Abstract
Lung ultrasound (LUS) has rapidly emerged in COVID-19 diagnosis and for the follow-up during the acute phase. LUS is not yet used routinely in lung damage follow-up after COVID-19 infection. We investigated the correlation between LUS score, and clinical and laboratory parameters of severity of SARS-COV-2 damage during hospitalization and at follow-up visit. Observational retrospective study including all the patients discharged from the COVID-19 wards, who attended the post-COVID outpatient clinic of the IRCCS Policlinico San Matteo in April–June 2020. 115 patients were enrolled. Follow-up visits with LUS score measurements were at a median of 38 days (IQR 28–48) after discharge. LUS scores were associated with the length of hospitalization (p < 0.001), patients’ age (p = 0.036), use of non-invasive ventilation (CPAP p < 0.001 or HFNC p = 0.018), administration of corticosteroids therapy (p = 0.030), and laboratory parameters during the acute phase (WBC p < 0.001, LDH p < 0.001, CRP p < 0.001, D-dimer p = 0.008, IL-6 p = 0.045), and inversely correlated with lymphocyte count (p = 0.007). We found correlation between LUS score and both LDH (p = 0.001) and the antibody anti-SARS-CoV-2 titers (p value = 0.008). Most of these finding were confirmed by dichothomizing the LUS score (≤ 9 or > 9 points). We found a significantly higher LUS score at the follow-up in the patients with persistent dyspnea (7.00, IQR 3.00–11.00) when compared to eupnoeic patients (3.00, IQR 0–7.00 p < 0.001). LUS score at follow-up visit correlates with more severe lung disease. These findings support the hypothesis that ultrasound could be a valid tool in the follow-up medium-term COVID-19 lung damage.
Keywords: Imaging, Lung, Radiology, Viral infection, Ultrasound
Introduction
Sonographic evaluation of COVID-19-induced lung damage has played a significant role since the first phases of the pandemic. Imaging follow-up of lung lesions after moderate-to-severe symptomatic COVID-19 has been suggested to help identifying patients who deserve a tighter clinical and rehabilitative care and who could benefit from further investigations or treatment [1]. Chest X-ray and Chest Computed Tomography Scan (CT-Scan) are the main imaging techniques used for such follow-up purposes [2] because of their wide use and the growing experience on SARS COV-2 infection patterns. Nevertheless, these techniques use ionizing radiation and their widespread use in large numbers of patients leads to a potential radiological harm [3]. Lung Ultrasound (LUS) has been widely used in emergency settings as a low cost, harm free and practical tool for ruling out respiratory failure differential diagnosis. LUS has rapidly emerged in COVID-19 diagnosis process and follow-up especially in the acute phase, when a rapid assessment of lung involvement is required at the critically ill patients’ bedside [4, 5]. However, LUS is not yet used routinely in follow-up of moderate-to-severe COVID-19 infection. The well-known operator dependability of ultrasound techniques can be at least partially addressed using scores and standardized lung scan procedures, as proposed by Soldati et al. [6]. In this study, we investigate the correlation between LUS scores and the severity of COVID-19 infection adding elements for the reliability of LUS as a potential substitute to chest X-ray and CT scan in COVID-19 patients’ follow-up.
The aim of the study is to evaluate the association between LUS score (considered on a continuous scale or dichotomized) and clinical and laboratory parameters of severity of SARS-COV-2 damage during hospitalization and at follow-up visit.
Materials and methods
Patients
In this retrospective study, we enrolled all consecutive outpatients, discharged from the Internal Medicine, Pneumology and Infectious Diseases wards, who attended the post-COVID outpatient clinic of the IRCCS Policlinico San Matteo, starting from April 27, 2020 until June 10, 2020. The inclusion criteria were having had an infection by SARS-CoV-2 during the first wave of the pandemic, an age greater than 18 years, and the ability to provide informed consent. Denial of informed consent was the only exclusion criterium. A total of 115 patients meeting the inclusion criteria were enrolled.
Demographic and clinical parameters
Clinical parameters considered were age, sex, length of hospital stay, time from discharge to outpatient evaluation, type of ventilation required (HFNC, CPAP), medical therapy during admission (corticosteroids, remdesivir, tocilizumab, hyperimmune plasma, heparin), and comorbidities already present upon admission. The above-mentioned parameters were obtained from the medical records.
Blood tests
Worst biomarkers levels were considered as follows:
Sonographic technique
The outpatient sonographic evaluation was performed by experienced sonographers (at least 3 years of lung ultrasound practice) using an Esaote MyLab Twice with a convex probe. According to the protocol, an abdominal preset was applied with the following features: 10 cm depth, total gain 50%, and placement of the focus on the pleural line.
LUS score
Patients underwent a sonographic assessment following the protocol proposed by Soldati et al. [6]. Briefly, 14 different areas of the chest wall were evaluated, and ten (10) s clips were recorded to allow further revision. Patients were in supine position, with a 45°angle grade of the bed for anterior and lateral scans and sitting position for posterior scans. Sonographers avoided cosmetic filters or specific presets, and did not use harmonic corrections, nor contrast nor compounding, the saturation phenomena were avoided by setting the gain and reducing mechanical index. During the sonographic examination, possible pleural effusion or cava vein dilatation was assessed. Each scan was associated with a given score from 0 to 3 according to the following:
Score 0: Regular pleural line. “A” lines are visible
Score 1: Small interruptions of the pleural line, isolated vertical artifacts underlying.
Score 2: Significant pleural line irregularities, pleura looks interrupted. Subpleural consolidations are documented associated with small white lung areas as pre-consolidative phase.
Score 3: Completely altered pleural line, diffuse white lung and tissue-like patterns.
At the end of the sonographic examination, the total LUS score was calculated by the sum of the 14 scores. Every clip was collected and consequently evaluated by an experienced sonographer with more than 15 years of experience in lung ultrasound.
Spirometry
The “Sensor Medics V MAX 22” spirometer was used at the post-COVID outpatient control for respiratory function tests (FEV1, FVC, FEV1/FVC, and DLCO measurements). Data analysis was conducted according the ATS/ERS guidelines [9, 10].
Statistical analysis
We used the Stata software, version 16.1, for all computations (StataCorp, College Station. TX, USA). All tests are two-sided and a p value < 0.05 is considered statistically significant. We described continuous data with the mean and standard deviation (SD) or the median and interquartile range (IQR) and categorical data as counts and percent. For the purpose of the analysis, to identify patients with the highest lung impairment, we dichotomized the total LUS score at its upper quartile (≤ 9/> 9). We computed the Spearman R and its 95% confidence interval (95% CI) to measure the association of continuous variables and the total LUS score and the Mann–Whitney U test to compare the LUS score between categorical variables. Finally, we compared patients with high and low LUS score with the Mann–Whiney U test or the Fisher exact test, for the continuous and categorical variables, respectively.
Ethical aspects
Patients signed informed consent on a form approved by the Ethical Committee of IRCCS Policlinico San Matteo, Pavia.
Results
Among the 115 patients enrolled in the study, 88 (76.5%) were male; age of patients and number of days between symptom onset, hospitalization, discharge, and follow-up are summarized in Table 1. Outpatients complained fatigue at the follow-up visit in 57% of cases and dyspnea in 37% of cases; patients less frequently (< 4%) complained other symptoms like chest pain, palpitations, cough, anosmia, and gastrointestinal disorders. Patients' median LUS score was 4, with an interquartile range (IQR) of 2–9. The distribution of LUS scores at the follow-up visit is represented in Fig. 1.
Table 1.
Description of enrolled population: age, number of comorbidities, days between symptom onset, hospitalization, discharge, and follow-up
| Age, mean ± SD | 62 ± 11.5 |
|---|---|
| Number of comorbidities, median (IQR) | 2 (1–2) |
| Days between symptom onset and hospitalization, median (IQR) | 7 (6–10) |
| Days of hospitalization, median (IQR) | 13 (10–21) |
| Days between discharge and follow-up, median (IQR) | 38 (28–48) |
| Days between hospitalization and follow-up, median (IQR) | 57 (43–65) |
SD standard deviation, IQR interquartile range
Fig. 1.
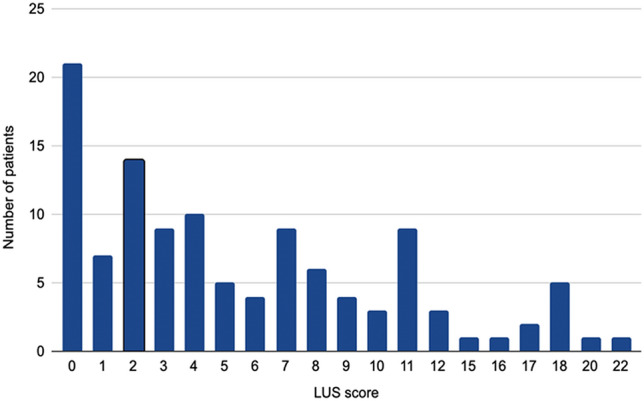
Distribution of the LUS score at the follow-up visit in enrolled patients
By dividing patients according to the LUS score (using the 75th percentile of 9 as the threshold), we obtained a group of 26 patients with LUS score > 9 and a group of 89 patients with LUS score ≤ 9. Median LUS score was higher in males than in females (5.0 IQR 2.00–9.50 vs. 3.00 IQR 1.00. 5.00), but not significantly (p value = 0.132). The proportion of patients with LUS score greater than 9 was also not significantly different among males and females (respectively, 25% and 15%, p value = 0.308). The correlation between age, length of stay, discharge-follow-up time, and LUS score by Spearman’s RHO and dichotomizing at LUS score more than 9 is summarized in Table 2.
Table 2.
Correlation between age, days of hospitalization and discharge-follow-up time, age expressed as mean ± standard deviation, length of stay, and discharge-follow-up time expressed in median (interquartile range)
| Variable | Spearman RHO (95% CI) | p Value | LUS score > 9 | LUS score ≤ 9 | p Value |
|---|---|---|---|---|---|
| Age (N = 115) | 0.195 (0.013–0.365) | 0.036 | 65.7 ± 11.2 | 61.4 ± 11.5 | 0.116 |
| Length of stay (days) (N = 115) | 0.34 (0.163–0.489) | < 0.001 | 16.50 (12.00–27.00) | 12 (9–18) | 0.022 |
| Discharge-follow-up time (days) (N = 114) | − 0.218 (− 0.386 to − 0.035) | 0.020 | 29.5 (20.0–40.0) | 40 (31.00–48.50) | 0.008 |
Among the risk factors, only onco-hematological disease at the admission is associated with the LUS score (p = 0.040). No association was observed with other risk factors nor with comorbidities (i.e., smoking status, hypertension, cardiovascular disease, cerebrovascular diseases, diabetes, chronic kidney diseases, asthma, COPD, tumors, immunodepression, iatrogenic immunosuppression, rheumatological diseases, psychiatric diseases, non-vascular neurological diseases, endocrine diseases excluding diabetes, and obesity).
Median LUS score at the follow-up visits for patients requiring non-invasive ventilation (CPAP and high-flow nasal cannula) is summarized in Table 3.
Table 3.
Average of the LUS score according to the ventilator therapies used at hospitalization
| Type of non-invasive ventilation | ||||
|---|---|---|---|---|
| CPAP | HFNC | |||
| Yes = 44 | No = 71 | Yes = 45 | No = 70 | |
| LUS score (median, IQR) | 6.00 (3.00–11.00) | 3.00 (0.00–8.00) | 5.00 (3.00–11.00) | 3.00 (0.00–8.00) |
| p Value | 0.008 | 0.018 | ||
CPAP continuous positive airway pressure, HFNC high-flow nasal cannula oxygen therapy, IQR interquartile range
Comparable results were observed by dichotomizing the LUS score > 9 and ≤ 9 (p value 0.01 for CPAP and 0.04 for HFNC). No significant differences were found between average LUS scores for patients undergoing different medical treatments (Tocilizumab, Remdesivir, Plasma, Steroid, Heparin in prophylactic dosage, Heparin in therapeutic dosage), except for those who were administered corticosteroids (median LUS score of 7.3 ± 5.6 vs. 5.1 ± 5.2 in untreated patients, p value = 0027. Most of the laboratory parameters (the worst value during hospitalization) showed a significant correlation with LUS score at the time of the first follow-up visit, as shown in Table 4. Similar significant associations were observed dichotomizing the LUS score (> 9 and ≤ 9) except for lymphocyte count and D-dimer. Conversely, blood tests performed in the follow-up visit did not show any correlation with LUS score except for LDH (Spearman’s Rho 2.93, p value = 0.0015), even by dichotomizing the score at > 9 o ≤ 9 (p = 0.035). We furthermore observed a correlation between the antibody titers and LUS score at follow-up (N = 91, Rho = 0.277, p value = 0.0078), also when dichotomized by LUS score > 9, p = 0.037. A higher median LUS score was assessed in patients with dyspnea ((7.00, IQR 3.00–11.00) when compared to eupnoeic patients (3.00, IQR 0–7.00 p < 0.001). The persistence of fatigue showed a non-significant association with the LUS score (6.62 ± 5.81 vs. 4.63 ± 4.67, p = 0.079). By dichotomizing the LUS score (> 9 vs. ≤ 9), we did not observe any significant correlation for persistent dyspnea or fatigue (p = 0.113 and p = 0.184, respectively).
Table 4.
Correlation between biochemical parameters (WBC, lymphocytes, LDH, CRP, D-dimer, fibrinogen, IL-6) at the worst value during hospitalization and LUS score at follow-up
| N | Rho spearman (95% CI) | p Value | |
|---|---|---|---|
| WBC (× 103/µl) | 114 | 0.333 (0.157–0.489) | < 0.001 |
| Lymphocytes (× 103/µl) | 114 | − 0.253 (− 0.417 to − 0.072) | 0.007 |
| LDH (mU/ml) | 109 | 0.343 (0.165–0.499) | < 0.001 |
| CRP (mg/dl) | 110 | 0.3151 (0.136–0.474) | < 0.001 |
| D-dimer (ug/l) | 24 | 0.5289 (0.159–0768) | 0.008 |
| Fibrinogen (mg/dl) | 27 | 0.1175 (− 0.275 to 0.476) | 0.559 |
| IL-6 (pg/ml) | 16 | 0.5060 (0.014–0.801) | 0.045 |
We did not find a correlation between LUS score and spirometry variables among the 50 patients that underwent the respiratory function test, as shown in Table 5. Criteria for execution of spirometry were persistence of dyspnea, evidence of oxygen saturation below 95% at rest, and necessity of oxygen support. Similar results were achieved by dichotomizing the LUS score by 9 (FEV1 p = 0.348, FEV1/FVC p = 0.630, DLCO p = 0.325).
Table 5.
Correlation of spirometric parameters with LUS score at follow-up
| N | Rho spearman (95% CI) | p Value | |
|---|---|---|---|
| FEV1 (% pred) | 50 | − 0.028 (− 0.304 to 0.252) | 0.848 |
| FEV1/FVC (%) | 50 | − 0.076 (− 0.347 to 0.207) | 0.599 |
| DLCO (%) | 49 | − 0.101 (− 0.372 to 0.185) | 0.488 |
FEV-1 forced expiratory volume in the first second, FVC forced vital capacity, DLCO diffusing capacity of lung for CO (carbon monoxide)
Discussion
According to our results, length of hospitalization was significantly correlated with the total LUS score. To explain this finding, it can be reasonably assumed that a longer hospital stay indicates a more severe disease and therefore a more critical lung damage, persisting up to follow-up.
An inverse correlation was found between LUS score and time from discharge to the outpatient visit, similarly to other studies [11] that described a complete resolution of lung damage (21% at 1 month, 69% at 3 months after hospital discharge). These findings highlight the progressive recovery process from COVID-19 lung damage after weeks and months from the acute disease. Within our study population, age was significantly correlated with total LUS score, and we speculate that elderly might likely be affected by several comorbidities (i.e., heart failure, lung diseases) and faced a more severe form of SARS COV-2 infection [12, 13].
Our data also suggest a statistically significant correlation between non-invasive ventilation (CPAP or HFNC) and LUS score at follow-up control. We speculate that patients requiring CPAP non-invasive ventilation suffered from more severe pneumonia; hence, lung damage sequelae might persist longer at a higher degree; a less likely theory is that parenchymal alterations might represent CPAP-induced lung damage; this hypothesis is even less likely for HFNC that less likely harm the lungs.
We did not consider the consequences of mechanical ventilation on lungs, because in this population, only 7 patients underwent oro-tracheal intubation and the mean age of that subgroup was lower than the mean age of the rest of the group undergoing other types of oxygen treatments. Moreover, intubated patients had longer hospital stay and a longer lapse to control visit; therefore, lung damage could have had more time to improve.
Among the COVID-19 infection treatments analyzed, only corticosteroids therapy correlates with LUS score, also when dichotomized, probably because steroids were mainly administered to patients with severe respiratory impairment before it became a standard treatment. Similarly, in those initial phases of the COVID-19 pandemic, patients with critical lung involvement were administered higher anticoagulant doses of heparin (LMWH) and that could explain the positive correlation with LUS score.
A very interesting finding is that almost all blood inflammation and lung damage biomarkers (WBC, CRP, LDH, D-Dimer, IL-6) correlate with LUS score, most of these also when score was dichotomized by 9 points. Only lymphocyte count had an inverse relationship with LUS score, which further confirms the hypothesis that LUS score is a valid measure of lung damage: in fact, lower lymphocyte count is associated with more severe COVID-19 as demonstrated in other studies [14, 15].
Previous studies showed that more severe pneumonia patients develop higher titers of anti-SARS-CoV2 [7, 8], and also in our limited data, we observe that neutralizing antibodies titer at the outpatient visit strongly correlates with higher LUS scores.
LUS score correlated also with persistent dyspnea at follow-up. When LUS score was dichotomized by 9, results did not show any relevant difference among the subgroups; on the other hand, when dichotomized by 7, LUS score allowed the identification of patients with and without dyspnea (p = 0.006). Using the same cut-off, fatigue appeared significantly more frequent in the > 7 LUS score patient subgroup (p = 0.041).
In contrast, spirometry parameters did not seem in correlation with LUS score; potential explanations for this are the following: a selection bias for more symptomatic patients that underwent the testing, the non-obstructive pattern of COVID-19 lung damage, and a similarity to other radiology test in which the lung imaging persists altered after lung function has restored [16].
Indeed, our study has some limitations. This is an observational study that can therefore only provide speculations for further studies. Population of the study was quite selected, as we included patients discharged alive and able to attend outpatient visits a probable bias toward more fit patients; nevertheless, the study shows interesting correlations between COVID-19 severity and LUS score that could have been even more evident if a wider range of severity of the disease had been represented. The follow-up visit was performed at various time intervals after discharge (median of 38 days, IQR 28–48 days) and, as we have shown that time influences the persistence of lung damage; nonetheless, this demonstrates that the associations of COVID-19 severity markers with LUS score remain true in a follow-up setting with a timing not strictly controlled. Additionally, only a minority of patients underwent CT scan, and thus, we were unable to compare this datum to the LUS score. The time-to-CT widely varied among patients, making it impossible to interpret any meaningful correlation with LUS sore. Only more symptomatic patients underwent CT, thus not representing the entire cohort. Future studies should focus on comparing LUS with CT scan. Finally, our data missed some other possible COVID-19 severity indexes (as P/F during admission or worst blood oxygen saturation); however, the multiple and consensual associations of many different severity markers with LUS score seems to us sufficiently convincing.
Our findings consistently suggest that LUS score at follow-up visit (1–2 months after the hospital admission) correlates with more severe lung disease, according to clinical and biochemical markers both during acute phase and at the follow-up. These findings support the hypothesis that ultrasound represents a valid tool for assessing medium-term COVID-19 lung damage. Further studies are needed to evaluate the utility of sonography in the long-term follow-up of COVID-19 patients. Our findings suggest that a possible cut-off to identify patients with more severe persistent lung damage could be in the 7–9 points range of LUS score. This cut-off needs to be prospectively validated in a large cohort of COVID-19 patients.
Abbreviations
- CPAP
Continuous positive airway pressure
- CRP
C-reactive protein
- CT
Computed tomography
- COVID-19
Coronavirus disease 2019
- DLCO
Diffusing capacity of lung for CO (carbon monoxide)
- FEV-1
Forced expiratory volume in the first second
- FVC
Forced vital capacity
- HFNC
High flow nasal cannula oxygen therapy
- LDH
Lactic de-hydrogenase
- LMWH
Low-molecular-weight heparin
- IL-6
InterLeukin-6
- LUS
Lung ultrasound
- WBC
White blood cells
Author contributions
All authors participated in the drafting of the manuscript or critical revision of the manuscript for important intellectual content and provided approval of the final submitted version. All authors approved the final version of the paper.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study was approved by the local ethics committee (Fondazione IRCCS Policlinico San Matteo) and all patients provided written informed consent.
Human and animal rights
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tiziano Perrone and Francesco Falaschi are co-first authors.
References
- 1.Lee KS, Wi YM. Residual lung lesions at 1-year CT after COVID-19. Radiology. 2021;302:720. doi: 10.1148/radiol.2021212350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larici AR, Cicchetti G, Marano R, et al. COVID-19 pneumonia: current evidence of chest imaging features, evolution and prognosis. Chin J Acad Radiol. 2021;4:229–240. doi: 10.1007/s42058-021-00068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 4.Gaspardone C, Meloni C, Preda A, et al. Lung ultrasound in COVID-19 a role beyond the acute phase? J Ultrasound Med. 2021;40:503–511. doi: 10.1002/jum.15425. [DOI] [PubMed] [Google Scholar]
- 5.Peixoto AO, Costa RM, Uzun R, et al. Applicability of lung ultrasound in COVID-19 diagnosis and evaluation of the disease progression: a systematic review. Pulmonology. 2021;27:529–562. doi: 10.1016/j.pulmoe.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19: a simple, quantitative, reproducible method. J Ultrasound Med. 2020;39:1413–1419. doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen CB, Jarlhelt I, Pérez-Alós L, et al. SARS-CoV-2 antibody responses are correlated to disease severity in COVID-19 convalescent individuals. J Immunol. 2021;206:109–117. doi: 10.4049/jimmunol.2000898. [DOI] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS task force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.Graham BL, Brusasco V, Burgos F, et al. ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;3:49. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Píriz A, Tung-Chen Y, Jiménez-Virumbrales D, et al. Importance of lung ultrasound follow-up in patients who had recovered from coronavirus disease 2019: results from a prospective study. J Clin Med. 2021;10:3196. doi: 10.3390/jcm10143196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smorenberg A, Peters EJ, van Daele P, et al. How does SARS-CoV-2 targets the elderly patients? A review on potential mechanisms increasing disease severity. Eur J Intern Med. 2021;83:1–5. doi: 10.1016/j.ejim.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherwani S, Khan MWA. Cytokine response in SARS-CoV-2 infection in the elderly. J Inflamm Res. 2020;13:737–747. doi: 10.2147/JIR.S276091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavakolpour S, Rakhshandehroo T, Wei EX, et al. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Park SS, Kim TY, et al. Lymphopenia as a biological predictor of outcomes in COVID-19 patients: a nationwide cohort study. Cancers. 2021;13:471. doi: 10.3390/cancers13030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruns AH, Oosterheert JJ, El Moussaoui R, et al. Pneumonia recovery: discrepancies in perspectives of the radiologist, physician and patient. J Gen Intern Med. 2010;25:203–206. doi: 10.1007/s11606-009-1182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


