Abstract
Introduction
Despite advances in treatment options and the management of patients with psoriasis, considerable unmet needs remain. Our objective was to identify ways to elevate the standard of care for patients with psoriasis by combining the perspectives of three important stakeholders: patients, clinicians and payors, and define ‘Calls to Action’ designed to achieve the identified changes.
Methods
Eight themes relevant to elevating the standard of care were identified from an insights-gathering questionnaire completed by all three stakeholder groups. A modified Delphi exercise gained consensus on statements informed by the insights. Statements were then used to inspire ‘Calls to Action’ – practical steps that could be taken to realise the desired changes and elevate the standard of care.
Results
In total, 18 European experts (10 dermatologists, 3 payors and 5 patient representatives) took part in the Delphi process. Consensus was reached on statements relating to all eight themes: improve healthcare systems to better support multidisciplinary team working and digital services, real-world data generation and optimal use, improve patient access, elevate quality-of-life measures as the most important outcomes, involve patients in patient-centred and personalised approaches to care, improve the relevance and reach of guidelines, education, and multistakeholder engagement. ‘Calls to Action’ common to all three stakeholder groups recognised the need to capitalise on the shift to digital healthcare, the need for consistent input into registries to generate real-world evidence to support guideline development, and the necessity of educating patients on the benefits of reporting outcomes to generate real-world data. The enormous quality-of-life burden and psychological impact of psoriasis, as well as the clinical needs of patients must be better understood, including by healthcare commissioners, so that funding priorities are assessed appropriately.
Conclusion
This unique initiative identified a practical ‘Call-to-Action Framework’ which, if implemented, could help improve the standard of care for patients with psoriasis.
Video Abstract (MP4 44777 KB)
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00846-3.
Keywords: Consensus, Delphi, Multistakeholder, Psoriasis, Standard of care
Plain Language Summary
Despite improvements in the management of psoriasis, there is room for the standard of care for patients to be improved further. The aim of the ‘Epicensus’ programme is to help realise improvements by bringing together three important stakeholder groups involved in the care of patients with psoriasis: dermatologists, payors and patient representatives. First, unmet needs were explored with these stakeholders and eight themes for change were identified: 1) improve healthcare systems to better support multidisciplinary team working and digital services; 2) optimise real-world data generation and use; 3) improve patient access; 4) elevate quality-of-life measures as the most important outcomes; 5) involve patients in people-centred and personalised approaches to care; 6) improve the relevance and reach of guidelines; 7) education; 8) multistakeholder engagement. Next, a panel of experts representing the three stakeholder groups took part in a consensus process (Delphi) to reach agreement on statements relating to each of the eight themes. The statements describe current problems and what needs to be changed to raise the standard of care for patients with psoriasis. Some of the problems identified are similar to those that existed a decade ago, showing that simply recognising what needs to change is not enough to bring about improvements: action must be taken. Therefore, the Epicensus participants met to produce specific ‘Calls to Action’– practical steps described in this publication that, if put into practice, should contribute to an improvement in the standard of care for patients with psoriasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00846-3.
Key Summary Points
| The treatment, care and management of patients with psoriasis remains suboptimal. |
| The Epicensus programme was designed to establish the current state of care from a uniquely multistakeholder perspective (dermatologists, payors and patient representatives). |
| A consensus exercise identified areas and aspects of care that need improving. |
| The results revealed that despite advances in treatment options and the management of patients with psoriasis, some unmet needs and barriers to improvements in the standard of care identified a decade ago still exist. |
| The aim of Epicensus is to effect positive change, and here we report ‘Calls to Action’ based on the consensus statements that, if implemented, should elevate the standard of care and therefore make progress towards optimal management of patients with psoriasis. |
Digital Features
This article is published with digital features, including a video abstract, infographic and plain language summary, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.23599077.
Introduction
Psoriasis, an immune-mediated, chronic inflammatory disease primarily affecting the skin and causing scaling, pain, itching and burning, is now widely accepted to be systemic in nature with manifestations beyond the skin [1, 2]. Patients with psoriasis can experience serious comorbidities including psoriatic arthritis (PsA), cardiometabolic syndrome, inflammatory bowel disease and psychological disorders [1–5].
Psoriasis is complex, requiring multidisciplinary, personalised care [4, 6–8]. Combined dermatology and rheumatology clinics can successfully improve the diagnosis and management of challenging cases and enable early detection and treatment of PsA [9]; however, this remains a significant practice gap [10, 11]. It has been suggested that the multidisciplinary team (MDT) approach should be the norm rather than the exception, potentially expanding beyond dermatology, rheumatology and gastroenterology [4]. Indeed, a study is currently recruiting to explore whether an interdisciplinary combined clinic (including nurses and psychologists) is more effective than usual siloed care [12, 13]. Furthermore, the coronavirus disease 2019 (COVID-19) pandemic has highlighted inadequacies in infrastructure and inequities in healthcare access [14]. Clinical pathways have had to adapt to continue to provide care for patients [15].
Despite advances in treatment options [16] and management [17], substantial unmet needs remain [6, 18, 19]. The physical and psychological multi-morbidities continue to exert far-reaching, negative consequences on patients and society including stigmatisation, work absenteeism, relationship difficulties and increasing out-of-pocket healthcare costs [2, 20–22]. Patients with psoriasis and physical or psychological comorbidities experience an increased clinical burden, worse quality of life (QoL) and greater economic burden than those without comorbidities [2]. Healthcare costs associated with psoriasis increase over time as the disease progresses [23], and compared with matched controls, patients with psoriasis experience higher healthcare costs and a greater negative impact on income and employment [24]. Disease burden is also affected by plaque location. Visible plaques and plaques in sexually-sensitive areas [2, 25] and plaques on the hands, feet or genitalia increase work absenteeism [21]. Low patient–physician concordance regarding satisfaction with psoriasis management often occurs [26] and is associated with increased disease symptoms and severity and reductions in QoL and work productivity [27].
Barriers to optimal care include the discrepancies between the heterogeneous patient population encountered in clinical practice and the uniform population enrolled in clinical trials [28, 29], and healthcare system differences including waiting times and variation in the availability of and access to advanced therapies including biologics and small molecules.
To alleviate the sustained burden, an elevation in the standard of care (SoC) is warranted but the range of unmet needs suggests that changes to achieve this would be multifaceted. A study by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and KPMG identified and explored areas in which care for patients with PsA could be improved and improvement indicators were defined [30]. Similarly, the aim of the Epicensus programme was to identify ways in which the SoC could be elevated from the perspective of three important stakeholders: patients, clinicians and payors. Here, we report a Delphi exercise performed to gain an up-to-date consensus on key themes related to elevating the SoC for patients with psoriasis from a unique, multistakeholder perspective. Having identified these areas, we also report ‘Calls to Action’ inspired by the consensus statements, which are designed to bring about the identified changes.
Methods
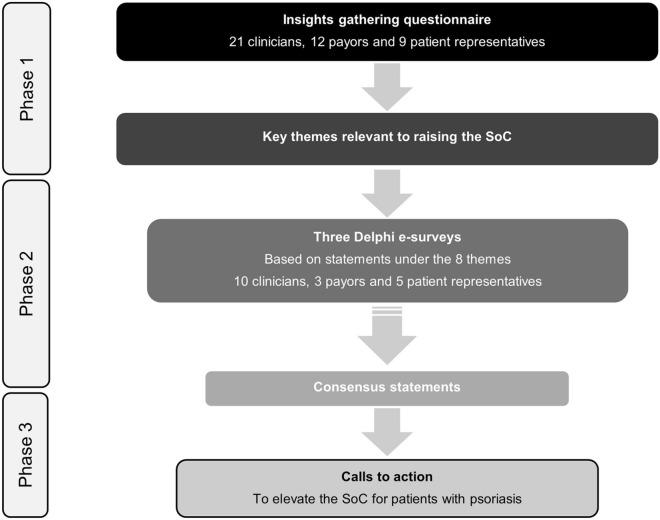
The Epicensus programme is a pan-European, multistakeholder initiative aimed at elevating the SoC for patients with psoriasis. By bringing together clinicians (dermatologists), payors and patient representatives, a broad picture of the current state of care was generated to inform how this could be elevated from the different stakeholder perspectives. The participating stakeholders are shown in the supplementary material. The goal was to generate ‘Calls to Action’ that, if implemented, could improve the overall SoC. The first phase of the programme was a questionnaire (see the electronic supplementary material) designed to gather insights around psoriasis care and changes needed to improve it, which was completed by all stakeholder groups. Responses were collated, analysed qualitatively and eight key themes relevant to elevating the SoC were identified (Table 1). This informed the second phase: a Delphi exercise to achieve consensus agreement on statements arising from the insights. Consensus was defined as ≥ 75% selecting ‘agree’ or ‘strongly agree’. Since the objective was to gain multistakeholder consensus, but the stakeholders were not equal in number, individual votes were weighted so that each stakeholder group had an equal impact on the overall percentage calculated. To progress towards an elevation in the standard of care, the third phase was a multistakeholder meeting in which ‘Calls to Action’, inspired by the consensus statements, were developed to achieve the improvements (Fig. 1). The electronic supplementary material contains further detailed information on the programme including the participants’ backgrounds (Table S1) and the Delphi voting.
Table 1.
Eight themes identified from the insights-gathering questionnaire that were used to inform the subsequent Delphi exercise
| Theme | Description of current situation and possible solutions/needs |
|---|---|
| Improve healthcare systems to better support MDT working and digital services |
• Healthcare systems are not designed to facilitate efficient diagnosis, referral, delivery and monitoring of treatment • Evidence-based changes to existing care pathways are needed to improve early diagnosis, referral, patient-centric and MDT care • Improvements are needed in MDT healthcare delivery at all points in the care pathway, from primary care to specialist clinics (digital and face-to-face), specialist pharmacists and nurses • Adopt digital healthcare to improve service provision |
| Real-world data generation |
• Robust datasets from well-designed studies and real-world data/evidence (including assessments of value over time including durability and the impact in difficult-to-treat patients) support the incorporation of novel medicines into treatment guidelines and their adoption as standards of care • Patients want to be involved in collaborative working to inform data generation, publications, guidelines and policies |
| Improve patient access | • All aspects of access need improvement from earlier, equitable and sustained access to innovative treatments as well as access to the right HCP at the right time to enhance the patient experience |
| Elevate quality of life measures as the most important outcomes |
• Clinicians and patients value outcomes that satisfy patient goals as the most important measures of treatment success • The elevation in the SoC in psoriasis should be a tangible improvement in the holistic burden of psoriasis on patients and carers |
| Involve patients in patient-centred and personalised approaches to care |
• Patients want to be partners at the centre of decision-making by being well informed and having their voices heard • Personalisation of care: an individualised approach to assessment and treatment, taking account of the holistic impact of psoriasis including tailored, clinical and cost-effective treatments to ensure timely intervention and value for patients |
| Improve relevance and reach of guidelines |
• Guidelines to support MDT approaches and integrated clinical and health economic guidelines are lacking • Real-world evidence, clinical and health economic guidelines and budget optimisation will facilitate the adoption of novel treatments • Guidelines must be supported by clinical data (trial and RWD), economic and operational considerations, and monitored to track the value over time |
| Education |
• Awareness of the disease and its comorbidities is lacking • A focus on broader healthcare professional education will drive elevations in standards of care • Patient misconceptions and anxiety can be alleviated by rapid provision of suitable information |
| Multistakeholder engagement |
• Recognise and explore optimal ways to establish multistakeholder initiatives • Engaging payors in partnership initiatives may be possible and strengthened when multistakeholder in nature • Strengthen the role of patients, patient advocacy groups (PAG) and payor input to research and development (R&D) and broader data generation initiatives |
The themes are presented in priority order as voted by the stakeholders
HCP healthcare professional, MDT multidisciplinary team, PAG patient advocacy group, R&D research and development, RWD real-world data, SoC standard of care
Fig. 1.
A flow chart to show the phases of the Epicensus programme. A large pool of stakeholders completed the questionnaire to gain as broad a perspective as possible. The number of stakeholders who completed the Delphi exercise was smaller but included some individuals new to the programme to test agreement with the statements with additional participants. SoC standard of care
Results
Respondents
One of the three payors only took part in the third Delphi e-survey while one clinician was unable to take part in the third Delphi e-survey. Therefore, 17 stakeholders answered each question except where an answer was omitted by one stakeholder and the percentage agreement was calculated out of 16 responses. The weighting used to calculate the consensus agreement was adjusted accordingly.
Consensus Statements and ‘Calls to Action’
Statements, percentage consensus agreement, and ‘Calls to Action’ common to all stakeholder groups are presented in the Tables. Statements used to inspire ‘Calls to Action’ are highlighted alongside the corresponding ‘Calls to Action’. These tables are complemented by and should be read in conjunction with the further information and responses to open-ended questions included in the ‘discussion points’ below for each theme. ‘Calls to Action’ that were common to only two groups are presented in the electronic supplementary material (Table S2). This manuscript is formed of the opinions of the authors themselves. There was no need to collect any type of patient data. Hence, the approval of an Ethics Committee was not required. Consent was obtained from all participants as they were contracted by UCB Pharma to take part in this programme.
Discussion Points
Improve Healthcare Systems to Better Support MDT Working and Digital Services (Table 2)
Table 2.
Consensus statements and ‘Calls to Action’ to improve healthcare systems to better support MDT working and digital services
| Statement | Weighted consensus agreement (%) | ‘Calls to Action’: all stakeholders must… |
|---|---|---|
| The existing psoriasis care pathway is entrenched but it needs to change to facilitate improvements in standards of care | 96.7 | NA |
| Evidence-based changes to existing care pathways are needed to improve the management of patients living with psoriasis | 100 | NA |
| An effective multidisciplinary approach requires a shift in mindset and a shift in infrastructure | 93.3 | NA |
| A focus of any multidisciplinary approach to elevating standards of care in psoriasis should be on the impact on the mental health status of patients | 73.3 | NA |
| Assessing the impact on the mental health status of patients should be one of the aims of any multidisciplinary approach to elevating standards of care for patients living with psoriasis | 96.7 | NA |
| Healthcare infrastructure needs to change to facilitate the establishment of clinics with enough adequately skilled HCPs from all essential specialities to create an efficient care pathway to manage patients with psoriatic disease effectively and in a timely manner | 100 | NA |
| Digital healthcare has a role to play in helping to elevate the SoC for patients living with psoriasis | 93.3 | NA |
| Digital healthcare needs to be carefully integrated into the care pathway | 96.7 | NA |
| Only certain patients living with psoriasis will be suitable for digital healthcare, while others would benefit from face-to-face appointments | 76.7 | NA |
| Digital healthcare encompasses the use of technology in its broadest sense to enhance and improve all aspects of healthcare/management of patients by supporting patients and HCPs through: facilitating a continuous dialogue between patients and their HCPs; supporting self-assessment and monitoring tools for patients to enable consistent recording of data; storing and sharing data and documentation; facilitating communication between HCPs of different specialities; appropriate use of telemedicine and virtual consultations; and facilitating administration for both HCPs and patients, at all stages of the patient journey | 95.8 | • Support adoption of digital care for equitable (and timely) access to MDT care |
| Digital healthcare should be made as accessible as possible to all patients with psoriatic disease and/or their caregivers to maximise the number of patients who can benefit from it | 100 |
Italics indicates a statement which did not reach consensus. Underline indicates ‘Calls to Action’ and the statements (in bold–underline) they were inspired by
HCP healthcare professional, MDT multidisciplinary team, NA not applicable
Consensus Statements and Open-Ended Questions
Consensus was achieved on all statements except one. Initial disagreement around the focus on mental health was resolved by modifying the statement to clarify that it should be one of the aims of an MDT approach. The composition of the MDT is discussed below (see ‘Improve the relevance and reach of guidelines’).
Open-ended questions on the topic of ‘digital healthcare’ revealed different interpretations of the term. It encompasses a broad range of activities as indicated by the penultimate consensus statement in Table 2. Stakeholders felt that teleconsultations and virtual consultations cannot fully replace face-to-face appointments; they must complement each other. While telephone consultations can be convenient, frequently constituting perfectly adequate follow up, virtual ‘Facetime’-style appointments are not always an improvement, proving awkward for physicians and patients alike if more intimate areas are involved. Appropriate patients for digital healthcare were identified as those comfortable with the technology (‘tech-savvy’), whose disease status is appropriate (e.g. stable) as well as those for whom travel is difficult (e.g. living in rural areas). Less suitable patients are usually older, unfamiliar with the technology or with disabilities or conditions (e.g. dementia) that prevent them from using digital media, as well as newly diagnosed patients or those with unstable disease. However, it was felt that these patients are in the minority and that sometimes it is stakeholders, not patients, who fail to embrace the digital shift.
‘Calls to Action’
Stakeholders agreed that supporting adoption of digital care will involve education, training, collaboration, and support among healthcare professionals (HCPs), payors and patients, not only in how to use the technology, but also to understand the benefits it provides to enhance engagement and support an elevation in the SoC.
Real-World Data (RWD) Generation and Optimal Use (Table 3)
Table 3.
Consensus statements and ‘Calls to Action’ for real-world data generation and optimal use
| Statement | Weighted consensus agreement (%) | ‘Calls to Action’: all stakeholders must… |
|---|---|---|
| Real-world data are needed to support the rapid incorporation of novel medicines into clinical guidelines and the refinement of clinical practice | 100 | • Support the development of national registries; ensure that data collection is regular and consistent to support the rapid incorporation of novel medicines into clinical guidelines and the refinement of clinical practice |
| Outcomes that are most important to patients are the most important to measure and collect in routine clinical practice | 90 | |
| RWD is vital to help inform clinicians of the real-world effectiveness and safety (including long-term data) of different treatments in ‘real’ patients, that is, those who are often excluded from clinical trials | 100 |
Underline indicates ‘Calls to Action’ and the statements (in bold–underline) they were inspired by
RWD real-world data
Consensus Statements and Open-Ended Questions
Stakeholders suggested that patients cared most about the effectiveness and sustained efficacy of treatments and QoL followed by the safety and tolerability of treatments and having clear skin. RWD was seen as critical in filling gaps in knowledge left by strict clinical trial inclusion criteria, resulting in a paucity of data in special populations (e.g. elderly, children, pregnant/breast-feeding women, those with cancer or other comorbid diseases).
‘Calls to Action’
Stakeholders advocated the development of national registries. Incentivising clinicians to report RWD consistently or making contribution mandatory, looking for opportunities to engage pharmacists in RWD generation activities, highlighting the importance of real-world evidence (RWE) to all stakeholders and educating patients on the value of RWE so that they proactively provide information were discussed. The need to capture a standard/minimal set of outcomes including patient-reported outcomes (PROs) was noted.
Improve Patient Access (Table 4)
Table 4.
Consensus statements and ‘Calls to Action’ to improve patient access
| Statement | Weighted consensus agreement (%) | ‘Calls to Action’: all stakeholders must… |
|---|---|---|
| Access to innovative treatments is currently suboptimal | 96.7 | NA |
| Access to psychological support services should be a mandatory part of the treatment pathway for all patients living with psoriasis | 76.7 | NA |
| Early intervention, with effective biologic treatments, may provide long-term value for patients and healthcare systems | 100 |
• Raise the profile of psoriasis so commissioners re-evaluate funding and convince them of the long-term cost savings from the balanced use of biologics if adopted early in treatment • Endorse a patient-centred approach to care based on the clinical needs of patients, backed by up-to-date evidence and data |
| Healthcare systems should be designed to deliver equitable access to dedicated specialist centres for all patients living with psoriasis | 100 | |
| The main barriers to equitable access to optimal psoriasis care are country-specific and operate at a national level. Therefore, they need to be addressed at a national level | 100 | |
| In my role, I have a key role in ensuring equity of access to optimal care for all patients living with psoriatic disease | 95.8 |
Underline indicates ‘Calls to Action’ and the statements (in bold–underline) they were inspired by
NA not applicable
Consensus Statements and Open-Ended Questions
Some stakeholders repeated their view that psychological support does not need to be mandatory; however, it should be available if needed. The right number of experienced staff, MDTs, good levels of funding and reimbursement, flexible prescribing, access to all/innovative medicines and involving patients in decisions were all identified as features of healthcare systems that offer equitable patient access.
‘Calls to Action’
Rapid access to well-informed, equitable care would be supported by ensuring that health-service commissioners better understand the clinical needs of patients with psoriasis, so they reassess funding priorities. Generating evidence of the value of biologics and biosimilars including their cost-effectiveness, especially in the long-term and when adopted early, was considered key to achieving this.
Elevate Quality-of-Life Measures as the Most Important Outcomes (Table 5)
Table 5.
Consensus statements and ‘Calls to Action’ to elevate quality of life measures as the most important outcomes
| Statement | Weighted consensus agreement (%) | ‘Calls to Action’: all stakeholders must… |
|---|---|---|
| The negative impact on quality of life and daily functioning is the greatest source of the cumulative burden of disease on patients living with psoriasis | 100 |
• Improve own knowledge of existing QoL tools and how to use them • Work towards developing newer QoL/PRO measurement tools to capture data consistently, including cumulative disease burden |
| QoL measures should be elevated as the most important clinical outcomes for patients with psoriasis assessed in clinical trials and clinical practice | 83.3 | |
| There are validated QoL tools and measurements in existence that should be used consistently to assess the QoL of patients living with psoriasis | 76.6 |
Underline indicates ‘Calls to Action’ and the statements (in bold–underline) they were inspired by
PRO patient-reported outcome, QoL quality of life
Consensus Statements and Open-Ended Questions
Consensus was reached that QoL measures should be elevated as the most important outcomes and that validated measures and tools to assess QoL exist and should be used consistently. Stakeholders identified the three most valuable measures as the Dermatology Life Quality Index followed by the Psoriasis Disability Index and the Psoriasis Index of QoL from a list of 11 suggestions with the option to add others. While most stakeholders (n = 14/17; 82.4%) agreed that the existing tools effectively and specifically capture all relevant aspects of QoL, a few dermatologists noted that existing tools (except the Patient Benefit Index (PBI)) fail to consider the patient perspective or psychological impact or that the visibility of lesions is not adequately or specifically assessed. While stakeholders acknowledged that the concept of Cumulative Life Course Impairment (CLCI) has potential clinical value, an actual measure to capture it is needed.
‘Calls to Action’
Stakeholders remain motivated to improve upon existing QoL tools possibly by incorporating them into a new, universal tool. Identifying and prioritising the most important existing tools, including those that are most relevant to payors, and agreeing on a consistent method of collecting high-quality data are crucial to address the burden psoriasis places on QoL and elevate the SoC.
Involve Patients in Patient-Centred and Personalised Approaches to Care (Table 6)
Table 6.
Consensus statements and ‘Calls to Action’ relating to involving patients in patient-centred and personalised approaches to care
| Statement | Weighted consensus agreement (%) | ‘Calls to Action’: all stakeholders must… |
|---|---|---|
| Patients living with psoriasis, and their families, need to be listened to and heard by ensuring they have the time and space to voice their concerns and opinion | 100 | NA |
| Care for patients living with psoriasis should be built on a holistic approach that puts the patient at the very centre and actively encourages patients to engage in all aspects of their care | 100 |
• Support patient involvement in health authority meetings, legislative processes and regulatory decisions • Capture RWD and patient-reported experiences to identify challenges, needs and issues for patients and carers |
| Patients living with psoriasis should play a valued role in decisions not only at the individual patient level but also at the system level | 100 | |
| Patients are a valuable, under-used source of vital information; for example, their experience of the patient pathway and administrative processes that determine medical care and treatment and how they could be improved, which could help elevate the SoC at the system (infrastructure) level | 100 |
Underline indicates ‘Calls to Action’ and the statements (in bold–underline) they were inspired by
NA not applicable, RWD real-world data, SoC standard of care
Consensus Statements and Open-Ended Questions
All statements relating to patient-centricity achieved 100% consensus, highlighting how critical patient-centricity and patient voice are. However, stakeholders noted that patients must be free to choose if they wish to participate in shared decision-making and their decision respected.
‘Calls to Action’
Stakeholders agreed that patients are uniquely placed to provide valuable information beyond that of their own disease and treatment progress. Their knowledge and experience of healthcare systems could provide information which may be utilised to help elevate the SoC. Establishing standard ways to capture their views is needed.
Improve the Relevance and Reach of Guidelines (Table 7)
Table 7.
Consensus statements and ‘Calls to Action’ relating to improving the relevance and reach of guidelines
| Statement | Weighted consensus agreement (%) | ‘Calls to Action’: all stakeholders must… |
|---|---|---|
| Guidelines that integrate clinical data (both trial and real-world) with health economic data are lacking | 93.3 | • Endorse development of integrated, harmonised, flexible and regularly updated guidelines |
| Standards of care in psoriasis would be elevated with the use of guidelines that consider clinical data, including real-world data, and health economic evidence | 96.7 | |
| Measuring the real-world clinical and health economic value of treatments in psoriasis over time is critical to ensure that standards of care can be elevated | 96.7 | |
| Standards of care would be improved if guidelines better reflected the need for a multidisciplinary/multi-stakeholder approach to the management of psoriasis | 100 | |
| Dermatologists and rheumatologists should share the management of patients with psoriasis | 86.6 | |
| Multidisciplinary guidance should be developed by a multidisciplinary team, including input from patients | 96.7 | |
| The practical gaps between guidelines and their implementation need to be addressed to elevate standards of care in psoriasis | 96.7 | NA |
| In my country, national/regional guidelines are more relevant to clinical practice than international guidelines | 93.3 | NA |
Underline indicates ‘Calls to Action’ and the statements (in bold–underline) they were inspired by
NA not applicable
Consensus Statements and Open-Ended Questions
Consensus was reached that dermatologists and rheumatologists should share the management of patients with psoriasis depending on disease manifestations. Essential MDT members needed to develop multidisciplinary guidance to support an MDT approach were identified as rheumatologists and dermatologists followed by psychologists, general practitioners, gastroenterologists and cardiologists. Some suggested that additional specialists were unnecessary, while others felt diabetologists/endocrinologists, obstetricians and gynaecologists would provide further valuable input to support guidance and thus improve the relevance and utility of guidelines. Barriers to implementing guidelines were identified as the organisation of the healthcare system (e.g. lack of MDT pathways), complexity of guidelines, the speed with which they are updated in a rapidly changing field and regional differences. Overall, 71% of stakeholders believed that changes at the healthcare system level should be prioritised to realise the most rapid elevation(s) in the SoC, while 29% would prioritise changes at the physician level.
‘Calls to Action’
To improve the relevance and reach of guidelines, a harmonised approach to their development is needed, encompassing all aspects of the disease (psychological, comorbidities etc.) and all patient subgroups (e.g. elderly) while incorporating the patient perspective and experiences and with regular reviews and updates to include clinical and health economic RWD (especially patient-relevant data).
Education (Table 8)
Table 8.
Consensus statements and ‘Calls to Action’ relating to education
| Statement | Weighted consensus agreement (%) | ‘Calls to Action’: all stakeholders must… |
|---|---|---|
| Patients should have access to high quality, comprehensive disease information that is available on demand in different formats with the opportunity to ask questions and seek clarification if required | 100 |
• Focus on a broader healthcare professional education adapted to the needs of the healthcare providers • Encourage and pursue multistakeholder collaborations and education opportunities • Support education and collaboration to better understand and manage comorbidities |
| Enhanced education and awareness of psoriasis in general practice is needed to improve appropriate referral to a dermatologist, timely diagnosis and earlier intervention through multidisciplinary, holistic care and effective treatments | 100 | |
| Dermatologists should be aware of the spectrum of comorbidities and their impact on patients living with psoriasis, so that further specialist advice can be sought in a timely manner | 100 | |
| Payors and healthcare professionals should better appreciate the total impact and cumulative disease burden posed by psoriasis over the lifetime of patients living with this disease | 100 |
Underline indicates ‘Calls to Action’ and the statements (in bold–underline) they were inspired by
Consensus Statements and Open-Ended Questions
The importance of education was highlighted by 100% consensus for all statements. Suggested education topics are summarised in Table 9.
Table 9.
A table to show the education topics* that each stakeholder would most benefit from
| Patient | GP | Payor | Dermatologist |
|---|---|---|---|
| Comorbidities | Availability and efficacy of innovative, systemic treatments | Cumulative life impact of psoriasis on patients and associated cost to society of such a chronic disease (absence from work, management of comorbidities) and the positive impact in the long term of early effective treatment initiation | New innovative treatments and their mechanism of action |
| Safety of treatments (current and innovative) | Comorbidities | PROs and the patient’s perspective and experience | |
| Efficacy of treatments (current and innovative) | Early diagnosis of PsA, when referral should be made, and the impact of delays on patients | Optimal use of treatment in subpopulations – women of childbearing age, planned pregnancy, children, different comorbidities (rheumatological conditions (particularly PsA), mental health issues, metabolic and cardiovascular comorbidities) | |
| Lifestyle information | Guidelines and treatment algorithms | ||
| Early recognition of PsA |
*Topics and comorbidities (for dermatologists) shown are those mentioned at least three times by stakeholders in response to open-ended questions and are arranged in descending order of frequency
PROs patient-reported outcomes, PsA psoriatic arthritis
‘Calls to Action’
The ‘Calls to Action’ reflect the consensus that all stakeholders would benefit from more education and indicate a preference for multistakeholder training and educational initiatives.
Multistakeholder Engagement (Table 10)
Table 10.
Consensus statements and ‘Calls to Action’ relating to multistakeholder engagement
| Statement | Weighted consensus agreement (%) | ‘Calls to Action’: all stakeholders must… |
|---|---|---|
| Multistakeholder initiatives are likely to be the most successful type of collaboration with the biopharmaceutical industry | 86.2 | • Encourage multistakeholder engagement as broadly as possible; for example, in planning processes, research, R&D initiatives, clinical trial design, decision-making and RWD generation while ensuring that patients are involved, heard and educated along the way |
| Engagement initiatives between payors, patients/patient groups and healthcare professionals that are focused on elevating standards of care in psoriasis can be supported by the industry, provided there are shared goals, clear governance and a sustained commitment from all parties | 100 | |
| R&D efforts and real-world data generation initiatives would be of more value if input from patients/patient advocacy groups was routinely sought | 100 | |
| Patients and patient advocacy groups are underutilised, their valuable and necessary input into R&D and RWD generation is lacking, and industry could do more to support such activities | 89.2 | |
| Industry should support patient/patient advocacy group involvement in R&D by facilitating their engagement in clinical trials from the beginning (planning and design stage) and beyond | 89.2 | NA |
| Industry should aid patient/patient advocacy group involvement in RWD generation by supporting registries and helping to inform and educate patients on the importance of contributing to (national/independent/transverse) registries | 89.2 | NA |
Underline indicates ‘Calls to Action’ and the statements (in bold–underline) they were inspired by
NA not applicable, R&D research and development, RWD real-world data
Consensus Statements and Open-Ended Questions
Current lack of patient involvement in many industry activities was confirmed. Stakeholders felt industry could best support patient involvement through funding in various forms (unrestricted grants, independent research, reimbursing patients’ time/expertise, sponsoring patient meetings to disseminate research findings, podcasts, publications, symposia for patients as well as training/education). Other suggestions included industry supporting registries and educating patients on the need to report data, supporting patients to help payors understand patient needs, promoting participation of patient research partners (patients who actively participate in research teams alongside and on an equal basis with professional researchers) in activities of groups such as GRAPPA and becoming involved in clinical trial design. However, this should complement but never replace industry support of physicians themselves.
‘Calls to Action’
Patients should be involved at all stages from (research and development) R&D to RWD and RWE generation, so that their knowledge, experience and perspectives can be harnessed to add value. Critical to the success of patient involvement is ensuring that outcomes and data are communicated back to patients appropriately within the local regulatory framework. This will enhance patient engagement and education, desirable factors in narrowing the gap between patients and industry.
Discussion
The ultimate aim of the pan-European Epicensus programme is to elevate the SoC for patients with psoriasis by creating a multistakeholder ‘Call-to-Action Framework’ to stimulate and drive positive change. Local adoption could encourage different stakeholders to collaborate and implement activities that align with the ‘Calls to Action’ to bring about these changes.
Insight gathering identified eight key themes that were explored through a modified Delphi technique. Consensus statements were used to inspire ‘Calls to Action’, developed by each stakeholder group separately but discussed together to find those common to all stakeholder groups. These common ‘Calls to Action’ are summarised in Table 11.
Table 11.
A summary of the ‘Calls to Action’ common to all stakeholders, per theme, to bring about an elevation in the SoC for patients with psoriasis
| Theme | ‘Calls to Action’ |
|---|---|
| Improve healthcare systems to better support MDT working and digital services | • Support adoption of digital care for equitable (and timely) access to MDT care |
| Real-world data generation and optimal use | • Support the development of national registries; ensure that data collection is regular and consistent to support the rapid incorporation of novel medicines into clinical guidelines and the refinement of clinical practice |
| Improve patient access |
• Raise the profile of psoriasis so commissioners re-evaluate funding and convince them of the long-term cost savings from the balanced use of biologics if adopted early in treatment • Endorse a patient-centred approach to care based on the clinical needs of patients, backed by up-to-date evidence and data |
| Elevate QoL measures as the most important outcomes |
• Improve own knowledge of existing QoL tools and how to use them • Work towards developing newer QoL/PRO measurement tools to capture data consistently, including cumulative disease burden |
| Involve patients in patient-centred and personalised approaches to care |
• Support patient involvement in health authority meetings, legislative processes and regulatory decisions • Capture RWD and patient-reported experiences to identify challenges, needs and issues for patients and carers |
| Improve the relevance and reach of guidelines | • Endorse development of integrated, harmonised, flexible and regularly updated guidelines |
| Education |
• Focus on a broader healthcare professional education adapted to the needs of the healthcare providers • Encourage and pursue multistakeholder collaborations and education opportunities • Support education and collaboration to better understand and manage comorbidities |
| Multistakeholder engagement | • Encourage multistakeholder engagement as broadly as possible; for example, in planning processes, research, R&D initiatives, clinical trial design, decision-making and RWD generation while ensuring that patients are involved, heard and educated along the way |
MDT multidisciplinary team, PRO patient-reported outcome, QoL quality of life, R&D research and development, RWD real-world data
During the COVID-19 pandemic, utilisation of digital healthcare increased and widespread adoption of various technologies accelerated [31]. Stakeholders agreed that capitalising on this shift to digital and wider technology will be key to elevating the SoC for patients with psoriasis by providing additional communication channel options as long as: it is made as widely accessible as possible (for clinicians and patients alike), patients understand its value, and it does not increase clinicians’ workload. This must be a collaborative effort between clinicians, payors and patients to maximise success. Enabling access and upskilling all users of the technologies is essential [31] and requires additional funding. Governance of ‘sensitive’ data is a potential hurdle to implementation.
A coordinated effort to set up or support national registries was a clear ‘Call to Action’. The coordinated development of collaborative databases systematically recording clinically relevant outcomes including PROs was considered key to supporting guidelines and clinical practice. In France, the Transparency Commission integrated data from the national real-life registry of biotherapies (PSOBIOTEQ), using it as the basis for its recommendations in January 2022 to move several biologics from third-line treatments into the second line [32]. Educating patients on the value of RWD and evidence so they proactively contribute is important, but adequate data protection must be in place.
In terms of assessing QoL measures, or more broadly, PROs, stakeholders agreed that existing tools could be used, but visibility of lesions is not adequately or specifically assessed. Given the impact of visible lesions on QoL and work absenteeism [2, 21, 25], this is one obvious omission to address with a new, freely accessible tool.
Giving patients the opportunity to be involved in shared decision-making was seen as important, but shared decision-making in psoriasis needs more research to overcome existing barriers and demonstrate potential benefits [33].
Opinion varied on additional specialists needed in the MDT, possibly reflecting differences between countries. In France, for instance, internists are often consulted when patients present with multi-organ diseases. Guidelines that incorporate input from all relevant parties are likely to be the most beneficial. However, as noted here and previously [34], barriers to their implementation, including incomplete knowledge of guidelines, their complexity, and the complexity of psoriasis itself must be overcome. Literature supports an MDT approach to psoriatic disease management. A report from Portugal showed the many different models of combined dermatology–rheumatology clinics in existence, but further evaluation of obstacles and benefits is needed to optimise care [35]. A working group explored barriers to best practice in PsA, concluding that a fully integrated, collaborative, multidisciplinary, patient-focused approach is required for optimal care [36], and in psoriasis, Eissing and colleagues suggest that addressing related barriers simultaneously is required to effect change [34].
All stakeholders require education on comorbidities; clinicians so they are aware of the full range and consult the appropriate specialists as needed, payors so they understand the broader implications of psoriasis on patients, and the healthcare system and patients so they understand the full extent of their condition. This agrees with a study examining whether patients with psoriasis or PsA recognised that they were being monitored for comorbidities associated with their condition. It concluded that patient education needs to be improved owing to discrepancies between patient responses and physician records about the presence and treatment of comorbidities [37].
Stakeholders agreed that multistakeholder initiatives are beneficial; performing activities in siloes only perpetuates existing knowledge gaps and barriers to collaborative working. Central to this is educating and motivating patients to participate in the widest range of activities, from R&D to clinical trials and beyond, with outcomes and data communicated back to patients. However, while patient advocacy groups (PAGs) have a role in clinical trial design, etc., careful planning is required, as their capacity is limited, and therefore, they are unable to be involved in all initiatives.
The ‘Calls to Action’ span a broad range of areas pertinent to psoriasis care and vary widely in their ease and speed of potential implementation. ‘Calls to Action’ relating to education could be quick to implement. Some aspects of digital healthcare and other technologies are already being utilised and these could be built upon and refined in line with the ‘Calls to Action’. Others, however, including the development of guidelines, may be more complex and time consuming to implement. Initial groundwork may be necessary, for example, to agree upon the specialist input needed as well as consultation with payors to agree upon the data needed to support early adoption of innovative therapies.
Several ‘Calls to Action’ are interconnected. The need for consistent recording of PROs links several themes. It is integral to improving digital healthcare, RWD and RWE generation and input into registries. Digitally upskilled patients consistently collecting PRO data may also contribute to the relevance of guidelines by providing data via registries or other RWE studies. Educational tools and reminders texted to patients improved clinical outcomes, adherence, and the patient–physician relationship in a pilot study [38], suggesting that improving education and digital healthcare could have positive effects. Another study compared a structured, multidisciplinary, patient education programme with usual psoriasis care and found that while QoL and disease severity did not differ between the groups, compared with usual care, the educational intervention improved patient knowledge of psoriasis and patient satisfaction with disease management which, nonetheless, are valuable outcomes [39]. This may lead to patients becoming more knowledgeable, engaged, and motivated to contribute to discussions aimed at improving MDT working and the patient pathway, thereby impacting other ‘Calls to Action’. Improved education and healthcare systems could lead to an improvement in patient access and enable other areas to then be tackled, such as multistakeholder engagement and the relevance and reach of guidelines.
The Epicensus programme has several strengths, notably the inclusion of three key stakeholder groups involved in the care and management of psoriasis: clinicians (dermatologists), payors and patient representatives, and over ten countries across Europe are represented, bringing a truly pan-European perspective. There was input from a large cohort of stakeholders and the opportunity for discussion both within and across stakeholder groups allowed for a unique depth of alignment on the ‘Calls to Action’. Taking place throughout 2021, this initiative has benefited from lessons learned during the COVID-19 pandemic in terms of innovative ways to optimise clinical practice, patient support and care. Limitations include the relatively large number of clinicians, represented solely by dermatologists, compared with the payors and patient representatives (mitigated by establishing a weighted consensus and having a lead from each stakeholder group to ensure equal representation in discussions). The low number of payors also meant that the diversity of European healthcare systems was not comprehensively covered, but extending discussion of the ‘Calls to Action’ to more members of the under-represented stakeholder groups would help to allay this limitation. Not all the consensus statements were discussed at the Consensus Council meetings. For example, appropriate integration of psychological screening or support so it is readily accessible to patients was agreed upon but ‘Calls to Action’ were not generated. A more complete ‘Call-to-Action Framework’ could be established if the remaining statements were used to generate ‘Calls to Action’. Having an industry sponsor can be seen as a limitation, but the questionnaires and Delphi e-surveys were completed by stakeholders independently and the sponsor did not take an active part in the Consensus Council meetings.
Themes highlighted by this initiative align well with other studies. Interviews conducted with dermatology outpatients with psoriasis revealed how the burden of psoriasis beyond the skin is not adequately addressed in consultations, including the psychosocial impact of the disease. The study concluded that patient education to improve knowledge and self-management is necessary and structural changes to dermatology services are needed [40]. Overlap with issues in psoriasis care identified a decade ago include the reach of guidelines, consistent use of assessment tools and improving access to new therapies and ongoing care [41], highlighting the enduring need for action to adequately address long-standing challenges. Results of a German national programme in psoriasis between 2004 and 2017 demonstrated that a coordinated programme targeting specific goals can successfully contribute to better quality of care [42, 43]. A similar systematic approach to determine improvements after implementation of initiatives designed to address the ‘Calls to Action’ presented here would be valuable.
Conclusions
The fact that this programme highlights that areas identified for intervention a decade ago remain unaddressed is a timely wake-up call – simply recognising an area of unmet need is not sufficient to effect change. Practical steps must now be taken to implement change that translates into improvements in the management of patients with psoriasis that contribute to an elevation in the standard of care. Therefore, the hope is that the Epicensus ‘Call-to-Action Framework’ provides the focus and impetus for all stakeholders involved in the care of people with psoriasis to engage in coordinated activities to address the ‘Calls to Action’ and elevate the standard of care. This next stage of the programme will take us nearer our vision of a truly integrated, collaborative, patient-centric approach to psoriasis management where patients are diagnosed early, managed by the right specialists incorporating digital healthcare as appropriate, supported by relevant education, and treated promptly with innovative therapies. In addition, patient-relevant data will be consistently collected using robust, specific tools to generate RWE to inform guidelines that are widely disseminated and followed, elevating the standard of care for patients with psoriasis. This in turn could help alleviate the long-term costs and cumulative life-long burden that this chronic, incurable disease places on healthcare systems and patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Anusha Patel for her participation in the Delphi voting, contributions at the Consensus Council meetings and for critical review of the manuscript. They would also like to thank Marcello Pani and David Trigos for their participation in the Delphi voting and contributions at the Consensus Council meetings. In addition, the authors would like to thank the clinicians, payors and patient representatives who completed the questionnaire providing valuable insights to initiate this programme. RBW is supported by the Manchester NIHR Biomedical Research Centre.
Funding
This programme, and the journal’s Rapid Service fee, was funded by UCB Pharma.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance were provided by Laura Harrington PhD, Ogilvy Health UK and funded by UCB Pharma.
Author Contributions
All the authors contributed to the Delphi exercise and participated in the Consensus Council meetings and the generation of Calls to Action. The first draft of the manuscript was written by Laura Harrington, PhD, under direction from the authors, and all authors critically reviewed the manuscript for important intellectual content and approved the final draft.
Disclosures
All authors received an honorarium from UCB for participating in the Delphi surveys and the Consensus Council meetings. Jan Koren, Valeria Corazza, Menno A de Rie, and Marius Grosser have no other competing interests to declare. Jo Lambert has received grants/contracts from AbbVie, Almirall, Celgene, Eli Lilly, Janssen-Cilag, LEO Pharma, Novartis, UCB; consulting fees from AbbVie, BMS, Celgene, Eli Lilly, Janssen-Cilag, LEO Pharma, Novartis and UCB; and payment/honoraria for activities such as lectures/presentations/speaker bureaus from AbbVie, Almirall, BMS, Janssen-Cilag, Pifzer and UCB. Simon F Thomsen has received research support from Janssen, LEO Pharma, Novartis and UCB; and has served on advisory boards for Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, UCB, Union Pharmaceuticals; and as a speaker for LEO Pharma, Novartis and UCB and has been the recipient of a travel grant to GUF and EADV from Novartis. Helen McAteer has received core funding support, paid to her employer, from Abbvie, Almirall, Amgen, Eli Lilly, Janssen, LEO Pharma, Novartis and UCB; and honoraria, paid to her employer, from Amgen and Novartis. Gabriella Fabbrocini has been principal investigator of clinical trials sponsored by and/or has received personal fees from AbbVie, abiogeny, Almirall, Celgene, Eli Lilly, LEO Pharma, Novartis, Sanofi and UCB. Denis Jullien reports personal fees from UCB, during the conduct of the study; personal fees and non-financial support from Abbvie, personal fees and non-financial support from Janssen-Cilag, personal fees and non-financial support from UCB, personal fees and non-financial support from Novartis, personal fees from Almirall, personal fees and non-financial support from Lilly, personal fees from MEDAC, personal fees from Celgene, personal fees from Bristol Myers Squibb, personal fees from Boehringer Ingelheim, outside the submitted work. Matthias Augustin has received consulting fees from AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GSK, Hexal, Janssen, LEO Pharma, Medac, Merck, MSD, Mundipharma, Novartis, Pfizer, Sandoz, UCB Pharma and Xenoport. Richard B Warren has received grants/contracts from AbbVie, Almirall, Janssen, Lilly, LEO, Novartis, Pfizer and UCB; consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Biogen, Boehringer Ingelheim, BMS, Celgene, DiCE, GSK, Janssen, Lilly, LEO, Novartis, Pfizer, Sanofi, Sun Pharma, UNION and UCB; payment/honoraria for activities such as lectures/presentations/speaker bureaus from AbbVie, Almirall, Boehringer Ingelheim, BMS, Celgene, Janssen, Lilly, LEO, Novartis, Pfizer, Sanofi, Sun Pharma and UCB. Elizabeth Lazaridou has received research grants to her department from AbbVie, Genesis Pharma, Janssen, LEO, Lilly, Novartis, Pfizer and UCB; honoraria for activities such as lectures/presentations/speaker bureaus from AbbVie, Genesis Pharma, Janssen, LEO, Lilly, Novartis and UCB; support for attending meetings and/or travel from AbbVie, Janssen, LEO, Lilly and UCB; and has participated on a Data Safety Monitoring Board or Advisory Board for AbbVie, Genesis Pharma, Janssen, LEO, Lilly, Novartis, Pfizer and UCB. Lluís Puig has received grants/support paid to his institution from AbbVie, Almirall, Amgen, Boehringer Ingelheim, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi and UCB; consulting fees from Abbvie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Leo-Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi, and UCB; and payment/honoraria for activities such as lectures/presentations/speaker bureaus for Janssen, Lilly and Novartis. Loïc Guillevin has served as a consultant for Boehringer Ingelheim, Certara, ESAI, GSK, Lilly, Novartis, Novo Nordisk, Roche, Sanofi, UCB Pharma. Wolf-Henning Boehncke has received payment/honoraria for activities such as lectures, presentations, speaker bureaus and has participated on Data Safety Monitoring Boards/Advisory Boards for AbbVie, Almirall, BMS, Celgene, Janssen, LEO, Lilly, Novartis, Pfizer and UCB.
Compliance with Ethics Guidelines
This manuscript is formed of the opinions of the authors themselves. There was no need to collect any type of patient data. Hence, the approval of an Ethics Committee was not required. Consent was obtained from all participants as they were contracted by UCB Pharma to take part in this programme.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1.Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol. 2018;9(579):20180405. doi: 10.3389/fimmu.2018.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths CEM, Jo SJ, Naldi L, et al. A multidimensional assessment of the burden of psoriasis: results from a multinational dermatologist and patient survey. Br J Dermatol. 2018;179:173–181. doi: 10.1111/bjd.16332. [DOI] [PubMed] [Google Scholar]
- 3.Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: national health and nutrition examination survey 2009–2012. JAMA Dermatol. 2016;152:73–79. doi: 10.1001/jamadermatol.2015.3605. [DOI] [PubMed] [Google Scholar]
- 4.Queiro R, Coto P. Multidisciplinary care for psoriatic disease: where we are and where we need to go. Rheumatology (Oxford) 2017;56:1829–1831. doi: 10.1093/rheumatology/kew485. [DOI] [PubMed] [Google Scholar]
- 5.Tillett W, Merola JF, Thaci D, et al. Disease characteristics and the burden of joint and skin involvement amongst people with psoriatic arthritis: a population survey. Rheumatol Ther. 2020;7(617–637):20200722. doi: 10.1007/s40744-020-00221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301–1315. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- 7.Grine L, de la Brassinne M, Ghislain PD, et al. A Belgian consensus on the definition of a treat-to-target outcome set in psoriasis management. J Eur Acad Dermatol Venereol. 2020;34(676–684):20200102. doi: 10.1111/jdv.16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maul JT, Navarini AA, Sommer R, et al. Gender and age significantly determine patient needs and treatment goals in psoriasis—a lesson for practice. J Eur Acad Dermatol Venereol. 2019;33(700–708):20190115. doi: 10.1111/jdv.15324. [DOI] [PubMed] [Google Scholar]
- 9.Luelmo J, Gratacos J, Moreno Martinez-Losa M, et al. Multidisciplinary psoriasis and psoriatic arthritis unit: report of 4 years' experience. Actas Dermosifiliogr. 2014;105(371–377):20131218. doi: 10.1016/j.ad.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Boehncke WH, Horvath R, Dalkilic E, et al. Association between clinical specialty setting and disease management in patients with psoriatic arthritis: results from LOOP, a cross-sectional, multi-country, observational study. J Eur Acad Dermatol Venereol. 2020;34(2035–2043):20200306. doi: 10.1111/jdv.16251. [DOI] [PubMed] [Google Scholar]
- 11.Baum EW, Schwartzman S. ALIGN PsA: advancing a multidisciplinary approach in PsA. Semin Cutan Med Surg. 2018;37:S125–S134. doi: 10.12788/j.sder.2018.057. [DOI] [PubMed] [Google Scholar]
- 12.Hjuler KF, Dige A, Agnholt J, et al. Effectiveness of interdisciplinary combined dermatology-gastroenterology-rheumatology clinical care compared to usual care in patients with immune-mediated inflammatory diseases: a parallel group, non-blinded, pragmatic randomised trial. BMJ Open. 2021;11(e041871):20210428. doi: 10.1136/bmjopen-2020-041871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clincialtrials.gov. Effectiveness of interdisciplinary care compared to usual care in patients with immune-mediated inflammatory diseases (NCAS-1). Available at: https://clinicaltrials.gov/ct2/show/NCT04200690 (last accessed 01 Mar 2022).
- 14.Brahmbhatt DH, Ross HJ, Moayedi Y. Digital technology application for improved responses to health care challenges: lessons learned from COVID-19. Can J Cardiol. 2022;38:279–291. doi: 10.1016/j.cjca.2021.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tysome JR. Improving clinical practice in ENT: lessons learnt from the COVID-19 pandemic. Clin Otolaryngol. 2021;46:295–296. doi: 10.1111/coa.13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sbidian E, Chaimani A, Afach S, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2020;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073–1113. doi: 10.1016/j.jaad.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Global Report on Psoriasis. 2016. Available at: https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf?sequence=1&isAllowed=y (last accessed 14 Mar 2022).
- 19.World Health Assembly. Resolution WHA67.9. 2014. Available at: https://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R9-en.pdf (last accessed: 14 Mar 2022).
- 20.Armstrong A, Jarvis S, Boehncke WH, et al. Patient perceptions of clear/almost clear skin in moderate-to-severe plaque psoriasis: results of the Clear About Psoriasis worldwide survey. J Eur Acad Dermatol Venereol. 2018;32(2200–2207):20180731. doi: 10.1111/jdv.15065. [DOI] [PubMed] [Google Scholar]
- 21.Jullien D, Paul C, Shourick J, et al. Psoriasis: frequency and reasons for absenteeism results from a study on 1609 active patients. J Eur Acad Dermatol Venereol. 2021;35:e301–e303. doi: 10.1111/jdv.17056. [DOI] [PubMed] [Google Scholar]
- 22.Richard MA, Paul C, De Pouvourville G, et al. Out-of-pocket expenditures in France to manage psoriasis in adult patients: results from an observational, cross-sectional, non-comparative, multicentre study. J Eur Acad Dermatol Venereol. 2021;35:912–918. doi: 10.1111/jdv.17000. [DOI] [PubMed] [Google Scholar]
- 23.Al Sawah S, Foster SA, Goldblum OM, et al. Healthcare costs in psoriasis and psoriasis sub-groups over time following psoriasis diagnosis. J Med Econ. 2017;20:982–990. doi: 10.1080/13696998.2017.1345749. [DOI] [PubMed] [Google Scholar]
- 24.Thomsen SF, Skov L, Dodge R, et al. Socioeconomic costs and health inequalities from psoriasis: a cohort study. Dermatology. 2019;235:372–379. doi: 10.1159/000499924. [DOI] [PubMed] [Google Scholar]
- 25.da Silva N, Augustin M, Langenbruch A, et al. Disease burden and treatment needs of patients with psoriasis in sexually-sensitive and visible body areas: results from a large-scale survey in routine care. Eur J Dermatol. 2020;30:267–278. doi: 10.1684/ejd.2020.3768. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths CEM, Augustin M, Naldi L, et al. Patient-dermatologist agreement in psoriasis severity, symptoms and satisfaction: results from a real-world multinational survey. J Eur Acad Dermatol Venereol. 2018;32:1523–1529. doi: 10.1111/jdv.14937. [DOI] [PubMed] [Google Scholar]
- 27.Korman NJ, Zhao Y, Pike J, et al. Satisfaction with current psoriasis treatment: misalignment between physician and patient perceptions. Dermatol Online J. 2016;22:20160715. [PubMed] [Google Scholar]
- 28.Cinelli E, Fabbrocini G, Megna M. Real-world experience versus clinical trials: pros and cons in psoriasis therapy evaluation. Int J Dermatol. 2022;61:e107–e108. doi: 10.1111/ijd.15644. [DOI] [PubMed] [Google Scholar]
- 29.Masson Regnault M, Castaneda-Sanabria J, Diep Tran MHT, et al. Users of biologics in clinical practice: would they be eligible for phase III clinical studies? Cohort study in the French Psoriasis Registry PSOBIOTEQ. J Eur Acad Dermatol Venereol. 2020;34:293–300. doi: 10.1111/jdv.15878. [DOI] [PubMed] [Google Scholar]
- 30.Helliwell PS, Favier G, Gladman DD, et al. Best-practice indicators in psoriatic disease care. J Rheumatol Suppl. 2019;95:38–45. doi: 10.3899/jrheum.190120. [DOI] [PubMed] [Google Scholar]
- 31.Golinelli D, Boetto E, Carullo G, et al. Adoption of digital technologies in health care during the COVID-19 pandemic: systematic review of early scientific literature. J Med Internet Res. 2020;22:e22280. doi: 10.2196/22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Transparency Commission. Journal officiel électronique authentifié n° 0020 du 25/01/2022. January 2022. Available at: https://www.legifrance.gouv.fr/download/pdf?id=u5ptDq3Zoqs1KEtFiRsvFXbDxD8eaE0g2h-finnZFg0= (last accessed 10 Feb 2022).
- 33.Larsen MH, Hagen KB, Krogstad AL, et al. Shared decision-making in psoriasis: a systematic review of quantitative and qualitative studies. Am J Clin Dermatol. 2019;20:13–29. doi: 10.1007/s40257-018-0390-5. [DOI] [PubMed] [Google Scholar]
- 34.Eissing L, Radtke MA, Zander N, et al. Barriers to guideline-compliant psoriasis care: analyses and concepts. J Eur Acad Dermatol Venereol. 2016;30:569–575. doi: 10.1111/jdv.13452. [DOI] [PubMed] [Google Scholar]
- 35.Mendes-Bastos P, Nero P, Ferreira P, et al. A multidisciplinary approach in psoriatic disease: the different models of dermatology-rheumatology collaborations in Portugal. Acta Reumatol Port. 2021;46:333–341. [PubMed] [Google Scholar]
- 36.Betteridge N, Boehncke WH, Bundy C, et al. Promoting patient-centred care in psoriatic arthritis: a multidisciplinary European perspective on improving the patient experience. J Eur Acad Dermatol Venereol. 2016;30:576–585. doi: 10.1111/jdv.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel P, Rosen CF, Chandran V, et al. Addressing comorbidities in psoriatic disease. Rheumatol Int. 2018;38:219–227. doi: 10.1007/s00296-017-3895-y. [DOI] [PubMed] [Google Scholar]
- 38.Balato N, Megna M, Di Costanzo L, et al. Educational and motivational support service: a pilot study for mobile-phone-based interventions in patients with psoriasis. Br J Dermatol. 2013;168:201–205. doi: 10.1111/j.1365-2133.2012.11205.x. [DOI] [PubMed] [Google Scholar]
- 39.Jendoubi F, Balica S, Richard MA, et al. A multicentre randomised controlled study evaluating the effect of a standardised education programme on quality of life, disease severity, and disease knowledge in patients with moderate-to-severe psoriasis: the EDUPSO study. Dermatology. 2021 doi: 10.1159/000520289. [DOI] [PubMed] [Google Scholar]
- 40.Khoury LR, Skov L, Moller T. Facing the dilemma of patient-centred psoriasis care: a qualitative study identifying patient needs in dermatological outpatient clinics. Br J Dermatol. 2017;177:436–444. doi: 10.1111/bjd.15292. [DOI] [PubMed] [Google Scholar]
- 41.Augustin M, Alvaro-Gracia JM, Bagot M, et al. A framework for improving the quality of care for people with psoriasis. J Eur Acad Dermatol Venereol. 2012;26(Suppl 4):1–16. doi: 10.1111/j.1468-3083.2012.04576.x. [DOI] [PubMed] [Google Scholar]
- 42.Augustin M, Eissing L, Langenbruch A, et al. The German national program on psoriasis health care 2005–2015: results and experiences. Arch Dermatol Res. 2016;308:389–400. doi: 10.1007/s00403-016-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langenbruch A, Mohr N, Kirsten N, et al. Quality of psoriasis care in Germany—results from the nationwide health care studies PsoHealth 2004–2017. J Eur Acad Dermatol Venereol. 2021;35:1536–1542. doi: 10.1111/jdv.17220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.