Abstract
Epstein-Barr virus (EBV), a γ-herpesvirus, is the first identified oncogenic virus, which establishes permanent infection in humans. EBV causes infectious mononucleosis and is also tightly linked to many malignant diseases. Various vaccine formulations underwent testing in different animals or in humans. However, none of them was able to prevent EBV infection and no vaccine has been approved to date. Current efforts focus on antigen selection, combination, and design to improve the efficacy of vaccines. EBV glycoproteins such as gH/gL, gp42, and gB show excellent immunogenicity in preclinical studies compared to the previously favored gp350 antigen. Combinations of multiple EBV proteins in various vaccine designs become more attractive approaches considering the complex life cycle and complicated infection mechanisms of EBV. Besides, rationally designed vaccines such as virus-like particles (VLPs) and protein scaffold-based vaccines elicited more potent immune responses than soluble antigens. In addition, humanized mice, rabbits, as well as nonhuman primates that can be infected by EBV significantly aid vaccine development. Innovative vaccine design approaches, including polymer-based nanoparticles, the development of effective adjuvants, and antibody-guided vaccine design, will further enhance the immunogenicity of vaccine candidates. In this review, we will summarize (i) the disease burden caused by EBV and the necessity of developing an EBV vaccine; (ii) previous EBV vaccine studies and available animal models; (iii) future trends of EBV vaccines, including activation of cellular immune responses, novel immunogen design, heterologous prime-boost approach, induction of mucosal immunity, application of nanoparticle delivery system, and modern adjuvant development.
Subject terms: Vaccines, Virology
Introduction
Epstein-Barr virus (EBV) is a γ-herpesvirus that contains a double-stranded DNA genome of approximately 172 kb and is the first identified human oncogenic virus1,2. The EBV particle has a typical three-layer structure: the outermost lipid envelope displaying multiple glycoproteins responsible for cell entry, the middle tegument containing 20–40 proteins, and the inner pseudo-icosahedral nucleocapsid surrounding the DNA genome. The complete atomic models of the EBV icosahedral capsid, dodecameric portal, and capsid-associated tegument complex were resolved recently3,4.
EBV infects more than 95% of humans and establishes a lifelong infection5. The target cells of EBV infection are B cells, epithelial cells, natural killer (NK)/T cells, and macrophages. In vitro, EBV infection leads to latent infection in B cells and a lytic infection in epithelial cells6. Although the mechanisms of EBV entry into B cells and epithelial cells are very distinct, the fusion triggering protein gH/gL and the fusion protein gB are involved in both processes7. EBV enters epithelial cells and B lymphoblastoid cells through fusion at the plasma membrane, while endocytosis is required for B lymphocyte infection8,9. To be specific, B cell entry is initiated by the most abundant membrane glycoprotein gp350/gp220 binding to the complement receptor-2 (CD21/CR2) or to CD35 (CR1)10–12. Following endocytosis into B lymphocytes9,13, binding of the gp42 C-terminal region of the heterotrimer gH/gL/gp42 to human leukocyte antigens class II (HLA-II), thereby causes the structure to become a “closed” state14,15. This conformational change is thought to allow gH/gL/gp42 complex to interact with gB to initialize fusion with the endosomal membrane16. The mechanism of entry into epithelial cells is less well-defined. The first step involves binding BMRF2 to integrins to lessen the distance between the virus and cell17. Then gH/gL binds to ephrin receptor A2 (EphA2) and this interaction is thought to allow gH/gL to induce gB initializing membrane fusion18,19. Besides EphA2, αvβ5/β6/β8-integrins may also contribute to this process, but their roles have not been clearly defined20. In addition, the interactions of gH/gL with nonmuscle myosin heavy chain IIA and gB with neuropilin-1 contribute to this process21,22.
The EBV life cycle is complex and involves the expression of approximately 80 viral proteins. The latent-lytic switch is a particularly significant event in the EBV life cycle, but its mechanism remains unknown. Only nine proteins that contribute to B cell transformation and tumorigenesis are expressed during latent infection. They include six EBV nuclear antigens (EBNA-1, -2, -3A, -3B, -3C, and -LP) and three latent membrane proteins (LMP-1, -2A, and -2B)23,24. Lytic infection involves numerous proteins, which can be divided into immediate early, early, and late proteins according to their expression during the different phases of viral replication25. Among these proteins, the immediate early proteins Zta (BZLF-1) and Rta (BRLF-1) act as triggers of the EBV lytic cycle25. Immediate early and early proteins control genome replication and expression of late proteins25. Many lytic gene products remain to be characterized as their functions are still unclear25.
Even though EBV infection is associated with different pathologies, prophylactic or therapeutic vaccines are not yet available. In this review, we will summarize (i) the disease burden caused by EBV and the necessity of developing an EBV vaccine; (ii) previous EBV vaccine studies, available animal models, and human clinical trials; (iii) future trends of EBV vaccine studies, including activation of cellular immune responses, novel immunogen design, heterologous prime-boost approach, induction of mucosal immunity, application of nanoparticle delivery system, and modern adjuvant development.
Why do we need an EBV vaccine?
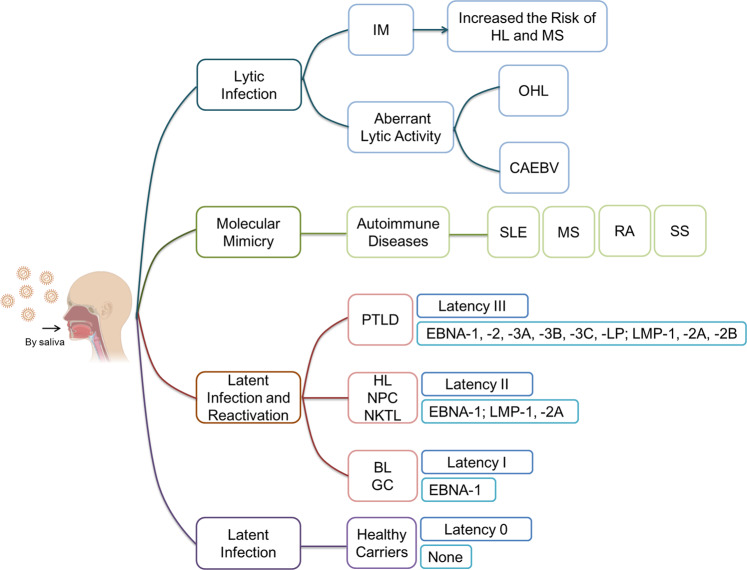
EBV is the pathogenic agent of diseases like infectious mononucleosis (IM), oral hairy leukoplakia (OHL), chronic active EBV disease (CAEBV), autoimmune diseases, and several malignancies (Fig. 1).
Fig. 1. EBV-associated diseases and latency states.
Primary EBV infection can cause infectious mononucleosis (IM), which also increases the risk of Hodgkin’s lymphoma (HL) and multiple sclerosis (MS). EBV aberrant lytic activity is associated with oral hairy leukoplakia (OHL), chronic active EBV disease (CAEBV). EBV infection and reactivation cause several autoimmune diseases, including rheumatoid arthritis (RA), Sjögren’s syndrome (SS), systemic lupus erythematosus (SLE), and MS through molecular mimicry. Besides, EBV is tightly associated with various lymphomas, including Burkitt’s lymphoma (BL), HL, natural killer/T-cell lymphoma (NKTL), as well as epithelial malignancies such as gastric carcinoma (GC) and nasopharyngeal carcinoma (NPC). The EBV latency state in HL, NKTL, and NPC is type II, while BL and GC display EBV latency type I. Besides these cancers, EBV latency is also associated to lymphoproliferative diseases in patients after stem cell transplantation or solid organ transplantation (PTLD). The figure was made from Biorender.com.
EBV primary lytic infection is associated with IM26. EBV primary infection in young children and adolescents remains asymptomatic, but young adults infected by EBV often develop IM, which also increases the risk of Hodgkin’s lymphoma (HL) and multiple sclerosis (MS)27,28. Boys with X-linked lymphoproliferative disease type I often develop severe IM after primary EBV infection, which may even become fatal29,30. Besides, EBV aberrant lytic activity is associated with OHL and CAEBV31,32.
Recently, as reported, EBV reactivation has been reported to enhance COVID-19 severity33–36. EBV infection and reactivation also contribute to the pathogenesis of several autoimmune diseases, including rheumatoid arthritis (RA), Sjögren’s syndrome (SS), systemic lupus erythematosus (SLE), and MS37,38. The EBV-specific antibodies and T cells generated during EBV infection and reactivation may react with autologous antigens and then causes autoimmune diseases37,38. Recently, a longitudinal study of a large cohort of subjects strongly supports the link between EBV infection and multiple sclerosis (MS)39. The risk of MS increased 32-fold after EBV infection39. Besides, antibodies against EBV nuclear antigen-1 (EBNA-1) induced during EBV infection cross-reacts with the central nervous system protein glial cell adhesion molecule and this molecular mimicry contributes to MS development40. However, molecular mimicry may be only one of the factors contributing to autoimmune disease pathogenesis.
EBV is tightly associated with various lymphomas, including Burkitt’s lymphoma (BL), HL, natural killer/T-cell lymphoma (NKTL), as well as epithelial malignancies such as gastric carcinoma (GC) and nasopharyngeal carcinoma (NPC)41,42. The oncogenic abilities of EBV latent proteins have been widely reported and EBV reactivation contributes to these malignancies43,44. EBV can adopt four latency states that are characterized by different protein expression patterns. Different malignancies are associated with different types of latent infections in the transformed cells (Fig. 1). The EBV latency state in HL, NKTL, and NPC is type II, while BL and GC display EBV latency type I. Beside these cancers, EBV latency is also associated with lymphoproliferative diseases in patients after stem cell transplantation or solid organ transplantation45,46.
Undoubtedly, EBV causes heavy global public health burdens. As reported, EBV caused 75,000 new cases/year of IM in the USA and 113,205, 105,554, 40,109, and 6318 new cases/year of GC, NPC, HL, and BL worldwide, respectively41,47. Remarkably, the geographical distribution of nasopharyngeal carcinoma is highly unbalanced and almost half of the new cases are in China, especially in South China48. In addition, the mortality of EBV-associated malignancies accounts for 1.8% of all cancer deaths47,49–51. Although NPC is highly sensitive to radiotherapy and chemotherapy, recurrence and metastasis are very common and linked to poor prognosis52,53. BL is a comparatively curable lymphoma, but the long-term survival rate is only 30–35% for relapsed or refractory BL in pediatric cases54. Hence, it is very necessary and urgent to develop an EBV prophylactic vaccine to prevent EBV infection and reduce the burden of all its associated diseases.
Where are we now?
Due to the inherent tumorigenicity of EBV and the difficulty to achieve high virus production in cell culture, inactivated or attenuated vaccines are not available. From the 1970s onwards, multiple EBV vaccine studies encompassed subunit vaccines, epitope vaccines, DNA vaccines, nanoparticle-based vaccines, viral vector vaccines, virus-like-particles (VLPs), or dendritic cells (DC) vaccines (Table 1). The animal models that can be infected by EBV include humanized mice, rabbits, rhesus macaques (Macaca mulatta), common marmosets (Callithrix jacchus), cottontop tamarins (Saguinus oedipus), and owl monkeys (Aotus trivirgatus). In addition, five human clinical trials were completed, but none of these vaccines successfully prevented EBV infection in humans.
Table 1.
Different types of EBV vaccine studies.
| Types | Antigen | Formulation | Ref. |
|---|---|---|---|
| Subunit vaccine | gp350 | Subunit gp350 adjuvanted with Freund’s adjuvant or alum | 57,63 |
| Subunit gp350 adjuvanted with Alum | 55 | ||
| Subunit gp350 adjuvanted with GLA/SE | 62 | ||
| Subunit gp350 adjuvanted with ISCOMs | 72 | ||
| Subunit gp350 adjuvanted with SAF-1 or alum | 60 | ||
| Subunit gp350 adjuvanted with SAF-1 | 61,70 | ||
| gp350 incorporated into liposomes | 58,59,71,74 | ||
| Tetrameric and monomeric gp3501–470 + CpG and alum | 56 | ||
| Fc-gp350 adjuvanted with CpG OND/Alum | 79 | ||
| gB | Trimeric gB adjuvanted with CpG and alum | 84 | |
| gH/gL | Trimeric and monomeric gH/gL adjuvanted with CpG and alum | 84 | |
| / | Glycoprotein complex | 83 | |
| EBNA-1 | αDEC-205-EBNA-1 adjuvanted with poly(I:C) | 95 | |
| Epitope vaccine | gp350 | CTL epitopes of gp350 and gH adjuvanted with TT and IFA | 67 |
| DC vaccine | BZLF-1 | DCs transfected to express BZLF-1 | 98 |
| DNA vaccine | gp350 | pCDNA3.1 plasmid encoding gp350 | 64 |
| gp350 | plasmid of tetrameric gp3501–470 | 56 | |
| mRNA vaccine | gH/gL/gp220/gp42 | mRNA-1189 (NCT05164094) | / |
| Nanoparticle vaccine | gH/gL/gp42 | ferritin-gH/gL/gp42 + SAS | 85 |
| gH/gL | ferritin-gH/gL + SAS | 85 | |
| nanoparticle displaying 60 copies of gH/gL | 86 | ||
| gp350 | ferritin-gp350 + SAS | 65 | |
| gp350 | LS- or I3-01- gp350 domain I/II/III adjuvanted with MF59 | 66 | |
| Virus-like particles (VLPs) | / | EBV-VLPs deleted EBNA-2, LMP-1, EBNA-3A, -B, -C and BZLF-1 | 102 |
| / | EBV-VLPs deleted BFLF-1/BFRF-1A or BBRF-1 | 103 | |
| gp350/gB/gp42/gH/gL | NDV-VLPs-gp350, gB, gp42, gH and gL | 87 | |
| gH/gL/EBNA-1 | NDV-VLPs-gH/gL-EBNA-1 | 100 | |
| gB/LMP-2 | NDV-VLPs- gB-LMP-2 | 100 | |
| gp350 | NDV-VLPs-gp350 | 75 | |
| HBc149 displaying immunodominant epitopes of gp350 | 78 | ||
| EBNA-1 | Immunogenic particles containing EBNA-1 + poly (I: C) | 104 | |
| Viral vector vaccines | gp350 | VV expressing gp350 | 68,69,73 |
| Adv expressing gp350 | 77 | ||
| gp350/gB/EBNA-2 or EBNA-3C | VV expressing gp350, gB, EBNA-2, or EBNA-3C | 99 | |
| EBNA-1 | Adv expressing EBNA-1 and VV-EBNA-1 | 96 |
GLA/SE glucopyranosyl lipid A incorporated into a stable emulsion, ISCOMs immune-stimulating complexes, SAF-1 Syntex adjuvant formulation, Fc crystallizable fragment, αDEC-205-EBNA-1 C-terminus of EBNA-1 fused with DEC-205 (a human endocytic receptor), CTL cytotoxic T lymphocytes, TT tetanus toxoid, IFA incomplete Freund’s adjuvant, DCs dendritic cells, SAS sigma adjuvant system, LS lumazine synthase, NDV Newcastle disease virus, HBc149 hepatitis B core antigen, VV vaccinia virus, Adv adenovirus.
Vaccine candidates
Considering the complexity of the EBV life cycle, EBV glycoproteins, lytic proteins, and latent proteins are all potential immunogens in EBV vaccine design. It is worth noting that the oncogenic potential of latent proteins should be avoided through proper modification of their immunogenic forms. It is likely that combinations of antigens will induce a more protective immune response, but much needs to be done to define the optimal selections of antigens or their combinations.
Vaccines using lytic glycoproteins as immunogens
gp350
gp350 is the most abundant glycoprotein on the EBV envelope and most previous vaccine studies focused on this antigen55–79. The selection of an adjuvant is one of the pivotal parts to develop an effective subunit and several combinations have been tested in various models.
Vaccines comprising monomeric gp350 (mono-gp350-based vaccines) have been combined with various adjuvants, including alum55,57,60,63, glucopyranosyl lipid A incorporated into the stable emulsion (GLA/SE)62, Syntex adjuvant formulation (SAF-1)60,61,70, immune-stimulating complexes (ISCOMs)72, Freund’s adjuvant 57,63, and incomplete Freund’s adjuvant (IFA)57,67 (Table 1).
The immune response to monomeric gp350 has been influenced by these different adjuvants. Mono-gp350 adjuvanted with alum protected three out of five cottontop tamarins from lymphoma and reduced secretion of EBV DNA in common marmosets55,57,60. Additionally, mono-gp350 adjuvanted with alum induced more robust protective responses than Freund’s adjuvant and IFA in common marmosets57. In a different study, mono-gp350 adjuvanted with alum elicited the same antibody levels in a rabbit model compared with SAF-160. Cottontop tamarins inoculated with mono-gp350 and SAF-1 were protected from lymphoma (two out of three were free of lymphoma61; four out of four were free of lymphoma70). Furthermore, vaccines incorporating mono-gp350 into glycoside Quil A-based ISCOMs required a lower antigen dose to protect four out of four cottontop tamarins from tumorigenesis after EBV challenge72. Besides, after inoculation of mono-gp350 with the Toll-like receptor 4 (TLR4) agonist GLA/SE, a gp350-specific T cell response was elicited, and anti-gp350 antibodies were detected for more than a year, indicating a durable immune response in vaccinated mice62. In addition, mono-gp350 adjuvanted with Freund’s adjuvant required less antigen dose and induced higher neutralizing titers than that adjuvanted with alum in rabbit63. Finally, sera from rabbits and owl monkeys inoculated with gp350 alone could mediate antibody-dependent cellular cytotoxicity (ADCC)76.
To sum up, although the various combinations with mono-gp350 were not systematically compared, they illustrate the critical role of adjuvants to address the requirements for lower gp350 antigen doses, less frequent inoculations, and durable immune responses. One study showed that levels of neutralizing antibodies do not reflect the protective effect of a vaccine in common marmosets57 while other studies emphasized the essential role of neutralizing antibodies at prevention tumor prevention in cottontop tamarins60,61. Among the adjuvants formulated with mono-gp350, SAF-1 seems to be better than alum to attain protection61,70. GLA/SE is beneficial to induce cellular immune responses62. Importantly, even with the applications of various adjuvants, these vaccines still need to be inoculated several times in order to elicit immune responses that protect animals from lymphoma. With the development of novel adjuvants, mono-gp350-based EBV vaccines may be successful in preventing EBV infection and associated diseases.
Sera of mice immunized with non-adjuvanted liposomes incorporating monomeric soluble gp350 (lipo-gp350) neutralize EBV infection in vitro74. However, multiple inoculations of lipo-gp350 adjuvanted with lipid A (fraction from E.coli lipopolysaccharide) induced high titers of neutralizing antibodies in mice and cottontop tamarins71. After 17 immunizations, cottontop tamarins vaccinated with lipo-gp350 were protected from lymphoma, while those immunized six times with lipo-gp350 still developed lymphoma58,59. Therefore, liposome delivery of gp350 combined with an efficient adjuvant may be another potential strategy to develop an effective EBV vaccine.
The use of multimeric gp350 has also been explored because of its higher immunogenicity compared to monomers. Mice immunized with tetrameric gp3501–470 using alum and CpG oligonucleotides (CpG ODN) as adjuvants elicited much higher anti-gp350 antibody and specific CD4+ T cell responses than mice immunized with monomeric gp35056. The enhanced immunogenicity may be due to enhanced B cell receptor (BCR) binding and signaling, vaccine uptake, or presentation and trapping by follicular dendritic cells. The first step of B cell activation is BCR recognition and cross-linking, thus, multimeric antigens are more effective because they better mimic the natural arrangement of multiple copies of the antigen on the virion surface. In addition, a heterodimeric antigen consisting of a mouse IgG2a crystallizable fragment (Fc) fragment and gp350 induced higher neutralizing antibody titers in mice compared to monomeric gp35079.
As different approaches to gp350-based EBV vaccination, vaccinia virus and adenovirus were used as viral vectors to express gp350. The WR strain of vaccinia virus expressing gp350 (VV-gp350) induced humoral immune responses in rabbits, cottontop tamarins, and common marmosets68,69,73. It is remarkable that although no anti-gp350 antibodies and low levels of neutralizing antibodies were detected in cottontop tamarins inoculated with VV-gp350, three out of four animals were still free of lymphoma after an EBV challenge with a dose of 105.3 lymphocytes-transforming doses that cause tumors in 100% of unvaccinated tamarins73. Similarly, all cottontop tamarins, which were vaccinated with a serotype 5 adenovirus expressing gp350 (Ad-gp350), were protected from lymphoma in vivo, even though their sera did not neutralize EBV in vitro77.
A DNA vaccine targeting antigen-presenting cells (APC) showed a good ability to elicit T-cell responses to gp350. Mice immunized with a recombinant pcDNA3.1 vector encoding gp350 induced not only gp350-specific antibodies but also cellular immune responses64. In a different study, sera from mice immunized with a plasmid expressing a gp3501–470 tetramer delivered with the PowderJect-XR-1 system showed higher antibody titers than those immunized with monomeric gp3501–47056. Those studies showed that nucleotide vaccines are attractive to improve immunogenicity and induce a stronger T-cell response that is crucial for killing EBV-infected cells. Note that only DNA vaccines have been studied and no data on RNA-based vaccines are currently available. mRNA vaccines for SARS-CoV-2 showed potent protective effects80,81. Recently, Moderna Inc. announced the initiation of a phase I study for its EBV mRNA vaccine mRNA-1189 (NCT05164094). The efficacy of such mRNA-based vaccines will likely influence the design of future EBV vaccines.
Other gp350-based vaccines include nanoparticle vaccines, epitope vaccines, and VLPs. Ferritin nanoparticles self-assemble to display 24 copies of gp350 (ferritin-gp350)65. These nanoparticles adjuvanted with Sigma Adjuvant System (SAS) elicited neutralizing antibodies in both mice and cynomolgus macaques65. Additionally, the immunized mice were protected from challenges with a recombinant vaccinia virus expressing gp35065. Nanoparticles of lumazine synthase (LS) or I3-01 displaying gp350 domain I/II/III induced higher titers of neutralizing antibodies than the monomeric form of gp35066.
Similarly, mice immunized with gp350 Cytotoxic T lymphocytes (CTL) epitopes combined with IFA and tetanus toxoid were also protected against the challenge of recombinant vaccinia virus expressing gp35067. The data highlight the importance of gp350 CTL epitopes and suggest that such epitopes are beneficial in the design of EBV vaccines.
Virus-like particles (VLPs) provide another attractive delivery system for EBV gp350 antigens. VLPs are multimeric self-assembled particles consisting of one or more structural proteins without a viral genome, which have no pathogenicity. Because their morphology and organization patterns are similar to natural viruses, VLPs can induce both cellular and humoral immune responses82. Chimeric VLPs based on the self-assembling hepatitis B capsid fragment hepatitis B core antigen (HBc149) were constructed to display three immunodominant epitopes of gp35078. In this system, these three peptides from the receptor binding domain of gp350 induced neutralizing antibodies in mice78. Interestingly, the humoral immune response was highly dependent on the sequential order in which these peptides were inserted in the HBc149 backbone78. VLPs based on Newcastle disease virus (NDV) capsid were constructed to display the ectodomain of gp350 (NDV-VLPs-gp350). These VLPs elicited a robust and durable neutralizing antibody response in mice75.
Other glycoproteins and combinations of glycoproteins
EBV entry into target cells is a well-organized and complex process. In addition to gp350, other glycoproteins are involved in virus entry and targeted by neutralizing antibodies. Central to the process of membrane fusion is the herpesvirus core fusion apparatus comprising gB trimers and gH/gL heterodimers16. Additionally, EBV B cell tropism is determined by the expression of gp4216. All these glycoproteins are potential antigens for vaccines aimed at neutralizing infection. Sera from rabbits inoculated with a mixture of glycoproteins prepared from the plasma membrane of EBV-positive P3HR-1 cells neutralized EBV in vitro83. Mice immunized with an epitope-based vaccine comprising gp85 (gH) and gp350 epitopes were protected from challenges with a recombinant vaccinia virus expressing gp85 or gp35067. Neutralizing titers of sera from rabbits immunized with trimeric or monomeric gH/gL, trimeric gB, and tetrameric gp3501–470 were much higher than those elicited by monomeric gp3501–470 (100-fold, 20-fold, 18-fold, and 4-fold higher, respectively)84. Ferritin nanoparticles containing gH/gL/gp42 elicited 2.5-fold higher neutralizing antibody levels against B cells infection and 250-fold higher neutralizing antibody titers against epithelial cells infection compared to ferritin-gp35085. In addition, nanoparticles displaying 60 copies of gH/gL instead of monomeric gH/gL induced neutralizing antibodies protected humanized mice from lethal EBV challenge86. Besides, a pentavalent vaccine based on NDV-VLPs containing EBV gp350, gB, gp42, gH, and gL was used with alum and monophosphoryl lipid A (MPLA) as adjuvants87. This cocktail induced neutralizing antibodies against infection of both B and epithelial cells in vitro87. Moreover, gH/gL/gp42 and gH/gL ferritin nanoparticles induced neutralizing antibodies in mice, ferrets, and nonhuman primates88. No immune competition was observed when combined with gp350D123 ferritin nanoparticles88. Besides, the passive transfer of antibodies purified from mice immunized with gH/gL/gp42 + gp350D123 or gH/gL + gp350D123 ferritin nanoparticles protected humanized mice from EBV-associated lymphoma88. These results clearly support the fact that gH/gL and gB are promising immunogen candidates. Recently, a clinical trial has been launched to evaluate an mRNA vaccine (mRNA-1189), which includes four mRNAs encoding gH, gL, gp42, and gp220 (NCT05164094). Overall, combining glycoprotein antigens is a promising approach for successful EBV vaccine development.
Vaccines using latent proteins and other lytic proteins as immunogens
Proteins that are not involved in virus entry should also be taken into consideration to develop effective vaccines for their expression in infected cells. These targets include other proteins of the lytic cycle as well as proteins expressed in various stages of latency.
In particular, EBNA-1 proved to be a robust immunogen. This antigen is expressed in almost all EBV-linked diseases and its role in maintaining the EBV genome in infected cells is a key factor in viral persistent infections89. EBNA-1 can be recognized by CD4+ T cells from almost all healthy carriers and EBNA-1-specific CD4+ and CD8+ T cells react with EBV-transformed B cells90–94. To use EBNA-1 as a vaccine, its C-terminus was fused with DEC-205 (a human endocytic receptor) and adjuvanted with poly (I:C)95. This vaccine candidate induced robust anti-EBNA-1 CD4+ and CD8+ T cell responses as well as anti-EBNA-1 IgM antibodies in humanized mice95. A heterologous prime-boost vaccination that combined a primary immunization with a recombinant adenovirus expressing EBNA-1 and a boost with a modified vaccinia virus Ankara (MVA) expressing EBNA-1 protected mice from EBNA-1 positive lymphoma after challenge96. Another nuclear antigen, EBNA-2 is one of the first viral proteins expressed during the initial stage of B cell immortalization97. EBV-infected B cells are recognized by EBNA-2-specific CD8+ T cells within 1-day post-infection and their proliferation can be prevented97.
BZLF-1 (Zta) has also been investigated as an immunogen. In a model of EBV-associated lymphoproliferative disease (LPD), survival rates of humanized mice significantly increased due to the specific CD8+ T cell response induced after inoculation of dendritic cells (DCs) transfected to express BZLF-198. This result suggests that the BZLF-1-based vaccine could potentially prevent or delay EBV-associated diseases98.
Combinations of membrane glycoproteins, latent, and lytic proteins
The above studies indicate that proteins involved in virus entry, lytic infection, as well as latency, can contribute to an effective vaccine against EBV. It is, therefore, worth considering different combinations of latent and lytic proteins to develop a comprehensive cocktail vaccine. Toward that goal, a multivalent vaccine was devised by combining recombinant vaccinia viruses, each expressing gp350, gp110, EBNA-2, or EBNA-3C99. This cocktail induced CD4+ T cell responses and antibody responses in mice, indicating that the combination of different EBV proteins into a single dose produces the desired immune response99.
Heterologous VLP is another platform of choice to combine various antigens. NDV-VLPs-gH/gL-EBNA-1 and NDV-VLPs-gB-LMP-2 induced potent neutralizing antibodies as well as EBV-specific cellular responses in mice100. A different approach is to produce EBV-VLPs in non-transforming, virus-free packaging cell lines, using EBV genomes with deletions of some genes101. For instance, EBV-VLPs lacking major oncoproteins EBNA-2, LMP-1, EBNA-3A, -B and -C, and BZLF-1 can be produced in engineered 293-VII+ cells102. Such EBV-VLPs elicited potent humoral and cellular responses in mice102. Alternatively, EBV-VLPs with deletions of BFLF-1/BFRF-1A or of BBRF-1 induced a CD4+ T cell response103. The above EBV-VLPs usually contain many lytic proteins instead of latent proteins. Van Zyl et al.104 constructed more immunogenic particles by overexpressing EBNA-1 in producer cells. Humanized mice immunized with these EBNA-1-VLPs were successfully protected against EBV challenge104. However, except for latent proteins, BNRF1 and viral particles can also induce genetic instability and chromosome defects in infected cells105,106. Hence, safety evaluation of genetic instability and chromosome defects is needed for VLPs generated from non-transforming, virus-free packaging cell lines.
Animal models
The lack of suitable animal models greatly hinders the research and development of EBV vaccines. Animal models which can be used to assess the protective effect of EBV vaccine candidates after EBV challenge, include humanized mice, rabbits, as well as nonhuman primates such as rhesus macaques, owl monkeys, cottontop tamarins, and common marmosets.
Humanized mice
Humanized mice are a novel model to investigate EBV infection and pathogenesis, study EBV-associated diseases as well as evaluate EBV vaccine candidates107. Humanized mice are based on immunodeficient mice, such as non-obese diabetic mice with scid, RAG, and/or IL-2 receptor γ chain mutations. These mice are transplanted with human CD34+ hematopoietic progenitor cells (HPCs) or peripheral blood mononuclear cells (PBMCs) from healthy donors. Infected cells in humanized mice express both latent and lytic EBV antigens after viral challenge108. Importantly, they can develop asymptomatic EBV infections, IM-like syndromes, or tumors depending on the EBV challenge dose, thus, they are useful models to study protection against EBV pathologies108,109. EBV-specific cellular immune responses are observed in humanized mice following EBV infection and the immune responses elicited by vaccines are similar to those of humans110,111. Furthermore, the innate immunity generated by reconstituted human NK cells also plays a significant role in the control of EBV lytic infections in this model112. However, humanized mice lack human epithelial cells, which are instrumental in the whole EBV infection cycle. Additionally, the development of “human” germinal centers and secondary lymphoid tissues is poor in this model109,113. Hence, the humoral immune responses cannot be reliably evaluated in the current humanized mice models. IgM antibody production against the viral capsid antigen BFRF-3 is detected in humanized mice114. Therefore, humanized mouse model is more suitable to evaluate the passive protective effect of antibodies purified from immunized mice, rabbits, or nonhuman primates. Studies using humanized mice to evaluate EBV vaccines are compiled in Table 2.
Table 2.
Humanized mice models for EBV vaccines.
| Year | Vaccine formulation and immunization route | Challenge strain | Results | Ref. |
|---|---|---|---|---|
| 2008 |
αDEC-205-EBNA-1(aa400-641) + poly(I:C) i.p. twice at one month interval |
None | EBNA-1 specific T cells and anti-EBNA-1 antibodies were detected | 95 |
| 2015 |
rAd5F35/BZLF-1-transduced human DCs i.p. once or twice at a 2-week interval |
NoneA | Prolonged survival to EBV-LPD | 98 |
| 2018 |
immunogenic particles containing EBNA-1 + poly(I:C) i.p. twice at a 4-week interval |
B95-8 | Significant protection against EBV challenge | 104 |
| 2022 |
Passive infusion of antibodies purified from mice immunized with gH/gL/gp42 + gp350D123 or gH/gL + gp350D123 ferritin nanoparticles 20 µg of mIgG per gram of mouse i.p. at day −1, day 0, and day 1 |
B95-8 | Only one of six mice in each group received immune IgG had transient low-level viremia | 88 |
| 2022 |
Passive infusion of antibodies purified from mice immunized with gH/gL 60 mer nanoparticle 500 mg of total IgG per mouse i.p. 48 h pre EBV challenge |
B95-8 | Purified antibodies from immunized mice protected humanized mice from lethal EBV challenge | 86 |
αDEC-205-EBNA-1 C-terminus of EBNA-1 fused with DEC-205 (a human endocytic receptor), None there is no challenge experiment. i.p. intraperitoneally, DCs dendritic cells. EBV-LPD EBV-associated lymphoproliferative diseases.
Ahumanized mice reconstituted with cells from an EBV-seropositive donor was used in this study.
Rabbits
Evidence showed that Japanese White rabbits can be persistently infected by EBV through intravenous inoculation since viral DNA and anti-EBV-VCA antibodies were both detected for 15 months115. Notably, persistent infections were also observed following infection of New Zealand White rabbits and Japanese White rabbits via the oral route, which is also the natural infection route in humans116,117. Furthermore, cells from New Zealand White rabbits infected intravenously proliferated in vivo following immunosuppression by cyclosporine A, which is reminiscent of observations in human post-transplantation lymphoproliferative disorder (PTLD) patients118. Together, these studies indicated that rabbit models are potential platforms for EBV vaccine evaluation.
Nonhuman primates
Rhesus lymphocryptovirus (rhLCV) is a homolog of EBV that only infects rhesus macaques and shares the same infectious features with EBV119. Experimental rhLCV infection in rhesus macaque causes either asymptomatic persistent latent infection or IM-like syndrome in immunocompetent macaques. However, in immunosuppressed macaques previously infected by simian immunodeficiency virus, rhLCV infection can lead to tumor formation119–121. Differences between rhLCV and EBV cannot be ignored, however, rhLCV vaccines and challenges performed in rhesus macaques can be considered as an indirect surrogate model to assess EBV vaccines122. Rhesus monkeys immunized with soluble rhLCV gp350 combined with alum as the adjuvant were protected against rhLCV oral challenge123. Interestingly, 72A1, a strong neutralizing monoclonal antibody targeting EBV gp350, protected rhesus macaques from oral challenge with a recombinant rhLCV carrying EBV gp350124. Such a chimeric virus may provide an interesting model to assess the in vivo protective effect of antibodies elicited by vaccine candidates.
Cottontop tamarins, common marmosets, and owl monkeys can be experimentally infected by EBV and recapitulate different aspects of human disease (Table 3). Cottontop tamarins are susceptible to experimental EBV infection and can develop malignant lymphomas after challenge with high doses of EBV125,126. Cleary and colleagues127 determined the 100% tumorigenesis dose of EBV strain B95-8 in cottontop tamarins and confirmed that tumors consisted of large-cell lymphomas with multiple copies of the EBV genome, which resembles the condition of PTLD patients. In addition, when cottontop tamarins recovered from tumors after the first challenge, cellular immune responses were observed after a second challenge, and these subjects remained healthy without any EBV-associated diseases128. Common marmosets can be infected by either the M81 strain (derived from an NPC patient) or the B95-8 strain (derived from an IM patient)129–132. The symptoms of infected common marmosets include lymphocytosis, the production of heterophile antibodies and the long-term production of EBV-specific antibodies are similar to those in humans132. After the EBV challenge, a persistent antibody response against EBV-VCA and early lytic proteins was observed132. However, antibodies against EBNA-1 were not detected and there were no viral antigens in the lymphocytes of infected animals, which differs from human cases133. In terms of pathologies, chronic infectious mononucleosis instead of LPD or lymphoma was observed in common marmosets. Owl monkeys also developed LPD after the experimental EBV challenge, and, interestingly, the EBV genome was found in a cell line established from an infected owl monkey134,135.
Table 3.
Outcomes of Nonhuman primate after EBV infection reflect different aspects of human diseases.
| Animal models | Reflection of human disease aspects |
|---|---|
| Cottontop tamarins | (a) EBV infection; (b) Malignant lymphomas after challenge with high doses of EBV; (c) Large-cell lymphomas with multiple copies of the EBV genome (resembles the condition of PTLD patients) |
| Common marmosets | (a) EBV infection; (b) Chronic infectious mononucleosis; (c) Lymphocytosis; (d) Production of heterophile antibodies; (e) long-term production of EBV-specific antibodies |
| Owl monkeys | (a) EBV infection; (b) LPD |
PTLD post-transplantation lymphoproliferative disorder, LPD lymphoproliferative disease.
From 1980 to 2000, various EBV vaccines were assessed in these nonhuman primate models for efficacy (Table 4). Notably, sterilizing immunity was not achieved in any of these studies. Another limitation lies in the fact that experimental infection in nonhuman primate models is quite different from natural routes in humans. In some studies, data showed that there was no direct correlation between neutralizing antibody levels and vaccine protective effects, for some of the immunized animals with high neutralizing antibody levels still developed lymphoma after a 100% tumorigenesis virus challenge while those without high neutralizing titers free of lymphoma59,73,77. However, another study demonstrated that neutralizing antibodies is one of the key attributes of tumorigenesis prevention58,60,61,70. Therefore, additional factors may be involved to confer complete protection, such as cellular immune responses and ADCC.
Table 4.
Nonhuman primate models for EBV vaccines.
| Year | Animal | Vaccine formulation and immunization route | Challenge strain | Results | Ref. |
|---|---|---|---|---|---|
| 1982 | Owl monkey |
Purified gp350 Two doses |
None | Sera had neutralizing and ADCC effects | 76 |
| 1984 | Cottontop tamarins |
Purified gp350 incorporated in liposomes + lipid A i.p. six times at a 3–9-week interval |
None | Neutralizing antibodies were detected | 71 |
| 1985 | Cottontop tamarins |
Purified gp350 incorporated in liposomes i.p. 17 times at a 2-week interval |
B95-8 | 2/2 were free of lymphoma after 100% tumorigenesis dose challenge | 58 |
| 1986 | Cottontop tamarins |
Purified gp350 incorporated into liposomes i.p. 6 times at a 2-week interval |
B95-8 | 4/4 developed lymphoma after 100% tumorigenesis dose challenge | 59 |
| 1988 | Cottontop tamarins |
Recombinant vaccinia viruses expressing gp350 (WR and Wyeth strains) i.d. 1 or 2 times at a 2-week interval |
B95-8 | Only the recombinant WR strain protected 3/4 of animals from lymphoma after a 100% tumorigenesis dose challenge | 73 |
| 1988 | Cottontop tamarins |
Purified gp350 with ISCOMs s.c. three times at a 2-week interval |
B95-8 | 4/4 were free of lymphoma after 100% tumorigenesis dose challenge | 72 |
| 1989 | Common marmosets |
Purified gp350 with Freund’s or alum adjuvant i.m. 3 times at a 4-week interval |
B95-8 | Alum-adsorbed antigen-induced protection against virus challenge | 57 |
| 1989 | Cottontop tamarins |
Purified gp350 with SAF-1 s.c. five times at a 2-week interval |
B95-8 | 4/4 were free of lymphoma after 100% tumorigenesis dose challenge | 70 |
| 1992 | Cottontop tamarins |
recombinant gp350 with BPV + SAF-1 adjuvant i.m. four times at a 10-day interval |
B95-8 | 2/3 was free of lymphoma after 100% tumorigenesis dose challenge | 61 |
| 1993 | Cottontop tamarins |
Recombinant adenovirus (serotype 5) expressing gp350 i.m. three times at 0-5-13 weeks |
B95-8 | 4/4 were free of lymphoma after 100% tumorigenesis dose challenge | 77 |
| 1994 | Cottontop tamarins |
recombinant gp350 with BPV + alum i.m. four times at a 4-week interval |
B95-8 | 3/5 were free of lymphoma after 100% tumorigenesis dose challenge | 60 |
| 1996 | Common marmosets |
Recombinant vaccinia virus expressing gp350 i.d. twice at a 5-week interval |
M81 | Replication of the challenge virus was decreased | 69 |
| 1998 | Common marmosets |
recombinant gp350 with BPV + alum i.m. three times at a 4-week interval |
M81 | Replication of the challenge virus was decreased | 55 |
None there is no challenge experiment, ADCC antibody-dependent cell-mediated cytotoxicity. ISCOMs immune stimulation complexes, SAF-1 Syntex adjuvant formulation, BPV bovine papillomavirus expression vector, i.p. intraperitoneally, i.d. intradermally, s.c. subcutaneously, i.m. intramuscularly.
Finally, one should note that nonhuman primates are expensive and not necessarily amenable to large and preliminary studies of vaccine candidates. In addition, specific models for EBV, such as marmosets and owl monkeys, are rare and not readily accessible. Cottontop tamarins are not available since they are an endangered species.
Clinical trials
From 1990 onwards, seven human clinical trials have been launched utilizing EBV gp350 or EBNA-3. For instance, Gu et al.136 utilized a live recombinant vaccinia virus (Tien Tan strain) expressing gp350 to immunize three groups of volunteers, including 11 adults (EBV seropositive and vaccinia seropositive), six juveniles (EBV seropositive and vaccinia seronegative), and 19 infants (EBV seronegative and vaccinia seronegative). In the adult group, antibody titers against EBV did not change after inoculation, while neutralizing antibody titers increased in young children and infants. Three out of nine infants still became naturally infected by EBV later. Meanwhile, ten out of ten control infants also became naturally infected. Moutschen and colleagues137 compared three vaccine formulations (recombinant gp350 alone, recombinant gp350 with alum, or recombinant gp350 with AS04) in seronegative and seropositive youths. All formulations were safe and well-tolerated. The formulation containing gp350 alone showed the weakest immunogenicity. Despite the detection of neutralizing antibodies and cellular immune responses, some subjects still became naturally infected. These observations are partly consistent with results obtained in common marmosets and cottontop tamarins, as discussed above. A phase II trial enrolled 181 seronegative young volunteers to test an EBV vaccine formulated with recombinant EBV gp350 and AS04 as an adjuvant138. Although anti-gp350 antibodies were detected over 18 months, this vaccine only prevented IM but not asymptomatic EBV infection. Another phase I trial recruited children with chronic kidney disease waiting for organ transplantation139. After inoculating two different doses (12.5 and 25 μg) of recombinant gp350 with alum, specific IgGs were found in all subjects. However, neutralizing antibodies were only detected in 1/4 of subjects who received the low dose and in 3/9 of subjects who received the high dose. Nevertheless, titers dropped quickly and vaccination did not affect the post-transplant immune condition of these children. Recently, a phase I clinical trial for a gp350-ferritin nanoparticle vaccine started to recruit subjects to evaluate vaccine safety and immunogenicity (NCT04645147). Another phase I clinical trial for an mRNA-based vaccine (mRNA-1189) containing four mRNA encoding gH, gL, gp42, and gp220 has been launched to evaluate to safety and tolerability of EBV mRNA vaccine in healthy adults ages 18 to 30 (NCT05164094).
Aside from glycoprotein-based vaccines, an EBNA-3 epitope-based vaccine was tested in 14 HLA B*0801-positive EBV-seronegative adults in a phase I trial140. This vaccine consisted of an EBNA-3 CD8+ epitope (FLRGRAYGL) combined with tetanus toxoid as CD4+ T cell helper and Montanide ISA 720 as the adjuvant. The vaccine proved to be safe and epitope-specific responses were observed, however, some immunized subjects seroconverted asymptomatically.
What can we do?
Previous clinical trials failed to generate sterile immunity. However, all these efforts generated valuable information but also identified many barriers that need to be overcome to develop an effective EBV vaccine. Not only EBV has a complicated life cycle involving numerous proteins, but it also displays two distinct tropisms. Thus, the selection and design of the immunogen are still the key parts of EBV vaccine development. First of all, to improve current designs, T-cell epitopes should be taken into consideration. The balance of cellular and humoral immune responses is essential for an ideal vaccine, according to the previously successful herpesvirus vaccine against VZV141. Induction and evaluation of cellular immune responses will be necessary for novel EBV vaccine studies. Moreover, the rational optimization of immunogen combinations needs to be undertaken. Particle-based delivery systems and efficient modern adjuvants will help to improve immunogenicity, extend the duration of immune responses and reduce the inoculation doses. In addition, heterologous prime-boost immunization and induction of mucosal immunity are also promising. Finally, antibody-guided vaccine design will be worth trying according to the lessons learned from RSV and HIV vaccine studies142. We will briefly discuss these aspects.
Immunogen design is the key part of vaccine development
An effective vaccine against a human herpesvirus is ShingrixTM (GSK), which consists in glycoprotein E as the antigen and AS01B as the adjuvant. This vaccine induces potent humoral and cellular immune responses, and successfully prevents shingles in the elderly141,143. The cellular immune response induced by ShingrixTM plays a crucial role in disease prevention. It changes the paradigm that a robust humoral immune response could be sufficient for prophylactic vaccines against herpesviruses. Similarly, induction of T cell immune response is also essential and important for SARS-CoV-2 vaccine development144. Hence, T cell epitopes should be included for the balance of cellular and humoral immune response required from an effective EBV vaccine.
This realization is supported by data showing that immunized cottontop tamarins with high neutralizing antibodies titers can still develop lymphomas, indicating that induction of neutralizing antibodies alone is insufficient for disease prevention59,73,77. Cytotoxic and helper T-cell responses play a significant role in viral infection and disease prevention. Helper CD4+ T cells are essential for B cell and CD8+ T cell activation by providing secondary signals (e.g., CD28-B7) and cytokines secretion, respectively. In turn, cytokines secretion of CD4+ T cells determines the types of antibodies produced. Cytokines secreted by Th1 cells (e.g., IFN-γ) are helpful to produce IgG2a and IgG3 in mice or IgG1 in humans, while Th2 cytokines (e.g., IL-4) preferentially induce IgG1 and IgE in mice or IgG2 in humans145. CD8+ T and CD4+ T cells are especially important to kill infected cells146,147. Indeed, the CD8+ T cell response is the predominant response for eliminating EBV-infected cells during lytic and latent infections148. The important roles of T cells in controlling EBV infection warrant the inclusion of T cell epitopes in future EBV vaccines. Taylor et al. summarized the T cell epitopes of almost all EBV proteins, which provides a valuable resource to contribute to the design of future EBV vaccine candidates149.
Although there were strong justifications for using gp350, this limited antigen selection is likely one of the reasons for the failure to generate a sterile immunity in previous clinical trials. The EBV infection process is complicated and various glycoproteins (gp350, gH/gL, gp42, and gB for B cell infection and BMRF2, gH/gL, and gB for epithelial cell infection) are involved in EBV entry17. Anti-gHgL antibodies appear more effective since they neutralize ~75% of epithelial cell infection, while anti-gp350 antibodies neutralize ~57% of B cell infection85. Recent studies showed that gH/gL and gB elicited more potent neutralizing antibodies than monomeric gp3501–470, and ferritin-gH/gL/gp42 induced much higher neutralizing antibody titers compared to ferritin-gp35084,85. Additionally, passive transfer of antibodies induced by nanoparticles displaying 60 copies of gH/gL, gH/gL/gp42 + gp350D123, or by gH/gL + gp350D123 ferritin nanoparticles protected humanized mice from EBV-associated lymphoma86,88. Hence, gH/gL, gp42, and gB are effective and promising antigens for the development of prophylactic vaccines to be tested in clinical trials. Although the improved design may enhance gp350 immunogenicity, we believe that prophylactic vaccines with broader antigen spectra are more likely to be successful.
To broaden the spectrum of B cell and T cell epitopes, it is worth combining glycoproteins, latent and other lytic proteins into a single dose. Previous preclinical and clinical studies focusing on gp350 have been unsatisfactory, thus, other entry glycoproteins must be considered to enhance the production of neutralizing antibodies. In addition to well-characterized entry glycoproteins, the role of several membrane glycoproteins remains unknown, such as gp150 or gp78150, may eventually contribute to vaccine development. Besides, UV-inactivated EBV, used as a positive control in various experiments, elicited better neutralizing responses than NDV-based VLPs75,87,100. Hence, including more potential antigens in the appropriate vector will be crucial for the rational design of multivalent vaccines. However, it is important to note that not all formulations are effective and some even produce unanticipated effects. For example, sera from mice inoculated with both VLPs-gH/gL-EBNA-1 and VLPs-gB-LMP-2 led to increased EBV infection of epithelial cells rather than neutralizing infection as expected100. How to design the combination of EBV proteins is still a key issue.
Heterologous prime-boost approaches
The vaccination prime-boost strategy is also important for vaccine efficiency. Heterologous prime-boost strategies were shown to be more immunogenic than homologous prime-boost approaches for HIV, herpes simplex virus type 2 (HSV-2), influenza, malaria, and tuberculosis151. Clinical trials for heterologous prime-boost SARS-CoV-2 vaccines are underway152. Examples of HIV vaccines have adopted “DNA prime-protein boost” strategies, which were able to induce both humoral and cellular immune responses153–155. A “DNA prime-viral vector boost” formulation against HIV has also been evaluated for its ability to induce cellular immune responses156,157. A “DNA prime-protein boost” approach for HSV-2 induced potent antibodies and both Th1 and Th2 immune responses158. An EBNA-1-based vaccine has been proven to be effective through an “EBNA-1-expressing adenovirus prime-EBNA-1-encoding MVA boost” strategy96. Experience from other enveloped viruses clearly indicates that a heterologous prime-boost strategy should be envisaged in the development of vaccines against EBV glycoproteins.
Mucosal immunity
The mucosal immune response has to be considered an important aspect of the prevention of EBV infection since EBV primary infection occurs at oropharynx sites6. Vaccines designed to increase mucosal immunity are desirable to protect against EBV infection. Induction of IgA and tissue-resident T cells should be targeted and assessed in trials of EBV vaccines. Oral or intranasal immunizations are particularly effective at inducing mucosal immunity compared to injectable vaccines. Currently, the licensed mucosal vaccines comprise inactivated or attenuated viruses administrated orally or intranasally159. An inhaled SARS-CoV-2 vaccine (adenovirus type 5 vector), Convidecia AirTM, has been approved for Emergency Use Authorization as a booster dose160. A series of studies provide ample evidence supporting the use of intranasal and oral vaccines to trigger robust mucosal immune responses. Hence, oral or intranasal immunizations with the appropriate EBV antigens should be considered. For instance, an inactivated virus formulation could be developed as the main vaccine or as a booster after injectable vaccines.
Particle-based vaccine is a promising field
Given that the immunogenicity of subunit and epitope vaccines has been usually insufficient, particles such as VLPs, protein scaffold nanoparticles, and polymer-based nanoparticles have been investigated. This approach is generally beneficial to enhance antigen immunogenicity since particles more closely mimic the characteristics of pathogens161.
VLPs are most similar to native viruses and have been used as immunogens in licensed HPV, HBV, and HEV vaccines162. VLPs are promising EBV vaccine candidates due to their high immunogenicity and their ability to induce potent humoral and cellular immune responses simultaneously, even without an adjuvant. In addition, VLPs can be designed to present various antigens or could be combined in vaccine formulations to expand the antigenic spectrum. As described above, protein scaffold-based nanoparticles, including ferritin, LS-, and I3-01 are promising to present EBV glycoproteins since they can induce much stronger immune responses than the soluble forms of the antigen65,66,85.
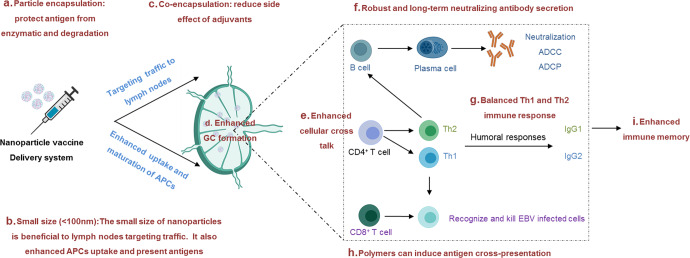
Polymer-based nanoparticle delivery systems are highly versatile. They can be prepared with various materials such as chitosan, polyanhydrides, lactic acid, and poly (lactic-co-glycolic acid), for instance. Those nanoparticles can accumulate into lymph nodes and enhance antigen and adjuvant uptake by APCs depending on their sizes, surface charge, shapes, and hydrophobicity163–166. Additionally, polymers can protect antigens from degradation before uptake into target cells. Therefore, polymer-based nanoparticle vaccines can simultaneously induce cellular and humoral immune responses and reduce side effects (Fig. 2). Under ideal conditions, most nanoparticles are delivered to lymph nodes, where they effectively elicit robust humoral and cellular immune responses through enhanced uptake by APCs, promotion of BCR cross-linkage, as well as antigen cross-presentation (Fig. 2). Studies of nanoparticle vaccine against hand-foot-and-mouth disease or influenza, demonstrated the potential of polymer-based nanoparticles167,168. This approach exhibits the most advantages considered critical for the design of EBV vaccines.
Fig. 2. Nanoparticle delivery system.
a Nanoparticle vaccine delivery systems can protect antigens from enzymatic degradation. b Depending on the size of the nanoparticles, they can be passively delivered to lymph nodes and enhance antigen-presenting cells (APCs) to take up and present antigens. d Hence, germinal center (GC) formation is enhanced. c, e–h Nanoparticle vaccine delivery systems can induce cellular and humoral immune responses simultaneously with minimum side effects. i Besides, nanoparticle vaccine delivery systems can improve the levels of antigen-specific memory T cells and B cells. The figure was made from Biorender.com.
Application of modern adjuvants can enhance immune responses to EBV antigens
Adjuvant selection is an important component of vaccine formulation since it affects the number of immunization and doses of antigens needed to obtain a protective immune response169,170.
Many adjuvants have been approved (such as Alum, MF59, IFA, AS04, AS03, AS01b, CpG ODN, and IMQ) or are currently being tested in clinical trials (such as flagellin, Matrix-M, GLA-SE, ISCOMs, and AS02). A key role of traditional adjuvants is to build an antigen depot and improve the exposure time of the antigen. Modern adjuvants, such as agonizts of pattern recognition receptors (PRRs), concentrate on the activation of innate immunity. In the presence of PRR agonizts, APCs are activated to aid both cellular and humoral immune responses. Some TLR agonizts also interact directly with B cells and provide the co-stimulatory signals to activate T cells171,172. MPLA, an agonist of TLR4, induces Th1 and Th2 responses, while CpG ODN, an agonist of TLR9, is biased to induce a Th1-dominant response173. The combination of PRRs agonizts and nano delivery systems to form nano-adjuvant is also a promising approach. For example, the AS01B adjuvant combines liposome as the delivery system and MPLA (TLR4 agonist) as the stimulating agent174. ShingrixTM, which effectively prevents shingles in the elderly, is an example of a vaccine using AS01B141,143. The application of the proper adjuvant or adjuvant system will greatly influence the success of subunit- and epitope-based vaccines against EBV.
Antibody-guided vaccine design
Finally, antibody-guided vaccine design is an innovative way to develop vaccines142. Briefly, vaccine candidates are developed based on information obtained from the characterization of effectively neutralizing antibodies and their epitopes. The availability of neutralizing antibody libraries against corresponding antigens facilitates this approach. Relevant current examples of this strategy are found in vaccines targeting viral fusion proteins. The structure of human respiratory syncytial virus (RSV) pre-fusion F protein, together with analyses of neutralizing antibody complex, led to the design of stable pre-fusion F protein vaccines. This approach led to the development of an RSV vaccine candidate, which proved to elicit neutralizing antibodies175,176. This approach was also instrumental in designing RNA vaccines against SARS-CoV-2 expressing a stable pre-fusion form of the viral spike, which is the major target of most potent neutralizing antibodies177,178. These advances open exciting prospects for vaccines targeting EBV gB and available technologies must be used to determine the pre-fusion form of gB as the main goal of structure-based vaccine design.
Many EBV-specific monoclonal antibodies have been isolated and characterized. Thus, the tools are available to rationally design EBV vaccines according to neutralizing epitopes recognized by these antibodies15,179–182.
Conclusion
EBV, as the first identified human oncogenic virus, causes a heavy health burden worldwide. It is imperative to develop an effective vaccine against EBV infection and EBV-associated diseases. Currently, none of the vaccine candidates are approved for clinical use, despite multiple attempts to develop an effective vaccine. Subunit vaccines, epitope vaccines, DNA vaccines, protein scaffold-based vaccines, viral vector vaccines, VLPs, and DC vaccines, all generated important information but generally failed to induce the required level of protection. Suitable animal models also need to be improved to study protection. Humanized mice, rabbits, rhesus macaques, and common marmosets are the most common animal models. However, each one of them has obvious limitations (as discussed above).
In human clinical trials, all vaccine candidates failed to prevent EBV infection. Induction of sterile immunity significantly correlates with a reduction of EBV-associated diseases. Hence, the ultimate goal of researchers is still to generate sterile immunity. Current efforts focus on antigen selection, combination, and design to improve the efficiency of vaccines. It is worth trying to develop a vaccine using new strategies for naïve pediatric populations to prevent the initial EBV infection. However, induction of sterile immunity may not be the only standard to evaluate the success of EBV vaccines. Preventing EBV-associated disease occurrence rather than EBV infection remains a valuable outcome when completely preventing EBV infection is not achieved. Vaccination with recombinant EBV gp350 adjuvanted with AS04 reduced the incidence of IM in seronegative subjects138. The incidence of HL and MS may be decreased accordingly27,28. Vaccination to reduce the incidence and severity of EBV-associated diseases is a valuable goal. Such goals were also established to evaluate the efficacy of SARS-CoV-2 vaccines in reducing symptomatic COVID-19183–185. Long-term clinical trials will be needed to assess the ability of the EBV vaccine to limit EBV-related diseases, in particular malignancies. Vaccination may also induce more potent cellular immune responses to control EBV reactivation in infected individuals148. Thus a vaccine that limits reactivation frequency and severity will have a valuable protective effect on infected individuals. This has been appreciated in the VZV vaccine ShingrixTM (GSK), which successfully protects latently infected individuals from shingles141,143. EBV latent proteins and reactivation events are tightly associated with EBV-associated malignancies43,44. Hence, vaccination of infected populations has the potential to reduce EBV-associated diseases burdens. In particular, the population of south China, which is at high risk of developing nasopharyngeal carcinoma, will benefit greatly from such vaccination186.
Besides, delayed infection may occur due to the non-sterile vaccination. As discussed above, one consequence of a delayed infection after vaccination is that the infection will not cause diseases anymore, or reduce the severity of diseases. From the public health point of view, this would be a desirable outcome of vaccination greatly. However, whether the incidence of EBV-linked malignancies or autoimmune response can be reduced remains unclear. As observed in SARS-CoV-2 vaccination and repeated infection, the delayed infection of the Wuhan strain or other variants of concern also boosted immune responses187. Hence, it is possible that a delayed infection, whether successful or not, may also boost anti-EBV immune responses, thereby reinforcing the individual’s protection.
Induction of robust, long-term, and balanced humoral and cellular immune responses should remain the primary goal in the development of a protective EBV vaccine. The antigen spectrum, the immunogenicity of selected antigens, and the breadth of immune responses are the key issues to achieve this goal. Over the years, immunogen selection has changed from glycoproteins, especially gp350, to a more extensive range, including lytic and latent proteins. The identification and characterization of B and T cell epitopes of EBV protein help to further optimize immunogen design. Nanoparticle-based systems showed potential for vaccine development and novel adjuvant formulations are promising to increase immunogenicity. In addition, antibody-guided vaccine design provides a framework to improve EBV vaccine development based on the knowledge of EBV-neutralization acquired over many years.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (81702001 to X.Z. and 81872228 to M.X.).
Author contributions
L.Z., C.K., W.Z., J.H., Q.F., Y.C., Q.Z., M-S.Z., Y-X.Z., M.X., and X.Z. wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ling Zhong, Claude Krummenacher.
Contributor Information
Miao Xu, Email: xumiao@sysucc.org.cn.
Xiao Zhang, Email: 103193@cqmu.edu.cn.
References
- 1.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/S0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 2.Baer R, et al. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, et al. Structures of capsid and capsid-associated tegument complex inside the Epstein-Barr virus. Nat. Microbiol. 2020;5:1285–1298. doi: 10.1038/s41564-020-0758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, et al. CryoEM structure of the tegumented capsid of Epstein-Barr virus. Cell Res. 2020;30:873–884. doi: 10.1038/s41422-020-0363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straus SE, Cohen JI, Tosato G, Meier J. NIH conference. Epstein-Barr virus infections: biology, pathogenesis, and management. Ann. Intern Med. 1993;118:45–58. doi: 10.7326/0003-4819-118-1-199301010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JI. Epstein-Barr virus infection. N. Engl. J. Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 7.Sathiyamoorthy K, Chen J, Longnecker R, Jardetzky TS. The COMPLEXity in herpesvirus entry. Curr. Opin. Virol. 2017;24:97–104. doi: 10.1016/j.coviro.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller N, Hutt-Fletcher LM. Epstein-Barr virus enters B cells and epithelial cells by different routes. J. Virol. 1992;66:3409–3414. doi: 10.1128/jvi.66.6.3409-3414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemerow GR, Cooper NR. Early events in the infection of human B lymphocytes by Epstein-Barr virus: the internalization process. Virology. 1984;132:186–198. doi: 10.1016/0042-6822(84)90102-8. [DOI] [PubMed] [Google Scholar]
- 10.Ogembo JG, et al. Human complement receptor type 1/CD35 is an Epstein-Barr Virus receptor. Cell Rep. 2013;3:371–385. doi: 10.1016/j.celrep.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young, K. A., Chen, X. S., Holers, V. M. & Hannan, J. P. Isolating the Epstein-Barr virus gp350/220 binding site on complement receptor type 2 (CR2/CD21. J. Biol. Chem.282, 36614–36625 (2007). [DOI] [PubMed]
- 12.Young KA, Herbert AP, Barlow PN, Holers VM, Hannan JP. Molecular basis of the interaction between complement receptor type 2 (CR2/CD21) and Epstein-Barr virus glycoprotein gp350. J. Virol. 2008;82:11217–11227. doi: 10.1128/JVI.01673-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanner J, Weis J, Fearon D, Whang Y, Kieff E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell. 1987;50:203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- 14.Sathiyamoorthy K, et al. Assembly and architecture of the EBV B cell entry triggering complex. PLoS Pathog. 2014;10:e1004309. doi: 10.1371/journal.ppat.1004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sathiyamoorthy K, et al. Structural basis for Epstein-Barr virus host cell tropism mediated by gp42 and gHgL entry glycoproteins. Nat. Commun. 2016;7:13557. doi: 10.1038/ncomms13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohl BS, Chen J, Longnecker R. Gammaherpesvirus entry and fusion: A tale how two human pathogenic viruses enter their host cells. Adv. Virus Res. 2019;104:313–343. doi: 10.1016/bs.aivir.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Mohl BS, Chen J, Sathiyamoorthy K, Jardetzky TS, Longnecker R. Structural and mechanistic insights into the tropism of Epstein-Barr virus. Mol. Cells. 2016;39:286–291. doi: 10.14348/molcells.2016.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, et al. Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. Nat. Microbiol. 2018;3:172–180. doi: 10.1038/s41564-017-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat. Microbiol. 2018;3:1–8. doi: 10.1038/s41564-017-0080-8. [DOI] [PubMed] [Google Scholar]
- 20.Chesnokova LS, Hutt-Fletcher LM. Fusion of Epstein-Barr virus with epithelial cells can be triggered by alphavbeta5 in addition to alphavbeta6 and alphavbeta8, and integrin binding triggers a conformational change in glycoproteins gHgL. J. Virol. 2011;85:13214–13223. doi: 10.1128/JVI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong D, et al. Nonmuscle myosin heavy chain IIA mediates Epstein-Barr virus infection of nasopharyngeal epithelial cells. Proc. Natl Acad. Sci. USA. 2015;112:11036–11041. doi: 10.1073/pnas.1513359112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HB, et al. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat. Commun. 2015;6:6240. doi: 10.1038/ncomms7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 24.Kanda T. EBV-encoded latent genes. Adv. Exp. Med. Biol. 2018;1045:377–394. doi: 10.1007/978-981-10-7230-7_17. [DOI] [PubMed] [Google Scholar]
- 25.Murata T. Encyclopedia of EBV-encoded lytic genes: an update. Adv. Exp. Med. Biol. 2018;1045:395–412. doi: 10.1007/978-981-10-7230-7_18. [DOI] [PubMed] [Google Scholar]
- 26.Luzuriaga K, Sullivan JL. Infectious mononucleosis. N. Engl. J. Med. 2010;362:1993–2000. doi: 10.1056/NEJMcp1001116. [DOI] [PubMed] [Google Scholar]
- 27.Hjalgrim H, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N. Engl. J. Med. 2003;349:1324–1332. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 28.Sheik-Ali S. Infectious mononucleosis and multiple sclerosis - Updated review on associated risk. Mult. Scler. Relat. Disord. 2017;14:56–59. doi: 10.1016/j.msard.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Coffey AJ, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 30.Seemayer TA, et al. X-linked lymphoproliferative disease: twenty-five years after the discovery. Pediatr. Res. 1995;38:471–478. doi: 10.1203/00006450-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Greenspan JS, Greenspan D, Webster-Cyriaque J. Hairy leukoplakia; lessons learned: 30-plus years. Oral. Dis. 2016;22:120–127. doi: 10.1111/odi.12393. [DOI] [PubMed] [Google Scholar]
- 32.Kimura H, Cohen JI. Chronic active Epstein-Barr virus disease. Front. Immunol. 2017;8:1867. doi: 10.3389/fimmu.2017.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paolucci S, et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int. J. Infect. Dis. 2021;104:315–319. doi: 10.1016/j.ijid.2020.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen T, Song J, Liu H, Zheng H, Chen C. Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 2021;11:10902. doi: 10.1038/s41598-021-90351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nadeem A, Suresh K, Awais H, Waseem S. Epstein-Barr virus coinfection in COVID-19. J. Investig. Med High. Impact Case Rep. 2021;9:23247096211040626. doi: 10.1177/23247096211040626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gold, J. E., Okyay, R. A., Licht, W. E. & Hurley, D. J. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens10.3390/pathogens10060763 (2021). [DOI] [PMC free article] [PubMed]
- 37.Niller HH, Wolf H, Minarovits J. Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity. 2008;41:298–328. doi: 10.1080/08916930802024772. [DOI] [PubMed] [Google Scholar]
- 38.Houen G, Trier NH. Epstein-Barr virus and systemic autoimmune diseases. Front. Immunol. 2020;11:587380. doi: 10.3389/fimmu.2020.587380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjornevik K, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375:296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 40.Lanz TV, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature. 2022;603:321–327. doi: 10.1038/s41586-022-04432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan G, Fitzmaurice C, Naghavi M, Ahmed LA. Global and regional incidence, mortality and disability-adjusted life-years for Epstein-Barr virus-attributable malignancies, 1990-2017. BMJ Open. 2020;10:e037505. doi: 10.1136/bmjopen-2020-037505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montes-Mojarro, I. A., Fend, F. & Quintanilla-Martinez, L. EBV and the Pathogenesis of NK/T Cell Lymphoma. Cancers10.3390/cancers13061414 (2021). [DOI] [PMC free article] [PubMed]
- 43.Luo Y, Liu Y, Wang C, Gan R. Signaling pathways of EBV-induced oncogenesis. Cancer Cell Int. 2021;21:93. doi: 10.1186/s12935-021-01793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young LS, Murray PG. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene. 2003;22:5108–5121. doi: 10.1038/sj.onc.1206556. [DOI] [PubMed] [Google Scholar]
- 45.Fujieda M, Hattori M. Cancer-infection interface in children after transplantation: posttransplant lymphoproliferative disorder and Epstein-Barr virus infection. Curr. Opin. Organ Transpl. 2013;18:549–554. doi: 10.1097/MOT.0b013e3283651b0d. [DOI] [PubMed] [Google Scholar]
- 46.Green M, Michaels MG. Epstein-Barr virus infection and posttransplant lymphoproliferative disorder. Am. J. Transpl. 2013;13:41–54. doi: 10.1111/ajt.12004. [DOI] [PubMed] [Google Scholar]
- 47.Cohen JI, Fauci AS, Varmus H, Nabel GJ. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci. Transl. Med. 2011;3:107fs107. doi: 10.1126/scitranslmed.3002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YP, et al. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 49.Naseem M, et al. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat. Rev. 2018;66:15–22. doi: 10.1016/j.ctrv.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J. Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 51.Khan G, Hashim MJ. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990-2010. Infect. Agent Cancer. 2014;9:38. doi: 10.1186/1750-9378-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng SH, et al. Long-term survival of nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:1323–1330. doi: 10.1016/S0360-3016(00)00779-3. [DOI] [PubMed] [Google Scholar]
- 53.Chua DT, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J. Clin. Oncol. 2005;23:1118–1124. doi: 10.1200/JCO.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 54.Short NJ, et al. Outcomes of adults with relapsed or refractory Burkitt and high-grade B-cell leukemia/lymphoma. Am. J. Hematol. 2017;92:E114–E117. doi: 10.1002/ajh.24720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox C, et al. Immunization of common marmosets with Epstein-Barr virus (EBV) envelope glycoprotein gp340: effect on viral shedding following EBV challenge. J. Med. Virol. 1998;55:255–261. doi: 10.1002/(SICI)1096-9071(199808)55:4<255::AID-JMV1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 56.Cui X, et al. A novel tetrameric gp350 1-470 as a potential Epstein-Barr virus. Vaccine. 2013;31:3039–3045. doi: 10.1016/j.vaccine.2013.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emini EA, Schleif WA, Silberklang M, Lehman D, Ellis RW. Vero cell-expressed Epstein-Barr virus (EBV) gp350/220 protects marmosets from EBV challenge. J. Med. Virol. 1989;27:120–123. doi: 10.1002/jmv.1890270210. [DOI] [PubMed] [Google Scholar]
- 58.Epstein MA, Morgan AJ, Finerty S, Randle BJ, Kirkwood JK. Protection of cottontop tamarins against Epstein-Barr virus-induced malignant lymphoma by a prototype subunit vaccine. Nature. 1985;318:287–289. doi: 10.1038/318287a0. [DOI] [PubMed] [Google Scholar]
- 59.Epstein MA, Randle BJ, Finerty S, Kirkwood JK. Not all potently neutralizing, vaccine-induced antibodies to Epstein-Barr virus ensure protection of susceptible experimental animals. Clin. Exp. Immunol. 1986;63:485–490. [PMC free article] [PubMed] [Google Scholar]
- 60.Finerty S, et al. Immunization of cottontop tamarins and rabbits with a candidate vaccine against the Epstein-Barr virus based on the major viral envelope glycoprotein gp340 and alum. Vaccine. 1994;12:1180–1184. doi: 10.1016/0264-410X(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 61.Finerty S, et al. Protective immunization against Epstein-Barr virus-induced disease in cottontop tamarins using the virus envelope glycoprotein gp340 produced from a bovine papillomavirus expression vector. J. Gen. Virol. 1992;73:449–453. doi: 10.1099/0022-1317-73-2-449. [DOI] [PubMed] [Google Scholar]
- 62.Heeke DS, et al. Identification of GLA/SE as an effective adjuvant for the induction of robust humoral and cell-mediated immune responses to EBV-gp350 in mice and rabbits. Vaccine. 2016;34:2562–2569. doi: 10.1016/j.vaccine.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 63.Jackman WT, Mann KA, Hoffmann HJ, Spaete RR. Expression of Epstein-Barr virus gp350 as a single chain glycoprotein for an EBV subunit vaccine. Vaccine. 1999;17:660–668. doi: 10.1016/S0264-410X(98)00248-5. [DOI] [PubMed] [Google Scholar]
- 64.Jung S, et al. DNA-mediated immunization of glycoprotein 350 of Epstein-Barr virus induces the effective humoral and cellular immune responses against the antigen. Mol. Cells. 2001;12:41–49. [PubMed] [Google Scholar]
- 65.Kanekiyo M, et al. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell. 2015;162:1090–1100. doi: 10.1016/j.cell.2015.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang YF, et al. Immunization with a self-assembled nanoparticle vaccine elicits potent neutralizing antibody responses against EBV infection. Nano Lett. 2021;21:2476–2486. doi: 10.1021/acs.nanolett.0c04687. [DOI] [PubMed] [Google Scholar]
- 67.Khanna R, Sherritt M, Burrows SR. EBV structural antigens, gp350 and gp85, as targets for ex vivo virus-specific CTL during acute infectious mononucleosis: potential use of gp350/gp85 CTL epitopes for vaccine design. J. Immunol. 1999;162:3063–3069. [PubMed] [Google Scholar]
- 68.Mackett M, Arrand JR. Recombinant vaccinia virus induces neutralising antibodies in rabbits against Epstein-Barr virus membrane antigen gp340. EMBO J. 1985;4:3229–3234. doi: 10.1002/j.1460-2075.1985.tb04070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mackett M, et al. Immunisation of common marmosets with vaccinia virus expressing Epstein-Barr virus (EBV) gp340 and challenge with EBV. J. Med. Virol. 1996;50:263–271. doi: 10.1002/(SICI)1096-9071(199611)50:3<263::AID-JMV9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 70.Morgan AJ, et al. Validation of a first-generation Epstein-Barr virus vaccine preparation suitable for human use. J. Med. Virol. 1989;29:74–78. doi: 10.1002/jmv.1890290114. [DOI] [PubMed] [Google Scholar]
- 71.Morgan AJ, Epstein MA, North JR. Comparative immunogenicity studies on Epstein-Barr virus membrane antigen (MA) gp340 with novel adjuvants in mice, rabbits, and cotton-top tamarins. J. Med. Virol. 1984;13:281–292. doi: 10.1002/jmv.1890130310. [DOI] [PubMed] [Google Scholar]
- 72.Morgan AJ, Finerty S, Lovgren K, Scullion FT, Morein B. Prevention of Epstein-Barr (EB) virus-induced lymphoma in cottontop tamarins by vaccination with the EB virus envelope glycoprotein gp340 incorporated into immune-stimulating complexes. J. Gen. Virol. 1988;69:2093–2096. doi: 10.1099/0022-1317-69-8-2093. [DOI] [PubMed] [Google Scholar]
- 73.Morgan AJ, et al. Recombinant vaccinia virus expressing Epstein-Barr virus glycoprotein gp340 protects cottontop tamarins against EB virus-induced malignant lymphomas. J. Med. Virol. 1988;25:189–195. doi: 10.1002/jmv.1890250209. [DOI] [PubMed] [Google Scholar]
- 74.North JR, Morgan AJ, Thompson JL, Epstein MA. Purified Epstein-Barr virus Mr 340,000 glycoprotein induces potent virus-neutralizing antibodies when incorporated in liposomes. Proc. Natl Acad. Sci. USA. 1982;79:7504–7508. doi: 10.1073/pnas.79.23.7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogembo JG, et al. A chimeric EBV gp350/220-based VLP replicates the virion B-cell attachment mechanism and elicits long-lasting neutralizing antibodies in mice. J. Transl. Med. 2015;13:50. doi: 10.1186/s12967-015-0415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qualtiere LF, Chase R, Pearson GR. Purification and biologic characterization of a major Epstein Barr virus-induced membrane glycoprotein. J. Immunol. 1982;129:814–818. [PubMed] [Google Scholar]
- 77.Ragot T, Finerty S, Watkins PE, Perricaudet M, Morgan AJ. Replication-defective recombinant adenovirus expressing the Epstein-Barr virus (EBV) envelope glycoprotein gp340/220 induces protective immunity against EBV-induced lymphomas in the cottontop tamarin. J. Gen. Virol. 1993;74:501–507. doi: 10.1099/0022-1317-74-3-501. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X, et al. A novel vaccine candidate based on chimeric virus-like particle displaying multiple conserved epitope peptides induced neutralizing antibodies against EBV infection. Theranostics. 2020;10:5704–5718. doi: 10.7150/thno.42494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao B, et al. Immunization with Fc-based recombinant Epstein-Barr virus gp350 elicits potent neutralizing humoral immune response in a BALB/c mice model. Front. Immunol. 2018;9:932. doi: 10.3389/fimmu.2018.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vogel AB, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 81.Corbett KS, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qian, C. et al. Recent progress on the versatility of virus-like particles. Vaccines10.3390/vaccines8010139 (2020). [DOI] [PMC free article] [PubMed]
- 83.Thorley-Lawson DA. A virus-free immunogen effective against Epstein-Barr virus. Nature. 1979;281:486–488. doi: 10.1038/281486a0. [DOI] [PubMed] [Google Scholar]
- 84.Cui X, et al. Rabbits immunized with Epstein-Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine. 2016;34:4050–4055. doi: 10.1016/j.vaccine.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 85.Bu W, et al. Immunization with components of the viral fusion apparatus elicits antibodies that neutralize Epstein-Barr virus in B cells and epithelial cells. Immunity. 2019;50:1305–1316 e1306. doi: 10.1016/j.immuni.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malhi H, et al. Immunization with a self-assembling nanoparticle vaccine displaying EBV gH/gL protects humanized mice against lethal viral challenge. Cell Rep. Med. 2022;3:100658. doi: 10.1016/j.xcrm.2022.100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Escalante, G. M. et al. A Pentavalent Epstein-Barr virus-like particle vaccine elicits high titers of neutralizing antibodies against Epstein-Barr virus infection in immunized rabbits. Vaccines10.3390/vaccines8020169 (2020). [DOI] [PMC free article] [PubMed]
- 88.Wei CJ, et al. A bivalent Epstein-Barr virus vaccine induces neutralizing antibodies that block infection and confer immunity in humanized mice. Sci. Transl. Med. 2022;14:eabf3685. doi: 10.1126/scitranslmed.abf3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chakravorty A, Sugden B. The AT-hook DNA binding ability of the Epstein Barr virus EBNA1 protein is necessary for the maintenance of viral genomes in latently infected cells. Virology. 2015;484:251–258. doi: 10.1016/j.virol.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blake N, et al. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 1997;7:791–802. doi: 10.1016/S1074-7613(00)80397-0. [DOI] [PubMed] [Google Scholar]
- 91.Long HM, et al. CD4+ T-cell responses to Epstein-Barr virus (EBV) latent-cycle antigens and the recognition of EBV-transformed lymphoblastoid cell lines. J. Virol. 2005;79:4896–4907. doi: 10.1128/JVI.79.8.4896-4907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munz C, et al. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J. Exp. Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nikiforow S, Bottomly K, Miller G, Munz C. Cytolytic CD4(+)-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation. J. Virol. 2003;77:12088–12104. doi: 10.1128/JVI.77.22.12088-12104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paludan C, et al. Epstein-Barr nuclear antigen 1-specific CD4(+) Th1 cells kill Burkitt’s lymphoma cells. J. Immunol. 2002;169:1593–1603. doi: 10.4049/jimmunol.169.3.1593. [DOI] [PubMed] [Google Scholar]
- 95.Gurer C, et al. Targeting the nuclear antigen 1 of Epstein-Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood. 2008;112:1231–1239. doi: 10.1182/blood-2008-03-148072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruhl J, et al. Heterologous prime-boost vaccination protects against EBV antigen-expressing lymphomas. J. Clin. Invest. 2019;129:2071–2087. doi: 10.1172/JCI125364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brooks JM, et al. Early T cell recognition of B cells following Epstein-Barr virus infection: identifying potential targets for prophylactic vaccination. PLoS Pathog. 2016;12:e1005549. doi: 10.1371/journal.ppat.1005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hartlage AS, et al. The Epstein-Barr virus lytic protein BZLF1 as a candidate target antigen for vaccine development. Cancer Immunol. Res. 2015;3:787–794. doi: 10.1158/2326-6066.CIR-14-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lockey TD, Zhan X, Surman S, Sample CE, Hurwitz JL. Epstein-Barr virus vaccine development: a lytic and latent protein cocktail. Front. Biosci. 2008;13:5916–5927. doi: 10.2741/3126. [DOI] [PubMed] [Google Scholar]
- 100.Perez EM, Foley J, Tison T, Silva R, Ogembo JG. Novel Epstein-Barr virus-like particles incorporating gH/gL-EBNA1 or gB-LMP2 induce high neutralizing antibody titers and EBV-specific T-cell responses in immunized mice. Oncotarget. 2017;8:19255–19273. doi: 10.18632/oncotarget.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hettich E, et al. Genetic design of an optimized packaging cell line for gene vectors transducing human B cells. Gene Ther. 2006;13:844–856. doi: 10.1038/sj.gt.3302714. [DOI] [PubMed] [Google Scholar]
- 102.Ruiss R, et al. A virus-like particle-based Epstein-Barr virus vaccine. J. Virol. 2011;85:13105–13113. doi: 10.1128/JVI.05598-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pavlova S, et al. An Epstein-Barr virus mutant produces immunogenic defective particles devoid of viral DNA. J. Virol. 2013;87:2011–2022. doi: 10.1128/JVI.02533-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Zyl DG, et al. Immunogenic particles with a broad antigenic spectrum stimulate cytolytic T cells and offer increased protection against EBV infection ex vivo and in mice. PLoS Pathog. 2018;14:e1007464. doi: 10.1371/journal.ppat.1007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shumilov A, et al. Epstein-Barr virus particles induce centrosome amplification and chromosomal instability. Nat. Commun. 2017;8:14257. doi: 10.1038/ncomms14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yiu SPT, Guo R, Zerbe C, Weekes MP, Gewurz BE. Epstein-Barr virus BNRF1 destabilizes SMC5/6 cohesin complexes to evade its restriction of replication compartments. Cell Rep. 2022;38:110411. doi: 10.1016/j.celrep.2022.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Munz C. Humanized mouse models for Epstein Barr virus infection. Curr. Opin. Virol. 2017;25:113–118. doi: 10.1016/j.coviro.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 108.Chen, H. et al. Dose-dependent outcome of EBV infection of humanized mice based on green Raji unit (GRU) doses. Viruses10.3390/v13112184 (2021). [DOI] [PMC free article] [PubMed]
- 109.Chatterjee B, Leung CS, Munz C. Animal models of Epstein Barr virus infection. J. Immunol. Methods. 2014;410:80–87. doi: 10.1016/j.jim.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 110.Fujiwara S, Imadome K, Takei M. Modeling EBV infection and pathogenesis in new-generation humanized mice. Exp. Mol. Med. 2015;47:e135. doi: 10.1038/emm.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Munz, C. Immune control and vaccination against the Epstein-Barr virus in humanized mice. Vaccines10.3390/vaccines7040217 (2019). [DOI] [PMC free article] [PubMed]
- 112.Chijioke O, et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013;5:1489–1498. doi: 10.1016/j.celrep.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Munz C. EBV infection of mice with reconstituted human immune system components. Curr. Top. Microbiol. Immunol. 2015;391:407–423. doi: 10.1007/978-3-319-22834-1_14. [DOI] [PubMed] [Google Scholar]
- 114.Yajima M, et al. A new humanized mouse model of Epstein-Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J. Infect. Dis. 2008;198:673–682. doi: 10.1086/590502. [DOI] [PubMed] [Google Scholar]
- 115.Takashima K, et al. A new animal model for primary and persistent Epstein-Barr virus infection: human EBV-infected rabbit characteristics determined using sequential imaging and pathological analysis. J. Med. Virol. 2008;80:455–466. doi: 10.1002/jmv.21102. [DOI] [PubMed] [Google Scholar]
- 116.Okuno K, et al. Epstein-Barr virus can infect rabbits by the intranasal or peroral route: an animal model for natural primary EBV infection in humans. J. Med. Virol. 2010;82:977–986. doi: 10.1002/jmv.21597. [DOI] [PubMed] [Google Scholar]
- 117.Rajcani J, et al. Epstein-Barr virus (HHV-4) inoculation to rabbits by intranasal and oral routes results in subacute and/or persistent infection dissimilar to human disease. Intervirology. 2014;57:254–269. doi: 10.1159/000360223. [DOI] [PubMed] [Google Scholar]
- 118.Khan G, Ahmed W, Philip PS, Ali MH, Adem A. Healthy rabbits are susceptible to Epstein-Barr virus infection and infected cells proliferate in immunosuppressed animals. Virol. J. 2015;12:28. doi: 10.1186/s12985-015-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moghaddam A, et al. An animal model for acute and persistent Epstein-Barr virus infection. Science. 1997;276:2030–2033. doi: 10.1126/science.276.5321.2030. [DOI] [PubMed] [Google Scholar]
- 120.Wang F. Nonhuman primate models for Epstein-Barr virus infection. Curr. Opin. Virol. 2013;3:233–237. doi: 10.1016/j.coviro.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rivailler P, et al. Experimental rhesus lymphocryptovirus infection in immunosuppressed macaques: an animal model for Epstein-Barr virus pathogenesis in the immunosuppressed host. Blood. 2004;104:1482–1489. doi: 10.1182/blood-2004-01-0342. [DOI] [PubMed] [Google Scholar]
- 122.Fujiwara S. Animal models of human gammaherpesvirus infections. Adv. Exp. Med. Biol. 2018;1045:413–436. doi: 10.1007/978-981-10-7230-7_19. [DOI] [PubMed] [Google Scholar]
- 123.Sashihara J, et al. Soluble rhesus lymphocryptovirus gp350 protects against infection and reduces viral loads in animals that become infected with virus after challenge. PLoS Pathog. 2011;7:e1002308. doi: 10.1371/journal.ppat.1002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muhe J, et al. Neutralizing antibodies against Epstein-Barr virus infection of B cells can protect from oral viral challenge in the rhesus macaque animal model. Cell Rep. Med. 2021;2:100352. doi: 10.1016/j.xcrm.2021.100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Werner J, Wolf H, Apodaca J, zur Hausen H. Lymphoproliferative disease in a cotton-top marmoset after inoculation with infectious mononucleosis-derived Epstein-Barr virus. Int. J. Cancer. 1975;15:1000–1008. doi: 10.1002/ijc.2910150617. [DOI] [PubMed] [Google Scholar]
- 126.Miller G, et al. Lymphoma in cotton-top marmosets after inoculation with Epstein-Barr virus: tumor incidence, histologic spectrum antibody responses, demonstration of viral DNA, and characterization of viruses. J. Exp. Med. 1977;145:948–967. doi: 10.1084/jem.145.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cleary ML, et al. Individual tumors of multifocal EB virus-induced malignant lymphomas in tamarins arise from different B-cell clones. Science. 1985;228:722–724. doi: 10.1126/science.2986287. [DOI] [PubMed] [Google Scholar]
- 128.Finerty S, Scullion FT, Morgan AJ. Demonstration in vitro of cell mediated immunity to Epstein-Barr virus in cotton-top tamarins. Clin. Exp. Immunol. 1988;73:181–185. [PMC free article] [PubMed] [Google Scholar]
- 129.Falk L, et al. Epstein-Barr virus: experimental infection of Callithrix jacchus marmosets. Int. J. Cancer. 1976;17:785–788. doi: 10.1002/ijc.2910170615. [DOI] [PubMed] [Google Scholar]
- 130.de-The G, et al. Natural antibodies to EBV-VCA antigens in common marmosets (Callithrix jacchus) and response after EBV inoculation. Intervirology. 1980;14:284–291. doi: 10.1159/000149198. [DOI] [PubMed] [Google Scholar]
- 131.Desgranges C, et al. In vitro transforming activity of EBV. I-Establishment and properties of two EBV strains (M81 and M72) produced by immortalized Callithrix jacchus lymphocytes. Biomedicine. 1976;25:349–352. [PubMed] [Google Scholar]
- 132.Wedderburn N, et al. Infectious mononucleosis-like response in common marmosets infected with Epstein-Barr virus. J. Infect. Dis. 1984;150:878–882. doi: 10.1093/infdis/150.6.878. [DOI] [PubMed] [Google Scholar]
- 133.Emini EA, et al. Establishment and characterization of a chronic infectious mononucleosislike syndrome in common marmosets. J. Med. Virol. 1986;18:369–379. doi: 10.1002/jmv.1890180410. [DOI] [PubMed] [Google Scholar]
- 134.Epstein MA, Hunt RD, Rabin H. Pilot experiments with EB virus in owl monkeys (Aotus trivirgatus). I Reticuloproliferative disease in an inoculated animal. Int. J. Cancer. 1973;12:309–318. doi: 10.1002/ijc.2910120202. [DOI] [PubMed] [Google Scholar]
- 135.Epstein MA, et al. Pilot experiments with EB virus in owl monkeys (Aotus trivirgatus). II. EB virus in a cell line from an animal with reticuloproliferative disease. Int. J. Cancer. 1973;12:319–332. doi: 10.1002/ijc.2910120203. [DOI] [PubMed] [Google Scholar]
- 136.Gu SY, et al. First EBV vaccine trial in humans using recombinant vaccinia virus expressing the major membrane antigen. Dev. Biol. Stand. 1995;84:171–177. [PubMed] [Google Scholar]
- 137.Moutschen M, et al. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein-Barr virus vaccine in healthy adults. Vaccine. 2007;25:4697–4705. doi: 10.1016/j.vaccine.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 138.Sokal EM, et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J. Infect. Dis. 2007;196:1749–1753. doi: 10.1086/523813. [DOI] [PubMed] [Google Scholar]
- 139.Rees L, et al. A phase I trial of epstein-barr virus gp350 vaccine for children with chronic kidney disease awaiting transplantation. Transplantation. 2009;88:1025–1029. doi: 10.1097/TP.0b013e3181b9d918. [DOI] [PubMed] [Google Scholar]
- 140.Elliott SL, et al. Phase I trial of a CD8+ T-cell peptide epitope-based vaccine for infectious mononucleosis. J. Virol. 2008;82:1448–1457. doi: 10.1128/JVI.01409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bharucha T, Ming D, Breuer J. A critical appraisal of ‘Shingrix’, a novel herpes zoster subunit vaccine (HZ/Su or GSK1437173A) for varicella zoster virus. Hum. Vaccin. Immunother. 2017;13:1789–1797. doi: 10.1080/21645515.2017.1317410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lanzavecchia A, Fruhwirth A, Perez L, Corti D. Antibody-guided vaccine design: identification of protective epitopes. Curr. Opin. Immunol. 2016;41:62–67. doi: 10.1016/j.coi.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 143.Cunningham AL, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N. Engl. J. Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 144.Moss P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022;23:186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 145.Li X, Wang X, Ito A. Tailoring inorganic nanoadjuvants towards next-generation vaccines. Chem. Soc. Rev. 2018;47:4954–4980. doi: 10.1039/C8CS00028J. [DOI] [PubMed] [Google Scholar]
- 146.Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. J. Biomed. Biotechnol. 2011;2011:954602. doi: 10.1155/2011/954602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rosendahl Huber S, van Beek J, de Jonge J, Luytjes W, van Baarle D. T cell responses to viral infections - opportunities for Peptide vaccination. Front. Immunol. 2014;5:171. doi: 10.3389/fimmu.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van Zyl DG, Mautner J, Delecluse HJ. Progress in EBV vaccines. Front. Oncol. 2019;9:104. doi: 10.3389/fonc.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein-Barr virus-induced disease. Annu. Rev. Immunol. 2015;33:787–821. doi: 10.1146/annurev-immunol-032414-112326. [DOI] [PubMed] [Google Scholar]
- 150.Hutt-Fletcher LM. EBV glycoproteins: where are we now? Future Virol. 2015;10:1155–1162. doi: 10.2217/fvl.15.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sapkota, B. et al. Heterologous prime-boost strategies for COVID-19 vaccines. J. Travel Med.10.1093/jtm/taab191 (2022). [DOI] [PMC free article] [PubMed]
- 153.Pal R, et al. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology. 2006;348:341–353. doi: 10.1016/j.virol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 154.Wang S, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26:1098–1110. doi: 10.1016/j.vaccine.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bansal A, et al. Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration. J. Virol. 2008;82:6458–6469. doi: 10.1128/JVI.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Amara RR, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.292.5514.69. [DOI] [PubMed] [Google Scholar]
- 157.Shiver JW, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 158.Sin JI, Bagarazzi M, Pachuk C, Weiner DB. DNA priming-protein boosting enhances both antigen-specific antibody and Th1-type cellular immune responses in a murine herpes simplex virus-2 gD vaccine model. DNA Cell Biol. 1999;18:771–779. doi: 10.1089/104454999314917. [DOI] [PubMed] [Google Scholar]
- 159.Lavelle EC, Ward RW. Mucosal vaccines - fortifying the frontiers. Nat. Rev. Immunol. 2022;22:236–250. doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu S, et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021;21:1654–1664. doi: 10.1016/S1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 162.Le, D. T. & Muller, K. M. In vitro assembly of virus-like particles and their applications. Life10.3390/life11040334 (2021). [DOI] [PMC free article] [PubMed]
- 163.Assis BRD, da Silva CD, Santiago MG, Ferreira LAM, Goulart GAC. Nanotechnology in adjuvants and vaccine development: what should we know? Nanomed. 2021;16:2565–2568. doi: 10.2217/nnm-2021-0360. [DOI] [PubMed] [Google Scholar]
- 164.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gutjahr, A. et al. Biodegradable polymeric nanoparticles-based vaccine adjuvants for lymph nodes targeting. Vaccines10.3390/vaccines4040034 (2016). [DOI] [PMC free article] [PubMed]
- 166.Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018;9:2224. doi: 10.3389/fimmu.2018.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zacharias ZR, et al. Polyanhydride nanovaccine induces robust pulmonary B and T cell immunity and confers protection against homologous and heterologous influenza A virus infections. Front. Immunol. 2018;9:1953. doi: 10.3389/fimmu.2018.01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qiao D, et al. Potency of a scalable nanoparticulate subunit vaccine. Nano Lett. 2018;18:3007–3016. doi: 10.1021/acs.nanolett.8b00478. [DOI] [PubMed] [Google Scholar]
- 169.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 170.Pulendran B, P SA, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Disco. 2021;20:454–475. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Imanishi T, Saito T. T cell co-stimulation and functional modulation by innate signals. Trends Immunol. 2020;41:200–212. doi: 10.1016/j.it.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 172.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 173.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat. Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr. Opin. Immunol. 2012;24:310–315. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McLellan JS, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McLellan JS, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang L, et al. A proof of concept for neutralizing antibody-guided vaccine design against SARS-CoV-2. Natl Sci. Rev. 2021;8:nwab053. doi: 10.1093/nsr/nwab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barnes CO, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Snijder J, et al. An antibody targeting the fusion machinery neutralizes dual-tropic infection and defines a site of vulnerability on Epstein-Barr virus. Immunity. 2018;48:799–811 e799. doi: 10.1016/j.immuni.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sathiyamoorthy K, et al. Inhibition of EBV-mediated membrane fusion by anti-gHgL antibodies. Proc. Natl Acad. Sci. USA. 2017;114:E8703–E8710. doi: 10.1073/pnas.1704661114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hong, J. et al. Antibody generation and immunogenicity analysis of EBV gp42 N-terminal region. Viruses10.3390/v13122380 (2021). [DOI] [PMC free article] [PubMed]
- 182.Zhang X, et al. Protective anti-gB neutralizing antibodies targeting two vulnerable sites for EBV-cell membrane fusion. Proc. Natl Acad. Sci. USA. 2022;119:e2202371119. doi: 10.1073/pnas.2202371119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N. Engl. J. Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tseng HF, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022;28:1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Andrews N, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou X, et al. A comprehensive risk score for effective risk stratification and screening of nasopharyngeal carcinoma. Nat. Commun. 2021;12:5189. doi: 10.1038/s41467-021-25402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Reynolds CJ, et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science. 2022;377:eabq1841. doi: 10.1126/science.abq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]