Abstract
Statins are known to block cholesterol synthesis in the liver. They also exhibit non-lipid pleiotropic effects due to the inhibition of protein prenylation, thereby modulating various signaling pathways of cellular homeostasis and integrity. Both lipid control and pleiotropic action of statins are clinically used, mainly for treatment of hypercholesterolemia and primary and secondary prevention of cardiovascular diseases. Because the prescription of statins is increasing and statin therapy is often lifelong, in particular in patients with other risk factors, safety issues being associated with polymorbidity and polypragmasia as well as the persistence with and adherence to statins are specific points of attention of clinicians and clinical pharmacologists. Furthermore, because skeletal myocytes have a cholesterol inhibitory sensitivity greater than hepatocytes, a choice of an appropriate statin based on its lipophilicity and the associated likelihood of its side effects on skeletal muscle cells and bone is warranted in such polymorbid patients. These approaches can effectively modulate the risk: benefit ratio and highlight a need for personalized therapy as much as possible, thereby minimizing risk of discontinuation of therapy and poor compliance.
Keywords: Statins, Pleiotropic action, Side effects, Lipophilicity, Skeletal muscles, Bone
Introduction
Statins are the most used class of lipid-modifying agents. In the United States, nearly 30% of adults 40 years and older are on a statin. In the world, an estimated 145.8 million people took statins in 2018 and the consumption of statins increased by 3.99% from 2013 to 2018 [1]. It is predicted that statin use will have a growing trend worldwide, although some specifications related to region, subregion, and country are expected.
Statins are powerful hypolipidemic drugs and called as “new penicillin” and “new aspirin”
Different statins have distinct hypolipidemic properties, with pravastatin and simvastatin providing less LDL-lowering power (25–35% reduction in LDL at 20 mg dosing) than newer statins, like rosuvastatin and atorvastatin (40–50% reduction in LDL at 20 mg dosing) [2]. Based on across-dose analyses, rosuvastatin (10–80 mg) has shown the most potent hypolipidemic activity which is followed by atorvastatin (10–80 mg), simvastatin (10–80 mg) and pravastatin (10–40 mg) [3]. The reduction of LDL levels has been indicated to be independent of the patient characteristics and doubling the dose of any statin has been able to generate on average only a further 6% decrease in LDL cholesterol [2, 4]. In addition to the effects on LDL levels, statins also increase HDL cholesterol levels by 6–12% and lower triacylglycerol levels [3]. The mechanism of action of hypolipidemic effects of statins involves the inhibition of the hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase) with resultant feedback effects on the transcription factor SREBP-2 (sterol-regulatory-element-binding protein 2) promoting the synthesis of LDL receptors on the surface of hepatocytes [5, 6]. As a result, the circulating LDL lipopoproteins are cleared from the blood. Statins-mediated decrease in the production of apolipoprotein B, which is the major constituent of the atherogenic particles, and the increase in the production of apolipoprotein A, which is the major apolipoprotein of HDL particles, also contribute to the hypolipidemic effects of these drugs [7]. Due to these effects of statins on lipid metabolism, they arrest the atherosclerosis process, and prevent further atherosclerotic plaque formation. Thus, statins have been suggested to be to atherosclerosis what penicillin was to infectious diseases [8]. Furthermore, because they have been shown to reduce cardiovascular events, including myocardial infarction, stroke, and death, statins have also been named as “new aspirin” [9]. Indeed, it has been reported that lowering LDL cholesterol by 2 mmol/L with any effective statin regimen can prevent major vascular events in about 10% patients at high risk of heart attacks and strokes (secondary prevention) and in 5% patients at lower risk (primary prevention) [10, 11].
Pleiotropic action of statins
Lipid modification alone cannot explain all benefits of statins on the cardiovascular system and non-lipid pleiotropic effects of statins significantly contribute to their cardioprotection. Namely, the inhibition of the synthesis of isoprenoids through a common pathway as cholesterol biosynthesis, that prevents post-translational modification of small GTP-binding proteins of the Ras/Rho family (Ras, Rac and Rho), has been shown to affect endothelial function [12, 13], oxidative stress [14–16], inflammation [17, 18], and thrombogenesis [19, 20]. Thus, reduction of the cardiovascular mortality and morbidity due to statin therapy can be a result of vascular endothelial function-improving, anti-inflammatory, anti-oxidant, platelet aggregation-inhibiting effects and plaque-stabilizing effects besides lowering cholesterol levels. Some of these pleiotropic effects can also modulate the pathomechanisms of cerebrovascular diseases, such as stroke, Alzheimer's disease, and Parkinson’s disease. However, both scenarios—the improvement of cognitive impairment and reversible decline in cognitive function due to statin therapy have been documented in clinical trials [21–24]. A very recent meta-analysis has indicated the absence of statins-induced neurocognitive risk and highlighted that treatment with statins shall be considered and not discontinued in elderly patients for primary and secondary prevention of cardiovascular disease [25]. Furthermore, it has been shown that statins due to pleiotropic action make the cancer cells more prone to apoptosis, and that inhibit their growth and differentiation, thereby eliciting the antitumor effects [25–27]. However under ischemic conditions, statins have been reported rather to retard apoptotic cell death and thereby mitigate post-ischemic organ damage [16, 28, 29]. Moreover, potential beneficial effects of statins due to pleiotropy have also been studied in diabetes mellitus, HIV [30], and multiple sclerosis [31]. It should be, however, mentioned that there was an intensive debate on the statin-induced modulation of glucose metabolism as a new-onset diabetes had occurred [32, 33]. The effects of statins have also been a subject of investigation in patients with COVID-19 infection. It has been indicated that statin use reduces risk of progressing to severe illness and in-hospital death in COVID-19 patients due to suppressing viral entry and replication, anti-inflammatory, anti-oxidative and immunomodulatory, as well as anti-thrombotic effects [34–36].
Classification of statins as the over-the counter drugs
The impressive cardioprotective effects of statins have instigated discussion of the re-classification of low doses of these drugs as the over-the counter (OTC) products. The sponsors of the applications proposed that a single-dose OTC statin should be indicated for individuals (i) who qualify for primary prevention under the third Adult Treatment Panel (ATP III) of the National Cholesterol Education Program, (ii) who are with multiple (≥ 2) risk factors and a 10-year coronary heart disease risk ≤ 20%, (iii) and who are with no contraindications to statins, and with no favorable likelihood of experiencing benefit versus risk [37, 38]. Initially, a low-dose of pravastatin and lovastatin had failed to secure OTC approval and a pharmaceutical company reapplied to the US Food and Drug Administration (FDA) for its non-prescription preparation in the United States. In 2004, a low-dose formulation of simvastatin (10-mg tablet) was approved as an OTC product in the United Kingdom. The anticipated effects of simvastatin 10 mg was a reduction of risk of a first major coronary event, such as non-fatal myocardial infarction or coronary artery disease death. It was estimated that by using such OTC products, an approximate 30% reduction in LDL cholesterol level results in an 11% diminution in risk of a major coronary artery disease event after 1 year of therapy, 24% after 2 years, and 33% after 3 years [39]. In spite of many positive ideas and ambitions for OTC classification of statins, various critical issues were raised. For instance, critics questioned the clinical efficacy of such a low dosage approved. Furthermore, the product marketing was challenged by the consumers groups. Lastly, it was retrospectively reported that OTC availability unlikely impacted on the level of general practice led management of patients at risk of coronary events. [40]. In addition to these factors, an increasing number of newly recognized adverse effects of statins forced the regulatory authorities to re-consider the OTC approval of statins and keep these hypolipidemic drugs as the prescription drugs in the majority of countries. In this regard, it can be mentioned that side effects of statins are usually considered as effects of a whole class while in certain cases they should be rather assigned to some types of statins only. In fact, side effects of an individual statin are determined by its pharmacokinetic properties and its chemical structure. This is of a particular importance in risk patients with other co-morbidities, in which the adherence to a certain statin over another one can be affected. In this paper, we address some side effects of statins on the skeletal muscle and bone and indicate their lipophilicity-associated individual differences. Furthermore, this review is also intended to help clinicians to manage patients on statins therapy in order to minimize the occurrence of side effects while not discontinuing the treatment of hypercholesterolemia and/or prevention of cardiovascular diseases.
Lipophilicity and chemical structure of statins
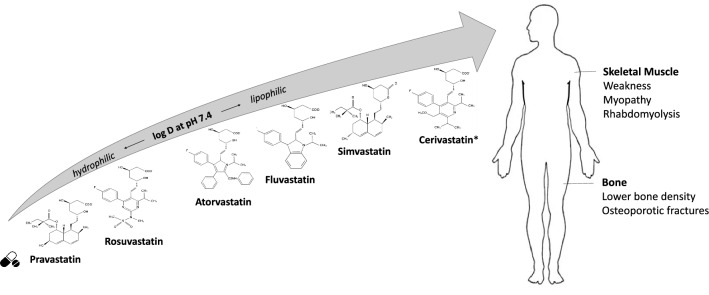
Dihydroxyheptanoic acid unit and a ring system with different side substituents are the two main structural components of statins. Within the former component, the modified hydroxyglutaric acid is similar to the endogenous substrate HMG-CoA and it is called the HMG-CoA analogue. The closed chain, which can be partially reduced naphthylene (like in lovastatin, simvastatin, and pravastatin), pyrrole (atorvastatin), indole (fluvastatin), pyrimidine (rosuvastatin), and quinoline (pitavastatin), is a part of the drug structure that binds to the target enzyme—HMG-CoA reductase [41]. Conversely, side chains determine the drug lipophilicity/hydrophilicity. Atorvastatin, fluvastatin, lovastatin and simvastatin are relatively lipophilic drugs, whereas pravastatin and rosuvastatin are more hydrophilic due to a polar hydroxyl and methylsulfonamide group [42, 43]. Cerivastatin was the most lipophilic statin—the statin with the highest oil:water ratio, while pravastatin is the most hydrophilic statin [44, 45]. Being aware of the complex physical and chemical properties of statins, which can affect the drug interaction with biological structures of cells (e.g., membrane diffusion, intracellular translocation, etc.), it is obvious that statins have different rate of drug absorption, organ penetration as well as action in a respective organ. Namely, hydrophilic statins require the carrier-mediated uptake into the liver, with the higher transport affinity and efficiency for rosuvastatin than that of pravastatin [46]. In contrast, lipophilic statins are capable of passive diffusion through the cell membrane indicating the decrease in their hepatoselectivity as they are also able to passively diffuse into other tissues [45]. Thus, lipophilicity/hydrophilicity is important for statin bioactivity as well as for bioavailability determined by drug metabolism, clearance and drug accumulation within the body. Pravastatin and rosuvastatin exhibit the efficacy and affinity mainly for the hepatic tissue, and a reduced potential for the uptake by peripheral cells. In contrast, non-hepatic cells are likely more exposed to simvastatin, atorvastatin, and fluvastatin than to hydrophilic statins. This paradigm can be adopted for both beneficial, therapeutic and adverse effects of statins. Beneficial non-lipid effects of statins due to affecting various cellular signaling pathways in non-hepatic tissues have been intensively studied and are nicely reviewed elsewhere [47–50]. In the following section of this manuscript, some side effects of statins on the skeletal muscle and bone, which are likely dependent on drug lipophilicity, are discussed. They are schematically drawn in Fig. 1.
Fig. 1.
A schematic picture indicating the increasing partition coefficients (water-octanol) at pH = 7.4 of some statins and highlighting the more incidence of side effects on the skeletal muscle and bone by taking lipophilic, rather than hydrophilic statins. Cerivastatin* was withdrawn from market in 2001
Some side effects of statins on skeletal muscle with respect to their lipophilicity
Statin muscle symptoms are well-known side effect of statins as the skeletal myocytes have cholesterol inhibitory sensitivity 40-times greater than hepatocytes [51]. A paper analyzing case reports of statin-induced rhabdomyolysis has indicated the cases associated mainly with simvastatin (40 mg/day) and atorvastatin therapy (10 mg/day). The majority of the statin-induced rhabdomyolysis cases occurred when simvastatin and atorvastatin were taken concomitantly with other medications, such as fibrates [52]. Cerivastatin is another statin known to produce various forms of myotoxicity ranging from mild forms, such as myopathy and myalgia, to fatal rhabdomyolysis. A combination therapy of cerivastatin with cyclosporine [53], bezafibrate [54], gemfibrozil [55, 56], influenza vaccine [54] as well as monotherapy of cerivastatin [57, 58] were reported to cause rhabdomyolysis. The incidence of cerivastatin-induced rhabdomyolysis appeared to be tenfold greater as compared to other statins [59]. About 100 rhabdomyolysis-related deaths were found to be associated with cerivastatin therapy till its deregistration by manufacturer in 2001. It can be, however, mentioned that cerivastatin exhibited remarkable hypolipidemic action. It was about 250-fold more potent than fluvastatin, 20-fold more potent than atorvastatin and 5.5-fold more potent than rosuvastatin. At a dose of 0.025 mg/day to 0.8 mg/day, cerivastatin caused LDL cholesterol decrease of 11.0–40.8%. These hypolipidemic effects were linear dose-related [60].
Clinical studies have inconsistently shown the association between statin use and risk of fracture. Both the prevention and higher probability of fractures due to statin therapy have been reported [61–67]. Patients taking high-potency statins, like atorvastatin and rosuvastatin, have been shown to be at a lower risk of developing osteoporotic fractures than those taking simvastatin [65]. In contrast, but in line with a concept presented in this paper referring to a higher probability of lipophilic statins to produce side effects on the non-hepatic tissue, it has been reported that females on lipophilic statins had statistically lower bone mineral density than those on hydrophilic statins. In addition, a high dose of atorvastatin reduced bone mineral density more than a low dose [68]. From our clinical experience, it is also apparent that atorvastatin and simvastatin increased the risk of fractures. Some studies have indicated no effects on the risk of fractures due to statin therapy [62, 63]. Contradictory to these observations, the increased increment in bone mineral density due to statin therapy has also been reported in some risk groups. In this regards, patients with osteoporosis and metabolic syndrome [66] and patients with diabetes mellitus would likely benefit from statin therapy [69]. Targeting cellular signaling pathways of inflammation and oxidative stress, which are common pathophysiological mechanisms for osteoporosis and metabolic syndrome and diabetes, can provide the rationale on multifactorial benefits in these specific subgroups of patients.
The above-discussed controversy on statins-mediated action on fractures could be explained by several factors. Firstly, many meta-analyses have been performed in elderly, and polymorbid patients. Thus, other co-existing risk factors, such as sedentary lifestyle, hypercholesterolaemia, disturbed calcium homeostasis, body mass index, age, gender, etc., in addition to polypragmasia could underlie heterogeneous and inconsistent data. A type of a statin, being either of the hydrophilic or lipophilic chemical structure, was also not specified in many of the above-discussed studies [61–66]. In fact, the enrollment of all statins irrespective of a type, and dose, instead of a comparative analysis among individual statins could also significantly affect findings.
Although side effects of statins on the skeletal muscles and bone can be a result of various above-discussed factors, lipophilicity and thereby non-selectively affecting non-hepatic tissues and increasing risk of toxicity in these tissues could play an important role. Thus, the administration of hydrophilic versus lipophilic statin should be carefully considered in this regard. This approach can also impact on the persistence with and adherence to statins, which are sex-dependent, and poor with a lower proportion for the primary prevention of cardiovascular diseases compared to secondary prevention populations [70–72].
Conclusion
In conclusion, statins-mediated pleiotropic effects largely account for clinical benefits. Because there is a growing need for statins to control high cholesterol and reduce the risk of cardiovascular disease a special attention is given on their safety in view of a short and long perspective. In addition, because there is the correlation between atherosclerosis and osteoporosis, independent of age, it is evident that many patients admitted to hospital at the orthopedics and traumatology department are on statin. Being mindful of all these facts, a personalized approach is highly warranted in the management of these patients. Besides generally known and advised methods, such as motivation and better communication between clinician and patient, the personalized intervention might include the recommendations which individual statin shall be used to achieve better compliance and not discontinuation of therapy.
Acknowledgements
The authors thank Dr. Horvath for his help with a graphical illustration.
Author contributions
Both authors contributed to the study conception and design and approved the final manuscript.
Funding
This study was supported by Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and The Slovak Research and Development Agency (APVV-20–0242, APPV-15–0607, VEGA 1/0016/20).
Data availability
Data availability statement is not applicable for this review.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is a review article and ethical approval is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrey Svec, Email: asvec@ru.unb.sk.
Adriana Adameova, Email: adameova@fpharm.uniba.sk.
References
- 1.Blais JE, Wei Y, Yap KKW, Alwafi H, Ma TT, Brauer R, Lau WCY, Man KKC, Siu CW, Tan KCB, Wong ICK, Wei L, Chan EW. Trends in lipid-modifying agent use in 83 countries. Atherosclerosis. 2021;328:44–51. doi: 10.1016/j.atherosclerosis.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, Cain VA, Blasetto JW, Group SS. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* trial) Am J Cardiol. 2003;92:152–60. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls SJ, Brandrup-Wognsen G, Palmer M, Barter PJ. Meta-analysis of comparative efficacy of increasing dose of atorvastatin versus rosuvastatin versus simvastatin on lowering levels of atherogenic lipids (from VOYAGER) Am J Cardiol. 2010;105:69–76. doi: 10.1016/j.amjcard.2009.08.651. [DOI] [PubMed] [Google Scholar]
- 5.Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol. 2002;67:491–498. doi: 10.1101/sqb.2002.67.491. [DOI] [PubMed] [Google Scholar]
- 6.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 7.Vega GL, Grundy SM. Effect of statins on metabolism of apo-B-containing lipoproteins in hypertriglyceridemic men. Am J Cardiol. 1998;81:36B–42B. doi: 10.1016/s0002-9149(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 8.Roberts WC. The underused miracle drugs: the statin drugs are to atherosclerosis what penicillin was to infectious disease. Am J Cardiol. 1996;78:377–378. doi: 10.1016/s0002-9149(96)00441-9. [DOI] [PubMed] [Google Scholar]
- 9.Veillard NR, Mach F. Statins: the new aspirin? Cell Mol Life Sci. 2002;59:1771–1786. doi: 10.1007/pl00012505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 11.Cholesterol Treatment Trialists C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113–118. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- 13.Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E-/- mice in vivo. Circulation. 2003;107:2480–2486. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- 14.Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, Itter G, Rosen R, Bohm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 15.Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, Linz W, Bohm M, Nickenig G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300–305. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh CC, Li CY, Hsu CH, Chen HL, Chen YH, Liu YP, Liu YR, Kuo HF, Liu PL. Mitochondrial protection by simvastatin against angiotensin II-mediated heart failure. Br J Pharmacol. 2019;176:3791–3804. doi: 10.1111/bph.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med. 2010;152(488–96):W174. doi: 10.7326/0003-4819-152-8-201004200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egashira K, Ni W, Inoue S, Kataoka C, Kitamoto S, Koyanagi M, Takeshita A. Pravastatin attenuates cardiovascular inflammatory and proliferative changes in a rat model of chronic inhibition of nitric oxide synthesis by its cholesterol-lowering independent actions. Hypertens Res. 2000;23:353–358. doi: 10.1291/hypres.23.353. [DOI] [PubMed] [Google Scholar]
- 19.Bourcier T, Libby P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:556–562. doi: 10.1161/01.atv.20.2.556. [DOI] [PubMed] [Google Scholar]
- 20.Dujovne CA, Harris WS, Altman R, Overhiser RW, Black DM. Effect of atorvastatin on hemorheologic-hemostatic parameters and serum fibrinogen levels in hyperlipidemic patients. Am J Cardiol. 2000;85:350–353. doi: 10.1016/s0002-9149(99)00745-6. [DOI] [PubMed] [Google Scholar]
- 21.Schultz BG, Patten DK, Berlau DJ. The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Transl Neurodegener. 2018;7:5. doi: 10.1186/s40035-018-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trompet S, van Vliet P, de Craen AJ, Jolles J, Buckley BM, Murphy MB, Ford I, Macfarlane PW, Sattar N, Packard CJ, Stott DJ, Shepherd J, Bollen EL, Blauw GJ, Jukema JW, Westendorp RG. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85–90. doi: 10.1007/s00415-009-5271-7. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG, PsgPSoPitEa R. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 24.Evans MA, Golomb BA. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29:800–811. doi: 10.1592/phco.29.7.800. [DOI] [PubMed] [Google Scholar]
- 25.Olmastroni E, Molari G, De Beni N, Colpani O, Galimberti F, Gazzotti M, Zambon A, Catapano AL, Casula M. Statin use and risk of dementia or alzheimer's disease: a systematic review and meta-analysis of observational studies. Eur J Prev Cardiol. 2022;29:804–814. doi: 10.1093/eurjpc/zwab208. [DOI] [PubMed] [Google Scholar]
- 26.Wong WW, Clendening JW, Martirosyan A, Boutros PC, Bros C, Khosravi F, Jurisica I, Stewart AK, Bergsagel PL, Penn LZ. Determinants of sensitivity to lovastatin-induced apoptosis in multiple myeloma. Mol Cancer Ther. 2007;6:1886–1897. doi: 10.1158/1535-7163.MCT-06-0745. [DOI] [PubMed] [Google Scholar]
- 27.Crescencio ME, Rodriguez E, Paez A, Masso FA, Montano LF, Lopez-Marure R. Statins inhibit the proliferation and induce cell death of human papilloma virus positive and negative cervical cancer cells. Int J Biomed Sci. 2009;5:411–420. [PMC free article] [PubMed] [Google Scholar]
- 28.Rajtik T, Carnicka S, Szobi A, Mesarosova L, Matus M, Svec P, Ravingerova T, Adameova A. Pleiotropic effects of simvastatin are associated with mitigation of apoptotic component of cell death upon lethal myocardial reperfusion-induced injury. Physiol Res. 2012;61(Suppl 2):S33–41. doi: 10.33549/physiolres.932420. [DOI] [PubMed] [Google Scholar]
- 29.Adameova A, Harcarova A, Matejikova J, Pancza D, Kuzelova M, Carnicka S, Svec P, Bartekova M, Styk J, Ravingerova T. Simvastatin alleviates myocardial contractile dysfunction and lethal ischemic injury in rat heart independent of cholesterol-lowering effects. Physiol Res. 2009;58:449–454. doi: 10.33549/physiolres.931751. [DOI] [PubMed] [Google Scholar]
- 30.Eckard AR, McComsey GA. The role of statins in the setting of HIV infection. Curr HIV/AIDS Rep. 2015;12:305–312. doi: 10.1007/s11904-015-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pihl-Jensen G, Tsakiri A, Frederiksen JL. Statin treatment in multiple sclerosis: a systematic review and meta-analysis. CNS Drugs. 2015;29:277–291. doi: 10.1007/s40263-015-0239-x. [DOI] [PubMed] [Google Scholar]
- 32.Zaharan NL, Williams D, Bennett K. Statins and risk of treated incident diabetes in a primary care population. Br J Clin Pharmacol. 2013;75:1118–1124. doi: 10.1111/j.1365-2125.2012.04403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. doi: 10.1136/bmj.f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi R, Talarico M, Coppi F, Boriani G. Protective role of statins in COVID 19 patients: importance of pharmacokinetic characteristics rather than intensity of action. Intern Emerg Med. 2020;15:1573–1576. doi: 10.1007/s11739-020-02504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song SL, Hays SB, Panton CE, Mylona EK, Kalligeros M, Shehadeh F, Mylonakis E. Statin use is associated with decreased risk of invasive mechanical ventilation in COVID-19 patients: a preliminary study. Pathogens. 2020 doi: 10.3390/pathogens9090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, Lei F, Chen MM, Yang H, Bai L, Song X, Lin L, Xia M, Zhou F, Zhou J, She ZG, Zhu L, Ma X, Xu Q, Ye P, Chen G, Liu L, Mao W, Yan Y, Xiao B, Lu Z, Peng G, Liu M, Yang J, Yang L, Zhang C, Lu H, Xia X, Wang D, Liao X, Wei X, Zhang BH, Zhang X, Yang J, Zhao GN, Zhang P, Liu PP, Loomba R, Ji YX, Xia J, Wang Y, Cai J, Guo J, Li H. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32(176–187):e4. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gotto AM., Jr Is it appropriate to make statins available over the counter? Over-the-counter statins are worth considering in primary prevention of cardiovascular disease. Circulation. 2006;114:1310–1314. doi: 10.1161/CIRCULATIONAHA.105.552257. [DOI] [PubMed] [Google Scholar]
- 38.Expert Panel on Detection E and Treatment of High Blood Cholesterol in A Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 39.Nash DB, Nash SA. Reclassification of simvastatin to over-the-counter status in the United Kingdom: a primary prevention strategy. Am J Cardiol. 2004;94:35F–39F. doi: 10.1016/j.amjcard.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 40.Paudyal V, Hansford D, Cunningham S, Stewart D. Pharmacists' perceived integration into practice of over-the-counter simvastatin five years post reclassification. Int J Clin Pharm. 2012;34:733–738. doi: 10.1007/s11096-012-9668-5. [DOI] [PubMed] [Google Scholar]
- 41.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 42.McTavish D, Sorkin EM. Pravastatin. A review of its pharmacological properties and therapeutic potential in hypercholesterolaemia. Drugs. 1991;42:65–89. doi: 10.2165/00003495-199142010-00005. [DOI] [PubMed] [Google Scholar]
- 43.McTaggart F, Buckett L, Davidson R, Holdgate G, McCormick A, Schneck D, Smith G, Warwick M. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol. 2001;87:28B–32B. doi: 10.1016/s0002-9149(01)01454-0. [DOI] [PubMed] [Google Scholar]
- 44.Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84:413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 45.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 46.Nezasa K, Higaki K, Takeuchi M, Nakano M, Koike M. Uptake of rosuvastatin by isolated rat hepatocytes: comparison with pravastatin. Xenobiotica. 2003;33:379–388. doi: 10.1080/0049825031000066259. [DOI] [PubMed] [Google Scholar]
- 47.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering–are they clinically relevant? Eur Heart J. 2003;24:225–248. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 48.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 50.Adameova A, Xu YJ, Duhamel TA, Tappia PS, Shan L, Dhalla NS. Anti-atherosclerotic molecules targeting oxidative stress and inflammation. Curr Pharm Des. 2009;15:3094–3107. doi: 10.2174/138161209789058048. [DOI] [PubMed] [Google Scholar]
- 51.Muntean DM, Thompson PD, Catapano AL, Stasiolek M, Fabis J, Muntner P, Serban MC, Banach M. Statin-associated myopathy and the quest for biomarkers: can we effectively predict statin-associated muscle symptoms? Drug Discov Today. 2017;22:85–96. doi: 10.1016/j.drudis.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Mendes P, Robles PG, Mathur S. Statin-induced rhabdomyolysis: a comprehensive review of case reports. Physiother Can. 2014;66:124–132. doi: 10.3138/ptc.2012-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez ML, Mora C, Navarro JF. Cerivastatin-induced rhabdomyolysis. Ann Intern Med. 2000;132:598. doi: 10.7326/0003-4819-132-7-200004040-00031. [DOI] [PubMed] [Google Scholar]
- 54.Plotkin E, Bernheim J, Ben-Chetrit S, Mor A, Korzets Z. Influenza vaccine–a possible trigger of rhabdomyolysis induced acute renal failure due to the combined use of cerivastatin and bezafibrate. Nephrol Dial Transplant. 2000;15:740–741. doi: 10.1093/ndt/15.5.740. [DOI] [PubMed] [Google Scholar]
- 55.Pogson GW, Kindred LH, Carper BG. Rhabdomyolysis and renal failure associated with cerivastatin-gemfibrozil combination therapy. Am J Cardiol. 1999;83:1146. doi: 10.1016/s0002-9149(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 56.Alexandridis G, Pappas GA, Elisaf MS. Rhabdomyolysis due to combination therapy with cerivastatin and gemfibrozil. Am J Med. 2000;109:261–262. doi: 10.1016/s0002-9343(00)00514-3. [DOI] [PubMed] [Google Scholar]
- 57.Gemici G, Toprak A, Oktay A. Rhabdomyolysis due to cerivastatin monotherapy. Am J Med. 2001;110:742. doi: 10.1016/s0002-9343(01)00730-6. [DOI] [PubMed] [Google Scholar]
- 58.Bakri R, Wang J, Wierzbicki AS, Goldsmith D. Cerivastatin monotherapy-induced muscle weakness, rhabdomyolysis and acute renal failure. Int J Cardiol. 2003;91:107–109. doi: 10.1016/s0167-5273(02)00581-8. [DOI] [PubMed] [Google Scholar]
- 59.Jamal SM, Eisenberg MJ, Christopoulos S. Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme A reductase inhibitors. Am Heart J. 2004;147:956–965. doi: 10.1016/j.ahj.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 60.Adams SP, Tiellet N, Alaeiilkhchi N, Wright JM. Cerivastatin for lowering lipids. Cochrane Database Syst Rev. 2020;1:CD012501. doi: 10.1002/14651858.CD012501.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pena JM, Aspberg S, MacFadyen J, Glynn RJ, Solomon DH, Ridker PM. Statin therapy and risk of fracture: results from the JUPITER randomized clinical trial. JAMA Intern Med. 2015;175:171–177. doi: 10.1001/jamainternmed.2014.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaCroix AZ, Cauley JA, Pettinger M, Hsia J, Bauer DC, McGowan J, Chen Z, Lewis CE, McNeeley SG, Passaro MD, Jackson RD. Statin use, clinical fracture, and bone density in postmenopausal women: results from the women's health initiative observational study. Ann Intern Med. 2003;139:97–104. doi: 10.7326/0003-4819-139-2-200307150-00009. [DOI] [PubMed] [Google Scholar]
- 63.van Staa TP, Wegman S, de Vries F, Leufkens B, Cooper C. Use of statins and risk of fractures. JAMA. 2001;285:1850–1855. doi: 10.1001/jama.285.14.1850. [DOI] [PubMed] [Google Scholar]
- 64.An T, Hao J, Sun S, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and meta-analysis. Osteoporos Int. 2017;28:47–57. doi: 10.1007/s00198-016-3844-8. [DOI] [PubMed] [Google Scholar]
- 65.Lin TK, Liou YS, Lin CH, Chou P, Jong GP. High-potency statins but not all statins decrease the risk of new-onset osteoporotic fractures: a nationwide population-based longitudinal cohort study. Clin Epidemiol. 2018;10:159–165. doi: 10.2147/CLEP.S145311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim KJ, Choi J, Kim JY, Bae JH, Kim KJ, Kim HY, Yoo HJ, Seo JA, Kim NH, Choi KM, Baik SH, Kim SG, Kim NH. Statin therapy and the risk of osteoporotic fractures in patients with metabolic syndrome: a nested case-control study. J Lipid Atheroscler. 2021;10:322–333. doi: 10.12997/jla.2021.10.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Del Chiaro A, Marchetti S, Parchi PD, Caprili G, Ipponi E, Scaglione M. Use of statins and hip fracture risk: a case-control study. Acta Chir Orthop Traumatol Cech. 2022;89:104–107. [PubMed] [Google Scholar]
- 68.Antonenko A, Leahy A, Babenko M, Lyons D. Low dose hydrophilic statins are the preferred agents for females at risk of osteoporosis. Bone Rep. 2022;16:101152. doi: 10.1016/j.bonr.2021.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung YS, Lee MD, Lee SK, Kim HM, Fitzpatrick LA. HMG-CoA reductase inhibitors increase BMD in type 2 diabetes mellitus patients. J Clin Endocrinol Metab. 2000;85:1137–1142. doi: 10.1210/jcem.85.3.6476. [DOI] [PubMed] [Google Scholar]
- 70.Olmastroni E, Boccalari MT, Tragni E, Rea F, Merlino L, Corrao G, Catapano AL, Casula M. Sex-differences in factors and outcomes associated with adherence to statin therapy in primary care: need for customisation strategies. Pharmacol Res. 2020;155:104514. doi: 10.1016/j.phrs.2019.104514. [DOI] [PubMed] [Google Scholar]
- 71.Hope HF, Binkley GM, Fenton S, Kitas GD, Verstappen SMM, Symmons DPM. Systematic review of the predictors of statin adherence for the primary prevention of cardiovascular disease. PLoS ONE. 2019;14:e0201196. doi: 10.1371/journal.pone.0201196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colantonio LD, Rosenson RS, Deng L, Monda KL, Dai Y, Farkouh ME, Safford MM, Philip K, Mues KE, Muntner P. Adherence to statin therapy among US adults between 2007 and 2014. J Am Heart Assoc. 2019;8:e010376. doi: 10.1161/JAHA.118.010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability statement is not applicable for this review.