an ethylenediamine with a similar structure to ethambutol but maintains good activity against ethambutol-resistant MTB strains. It inhibits mycobacterial membrane protein large 3 (MmpL3), which plays an important role in the transportation of mycolic acids, in the form of trehalose monomycolate, to the outer part of the cell wall, and may also reduce the arabinogalactan underneath [59]. It also targets other synthetic enzymes for energy production and efflux pump mechanisms [40, 60]. It has been demonstrated to have in vitro activity against both DS- and drug-resistant (DR)-TB. An in vitro study demonstrated an excellent rate of killing that was superior to single drug use of sutezolid (PNU-100480); however, combination of the two showed additive efficacy even at sub-MIC concentrations [61].
Although safe and well tolerated, in combination with rifampin SQ109 did not enhance early bactericidal activity (EBA) over the first 4 days of treatment, likely because rifampin accelerated its metabolism [62]. Additionally, treatment with SQ109 alone did not show any significant activity in vivo. This is in contrast to a prior in vitro study suggesting synergy with rifampin [63]. In comparison, in combination with bedaquiline, SQ109 improved EBA four- to eightfold [63]. In a multi-arm study, the median time to sputum culture conversion did not improve by substituting SQ109 for ethambutol in standard therapy with rifampin, isoniazid, and pyrazinamide, and this arm of the trial was stopped early for lack of efficacy [64, 65, 66]. SQ109 is generally well tolerated, and rates of adverse effects were similar to those seen in the two treatment groups. There are no active studies with SQ109 listed on the clinicaltrials.gov website (accessed 4 June 2022).
Arabinogalactan Middle Layer
The middle layer of the cell wall consists of branched arabinogalactan, a polysaccharide chain consisting of galactose and arabinose sugars in a furanose form, covalently linked to both the adjacent peptidoglycan layer and the outer mycolic acid layers [8, 40]. Ethambutol and its analogues, capreomycin and DprE1 enzyme inhibitors, interfere with arabinogalactan metabolism [22, 38, 63, 64]. Ethambutol disrupts arabinogalactan synthesis by inhibiting the enzyme arabinosyl transferase [67]. Capreomycin is believed to alter mycolic acid synthesis in addition to inhibiting protein synthesis [68].
DprE1 is an essential flavo-enzyme required for the formation of D-arabinofuranose, a component of arabinogalactan and lipoarabinomannan [7, 69]. In combination with DprE2, it epimerizes decaprenylphosphoryl ribose to decaprenylphosphoryl arabinose, an essential step for arabinogalactan and lipoarabinomannan biosynthesis [70, 71].
Several drug classes are undergoing development that target this essential enzyme for cell wall synthesis including the benzothiazinones, a carbostyril derivative, and azaindoles [72–74]. Drugs that target DprE1 benefit from it being situated in the periplasmic space of the cell wall, thereby avoiding efflux and cytoplasmic resistance mechanisms in resistant strains [75].
BTZ-043
BTZ-043 is one of the lead compounds of the class of medications called benzothiazinones first discovered in 2009 that are suicide inhibitors of DprE1 [76]. It acts by forming an adduct with the Cys387cysteine residue [77]. BTZ-043 inhibits mycobacterial cell wall synthesis by blocking DprE1 and the subsequent formation of arabinogalactan and arabinomannan in the middle arabinogalactan component of the TB cell wall [8]. It is one of the most highly bactericidal agents in vitro with nanomolar concentrations producing significant bacterial inhibition and it is active against drug-resistant (DR)-TB as well [78]. The bactericidal effect of BTZ-043 is additive in combination with most other TB drugs but appears to be synergistic with bedaquiline [79]. A randomized, double-blind, placebo-controlled single-ascending dose study was completed in March 2019 that evaluated the safety, tolerability, and pharmacokinetics of single doses of BTZ-043 in healthy volunteers [80]. A multiple-ascending dose phase Ib/IIa study of BTZ-043 to evaluate safety, tolerability, and EBA over 14 days was started in August 2019 and currently is active but not recruiting [81].
PBTZ 169
PBTZ-169 (piperazine-BTZ; macozinone) is a derivative of benzothiazinone, similar in structure to BTZ-043 with the addition of a piperazine moiety, and yet is even more potent at nanomolar concentrations [78]. Similar to BTZ-043, it inhibits DprE1, but may be tenfold less cytotoxic [76]. Preclinical study has demonstrated in vitro activity against both MDR- and XDR-TB, and it shows excellent additive activity when combined with agents such as isoniazid, pretomanid, moxifloxacin, rifampin, and SQ109, and synergy when combined with bedaquiline [78]. The combination with bedaquiline was further assessed in the chronic TB mouse model and found to reduce the burden of TB (as measured by the reduction in CFU in lungs and spleen) to a greater degree than the standard combination of isoniazid, rifampin, and pyrazinamide. One postulate is that through inhibition of DprE1, PBTZ-169 may weaken the cell wall and thereby improve target access for bedaquiline [78]. A phase 1 clinical trial investigating single-ascending doses of a new bio-enhanced formulation of PBTZ-169 that included 32 healthy male volunteers was completed in April 2018 [82]. Another phase I trial in healthy male volunteers, aged 18–45 years with body mass indices (BMIs) 18.5–25 kg/m2, included seven cohorts who were given doses ranging from 40 to 640 mg of PBTZ-169 daily for 14 days [83]. The primary outcome was safety and tolerability. All 40 volunteers were able to complete the 14-day study. In 2017, a phase IIa EBA study of monotherapy for 14 days was started in DS-TB patients in Russia and Belarus. It was completed in February 2018 with 16 enrolled patients [84]. The EBA of three doses of PBTZ-169, 160 mg/day, 320 mg/day, and 640 mg/day, were all greater than INH 600 mg/day at 14 days as measured by a reduction in the number of CFU/mL [84]. Solubility issues are delaying further development of the drug [7].
An analogue of PBTZ-169, TZY-584, demonstrated lower MICs against a number of DS- and DR-TB strains than INH, rifampin, and bedaquiline, and results were similar to PBTZ-169. It was also effective against intracellular organisms in infected macrophages [75]. There are no active studies currently listed at clinicaltrials.gov (accessed 6 June 2022).
OPC-167832
OPC-167832 is also an inhibitor of mycobacterial cell wall synthesis. It is a carbostyril derivative that inhibits DprE1 [76]. It is highly potent, and MICs have been demonstrated that are up to 1000-fold lower than many currently used anti-TB medications [73]. Combined with its favorable distribution from plasma to lung and to TB lesions, sustained drug levels above the MIC for a prolonged period suggest it may have very promising activity against TB [76]. It is effective against intracellular TB and has preserved activity against MDR- and XDR-TB, and has no significant activity against standard Gram-positive and Gram-negative bacteria, limiting any significant impact on human bacterial flora. The combination of OPC-167832 with moxifloxacin, bedaquiline, and delamanid showed excellent sterilizing activity in the mouse model, predicting a low rate of relapse [73]. In a checkerboard assay there was no antagonism when it was combined with delamanid, bedaquiline, the fluoroquinolones, levofloxacin and moxifloxacin, or linezolid [73]. There is an ongoing phase 1/2b safety/efficacy trial [85] and phase 2b dose-finding trial in combination with bedaquiline and delamanid for DS pulmonary TB [86].
TBA-7371
TBA-7371 is another inhibitor of DprE1 developed in collaboration with TB Alliance. It is a derivative of 1,4-azaindoles with an MIC of 0.64 μg/mL against TB with excellent solubility and clearance, limiting its toxicity [76, 87]. It also inhibits phosphodiesterase VI, the enzyme that normally inactivates it [7]. It has demonstrated notable reduction in CFU in the lungs in both the acute and chronic mouse models. After 8 weeks of treatment in mice, it showed an average 1.5 log10CFU reduction in the lungs [76]. Despite having higher MICs compared to PBTZ-169 and OPC-167832, it appeared to be as efficacious as PBTZ-169. A phase 2a dose-finding and EBA study is currently underway [88].
Inner Peptidoglycan Layer
The inner layer of the cell wall consists of crosslinked peptidoglycan, alternating N-acetyl glucosamine and N-acetyl muramic acid moieties, attached to short peptide side chains [40, 89]. Cycloserine, terizidone, and carbapenems are second-line antibiotics that interfere with peptidoglycan synthesis and are generally used to treat DR-TB [8]. Cycloserine and terizidone target D-alanine-D-alanine ligase, an essential enzyme that joins two D-alanine moieties together that are then attached to peptidoglycan [90]. Unfortunately, adverse effects associated with cycloserine are not uncommon, and include seizures, psychosis, and peripheral neuropathy [91].
Despite MTB containing a broad-spectrum β lactamase, BlaC, it was found to not be highly active against carbapenem antibiotics, particularly meropenem [92]. Further, BlaC is an Ambler Class A ß-lactamase that can be stably inactivated by the ß-lactamase inhibitor clavulanate, but not with others such as tazobactam and sulbactam [93]. The combination of meropenem with clavulanate has potent MTB in vitro activity [94], but unfortunately this combination is not available, therefore requiring meropenem to be co-administered along with the readily available combination of amoxicillin-clavulanate. Nevertheless, in a 14-day EBA study in patients with smear-positive pulmonary TB with meropenem, in combination with amoxicillin-clavulanate, meropenem reduced the mycobacterial sputum load by 1.5 orders of magnitude, similar to the reduction expected with rifampin and pyrazinamide [92]. Carbapenems inhibit transpeptidases, essential enzymes that crosslink peptidoglycan [95]. Meropenem is only available as an intravenous medication. A recently published trial demonstrated that it has to be given three times daily to be effective, which is impractical for outpatients requiring the medication for 6 months, and it was frequently associated with gastrointestinal side effects [96].
Sanfetrinem Cilexetil
Sanfetrinem cilexetil is an oral tricyclic carbapenem that was first investigated in the 1990s but development was suspended prior to phase III studies for financial reasons, and only recently has been repurposed as a drug to treat TB [7, 97]. Sanfetrinem is effective intracellularly with a lower range of MIC90 than meropenem: 1–4 μg/mL versus 2–64 μg/mL, respectively [7]. A phase II study is underway that plans to recruit 105 patients with rifampin-susceptible pulmonary TB [98]. Sanfetrinem powder will be given as a suspension in water at a dose of 1.6 g every 12 h with various combinations of rifampin and amoxicillin–clavulanate, with the primary outcome being EBA as determined by the change in CFU from pretreatment values to day 14 on solid media and secondary outcomes will be EBA as measured by a change in time-to-positivity by day 14 in BACTEC MGIT 960 liquid culture and the number of patients with treatment-emergent adverse effects [98]. There are no current studies underway for alternative oral carbapenem therapy (clinicaltrials.gov accessed 31 October 2022).
Protein Inhibition
Linezolid and Other Oxazolidinones
Oxazolidinones inhibit protein synthesis. The bacterial 70S ribosome comprises two subunits: a 30S and a 50S subunit with a bridging surface area that contains three binding sites for t-RNA named A, P, and E. Oxazolidinones bind the A site and block the attachment of t-RNA to the ribosome, precluding protein synthesis [40]. Oxazolidinones are active against MTB and several are currently in development, and linezolid is already being used, to treat TB. In addition to linezolid, tedizolid, sutezolid, delpazolid, and TB-223 are all members of the oxazolidinone class.
Linezolid
Linezolid is a repurposed drug originally developed to treat Gram-positive infections with difficult drug-resistance profiles, and is now included in oral regimens to treat MDR-TB including XDR-TB [28]. Treatment regimens for MDR-TB containing linezolid were associated with treatment success and reduced mortality [99]. The WHO has since reclassified it as a group A drug for the treatment of MDR- and XDR-TB [28]. The standard dose to treat Gram-positive infections is 600 mg twice daily, but prolonged treatment with this dose is associated with a high risk of bone marrow suppression and neuropathy, and the risk is increased if the treatment course is extended beyond 10 days. Myelosuppression is due to linezolid inhibiting mitochondrial protein synthesis in bone marrow precursor cells [100]. Linezolid can also cause optic and peripheral neuropathy, lactic acidosis, pancreatitis, and serotonin syndrome in those on selective serotonin reuptake inhibitors [101–103]. A linezolid dose of 600 mg daily is sufficient to treat TB but is potentially required for up to 18 months, and is often associated with side effects [99]. In the NIX-TB trial, 81% of the patients experienced myelosuppression, either anemia or thrombocytopenia, 41% peripheral neuropathy, and 2% optic neuritis [16]. In another cohort of 472 patients, of whom 90% received linezolid, 28.4% developed peripheral neuropathy and 5.1% myelosuppression [104]. However, the results of the ZeNix trial were recently published and highlight that in combination with bedaquiline and pretomanid, a reduced dose of linezolid (compared to the doses used in Nix-TB) of 600 mg daily for 26 weeks provided similarly excellent efficacy outcomes [105]. Additionally, the reduced dose demonstrated significantly improved safety outcomes, with only 4% developing myelosuppression at this dose and 13% requiring dose modification for any cause. Other oxazolidinones are being developed with the hope of achieving the therapeutic benefits of linezolid without its adverse effects. Alternatively, there are compelling data that mitochondrial toxicity that leads to neuropathy and myelosuppression is associated with linezolid trough concentrations >2 μg/mL and may be mitigated with therapeutic drug monitoring [106, 107].
Tedizolid
Tedizolid is a newer oxazolidinone prodrug converted in serum to its active form, and subsequently inhibits the 50S subunit of the ribosome, impairing protein synthesis [108]. It exhibits excellent penetration into cutaneous and pulmonary tissue (superior to linezolid), making it an optimistic option for treatment of TB [109]. It demonstrates vast Gram-positive inhibitory activity, and in addition demonstrated activity against both rapidly and slowly growing nontuberculous mycobacteria, including TB [109]. It demonstrates the most potent in vitro activity of any of the oxazolidinones against rapidly-growing M. abscessus [110].
An in vitro study has demonstrated efficacy of tedizolid against nonreplicating persisting mycobacteria with similar efficacy to standard first-line therapy [111], with superior sterilizing activity compared to linezolid in the hollow fibre model [112, 113] and lower MICs even in linezolid-resistant isolates, highlighting the potential for greater potency [108, 114]. One in vitro study found a > 10,000-fold CFU/mL difference for intracellular activity between linezolid and tedizolid, making it a potentially superior agent for cavitary and disseminated disease in children [112]. In vitro efficacy that may be superior to linezolid has been demonstrated in both DS and DR isolates, including MDR-TB [108, 114].
Tedizolid seems to have a superior safety profile to linezolid [109]. Its more common side effects were mild AEs including headache and gastrointestinal (GI) intolerance, and it seems to have a better hematologic safety profile to that shown by linezolid [96, 102]. Mouse studies have suggested that the risk of serotonin syndrome with tedizolid in combination with other serotonergic chemicals may be lower than that identified with linezolid [103].
The main challenge with prolonged use of linezolid, as seen in TB treatment regimens, is toxicity associated with mitochondrial inhibition. Models have suggested that toxicity from tedizolid may be less and intermittent dosing strategies may be possible that maintain effectiveness, while reducing the potential for toxicity [100]. However, there are no clinical studies utilizing tedizolid for TB to date (clinicaltrials.gov accessed 1 November 2022), and data indicating the reduced toxicity of tedizolid relative to linezolid are largely limited to maximal durations of 12 weeks in non-TB bacterial infections or case series [116, 117, 118]. Therefore, it is yet to be confirmed if the toxicity of tedizolid is indeed less with the durations of treatment required for TB.
Sutezolid (PNU-100480)
Sutezolid is a thiomorpholinyl analog of linezolid. In vitro testing has suggested that sutezolid has the strongest activity among oxazolidinones against slowly growing nontuberculous mycobacterial species, as well as TB, and that it is superior to linezolid [119, 120]. Activity is evident against both DS- and DR-TB [106].
The sterilizing activity of sutezolid increases its potential to shorten TB therapy. Comparative studies in mice demonstrated that the addition of sutezolid to shortened (4-month) standard first-line therapy improved its efficacy and reduced the risk of relapse significantly compared to regimens without it [122].
In a small trial, 59 patients with pulmonary TB were randomized to two different doses of sutezolid for 14 days. Notable EBA was evident on sputum CFU count as well as time to positivity in blood culture inoculated with MTB for whole blood bactericidal activity. Treatment was also safe, with no subject requiring dose adjustment or discontinuation [123]. The most common reported adverse effect was asymptomatic aminotransferase elevation [123].
A phase IIb dose-finding/efficacy study is currently underway, as added therapy to bedaquiline, delamanid, and moxifloxacin [124].
Delpazolid (LCB01-0371)
Delpazolid is a novel oxazolidinone that contains a cyclic amidrazone group. It is highly bactericidal, and similar to other oxazolidinones exerts its antimycobacterial function by inhibiting protein synthesis at the 23s rRNA [109]. Similar to other oxazolidinones, it possesses broad Gram-positive activity and also seems to have potent activity against M. abscessus [40].
Mixed results have been evident in in vitro studies against DR-TB. Specifically, Zong et al. identified that delpazolid had a lower MIC against MDR-TB with a lower proportion with resistance, relative to linezolid, but higher MICs in XDR-TB [125]. In those with linezolid-resistant isolates, 50% were still susceptible to delpazolid. This study also highlighted that high-level resistance to both linezolid and delpazolid was mediated by mutations in 23s rRNA. Their data identified similar anti-tuberculosis activity to that of linezolid. Notably, there were more linezolid-resistant MDR-TB strains compared to delpazolid, including a novel ribosomal protein mutation (rplD) rendering high-level resistance to linezolid but preserved low MIC to delpazolid [125]. However, a significant proportion of resistant isolates to either linezolid or delpazolid did not have mutations in recognized target genes that may implicate other alterations in the 23s rRNA or perhaps efflux pumps as the cause [125]. They identified that only 2.9% of MDR isolates were resistant to delpazolid, providing the opportunity for its use as a component in MDR-TB treatment [126].
Another in vitro study demonstrated comparable EBA to that of linezolid [110, 127]. It appears to come with an acceptable hematologic safety profile in a short study and it is argued this may be due to its shorter half-life leading to reduced impact on mitochondrial toxicity [128]. There are no longer-term clinical use data or comparative data with linezolid.
TBI-223
TBI-223, another oxazolidinone, has several advantageous properties including improved stability in microsomes and hepatocytes, does not induce or inhibit the CYP enzyme system, has good oral bioavailability, and is active against both DS- and DR-TB strains [7]. Its in vitro activity is similar to linezolid, but appears to have a superior safety profile [40]. Its phase I study has been completed but the results are not yet available [129].
OTB-658
OTB-658 is another oxazolidinone in pre-clinical study that has shown promising in vitro and in vivo activity with possible improvement compared to linezolid [130]. The MIC against MTB was found to be lower than that with linezolid with retained effectiveness against intracellular bacilli. It also has exhibited low spontaneous mutation frequency, rendering it a promising agent against resistant MTB and inviting the opportunity for further exploration in clinical study [131].
Posizolid (AZD5847)
Posizolid was developed by AstraZeneca and found in preclinical studies to have an improved safety profile and in vitro potency over linezolid in treating both intracellular and extracellular TB, including MDR-TB [132]. However, EBA was only modest with twice-daily dosing and there were two serious AEs, including one in a patient developing severe thrombocytopenia [133]. The drug was subsequently withdrawn from development in 2016.
Aminoacyl-tRNA Synthetases
Inhibition of protein production at alternative targets not yet established offers potential utility in attenuating mycobacterial growth in DR-TB. Aminoacyl-tRNA synthetases are imperative enzymes for protein synthesis/translation and one such recognized enzyme is the isoleucyl-tRNA synthetase, which is inhibited by the topical antibacterial mupirocin [134, 135]. Alternatively, leucyl-tRNA synthetases may be inhibited by boron-containing compounds known as oxaboroles. Tevaborale is an irreversible leucyl-tRNA synthetase inhibitor with activity against Candida albicans [115]. Several putative compounds have been constructed and identified using X-ray crystallography and compared in in vitro and in vivo murine models against mycobacteria [134]. The most optimistic compound to date is GSK656 (‘Compound 8’) in phase IIa trials targeting TB with highly potent in vitro activity [135–137]. Several other analogues with slightly different structure are being explored for putative use in TB [135].
RNA Polymerase Inhibition
Rifampin
Despite almost 60 years since its introduction, rifampin remains an indispensable molecule against DS-TB, whose value has paved the way for attempts at further optimized rifamycins such as rifapentine. By inhibiting the mycobacterial RNA polymerase, rifampin impairs RNA synthesis providing bactericidal activity in a concentration-dependent manner [12]. However, it is the sterilizing capabilities of rifampin against slowly replicating bacilli with prolonged post-antibiotic effect that prevents relapse of disease and has allowed significant shortening of the treatment duration of DS-TB [12]. The primary drawback of rifampin is its induction of the hepatic cytochrome P450 system leading to numerous drug-drug interactions.
Rifapentine
Rifapentine is a semi-synthetic derivative of rifampin that inhibits the DNA-dependent RNA polymerase necessary for transcription. In contrast to rifampin, rifapentine has a cyclopentyl side chain ring instead of a methyl group, which significantly increases protein binding and half-life [12]. Interestingly, increasing dose and repeated dosing have suggested decreased bioavailability and increased clearance, respectively, favoring the possibility of autoinduction [138]. Preclinical studies favored an even lower MIC in TB than rifampin, and due to its strong binding to RNA polymerase even in low enzyme activity, a promising option for LTBI [12, 139]. Rifapentine shares similar risks for AEs to rifampin, including hepatotoxicity and influenza-like reaction, which is typically more common with intermittent administration [12]. Cross-resistance between rifampin and rifapentine is predicted almost entirely and is due to point mutations in the rpoB gene [12].
The potency of rifapentine against TB combined with its long half-life has found once-weekly rifapentine combined with isoniazid to be an encouraging option for LTBI treatment that is now a first-line option [140, 141]. Additionally, rifapentine is being investigated as single-drug therapy for LTBI [142]. In TB disease, mouse model data supported the possibility of daily administration that could shorten therapy [143]. The optimism that rifapentine might lead to treatment shortening led to a recent randomized, non-inferiority trial that showed that 4 months of rifapentine combined with moxifloxacin, isoniazid, and pyrazinamide was non-inferior to standard 6-month therapy in a microbiologically eligible population for unfavorable outcomes (15.5% vs. 14.6%; 1.0% difference, 95% CI − 2.6 to 4.5). The rates of AE rates were no higher [144]. This has led to the recent recommendation of this regimen as a treatment alternative in DS-TB [145].
Impaired Energy Metabolism, Including ATP synthesis, Cytochrome Complex, and Electron Transport Chain Function, Membrane Destabilization/Reactive Oxygen Species
Bedaquiline
Bedaquiline is the first FDA-approved novel agent against TB since 1971 and the first-in-class of diarylquinolines inhibiting mycobacterial ATP synthesis mediated along the electron transport chain. It accomplishes this by inhibiting F-ATP synthase activity, depleting bacterial ATP, which subsequently inhibits DNA and protein synthesis and nitrogen metabolism [18]. These mechanisms produce in vitro activity against both replicating and non-replicating bacilli, reducing the number of bacilli during log phase growth and offering sterilizing activity, the ability to prevent post-treatment relapse.
In 2012, it received accelerated approval from the FDA for use in MDR-TB [5]. The promotion of this agent was based on expedited sputum culture conversion (83 days vs. 125 days) when added to standard of care for MDR-TB [146]. There were also more patients found to have converted their sputum to culture negative (79% vs. 58%) by 24 weeks, and this difference was sustained at 120 weeks. The relationship of culture conversion at 24 weeks and long-term outcomes at 120 weeks have been supported and reliable [147]. Tolerance of bedaquiline was adequate, with no increased risk of AEs compared to standard of care alone. However, there was an excess number of deaths in the bedaquiline group relative to placebo (ten patients (12.6%) vs. two patients (2.5%), p = 0.02) of unclear etiology, which led to a Black Box warning being required on the product monograph by the FDA [146, 148].
Nevertheless, following these promising efficacy results there was an extensive roll-out of bedaquiline in 2015 nationally in South Africa, through combined compassionate drug access and local approval, where it rapidly was incorporated into all DR-TB oral regimens. Outcomes were significantly improved. Among more than 19,000 patients with MDR- or XDR-TB studied between 2014 and 2016, bedaquiline was given to 5.2%. All-cause mortality was significantly reduced in the bedaquiline cohort with MDR-TB (HR 0.35; 95% CI 0.28–0.46) and XDR-TB (HR 0.26; 95% CI 0.18–0.38) and overall mortality was more in line with usual rates of death in patients with DS-TB [149]. Subsequently, the WHO recommended the inclusion of bedaquiline in an all-oral regimen as preferred initial treatment for MDR- or XDR-TB [13].
A large retrospective analysis of 428 patients across 25 centres and in 15 countries found excellent rates of treatment success (71.3%) and culture conversion (91.8%) with bedaquiline-containing regimens in MDR-TB. QTc prolongation beyond 500 ms occurred in 9.7% of the patients, including one of the 33 who died, but that death was felt to unlikely be due to bedaquiline [150].
Available clinical data support the effectiveness of bedaquiline when added to guideline-based therapy or more recently in combination with other agents. The Nix-TB trial demonstrated the efficacy of the combination of bedaquiline and pretomanid in conjunction with the bactericidal activity of linezolid [16]. A phase III follow-up study, ZeNix, found that this same combination along with a reduced dose of linezolid at 600 mg for 26 weeks produced the optimal balance of efficacy and safety [105]. The NExT Study was recently published and compared a 6- to 9-month interventional regimen of bedaquiline, levofloxacin, and linezolid with two other drugs (among ethionamide, high-dose isoniazid, terizidone) to the standardized ≥ 9-month injectable-based regimen for MDR-TB [151]. At 24 months, although suboptimal outcomes overall, those in the interventional arm were twice as likely to have a favorable treatment outcome (51% vs. 22.7%; RR 2.2; 95% CI 1.2–4.1; p = 0.006). Specifically, this combination produced fewer unfavorable outcomes (HR 0.4; 95% CI 0.2–0.5; p < 0.001), greater culture conversion rates when censored for those who had bedaquiline substituted into the standardized regimen (HR 2.6; 95% CI 1.4–4.9; p = 0.003), and were more likely to complete therapy without drug substitution. There were more AEs experienced with this combination, mainly driven by linezolid toxicity (hematologic and neurologic).
A systematic review and meta-analysis explored the results of eight studies (two randomized controlled trials and six observational studies) with 1,784 patients who received bedaquiline added to a background MDR-TB regimen, compared with 20,000 patients who did not [152]. Those who received bedaquiline had higher rates of culture conversion (RR 1.272; 95% CI 1.165–1389; p < 0.001). Those receiving bedaquiline demonstrated lower all-cause mortality (RR 0.529; 95% CI 0.454–0.616; p < 0.001), but no difference in treatment success rates (RR 0.98; 95% CI 0.948–1.1013; p = 0.234). There was significant heterogeneity in the included studies.
In a phase IIb trial, the combination of bedaquiline and delamanid with pyrazinamide plus or minus moxifloxacin was shown to have higher EBA, as judged by time-to-culture positivity, relative to standard first-line therapy in both DS- and MDR-TB, respectively, with similar rates of AEs [153]. This creates the opportunity for further phase III exploration of this combination. Both bedaquiline and delamanid risk QTc prolongation as potential side effects. However, the mean increase in QTc is additive but typically manageable, at around 20 ms above baseline [154]. A modelling study suggested that preventative treatment of pediatric household contacts of MDR-TB cases with bedaquiline or delamanid would reduce the number of secondary cases and mortality compared to treatment with fluoroquinolones [155]. Limiting treatment to children under 5 years of age and to those co-infected with HIV would reduce the number of cases and mortality prevented but would be more cost-effectiveness [130].
With clinical experience, bedaquiline is generally well tolerated. The main AEs reported include nausea/vomiting, headache, and arthralgia [146]. As mentioned, the initial phase IIb trial had an increased number of deaths compared to placebo (12.6% vs. 2.5%), though none were clearly attributed to the drug. This unexplained increased risk of death with bedaquiline has not been borne out in subsequent studies. A subsequent multicentre, single-arm clinical trial identified 16 deaths (6.9%), and none were deemed to be related to bedaquiline [146]. Nine of the 10 deaths occurred after the 24-week bedaquiline treatment period; six were due to progression of MDR-TB, and one each was due to acute alcohol poisoning, stroke, motor vehicle accident, and cirrhosis, respectively [146]. The M2 bedaquiline metabolite inhibits KCNH2 (hERG), interfering with potassium channel function and consequently interfering with normal cardiac repolarization and prolonging the QT interval [156]. Because of the known increase of the QTc, this has led to the question of whether these deaths may have been cardiac. The mean change in QTc has been reported to be between 12 and 15 ms [146, 154]; however, this is generally in combination with other medications that may further prolong the QTc, such as clofazimine and fluoroquinolones. Nevertheless, the reported incidence of QTc beyond 500 ms was 3% [157].
However, reports of resistance have been noted and it has been suggested that acquired resistance has the opportunity to occur at low serum levels [158]. Since it has a very long half-life, stopping it well before the other anti-tubercular agents is recommended to prevent selection of bedaquiline-resistant mutants [159]. Even with the potent combination of bedaquiline and pretomanid, as use of bedaquiline increases, so too does the anticipated risk of evolving resistant pathogens. Mouse models of infection with the bedaquiline loss-of-function mutation Rv0678 are less susceptible to the drug and may be selected for along with other drug resistance, even in the presence of other highly effective drugs like pretomanid [160]. Bedaquiline is metabolized by the cytochrome P450 CPY3A4 enzyme and inducers of this enzyme such as rifampin and rifapentine reduce exposure to bedaquiline, whereas inhibitors, including azoles and some antiretrovirals, increase exposure [161, 162].
With the success and broadly recommended inclusion of bedaquiline into all regimens against MDR-TB, similar in class molecules are being explored. ATP synthase inhibition appears to provide effective sterilizing activity. A prominent site of resistance recognized with bedaquiline is the Rv0678 gene, which encodes a repressor of the MmpS5–MmpL5 efflux transporter. Mutations of the gene thereby lead to increased efflux of the molecule [163]. Two novel diarylquinolines, TBAJ-587 and TBAJ-876, are being explored that may have enhanced efficacy against bedaquiline-resistant TB strains [160, 163].
TBAJ-876
TBAJ-876 is a structurally distinct diarylquinoline analogue of bedaquiline that also inhibits the F-ATP synthase [18]. Despite not possessing the same electron transport chain uncoupling activity of its predecessor bedaquiline, it demonstrates equivalent in vitro bactericidal activity to that of bedaquiline [19].
In a murine model, TBAJ-876 at low doses demonstrated similar activity to bedaquiline and at higher doses appeared more bactericidal with greater effect at reducing the CFU burden [163]. This improved efficacy was retained over bedaquiline in strains with the Rv0678 mutation. There is currently a phase I study underway to assess its safety and tolerability [164].
TBAJ-587
Another bedaquiline analogue, TBAJ-587, has demonstrated more potent in vitro activity than bedaquiline while possibly having an improved safety profile in terms of lower cardiac risk [165]. In the mouse model, it was found to be significantly more bactericidal than bedaquiline, and offers the theoretical potential to shorten treatment durations [160]. It is currently undergoing a phase I clinical trial [166].
Sudapyridine (WX-081)
Sudapyridine is another diarylquinoline with comparable efficacy against DS- and DR-TB to that of bedaquiline, but is less lipophilic with a better pharmacokinetic profile, reducing the risk of QTc prolongation [167]. An in vitro assay of the effects of sudapyridine on the human hERG ion channel was reassuring and, while data are limited, sudapyridine has also been reported to have less evidence of qualitative and quantitative impact on the ECG, potentially due to reduced generation of its cardiac-relevant metabolite (WX-081-M3) compared to bedaquiline [167]. A full phase I study has been completed and a phase II trial to determine EBA and safety is underway and is almost complete [7, 168]. The phase IIa study includes 84 patients in five different treatment arms. Patients with newly treated drug-susceptible pulmonary TB are randomized into one of four arms to receive guideline-based multidrug treatment and 150 mg, 300 mg, 450 mg, or no sudapyridine daily for 2 weeks. The fifth arm consists of patients with DR-TB receiving sudapyridine 400 mg daily for 2 weeks, followed by 150 mg daily for 6 weeks, along with multi-drug background treatment (accessed 9 July 2022). Primary outcomes in this phase IIa study are time to sputum culture positivity in liquid culture medium and EBA is measured as the rate of CFU/mL of sputum on solid medium [168].
Imidazopyridine
The electron transport chain provides the energy required for ATP synthesis [169]. Electron transfer through various cytochromes is initiated by the oxidation of organic material creating an electric potential resulting in a proton flow and eventually the enzyme, ATP synthase, phosphorylates adenosine diphosphate (ADP) to ATP [22]. The cytochrome bc1 is an essential component of the electron transport chain in the oxidative phosphorylation pathway [170].
Telacebec (Q203)
Ending in cebec indicates the activity of telacebec is a cytochrome bc1 complex inhibitor of tuberculosis, impairing energy metabolism [170]. It is an imidazopyridine and the mode of action of telacebec is different from other drugs undergoing investigation since it inhibits MTB growth, both DS- and DR-TB, by targeting the QcrB subunit of respiratory cytochrome bc1 complex at a low concentration [67, 169]. The MIC50 against MTB is 2.7 nM in culture medium and 0.28 nM inside macrophages [169].
Telacebec has several attractive pharmacologic features including an oral bioavailability of 90%, a terminal half-life of 23.4 h, and it does not inhibit the cytochrome P450 isoenzymes, so drug-drug interactions should not be a concern [169].
This unique mode of action of telacebec allows MDR- and XDR-TB to be easily targeted. The drug was well tolerated during a phase I trial and there were no serious AEs with single oral doses ranging from 10 to 800 mg [171]. In the phase IIa study [172], treatment-naïve patients with pulmonary DS-TB were given 100 mg, 200 mg, or 300 mg of telacebec for 14 days with a reduction in the sputum mycobacterial load proportional to the dose of telacebec, as determined by the time-to-culture positivity in MGIT 960 system liquid media [173]. Telacebec also has excellent activity against Mycobacterium ulcerans, the causative organism of Buruli ulcer, and Mycobacterium leprae [174].
Pyrifazimine (TBI-166)
Clofazimine, the currently available riminophenazine, has potent antimycobacterial activity. The mechanism of action of clofazimine is not entirely clear due to its predominantly intracellular activity. It is favored to produce intracellular reactive oxygen species, competition for electrons along the mycobacterial respiratory chain, and membrane disruption [175, 176]. Pyrifazimine is a second-generation riminophenazine, similar to clofazimine, that has been shown to have potent anti-TB activity, especially in DR-TB. Pyrifazimine was derived based on optimization of other potential riminophenazine analogues. Specifically, by ensuring a substituent with a 2-methoxypyridylamino group at the C-2 position of the phenazine, it was found to have improved pharmacokinetic properties with increased antimycobacterial potency compared to clofazimine without evidence of skin discoloration, which is almost ubiquitously encountered with clofazimine [66, 177–179]. The reduction in lipophilicity is a significant driver in reducing tissue deposition and subsequent pigmentation change [179]. Following the in vitro and in vivo studies in murine models, success of this chemical prompted its promotion as a drug candidate. A phase I clinical trial was initiated in 2018 in China. A phase II bactericidal activity and comparative safety study of increasing doses of pyrifazimine is underway [180]. In synergistic testing in mouse models, the combination of pyrifazimine with bedaquiline and linezolid was highly bactericidal compared to a usual first-line control regimen and the most effective combination compared to others. The regimen was recommended for further phase II testing [177]. Synergy was also found between pyrifazimine, bedaquiline, SQ109, and PBTZ169 [181].
DNA Gyrase and Topoisomerase
Fluoroquinolones
The fluoroquinolones levofloxacin and moxifloxacin have been repurposed as Group A drugs for the treatment of MDR-TB [28]. The pronounced efficacy of these drugs for treatment of MDR-TB has been highlighted in an individual patient-data level meta-analysis that showed that their use is associated with significantly improved treatment success and survival [99]. Several trials assessed if substituting moxifloxacin for either INH or ethambutol or substituting ethambutol with gatifloxacin could shorten the treatment regimen for DS-pulmonary TB from 6 to 4 months, but found that the 4-month regimens were inferior to standard therapy [182, 183]. Gatifloxacin is no longer available because of its dysglycemic effects. However, more recent data have offered the first positive outcomes supporting a 4-month treatment course in DS-TB [144] and led to an updated conditional recommendation for its use from the WHO [145].
Fodrepodacin (SPR720)
SPR720, renamed fodrepodacin, is a prodrug that is converted to SPR719, an oral bacterial DNA gyrase (gyrB) inhibitor that interferes with DNA replication. It demonstrated activity against both MTB and nontuberculous mycobacteria in in vitro and murine studies [184]. A regimen of SPR720, rifampin, and pyrazinamide showed comparable efficacy to a moxifloxacin, rifampin, and pyrazinamide regimen (Stop TB Partnership). The FDA placed a clinical trial hold on SPR720 following a mortality data review from a nonhuman toxicology study but subsequently lifted it in January 2022 [185]. It appears that Spero therapeutics, the company developing the molecule, will focus research on treatment of nontuberculous mycobacteria [7, 186] (accessed 29 June 2022).
VXc-486
VXc-486 is a new aminobenzimidazole that also targets gyrase B and is bactericidal against both DS- and DR-TB, with MICs ranging from 0.03 to 0.30 mg/mL and 0.08 to 5.48 mg/mL, respectively [7]. This activity was preserved even in XDR-TB, which was also resistant to fluoroquinolones, inhibitory against intracellular TB, and had suggested synergy with linezolid, bedaquiline, and clofazimine. It is also effective against M. avium, M. abscessus, and M. kansasii [187]. No trials are currently listed on clinicaltrials.gov (accessed 15 August 2022).
GSK2556286
GSK2556286 is a pyrimidine-2,4-dione that interferes with MTB’s ability to catabolize cholesterol from the host and utilize it as a carbon source. It selectively kills intracellular MTB in macrophages, MIC50 < 0.1 μm, and is considered to be of moderate efficacy but more effective when combined with other drugs [22]. It is efficacious in cholesterol-containing media and does not cause cross-resistance with other antitubercular agents [188]. A phase I double-blind, randomized, sequential, parallel-dose cohort study in 120 healthy adult participants is currently underway [189].
Drugs That Inhibit Drug Efflux
The use of efflux-pump inhibitors to address TB drug resistance is also being investigated and there have been reports of success when an otherwise effective treatment regimen could not be provided [190]. Phenothiazines inhibit bacterial efflux and the resulting high drug concentrations are toxic to both intracellular DS-and DR-TB [191]. They would be attractive options for the management of DR-TB but their CNS effects are problematic. Prior to the introduction of the new and repurposed drugs to treat DR-TB, there were reports of successful treatment of MDR- and even XDR-TB with thioridazine [192]. Thioridazine was felt to be the phenothiazine with the best risk/benefit profile but it produces CNS and cardiac adverse effects including QT interval prolongation and, rarely, torsade de pointes [193, 194]. Subsequent reports suggest that low enough doses to avoid most adverse effects are effective against MTB, but there are no currently registered clinical trials [195].
Verapamil
Verapamil is also an efflux pump inhibitor. It accelerated bacterial clearance in a murine model of treated TB [196]. It is not active against extracellular MTB but increases intracellular antibiotic concentrations and possibly reduces the risk of the development of drug resistance [24]. There are no current registered clinical studies utilizing verapamil for TB (clinical trials.gov accessed 31 October 2022).
FNDR-20081
While the site of action is not entirely clear, resistance mapping for FNDR-20081 seemed to localize it to MmpL5, which regulates efflux transport, or to Rv3683, a metallophosphoesterase [197]. It is a promising first-in-class quinoline derivative combined with oxadiazole and piperazine in tandem with potent inhibitory activity against both DS and DR-TB strains. When combined with other common anti-mycobacterials, no antagonism was identified, additive activity was found with bedaquiline, pretomanid, and linezolid, and synergy identified with clofazimine and ethambutol. This has offered promise in its clinical effectiveness, inviting the opportunity for clinical exploration [197].
Conclusion
In conclusion, due to the prolonged nature of TB treatment that is rife with AEs, exploration of novel and repurposed therapies that might continue to improve treatment duration and toleration is an emphasis in global TB research. Herein, we have highlighted several such compounds and their unique sites of activity. Sequencing the MTB genome has identified numerous potential targets for drug development in both DS- and DR-strains. If current treatment regimens are given with a daily observed therapy protocol, results against DS-TB strains are excellent. The success rates in patients with DR-, particularly RR-TB and more complex resistance patterns, are inferior. Treatment regimens for DR-TB are more complex, considerably longer, associated with more adverse effects, and are much more expensive to provide [99].
Collaboration and global TB foundation funding have encouraged the recent discovery of novel TB targets, including mycobacterial ATP synthase and the arabinogalactan portion of the cell wall, which offer promising opportunities. Subsequent preclinical development has provided some of the most potent compounds to date in in vitro study. Despite this, it is well recognized that many of these compounds described will be delayed or not make it to the broader therapeutic market. Experiences with agents such as posizolid and sanfetrinem highlight the fact that promising in vitro and early clinical studies do not always translate into clinical efficacy, or finances may limit further development.
Despite these major advances, infection rates and mortality from TB remain stubbornly high. Cost and lack of resources, primarily in the developing world, remain major impediments to the global effort to eliminate the disease. Eliminating this disease will require many things, but the provision of effective medications that are inexpensive, orally active, allow shortening of treatment regimens for both DS-and DR-TB, have infrequent and tolerable side effects, and do not require regular laboratory monitoring will help to achieve the goal of defeating this ongoing pandemic. Therefore, ongoing pursuit and exploitation of novel and repurposed sites are imperative, with the ongoing financial support of global TB research communities to ensure the pipeline does not dry up.
Declarations
Funding
No funding was received for this review.
Conflicts of interest/competing interest
Neither Brett Edwards nor Stephen Field has any conflicts of interest related to the contents of this article.
Authors’ contributions
Both Brett Edwards and Stephen Field shared equally in the research, composition, and revision of this article.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Data availability
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
References
- 1.Daniel TM. The history of tuberculosis. Respir Med. 2006;100(11):1862–1870. doi: 10.1016/j.rmed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Herzog H. History of tuberculosis. Respiration. 1998;65(1):5–15. doi: 10.1159/000029220. [DOI] [PubMed] [Google Scholar]
- 3.Bunyan J. Life and Death of Mr. Badman. Nathaniel Ponder Publishing; 1680.
- 4.Global Tuberculosis Report [Internet]. World Health Organization. 2021. https://www.who.int/publications/i/item/9789240037021. Accessed 17 Jun 2021.
- 5.Bedaquiline [Internet]. FDA. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/204384s000lbl.pdf. Accessed 6 Feb 2020.
- 6.Deltyba | European Medicines Agency [Internet]. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/deltyba#authorisation-details-section. Accessed 2 Jun 2022.
- 7.Clinical Pipeline [Internet]. Working Group on New TB Drugs—Stop TB Partnership. 2022. https://www.newtbdrugs.org. Accessed 22 Apr 2022.
- 8.Shanib Bhat Z, Ahmad Rather M, Maqbool M, Lah HU, Khalid Yousuf S, Ahmad Z. Cell wall: a versatile fountain of drug targets in Mycobacterium tuberculosis. Biomed Pharmacother. 2017;95:1520–1534. doi: 10.1016/j.biopha.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 9.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Executive summary: official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis. 2016;63(7):853–867. doi: 10.1093/cid/ciw566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston JC, Cooper R, Menzies D. Chapter 5: treatment of tuberculosis disease. Can J Respir Crit Care Sleep Med. 2022;6(1):66–76. [Google Scholar]
- 11.Gopal P, Sarathy JP, Yee M, Ragunathan P, Shin J, Bhushan S, et al. Pyrazinamide triggers degradation of its target aspartate decarboxylase. Nat Commun. 2020;11(1):1661. doi: 10.1038/s41467-020-15516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfarisi O, Alghamdi WA, Al-Shaer MH, Dooley KE, Peloquin CA. Rifampin vs. rifapentine: what is the preferred rifamycin for tuberculosis? Expert Rev Clin Pharmacol. 2017;10(10):1027–1036. doi: 10.1080/17512433.2017.1366311. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment [Internet]. World Health Organization. 2019. p. 1–104. https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/. Accessed 1 Aug 2019.
- 14.Nahid P, Mase SR, Migliori GB, Sotgiu G, BothamLey GH, Brozek JL, et al. Treatment of drug-resistant tuberculosis an official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019;200(10):93–142. doi: 10.1164/rccm.201909-1874ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brode SK, Dwilow R, Kunimoto D, Menzies D, Khan FA. Drug-resistant tuberculosis. Can J Respir Crit Care, Sleep Med. 2022;6(1):109–128. [Google Scholar]
- 16.Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry C, du Cros P, Fielding K, Gajewski S, Kazounis E, McHugh TD, et al. TB-PRACTECAL: study protocol for a randomised, controlled, open-label, phase II-III trial to evaluate the safety and efficacy of regimens containing bedaquiline and pretomanid for the treatment of adult patients with pulmonary multidrug-resistant tubercul. Trials [Internet]. 2022;23(1). [DOI] [PMC free article] [PubMed]
- 18.Sarathy JP, Gruber G, Dick T. Re-understanding the mechanisms of action of the anti-mycobacterial drug bedaquiline. Antibiotics. 2019;8(4):261. doi: 10.3390/antibiotics8040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarathy JP, Ragunathan P, Cooper CB, Upton AM, Grüber G, Dick T. TBAJ-876 Displays bedaquiline-like mycobactericidal potency without retaining the parental drug’s uncoupler activity. Antimicrob Agents Chemother. 2020;64:e01540–19. doi: 10.1128/AAC.01540-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrahams KA, Besra GS. Mycobacterial cell wall biosynthesis: a multifaceted antibiotic target. Parasitology. 2018;145:116–133. doi: 10.1017/S0031182016002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Zhao Y, Gao Y, Wu L, Gao R, Zhang Q, et al. Structures of cell wall arabinosyltransferases with the anti-tuberculosis drug ethambutol. Science (80-). 2020;368(6496):1211–1219. doi: 10.1126/science.aba9102. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes GFS, Thompson AM, Castagnolo D, Denny WA, Dos SJL. Tuberculosis drug discovery: challenges and new horizons. J Med Chem. 2022;65(11):7489–7531. doi: 10.1021/acs.jmedchem.2c00227. [DOI] [PubMed] [Google Scholar]
- 23.Jankute M, Cox JAG, Harrison J, Besra GS. Assembly of the mycobacterial cell wall. Annu Rev Microbiol. 2015;69(1):405–423. doi: 10.1146/annurev-micro-091014-104121. [DOI] [PubMed] [Google Scholar]
- 24.Singh V, Chibale K. Strategies to combat multi-drug resistance in tuberculosis. Acc Chem Res. 2021;54(10):2361–2376. doi: 10.1021/acs.accounts.0c00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayama K, Wang C, Besra GS. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin Microbiol Rev. 2005;18(1):81–101. doi: 10.1128/CMR.18.1.81-101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unissa AN, Subbian S, Hanna LE, Selvakumar N. Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect Genet Evol. 2016;45:474–492. doi: 10.1016/j.meegid.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Vilchèze C, Jacobs WR. The mechanism of isoniazid killing: clarity through the scope of genetics. Annu Rev Microbiol. 2007;61:35–50. doi: 10.1146/annurev.micro.61.111606.122346. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO consolidated guidelines on tuberculosis Module 4: Treatment Drug-resistant tuberculosis treatment [Internet]. 2020. https://www.who.int/publications/i/item/9789240007048. Accessed 1 Jul 2022. [PubMed]
- 29.Tornheim JA, Dooley KE. The global landscape of tuberculosis therapeutics. Annu Rev Med. 2019;70:105–120. doi: 10.1146/annurev-med-040717-051150. [DOI] [PubMed] [Google Scholar]
- 30.Dean AS, Zignol M, Cabibbe AM, Falzon D, Glaziou P, Cirillo DM, et al. Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: a multicountry analysis of cross-sectional data. PLoS Med. 2020;17(1):e1003008. doi: 10.1371/journal.pmed.1003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um KS, Wilson T, et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263(5144):227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 32.Morlock GP, Metchock B, Sikes D, Crawford JT, Cooksey RC. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother. 2003;47(12):3799–3805. doi: 10.1128/AAC.47.12.3799-3805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scardigli A, Caminero JA, Sotgiu G, Centis R, D’Ambrosio L, Migliori GB. Efficacy and tolerability of ethionamide versus prothionamide: a systematic review. Eur Respir J. 2016;48(3):946–952. doi: 10.1183/13993003.00438-2016. [DOI] [PubMed] [Google Scholar]
- 34.Guieu B, Jourdan JP, Dreneau A, Willand N, Rochais C, Dallemagne P. Desirable drug–drug interactions or when a matter of concern becomes a renewed therapeutic strategy. Drug Discov Today. 2021;26(2):315–328. doi: 10.1016/j.drudis.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Clinical Trial to Investigate the Safety, Tolerability and Pharmacokinetics of BVL-GSK098 in Healthy Volunteers [Internet]. 2022. https://clinicaltrials.gov/ct2/results?cond=&term=NCT04654143&cntry=&state=&city=&dist=. Accessed 20 Aug 2022.
- 36.Falzon D, Hill G, Pal SN, Suwankesawong W, Jaramillo E. Pharmacovigilance and tuberculosis: applying the lessons of thioacetazone. Bull World Health Organ. 2014;92(12):918–919. doi: 10.2471/BLT.14.142570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez-Hoyos M, Perez-Herran E, Gulten G, Encinas L, Álvarez-Gómez D, Alvarez E, et al. Antitubercular drugs for an old target: GSK693 as a promising InhA direct inhibitor. EBioMedicine. 2016;8:291–301. doi: 10.1016/j.ebiom.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki H, Haraguchi Y, Itotani M, Kuroda H, Hashizume H, Tomishige T, et al. Synthesis and antituberculosis activity of a novel series of optically active 6-nitro-2,3-dihydroimidazo[2,1-b]oxazoles. J Med Chem. 2006;49(26):7854–7860. doi: 10.1021/jm060957y. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Matsumoto M, Ishida H, Ohguro K, Yoshitake M, Gupta R, et al. Delamanid: from discovery to its use for pulmonary multidrug-resistant tuberculosis (MDR-TB) Tuberculosis. 2018;111:20–30. doi: 10.1016/j.tube.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Shetye GS, Franzblau SG, Cho S. New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Transl Res. 2020;220:68–97. doi: 10.1016/j.trsl.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Wen S, Jing W, Zhang T, Zong Z, Xue Y, Shang Y, et al. Comparison of in vitro activity of the nitroimidazoles delamanid and pretomanid against multidrug-resistant and extensively drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2019;38:1293–1296. doi: 10.1007/s10096-019-03551-w. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen TVA, Anthony RM, Cao TTH, Bañuls AL, Nguyen VAT, Vu DH, et al. Delamanid resistance: update and clinical management. Clin Infect Dis. 2020;71(12):3252–3259. doi: 10.1093/cid/ciaa755. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3:2131–2144. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujiwara M, Kawasaki M, Hariguchi N, Liu Y, Matsumoto M. Mechanisms of resistance to delamanid, a drug for Mycobacterium tuberculosis. Tuberculosis. 2018;108:186–194. doi: 10.1016/j.tube.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Ryan NJ, Lo JH. Delamanid: first global approval. Drugs. 2014;74(9):1041–1045. doi: 10.1007/s40265-014-0241-5. [DOI] [PubMed] [Google Scholar]
- 46.Khoshnood S, Taki E, Sadeghifard N, Kaviar VH, Haddadi MH, Farshadzadeh Z, et al. Mechanism of action, resistance, synergism, and clinical implications of delamanid against multidrug-resistant Mycobacterium tuberculosis. Front Microbiol. 2021;12:717045. doi: 10.3389/fmicb.2021.717045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimokawa Y, Sasahara K, Yoda N, Mizuno K, Umehara K. Delamanid does not inhibit or induce cytochrome P450 enzymes in vitro. Biol Pharm Bull. 2014;37(11):1727–1735. doi: 10.1248/bpb.b14-00311. [DOI] [PubMed] [Google Scholar]
- 48.Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 49.Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, Kummik T, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41(6):1393–1400. doi: 10.1183/09031936.00125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hafkin J, Hittel N, Martin A, Gupta R. Early outcomes in MDR-TB and XDR-TB patients treated with delamanid under compassionate use. Eur Respir J. 2017;50(1):1700311. doi: 10.1183/13993003.00311-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hewison C, Ferlazzo G, Avaliani Z, Hayrapetyan A, Jonckheere S, Khaidarkhanova Z, et al. Six-month response to delamanid treatment in MDR TB patients—volume 23, number 10—October 2017—emerging Infectious Diseases journal—CDC. Emerg Infect Dis. 2017;23(10):1746–1748. doi: 10.3201/eid2310.170468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Von Groote-Bidlingmaier F, Patientia R, Sanchez E, Balanag V, Jr, Ticona E, Segura P, et al. Efficacy and safety of delamanid in combination with an optimised background regimen for treatment of multidrug-resistant tuberculosis: a multicentre, randomised, double-blind, placebo-controlled, parallel group phase 3 trial. Lancet Respir Med. 2019;7(3):249–259. doi: 10.1016/S2213-2600(18)30426-0. [DOI] [PubMed] [Google Scholar]
- 53.Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, et al. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science (80-). 2008;322(5906):1392–1395. doi: 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lenaerts AJ, Gruppo V, Marietta KS, Johnson CM, Driscoll DK, Tompkins NM, et al. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob Agents Chemother. 2005;49(6):2294–2301. doi: 10.1128/AAC.49.6.2294-2301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rifat D, Li SY, Ioerger T, Shah K, Lanoix JP, Lee J, et al. Mutations in fbiD (Rv2983) as a novel determinant of resistance to pretomanid and delamanid in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2021;65(1):e01948-20. doi: 10.1128/AAC.01948-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haver HL, Chua A, Ghode P, Lakshminarayana SB, Singhal A, Mathema B, et al. Mutations in genes for the F420 biosynthetic pathway and a nitroreductase enzyme are the primary resistance determinants in spontaneous in vitro-selected PA-824-resistant mutants of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2015;59(9):5316–5323. doi: 10.1128/AAC.00308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu J, Li SY, Almeida DV, Tasneen R, Barnes-Boyle K, Converse PJ, et al. Contribution of pretomanid to novel regimens containing bedaquiline with either linezolid or moxifloxacin and pyrazinamide in murine models of tuberculosis. Antimicrob Agents Chemother. 2019;63(5):e00021-19. doi: 10.1128/AAC.00021-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trial to Evaluate the Efficacy, Safety and Tolerability of BPaMZ in Drug-Sensitive (DS-TB) Adult Patients and Drug-Resistant (DR-TB) Adult Patients [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03338621?term=NCT03338621&draw=2&rank=1. Accessed 13 May 2022.
- 59.Sacksteder KA, Protopopova M, Barry CE, Andries K, Nacy CA. Discovery and development of SQ109: a new antitubercular drug with a novel mechanism of action. Future Microbiol. 2012;7(7):823–837. doi: 10.2217/fmb.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malwal SR, Zimmerman MD, Alvarez N, Sarathy JP, Dartois V, Nacy CA, et al. Structure, in Vivo Detection, and Antibacterial Activity of Metabolites of SQ109, an Anti-Infective Drug Candidate. ACS Infect Dis. 2021;7(8):2492–2507. doi: 10.1021/acsinfecdis.1c00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddy VM, Dubuisson T, Einck L, Wallis RS, Jakubiec W, Ladukto L, et al. SQ109 and PNU-100480 interact to kill Mycobacterium tuberculosis in vitro. J Antimicrob Chemother. 2012;67:1163–1166. doi: 10.1093/jac/dkr589. [DOI] [PubMed] [Google Scholar]
- 62.Heinrich N, Dawson R, Du Bois J, Narunsky K, Horwith G, Phipps AJ, et al. Early phase evaluation of SQ109 alone and in combination with rifampicin in pulmonary TB patients. J Antimicrob Chemother. 2015;70(5):1558–1566. doi: 10.1093/jac/dku553. [DOI] [PubMed] [Google Scholar]
- 63.Reddy VM, Einck L, Andries K, Nacy CA. In vitro interactions between new antitubercular drug candidates SQ109 and TMC207. Antimicrob Agents Chemother. 2010;54(7):2840–2846. doi: 10.1128/AAC.01601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis. 2017;17(1):39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Black TA, Buchwalde UK. The pipeline of new molecules and regimens against drug-resistant tuberculosis. J Clin Tuberc Other Mycobact Dis. 2021;25:100285. doi: 10.1016/j.jctube.2021.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tetali SR, Kunapaeddi E, Mailavaram RP, Singh V, Borah P, Deb PK, et al. Current advances in the clinical development of anti-tubercular agents. Tuberculosis. 2020;125:101989. doi: 10.1016/j.tube.2020.101989. [DOI] [PubMed] [Google Scholar]
- 67.Oh S, Trifonov L, Yadav VD, Barry CE, Boshoff HI. Tuberculosis drug discovery: a decade of hit assessment for defined targets. Front Cell Infect Microbiol. 2021;11:e611304. doi: 10.3389/fcimb.2021.611304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Man DKW, Kanno T, Manzo G, Robertson BD, Lam JKW, Mason AJ. Rifampin- or capreomycin-induced remodeling of the mycobacterium smegmatis mycolic acid layer is mitigated in synergistic combinations with cationic antimicrobial peptides. mSphere. 2018;3(4):e00218-18. doi: 10.1128/mSphere.00218-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chikhale RV, Barmade MA, Murumkar PR, Yadav MR. Overview of the development of DprE1 inhibitors for combating the menace of tuberculosis. J Med Chem. 2018;61(19):8563–8593. doi: 10.1021/acs.jmedchem.8b00281. [DOI] [PubMed] [Google Scholar]
- 70.Mikušová K, Huang H, Yagi T, Holsters M, Vereecke D, D’Haeze W, et al. Decaprenylphosphoryl arabinofuranose, the donor of the D-arabinofuranosyl residues of mycobacterial arabinan, is formed via a two-step epimerization of decaprenylphosphoryl ribose. J Bacteriol. 2005;187(23):8020–8025. doi: 10.1128/JB.187.23.8020-8025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brecik M, Centárová I, Mukherjee R, Kolly GS, Huszár S, Bobovská A, et al. DprE1 Is a vulnerable tuberculosis drug target due to its cell wall localization. ACS Chem Biol. 2015;10(7):1631–1636. doi: 10.1021/acschembio.5b00237. [DOI] [PubMed] [Google Scholar]
- 72.Makarov V, Manina G, Mikusova K, Möllmann U, Ryabova O, Saint-Joanis B, et al. Benzothiazinones kill Mycobacterium tuberculosis by blocking Arabinan synthesis. Science. 2009;324(5928):801–804. doi: 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hariguchi N, Chen X, Hayashi Y, Kawano Y, Fujiwara M, Matsuba M, et al. OPC-167832, a novel carbostyril derivative with potent antituberculosis activity as a DprE1 inhibitor. Antimicrob Agents Chemother. 2020;64(6):e02020–19. doi: 10.1128/AAC.02020-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chatterji M, Shandil R, Manjunatha MR, Solapure S, Ramachandran V, Kumar N, et al. 1,4-azaindole, a potential drug candidate for treatment of tuberculosis. Antimicrob Agents Chemother. 2014;58(9):5325–5331. doi: 10.1128/AAC.03233-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo S, Fu L, Wang B, Chen X, Zhao J, Liu M, et al. In vitro and in vivo antimicrobial activities of a novel piperazine-containing benzothiazinones candidate TZY-5-84 against Mycobacterium tuberculosis. Biomed Pharmacother. 2020;131:110777. doi: 10.1016/j.biopha.2020.110777. [DOI] [PubMed] [Google Scholar]
- 76.Robertson GT, Ramey ME, Massoudi LM, Carter CL, Zimmerman M, Kaya F, et al. Comparative analysis of pharmacodynamics in the c3heb/fej mouse tuberculosis model for DprE1 inhibitors TBA-7371, PBTZ169, and OPC-167832. Antimicrob Agents Chemother. 2021;65(11):e00583–21. doi: 10.1128/AAC.00583-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Makarov V, Neres J, Hartkoorn RC, Ryabova OB, Kazakova E, Šarkan M, et al. The 8-pyrrole-benzothiazinones are noncovalent inhibitors of DprE1 from Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2015;59(8):4446–4452. doi: 10.1128/AAC.00778-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Makarov V, Lechartier B, Zhang M, Neres J, van der Sar AM, Raadsen SA, et al. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol Med. 2014;6(3):372–383. doi: 10.1002/emmm.201303575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lechartier B, Hartkoorn RC, Cole ST. In vitro combination studies of benzothiazinone lead compound BTZ043 against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56(11):5790–5793. doi: 10.1128/AAC.01476-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.A Single Ascending Dose Study of BTZ043 [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03590600?term=NCT03590600&draw=2&rank=1. Accessed 13 Aug 2022.
- 81.BTZ-043—Multiple Ascending Dose (MAD) to Evaluate Safety, Tolerability and Early Bactericidal Activity (EBA) [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT04044001?term=NCT04044001&draw=2&rank=1. Accessed 13 Aug 2022.
- 82.Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Ex-vivo Antitubercular Activity of PBTZ169 Formulation [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03423030?term=NCT03423030&draw=2&rank=1. Accessed 13 Aug 2022.
- 83.Phase 1 Study of PBTZ169 [Internet]. 2022. https://clinicaltrials.gov/ct2/results?cond=&term=NCT03036163&cntry=&state=&city=&dist=. Accessed 13 Aug 2022.
- 84.Phase 2a Study of PBTZ169 [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03334734?term=NCT03334734&draw=2&rank=1. Accessed 13 Aug 2022.
- 85.A Phase 1/2 Trial of Multiple Oral Doses of OPC-167832 for Uncomplicated Pulmonary Tuberculosis [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03678688?term=NCT03678688&draw=2&rank=1. Accessed 13 Aug 2022.
- 86.Safety and Efficacy Evaluation of 4-month Regimen of OPC-167832, Delamanid and Bedaquiline in Participants With Drug-Susceptible Pulmonary TB [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT05221502?term=NCT05221502&draw=2&rank=1. Accessed 13 Aug 2022.
- 87.Shirude PS, Shandil R, Sadler C, Naik M, Hosagrahara V, Hameed S, et al. Azaindoles: noncovalent DprE1 inhibitors from scaffold morphing efforts, kill Mycobacterium tuberculosis and are efficacious in vivo. J Med Chem. 2013;56(23):9701–9708. doi: 10.1021/jm401382v. [DOI] [PubMed] [Google Scholar]
- 88.Early Bactericidal Activity of TBA-7371 in Pulmonary Tuberculosis [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT04176250?term=NCT04176250&draw=2&rank=1. Accessed 13 Aug 2022.
- 89.van Heijenoort J. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology. 2001;11(3):25R–36R. [DOI] [PubMed]
- 90.Batson S, De Chiara C, Majce V, Lloyd AJ, Gobec S, Rea D, et al. Inhibition of D-Ala:D-Ala ligase through a phosphorylated form of the antibiotic D-cycloserine. Nat Commun. 2017;8(1):1939. doi: 10.1038/s41467-017-02118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lan Z, Ahmad N, Baghaei P, Barkane L, Benedetti A, Brode SK, et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8(4):383–394. doi: 10.1016/S2213-2600(20)30047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diacon AH, van der Merwe L, Barnard M, von Groote-Bidlingmaier F, Lange C, García-Basteiro AL, et al. β-Lactams against Tuberculosis-new trick for an old dog? N Engl J Med. 2016;375(4):393–394. doi: 10.1056/NEJMc1513236. [DOI] [PubMed] [Google Scholar]
- 93.Hugonnet JE, Blanchard JS. Irreversible inhibition of the Mycobacterium tuberculosis β-lactamase by clavulanate. Biochemistry. 2007;46(43):11998–12004. doi: 10.1021/bi701506h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, Blanchard JS. Meropenem-Clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science. 2009;323(5918):1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cordillot M, Dubée V, Triboulet S, Dubost L, Marie A, Hugonnet JE, et al. In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by L, D-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother. 2013;57(12):5940–5945. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Jager V, Gupte N, Nunes S, Barnes GL, van Wijk RC, Mostert J, et al. Early bactericidal activity of meropenem plus clavulanate (with or without rifampin) for tuberculosis: the COMRADE randomized, phase 2A clinical trial. Am J Respir Crit Care Med. 2022;205(10):1228–1235. doi: 10.1164/rccm.202108-1976OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doern GV, Pierce G, Brueggemann AB. In vitro activity of sanfetrinem (GV104326), a new trinem antimicrobial agent, versus Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Diagn Microbiol Infect Dis. 1996;26(1):39–42. doi: 10.1016/S0732-8893(96)00173-3. [DOI] [PubMed] [Google Scholar]
- 98.EBA, Safety and Tolerability of Sanfetrinem Cilexetil [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT05388448?term=NCT05388448&draw=2&rank=1. Accessed 13 Aug 2022.
- 99.Ahmad N, Ahuja SD, Akkerman OW, Alffenaar J, Anderson L, Baghaei P. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–834. doi: 10.1016/S0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oehadian A, Santoso P, Menzies D, Ruslami R. Concise clinical review of hematologic toxicity of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis: role of mitochondria. Tuberc Respir Dis (Seoul). 2022;85(2):111–121. doi: 10.4046/trd.2021.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Narita M, Tsuji BT, Yu VL. Linezolid-associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome. Pharmacotherapy. 2007;27(8):1189–1197. doi: 10.1592/phco.27.8.1189. [DOI] [PubMed] [Google Scholar]
- 102.Kishor K, Dhasmana N, Kamble S, Sahu R. Linezolid induced adverse drug reactions—an update. Curr Drug Metab. 2015;16(7):553–559. doi: 10.2174/1389200216666151001121004. [DOI] [PubMed] [Google Scholar]
- 103.Pfizer. Zyvoxam Product Monograph [Internet]. https://www.pfizer.ca/sites/default/files/202203/ZYVOXAM_PM_EN_256427_27-Jan-2022.pdf
- 104.Huerga H, Khan U, Bastard M, Mitnick CD, Lachenal N, Khan PY, et al. Safety and effectiveness outcomes from a 14-country cohort of patients with multi-drug resistant tuberculosis treated concomitantly with bedaquiline, delamanid and other second-line drugs. Clin Infect Dis. 2022;ciac176. [DOI] [PMC free article] [PubMed]
- 105.Conradie F, Bagdasaryan TR, Borisov S, Howell P, Mikiashvili L, Ngubane N, et al. Bedaquiline–pretomanid–linezolid regimens for drug-resistant tuberculosis. N Engl J Med. 2022;387(9):810–823. doi: 10.1056/NEJMoa2119430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown AN, Drusano GL, Adams JR, Rodriquez JL, Jambunathan K, Baluya DL, et al. Preclinical evaluations to identify optimal linezolid regimens for tuberculosis therapy. MBio. 2015;6(6):e01741–15. doi: 10.1128/mBio.01741-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song T, Lee M, Jeon HS, Park Y, Dodd LE, Dartois V, et al. Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. EBioMedicine. 2015;2(11):1627–1633. doi: 10.1016/j.ebiom.2015.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruiz P, Causse M, Vaquero M, Casal M. In vitro activity of tedizolid against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2019;63:e01939–e2018. doi: 10.1128/AAC.01939-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iqbal K, Milioudi A, Wicha SG. Pharmacokinetics and pharmacodynamics of tedizolid. Clin Pharmacokinet. 2022;61(4):489–503. doi: 10.1007/s40262-021-01099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wen S, Gao X, Zhao W, Huo F, Jiang G, Dong L, et al. Comparison of the in vitro activity of linezolid, tedizolid, sutezolid, and delpazolid against rapidly growing mycobacteria isolated in Beijing. China. Int J Infect Dis. 2021;109:253–260. doi: 10.1016/j.ijid.2021.06.055. [DOI] [PubMed] [Google Scholar]
- 111.Srivastava S, Cirrincione KN, Deshpande D, Gumbo T, Friberg LE, Van WR, et al. Tedizolid, faropenem, and moxifloxacin combination with potential activity against nonreplicating Mycobacterium tuberculosis. Front Pharmacol. 2021;11:616294. doi: 10.3389/fphar.2020.616294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deshpande D, Srivastava S, Nuermberger E, Koeuth T, Martin KR, Cirrincione KN, et al. Multiparameter responses to tedizolid monotherapy and moxifloxacin combination therapy models of children with intracellular tuberculosis. Clin Infect Dis. 2018;67(Suppl 3):S342–S348. doi: 10.1093/cid/ciy612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srivastava S, Deshpande D, Nuermberger E, Lee PS, Cirrincione K, Dheda K, et al. The sterilizing effect of intermittent tedizolid for pulmonary tuberculosis. Clin Infect Dis. 2018;2018(3):336–377. doi: 10.1093/cid/ciy626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aono A, Murase Y, Chikamatsu K, Igarashi Y, Shimomura Y, Hosoya M, et al. In vitro activity of tedizolid and linezolid against multidrug-resistant Mycobacterium tuberculosis: a comparative study using microdilution broth assay and genomics. Diagnostic Microbiol Infect Dis. 2022;103:115714. doi: 10.1016/j.diagmicrobio.2022.115714. [DOI] [PubMed] [Google Scholar]
- 115.Mikamo H, Takesue Y, Iwamoto Y, Tanigawa T, Kato M, Tanimura Y, et al. Efficacy, safety and pharmacokinetics of tedizolid versus linezolid in patients with skin and soft tissue infections in Japan—results of a randomised, multicentre phase 3 study. J Infect Chemother. 2018;24(6):434–442. doi: 10.1016/j.jiac.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 116.Flanagan S, Bartizal K, Minassian SL, Fang E, Prokocimer P. In vitro, in vivo, and clinical studies of tedizolid to assess the potential for peripheral or central monoamine oxidase interactions. Antimicrob Agents Chemother. 2013;57(7):3060–3066. doi: 10.1128/AAC.00431-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lletí MS, García-Bustos V, Ruiz LM, Cabañero-Navalon MD. Tedizolid: new data and experiences for clinical practice. Rev Española Quimioter. 2021;34(Suppl1):22–25. doi: 10.37201/req/s01.06.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Poon YK, La Hoz RM, Hynan LS, Sanders J, Monogue ML. Tedizolid vs Linezolid for the Treatment of Nontuberculous Mycobacteria Infections in Solid Organ Transplant Recipients. Open Forum Infect Dis. 2021;8(4):ofab093. [DOI] [PMC free article] [PubMed]
- 119.Yu X, Huo F, Wang F, Wen S, Jiang G, Xue Y, et al. In vitro antimicrobial activity comparison of linezolid, tedizolid, sutezolid and delpazolid against slowly growing mycobacteria isolated in Beijing. China. Infect Drug Resist. 2021;14:4689–4697. doi: 10.2147/IDR.S332835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang M, Sala C, Dhar N, Vocat A, Sambandamurthy VK, Sharma S, et al. In vitro and in vivo activities of three oxazolidinones against nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58(6):3217–3223. doi: 10.1128/AAC.02410-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yip PCW, Kam KM, Lam ETK, Chan RCY, Yew WW. In vitro activities of PNU-100480 and linezolid against drug-susceptible and drug-resistant Mycobacterium tuberculosis isolates. Int J Antimicrob Agents. 2013;42(1):96–97. doi: 10.1016/j.ijantimicag.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 122.Williams KN, Brickner SJ, Stover CK, Zhu T, Ogden A, Tasneen R, et al. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am J Respir Crit Care Med. 2009;180(4):371–376. doi: 10.1164/rccm.200904-0611OC. [DOI] [PubMed] [Google Scholar]
- 123.Wallis RS, Dawson R, Friedrich SO, Venter A, Paige D. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of Patients with pulmonary tuberculosis. PLoS ONE. 2014;9(4):94462. doi: 10.1371/journal.pone.0094462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.PanACEA Sutezolid Dose-finding and Combination Evaluation (SUDOCU) [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03959566?term=NCT03959566&draw=2&rank=1. Accessed 13 Aug 2022.
- 125.Zong Z, Jing W, Shi J, Wen S, Zhang T, Huo F, et al. Comparison of in vitro activity and MIC distributions between the novel oxazolidinone delpazolid and linezolid against multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis in China. Antimicrob Agents Chemother. 2018;62(8):e00165-18. doi: 10.1128/AAC.00165-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.PanACEA DElpazolid Dose-finding and COmbination DEvelopment (DECODE) (DECODE) [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT04550832?term=delpazolid&cond=tuberculosis&draw=2&rank=2. Accessed 13 Aug 2022.
- 127.Sang Kim J, Kim YH, Haak Lee S, Hyung Kim Y, Kim JW, Young Kang J, et al. Early bactericidal activity of delpazolid (LCB01-0371) in patients with pulmonary tuberculosis. Antimicrob Agents Chemother. 2022;66(2):e01684–21. doi: 10.1128/aac.01684-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Choi Y, Lee SW, Kim A, Jang K, Nam H, Cho YL, et al. Safety, tolerability and pharmacokinetics of 21 day multiple oral administration of a new oxazolidinone antibiotic, LCB01-0371, in healthy male subjects. J Antimicrob Chemother. 2018;73:183–190. doi: 10.1093/jac/dkx367. [DOI] [PubMed] [Google Scholar]
- 129.A Phase 1 Study to Evaluate Safety, Tolerability, and Pharmacokinetics of TBI-223 in Healthy Adults [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03758612?term=NCT03758612&draw=2&rank=1. Accessed 19 Jun 2022.
- 130.Jiang J, Liu Y, Liu X, Zhang D, Huang H, Wang B, et al. Simultaneous determination of a novel oxazolidinone anti-tuberculosis OTB-658 and its metabolites in monkey blood by LC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci. 2020;2021(1167):122552. doi: 10.1016/j.jchromb.2021.122552. [DOI] [PubMed] [Google Scholar]
- 131.Guo S, Wang B, Fu L, Chen X, Zhang W, Huang H, et al. In vitro and in vivo activity of oxazolidinone candidate OTB-658 against mycobacterium tuberculosis. Antimicrob Agents Chemother. 2021;65(11):e00974–21. doi: 10.1128/AAC.00974-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Balasubramanian V, Solapure S, Iyer H, Ghosh A, Sharma S, Kaur P, et al. Bactericidal activity and mechanism of action of AZD5847, a Novel oxazolidinone for treatment of tuberculosis. Antimicrob Agents Chemother. 2014;58(1):495–502. doi: 10.1128/AAC.01903-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Furin JJ, Du Bois J, Van Brakel E, Chheng P, Venter A, Peloquin CA, et al. Early Bactericidal activity of AZD5847 in patients with pulmonary tuberculosis. Antimicrob Agents Chemother. 2016;60(11):6591–6599. doi: 10.1128/AAC.01163-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Palencia A, Li X, Bu W, Choi W, Ding CZ, Easom EE, et al. Discovery of novel oral protein synthesis inhibitors of Mycobacterium tuberculosis that target Leucyl-tRNA synthetase. Antimicrob Agents Chemother. 2016;60(10):6271–6280. doi: 10.1128/AAC.01339-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bouz G, Zitko J. Inhibitors of aminoacyl-tRNA synthetases as antimycobacterial compounds: an up-to-date review. Bioorg Chem. 2021;110:104806. doi: 10.1016/j.bioorg.2021.104806. [DOI] [PubMed] [Google Scholar]
- 136.An Early Bactericidal Activity, Safety and Tolerability of GSK3036656 in Subjects With Drug-sensitive Pulmonary Tuberculosis [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03557281?term=NCT03557281&draw=2&rank=1. Accessed 21 Jun 2022.
- 137.Li X, Hernandez V, Rock FL, Choi W, Mak YSL, Mohan M, et al. Discovery of a potent and specific M. tuberculosis Leucyl-tRNA synthetase inhibitor: (S)-3-(Aminomethyl)-4-chloro-7-(2-hydroxyethoxy)benzo[c][1,2]oxaborol-1(3H)-ol (GSK656) J Med Chem. 2017;60(19):8011–8026. doi: 10.1021/acs.jmedchem.7b00631. [DOI] [PubMed] [Google Scholar]
- 138.Dooley KE, Bliven-Sizemore E, Weiner M, Lu Y, Nuermberger EL, Hubbard W, et al. Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers. Clin Pharmacol Ther. 2012;91(5):881–888. doi: 10.1038/clpt.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heifets L, Lindholm-Levy P, Flora M. Bactericidal activity in vitro of various rifamycins against Mycobacterium avium and Mycobacterium tuberculosis. Am Rev Respir Dis. 1990;141(3):626–630. doi: 10.1164/ajrccm/141.3.626. [DOI] [PubMed] [Google Scholar]
- 140.Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 141.Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the national tuberculosis controllers association and CDC, 2020. MMWR Recomm Rep. 2020;69(RR-1):1–11. doi: 10.15585/mmwr.rr6901a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Assessment of the safety, tolerability, and effectiveness of rifapentine given daily for LTBI (ASTERoiD) [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03474029?term=latent+tuberculosis&cond=rifapentine&draw=2&rank=1. Accessed 5 Nov 2022.
- 143.Rosenthal I, Zhang M, Williams K, Peloquin CA, Tyagi S, Vernon A, et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4(12):e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dorman SE, Nahid P, Kurbatova EV, Phillips PPJ, Bryant K, Dooley KE, et al. Four-month rifapentine regimens with or without moxifloxacin for Tuberculosis. N Engl J Med. 2021;384(18):1705–1718. doi: 10.1056/NEJMoa2033400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: drug-susceptible tuberculosis treatment. 2022. p. 1–71. [PubMed]
- 146.Diacon AH, Pym A, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371(8):723–732. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 147.Meyvisch P, Kambili C, Andries K, Lounis N, Theeuwes M, Dannemann B, et al. Evaluation of six months sputum culture conversion as a surrogate endpoint in a multidrug resistant-tuberculosis trial. PLoS ONE. 2018;13(7):e0200539. doi: 10.1371/journal.pone.0200539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Janssen. Bedaquiline: Product Monograph [Internet]. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/204384s000lbl.pdf. Accessed 6 Nov 2022
- 149.Schnippel K, Ndjeka N, Maartens G, Meintjes G, Master I, Ismail N, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med. 2018;6(9):699–706. doi: 10.1016/S2213-2600(18)30235-2. [DOI] [PubMed] [Google Scholar]
- 150.Borisov SE, Dheda K, Enwerem M, Leyet RR, D’Ambrosio L, Centis R, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49(5):e1700387. doi: 10.1183/13993003.00387-2017. [DOI] [PubMed] [Google Scholar]
- 151.Esmail A, Oelofse S, Lombard C, Perumal R, Mbuthini L, Mahomed AG, et al. An all-oral 6-month regimen for multidrug-resistant tuberculosis a multicenter, randomized controlled clinical trial (the NExT study) Am J Respir Crit Care Med. 2022;205(10):1214–1227. doi: 10.1164/rccm.202107-1779OC. [DOI] [PubMed] [Google Scholar]
- 152.Wang MG, Wu SQ, He JQ. Efficacy of bedaquiline in the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):970. doi: 10.1186/s12879-021-06666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tweed CD, Dawson R, Burger DA, Conradie A, Crook AM, Mendel CM, et al. Bedaquiline, moxifloxacin, pretomanid, and pyrazinamide during the first 8 weeks of treatment of patients with drug-susceptible or drug-resistant pulmonary tuberculosis: a multicentre, open-label, partially randomised, phase 2b trial. Lancet Respir Med. 2019;7(12):1048–1058. doi: 10.1016/S2213-2600(19)30366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dooley KE, Rosenkranz SL, Conradie F, Moran L, Hafner R, von Groote-Bidlingmaier F, et al. QT effects of bedaquiline, delamanid, or both in patients with rifampicin-resistant tuberculosis: a phase 2, open-label, randomised, controlled trial. Lancet Infect Dis. 2021;21(7):975–983. doi: 10.1016/S1473-3099(20)30770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dodd PJ, Mafirakureva N, Seddon JA, McQuaid CF. The global impact of household contact management for children on multidrug-resistant and rifampicin-resistant tuberculosis cases, deaths, and health-system costs in 2019: a modelling study. Lancet Glob Heal. 2022;10(7):e1034–e1044. doi: 10.1016/S2214-109X(22)00113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ahmad I, Jadhav H, Shinde Y, Jagtap V, Girase R, Patel H. Optimizing Bedaquiline for cardiotoxicity by structure based virtual screening, DFT analysis and molecular dynamic simulation studies to identify selective MDR-TB inhibitors. Silico Pharmacol. 2021;9(1):23. doi: 10.1007/s40203-021-00086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pontali E, Sotgiu G, Tiberi S, D’Ambrosio L, Centis R, Migliori GB. Cardiac safety of bedaquiline: a systematic and critical analysis of the evidence. Eur Respir J. 2017;50(5):1701462. doi: 10.1183/13993003.01462-2017. [DOI] [PubMed] [Google Scholar]
- 158.Mase S, Chorba T, Lobue P, Castro KG. Provisional CDC guidelines for the use and safety monitoring of bedaquiline fumarate (sirturo) for the treatment of multidrug-resistant tuberculosis. MMWR Reports Recomm. 2013;62(RR09):1–12. [PubMed] [Google Scholar]
- 159.Fox GJ, Menzies D. A review of the evidence for using bedaquiline (TMC207) to treat multi-drug resistant tuberculosis. Infect Dis Ther. 2013;2(2):123–144. doi: 10.1007/s40121-013-0009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu J, Converse PJ, Upton AM, Mdluli K, Fotouhi N, Nuermberger E. Comparative efficacy of the novel diarylquinoline TBAJ-587 and bedaquiline against a resistant Rv0678 mutant in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2021;65(4):e02418–20. doi: 10.1128/AAC.02418-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Svensson EM, Murray S, Karlsson MO, Dooley KE. Rifampicin and rifapentine significantly reduce concentrations of bedaquiline, a new anti-TB drug. J Antimicrob Chemother. 2015;70(4):1106–1114. doi: 10.1093/jac/dku504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Svensson EM, Dooley KE, Karlsson MO. Impact of lopinavir-ritonavir or nevirapine on bedaquiline exposures and potential implications for patients with tuberculosis-HIV coinfection. Antimicrob Agents Chemother. 2014;58(11):6406–6412. doi: 10.1128/AAC.03246-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Almeida D, Converse PJ, Li SY, Upton AM, Fotouhi N, Nuermberger EL. Comparative efficacy of the novel diarylquinoline TBAJ-876 and bedaquiline against a resistant Rv0678 mutant in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2021;65(12):e0141221. doi: 10.1128/AAC.01412-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Evaluate Safety, Tolerability, PK of TBAJ-876 in Healthy Adults [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT04493671?term=NCT04493671&draw=2&rank=1. Accessed 13 Jun 2022.
- 165.Sutherland HS, Tong AST, Choi PJ, Blaser A, Franzblau SG, Cooper CB, et al. Variations in the C-unit of bedaquiline provides analogues with improved biology and pharmacology. Bioorg Med Chem. 2020;28(1):115213. doi: 10.1016/j.bmc.2019.115213. [DOI] [PubMed] [Google Scholar]
- 166.Evaluation of the Safety, Tolerability, PK of TBAJ-587 in Healthy Adults [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT04890535?term=tbaj587&cond=tuberculosis&draw=2&rank=1. Accessed 12 Aug 2022.
- 167.Yao R, Wang B, Fu L, Li L, You K, Li YG, et al. Sudapyridine (WX-081), a novel compound against Mycobacterium tuberculosis. Microbiol Spectr. 2022;10(1):e02477–21. doi: 10.1128/spectrum.02477-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Evaluation of Early Bactericidal Activity and Safety in Pulmonary Tuberculosis With WX-081 (WX-081) [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT04608955?term=NCT04608955&draw=2&rank=1. Accessed 28 May 2022.
- 169.Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, et al. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med. 2013;19:1157–1160. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 170.Lee BS, Pethe K. Telacebec: an investigational antibacterial for the treatment of tuberculosis (TB) Expert Opin Investig Drugs. 2022;31(2):139–144. doi: 10.1080/13543784.2022.2030309. [DOI] [PubMed] [Google Scholar]
- 171.Kim J, Choi J, Kang H, Ahn J, Hutchings J, van Niekerk C, et al. Safety, tolerability, and pharmacokinetics of telacebec (Q203), a new antituberculosis agent, in healthy subjects. Antimicrob Agents Chemother. 2022;66(1):e01436–21. doi: 10.1128/AAC.01436-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.A Phase 2 Study to Evaluate Early Bactericidal Activity, Safety, Tolerability, and Pharmacokinetics of Multiple Oral Doses of Telacebec (Q203) [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT03563599?term=NCT03563599&draw=1&rank=1. Accessed 2 Jun 2022.
- 173.de Jager VR, Dawson R, van Niekerk C, Hutchings J, Kim J, Vanker N, et al. Telacebec (Q203), a new antituberculosis agent. N Engl J Med. 2020;382(13):1280–1281. doi: 10.1056/NEJMc1913327. [DOI] [PubMed] [Google Scholar]
- 174.Almeida DV, Converse PJ, Omansen TF, Tyagi S, Tasneen R, Kim J, et al. Telacebec for ultrashort treatment of buruli ulcer in a mouse model. Antimicrob Agents Chemother. 2020;64(6):e00259–20. doi: 10.1128/AAC.00259-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bvumbi MV. Activity of riminophenazines against Mycobacterium tuberculosis: a review of studies that might be contenders for use as antituberculosis agents. Chem Med Chem. 2020;15(23):2207–2219. doi: 10.1002/cmdc.202000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cholo MC, Mothiba MT, Fourie B, Anderson R. Mechanisms of action and therapeutic efficacies of the lipophilic antimycobacterial agents clofazimine and bedaquiline. J Antimicrob Chemother. 2017;72(2):338–353. doi: 10.1093/jac/dkw426. [DOI] [PubMed] [Google Scholar]
- 177.Zhang Y, Zhu H, Fu L, Wang B, Guo S, Chen X, et al. Identifying regimens containing TBI-166, a new drug candidate against Mycobacterium tuberculosis in vitro and in vivo. Antimicrob Agents Chemother. 2019;63(7):e02496–18. doi: 10.1128/AAC.02496-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lu Y, Wang B, Fu L, Zhu H, Guo S, Huang H, et al. Clofazimine analogs with efficacy against experimental tuberculosis and reduced potential for accumulation. Antimicrob Agents Chemother. 2011;55:5185–5193. doi: 10.1128/AAC.00699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang D, Liu Y, Zhang C, Zhang H, Wang B, Xu J, et al. Synthesis and biological evaluation of novel 2-methoxypyridylamino-substituted riminophenazine derivatives as antituberculosis agents. Molecules. 2014;19(4):4380–4394. doi: 10.3390/molecules19044380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Early Bactericidal Activity Safety Pulmonary Tuberculosis Pyrifazimine (TBI-166) [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT04670120?term=NCT04670120&draw=2&rank=1. Accessed 14 Jun 2022.
- 181.Liu H, Fu L, Wang B, Wang N, Li D, Ding Y, et al. Study on the pharmacodynamic activity of combinations with the new anti-tuberculosis drug pyrifazimine in vitro and in vivo in mouse. Zhonghua Zhong Liu Za Zhi. 2022;45(6):560–566. doi: 10.3760/cma.j.cn112147-20211008-00697. [DOI] [PubMed] [Google Scholar]
- 182.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med. 2014;371(17):1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Merle CS, Fielding K, Sow OB, Gninafon M, Lo MB, Mthiyane T, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med. 2014;371(17):1588–1598. doi: 10.1056/NEJMoa1315817. [DOI] [PubMed] [Google Scholar]
- 184.Talley AK, Thurston A, Moore G, Gupta VK, Satterfield M, Manyak E, et al. First-in-human evaluation of the safety, tolerability, and pharmacokinetics of SPR720, a novel oral bacterial DNA gyrase (GyrB) inhibitor for mycobacterial infections. Antimicrob Agents Chemother. 2021;65(11):e012021. [DOI] [PMC free article] [PubMed]
- 185.SperoTherapeutics. Spero Therapeutics Announces Lifting of FDA Clinical Trial Hold on SPR720 [Internet]. 2022. https://investors.sperotherapeutics.com/news-releases/news-release-details/spero-therapeutics-announces-lifting-fda-clinical-trial-hold. Accessed 22 Jun 2022.
- 186.SperoTherapeutics. SPR720: An Oral Antibiotic Designed to Treat Nontuberculous Mycobacterial Pulmonary Disease, a Rare Orphan Disease [Internet]. 2022. https://sperotherapeutics.com/pipeline/spr720-non-tuberculosis-mycobacterium/. Accessed 22 Jun 2022.
- 187.Locher CP, Jones SM, Hanzelka BL, Perola E, Shoen CM, Cynamon MH, et al. A novel inhibitor of gyrase B is a potent drug candidate for treatment of tuberculosis and nontuberculosis mycobacterial infections. Antimicrob Agents Chemother. 2015;59(3):1455–1465. doi: 10.1128/AAC.04347-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nuermberger EL, Martínez-Martínez MS, Sanz O, Urones B, Esquivias J, Soni H, et al. GSK2556286 is a novel antitubercular drug candidate effective in vivo with the potential to shorten tuberculosis treatment. Antimicrob Agents Chemother. 2022;66(6):e0013222. doi: 10.1128/aac.00132-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.A Study to Evaluate Safety, Tolerability and Pharmacokinetics of GSK2556286 in Healthy Adult Participants [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT04472897?term=NCT04472897&draw=2&rank=1. Accessed 2 Jun 2022.
- 190.Amaral L, Boeree MJ, Gillespie SH, Udwadia ZF, Van SD. Thioridazine cures extensively drug-resistant (XDR-TB) and the need for global trials is now! Int J Antimicrob Agents. 2010;35:524–526. doi: 10.1016/j.ijantimicag.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 191.Vesenbeckh S, Krieger D, Bettermann G, Schönfeld N, Bauer TT, Rüssmann H, et al. Neuroleptic drugs in the treatment of tuberculosis: minimal inhibitory concentrations of different phenothiazines against Mycobacterium tuberculosis. Tuberculosis (Edinb) 2016;98:27–29. doi: 10.1016/j.tube.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 192.Field SK, Fisher D, Jarand JM, Cowie RL. New treatment options for multidrug-resistant tuberculosis. Ther Adv Respir Dis. 2012;6(5):255–268. doi: 10.1177/1753465812452193. [DOI] [PubMed] [Google Scholar]
- 193.Liu Y, Xu X, Zhang Y, Li M, Guo J, Yan C, et al. Thioridazine induces cardiotoxicity via reactive oxygen species-mediated herg channel deficiency and L-type calcium channel activation. Oxid Med Cell Longev. 2020;2020:3690123 [DOI] [PMC free article] [PubMed]
- 194.Martins M, Schelz Z, Martins A, Molnar J, Hajös G, Riedl Z, et al. In vitro and ex vivo activity of thioridazine derivatives against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2007;29(3):338–340. doi: 10.1016/j.ijantimicag.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 195.Kristiansen JE, Dastidar SG, Palchoudhuri S, Roy DS, Das S, Hendricks O, et al. Phenothiazines as a solution for multidrug resistant tuberculosis: from the origin to present. Int Microbiol. 2015;18(1):1–12. doi: 10.2436/20.1501.01.229. [DOI] [PubMed] [Google Scholar]
- 196.Gupta S, Tyagi S, Almeida DV, Maiga MC, Ammerman NC, Bishai WR. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am J Respir Crit Care Med. 2013;188(5):600–607. doi: 10.1164/rccm.201304-0650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaur P, Potluri V, Ahuja VK, Naveenkumar CN, Krishnamurthy RV, Gangadharaiah ST, et al. A multi-targeting pre-clinical candidate against drug-resistant tuberculosis. Tuberculosis. 2021;129(March):102104. doi: 10.1016/j.tube.2021.102104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.