Abstract
Gut microbiota and fecal bile acids were analyzed in 278 patients with α-synucleinopathies, which were comprised of 28 patients with dementia with Lewy bodies (DLB), 224 patients with Parkinson’s disease (PD), and 26 patients with idiopathic rapid eye movement sleep behavior disorder (iRBD). Similarly to PD, short-chain fatty acids-producing genera were decreased in DLB. Additionally, Ruminococcus torques and Collinsella were increased in DLB, which were not changed in PD. Random forest models to differentiate DLB and PD showed that high Ruminococcus torques and high Collinsella, which presumably increase intestinal permeability, as well as low Bifidobacterium, which are also observed in Alzheimer’s disease, were predictive of DLB. As Ruminococcus torques and Collinsella are also major secondary bile acids-producing bacteria, we quantified fecal bile acids and found that the production of ursodeoxycholic acid (UDCA) was high in DLB. Increased UDCA in DLB may mitigate neuroinflammation at the substantia nigra, whereas neuroinflammation may not be critical at the neocortex. Theraeutic intervention to increase Bifidobacteirum and its metabolites may retard the development and progression of DLB.
Subject terms: Parkinson's disease, Epidemiology, Dementia
Introduction
α-Synucleinopathies are a group of neurodegenerative disorders characterized by abnormal aggregation of α-synuclein fibrils (Lewy bodies) in the brain, and is comprised of iRBD, PD, and DLB1. Multiple system atrophy (MSA) is attributed to another species of abnormal aggregation of α-synuclein fibrils2, and will not be addressed in this communication. More than 90% of iRBD patients develop other forms of α-synucleinopathies in ten or more years3. PD patients develop motor symptoms without dementia at first. Some PD patients later develop dementia, which is called PD dementia (PDD)4. In contrast, DLB patients develop dementia before or less than one year after the onset of motor symptoms5. DLB is a type of dementia characterized by visual hallucinations, fluctuating cognitive impairment, sleep disturbance, movement disorders (parkinsonism), and autonomic dysfunctions5,6. DLB accounts for about twenty percent of dementia and is the second most common dementia after Alzheimer’s disease5,6. The signs, symptoms, and cognitive profiles of PDD are similar to those of DLB7, and there is no essential difference in the pathology of autopsied cases, but unidentified factor(s) should differentiate DLB and PDD. Gut microbiota could be one of the differentiating factors. In α-synucleinopathies, Lewy bodies are observed in the lower brainstem, the cerebral cortex8, the olfactory bulb9, the salivary glands10, the skin11, the autonomic nervous system12, and the intestine10,13,14. In 2003, Braak proposed a hypothesis that abnormal α-synuclein fibrils start from the nucleus tractus solitarius of the vagal nerve and gradually ascend to the substantia nigra9,15–17. PD patients sometimes develop constipation, iRBD, and depression about 20, 10, and 5 years before the onset of motor symptoms18, which is in accordance with Braak’s hypothesis.
There are more than 20 studies on gut microbiota in patients with PD and iRBD reported by us19–22 and others23–40, but gut microbiota in DLB has not been reported to the best of our knowledge. We previously showed by meta-analysis of gut microbiota in different countries that mucin-degrading genus Akkermansia was increased in PD and iRBD, while short chain fatty acids (SCFA)-producing genera Faecalibacterium and Roseburia were decreased in PD but not in iRBD20,21. In this study, we analyzed gut microbiota in DLB, which was compared with controls, iRBD, and PD with or without cognitive decline.
Results
Analysis of each taxon between controls and DLB, and controls and PD
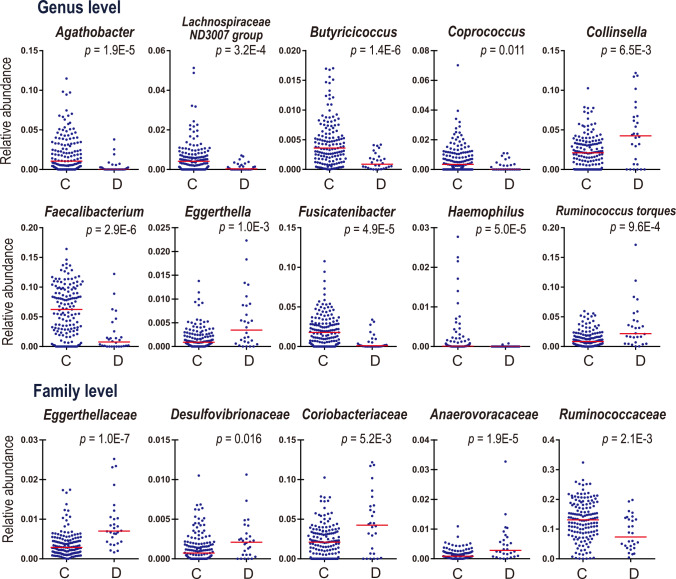
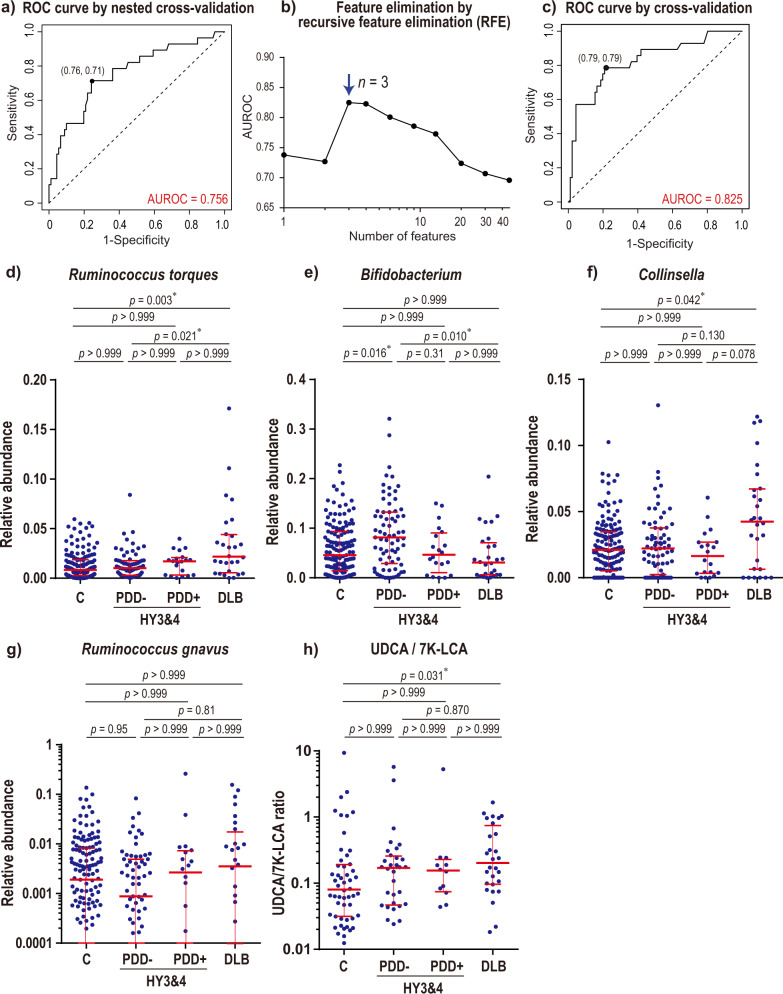
We obtained fecal samples in 224 PD patients, 26 iRBD patients, 28 DLB patients, and 147 controls. The numbers of PD patients at Hoehn & Yahr stages 1 to 5 with or without dementia are indicated in Supplementary Table 1. The collation of the demographic and clinical features between (i) controls and DLB, (ii) controls and PD, and (iii) controls and iRBD is indicated in Table 1. Five to six features out of the seven collated features were statistically different in either DLB, PD, or iRBD compared to controls. Next, we examined taxonomic differences between controls and DLB using Analysis of Compositions of Microbiomes with Bias Correction (ANCOM-BC), which examines taxonomic differences in two groups41, and Wilcoxon rank sum test (Supplementary Table 2a at the genus level and 2b at the family level). In ANCOM-BC, five confounding factors (age, sex, BMI, constipation, and PPI) were included in the analysis. In DLB, at the genus level, three genera were increased (Collinsella, Eggerthella, and Ruminococcus torques) and seven genera were decreased (Agathobacter, Lachnospiraceae ND3007 group, Butyricicoccus, Coprococcus, Faecalibacterium, Fusicatenibacter, and Haemophilus) after adjusting for the confounding factors (Fig. 1 and Supplementary Table 2a). In DLB, at the family level, four families were increased (Eggerthellaceae, Desulfovibrionaceae, Coriobacteriaceae, and Anaerovoracaceae) and one family was decreased (Ruminococcaceae) after adjusting for the confounding factors (Fig. 1 and Supplementary Table 2b). Nested cross-validation of random forest models to differentiate controls and DLB gave rise to the area under the receiver operating characteristic curve (AUROC) of 0.816 (95% confidence interval: 0.714–0.917) (Supplementary Fig. 1), indicating that gut bacteria were able to differentiate controls and DLB efficiently. Fifteen genera made the maximum AUROC by leave-one-out cross-validation in recursive feature elimination (Supplementary Fig. 1 and Supplementary Table 4). The three genera (Collinsella, Eggerthella, and Ruminococcus torques) that were significantly increased in ANCOM-BC and Wilcoxon rank sum test were also essential determinants in random forest models.
Table 1.
Clinical and demographic features of controls, DLB, PD, and iRBD patients.
| Control (n = 147)a | DLB (n = 28)a | PD (n = 224)a | iRBD (n = 26)a | ||||
|---|---|---|---|---|---|---|---|
| P-valueb | P-valueb | P-valueb | |||||
| Age (years) | 68.3 ± 9.9 | 77.5 ± 5.9 | 5.0E-6* | 68.2 ± 8.6 | 0.93 | 74.5 ± 6.4 | 2.3E-3* |
| # Females | 69 | 14 | 0.84 | 130 | 0.043* | 6 | 0.031* |
| Body mass index (BMI) | 22.9 ± 3.0 | 20.9 ± 3.5 | 2.9E-3* | 21.7 ± 3.0 | 1.9E-4* | 24.4 ± 2.4 | 0.016* |
| # Constipation (≤ twice a week) | 6 | 12 | 3.6E-7* | 80 | 3.3E-14* | 9 | 2.8E-5* |
| Stool frequency/week | 7.9 ± 4.3 | 4.8 ± 4.6 | 7.7E-4* | 4.7 ± 4.1 | 6.8E-12* | 5.8 ± 5.7 | 0.034* |
| Disease duration (years) | – | 2.1 ± 2.2 | – | 7.5 ± 6.1 | – | 6.4 ± 4.8 | – |
| # Proton pump inhibitor | 12 | 6 | 0.045* | 35 | 0.038* | 7 | 0.011* |
| # H2 blocker | 6 | 3 | 0.16 | 8 | 0.79 | 1 | 1.00 |
| MDS-UPDRS | – | 59.2 ± 36.2 | – | 50.1 ± 23.1 | – | 7.6 ± 5.5 | – |
| MDS-UPDRS III | – | 31.0 ± 21.0 | – | 26.4 ± 13.5 | – | 2.0 ± 2.6 | – |
aMean and SD are indicated when applicable. bEither Student’s t-test or Fisher’s exact test is applied to be compared to controls. *P < 0.05.
Fig. 1. Plots of ten genera and five families that were significantly changed between controls (C) and DLB (D).
Medians are indicted by red bars. P-values are calculated by Wilcoxon rank sum test. Q-values by the Benjamini-Hochberg method are indicated in Supplementary Table 2a at the genus level and 2b at the family level.
We previously analyzed almost identical fecal samples in controls and PD using ANCOM42 and Wilcoxon rank sum test20. We then analyzed confounding factors in 18 genera and 5 families with generalized linear modeling (GLM)20. Here, we compared controls and PD using ANCOM-BC by simultaneously adjusting for the five confounding factors. We previously showed that eight genera (Christensenellaceae R-7 group, Ruminococcaceae_anonymous, UBA1819, Oscillibacter, Family XIII_anonymous, Alistipes, Akkermansia, and Family XIII AD3011 group) were increased in PD (see Supporting Information Fig. S2 in our previous report20), whereas only two genera, Akkermansia and Oscillibacter, which were a subset of the eight previous genera, were increased in our current analysis (Supplementary Table 3a). Similarly, we previously showed that seven genera (Fusicatenibacter, Butyricicoccus, Lachnospiraceae ND3007 group, Faecalibacteriumb, Roseburia, Blautia, and Ruminococcaceae UCG-013) were decreased in PD (see Supporting Information Fig. S2 in our previous report20), while four previous genera (Butyricicoccus, Blautia, Fusicatenibacter, and Lachnospiraceae ND3007 group) and three new genera (Coprococcus, Monoglobus, and Agathobacter) were decreased in our current analysis (Supplementary Table 3a). We previously concluded by additionally performing meta-analysis of gut microbiota in PD in five countries that PD patients had increased Akkermansia and decreased SCFA-producing genera20. The changes in these genera were indeed shared between our previous and current analyses. Nested cross-validation of random forest models to differentiate controls and PD, which were not generated in our previous report20, yielded the AUROC of 0.762 (0.714–0.810) (Supplementary Fig. 2). Twenty-five genera made the maximum AUROC by leave-one-out cross validation in recursive feature elimination (Supplementary Fig. 2 and Supplementary Table 5).
When DLB and PD were compared, five out of the seven decreased genera in DLB (Agathobacter, Lachnospiraceae ND3007 group, Butyricicoccus, Coprococcus, and Fusicatenibacter) were also decreased in PD, whereas none of the three increased genera in DLB were increased in PD. Random forest modeling showed that gut bacteria differentiated controls and PD less efficiently than controls and DLB, which was likely due to a broad spectrum of disease severities in PD compared to those in DLB.
Analysis of the overall composition of gut microbiota
We performed PERMANOVA to examine the overall composition of gut microbiota in controls and DLB (Table 2). The overall composition of gut microbiota between controls and DLB was statistically different by all three distance metrics (Table 2a). We also found that age, sex, and PPI affected the overall composition of gut microbiota (Table 2b). Donepezil and memantine, both of which were used to treat dementia, did not affect the overall composition of gut microbiota in DLB patients (Table 2c). PERMANOVA analyses between controls and PD20 and between controls and iRBD21 were performed previously using almost the same samples, and were not repeated in this communication.
Table 2.
PERMANOVA to examine the effect of each factor on the overall bacterial composition.
| # DLB patients | # Controls | P-value | |||
|---|---|---|---|---|---|
| Chao | Weighted UniFrac | Unweighted UniFrac | |||
| (a) | 28 | 147 | |||
| DLB | 1.0E-7* | 1.0E-7* | 1.20E-04* | ||
| (b) | 28 | 142a | |||
| DLB | 1.0E-6* | 1.0E-6* | 1.3E-4* | ||
| Age | 4.8E-3* | 1.3E-3* | 2.8E-3* | ||
| Sex | 0.045* | 0.015* | 0.15 | ||
| BMI | 0.54 | 0.43 | 0.47 | ||
| Constipation | 0.13 | 0.43 | 0.52 | ||
| PPI | 1.2E-3* | *2.6E-4* | 0.12 | ||
| (c) | 28 | – | |||
| Donepezil | 0.88 | 0.92 | 0.13 | ||
| Memantine | 0.11 | 0.20 | 0.10 | ||
P-values of three distance metrics (Chao, weighted-UniFrac, and unweighted-UniFrac) are indicated. (a) Analysis of the effect of DLB on the overall microbial composition without considering other covariates in DLB and controls. (b) Analysis of the effects of DLB, age, sex, BMI, constipation, and PPI on the overall microbial composition in DLB and controls. (c) Analysis of the effects of donepezil and memantine, drugs for dementia, on the overall microbial composition in DLB.
PCoA analysis, as well as integrated topological analysis with tmap for simultaneous mapping of the overall gut microbiota, disease states, and clinical features
PCoA to examine the difference in the overall composition of gut microbiota revealed that the centers of gravity were shifted from the lower right to the upper left with the disease progression in PD, and that the center of gravity in DLB was close to those in Hoehn & Yahr stages 3 and 4 in PD (HY3&4) and PD with Mini-Mental State Examination (MMSE) < 26 (PD with cognitive decline, PDD+) (Fig. 2a). Next, we performed tmap43 to examine the relationship between taxonomic abundances, disease states, and clinical features in the same dimensions. The tmap analysis revealed that controls were closely located to SCFA-producing genera (Faecalibacterium, Coprococcus, Anaerostipes, Lachnospiraceae ND 3007 group, and Fusicatenibacter), indicating that controls were rich in SCFA-producing genera (Fig. 2b). In addition, DLB was closely located to PDD+ and HY3&4 (Fig. 2b), which was in accordance with the PCoA analysis (Fig. 2a).
Fig. 2. PCoA and tmap plots.
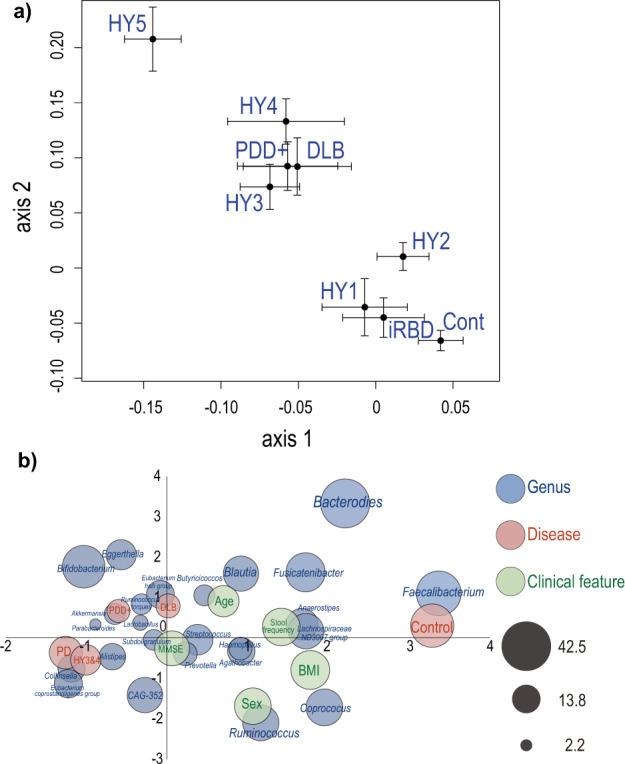
a PCoA plot showing the centers of gravity and the standard errors of the overall compositions of gut microbiota in nine disease states. Bray-Curtis distance was used as a distance metric. b An integrated topological map, tmap, showing how close genera, disease states, and clinical features are to each other. Blue, red, and green circles indicate genera, disease states, and clinical features, respectively. The size of circles indicates the SAFE score, which represents the network-level association of a target feature and is used as an effect size.
Random forest models to differentiate DLB and HY3&4, as well as DLB and PDD+
According to PCoA and tmap, the overall composition of gut microbiota in DLB was similar to those of HY3&4 and PDD+ . In order to identify bacteria that were uniquely changed in DLB, we made random forest models to differentiate DLB (n = 28) and HY3&4 (n = 91) (including both PDD− and PDD+), as well as DLB (n = 28) and PDD+ (n = 31) (including all HY stages). The AUROC to differentiate DLB and HY3&4 was 0.756 (95% confidence interval: 0.649–0.864) (Fig. 3a) by nested cross-validation. Three genera (Ruminococcus torques, Bifidobacterium, and Collinsella) made the maximum AUROC by leave-one-out cross-validation in recursive feature elimination (Fig. 3b). The top ten genera remained in recursive feature elimination are indicated in Supplementary Table 6. We analyzed taxonomic differences between DLB and HY3&4 by ANCOM-BC and Wilcoxon rank sum test (Supplementary Table 7a). Wilcoxon rank sum test showed that Ruminococcus torques, Bifidobacterium, and Collinsella were ranked first, third, and seventh, respectively. None of the 94 analyzed genera, however, were significantly changed after being corrected for multiple comparisons.
Fig. 3. Random forest models and essential intestinal genera to differentiate DLB and Hoehn & Yahr stages 3 and 4 (HY3&4) including both PDD− and PDD+ , and plots of fecal bile acids.
a ROC curves of nested cross-validation of random forest models to differentiate DLB and HY3&4 (both PDD− and PDD+). The optimal point by Youden index is indicated by a dot with the specificity and sensitivity in parentheses. b AUROCs by leave-one-out cross-validation of random forest models while genera were recursively eliminated. An arrow points to the maximum AUROC with the number of genera. The top ten genera that differentiated DLB and HY3&4 (both PDD− and PDD+), as well as the exact AUROC values, are indicated in Supplementary Table 6. c ROC curves of leave-one-out cross-validation of random forest models generated with three genera indicated by an arrow in b. The optimal point by Youden index is indicated by a dot with the specificity and sensitivity in parentheses. d, e, f Relative abundances of three genera indicated by an arrow in b in controls (n = 147), HY3&4 with MMSE ≥ 26116 (PDD−; n = 71), HY3&4 with MMSE < 26116 (PDD+ ; n = 20), and DLB (n = 28). P-values by Kruskal-Wallis test were all less than 0.05. P-values by Dunn’s post hoc test are indicated with an asterisk for p < 0.05. g Relative abundance of Ruminococcus gnavus, which also produces ursodeoxycholic acid (UDCA) from 7-ketolithocholic acid (7K-LCA), in the four categories. Although p-value by Kruskal-Wallis test was 0.40, p-values by Dunn’s post hoc test are indicated. Note that relative abundance is plotted on a logarithmic scale to clearly indicate medians and interquartile range. h Fecal UDCA/7K-LCA ratios in the four categories. P-value by Kruskal-Wallis test was 0.044. P-values by Dunn’s post hoc test are indicated with an asterisk for p < 0.05. UDCA and 7K-LCA were randomly measured in available fecal samples. (d, e, f, g, h) Median and interquartile range are indicated in red.
In contrast to a model to differentiate DLB and HY3&4, the AUROC to differentiate DLB and PDD+ was 0.603 (0.451–0.754) by nested cross-validation, which indicated that gut microbiota could not efficiently differentiate DLB and PDD+. Taxonomic differences between DLB and PDD+ by ANCOM-BC and Wilcoxon rank sum test were indicated in Supplementary Table 7b. Collinsella was the only genera that was significantly increased in DLB compared to PDD+ by ANCOM-BC.
Analysis of three genera in patients with or without cognitive decline
As shown above (Fig. 3b), three genera, Ruminococcus torques, Bifidobacterium, and Collinsella, were essential determinants to differentiate DLB and HY3&4. When relative abundances of the three genera were compared in controls (n = 147), PDD− at HY3&4 (n = 71), PDD+ at HY3&4 (n = 20), and DLB (n = 28), (i) Ruminococcus torques was increased in DLB compared to controls, (ii) Bifidobacterium was decreased in DLB compared to PDD−, and (iii) Collinsella was increased in DLB compared to controls (Fig. 3d–f). Thus, increased Ruminococcus torques, decreased Bifidobacterium, and increased Collinsella were unique to DLB.
Correlation between five clinical features and bacterial abundances in DLB
We calculated Spearman’s rank correlation coefficients between five clinical features [age, disease duration, MMSE, total Movement Disorder Society’s (MDS) version of the Unified Parkinson’s Disease Rating Scale (UPDRS), MDS-UPDRS III] and the abundances of ten genera that were significantly changed in DLB compared to controls (Supplementary Table 8). Ruminococcus torques was negatively correlated with MMSE. Eggerthella and Coprococcus were positively and negatively correlated with total MDS-UPDRS, respectively. Thus, Ruminococcus torques was likely to be increased in dementia, whereas Eggerthella was likely to be increased and Coprococcus was likely to be decreased with the progression of parkinsonism in DLB. In contrast to DLB, neither of the three genera was significantly changed in PD in our meta-analysis of five countries20.
Quantification of fecal bile acids
Three genera (Ruminococcus torques, Collinsella, and Ruminococcus gnavus), which had relative abundances of more than 0.5% in our cohort, carry 7β-hydroxysteroid dehydrogenase (7BHD) [EC 1.1.1.201] to catalyze bidirectional reactions between 7-ketolithocholic acid (7K-LCA) and ursodeoxycholic acid (UDCA) according to KEGG and UniRef90. We showed above that both Ruminococcus torques and Collinsella were high in DLB (Fig. 3d, f). Ruminococcus gnavus tended to be high in DLB and PDD+ at HY3&4 (Fig. 3g), which was similar to Ruminococcus torques. We quantified fecal UDCA and 7K-LCA concentrations, and calculated the ratio of UDCA/7K-LCA to estimate the activity of 7BHD. The UDCA/7K-LCA ratio was significantly increased in DLB compared to controls (Fig. 3h). The median of the UDCA/7K-LCA ratios was high in PDD− and PDD+ at HY3&4 compared to controls, but p-values were both greater than 0.999 (Fig. 3h). Spearman’s rank correlation coefficients between the UDCA/7K-LCA ratios and Ruminococcus torques, Collinsella, and Ruminococcus gnavus were −0.009 (p = 0.922), −0.189 (p = 0.036), and 0.396 (p < 0.0001), respectively.
Comparison of four genera (Ruminococcus torques, Bifidobacterium, Collinsella, and Ruminococcus gnavus) between controls, iRBD, PD, and DLB
We additionally plotted the four genera (Ruminococcus torques, Bifidobacterium, Collinsella, and Ruminococcus gnavus) indicated in Fig. 3 in controls, iRBD, PD, and DLB (Supplementary Fig. 3). As we observed in the comparisons between DLB and HY3&4 (PDD− and PDD+) (Fig. 3d–g), Ruminococcus torques, Collinsella, and Ruminococcus gnavus were increased in DLB, and Bifidobacterium was increased in PD, although statistical significance was not always observed. In addition, the abundances of the four genera in iRBD were similar to those in controls.
Discussion
We analyzed gut microbiota in DLB to examine whether any intestinal bacteria are unique to DLB, as well as to both DLB and PD with cognitive decline (PDD+). Analysis of each taxon between DLB and controls revealed that seven genera (Agathobacter, Lachnospiraceae ND3007 group, Butyricicoccus, Coprococcus, Faecalibacterium, Fusicatenibacter, and Haemophilus) were significantly decreased, and three genera (Collinsella, Eggerthella, and Ruminococcus torques) were significantly increased in DLB (Fig. 1 and Supplementary Table 2a). Correlation analysis of gut microbiota and clinical features in DLB revealed that decreased Coprococcus and increased Eggerthella were likely to be associated with the progression of parkinsonism, whereas increased Ruminococcus torques was likely to be associated with dementia (Supplementary Table 8). Six of the seven decreased genera excluding Haemophilus were SCFA-producing bacteria. Genera that were significantly decreased in DLB were similar to those in PD (Supplementary Tables 2a, 3a). Decreases of SCFA-producing bacteria have been repeatedly reported in PD20,24,26, Alzheimer’s disease44–47, and ALS48,49, and are likely to be a shared feature in neurodegenerative diseases. SCFA, especially butyrate, ameliorates mucosal inflammation and oxidative status, increases the intestinal mucin layer, and induces regulatory T cells by suppressing histone deacetylases50–52. Two of the three increased genera (Collinsella and Ruminococcus torques) were essential to differentiate DLB and HY3&4, and will be addressed later. To summarize, SCFA-producing genera were decreased in DLB, as has been observed in PD. In contrast, the three genera that were increased in DLB, were not changed in PD.
The overall composition of gut microbiota was significantly different in DLB compared to controls according to PERMANOVA (Table 2a). In addition, age, sex, and PPI, but not BMI, constipation, donepezil, or memantine, affected the overall composition of gut microbiota (Table 2b, c). The effects of age, sex, and PPI on gut microbiota have been previously reported: (i) aging decreases Bifidobacterium53, and increases Bacteroides, Eubacterium, and Clostridiaceae54; (ii) females have higher α-diversity of intestinal microbiota55–57; (iii) males show decreased Bacteroides and increased Prevotella in the Human Microbiome Project (HMP) Consortium58; and (iv) PPI increases Streptococcus and decreases Faecalibacterium59. Thus, the change in the overall composition of gut microbiota in DLB was also accounted for by the effects of age, sex, and PPI on specific bacteria.
As indicated in the introduction, α-synucleinopathies are comprised of iRBD, PD, and DLB, and more than 90% of iRBD patients later develop other forms of α-synucleinopathies3. DLB develops dementia first, whereas PDD+ develops dementia in the course of the progression of PD. PCoA showed that the centers of gravity were shifted with the progression of PD (Fig. 2a). PCoA is also consistent with the notion that iRBD is prodromal to PD and DLB. Clustering of the centers of gravity in DLB, HY3&4, and PDD+ prompted us to compare DLB vs HY3&4, as well as DLB vs PDD+ . Although random forest modeling failed to differentiate DLB and PDD+ , genus Collinsella was significantly increased in DLB compared to PDD+ (q-value by ANCOM-BC = 0.044, Supplementary Table 7b). On the other hand, random forest modeling to differentiate DLB and HY3&4 showed that three genera (Ruminococcus torques, Bifidobacterium, and Collinsella) were essential determinants (Fig. 3b).
Ruminococcus torques and Collinsella were both increased in DLB (Fig. 3d, f). Bifidobacterium will be discussed later. Ruminococcus torques is also increased in ulcerative colitis and Crohn’s disease60. Ruminococcus torques is the most efficient bacterium that degrades mucin 2 (MUC2), which constitutes the cell surface mucin in the colon60. Collinsella is also increased in rheumatoid arthritis61. Collinsella enhances gut permeability by decreasing the tight junction protein ZO-1 in a mouse model of rheumatoid arthritis61. Collinsella also increases the production of the proinflammatory cytokine IL-17A in human intestinal epithelial cell lines61. Increased Ruminococcus torques and Collinsella in DLB are thus likely to increase gut permeability. Increased gut permeability may cause exposure of the intestinal neural plexus to pesticides/herbicides and lipopolysaccharide (LPS), both of which are likely to predispose the neural plexus to oxidative stress and inflammation. Increased risks of PD by pesticides/herbicides have been repeatedly reported62. Increased intestinal permeability in PD has been demonstrated by decreased serum lipopolysaccharide (LPS)-binding protein19,63, as well as increased intestinal staining for nitrotyrosine and E. coli63. Pesticides/herbicides and LPS may potentiate the formation of abnormal α-synuclein fibrils in PD, and similar mechanisms may be operational in DLB.
Collinsella is also increased in atherosclerosis64 and coronary artery disease65, but its relevance to DLB remains unknown. In contrast to high Collinsella in DLB, rheumatoid arthritis61, atherosclerosis64, and coronary artery disease65, low Collinsella was associated with high mortality rates of COVID-19 in 953 healthy subjects in ten countries66. Indeed, Collinsella was low in patients with patients with COVID-19 in three reports67–69, although this observation was not confirmed in another report70. The reason for the apparently discordant effects of Collinsella on different diseases remains elusive.
We observed a statistically significant increase of the fecal UDCA/7K-LCA ratio only in DLB (Fig. 3h). Ruminococcus torques, Collinsella, and Ruminococcus gnavus are major intestinal bacteria carrying 7BHD (EC 1.1.1.201) that catalyzes bidirectional reactions between 7K-LCA and UDCA71. Interestingly, Ruminococcus torques, Collinsella, and Ruminococcus gnavus were ranked first, third, and sixth in recursive feature elimination to differentiate DLB and HY3&4 in random forest modeling (Supplementary Table 6). UDCA is a major secondary bile acid in the enterohepatic circulation72. UDCA suppresses pro-inflammatory cytokines like TNF-α, IL-1β, IL-2, IL-4, and IL-673,74, and have anti-oxidant and anti-apoptotic effects75,76. UDCA and its taurine conjugate, tauroursodeoxycholate, inhibit Aβ-induced apoptosis and have mitochondrial protective effects in mouse models of Alzheimer’s disease77–79 and in fibroblasts derived from patients with Alzheimer’s disease80. The effects of UDCA on PD have also been repeatedly reported81–83. The increase of UDCA may mitigate inflammation-mediated dopaminergic cell death at the substantia nigra. In the neocortex, however, neuroinflammation may not critically trigger neuronal cell death, and suppression of neuroinflammation by UDCA may fail to mitigate the development of DLB. Indeed, intraperitoneal injection of LPS causes P2Y6 receptor-mediated activation of microglia and inflammatory neuronal loss in the substantia nigra, but not in the cortex or hippocampus84. Delayed neuronal cell death in the substantia nigra due to suppressed neuroinflammation in DLB masks the ɑ-synuclein pathology in the substantia nigra, which also accounts for the delayed age of onset of DLB compared to that of PD6.
In addition to Ruminococcus torques and Collinsella, Eggerthella was also increased in DLB compared to controls (Fig. 1, Supplementary Table 2a). Although Eggerthella does not have 7BHD (EC 1.1.1.201), Eggerthella also catalyzes secondary bile acids85,86. As Eggerthella inhibits inflammation in the gut by producing bile acids85, Eggerthella may have a similar effect as Ruminococcus torques and Collinsella.
We previously reported that increased Lactobacillus in PD was accounted for not by PD but by COMT inhibitors, drugs for PD20. Similarly, we here showed that increased Bifidobacterium in PD was accounted for not by PD but by COMT inhibitors (Supplementary Fig. 4a). Bifidobacterium was previously reported to be increased in PD in three meta-analyses including ours20,87,88, but the increase of Bifidobacterium might be due to COMT inhibitors. We showed that Bifidobacterium tended to be lower in PDD− compared to PDD+ (Fig. 3e). As the ratios of COMT inhibitor intake were not different between PDD− and PDD+ (p = 0.53 by Fisher’s exact test), the presence of dementia might have lowered Bifidobacterium in PDD+ . Similarly, the median of Bifidobacterium in DLB was lower than that in controls (p = 0.194 by Wilcoxon rank sum test, which became p > 0.999 after correcting for multiple comparisons in Fig. 3e), while nobody in DLB or controls was taking COMT inhibitors. In addition, Bifidobacterium was positively correlated with MMSE in patients with PD and DLB, who were not taking COMT inhibitors (Supplementary Fig. 4b). Thus, Bifidobacterium was likely to be increased by COMT inhibitors and decreased by dementia. Decreased Bifidobacterium is observed in Alzheimer’s disease89,90 and is predictive of rapid progression of non-motor symptoms including cognitive decline in PD91. Frequent coexistence of tauopathy in Alzheimer’s disease and DLB92,93 is also in accordance with the notion that Bifidobacterium is decreased in dementia. Administration of Bifidobacterium ameliorates cognitive dysfunction in a mouse model of Alzheimer’s disease94,95, as well as in humans96,97. Oral administration of Bifidobacterium elevates brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, in the brain of rodents98. BDNF plays a significant role in neurogenesis99 and is decreased in the autopsied brain of Alzheimer’s disease100. Similarly, decreased serum BDNF is related to the development of Alzheimer’s disease101,102 and the dopaminergic cell death in PD102,103. Serum BDNF, however, is paradoxically increased in Alzheimer’s disease104 and PD105,106, which is likely to represent compensatory mechanisms104–106. Thus, decreased Bifidobacterium in DLB and PDD+ may be causally associated with cognitive decline via decreased BDNF.
Our study has two limitations. First, the number of fecal samples of DLB patients was limited to 28, which disabled subgroup analysis, although this is a first report of gut microbiota in DLB. Second, we could not show whether the change of gut microbiota in DLB was the cause or the consequence. In future studies, more fecal samples of DLB patients and longitudinal analysis will be required.
In conclusion, similarly to PD, SCFA-producing genera were decreased in DLB. Additionally, Ruminococcus torques and Collinsella were increased in DLB, which were not changed in PD. High Ruminococcus torques and high Collinsella, which were predicted to increase intestinal permeability and to increase secondary bile acids, as well as low Bifidobacterium, which were observed in Alzheimer’s disease, were predictive of DLB in random forest models. Indeed, the production of UDCA was high in DLB, and increased UDCA in DLB may mitigate neuroinflammation at the substantia nigra. Therapeutic intervention to increase Bifidobacterium potentially retards the development and progression of DLB.
Methods
Patients
All studies were approved by the Ethical Review Committees of the Nagoya University Graduate School of Medicine (approval #2016-0151), Iwate Medical University (approval #H28-123), Okayama Kyokuto Hospital (approval #kyoIR-2016002), and Fukuoka University School of Medicine (approval #2016M027). We got written informed consent from all recruited subjects.
We obtained fecal samples in 224 PD patients, 26 iRBD patients, 28 DLB patients, and 147 controls (November 2016 to May 2019). DLB patients were diagnosed according to the Dementia with Lewy Bodies Consortium5. We excluded DLB patients with other chronic diseases including diabetes mellitus, heart failure, liver cirrhosis, malignancy, hematological diseases, and autoimmune diseases. Similarly, we excluded DLB patients who claimed to have taken antibiotics in the past one month.
DNA isolation and 16S rRNA V3-V4 gene amplicon sequencing
The samples were transported from the participant’s home to Nagoya University below 4˚C, freeze-dried107, and subjected to DNA isolation and sequencing of the 16S rRNA V3–V4 region using a pair of primers (341F, 5′-CCTACGGGNGGCWGCAG-3′ and 805R, 5′-GACTACHVGGGTATCTAATCC-3′).20,21 Paired-end sequencing of 300-nucleotide fragments was performed using the MiSeq reagent kit V3 on a MiSeq System (Illumina). The 16S rRNA gene amplicon sequencing data were analyzed by QIIME2108 with DADA2 using the SILVA taxonomy database release 138109,110.
Possible confounding factors
We compared six demographic and clinical features [age, sex, body mass index (BMI), constipation, proton pump inhibitor intake (PPI), and H2 blocker intake] between (i) controls and DLB, (ii) controls and PD, and (iii) controls and iRBD. Subjects with the stool frequency twice a week or less were defined to be constipated111.
We further analyzed the effects of (i) DLB, (ii) DLB, age, sex, BMI, constipation, and PPI, and (iii) donepezil and memantine in DLB patients, on the overall composition of gut microbiota with PERMANOVA112. All genera were included in this analysis. The effect of each feature was evaluated by three distance metrics of Chao113, unweighted-UniFrac114, and weighted-UniFrac114. Chao and unweighted/weighted-UniFrac were calculated with the R package vegan and QIIME2, respectively.
Analysis of each taxon between (i) controls and DLB, (ii) controls and PD, (iii) DLB and HY3&4 (including both PDD− and PDD+), and (iv) DLB and PDD+ at any HY stages
Taxa were filtered at the genus and family levels using the following two conditions. First, for each taxon, we counted the number of samples in which the relative abundance of the taxon of interest was greater than 1E-4. The number of such samples should constitute more than 20% of all samples. Second, we chose taxa with the average relative abundance of more than 0.001.
For each pair of (i) controls and DLB, (ii) controls and PD, (iii) DLB and HY3&4 (including both PDD− and PDD+), and (iv) DLB and PDD+ at any HY stages, we tested the difference of each taxon using Analysis of Compositions of Microbiomes with Bias Correction (ANCOM-BC)41 and the Wilcoxon rank sum test. Five confounding factors (age, sex, BMI, constipation, and PPI) were included in the analysis with ANCOM-BC on R version 4.2.1. The Wilcoxon rank sum test was calculated with the mannwhitneyu functionality of scipy.stat on Python 3.8.2. The false discovery rate (FDR) by the Benjamini-Hochberg method115 of both ANCOM-BC and Wilcoxon rank sum test less than 0.05 was considered to be significant. We made random forest models using the RandomForestRegressor functionality of sklearn.ensemble on Python 3.8.2 to identify essential bacteria to differentiate each pair of (i), (ii), (iii), and (iv) by leave-one-out cross-validation, and to calculate AUROC by nested cross validation22.
Analysis of the overall gut microbiota in controls, DLB, iRBD, PD at any HY stages, and PDD+ at any HY stages
PD patients with MMSE lower than 26116 were arbitrarily defined as PDD+ , as there is no definite criteria for PDD4,117. PD patients with MMSE ≥ 26 are indicated by PDD−. Microbiota data in controls, PD, and iRBD in our previous report20,21 were included in the overall analysis. We performed Principal Coordinates Analysis (PCoA) using controls, iRBD, PD at any HY stages, PDD+ at any HY stages, and DLB. Next, we performed tmap43, an integrative map based on topological data analysis for interpreting microbiome data and metadata simultaneously. Although we used all genera in this analysis, we plotted the 20 most abundant genera, as well as genera that were significantly changed in DLB. By tmap, we plotted bacteria, clinical features, and disease states on an identical two-dimensional plane and examined which features were close to each other.
Quantification of fecal bile acids
The quantitative determination using a liquid chromatography with tandem mass spectrometry (LC-MS/MS) was performed to determined fecal concentrations of 7K-LCA and UDCA in 52 controls, 44 patients with HY3&4 (PDD− and PDD+), and 28 patients with DLB. 7K-LCA (> 97% purity) and UDCA (> 96% purity) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and FUJIFILM Wako Pure Chemical Co. (Osaka, Japan), respectively. UDCA-D4 was purchased from Sigma Aldrich (St. Louis, MO, USA) and used as an internal standard.
Briefly, 20 mg of freeze-dried fecal samples were mixed with 1 ml of 70% ethanol and internal standard solution. After vigorous shaking and centrifugation, the supernatants were transferred into a solid phase extraction column (strong anion exchange column). The bile acids were eluted by 1 ml of 2% formic acid in acetonitrile. The eluates were injected into LC − MS/MS, which was composed of Agilent 1200 Infinity LC coupled with an Agilent Ultivo Triple Quadrupole LC/MS System (Agilent Technologies). The operating conditions of LC were as follows: LC column, Cadenza CD-C18 (Imtakt, Kyoto, Japan), 150 × 2 mm i.d., 3 μm silica; mobile phase A, H2O containing 5 mmol/l of formic acid; mobile phase B, 100 % of acetonitrile; and injection volume, 10 μL. A freeze-dried quality control (QC) sample was quantified every 20 freeze-dried samples. The precision of the QC was less than 5.4% relative standard deviation (%RSD).
Supplementary information
Acknowledgements
We acknowledge Keiichi Takimoto, Keigo Otsuka, Karin Ozeki, Harumi Kodama, and Tomomi Yamada at the Nagoya University Graduate School of Medicine for their technical assistance. We also acknowledge Division for Medical Research Engineering, Nagoya University Graduate School of Medicine for their technical support on 16S rRNA-seq analysis. This study was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (JP21H03561, JP20K06925, and JP22K15394); the Ministry of Health, Labour and Welfare of Japan (20FC1036); the Japan Agency for Medical Research and Development (JP21ek0109488, and JP21bm0804005), the National Center of Neurology and Psychiatry (2–5), and the Hori Sciences and Arts Foundation.
Author contributions
H.N., M.H., and K.O. conceived the study. H.N. performed data science analyses with the help of T.Y., H.H., and I.T. M.I., T.H., J.U., and M.H. performed microbiota analyses. T.M., K.K., Y.T., M.K., and M.H. provided fecal samples and clinical data. All authors critically revised and approved the manuscript.
Data availability
FASTQ files of our dataset are available at the DNA Data Bank of Japan (DDBJ) under the accession numbers of “DRA009229” for PD and controls and “DRA012438” for PD and conrols, and “DRA009322” for iRBD and “DRA011417” for iRBD.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Masaaki Hirayama, Email: hirasan@met.nagoya-u.ac.jp.
Kinji Ohno, Email: ohnok@med.nagoya-u.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-022-00428-2.
References
- 1.McCann H, Stevens CH, Cartwright H, Halliday GM. alpha-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 2014;20:S62–S67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- 2.Fanciulli A, Wenning GK. Multiple-system atrophy. N. Engl. J. Med. 2015;372:249–263. doi: 10.1056/NEJMra1311488. [DOI] [PubMed] [Google Scholar]
- 3.Dauvilliers Y, et al. REM sleep behaviour disorder. Nat. Rev. Dis. Prim. 2018;4:19. doi: 10.1038/s41572-018-0016-5. [DOI] [PubMed] [Google Scholar]
- 4.Emre M, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 5.McKeith IG, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Outeiro TF, et al. Dementia with Lewy bodies: an update and outlook. Mol. Neurodegener. 2019;14:5. doi: 10.1186/s13024-019-0306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomperts SN. Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia. Contin. (Minneap. Minn.) 2016;22:435–463. doi: 10.1212/CON.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 9.Chiang HL, Lin CH. Altered Gut Microbiome and Intestinal Pathology in Parkinson’s Disease. J. Mov. Disord. 2019;12:67–83. doi: 10.14802/jmd.18067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cersosimo MG. Gastrointestinal Biopsies for the Diagnosis of Alpha-Synuclein Pathology in Parkinson’s Disease. Gastroenterol. Res Pr. 2015;2015:476041. doi: 10.1155/2015/476041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbons CH, Garcia J, Wang N, Shih LC, Freeman R. The diagnostic discrimination of cutaneous alpha-synuclein deposition in Parkinson disease. Neurology. 2016;87:505–512. doi: 10.1212/WNL.0000000000002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol. Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov. Disord. 2012;27:716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. (Vienna) 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 16.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol. Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: the dual hit theory revisited. Ann. N. Y Acad. Sci. 2009;1170:615–622. doi: 10.1111/j.1749-6632.2009.04365.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa S, et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS One. 2015;10:e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiwaki H, et al. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov. Disord. 2020;35:1626–1635. doi: 10.1002/mds.28119. [DOI] [PubMed] [Google Scholar]
- 21.Nishiwaki, H. et al. Short-Chain Fatty Acid-Producing Gut Microbiota Is Decreased in Parkinson’s Disease but Not in Rapid-Eye-Movement Sleep Behavior Disorder. mSystems5, 10.1128/mSystems.00797-20 (2020). [DOI] [PMC free article] [PubMed]
- 22.Nishiwaki H, et al. Short chain fatty acids-producing and mucin-degrading intestinal bacteria predict the progression of early Parkinson’s disease. NPJ Parkinsons Dis. 2022;8:65. doi: 10.1038/s41531-022-00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheperjans F, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 24.Keshavarzian A, et al. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 25.Unger MM, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Hill-Burns EM, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017;32:739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrov VA, et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017;162:734–737. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 28.Bedarf JR, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med. 2017;9:39. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopfner F, et al. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017;1667:41–45. doi: 10.1016/j.brainres.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Li W, et al. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 2017;60:1223–1233. doi: 10.1007/s11427-016-9001-4. [DOI] [PubMed] [Google Scholar]
- 31.Qian Y, et al. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018;70:194–202. doi: 10.1016/j.bbi.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Lin A, et al. Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat. Disord. 2018;53:82–88. doi: 10.1016/j.parkreldis.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Heintz-Buschart A, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018;33:88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan AH, et al. Unveiling the function of altered gut microbiota composition in Parkinson’s disease. Mov. Disord. 2018;33:S783–S784. [Google Scholar]
- 35.Barichella M, et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019;34:396–405. doi: 10.1002/mds.27581. [DOI] [PubMed] [Google Scholar]
- 36.Pietrucci, D. et al. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Parkinsonism Relat Disord, 10.1016/j.parkreldis.2019.06.003 (2019). [DOI] [PubMed]
- 37.Cirstea MS, et al. Microbiota Composition and Metabolism Are Associated With Gut Function in Parkinson’s Disease. Mov. Disord. 2020;35:1208–1217. doi: 10.1002/mds.28052. [DOI] [PubMed] [Google Scholar]
- 38.Li C, et al. Gut Microbiota Differs Between Parkinson’s Disease Patients and Healthy Controls in Northeast China. Front Mol. Neurosci. 2019;12:171. doi: 10.3389/fnmol.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vascellari, S. et al. Gut Microbiota and Metabolome Alterations Associated with Parkinson’s Disease. mSystems5, 10.1128/mSystems.00561-20 (2020). [DOI] [PMC free article] [PubMed]
- 40.Aho VTE, et al. Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707. doi: 10.1016/j.ebiom.2019.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin H, Peddada SD. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020;11:3514. doi: 10.1038/s41467-020-17041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandal S, et al. Analysis of composition of microbiomes: a novel method for studying microbial composition. Micro. Ecol. Health Dis. 2015;26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao T, Wei Y, Luo M, Zhao GP, Zhou H. tmap: an integrative framework based on topological data analysis for population-scale microbiome stratification and association studies. Genome Biol. 2019;20:293. doi: 10.1186/s13059-019-1871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hung CC, Chang CC, Huang CW, Nouchi R, Cheng CH. Gut microbiota in patients with Alzheimer’s disease spectrum: a systematic review and meta-analysis. Aging (Albany NY) 2022;14:477–496. doi: 10.18632/aging.203826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li B, et al. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019;15:1357–1366. doi: 10.1016/j.jalz.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Liu P, et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019;80:633–643. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Vogt NM, et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholson K, et al. The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 2021;22:186–194. doi: 10.1080/21678421.2020.1828475. [DOI] [PubMed] [Google Scholar]
- 49.Fang X, et al. Evaluation of the Microbial Diversity in Amyotrophic Lateral Sclerosis Using High-Throughput Sequencing. Front Microbiol. 2016;7:1479. doi: 10.3389/fmicb.2016.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canani RB, et al. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 52.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 53.Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Odamaki T, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YS, Unno T, Kim BY, Park MS. Sex Differences in Gut Microbiota. World J. Mens. Health. 2020;38:48–60. doi: 10.5534/wjmh.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borgo F, et al. Body Mass Index and Sex Affect Diverse Microbial Niches within the Gut. Front Microbiol. 2018;9:213. doi: 10.3389/fmicb.2018.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao X, et al. Body Mass Index Differences in the Gut Microbiota Are Gender Specific. Front Microbiol. 2018;9:1250. doi: 10.3389/fmicb.2018.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takagi T, et al. The influence of long-term use of proton pump inhibitors on the gut microbiota: an age-sex-matched case-control study. J. Clin. Biochem Nutr. 2018;62:100–105. doi: 10.3164/jcbn.17-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Png CW, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 61.Chen J, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016;8:43. doi: 10.1186/s13073-016-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Islam, M. S. et al. Pesticides and Parkinson’s disease: Current and future perspective. J Chem Neuroanat, 101966, 10.1016/j.jchemneu.2021.101966 (2021). [DOI] [PubMed]
- 63.Forsyth CB, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karlsson FH, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Z, et al. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis. 2019;284:121–128. doi: 10.1016/j.atherosclerosis.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 66.Hirayama M, et al. Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS One. 2021;16:e0260451. doi: 10.1371/journal.pone.0260451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu S, et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020;71:2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeoh YK, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Y, et al. Altered oral and gut microbiota and its association with SARS-CoV-2 viral load in COVID-19 patients during hospitalization. NPJ Biofilms Microbiomes. 2021;7:61. doi: 10.1038/s41522-021-00232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim, H. N. et al. Reversion of Gut Microbiota during the Recovery Phase in Patients with Asymptomatic or Mild COVID-19: Longitudinal Study. Microorganisms9, 10.3390/microorganisms9061237 (2021). [DOI] [PMC free article] [PubMed]
- 71.Lee JY, et al. Contribution of the 7beta-hydroxysteroid dehydrogenase from Ruminococcus gnavus N53 to ursodeoxycholic acid formation in the human colon. J. Lipid Res. 2013;54:3062–3069. doi: 10.1194/jlr.M039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, et al. Ursodeoxycholic acid accelerates bile acid enterohepatic circulation. Br. J. Pharm. 2019;176:2848–2863. doi: 10.1111/bph.14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ko WK, et al. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS One. 2017;12:e0180673. doi: 10.1371/journal.pone.0180673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ko WK, et al. Ursodeoxycholic Acid Inhibits Inflammatory Responses and Promotes Functional Recovery After Spinal Cord Injury in Rats. Mol. Neurobiol. 2019;56:267–277. doi: 10.1007/s12035-018-0994-z. [DOI] [PubMed] [Google Scholar]
- 75.Lapenna D, et al. Antioxidant properties of ursodeoxycholic acid. Biochem Pharm. 2002;64:1661–1667. doi: 10.1016/S0006-2952(02)01391-6. [DOI] [PubMed] [Google Scholar]
- 76.Kim YJ, Jeong SH, Kim EK, Kim EJ, Cho JH. Ursodeoxycholic acid suppresses epithelial-mesenchymal transition and cancer stem cell formation by reducing the levels of peroxiredoxin II and reactive oxygen species in pancreatic cancer cells. Oncol. Rep. 2017;38:3632–3638. doi: 10.3892/or.2017.6045. [DOI] [PubMed] [Google Scholar]
- 77.Lo AC, Callaerts-Vegh Z, Nunes AF, Rodrigues CM, D’Hooge R. Tauroursodeoxycholic acid (TUDCA) supplementation prevents cognitive impairment and amyloid deposition in APP/PS1 mice. Neurobiol. Dis. 2013;50:21–29. doi: 10.1016/j.nbd.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Vang S, Longley K, Steer CJ, Low WC. The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases. Glob. Adv. Health Med. 2014;3:58–69. doi: 10.7453/gahmj.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nunes AF, et al. TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-beta deposition in APP/PS1 mice. Mol. Neurobiol. 2012;45:440–454. doi: 10.1007/s12035-012-8256-y. [DOI] [PubMed] [Google Scholar]
- 80.Bell SM, et al. Ursodeoxycholic Acid Improves Mitochondrial Function and Redistributes Drp1 in Fibroblasts from Patients with Either Sporadic or Familial Alzheimer’s Disease. J. Mol. Biol. 2018;430:3942–3953. doi: 10.1016/j.jmb.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mortiboys H, et al. UDCA exerts beneficial effect on mitochondrial dysfunction in LRRK2(G2019S) carriers and in vivo. Neurology. 2015;85:846–852. doi: 10.1212/WNL.0000000000001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qi H, Shen D, Jiang C, Wang H, Chang M. Ursodeoxycholic acid protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis in MPTP/MPP(+)-induced Parkinson’s disease. Neurosci. Lett. 2021;741:135493. doi: 10.1016/j.neulet.2020.135493. [DOI] [PubMed] [Google Scholar]
- 83.Huang F, Pariante CM, Borsini A. From dried bear bile to molecular investigation: A systematic review of the effect of bile acids on cell apoptosis, oxidative stress and inflammation in the brain, across pre-clinical models of neurological, neurodegenerative and neuropsychiatric disorders. Brain Behav. Immun. 2022;99:132–146. doi: 10.1016/j.bbi.2021.09.021. [DOI] [PubMed] [Google Scholar]
- 84.Milde S, et al. Inflammatory neuronal loss in the substantia nigra induced by systemic lipopolysaccharide is prevented by knockout of the P2Y6 receptor in mice. J. Neuroinflammation. 2021;18:225. doi: 10.1186/s12974-021-02280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paik D, et al. Human gut bacteria produce TauEta17-modulating bile acid metabolites. Nature. 2022;603:907–912. doi: 10.1038/s41586-022-04480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. 2021;9:140. doi: 10.1186/s40168-021-01101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Romano S, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7:27. doi: 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wallen ZD. Comparison study of differential abundance testing methods using two large Parkinson disease gut microbiome datasets derived from 16S amplicon sequencing. BMC Bioinforma. 2021;22:265. doi: 10.1186/s12859-021-04193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhuang ZQ, et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2018;63:1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 90.Haran, J. P. et al. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio10, 10.1128/mBio.00632-19 (2019). [DOI] [PMC free article] [PubMed]
- 91.Minato T, et al. Progression of Parkinson’s disease is associated with gut dysbiosis: Two-year follow-up study. PLoS One. 2017;12:e0187307. doi: 10.1371/journal.pone.0187307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X, et al. Tau Pathology in Parkinson’s Disease. Front Neurol. 2018;9:809. doi: 10.3389/fneur.2018.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jellinger KA. Are there morphological differences between Parkinson’s disease-dementia and dementia with Lewy bodies? Parkinsonism Relat. Disord. 2022;100:24–32. doi: 10.1016/j.parkreldis.2022.05.024. [DOI] [PubMed] [Google Scholar]
- 94.Kobayashi Y, et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017;7:13510. doi: 10.1038/s41598-017-13368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee HJ, Lee KE, Kim JK, Kim DH. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019;9:11814. doi: 10.1038/s41598-019-48342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobayashi Y, Kuhara T, Oki M, Xiao JZ. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef. Microbes. 2019;10:511–520. doi: 10.3920/BM2018.0170. [DOI] [PubMed] [Google Scholar]
- 97.Kobayashi Y, et al. Bifidobacterium Breve A1 Supplementation Improved Cognitive Decline in Older Adults with Mild Cognitive Impairment: An Open-Label, Single-Arm Study. J. Prev. Alzheimers Dis. 2019;6:70–75. doi: 10.14283/jpad.2018.32. [DOI] [PubMed] [Google Scholar]
- 98.Jang, H. M., Lee, K. E. & Kim, D. H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients11, 10.3390/nu11040819 (2019). [DOI] [PMC free article] [PubMed]
- 99.Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front Cell Neurosci. 2019;13:363. doi: 10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Phillips HS, et al. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 101.Gezen-Ak D, et al. BDNF, TNFalpha, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J. Alzheimers Dis. 2013;37:185–195. doi: 10.3233/JAD-130497. [DOI] [PubMed] [Google Scholar]
- 102.Lima Giacobbo B, et al. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019;56:3295–3312. doi: 10.1007/s12035-018-1283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ziebell M, et al. Striatal dopamine transporter binding correlates with serum BDNF levels in patients with striatal dopaminergic neurodegeneration. Neurobiol. Aging. 2012;33:428 e421–425. doi: 10.1016/j.neurobiolaging.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 104.Angelucci F, et al. Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI) patients are characterized by increased BDNF serum levels. Curr. Alzheimer Res. 2010;7:15–20. doi: 10.2174/156720510790274473. [DOI] [PubMed] [Google Scholar]
- 105.Ventriglia M, et al. Serum brain-derived neurotrophic factor levels in different neurological diseases. Biomed. Res Int. 2013;2013:901082. doi: 10.1155/2013/901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scalzo P, Kummer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol. 2010;257:540–545. doi: 10.1007/s00415-009-5357-2. [DOI] [PubMed] [Google Scholar]
- 107.Ueyama J, et al. Freeze-drying enables homogeneous and stable sample preparation for determination of fecal short-chain fatty acids. Anal. Biochem. 2020;589:113508. doi: 10.1016/j.ab.2019.113508. [DOI] [PubMed] [Google Scholar]
- 108.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yilmaz P, et al. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014;42:D643–D648. doi: 10.1093/nar/gkt1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jamshed N, Lee ZE, Olden KW. Diagnostic approach to chronic constipation in adults. Am. Fam. Physician. 2011;84:299–306. [PubMed] [Google Scholar]
- 112.Anderson MJ. A new method for non‐parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- 113.Chao A, Chazdon RL, Colwell RK, Shen TJ. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005;8:148–159. doi: 10.1111/j.1461-0248.2004.00707.x. [DOI] [Google Scholar]
- 114.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Benjamini Y, Hochberg Y. Controlling The False Discovery Rate - A Practical And Powerful Approach To Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- 116.Goetz CG, Emre M, Dubois B. Parkinson’s disease dementia: definitions, guidelines, and research perspectives in diagnosis. Ann. Neurol. 2008;64:S81–S92. doi: 10.1002/ana.21455. [DOI] [PubMed] [Google Scholar]
- 117.Poewe W, et al. Diagnosis and management of Parkinson’s disease dementia. Int J. Clin. Pr. 2008;62:1581–1587. doi: 10.1111/j.1742-1241.2008.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
FASTQ files of our dataset are available at the DNA Data Bank of Japan (DDBJ) under the accession numbers of “DRA009229” for PD and controls and “DRA012438” for PD and conrols, and “DRA009322” for iRBD and “DRA011417” for iRBD.